Abstract
Members of the solute carrier (SLC) 36 family are involved in transmembrane movement of amino acids and derivatives. SLC36 consists of four members. SLC36A1 and SLC36A2 both function as H+-coupled amino acid symporters. SLC36A1 is expressed at the luminal surface of the small intestine but is also commonly found in lysosomes in many cell types (including neurones), suggesting that it is a multipurpose carrier with distinct roles in different cells including absorption in the small intestine and as an efflux pathway following intralysosomal protein breakdown. SLC36A1 has a relatively low affinity (Km 1–10 mM) for its substrates, which include zwitterionic amino and imino acids, heterocyclic amino acids and amino acid-based drugs and derivatives used experimentally and/or clinically to treat epilepsy, schizophrenia, bacterial infections, hyperglycaemia and cancer. SLC36A2 is expressed at the apical surface of the human renal proximal tubule where it functions in the reabsorption of glycine, proline and hydroxyproline. SLC36A2 also transports amino acid derivatives but has a narrower substrate selectivity and higher affinity (Km 0.1–0.7 mM) than SLC36A1. Mutations in SLC36A2 lead to hyperglycinuria and iminoglycinuria. SLC36A3 is expressed only in testes and is an orphan transporter with no known function. SLC36A4 is widely distributed at the mRNA level and is a high-affinity (Km 2–3 µM) transporter for proline and tryptophan. We have much to learn about this family of transporters, but from current knowledge, it seems likely that their function will influence the pharmacokinetic profiles of amino acid-based drugs by mediating transport in both the small intestine and kidney.
LINKED ARTICLES
This article is part of a themed section on Transporters. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2011.164.issue-7
Keywords: SLC36, PAT1, PAT2, amino acid, drug transport, membrane transport, absorption, small intestine, kidney, proton
Introduction
To date, the Human Genome Organisation (HUGO) has categorized 378 membrane transporters into 51 solute carrier (SLC) families (Hediger et al., 2004). A transporter is assigned to a specific SLC family if it has 20% or more amino acid sequence identity to the other members of that family (Hediger et al., 2004). The ability to transport amino acids across cellular membranes is a fundamental function of all animal and plant cells as amino acids play important roles in many essential biological functions such as protein synthesis, neurotransmission, cell growth and nitrogen metabolism. Historically, amino acid transport systems have been identified and characterized functionally and thus named due to some key functional characteristic (such as substrate specificity, ion dependency or exchange properties) (Christensen, 1990; Palacin et al., 1998). More recently, amino acid transporters have been identified at the molecular level (Palacin et al., 1998; Bröer, 2008a,b;). Current analysis of the SLC families identifies, in mammals, 59 functional amino acid transport proteins that are grouped into 12 separate SLC families. Orphan transporters are present in many of these families, suggesting that the total number of mammalian amino acid transport proteins is actually much greater than the 59 identified to date.
The purpose of this review is to summarize current knowledge of the SLC36 family of amino acid transporters. This family consists of four members, two of which, SLC36A1 and SLC36A2, have been characterized in some detail. The SLC36A1 and SLC36A2 transporters are unusual both in their ion coupling and substrate selectivity. They function as H+/amino acid symporters, unlike the vast majority of mammalian amino acid transporters that function as exchangers or Na+/amino acid symporters. SLC36A1 is the most thoroughly characterized of the SLC36 transporters and is expressed at the luminal surface of the human small intestine. SLC36A1 transports a broad range of amino acids and many orally-active amino acid-based drugs and derivatives (including a large number of GABA- and proline-related compounds) (Table 1) that can be used experimentally and/or clinically to treat infections, diabetes, epilepsy, schizophrenia, homocystinuria, cancer cell growth (in vitro) and in photodynamic therapy, fluorescent diagnosis and fluorescent-guided resection of cancer. SLC36A2 is expressed at the apical membrane in the renal proximal tubule and is involved in reabsorption of amino acids and derivatives from the renal filtrate. Defects in SLC36A2 cause iminoglycinuria (excess urinary proline, hydroxyproline and glycine) and hyperglycinuria (isolated excess urinary glycine). It seems likely that function of both transporters will influence the pharmacokinetic profiles of amino acid-based drugs by mediating absorption in the small intestine and reabsorption in the kidney.
Table 1.
The SLC36 family of mammalian amino acid transporters
| Gene* | Protein | Aliases | Examples of natural substrates, drugs and related derivatives |
|---|---|---|---|
| SLC36A1 | PAT1 | imino acid carrier | Small, unbranched, zwitterionic α-, β- and γ-amino and imino acids; N-methylated amino acids; heterocyclic amino acids containing four- to six-membered rings. Substrates include glycine, proline, alanine, trans-4-hydroxy-proline, β-alanine, taurine, sarcosine, betaine, GABA, d-proline, d-alanine, d-cysteine, d-serine, MeAIB, AIB, β-ABA, nipecotic acid, guvacine, THPO, isonipecotic acid, muscimol, gaboxadol, vigabatrin, 1-aminocyclopropanecarboxylic acid, 3-amino-1-propanesulphonic acid, l-azetidine-2-carboxylic acid, 3,4-dehydro-d,l-proline, l- and d-cycloserine, 5-aminolevulinic acid, β-guanidinopropionic acid, and short-chain fatty acids (butyrate, acetate, propionate). |
| LYAAT-1 | |||
| Tramdorin 3 | |||
| SLC36A2 | PAT2 | Tramdorin 1 | Small, unbranched, dipolar amino and imino acids and heterocyclic amino acids containing four- to five-membered rings. Substrates include glycine, alanine, l- and d-proline, trans-4-hydroxy-proline, sarcosine, MeAIB, d- and l-cycloserine, l-azetidine-2-carboxylic acid, 3,4-dehydro-d,l-proline, and short-chain fatty acids (acetate, propionate, butyrate). |
| SLC36A3 | PAT3 | Tramdorin 2 | |
| SLC36A4 | PAT4 | LYAAT-2 | Proline, tryptophan |
The full list of SLC tables can be found at the HUGO Gene Nomenclature Committee (HGNC) Database at: http://www.bioparadigms.org/slc/intro.htm
Nomenclature
The first cDNA corresponding to a member of SLC36 (SLC36A1) was isolated from rat brain and named LYAAT1 (for lysosomal amino acid transporter 1) (Sagnéet al., 2001; Wreden et al., 2003). SLC36A1 has since been cloned from mouse (Boll et al., 2002), human (Chen et al., 2003a) and rabbit (Miyauchi et al., 2005), and the transport system is most commonly known as PAT1 for proton-coupled amino acid transporter 1 (see Table 1). The carrier was named PAT1 because it represents the molecular identification of a H+-coupled amino acid transporter, named system PAT, characterized previously at the brush-border membrane of monolayers of the human intestinal epithelial cell line Caco-2 (Thwaites et al., 1993a; 1993b; 1994; 1995a; 2000; Ranaldi et al., 1994; Thwaites and Stevens, 1999). System PAT has the same substrate specificity and relative selectivity as the imino acid carrier (Anderson et al., 2004), a transport system characterized at the luminal surface of the rat small intestine (Munck, 1966; Munck et al., 1994), suggesting that they represent the human and rat forms of the same carrier. The nomenclature in this field has been used inconsistently over the years, and the rat imino acid carrier has been known by many different names including the glycine–proline carrier, sarcosine carrier, system 4 and methionine-insensitive ‘sarcosine–glycine–proline’ system (Newey and Smyth, 1964; Wiseman, 1968; Daniels et al., 1969a,b; Thompson et al., 1970; De la Noüe et al., 1971). A detailed review of the physiology, function and history of SLC36A1, system PAT and the imino acid carrier, can be found in Thwaites and Anderson (2007a).
SLC36A2 was cloned from mouse (Boll et al., 2002), rat (Bermingham et al., 2002; Chen et al., 2003b) and human (Boll et al., 2003b). The most commonly used abbreviation is PAT2 (for proton-coupled amino acid transporter 2), although it has also been named Tramdorin 1 for transmembrane domain rich protein 1 (Table 1). The clearest evidence for a physiological role of PAT2 is in the reabsorption of amino acids in the renal proximal tubule as defective PAT2 function leads to the amino acid uria iminoglycinuria [Online Mendelian Inheritance in Man (OMIM) 242600] (Bröer et al., 2008). Prior to molecular identification, there was little physiological evidence for a PAT2-like transporter except that the functional characteristics of PAT2 are similar to a transport system detected in preparations (cerebral cortical slices, homogenates, synaptosomes) of rat brain (Johnston and Iversen, 1971; Logan and Snyder, 1971; Bennett et al., 1974; Rubio-Aliaga et al., 2004; Kennedy et al., 2005).
There are two additional members of the SLC36 family, SLC36A3 and SLC36A4 (Boll et al., 2003b; Chen et al., 2003b; Wreden et al., 2003; Bermingham and Pennington, 2004). SLC36A3 was cloned from mouse and named PAT3 (Boll et al., 2003b) and remains an orphan transporter with no known function. SLC36A4 was cloned from rat and named LYAAT2 (Wreden et al., 2003) but is more commonly known as PAT4 (see Table 1). PAT4 was actually one of the earliest SLC36 sequences to be identified from the mouse, rat and human genomes (Boll et al., 2003b; Chen et al., 2003b; Wreden et al., 2003). Nevertheless, despite intense study by a number of investigators, the transport function of PAT4 has remained unknown until recently. The results of a recent study (Pillai and Meredith, 2011) suggest that human PAT4 might also function as an amino acid transporter.
The use of abbreviations in the literature can unintentionally cause confusion for the reader as the PAT1 abbreviation is used to describe several other genes and proteins. For example, an anion exchanger within the SLC26 family (SLC26A6) is known not only as CFEX for chloride-formate exchanger (Knauf et al., 2001) but also PAT1 for putative anion transporter (Greeley et al., 2001). To prevent further confusion in the literature, we advocate the use of SLC names alongside the more commonly used transporter names and abbreviations.
Pharmacology and function
Amino acid and drug transport via members of the SLC36 family are usually measured by uptake of [3H] or [14C] labelled ligands (e.g. Thwaites et al., 1993a, 1995c; Sagnéet al., 2001; Boll et al., 2002; Chen et al., 2003a; 2003b; Wreden et al., 2003; Anderson et al., 2004). If a compound is unavailable in radiolabelled form, evidence for its transport via the carrier can be determined by the relative ability of the compound to cis-inhibit (when present in the ipsilateral compartment) and trans-stimulate (when present at the contralateral side of the membrane) the uptake of a standard substrate (e.g. Thwaites et al., 1995c; 2000; Boll et al., 2002; 2003a; Anderson et al., 2004, 2010; Foltz et al., 2004b; 2005; Kennedy et al., 2005; Metzner et al., 2006; Edwards et al., 2011).
Both PAT1 and PAT2 transport zwitterionic amino acids in a 1:1 stoichiometry with a H+ (Thwaites et al., 1994; Boll et al., 2002; Wreden et al., 2003), meaning that both carriers are rheogenic (i.e. able to generate current) and membrane potential sensitive. The movement of the coupling ion (in this case a H+) has been used to measure PAT1 function at the apical membrane of human intestinal epithelial Caco-2 cell monolayers where PAT1-mediated H+-influx has been measured as a decrease in pHi in cells loaded with the pH-sensitive dye 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF) (e.g. Thwaites et al., 1993a; 1995c; 2000; Anderson et al., 2004; Abbot et al., 2006); an increase in inward short-circuit current in voltage-clamped Caco-2 cell monolayers in Ussing chambers (e.g. Thwaites et al., 1993a,b; 1994; 1995b,c; 2000;); a change in membrane potential using a red membrane potential-sensitive dye (Metzner et al., 2005, 2006). In cultured isolated rat hippocampal neurones, PAT1 function has been detected using pH-sensitive green fluorescent protein (Wreden et al., 2003).
To allow detailed functional characterization of either PAT1 or PAT2 in isolation, the transporters have been expressed heterologously either in mammalian cell lines (e.g. COS-7, HRPE or HeLa cells) (Sagnéet al., 2001; Chen et al., 2003a; 2003b; Wreden et al., 2003; Miyauchi et al., 2005) or in Xenopus laevis oocytes (e.g. see Boll et al., 2002; 2003a; Chen et al., 2003b; Wreden et al., 2003; Anderson et al., 2004, 2009, 2010; Foltz et al., 2004a, 2005; Kennedy et al., 2005; Metzner et al., 2005; 2009; Abbot et al., 2006; Edwards et al., 2011). Expression in X. laevis oocytes allows detailed kinetic analysis of transporter function by measurement of substrate-induced inward current (H+ movement) using electrophysiological techniques such as two-electrode voltage-clamp (TEVC) or giant patch clamp (Boll et al., 2002; 2003a; Wreden et al., 2003; Anderson et al., 2004; 2010; Foltz et al., 2004a; 2005; Kennedy et al., 2005). H+ movement has also been measured using H+-sensitive intracellular electrodes in oocytes (Boll et al., 2002; Foltz et al., 2004a) or BCECF in PAT1-transfected HeLa cells (Wreden et al., 2003).
SLC36A1
PAT1 transport has been characterized using cDNA clones from human, rat, mouse and rabbit, and no marked differences in transport (substrate specificity or substrate affinity) have been observed between these orthologues. PAT1 is a H+-coupled, pH-dependent, low-affinity transporter (Km approximately 1–10 mM) of small, unbranched, zwitterionic α-, β- and γ-amino and imino acids; N-methylated amino acids; and heterocyclic amino acids containing four- to six-membered rings (Thwaites and Anderson, 2007a,b;). Substrates include glycine, proline, alanine, trans-4-hydroxy-proline, β-alanine, taurine, sarcosine, betaine and GABA; the d-amino acids d-proline, d-alanine, d-cysteine and d-serine; and the amino acid derivatives α-(methylamino)isobutyric acid (MeAIB), α-aminoisobutyric acid (AIB) and β-aminobutyric acid (β-ABA) (see Table 1) (Sagnéet al., 2001; Boll et al., 2002; 2003a; Chen et al., 2003a; Wreden et al., 2003; Anderson et al., 2004; 2009; Miyauchi et al., 2005; Abbot et al., 2006). This substrate specificity is identical to that described for system PAT at the apical membrane of human Caco-2 cell monolayers (Thwaites et al., 1993a; 1994; 1995b,c; Thwaites and Stevens, 1999; Anderson et al., 2004, 2009; Metzner et al., 2004, 2006) and the imino acid carrier at the luminal surface of the rat small intestine (Newey and Smyth, 1964; Munck, 1966; Wiseman, 1968; Daniels et al., 1969a,b; Thompson et al., 1970; De la Noüe et al., 1971; Munck et al., 1994; Anderson et al., 2004; Iñigo et al., 2006). A comprehensive review of the relationship between the imino acid carrier, system PAT and PAT1 can be found in Thwaites and Anderson (2007a). The most detailed investigations of the substrate specificity of this carrier can be found in Thwaites et al. (1995c), Boll et al. (2003a) and Metzner et al. (2006). In addition to rheogenic H+/zwitterionic amino acid transport, PAT1 can also function as an electroneutral transport system for H+ and fatty acids including acetate, propionate and butyrate (Foltz et al., 2004a).
The low-affinity, high-capacity, transport characteristics and relatively broad substrate specificity identify PAT1 as a potential pathway for transport of orally delivered hydrophilic amino acid-based drugs across the luminal surface of the human small intestinal wall, the initial barrier to effective drug delivery (Figure 1). In particular a number of GABA- and proline-related compounds have been identified as PAT1 substrates (Ranaldi et al., 1994; Thwaites et al., 1995a; 2000; Anderson et al., 2004; Metzner et al., 2004; 2006; 2009; Abbot et al., 2006; Larsen et al., 2008; 2009; 2010;). PAT1 substrates with therapeutic, or potential therapeutic, value include nipecotic acid, guvacine and THPO (GAT transporter inhibitors) (Thwaites et al., 2000; Anderson et al., 2004; Abbot et al., 2006; Larsen et al., 2008); isonipecotic acid, muscimol and gaboxadol (GABAA receptor agonists) (Thwaites et al., 2000; Anderson et al., 2004; Larsen et al., 2008; 2009; 2010;); vigabatrin (a GABA-transaminase inhibitor) (Abbot et al., 2006); 1-aminocyclopropanecarboxylic acid (a partial agonist for the NMDA receptor) (Thwaites et al., 2000); 3-amino-1-propanesulphonic acid (an anticraving agent) (Thwaites et al., 2000; Metzner et al., 2004); l-azetidine-2-carboxylic acid and 3,4-dehydro-d,l-proline (inhibitors of collagen synthesis) (Metzner et al., 2004, 2006); l-cycloserine (an inhibitor of cancer cell growth in vitro) (Anderson et al., 2004; Metzner et al., 2006); 5-aminolevulinic acid (used in photodynamic therapy, fluorescent diagnosis and fluorescent-guided resection of cancer) (Anderson et al., 2010); d-serine (used to treat schizophrenia) (Thwaites et al., 1995a,c; Boll et al., 2002; Chen et al., 2003a); d-cycloserine (used as an antibiotic and in the treatment of schizophrenia) (Ranaldi et al., 1994; Thwaites et al., 1995a; Anderson et al., 2004); betaine (used in the treatment of homocystinuria) (Thwaites et al., 1995c; Boll et al., 2003a); and β-guanidinopropionic acid (an anti-hyperglycaemic agent) (Metzner et al., 2009) (Figure 1; Table 1).
Figure 1.
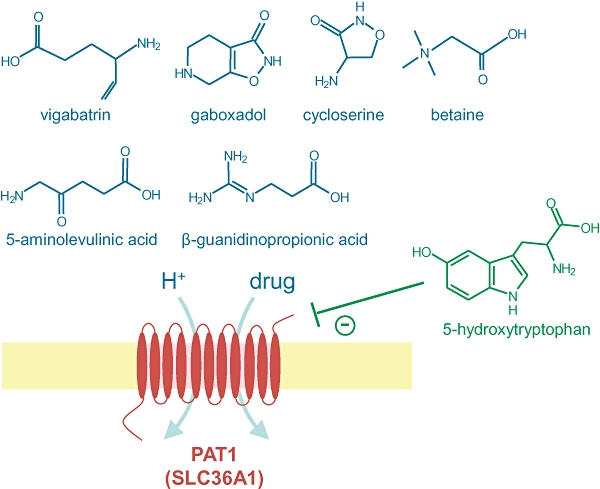
The H+-coupled amino acid transporter PAT1 (SLC36A1) can transport a number of compounds with potential therapeutic value (shown in blue). Several analogues of tryptophan such as 5-hydroxy-l-tryptophan (shown in green) are non-transported inhibitors of PAT1.
The compounds listed above are all transported substrates of PAT1 and will thus compete for transport. In contrast, Brandsch and colleagues identified that l-tryptophan and the derivatives tryptamine, 5-hydroxy-l-tryptophan, serotonin and indole-3-propionic acid are non-transported, competitive inhibitors of PAT1 (Ki 0.9–6.1 mM) (Figure 1) (Metzner et al., 2005). 5-Hydroxy-l-tryptophan has been used to identify the relative contribution of PAT1 and the di/tripeptide transporter PepT1 (SLC15A1) to uptake of 5-aminolevulinic acid at the apical membrane of human intestinal Caco-2 cell monolayers (Anderson et al., 2010). In addition, l-tryptophan and 5-hydroxy-l-tryptophan have been used in vivo in studies in dogs and rats, respectively, to reduce gaboxadol absorption (Larsen et al., 2009, 2010). These non-transported inhibitors are likely to prove highly useful tools to aid in the identification of the relative contribution of PAT1 to substrate transport in membranes and tissues expressing multiple transporters with overlapping substrate specificity. However, the absolute value of the compounds may be restricted to some degree as the affinity for PAT1 is relatively low and, as yet, it is not known how many other carriers may also be inhibited by these compounds. For example, another tryptophan derivative, α-methyl-d,l-tryptophan, acts as a relatively high affinity (IC50 250 µM) non-transported inhibitor of the amino acid transporter ATB0,+ (SLC6A14) (Karunakaran et al., 2008).
PAT1 can be considered as a low affinity multipurpose transporter with substrate specificity that overlaps with many of the SLC6 family of Na+- and Cl--dependent neurotransmitter transporters including GAT1 (SLC6A1), GlyT2 (SLC6A5), TauT (SLC6A6), PROT (SLC6A7), CT1 (SLC6A8), GlyT1 (SLC6A9), GAT3 (SLC6A11), BGT1 (SLC6A12), GAT2 (SLC6A13), ATB0,+ (SLC6A14), SBAT1 (SLC6A15), NTT4 (SLC6A17), XT2 (SLC6A18), B0AT1 (SLC6A19) and SIT1 (SLC6A20). In general, the SLC6 transporters are highly selective and have a much higher affinity for their substrates compared to PAT1. Therefore, we can speculate that the role of PAT1 is maybe to function as a high-capacity route for mass transfer of substrates in tissues where the high-affinity SLC6 carriers might be saturated. Evidence to date suggests that PAT1 is not generally co-expressed at the same membrane surfaces as SLC6 members except at the luminal surface of the small intestine where TauT, CT1, ATB0,+, B0AT1 and SIT1 are expressed to varying degrees. The relative roles of PAT1 and other carriers in neutral amino acid transport in the intestine and kidney are reviewed elsewhere (Thwaites and Anderson, 2007a; Bröer, 2008a,b;).
SLC36A2
PAT2, like PAT1, is a rheogenic H+-coupled, pH-dependent, transporter of small, unbranched, dipolar amino and imino acids but has a higher affinity (Km 0.1–0.7 mM) than PAT1 and has a relatively restricted substrate profile (Table 1). PAT2 substrates include glycine, alanine, l- and d-proline, trans-4-hydroxy-proline, sarcosine and the amino acid derivative MeAIB (Boll et al., 2002; Chen et al., 2003b; Rubio-Aliaga et al., 2004; Foltz et al., 2004b; Kennedy et al., 2005; Bröer et al., 2008; Edwards et al., 2011). Like PAT1, PAT2 also transports amino acids containing a free carboxyl group and small side chain but has a preference for the amino group in the α position, is more selective for l- over d-enantiomers and is less tolerant of N-methylation (Foltz et al., 2004b; Kennedy et al., 2005). The Km values estimated from transport measurements using the mouse, rat or human PAT2 transporters are almost identical. PAT2 transports a number of heterocyclic compounds with four- or five-membered ring structures including some with potential in therapy such as d-cycloserine, l-cycloserine, l-azetidine-2-carboxylic acid and 3,4-dehydro-d,l-proline, all mentioned above (Table 1) (Kennedy et al., 2005; Edwards et al., 2011). Like PAT1, PAT2 can also function in an electroneutral mode whereby it cotransports a H+ and a short-chain fatty acid (such as acetate, propionate or butyrate) but with relatively low affinity (Foltz et al., 2004a). As observed with PAT1, a number of tryptophan derivatives can also inhibit PAT2 in a non-transported manner, with 5-hydroxy-l-tryptophan and α-methyl-d,l-tryptophan having the greatest affinities (IC50 1.6 and 3.5 mM respectively) (Edwards et al., 2011). Although relatively low affinity inhibitors, these two tryptophan derivatives should help discriminate between PAT1 and PAT2 transport, as 5-hydroxy-l-tryptophan has a preference for PAT1, whereas the two compounds inhibit PAT2 with similar potency (Edwards et al., 2011). The most detailed investigations of the substrate specificity of PAT2 can be found in Boll et al. (2002), Foltz et al. (2004b), Kennedy et al. (2005) and Edwards et al. (2011).
In a similar manner to PAT1 in the small intestine, the function of PAT2 in the renal proximal tubule is likely to overlap to some extent with members of the SLC6 family including XT2 (SLC6A18), B0AT1 (SLC6A19) and SIT1 (SLC6A20), all of which are likely to function in the reabsorption of glycine and imino acids from the renal filtrate.
SLC36A4
PAT4 function has not been characterized in as much detail as PAT1 and PAT2. However, PAT4 appears to function by facilitated diffusion in an electroneutral, Na+-independent, manner with a relatively high affinity for its substrates proline (Km 3 µM) and tryptophan (Km 2 µM). The pH dependence is distinct from PAT1 and PAT2, with transport being maximal at pH 7.4 and reduced at pH 5.5 and pH 8.4 (Pillai and Meredith, 2011).
Distribution: localization and function
SLC36A1
As determined by Northern blot and RT-PCR, PAT1 mRNA expression appears to be ubiquitous in human (Chen et al., 2003a; Anderson et al., 2004, 2009; Bermingham and Pennington, 2004), rat (Sagnéet al., 2001), mouse (Boll et al., 2002; 2003b;) and rabbit (Miyauchi et al., 2005) tissues. High levels of expression are found in both small intestine and brain in human and mouse (Boll et al., 2002; Chen et al., 2003a; Bermingham and Pennington, 2004). PAT1 mRNA was expressed in all paired normal and tumour samples from colonic tissue from 18 patients (Anderson et al., 2010). PAT1 mRNA is expressed in neurones in most areas of the rat CNS (Agulhon et al., 2003). In situ hybridization in rat brain identified the highest PAT1 mRNA expression in the cortex, thalamus, pyramidal cells of the hippocampus and Purkinje cells of the cerebellum, suggesting that PAT1 is expressed in both glutamatergic and GABAergic neurones (Sagnéet al., 2001; Wreden et al., 2003). For a detailed description of PAT1 mRNA distribution in the brain, see Agulhon et al. (2003).
PAT1 protein is expressed at the brush-border membrane in human small intestine, rat small intestine and human intestinal epithelial Caco-2 cell monolayers (Chen et al., 2003a; Anderson et al., 2004). In contrast, in the mouse kidney, PAT1 protein is found in sub-apical compartments within the S1 segment of the proximal tubule (Vanslambrouck et al., 2010). This intracellular localization is consistent with the earlier demonstration of predominant lysosomal localization in neuronal tissues in rat brain, although PAT1 immunoreactivity was also found in other intracellular organelles (e.g. Golgi, endoplasmic reticulum) and occasionally the plasma membrane (Sagnéet al., 2001; Agulhon et al., 2003). In rat hippocampal neurones in culture, PAT1 immunoreactivity is also colocalized at the plasma membrane with markers for the exocyst (a complex involved in targeting and tethering secretory vesicles to the plasma membrane) (Wreden et al., 2003). Lysosomal localization of PAT1 is also observed in the mouse hypothalamic GT1-7 and neuroblastoma N2a cell lines (Pimpinelli et al., 2005). In human macrophages and NIH3T3 cells, PAT1 colocalized with the haematopoietic cell kinase p61Hck in lysosomes and also, partially, in podosomes (Cougoule et al., 2005). In PAT1-transfected cells (HeLa, PC12 and COS7), PAT1 colocalizes with the lysosomal protein LAMP1 (Wreden et al., 2003).
A role for PAT1 in amino acid and drug absorption across the small intestinal wall is supported by evidence for PAT1 function at the brush-border membrane from studies in human Caco-2 cell monolayers (Nicklin et al., 1992; Thwaites et al., 1993a,b; 1994; 1995a; 2000; Ranaldi et al., 1994; Thwaites and Stevens, 1999; Chen et al., 2003a; Boll et al., 2003a; Anderson et al., 2004; 2009; 2010; Metzner et al., 2004; 2005; 2006; 2009; Anderson and Thwaites, 2005; 2007; Abbot et al., 2006; Larsen et al., 2008; 2009;), rat small intestine (Newey and Smyth, 1964; Munck, 1966; Wiseman, 1968; Daniels et al., 1969a,b; Thompson et al., 1970; De la Noüe et al., 1971; Munck et al., 1994; Anderson et al., 2004; Iñigo et al., 2006), lizard duodenum (Díaz et al., 2000) and eel intestine (Ingrosso et al., 2000); for a review, see Thwaites and Anderson (2007a). However, PAT1 may not be functional at the apical membrane of rabbit and guinea pig small intestine (Anderson et al., 2004; for review of species differences in intestinal amino acid absorption, see Munck and Munck, 1994; Thwaites and Anderson, 2007a).
There is evidence for PAT1-like transport in brush-border membrane vesicles (BBMV) prepared from rabbit renal pars convoluta (Røigaard-Petersen and Sheikh, 1984; Rajendran et al., 1987; Røigaard-Petersen et al., 1987; 1989; 1990; Jessen et al., 1988a,b; 1989; 1991; Jessen and Sheikh, 1991; Wunz and Wright, 1993; Miyauchi et al., 2005), although these observations are discussed below in more detail in relation to the recent identification of PAT2 function in the mouse and human kidney (Bröer et al., 2008; Vanslambrouck et al., 2010).
PAT1 function has been measured at the plasma membrane of dissociated rat hippocampal neurones in culture, suggesting that it could play a role in neurotransmitter transport in the brain (Wreden et al., 2003). However, the dominant lysosomal localization in most neurones, and other cells, points to a more general role in efflux of lysosomal proteolysis products into the cytosol (Sagnéet al., 2001).
PAT1 is thought to be involved in the control of growth and proliferation. Over expression of human PAT1 in the differentiating eye of Drosophila melanogaster leads to an increase in ommatidial size (Heublein et al., 2010), as observed previously with PAT-like Drosophila transporters (Goberdhan et al., 2005). In the human MCF-7 and HEK-293 cell lines, PAT1 protein is intracellular and is partially associated with an endosomal compartment (Heublein et al., 2010). siRNA knockdown of PAT1 expression decreased cell proliferation possibly through a decrease in mTORC1 signalling, whereas overexpression of PAT1 led to an increased rate of proliferation. Thus, an additional proposed function of PAT1 is as an intracellular amino acid sensor, similar observations being made with human PAT4 (Heublein et al., 2010).
SLC36A2
PAT2 mRNA expression, as detected by Northern blot, RT-PCR and qPCR, is less widespread than PAT1 but is detected in heart, lung, kidney, skeletal muscle, spleen and brain in mouse (Boll et al., 2002; 2003b; Rubio-Aliaga et al., 2004; Vanslambrouck et al., 2010); adipose tissue, spleen, lung, skeletal muscle, kidney, heart and brain in rat (Chen et al., 2003b; Sundberg et al., 2008); and kidney and skeletal muscle in human (Bermingham and Pennington, 2004; Nishimura and Naito, 2005). In situ hybridization localized PAT2 mRNA to bone marrow and fat pad in mouse (Bermingham and Pennington, 2004).
PAT2 immunoreactivity has been detected at the brush-border membrane of the S1 segment of the proximal tubule in human kidney (Bröer et al., 2008) and along the length of the proximal tubule (S1–S3 segments) in mouse (Vanslambrouck et al., 2010). PAT2 protein has been localized to paranodes and incisures in myelinated fibres of rat sciatic nerve (Bermingham et al., 2002). In mouse spinal cord and brain, PAT2 protein was absent from lysosomes but was detected in endoplasmic reticulum, recycling endosomes and other cellular compartments including the plasma membrane (Rubio-Aliaga et al., 2004). Transfection of a C-terminally tagged version of mouse PAT2 in HeLa cells led to PAT2 expression at the plasma membrane and a perinuclear, possibly endosomal, compartment whereas PAT2 was absent from lysosomes demonstrating a distinct sub-cellular distribution compared with PAT1 (Boll et al., 2002). Bermingham and colleagues have suggested that this distinct distribution is due to the presence of a lysosomal targeting motif in PAT1, which is absent in PAT2 (Bermingham et al., 2002).
There is less evidence for endogenous PAT2 function, compared with PAT1, although, as stated above, the transport characteristics of PAT2 (ion dependency, substrate specificity, substrate affinity) are very similar to those described in a series of studies using preparations of rat cerebral cortex and spinal cord (Johnston and Iversen, 1971; Logan and Snyder, 1971; Bennett et al., 1974; see discussion sections in Rubio-Aliaga et al., 2004; Kennedy et al., 2005).
The loss or reduction of function observed in human PAT2 patients with iminoglycinuria is, at least in part, responsible for the increased loss of glycine, proline and hydroxyproline in urine, suggesting that an important role of PAT2 in humans is in the recovery of amino acids from the renal filtrate (Bröer et al., 2008). This is supported by the immunocytochemical localization of PAT2 protein to the apical membrane of renal proximal tubule in both human and mouse (Bröer et al., 2008; Vanslambrouck et al., 2010).
A series of studies using BBMV prepared from the pars convoluta of the rabbit kidney have identified function of a PAT-like transport system indicative of a role in the renal reabsorption of amino acids (Røigaard-Petersen and Sheikh, 1984; Rajendran et al., 1987; Røigaard-Petersen et al., 1987; 1989; 1990; Jessen et al., 1988a,b; 1989; 1991; Jessen and Sheikh, 1991; Wunz and Wright, 1993; Miyauchi et al., 2005). The ion dependency is similar to that observed for both PAT1 and PAT2, but the substrate specificity and substrate affinity (Km 2.8–9.7 mM) are more like PAT1 than PAT2. Indeed, for a number of years, it has been considered that these studies were reporting transport via the same carrier (PAT1) characterized in human intestinal Caco-2 cell monolayers (for review, see Thwaites and Anderson, 2007a). However, the recent studies of PAT2 by Bröer and colleagues (Bröer et al., 2008; Vanslambrouck et al., 2010) emphasize the need to revaluate the rabbit renal BBMV studies. It is clear that in human and mouse, PAT2 is localized at the proximal tubule brush-border, whereas in mouse, PAT1 has a sub-cellular localization. The reason for this apparent discrepancy between the different studies is not clear but could be due to a number of reasons. It is possible that the expression of PAT1 and PAT2 in the proximal tubule show variation in different species such that PAT2 may be the carrier in human and mouse, whereas PAT1 fulfils the same function in rabbit. It is also possible that the BBMV preparations used in the rabbit studies were contaminated with a PAT1-containing sub-cellular membrane. However, if that is the case, it might be expected that similar PAT1 contamination would be observed in the studies using BBMV prepared from the pars recta, but no PAT-like function was observed in those BBMV (e.g. Jessen et al., 1988a; 1988b; 1989; 1991; Jessen and Sheikh, 1991). It may be that PAT1 is localized exclusively within a sub-cellular compartment only within the S1 segment, and this possibility is not inconsistent with the observations in mouse kidney by Vanslambrouck et al. (2010). The lack of apparent PAT2 function in the rabbit BBMV studies could be because PAT2 is not expressed at the brush-border in rabbit proximal tubule, or that the transport system could not be detected under the experimental conditions used. These possibilities require further study, but it seems clear that in both human and mouse, it is PAT2, and not PAT1, that is involved in the renal reabsorption of glycine, proline and hydroxyproline (Bröer et al., 2008; Vanslambrouck et al., 2010).
SLC36A3
PAT3 mRNA (as detected by Northern blot and RT-PCR) is exclusively expressed in the testes in human (Bermingham and Pennington, 2004), mouse (Boll et al., 2003b) and rat (Sundberg et al., 2008).
SLC36A4
PAT4 mRNA expression appears to be ubiquitous in rat and human tissues (Nishimura and Naito, 2005; Sundberg et al., 2008) and human cancer cell lines (Heublein et al., 2010).
SLC36-related transporters in non-mammalian organisms
Although there are four SLC36 members in all mammalian genomes investigated thus far, there are greater numbers of SLC36-like genes in the genomes of some other organisms. Both Sagnéet al. (2001) and Boll et al. (2003b) noted that there are a number of predicted PAT-like proteins in the fruit fly D. melanogaster. There are 11 members of the Drosophila PAT family, and the predicted proteins show 23–40% identity with human PAT1–4. Two of the Drosophila PAT-like transporters have been characterized following expression in Xenopus oocytes (Goberdhan et al., 2005). Like mammalian forms of PAT1 and PAT2, one of the Drosophila transporters, CG1139 or PAT, transported alanine, glycine and proline, transport being increased by a decrease in extracellular pH. The affinity of CG1139 (Km 1.2–8.0 mM) was similar to mammalian forms of PAT1 (Goberdhan et al., 2005). CG1139 mRNA (as detected by Northern blot) is enriched within the Drosophila body, suggesting that, like human PAT1, this PAT transporter could be expressed in the gut (Romero-Calderón et al., 2007). In contrast, mRNA for the second transporter investigated, CG3424 (or PATH, for pathetic as a mutation led to reduced growth in flies), showed a ubiquitous expression, and in situ data, available through the Berkeley Drosophila Genome Project (BDGP), demonstrate expression in brain, muscle, the endocrine system and trachea (Romero-Calderón et al., 2007). PATH showed different transport characteristics (alanine transport decreased as extracellular pH was lowered) and a higher affinity (Km 3 µM) compared with PAT1 and PAT2 but does have some similarity with PAT4. Modulation of both CG1139 and PATH expression in Drosophila is characterized by changes in eye and wing size, suggesting a role for the carriers in control of cell growth (Goberdhan et al., 2005).
Following a blood meal, there is a change in mRNA levels in the midgut of the mosquito Aedes aegypti (Sanders et al., 2003). Microarray analysis indicated an increase in expression of mRNA for AAEL007191, and this was confirmed by qPCR, which demonstrates a 16-fold increase in mRNA at 3 and 24 h post feeding (Evans et al., 2009). Injection of this mRNA into Xenopus oocytes led to expression of a PAT-like amino acid transporter named AePAT1. Like mammalian forms of PAT1, AePAT1 is a low-affinity, Na+-independent, pH-dependent, rheogenic H+/amino acid symporter, but the substrate selectivity is distinct from mammalian PAT1 as AePAT1 transports glutamine (Km 7.2 mM), tryptophan, serine and cysteine as well as the mammalian PAT1 substrate proline (Evans et al., 2009). However, despite some functional differences, AePAT1 may well perform a similar function to human PAT1 in absorption of amino acids from diet, as AePAT1 protein is expressed at the apical membrane in both A. aegypti adult midgut (both male and female) and larval caecae. Western blot analysis of protein from adult midgut identifies a single band of approximately 51 kDa, which is similar to the predicted size of 53 kDa (Evans et al., 2009). In females, a blood meal is required to initiate vitellogenesis, and it is likely that AePAT1 is important in providing nutrients for egg production and mosquito survival (Evans et al., 2009).
It is worth emphasizing that there are many more PAT-like transporters, or genes, in invertebrates such as D. melanogaster and A. aegypti than the four found in mammals (Sagnéet al., 2001; Boll et al., 2003b; Goberdhan et al., 2005; Evans et al., 2009). Currently, we do not know whether any of the invertebrate transporters share the exact same functional characteristics as the mammalian transporters as, at present, only six different PAT-like transporters have been identified functionally (Table 2). Therefore, we do need to take care when assigning names and numbers to these transporters to avoid creating confusion in the literature. For example, AePAT1 functions in a similar way to mammalian forms of PAT1 and is expressed in a similar location in the gut. However, there are also marked differences in the substrate specificity, and we do not yet know whether or not any of the other mosquito transporters behave more like mammalian PAT1 than AePAT1.
Table 2.
Functional PAT-related solute transporters in mammals and other animals
| Protein | Species | Gene | Transport classification database ID* |
|---|---|---|---|
| PAT1 | Rat, mouse, human, rabbit | SLC36A1 | 2.A.18.8.1 |
| PAT2 | Mouse, rat, human | SLC36A2 | 2.A.18.8.2 |
| PAT4 | Human | SLC36A4 | |
| PAT | Drosophila melanogaster | CG1139 | |
| PATH | Drosophila melanogaster | CG3424 | 2.A.18.8.3 |
| AePAT1 | Aedes aegypti | AAEL007191 |
Transport Classification Database (Saier et al., 2006) at: http://www.tcdb.org/
Regulation
Acute influences on transporter activity
The function of any ion-coupled cotransporter that is driven by transmembrane ionic gradients depends exquisitely upon the ionic composition within the local microdomains bathing either side of the cell membrane in which the protein is resident. Thus, the function of a transport system, such as PAT1, which is driven by the H+-electrochemical gradient, will depend upon the local pH (chemical H+ gradient) at both intracellular and extracellular faces of the membrane and the membrane potential (electrical gradient) across that membrane. Activity of the transporter will thus be modulated rapidly by changes in either pH or membrane potential.
SLC36A1
The activity of PAT1 at the apical membrane of Caco-2 cell monolayers increases as the apical (extracellular) pH is reduced (e.g. Thwaites et al., 1993a, 1995c; Thwaites and Stevens, 1999). When PAT1 (rat, mouse, human and rabbit forms) is expressed heterologously it functions as a pH-dependent, H+-coupled, Na+-independent amino acid transporter (Sagnéet al., 2001; Boll et al., 2002; Chen et al., 2003a; Miyauchi et al., 2005). Clearly, the maintenance of the driving force (H+-electrochemical gradient) for PAT1 function is important as a reduction, through use of either the NH4Cl prepulse-and-release manoeuvre (which will acidify the cell) (Thwaites and Stevens, 1999) or the protonophore nigericin (which will dissipate the gradient) (Sagnéet al., 2001; Wreden et al., 2003), will reduce transport. The relationship between PAT1 function, pH and the driving force is, however, complex. A detailed investigation by Daniel and colleagues (Foltz et al., 2005) using mouse PAT1 expressed in Xenopus oocytes indicates that the H+ binds first to the transport protein, which increases the affinity for the amino acid substrate. Hyperpolarization of the membrane potential increases substrate affinity and maximal transport. In addition, a decrease in extracellular pH decreases the Km value for amino acid substrate binding (Foltz et al., 2005). Therefore, both proton concentration and membrane potential control the affinity of the PAT1 transport protein for its substrates. PAT1 can also function bidirectionally, and the direction of movement will depend upon the local H+ and substrate gradients, with the transporter having a greater affinity for substrates in the influx mode (Foltz et al., 2005).
In human Caco-2 cell monolayers, PAT1-mediated amino acid-coupled H+-influx across the apical membrane leads to an intracellular acidification. This apical acidification activates the apical Na+/H+ exchanger NHE3 (SLC9A3), which recycles protons back across the apical membrane to maintain pHi and the H+-electrochemical gradient (Thwaites et al., 1994; 1995a; 1999; 2000; 2002;). This functional coupling with NHE3 (known as ‘functional cooperativity’) means that in intact epithelia, such as human Caco-2 cells or rat small intestine, the Na+-independent PAT1 becomes partially dependent upon extracellular Na+ (Munck et al., 1994; Anderson et al., 2004) as NHE3 cannot function in the absence of Na+. Thus, any factor that decreases NHE3 activity will indirectly inhibit PAT1 function by decreasing maximal transport that, in turn, will ultimately reduce the intestinal epithelial absorptive capacity for amino acids and amino acid-based therapeutics (Anderson et al., 2004; Anderson and Thwaites, 2005, 2007; for review, see Thwaites and Anderson, 2007a,b; Anderson and Thwaites, 2010). Such a reduction in PAT1 capacity is observed at the apical surface of Caco-2 cell monolayers in the presence of a variety of factors that inhibit NHE3 including the selective NHE3 inhibitor 3-[2-(3-guanidino-2-methyl-3-oxo-propenyl)-phenyl]-N-isopropylidene-2-methyl-acrylamide dihydro-chloride (S1611; Schwark et al., 1998) and the amiloride analogue 5-(N-ethyl-N-isopropyl)-amiloride (EIPA), and any factor that will increase [cAMP]i including forskolin, 8-bromoadenosine-3′,5′-cyclic monophosphate (8-br-cAMP), the neurotransmitters vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP), and phosphodiesterase inhibitors such as caffeine (Anderson et al., 2004; Anderson and Thwaites, 2005, 2007).
SLC36A2
The pH dependence of PAT2 transport is distinct from that of PAT1 (Boll et al., 2002; Kennedy et al., 2005). Half-maximal transport via PAT2 is observed at extracellular pH values as high as pH 7.4–9.3 (Boll et al., 2002; Rubio-Aliaga et al., 2004; Foltz et al., 2004b; Kennedy et al., 2005), suggesting that maximal transport is not likely to be altered greatly by changes within the physiological pH range. Foltz et al. (2004b) investigated the pH dependence and membrane potential sensitivity of mouse PAT2 in some detail, and the greatest influence on PAT2 appears to be the effect of changes in membrane voltage where a large decrease in proton binding affinity is observed as the membrane is depolarized from −120 to −20 mV (Foltz et al., 2004b). In addition, a decrease in substrate affinity was observed as extracellular pH was increased to neutral and alkaline pH values when membrane potential was held at −60 mV (Foltz et al., 2004b). The extracellular pH in the proximal tubule can be as low as pH 6.7–6.8 (Vieira and Malnic, 1968), suggesting that the transporter may function relatively independently of pH at these values but will be modulated greatly by changes in membrane voltage (Foltz et al., 2004b). As observed with PAT1, PAT2 can also function bidirectionally, and the direction of movement (influx or efflux) will depend upon the prevailing substrate concentration gradient, pH gradient and membrane potential (Rubio-Aliaga et al., 2004; Kennedy et al., 2005; Edwards et al., 2011).
Influences on gene expression of the transporter(s)
SLC36A2
This is an area where there is relatively little information in the literature, although a few studies have identified regulated expression of PAT2. The POU domain transcription factor pou3f1 is required for normal differentiation of myelinating Schwann cells. The PAT2 gene is one of six genes that are down-regulated in the sciatic nerves of pou3f1-null mice, suggesting a role for PAT2 in amino acid transport in differentiating Schwann cells (Bermingham et al., 2002).
Developmental iminoglycinuria is common in infant mammals and is associated with hyperexcretion of glycine, proline and hydroxyproline. This failure to adequately reabsorb filtered amino acids in the renal proximal tubule is due to the delayed expression of a number of amino acid transporters including PAT2, XT2 (SLC6A18), B0AT1 (SLC6A19) and SIT1 (SLC6A20) (Vanslambrouck et al., 2010). In developing mouse kidney, there is a 5.3-fold increase in PAT2 mRNA expression (as determined by qPCR) between birth and day 7. The level of PAT2 mRNA then decreases at day 14 (20% of the level at day 7) before recovering to adult levels (Vanslambrouck et al., 2010).
Embryo–maternal communication is critical in the preparation of the uterus for implantation of the developing conceptus. In a study investigating endometrial tissues from non-pregnant and pregnant (pre-implantation) horses, PAT2 was one of the most highly up-regulated genes (15.5-fold), suggesting that PAT2 has an important role to play in nutrient supply in early embryo development (Klein et al., 2010).
SLC36A4
In rat brainstem, PAT4 mRNA levels decrease following either a 24 h starvation or a 3 day treatment with l-DOPA (Sundberg et al., 2008).
Biochemistry and genetics
In all mammalian genomes investigated thus far, there are four SLC36-related genes. In each genome, the genes for PAT1 (SLC36A1), PAT2 (SLC36A2) and PAT3 (SLC36A3) are clustered together (e.g. on human chromosome 5q33.1), whereas the gene for PAT4 (SLC36A4) has a distinct location (human chromosome 11q21) (Boll et al., 2003b; Chen et al., 2003b; Bermingham and Pennington, 2004; Thwaites and Anderson, 2007a). The gene cluster, when considered alongside the observation that the coding region for each of the three genes SLC36A1, SLC36A2 and SLC36A3 is each contained within 10 exons, suggests that the transporter genes have diverged from a common ancestor (Boll et al., 2003b; Chen et al., 2003b).
The human PAT proteins are 476 (PAT1), 483 (PAT2), 470 (PAT3) and 504 (PAT4) amino acids in length. Over the aligned regions, the human PAT1 sequence shares with its paralogues approximately 73% identity (84% similarity) with PAT2, 67% identity (83% similarity) with PAT3 and 53% similarity (70% identity) with PAT4. A high degree of likeness (78–88% identity, 88–94% similarity) is observed between human, mouse and rat orthologues over the aligned regions.
PAT1 and PAT2 are both predicted to have 11 transmembrane domains, each with a cytosolic N-terminus. The C-terminus of each transport protein will have an extracellular location when expressed at the plasma membrane and an intravesicular location when expressed at the membranes of intracellular organelles such as lysosomes and endosomes.
SLC36A1
A number of amino acid residues within the PAT1 sequence at sites likely to be involved in either proton sensitivity (histidine residues) or post-translational modification by N-glycosylation (asparagine residues) were predicted by Boll et al. (2003b) and Bermingham and Pennington (2004). These predictions have been tested by Brandsch and colleagues using site-directed mutagenesis of human PAT1 (Metzner et al., 2008; Dorn et al., 2009a). Mutation of His55, which is conserved in human PAT1–4, to either Ala or Asn resulted in loss of PAT1 activity, suggesting that His55 is obligatory for PAT1 function (Metzner et al., 2008). This histidine residue (His55) is predicted to lie close to the intracellular surface of transmembrane domain 1 within the PAT1 protein, and it is proposed to be involved in binding and translocation of the H+ during H+/amino acid cotransport (Metzner et al., 2008).
N-glycosylation can be important in correct membrane targeting of proteins. Following expression in Xenopus oocytes, PAT1 protein was found to be glycosylated at three sites, Asn174 and Asn183 in the second extracellular loop (between transmembrane domains 3 and 4) and Asn470 located towards the C-terminus (Dorn et al., 2009a). Glycosylation was lost when the asparagine residues were substituted with glutamine, but there was no reduction in PAT1 transport in the mutants containing a single substitution. In the triple Asn174/183/470Gln PAT1 mutant, transport was reduced to 29% of wild type. The similar decrease in plasma membrane PAT1 protein levels suggests that the loss of transport is due to decreased PAT1 abundance rather than any effect on transporter function per se (Dorn et al., 2009a). Western blot analysis of an N-terminally HA-tagged human PAT1 protein recovered from injected Xenopus oocytes identifies a single band of approximately 50 kDa that is reduced to approximately 45 kDa following PNGase F treatment (Dorn et al., 2009a). These observations are broadly consistent with the earlier study by Wreden and colleagues, which demonstrated the presence of a number of bands in protein from rat PAT1-transfected HeLa cells that were absent from the control cells (Wreden et al., 2003). A strong band was observed at approximately 55 kDa and a weaker band at approximately 45 kDa. In addition, in protein from rat PAT1-transfected HeLa cells, a smear is observed around 75 kDa (Wreden et al., 2003), which suggests that PAT1 may undergo greater glycosylation when expressed in human cells compared to Xenopus oocytes.
Once resident within the membrane some membrane proteins are stabilized by disulphide bond formation. Dorn et al. (2009b) have demonstrated that the function, but not membrane localization, of human PAT1 is dependent upon the formation of a disulphide bridge between Cys180 in the second extracellular loop (between transmembrane domains 3 and 4) and Cys329 in the fourth extracellular loop (between transmembrane domains 7 and 8). Formation of this bridge likely stabilizes the substrate binding site of PAT1 (Dorn et al., 2009b).
Relatively little is known about the effects of natural mutations or single nucleotide polymorphisms on function of the members of the SLC36 family. However, a single change in the second exon of the horse PAT1 gene produces a Thr63Arg change in horse PAT1 (contained within transmembrane domain 1), which is responsible for a change in skin pigmentation known as the champagne dilution phenotype (Cook et al., 2008). The effect on horse PAT1 function of the Thr63Arg mutation is not known, although its location within transmembrane domain 1 suggests that it will lie close to the substrate binding pocket.
SLC36A2
Less is known about the importance of specific amino residues in PAT2 function and localization. Western blot analysis of protein from both rat sciatic nerve (Bermingham et al., 2002) and mouse brain (Rubio-Aliaga et al., 2004) using PAT2-specific antisera identify a single band approximately 52–55 kDa in size. Although this band is consistent with the predicted size of the unglycosylated protein, no deglycosylation measurements have been made, so it is unclear as to whether the PAT2 protein is glycosylated or not. Examination of protein from mouse PAT2-transfected HEK293 cells reveals specific bands at both 52 and 45 kDa (Bermingham et al., 2002), which could be interpreted as representing both glycosylated and unglycosylated forms of the protein. It could be that the relative abundance of the glycosylated (relative to the unglycosylated) form is much greater in normal tissues compared with transfected cells, which could reflect a different pattern of sub-cellular distribution of the PAT2 protein.
In patients with the inherited renal disorder iminoglycinuria (excess urinary proline, hydroxyproline and glycine), two changes in the human PAT2 gene have been shown to produce altered proteins (Bröer et al., 2008). A single mutation in the PAT2 gene produces a PAT2 transport protein with a Gly87Val substitution in transmembrane domain 2. This Gly87Val PAT2 had reduced transport activity versus wild-type PAT2 due to an increase in Km values for both proline (from 140 to 390 µM) and glycine (from 490 to 2350 µM), whereas maximal transport capacity and membrane expression are unaffected (Bröer et al., 2008). It is estimated that homozygosity for this mutation will occur at a frequency of 1:6900. In a separate pedigree, a splice donor site mutation in the first intron of the PAT2 gene was identified, which produced a non-functional truncated protein (Bröer et al., 2008). Homozygosity for this mutation will occur at approximately 1:62 500.
SLC36A4
A single study using a PAT4-specific antiserum has demonstrated the presence of several bands in protein from rat PAT4-transfected HeLa cells with the strongest band at approximately 55 kDa, consistent with the predicted size of the rat PAT4 protein (Wreden et al., 2003).
Pathology and clinical significance
Transporter dysfunction and its impact
SLC36A1
The champagne dilution phenotype in horses is characterized by a dilution of hair colour intensity in both red and black pigments such that horse skin colour changes from chestnut to gold champagne, bay to amber champagne and black to classic champagne (Cook et al., 2008). In the horses expressing the PAT1 variant, changes were observed in eye, skin and hair pigmentation (Cook et al., 2008). Further investigation is required to identify the role of the PAT1 mutation in this change.
SLC36A2
It has been proposed that three transport systems are responsible predominantly for the reabsorption of glycine and imino acids in the renal proximal tubule (Lasley and Scriver, 1979), and that defects in one or more of these carriers would be responsible for iminoglycinuria (excess urinary proline, hydroxyproline and glycine) and/or hyperglycinuria (OMIM 138500) (isolated excess urinary glycine). The suggestion that these transport systems would include a common transport system as well as specific transport systems for glycine and imino acids seems consistent with current knowledge with PAT2 fulfilling the role of the common transporter, and the IMINO transporter SIT1 (SLC6A20) (Kowalczuk et al., 2005; Takanaga et al., 2005) and XT2 (SLC6A18) (Singer et al., 2009) being the imino acid and glycine-specific carriers respectively. Either of the PAT2 mutations identified by Bröer et al. (2008) will lead to a reduction in reabsorption of amino acids in the renal proximal tubule and hyperexcretion of amino acids in urine (Bröer et al., 2008). However, the inheritance pattern of mutations leading to production of either an iminoglycinuria or hyperglycinuria phenotype is complex. Iminoglycinuria is observed in an individual homozygous for the truncated (non-functional) form of PAT2. However, in individuals homozygous for the mutated Gly87Val PAT2 (which has reduced function), only hyperglycinuria is observed, unless the individual also shows haploinsufficiency in the proline transporter SIT1, where a single nucleotide polymorphism in SLC6A20 produces a threonine to methionine change (Thr199Met) in the fifth transmembrane domain of the SIT1 protein (Bröer et al., 2008). This Thr199Met SIT1 protein has a much reduced transport capacity compared with the wild-type protein (Bröer et al., 2008). Iminoglycinuria produced by a combination of mutations in PAT2 and SIT1 is estimated to occur at a combined frequency of 1:92 600. In both iminoglycinuria and hyperglycinuria individuals, additional mutations were also described in both XT2 (SLC6A18) and B0AT1 (SLC6A19), but no mutations were found in PAT1 (Bröer et al., 2008). The complexity of these disorders suggests that other pedigrees may have different inheritance patterns involving a variety of SLC36 and SLC6 transporters, which also emphasizes the overlap in substrate specificity and function of these carriers. Clearly, the transport of any drug via these mutated SLC36 and SLC6 carriers will be reduced, which, in turn, will alter renal reabsorption and the pharmacokinetics of each drug compound.
Indirect influence of disease and drugs on transporter function
An area of low pH (pH 6.1–6.8) is present adjacent to the luminal surface of the mammalian small intestine (McEwan et al., 1988; Daniel et al., 1989), and this low pH environment (the acid microclimate) provides the pH gradient that is utilized by absorptive transporters such as the amino acid transporter PAT1. Thus, any pathophysiological or pharmacological modulation (alkalinization) of this environment will indirectly affect the efficiency of H+-coupled transporters involved in nutrient and drug absorption. The acid microclimate is alkalinized in coeliac disease and following exposure to enterotoxins, cyclic nucleotides and phorbol esters (Lucas et al., 1978; McEwan et al., 1988; Shimada and Hoshi, 1988). In studies using human intestinal epithelial Caco-2 cell monolayers, H+-coupled nutrient and drug transport (e.g. PAT1) is reduced indirectly by a reduction in NHE3 activity following exposure to amiloride-related drugs, cyclic nucleotides, phorbol esters and phosphodiesterase inhibitors found in diet (caffeine, theophylline) and those delivered orally such as pentoxifylline (used to treat intermittent claudication) (Anderson et al., 2004; Anderson and Thwaites, 2005; 2007; 2010; Thwaites and Anderson, 2007a,b;).
Conclusion
It seems likely that the historical view that most drug molecules are transported passively across biological membranes by simple diffusion is incorrect. Rather, many drug molecules are transported across cell membranes in the human body via movement through a diverse range of carrier proteins each possessing a unique substrate selectivity and mode of action (Hagenbuch and Meier, 2003; Mizuno et al., 2003; Schinkel and Jonker, 2003; Koepsell et al., 2007; Thwaites and Anderson, 2007b; Dobson and Kell, 2008; Rubio-Aliaga and Daniel, 2008; Anderson and Thwaites, 2010; Giacomini et al., 2010). Many of these mammalian transporters are members of the SLC families of proteins (a list of which can be found at the HGNC Database at: http://www.bioparadigms.org) (Hediger et al., 2004). The relative concentration of any drug in any tissue will depend in part on the rates of influx and efflux mediated by various SLC and ABC transporters and the relative levels of expression of each transport protein in each membrane. To aid rational drug design and delivery, knowledge of the substrate specificity, ion-coupling and tissue and membrane distribution of each solute transporter is required. In this review, we summarize the current state of knowledge relating to the SLC36 family of amino acid transporters. Although relatively little is known about SLC36 transporter function, in comparison with some other SLC families, it is clear that members of SLC36 have great potential as routes for drug delivery in the small intestine and reabsorption in the kidney of a variety of amino acid derivatives and analogues, including neuromodulatory agents, anti-bacterials, anti-hyperglycaemics and anti-cancer agents.
Acknowledgments
This work was supported by the Wellcome Trust (grant number 078640/Z/05/Z).
Glossary
Abbreviations
- AIB
α-aminoisobutyric acid or 2-aminoisobutyric acid
- BBMV
brush-border membrane vesicles
- BCECF
2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein
- BDGP
Berkeley Drosophila Genome Project
- β-ABA
β-aminobutyric acid
- EIPA
5-(N-ethyl-N-isopropyl)-amiloride
- HGNC
HUGO Gene Nomenclature Committee
- HUGO
Human Genome Organisation
- MeAIB
α-(methylamino)isobutyric acid or 2-(methylamino)isobutyric acid
- OMIM
Online Mendelian Inheritance in Man
- PACAP
pituitary adenylate cyclase-activating polypeptide
- S1611
3-[2-(3-guanidino-2-methyl-3-oxo-propenyl)-phenyl]-N-isopropylidene-2-methyl-acrylamide dihydro-chloride
- SLC
solute carrier
- TEVC
two-electrode voltage-clamp
- THPO
4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol
- VIP
vasoactive intestinal peptide
- 8-br-cAMP
8-bromoadenosine-3′,5′-cyclic monophosphate
Conflict of interest
The authors state no conflicts of interest.
References
- Abbot EL, Grenade DS, Kennedy DJ, Gatfield KM, Thwaites DT. Vigabatrin transport across the human intestinal epithelial (Caco-2) brush-border membrane is via the H+-coupled amino acid transporter hPAT1. Br J Pharmacol. 2006;147:298–306. doi: 10.1038/sj.bjp.0706557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Rostaing P, Ravassard P, Sagné C, Triller A, Giros B. Lysosomal amino acid transporter LYAAT-1 in the rat central nervous system: an in situ hybridization and immunohistochemical study. J Comp Neurol. 2003;462:71–89. doi: 10.1002/cne.10712. [DOI] [PubMed] [Google Scholar]
- Anderson CMH, Thwaites DT. Indirect regulation of the intestinal H+-coupled amino acid transporter hPAT1 (SLC36A1) J Cell Physiol. 2005;204:604–613. doi: 10.1002/jcp.20337. [DOI] [PubMed] [Google Scholar]
- Anderson CMH, Thwaites DT. Regulation of intestinal hPepT1 (SLC15A1) activity by phosphodiesterase inhibitors is via inhibition of NHE3 (SLC9A3) Biochim Biophys Acta. 2007;1768:1822–1829. doi: 10.1016/j.bbamem.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CMH, Thwaites DT. Hijacking solute carriers for proton-coupled drug transport. Physiology. 2010;25:364–377. doi: 10.1152/physiol.00027.2010. [DOI] [PubMed] [Google Scholar]
- Anderson CMH, Grenade DS, Boll M, Foltz M, Wake KA, Kennedy DJ, et al. H+/amino acid transporter 1 (PAT1) is the imino acid carrier: an intestinal nutrient/drug transporter in human and rat. Gastroenterology. 2004;127:1410–1422. doi: 10.1053/j.gastro.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Anderson CMH, Howard A, Walters JRF, Ganapathy V, Thwaites DT. Taurine uptake across the human intestinal brush-border membrane is via two transporters: H+-coupled PAT1 (SLC36A1) and Na+ and Cl- dependent TauT (SLC6A6) J Physiol. 2009;587:731–744. doi: 10.1113/jphysiol.2008.164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CMH, Jevons M, Thangaraju M, Edwards N, Conlon NJ, Woods S, et al. Transport of the photodynamic agent 5-aminolevulinic acid by distinct H+-coupled nutrient carriers coexpressed in the small intestine. J Pharmacol Exp Ther. 2010;332:220–228. doi: 10.1124/jpet.109.159822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JP, Mulder AH, Snyder SH. Neurochemical correlates of synaptically active amino acids. Life Sci. 1974;15:1045–1056. doi: 10.1016/s0024-3205(74)80002-0. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Pennington J. Organization and expression of the SLC36 cluster of amino acid transporter genes. Mamm Genome. 2004;14:114–125. doi: 10.1007/s00335-003-2319-3. [DOI] [PubMed] [Google Scholar]
- Bermingham JR, Shumas S, Whisenhunt T, Sirkowski EE, O'Connell S, Scherrer SS, et al. Identification of genes that are downregulated in the absence of the POU domain transcription factor pou3f1 (Oct-6, Tst-1, SCIP) in sciatic nerve. J Neurosci. 2002;22:10217–10231. doi: 10.1523/JNEUROSCI.22-23-10217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll M, Foltz M, Rubio-Aliaga I, Kottra G, Daniel H. Functional characterization of two novel mammalian electrogenic proton-dependent amino acid cotransporters. J Biol Chem. 2002;277:22966–22973. doi: 10.1074/jbc.M200374200. [DOI] [PubMed] [Google Scholar]
- Boll M, Foltz M, Anderson CMH, Oechsler C, Kottra G, Thwaites DT, et al. Substrate recognition by the mammalian proton-dependent amino acid transporter PAT1. Mol Membr Biol. 2003a;20:261–269. doi: 10.1080/0968768031000100759. [DOI] [PubMed] [Google Scholar]
- Boll M, Foltz M, Rubio-Aliaga I, Daniel H. A cluster of proton/amino acid transporter genes in the human and mouse genomes. Genomics. 2003b;82:47–56. doi: 10.1016/s0888-7543(03)00099-5. [DOI] [PubMed] [Google Scholar]
- Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. 2008a;88:249–286. doi: 10.1152/physrev.00018.2006. [DOI] [PubMed] [Google Scholar]
- Bröer S. Apical transporters for neutral amino acids: physiology and pathophysiology. Physiology. 2008b;23:95–103. doi: 10.1152/physiol.00045.2007. [DOI] [PubMed] [Google Scholar]
- Bröer S, Bailey CG, Kowalczuk S, Ng C, Vanslambrouck JM, Rodgers H, et al. Iminoglycinuria and hyperglycinuria are discrete human phenotypes resulting from complex mutations in proline and glycine transporters. J Clin Invest. 2008;118:3881–3892. doi: 10.1172/JCI36625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Fei YJ, Anderson CMH, Wake KA, Miyauchi S, Huang W, et al. Structure, function and immunolocalization of a proton-coupled amino acid transporter (hPAT1) in the human intestinal cell line Caco-2. J Physiol. 2003a;546:349–361. doi: 10.1113/jphysiol.2002.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kennedy DJ, Wake KA, Zhuang L, Ganapathy V, Thwaites DT. Structure, tissue expression pattern, and function of the amino acid transporter rat PAT2. Biochem Biophys Res Commun. 2003b;304:747–754. doi: 10.1016/s0006-291x(03)00648-x. [DOI] [PubMed] [Google Scholar]
- Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Cook D, Brooks S, Bellone R, Bailey E. Missense mutation in exon 2 of SLC36A1 responsible for champagne dilution in horses. PLoS Genet. 2008;4:e1000195. doi: 10.1371/journal.pgen.1000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougoule C, Carréno S, Castandet J, Labrousse A, Astarie-Dequeker C, Poincloux R, et al. Activation of the lysosome-associated p61Hck isoform triggers the biogenesis of podosomes. Traffic. 2005;6:682–694. doi: 10.1111/j.1600-0854.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- Daniel H, Fett C, Kratz A. Demonstration and modification of intervillous pH profiles in rat small intestine in vitro. Am J Physiol. 1989;20:G489–G495. doi: 10.1152/ajpgi.1989.257.4.G489. [DOI] [PubMed] [Google Scholar]
- Daniels VG, Dawson AG, Newey H, Smyth DH. Effect of carbon chain length and amino group position on neutral amino acid transport systems in rat small intestine. Biochim Biophys Acta. 1969a;173:575–577. doi: 10.1016/0005-2736(69)90025-x. [DOI] [PubMed] [Google Scholar]
- Daniels VG, Newey H, Smyth DH. Stereochemical specificity of neutral amino acid transfer systems in rat small intestine. Biochim Biophys Acta. 1969b;183:637–639. doi: 10.1016/0005-2736(69)90177-1. [DOI] [PubMed] [Google Scholar]
- De la Noüe J, Newey H, Smyth DH. Transfer of alanine isomers by rat small intestine. J Physiol. 1971;214:105–114. doi: 10.1113/jphysiol.1971.sp009421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz M, Medina V, Gómez T, Lorenzo A. Membrane mechanisms for electrogenic Na+-independent l-alanine transport in the lizard duodenal mucosa. Am J Physiol. 2000;279:R925–R935. doi: 10.1152/ajpregu.2000.279.3.R925. [DOI] [PubMed] [Google Scholar]
- Dobson PD, Kell DB. Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule? Nat Rev Drug Discov. 2008;7:205–220. doi: 10.1038/nrd2438. [DOI] [PubMed] [Google Scholar]
- Dorn M, Jaehme M, Weiwad M, Markwardt F, Rudolph R, Brandsch M, et al. The role of N-glycosylation in transport function and surface targeting of the human solute carrier PAT1. FEBS Lett. 2009a;583:1631–1636. doi: 10.1016/j.febslet.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Dorn M, Weiwad M, Markwardt F, Laug L, Rudolph R, Brandsch M, et al. Identification of a disulfide bridge essential for transport function of the human proton-coupled amino acid transporter hPAT1. J Biol Chem. 2009b;284:22123–22132. doi: 10.1074/jbc.M109.023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards N, Anderson CMH, Gatfield KM, Jevons MP, Ganapathy V, Thwaites DT. Amino acid derivatives are substrates or non-transported inhibitors of the amino acid transporter PAT2 (slc36a2) Biochim Biophys Acta. 2011;1808:260–270. doi: 10.1016/j.bbamem.2010.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans AM, Aimanova KG, Gill SS. Characterization of a blood-meal-responsive proton-dependent amino acid transporter in the disease vector, Aedes aegypti. J Exp Biol. 2009;212:3263–3271. doi: 10.1242/jeb.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz M, Boll M, Raschka L, Kottra G, Daniel H. A novel bifunctionality: PAT1 and PAT2 mediate electrogenic proton/amino acid and electroneutral proton/fatty acid symport. FASEB J. 2004a;18:1758–1760. doi: 10.1096/fj.03-1387fje. [DOI] [PubMed] [Google Scholar]
- Foltz M, Oechsler C, Boll M, Kottra G, Daniel H. Substrate specificity and transport mode of the proton-dependent amino acid transporter mPAT2. Eur J Biochem. 2004b;271:3340–3347. doi: 10.1111/j.1432-1033.2004.04268.x. [DOI] [PubMed] [Google Scholar]
- Foltz M, Mertl M, Dietz V, Boll M, Kottra G, Daniel H. Kinetics of bidirectional H+ and substrate transport by the proton-dependent amino acid symporter PAT1. Biochem J. 2005;386:607–616. doi: 10.1042/BJ20041519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberdhan DCI, Meredith D, Boyd CAR, Wilson C. PAT-related amino acid transporters regulate growth via a novel mechanism that does not require bulk transport of amino acids. Development. 2005;132:2365–2375. doi: 10.1242/dev.01821. [DOI] [PubMed] [Google Scholar]
- Greeley T, Shumaker H, Wang Z, Schweinfest CW, Soleimani M. Downregulated in adenoma and putative anion transporter are regulated by CFTR in cultured pancreatic duct cells. Am J Physiol. 2001;281:G1301–G1308. doi: 10.1152/ajpgi.2001.281.5.G1301. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteins. Pflügers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- Heublein S, Kazi S, Ogmundsdóttir MH, Attwood EV, Kala S, Boyd CAR, et al. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene. 2010;29:4068–4079. doi: 10.1038/onc.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingrosso L, Marsigliante S, Zonno V, Storelli C, Vilella S. An l-proline-dependent proton flux is located at the apical membrane level of the eel enterocytes. Am J Physiol. 2000;279:R1619–R1624. doi: 10.1152/ajpregu.2000.279.5.R1619. [DOI] [PubMed] [Google Scholar]
- Iñigo C, Barber A, Lostao MP. Na+ and pH dependence of proline and β-alanine absorption in rat small intestine. Acta Physiol. 2006;186:271–278. doi: 10.1111/j.1748-1716.2006.01538.x. [DOI] [PubMed] [Google Scholar]
- Jessen H, Sheikh MI. Renal transport of taurine in luminal membrane vesicles from rabbit proximal tubule. Biochim Biophys Acta. 1991;1064:189–198. doi: 10.1016/0005-2736(91)90301-n. [DOI] [PubMed] [Google Scholar]
- Jessen H, Vorum H, Jorgensen KE, Sheikh MI. Characteristics of d-alanine transport by luminal membrane vesicles from pars convoluta and pars recta of rabbit proximal tubule. Biochim Biophys Acta. 1988a;942:262–270. doi: 10.1016/0005-2736(88)90028-4. [DOI] [PubMed] [Google Scholar]
- Jessen H, Vorum H, Jorgensen KE, Sheikh MI. Energetics of renal Na+ and H+/l-alanine co-transport systems. Biochem J. 1988b;256:299–302. doi: 10.1042/bj2560299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen H, Jorgensen KE, Røigaard-Petersen H, Sheikh MI. Demonstration of H+- and Na+-coupled cotransport of β-alanine by luminal membrane vesicles of rabbit proximal tubule. J Physiol. 1989;411:517–528. doi: 10.1113/jphysiol.1989.sp017587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen H, Vorum H, Jorgensen KE, Sheikh MI. Na+- and H+-gradient-dependent transport of α-aminoisobutyrate by luminal membrane vesicles from rabbit proximal tubule. J Physiol. 1991;436:149–167. doi: 10.1113/jphysiol.1991.sp018544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GAR, Iversen LL. Glycine uptake in rat central nervous system slices and homogenates: evidence for different uptake systems in spinal cord and cerebral cortex. J Neurochem. 1971;18:1951–1961. doi: 10.1111/j.1471-4159.1971.tb09601.x. [DOI] [PubMed] [Google Scholar]
- Karunakaran S, Umapathy NS, Thangaraju M, Hatanaka T, Itagaki S, Munn DH, et al. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem J. 2008;414:343–355. doi: 10.1042/BJ20080622. [DOI] [PubMed] [Google Scholar]
- Kennedy DJ, Gatfield KM, Winpenny JP, Ganapathy V, Thwaites DT. Substrate specificity and functional characterisation of the H+/amino acid transporter rat PAT2 (Slc36a2) Br J Pharmacol. 2005;144:28–41. doi: 10.1038/sj.bjp.0706029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Scoggin KE, Ealy AD, Troedsson MH. Transcriptional profiling of equine endometrium during the time of maternal recognition of pregnancy. Biol Reprod. 2010;83:102–113. doi: 10.1095/biolreprod.109.081612. [DOI] [PubMed] [Google Scholar]
- Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS. Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci USA. 2001;98:9425–9430. doi: 10.1073/pnas.141241098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Kowalczuk S, Bröer A, Munzinger M, Tietze N, Klingel K, Bröer S. Molecular cloning of the mouse IMINO system: an Na+- and Cl--dependent proline transporter. Biochem J. 2005;386:417–422. doi: 10.1042/BJ20050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Larsen BB, Frølund B, Nielsen CU. Transport of amino acids and GABA analogues via the human proton-coupled amino acid transporter, hPAT1: characterization of conditions for affinity and transport experiments in Caco-2 cells. Eur J Pharm Sci. 2008;35:86–95. doi: 10.1016/j.ejps.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Larsen M, Holm R, Jensen KG, Brodin B, Nielsen CU. Intestinal gaboxadol absorption via PAT1 (SLC36A1): modified absorption in vivo following co-administration of l-tryptophan. Br J Pharmacol. 2009;157:1380–1389. doi: 10.1111/j.1476-5381.2009.00253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M, Holm R, Jensen KG, Sveigaard C, Brodin B, Nielsen CU. 5-Hydroxy-l-tryptophan alters gaboxadol pharmacokinetics in rats: involvement of PAT1 and rOat1 in gaboxadol absorption and elimination. Eur J Pharm Sci. 2010;39:68–75. doi: 10.1016/j.ejps.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Lasley L, Scriver CR. Ontogeny of amino acid reabsorption in human kidney. Evidence from the homozygous infant with familial renal iminoglycinuria for multiple proline and glycine systems. Pediatr Res. 1979;13:65–70. doi: 10.1203/00006450-197901000-00014. [DOI] [PubMed] [Google Scholar]
- Logan WJ, Snyder SH. Unique high affinity uptake systems for glycine, glutamic and aspartic acids in central nervous tissue of the rat. Nature. 1971;234:297–299. doi: 10.1038/234297b0. [DOI] [PubMed] [Google Scholar]
- Lucas ML, Cooper BT, Lei FH, Johnson IT, Holmes GKT, Blair JA, et al. Acid microclimate in coeliac and Crohn's disease: a model for folate malabsorption. Gut. 1978;19:735–742. doi: 10.1136/gut.19.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan GTA, Daniel H, Fett C, Burgess MN, Lucas ML. The effect of Escherichia coli STa enterotoxin and other secretagogues on mucosal surface pH of rat small intestine in vivo. Proc R Soc Lond B. 1988;234:219–237. doi: 10.1098/rspb.1988.0045. [DOI] [PubMed] [Google Scholar]
- Metzner L, Kalbitz J, Brandsch M. Transport of pharmacologically active proline derivatives by the human proton-coupled amino acid transporter hPAT1. J Pharmacol Exp Ther. 2004;309:28–35. doi: 10.1124/jpet.103.059014. [DOI] [PubMed] [Google Scholar]
- Metzner L, Kottra G, Neubert K, Daniel H, Brandsch M. Serotonin, l-tryptophan, and tryptamine are effective inhibitors of the amino acid transport system PAT1. FASEB J. 2005;19:1468–1473. doi: 10.1096/fj.05-3683com. [DOI] [PubMed] [Google Scholar]
- Metzner L, Neubert K, Brandsch M. Substrate specificity of the amino acid transporter PAT1. Amino Acids. 2006;31:111–117. doi: 10.1007/s00726-005-0314-6. [DOI] [PubMed] [Google Scholar]
- Metzner L, Natho K, Zebisch K, Dorn M, Bosse-Doenecke E, Ganapathy V, et al. Mutational analysis of histidine residues in the human proton-coupled amino acid transporter PAT1. Biochim Biophys Acta. 2008;1778:1042–1050. doi: 10.1016/j.bbamem.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Metzner L, Dorn M, Markwardt F, Brandsch M. The orally active antihyperglycemic drug β-guanidinopropionic acid is transported by the human proton-coupled amino acid transporter hPAT1. Mol Pharm. 2009;6:1006–1011. doi: 10.1021/mp9000684. [DOI] [PubMed] [Google Scholar]
- Miyauchi S, Abbot EL, Zhuang L, Subramanian R, Ganapathy V, Thwaites DT. Isolation and function of the amino acid transporter PAT1 (slc36a1) from rabbit and discrimination between transport via PAT1 and system IMINO in renal brush-border membrane vesicles. Mol Membr Biol. 2005;22:549–559. doi: 10.1080/09687860500421779. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Niwa T, Yotsumoto Y, Sugiyama Y. Impact of drug transporter studies on drug discovery and development. Pharmacol Rev. 2003;55:425–461. doi: 10.1124/pr.55.3.1. [DOI] [PubMed] [Google Scholar]
- Munck BG. Amino acid transport by the small intestine of the rat. The existence and specificity of the transport mechanism of imino acids and its relation to the transport of glycine. Biochim Biophys Acta. 1966;120:97–103. doi: 10.1016/0926-6585(66)90281-0. [DOI] [PubMed] [Google Scholar]
- Munck LK, Munck BG. Amino acid transport in the small intestine. Physiol Res. 1994;43:335–346. [PubMed] [Google Scholar]
- Munck BG, Munck LK, Rasmussen SN, Polache A. Specificity of the imino acid carrier in rat small intestine. Am J Physiol. 1994;266:R1154–R1161. doi: 10.1152/ajpregu.1994.266.4.R1154. [DOI] [PubMed] [Google Scholar]
- Newey H, Smyth DH. The transfer system for neutral amino acids in the rat small intestine. J Physiol. 1964;170:328–343. doi: 10.1113/jphysiol.1964.sp007334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin PL, Irwin WJ, Hassan IF, Mackay M. Proline uptake by monolayers of human intestinal absorptive (Caco-2) cells in vitro. Biochim Biophys Acta. 1992;1104:283–292. doi: 10.1016/0005-2736(92)90042-k. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter super families. Drug Metab Pharmacokinet. 2005;20:452–477. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- Palacin M, Estevez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiol Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Pillai SM, Meredith D. SLC36A4 (hPAT4) is a high affinity amino acid transporter when expressed in Xenopus laevis oocytes. J Biol Chem. 2011;286:2455–2460. doi: 10.1074/jbc.M110.172403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpinelli F, Lehmann S, Maridonneau-Parini I. The scrapie prion protein is present in flotillin-1-positive vesicles in central- but not peripheral-derived neuronal cell lines. Eur J Neurosci. 2005;21:2063–2072. doi: 10.1111/j.1460-9568.2005.04049.x. [DOI] [PubMed] [Google Scholar]
- Rajendran VM, Barry JA, Kleinman JG, Ramaswamy K. Proton-gradient dependent transport of glycine in rabbit renal brush border membrane vesicles. J Biol Chem. 1987;262:14974–14977. [PubMed] [Google Scholar]
- Ranaldi G, Islam K, Sambuy Y. d-Cycloserine uses an active transport mechanism in the human intestinal cell line Caco-2. Antimicrob Agents Chemother. 1994;38:1239–1245. doi: 10.1128/aac.38.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Røigaard-Petersen H, Sheikh MI. Renal transport of neutral amino acids. Biochem J. 1984;220:25–33. doi: 10.1042/bj2200025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Røigaard-Petersen H, Jacobsen C, Sheikh MI. H+-l-proline cotransport by vesicles from pars convoluta of rabbit proximal tubule. Am J Physiol. 1987;253:F15–F20. doi: 10.1152/ajprenal.1987.253.1.F15. [DOI] [PubMed] [Google Scholar]
- Røigaard-Petersen H, Jacobsen C, Jessen H, Mollerup S, Sheikh MI. Electrogenic uptake of d-imino acids by luminal membrane vesicles from rabbit kidney proximal tubule. Biochim Biophys Acta. 1989;984:231–237. doi: 10.1016/0005-2736(89)90221-6. [DOI] [PubMed] [Google Scholar]
- Røigaard-Petersen H, Jessen H, Mollerup S, Jorgensen KE, Jacobsen C, Sheikh MI. Proton-gradient dependent renal transport of glycine: evidence from vesicle studies. Am J Physiol. 1990;258:F388–F396. doi: 10.1152/ajprenal.1990.258.2.F388. [DOI] [PubMed] [Google Scholar]
- Romero-Calderón R, Shome RM, Simon AF, Daniels RW, DiAntonio A, Krantz DE. A screen for neurotransmitter transporters expressed in the visual system of Drosophila melanogaster identifies three novel genes. Dev Neurobiol. 2007;67:550–569. doi: 10.1002/dneu.20342. [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Daniel H. Peptide transporters and their roles in physiological processes and drug disposition. Xenobiotica. 2008;38:1022–1042. doi: 10.1080/00498250701875254. [DOI] [PubMed] [Google Scholar]
- Rubio-Aliaga I, Boll M, Vogt-Weisenhorn DM, Foltz M, Kottra G, Daniel H. The proton/amino acid cotransporter PAT2 is expressed in neurons with a different subcellular localization than its paralog PAT1. J Biol Chem. 2004;279:2754–2760. doi: 10.1074/jbc.M305556200. [DOI] [PubMed] [Google Scholar]
- Sagné C, Agulhon C, Ravassard P, Darmon M, Hamon M, El Mestikawy S, et al. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc Natl Acad Sci USA. 2001;98:7206–7211. doi: 10.1073/pnas.121183498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saier MH, Jr, Tran CV, Barabote RD. TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006;34:D181–D186. doi: 10.1093/nar/gkj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders HR, Evans AM, Ross LS, Gill SS. Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochem Mol Biol. 2003;33:1105–1122. doi: 10.1016/s0965-1748(03)00124-3. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55:3–29. doi: 10.1016/s0169-409x(02)00169-2. [DOI] [PubMed] [Google Scholar]
- Schwark JR, Jansen HW, Lang HJ, Krick W, Burckhardt G, Hropot M. S3226, a novel inhibitor of Na+/H+ exchanger subtype 3 in various cell types. Pflügers Arch. 1998;436:797–800. doi: 10.1007/s004240050704. [DOI] [PubMed] [Google Scholar]
- Shimada T, Hoshi T. Na+-dependent elevation of the acidic cell surface pH (microclimate pH) of rat jejunal villus cells induced by cyclic nucleotides and phorbol ester: possible mediators of the regulation of the Na+/H+ antiporter. Biochim Biophys Acta. 1988;937:328–334. doi: 10.1016/0005-2736(88)90255-6. [DOI] [PubMed] [Google Scholar]
- Singer D, Camargo SM, Huggel K, Romeo E, Danilczyk U, Kuba K, et al. Orphan transporter SLC6A18 is renal neutral amino acid transporter B0AT3. J Biol Chem. 2009;284:19953–19960. doi: 10.1074/jbc.M109.011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg BE, Wååg E, Jacobsson JA, Stephansson O, Rumaks J, Svirskis S, et al. The evolutionary history and tissue mapping of amino acid transporters belonging to solute carrier families SLC32, SLC36, and SLC38. J Mol Neurosci. 2008;35:179–193. doi: 10.1007/s12031-008-9046-x. [DOI] [PubMed] [Google Scholar]
- Takanaga H, Mackenzie B, Suzuki Y, Hediger MA. Identification of mammalian proline transporter SIT1 (SLC6A20) with characteristics of classical system imino. J Biol Chem. 2005;280:8974–8984. doi: 10.1074/jbc.M413027200. [DOI] [PubMed] [Google Scholar]
- Thompson E, Levin RJ, Jackson MJ. The stimulating effect of low pH on the amino acid transferring systems of the small intestine. Biochim Biophys Acta. 1970;196:120–122. doi: 10.1016/0005-2736(70)90175-6. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CMH. Deciphering the mechanisms of intestinal imino (and amino) acid transport: the redemption of SLC36A1. Biochim Biophys Acta. 2007a;1768:179–197. doi: 10.1016/j.bbamem.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Anderson CMH. H+-coupled nutrient, micronutrient and drug transporters in the mammalian small intestine. Exp Physiol. 2007b;92:603–619. doi: 10.1113/expphysiol.2005.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Stevens BC. H+/zwitterionic amino acid symport at the brush-border membrane of human intestinal epithelial (Caco-2) cells. Exp Physiol. 1999;84:275–284. [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Brown CDA, Hirst BH, Simmons NL. Na+-independent, H+-coupled transepithelial beta-alanine absorption by human intestinal Caco-2 cell monolayers. J Biol Chem. 1993a;268:18438–18441. [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Cook MJ, Hirst BH, Simmons NL. H+-coupled (Na+-independent) proline transport in human intestinal (Caco-2) epithelial cell monolayers. FEBS Lett. 1993b;333:78–82. doi: 10.1016/0014-5793(93)80378-8. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Brown CDA, Hirst BH, Simmons NL. l-Alanine absorption in human intestinal Caco-2 cells driven by the proton electrochemical gradient. J Membr Biol. 1994;140:143–151. doi: 10.1007/BF00232902. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Armstrong G, Hirst BH, Simmons NL. d-Cycloserine transport in human intestinal epithelial (Caco-2) cells: mediation by a H+-coupled amino acid transporter. Br J Pharmacol. 1995a;115:761–766. doi: 10.1111/j.1476-5381.1995.tb14998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Hirst BH, Simmons NL. H+-coupled α-methylaminoisobutyric acid transport in human intestinal Caco-2 cells. Biochim Biophys Acta. 1995b;1234:111–118. doi: 10.1016/0005-2736(94)00268-t. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, McEwan GTA, Simmons NL. The role of the proton electrochemical gradient in the transepithelial absorption of amino acids by human intestinal Caco-2 cell monolayers. J Membr Biol. 1995c;145:245–256. doi: 10.1007/BF00232716. [DOI] [PubMed] [Google Scholar]
- Thwaites DT, Ford D, Glanville M, Simmons NL. H+/solute-induced intracellular acidification leads to selective activation of apical Na+/H+ exchange in human intestinal epithelial cells. J Clin Invest. 1999;104:629–635. doi: 10.1172/JCI7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Basterfield L, McCleave PMJ, Carter SM, Simmons NL. Gamma-aminobutyric acid (GABA) transport across human intestinal epithelial (Caco-2) cell monolayers. Br J Pharmacol. 2000;129:629–635. doi: 10.1038/sj.bjp.0703069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thwaites DT, Kennedy DJ, Raldua D, Anderson CMH, Mendoza ME, Bladen CL, et al. H+/dipeptide absorption across the human intestinal epithelium is controlled indirectly via a functional Na+/H+ exchanger. Gastroenterology. 2002;122:1322–1333. doi: 10.1053/gast.2002.32992. [DOI] [PubMed] [Google Scholar]
- Vanslambrouck JM, Bröer A, Thavyogarajah T, Holst J, Bailey CG, Bröer S, et al. Renal imino acid and glycine transport system ontogeny and involvement in developmental iminoglycinuria. Biochem J. 2010;428:397–407. doi: 10.1042/BJ20091667. [DOI] [PubMed] [Google Scholar]
- Vieira FL, Malnic G. Hydrogen ion secretion by rat renal cortical tubules as studied by an antimony microelectrode. Am J Physiol. 1968;214:710–718. doi: 10.1152/ajplegacy.1968.214.4.710. [DOI] [PubMed] [Google Scholar]
- Wiseman G. Absorption of amino acids. In: Code DF, editor. Handbook of Physiology Section 6 Alimentary Canal. Vol. 3. Washington, DC: American Physiological Society; 1968. pp. 1277–1307. [Google Scholar]
- Wreden CC, Johnson J, Tran C, Seal RP, Copenhagen DR, Reimer RJ, et al. The H+-coupled electrogenic lysosomal amino acid transporter LYAAT1 localizes to the axon and plasma membrane of hippocampal neurons. J Neurosci. 2003;23:1265–1275. doi: 10.1523/JNEUROSCI.23-04-01265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wunz TM, Wright SH. Betaine transport in rabbit renal brush-border membrane vesicles. Am J Physiol. 1993;264:F948–F955. doi: 10.1152/ajprenal.1993.264.6.F948. [DOI] [PubMed] [Google Scholar]


