Abstract
Inner ear hair cells convert hair bundle deflection into mechanical force sensed by ion channels via extracellular tip links between adjacent stereocilia. In this Neuron issue, Grillet and colleagues show the protein harmonin mechanically reinforces tip link upper insertion sites. Harmonin loss at this site reduces mechanotransduction kinetics and sensitivity.
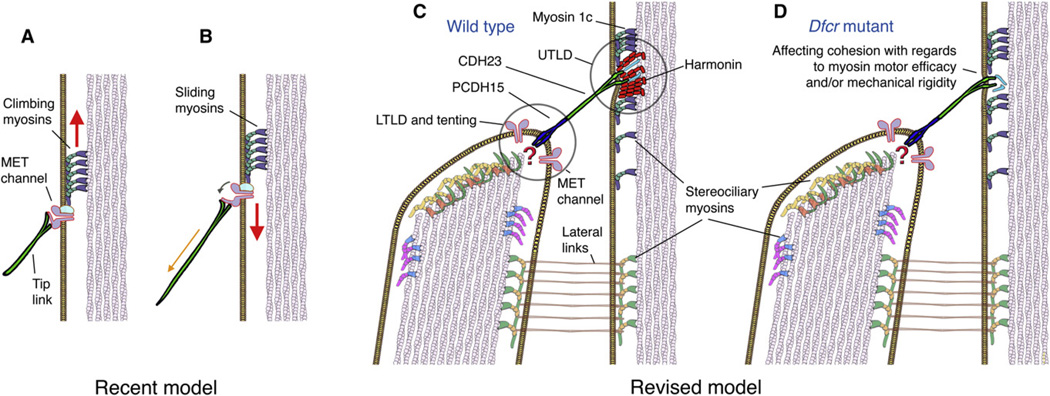
Hair cells, the inner ear’s mechanosensory cells that convert mechanical stimuli into electrical signals, are named for their apical protrusions of actin-rich stereocilia, organized in staircase-like rows of increasing heights. Stereocilia are interconnected with different types of filaments, the most important of these being the tip link. Tip links emerge from the tented tips of shorter stereocilia and stretch over a distance of 150–200 nm to the sides of neighboring taller stereocilia, where they are thought to be the mechanical links conveying shearing motion between stereocilia into force exerted onto the mechanoelectrical transduction (MET) channels (Pickles et al., 1984). A recent view of the transduction apparatus (Holt et al., 2002) (Figure 1A) places the upper end of the tip link at or near the gate of the MET channels, where increased tension in the tip links leads to channel opening (Figure 1B). Adaptation constantly readjusts the channel location along the side of the stereocilium so that it is poised at a maximally sensitive position even during sustained stimuli. Fast adaptation is attributed to rapid channel closure, likely mediated by binding of Ca2+ to sites at or near the channel, whereas slow adaptation is a Ca2+-dependent process thought to involve myosin motors (Vollrath et al., 2007). The motor model of adaptation involves attachment of the channel to a motor complex that is regulated by local [Ca2+]i; at low concentrations, the motor climbs (Figure 1A), increasing tension, and when Ca2+ enters through the transduction channels, the motor slides (Figure 1B), thereby decreasing tension in the tip link, leading to channel reclosure.
Figure 1. Recent and Revised Schematic Models of the Stereociliary Mechanotransduction Apparatus.
(A and B) In a recent model, the upper end of the tip link connects directly or indirectly (not shown) with the mechanoelectrical transduction (MET) channel(s). Slow adaptation involving the [Ca2+]i-dependent climbing and sliding of myosin motors is indicated by the arrows.
(C) A revised model taking into account the transmembrane proteins PCDH15 and CDH23. Major building blocks of the upper tip-link density (UTLD) are harmonins, which provide a sturdy anchor point for the tip link with the cytoskeleton. MET channels have recently been shown to be associated with the lower tip-link density (LTLD), but their mechanical link to the lower insertion point of the tip link is unclear (question mark). Tenting of the membrane, however, would be consistent with activation by membrane stretch.
(D) In dfcr mutant mice, harmonin is absent from the upper tip link insertion point, affecting the transduction apparatus’ mechanical characteristics. It is not clear whether other proteins (indicated in turquoise) that have been proposed in this region are affected.
A series of recent findings now change our view of how the known parts of the mechanotransduction complex physically line up with respect to each other. Tip links are formed by protocadherin 15 (PCDH15) and cadherin 23 (CDH23), which constitute their lower and upper parts, respectively (Kazmierczak et al., 2007; Siemens et al., 2004). Because CDH23 traverses the plasma membrane at the upper tip link insertion site, it is unlikely that the upper part of the tip link is directly associated with the transduction channel in the manner shown in Figures 1A and 1B. Moreover, MET channels localize at the stereociliary tips, near the lower insertion point of the PCDH15 portion of tip link (Beurg et al., 2009) (Figure 1C). The stereociliary plasma membranes at the upper and lower tip link insertion sites display obvious electron-dense plaques that can be revealed by electron microscopy (Furness and Hackney, 1985). Grillet et al. in this issue of Neuron now place the protein harmonin, a scaffolding protein, in a functional relationship with the known components of the transduction apparatus. They provide compelling evidence that harmonin is a major constituent of the intracellular electron-dense area juxtaposed at the site where CDH23, the upper tip-link component, enters the plasma membrane (Figure 1C). This area, now termed upper tip-link density (UTLD), had previously been brought in context with localization of harmonin during stereociliary development (Lefèvre et al., 2008). The authors now demonstrate with electron microscopic resolution that in mature hair cells immunoreactivity for harmonin is highly enriched at the UTLD. Because of its multiple protein interacting sites, harmonin is a good candidate for linking the intracellular domain of CDH23 with actin filaments and it has been hypothesized that the protein plays an important role in coupling mechanotransduction machinery proteins, such as the tip link, with the F-actin backbone of stereocilia (Boëda et al., 2002).
Functional investigation of stereociliary proteins is a difficult endeavor because these proteins appear to be fulfilling distinct roles at different locations during development. Consequently, null alleles of these proteins often result in grossly disorganized hair bundles before the onset of mechanoelectrical transduction, which hinders a refined analysis of their specific function in the mechanoelectrical transduction process (Vrijens et al., 2008). Grillet and colleagues now report a very elegant physioanatomical analysis of two specific mutant harmonin isoforms that are much less disturbing to hair bundle development than a null allele. The first isoform was generated by altering harmonin’s PDZ2 domain (knockin allele harmonin-PDZ2AAA) so that it is no longer able to bind to the intracellular domain of CDH23. Mice homozygous for the harmonin PDZAAA mutation were profoundly deaf and displayed a diffuse distribution of harmonin immunoreactivity in stereocilia, whereas the concentrated accumulation of harmonin at the UTLDs was not detectable. These findings confirm the importance of harmonin’s PDZ2 interaction site for proper localization of the protein at the UTLDs.
The second harmonin isoform characterized by the authors came from the previously described “deaf circler” (dfcr) mouse that carries a deletion of the two coiled-coil domains and the proline, serine, and threonine-rich (PST) domain, resulting in loss of capacity of harmonin to interact with actin filaments. The authors found that previously reported developmental hair bundle defects in dfcr mice (Johnson et al., 2003) are much less obvious in the C57Bl/6 background used in their study, which allowed them to analyze the effects of this mutation in the context of morphologically intact hair bundles. Instead of accumulating at the UTLDs, dfcr-harmonin accumulated near the stereocilia tips. Scanning and transmission electron microscopy (SEM and TEM) revealed that tip links were present in homozygous dfcr mice, but the dense staining of UTLDs visible in TEM micrographs of wild-type stereocilia was absent in dfcr stereocilia (Figure 1D). These subtle changes in a previously uninvestigated structure of the mechanotransduction complex provided the opportunity to measure responses of dfcr cochlear outer hair cells to mechanical stimulation. No changes of maximum transduction current amplitudes were detectable in dfcr hair cells when compared with those of wild-type controls, indicating that all transduction channels were functional. Nevertheless, current displacement functions were shifted rightward and the sensitivity (slope) of the dfcr responses was reduced, meaning larger stimuli were required to obtain comparable responses. Interestingly, the activation of transduction was slowed in mutant hair cells, possibly indicating a slowing of force transmission. The dfcr mutation also decreased the time course of slow and fast adaptation of the transduction current. Probably the best evidence for being part of the adaptation machinery exists for myosin Ic (Holt et al., 2002), though no direct causal relationship has been established (but see work on other hair bundle myosins, for example by Kros et al., 2002 and Stepanyan and Frolenkov, 2009). The authors show that the distribution of myosin Ic is not affected in dfcr hair cells, indicating that although UTLDs are no longer visible by TEM and harmonin is absent, there are still proteins, such as myosin Ic, present in this region (Figure 1D). Other scaffolding proteins, such as MAGI1, a CDH23 binding protein (Xu et al., 2008), could be involved in assembly and maintenance of the UTLD and its role augmented in the absence of harmonin.
The authors propose a model in which harmonin at the UTLD directly or indirectly regulates the motor complex; for example, in the dfcr mutant, myosin Ic could be affected in a way such that the transduction/adaptation apparatus is less efficient, leading to reduced force production at rest, which in turn decreases the average open probability of the transduction channels at rest. With the information at hand, it is difficult to ascertain how a lack of harmonin at the UTLD is affecting the adaptation process directly and whether the proposed deficit in force production is causal for the slowed activation kinetics in dfcr hair cells. The authors acknowledge that the situation is likely more complex than their simplified model. The domain structure of harmonin, consisting of three PDZ domains, two coiled-coil domains, and a PST domain, indicates a function as a scaffolding protein, an observation supported by its involvement in binding stereociliary proteins such as CDH23 and its ability to oligomerize. It is therefore equally possible, as also noted by the authors, that the absence of harmonin weakens the rigidity of the UTLD. This weakening could alter force transmission along the link, adding a compliant component that slows MET activation and adaptation. Intracellularly, the lack of integrity could promote detachment of the whole complex from the cytoskeleton, decreasing the efficiency of myosin motors and resulting in lowered open probability at rest.
Recent findings, including the one reported by Grillet and colleagues, require a refining of the prevailing model of mechanotransduction (Figure 1). Transduction channels, for example, appear to be exclusively present at the tops of stereocilia (Beurg et al., 2009), not at the UTLD, putting them at a distance from harmonin, CDH23, and myosin Ic. Given that force is likely constant throughout the mechanotransduction machinery from proteins coupled at the lower tip-link density (LTLD) to those in the UTLD, alterations in any of these proteins would have ramifications for the MET response despite being located at a distance. Precedence for this exists with the myosin VIIa mutants, where activation curves are dramatically shifted to the right despite a lack of myosin VIIa localization near the transduction channels (Kros et al., 2002). It does seem that the interactions of the tip link with the cytoskeleton and the plasma membrane differ at the UTLD and LTLD despite sensing a similar tension. The LTLD experiences plasma membrane stretch (tenting) in the resting position (Figure 1C); stretch that may be conveyed to the MET channels, whereas this same tension is conveyed to the UTLD by the same tip link; however, the upper tip link insertion site shows no tenting. In fact, there is often an indentation visible at the site of attachment of the upper tip link to the side of the next taller stereocilium. This “harbor-like” structure appears to be mechanically reinforced intracellularly by the UTLD, which consists of harmonin and other proteins (Grillet et al., 2009). Weakening of these reinforcements could make the upper tip link insertion site more compliant and indirectly affect the dynamics of force transmission, thereby altering the activation dynamics of the transduction current in dfcr hair cells. A structurally weakened insertion site is likely more sensitive to stimulation, which may ultimately lead to destruction of the site and could be reflected in the profound deafness in 4-week-old dfcr mice. The lack of coordination could stochastically alter synchrony of the opening and reclosure of the 50–100 transduction channels in a hair bundle, resulting in the slowed kinetics observed by the authors. It appears that the textbooks have to be revised and that the next years will bring additional refinements of our view of the molecular machinery that is responsible for hair cell mechanoreception. A prerequisite to the revision of existing models is the identification of the key components of the mechanotransduction machinery and the functional consequences associated with modification of these proteins. Grillet et al. present an excellent example of the power of the multidisciplinary approach that will be needed to finally unravel the complexity of the sensory hair bundle. Overall, it is fascinating that the whole process involves the mechanical cohesion of many parts that are organized in series to each other.
REFERENCES
- Beurg M, Fettiplace R, Nam JH, Ricci AJ. Nat Neurosci. 2009;12:553–558. doi: 10.1038/nn.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boëda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, Perfettini I, Fath KR, Shorte S, Reiners J, et al. EMBO J. 2002;21:6689–6699. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Hackney CM. Hear. Res. 1985;18:177–188. doi: 10.1016/0378-5955(85)90010-3. [DOI] [PubMed] [Google Scholar]
- Grillet N, Xiong W, Reynolds A, Kazmierczak P, Sato T, Lillo C, Dumont RA, Hintermann E, Sczaniecka A, Schwander M, et al. Neuron. 2009;62:375–387. doi: 10.1016/j.neuron.2009.04.006. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, Corey DP, Mercer JA, Gillespie PG. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Gagnon LH, Webb LS, Peters LL, Hawes NL, Chang B, Zheng QY. Hum. Mol. Genet. 2003;12:3075–3086. doi: 10.1093/hmg/ddg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Marcotti W, van Netten SM, Self TJ, Libby RT, Brown SD, Richardson GP, Steel KP. Nat. Neurosci. 2002;5:41–47. doi: 10.1038/nn784. [DOI] [PubMed] [Google Scholar]
- Lefèvre G, Michel V, Weil D, Lepelletier L, Bizard E, Wolfrum U, Hardelin J, Petit C. Development. 2008;135:1427–1437. doi: 10.1242/dev.012922. [DOI] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, Osborne MP. Hear. Res. 1984;15:103–112. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, Müller U. Nature. 2004;428:950–955. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- Stepanyan R, Frolenkov GI. J. Neurosci. 2009;29:4023–4034. doi: 10.1523/JNEUROSCI.4566-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath MA, Kwan KY, Corey DP. Annu. Rev. Neurosci. 2007;30:339–365. doi: 10.1146/annurev.neuro.29.051605.112917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijens K, Van Laer L, Van Camp G. Hum. Genet. 2008;124:325–348. doi: 10.1007/s00439-008-0556-y. [DOI] [PubMed] [Google Scholar]
- Xu Z, Peng AW, Oshima K, Heller S. J. Neurosci. 2008;28:11269–11276. doi: 10.1523/JNEUROSCI.3833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]