Background: P2X receptors show marked variation in the time-course of responses.
Results: Amino-terminal chimeric and mutant P2X1 and -2 receptors had reciprocal effects on desensitization.
Conclusion: The intracellular amino terminus plays a dominant role in regulation of the time-course of ATP currents.
Significance: The P2X receptor channel pore region formed by the transmembrane segments is regulated by the amino terminus.
Keywords: ATP, Gating, Ion Channels, Mutagenesis, Purinergic Receptor
Abstract
P2X receptors show marked variations in the time-course of response to ATP application from rapidly desensitizing P2X1 receptors to relatively sustained P2X2 receptors. In this study we have used chimeras between human P2X1 and P2X2 receptors in combination with mutagenesis to address the contribution of the extracellular ligand binding loop, the transmembrane channel, and the intracellular regions to receptor time-course. Swapping either the extracellular loop or both transmembrane domains (TM1 and -2) between the P2X1 and P2X2 receptors had no effect on the time-course of ATP currents in the recipient receptor. These results suggest that the agonist binding and channel-forming portions of the receptor do not play a major role in the control of the time-course. In contrast replacing the amino terminus of the P2X1 receptor with that from the non-desensitizing P2X2 receptor (P2X1-2N) slowed desensitization, and the mirror chimera induced rapid desensitization in the P2X2-1N chimera. These reciprocal effects on time-course can be replicated by changing four variant amino acids just before the first transmembrane (TM1) segment. These pre-TM1 residues also had a dominant effect on chimeras where both TMs had been transferred; mutating the variant amino acids 21–23 to those found in the P2X2 receptor removed desensitization from the P2X1-2TM1/-2 chimera, and the reciprocal mutants induced rapid desensitization in the non-desensitizing P2X2-1TM1/-2 chimera. These results suggest that the intracellular amino terminus, in particular the region just before TM1, plays a dominant role in the regulation of the time-course of ATP evoked P2X receptor currents.
Introduction
Purinergic signaling was initially proposed in the 1970s by Burnstock (1), and it is now clear that ATP-gated P2X receptor cation channels have widespread functional roles within the body (2). For example P2X1 receptors make substantial contributions to platelet activation/thrombus formation (3, 4), the neuronal control of smooth muscle function (5–7), and regulation of neutrophil migration (8), while P2X2 receptors are expressed in the nervous system, respond to ATP released from neurons and glial cells (9–11), and can mediate synaptic transmission (12, 13). The seven mammalian P2X receptor subunits (P2X1–7) form functional homo- or heterotrimeric receptors (14, 15). This variety of subunit combinations gives rise to a wide range of phenotypes with ligand profiles ranging from nanomolar ATP sensitivity for P2X1/5 receptors (16), increasing to millimolar levels for P2X7 receptors (17). The P2X receptor subtypes also show variations in time-course (to the continued application of a maximal concentration of ligand) from rapid transient responses that decay (referred to as desensitization) with time constants of hundreds of milliseconds for P2X1 and P2X3 receptors to those where there is little decline in response, e.g. P2X2 and P2X7 receptors (18).
The time-course of response plays an important role in receptor signaling. The desensitization of the P2X1 receptor to high concentrations of ATP restricts signaling to a brief pulse of depolarization and calcium influx (∼ 5–10% of the current passing through the P2X1 receptor channel under physiological conditions is calcium (19–21)). Recovery from desensitization (on removal of ATP) takes ∼5 min and involves regulatory and trafficking events (22, 23). However lower concentrations of ATP may give rise to more sustained signaling, albeit at a substantially reduced level due to the cumulative activation (and desensitization) of the receptors. Desensitization, therefore, provides a mechanism whereby the concentration of a “bolus” of agonist can shape the temporal response from small relatively sustained responses to large transient responses (24). In contrast, P2X2 receptor currents show little desensitization during continued activation over several seconds, and their prolonged application can lead to increases in ionic permeation/pore dilation (18). P2X2 receptors, therefore, can function as effective sensors for variations in ATP levels, for example providing a mechanism for the regulation of synaptic transmission (2) either directly as routes for presynaptic calcium influx (25) and/or modulating the activity of other neuronal ligand gated channels, e.g. nicotinic acetylcholine receptors (26, 27).
The crystallization of the zebrafish P2X4 receptor confirmed that P2X receptors constitute a structurally distinct class of ligand gated ion channels formed from the trimeric assembly of subunits with two transmembrane regions (TM12 and TM2), a large extracellular ligand binding loop and intracellular amino and carboxyl termini (28–30). Many studies have focused on the ionic permeation pathway and shown that the three TM2 segments line the pore of the channel and are encircled by the TM1 segments (31–36). Mutations in either TM can modify agonist potency (36) and the time-course of P2X receptor currents, even leading to constitutively open channels (31–33, 37, 38). These studies clearly indicate that perturbation of the transmembrane regions can regulate channel gating and time-course. Splice variants of the P2X2 receptor suggested that the carboxyl terminal can also play a role in the control of time-course (39, 40). Subsequent work has identified residues in both the amino and carboxyl termini that when mutated change the time-course of response (41–44). More than 15 years ago a study using chimeric P2X receptors showed that the transmembrane segments and adjacent intracellular domains (45) contribute to the regulation of time-course; however; the contribution of just the TM or intracellular segments and the interaction between the intracellular and TM regions was unclear.
To address the role of the transmembrane and intracellular regions in the gating of P2X receptors, we generated a series of chimeras and mutations between human P2X1 (desensitizing) and P2X2 (non-desensitizing) receptors. We found that replacing both transmembrane domains of the P2X1 receptor with those from the P2X2 receptor or vice versa had no effect on the time-course. However, swapping the intracellular amino or carboxyl terminal sequences adjacent to the TMs could have profound effects on the time-course of the response (16 amino acids before TM1 had reciprocal effects in simple and complex chimeras). These results suggest that it is not the TM segments per se but their interaction with the intracellular portions, in particular the pre-TM1 intracellular region, that regulates channel behavior. This study extends our understanding of the control of channel properties and provides a mechanistic insight into how P2X receptor function could be regulated by intracellular accessory proteins.
EXPERIMENTAL PROCEDURES
Generation of Chimeric P2X Receptors and Point Mutations
Chimeric receptors were generated by mega-primer-mediated domain-swapping. To generate the mega-primers, the smaller of the two receptor components required for the chimera needed to be amplified by PCR. Forward and reverse primers (Sigma) were designed to encode 25 base pairs of the region of interest and also a 25-base pair overhang corresponding to the desired insertion location within the body of the receptor chimera. Each 50-μl PCR reaction contained 50 ng of template P2X receptor, 250 pmol of each primer, 100 μm dNTPs, and 2.5 units/ml PfuTurbo DNA polymerase (Agilent Technologies, Cheshire, UK). PCR parameters included an initial denaturation at 95 °C for 3 min followed by 30 cycles (95 °C for 30 s, 55 °C for 60 s, 72 °C for 60 s) and a final elongation at 72 °C for 10 min. The product from this first PCR was purified (Qiagen, Sussex, UK), and 1 μl was used as the mega-primer for the second PCR whereby the larger of the two receptor components required for the chimera was used as template DNA (50 ng of template, 1 μl of mega-primer, 100 μm dNTPs, and 2.5 units of PfuTurbo DNA Polymerase). After initial denaturation at 95 °C for 3 min, DNA fragments were amplified through 16 cycles (95 °C for 30 s, 55 °C for 60 s, 68 °C for 16 min) followed by elongation at 72 °C for 10 min. An aliquot of the reaction was separated by agarose gel electrophoresis whereby a band corresponding to the size of the chimeric gene could be seen. The remaining reaction was treated with Dpn-1 (Agilent) to remove any remaining template DNA and then transformed into XL1-Blue-competent cells (Agilent). The sequence of the resulting clones was verified by sequencing using Leicester University PNACL services.
Substitution of smaller regions of the receptors was performed by the QuikChangeTM mutagenesis kit (Stratagene, La Jolla, CA). Forward and reverse primers were designed to incorporate the change and the PCR reaction set up as per the conditions stated above for the second PCR reaction. Production of the correct mutations and the absence of coding errors in the P2X1 mutant constructs were verified by DNA sequencing.
Expression in Xenopus laevis Oocytes
Wild type, chimeric, and mutant constructs were transcribed to produce sense strand cRNA (mMessage mMachineTM, Ambion, Austin, TX) as described previously (46). Manually defolliculated stage V X. laevis oocytes were injected with 50 nl (50 ng) of cRNA using an Inject + Matic microinjector (J. Alejandro Gaby, Genéva, Switzerland) and stored at 18 °C in ND96 buffer (96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 5 mm sodium pyruvate, 5 mm HEPES, pH 7.6). Media were changed daily before recording 3–7 days later.
Electrophysiological Recordings
Two-electrode voltage clamp recordings (at a holding potential of −60 mV) were carried out on cRNA-injected oocytes using a GeneClamp 500B amplifier with a Digidata 1322 analog-to-digital converter and pClamp 8.2 acquisition software (Axon Instruments, Molecular Devices, Foster City, CA) as previously described (46). Native oocyte calcium-activated chloride currents in response to P2X receptor stimulation were reduced by replacing 1.8 mm CaCl2 with 1.8 mm BaCl2 in the ND96 bath solution. ATP (Mg2+ salt) was applied via a U-tube perfusion system (Sigma). ATP was applied at 5-min intervals to evoke reproducible ATP responses.
The peak amplitude of ATP evoked currents at the chimeras P2X1-2C, P2X1-2Cα, P2X1-2TM2 Cα, P2X1-2C NKVYSH, and P2X2-1-(21–23) TM1/-2 were significantly smaller compared with WT (Table 1). When stored in ND96 with the P2X receptor antagonist suramin (10 μm), after subsequent washing, responses to 100 μm ATP were increased for P2X1-2C (20-fold), P2X1-2Cα (2.5-fold), P2X1-2TM2Cα (5-fold), and P2X2-1-(21–23) TM1/-2 (1.9-fold). Peak ATP evoked currents were also increased after 3 h of incubation in ND96 containing apyrase (3.3 units/ml, to break down endogenously released ATP) for the chimers P2X1-2C (3-fold), P2X1-2Cα (5-fold), and P2X2-1-(21–23) TM1/-2 (3-fold). Peak currents for the P2X1-2C NKVYSH mutant were not changed by either suramin or apyrase treatment. For WT P2X1 receptors apyrase treatment produced a small 0.2-fold increase in peak amplitude. These results suggest that the reduction in peak current amplitudes for the majority of chimeras resulted at least in part from desensitization of the receptor by endogenously released ATP.
TABLE 1.
Summary of effect of chimeras and point mutations on a human P2X1 receptor background
Mean data are shown for peak current amplitude to a maximal concentration of ATP (usually 100 μm) as well as the time-course for the peak current to decay by 50%. For mutants that did not decay by 50% during a 20-s application of a maximal concentration of ATP, the % of peak current remaining at 20 s is given. The decline in current amplitude on washout of ATP (deactivation) is expressed as the % of the current remaining at a 50-s washout. ATP potency is given as pEC50 for some mutants. n = 3–20.
| Predominant P2X1 chimera/mutant | Peak current | pEC50 | In the presence of 100 μm ATP |
Washout of ATP % remaining at 50 s washout | |
|---|---|---|---|---|---|
| Desensitization to 50% peak | % remaining at 20 s | ||||
| μA | s | ||||
| P2X1 | 10.8 ± 1.3 | 6.3 ± 0.02 | 1.1 ± 0.13 | 4.8 ± 1.00 | 0.06 ± 0.03 |
| 2-N | 8.4 ± 3.8 | 6.3 ± 0.13 | 3.7 ± 0.40a | 0.21 ± 0.08 | |
| 2-Nα | 6.4 ± 0.9 | 2.3 ± 0. 22a | |||
| 2-Nβ | 13.9 ± 0.7 | 7.6 ± 0. 56a | |||
| Triple (T) | 9.0 ± 0.8 | 2.5 ± 0.29 | 9.2 ± 1.60 | ||
| T+ 32–35 | 8.9 ± 0.8 | 4.1 ± 0.15 | 13.2 ± 2.65b | ||
| T+ 36–44 | 10.0 ± 0.6 | 2.7 ± 0.07 | 13.9 ± 2.02b | ||
| T+ 45–47 | 11.2 ± 1.5 | 4.0 ± 0.65 | 19.6 ± 0.76a | ||
| 2(D17E) | 14.8 ± 0.9 | 1.8 ± 0.23a | 3.8 ± 3.80 | ||
| 2-(20–23) | 6.4 ± 0.9 | 1.6 ± 0.20a | 11.2 ± 1.16b | ||
| 2-(27–29) | 4.8 ± 0.7 | 0.8 ± 0.06b | 0.4 ± 0.28 | ||
| 2-(D17E) + 20–23 | 6.6 ± 0.1 | 1.9 ± 0.24a | 7.6 ± 1.24 | ||
| 2-(D17E) + 27–29 | 7.0 ± 1.2 | 3.8 ± 0.14a | 0.5 ± 0.13 | ||
| 2-(20–23) + (27–29) | 11.8 ± 1.2 | 3.5 ± 0.73a | 5.9 ± 0.54 | ||
| 2-TM1 | 11.5 ± 2.3 | 7.3 ± 0.09a | 78.1 ± 8.50a | 41.8 ± 2.80a | |
| 2-EXT | 3.4 ± 1.1 | 6.5 ± 0.07b | 0.7 ± 0.03 | 0.9 ± 0.48 | 24.2 ± 0.74a |
| 2-TM2 | 5.5 ± 1.3 | 6.0 ± 0.09 | 67.5 ± 7.19a | 48.3 ± 2.80a | |
| 2-TM1 and -2 | 10.0 ± 1.3 | 6.5 ± 0.16 | 0.6 ± 0.11 | 5.7 ± 0.69 | |
| 2-C | 0.1 ± 0.04c | 5.7 ± 0.14b | 0.07 ± 0.01a,c | 0.04 ± 0.04 | |
| 2-Cα | 1.3 ± 0.4c | 0.1 ± 0.02a | |||
| 2-Cβ | 5.7 ± 0.4 | 0.4 ± 0.08b | |||
| 2-C(NKVYS) | 6.2 ± 1.3 | 0.08 ± 0.02c | 0.08 ± 0.08 | ||
| 2-C(NKVYSH) | 0.5 ± 0.1c | 0.09 ± 0.02c | 0.20 ± 0.20 | ||
| 2-C(TMFM) | 8.0 ± 0.7 | 0.2 ± 0.03b | 0.21 ± 0.07 | ||
| 2-NC | 18.6 ± 1.2a | 5.6 ± 0.14b | 4.7 ± 0.42a | 1.48 ± 0.67 | |
| 2-Nβ Cα | 10.5 ± 1.0 | 1.1 ± 0.07 | 5.3 ± 0.61 | ||
| 2-Nβ TM1 | 6.1 ± 1.4 | 7.0 ± 0.05b | 98.4 ± 0.62a | 47.9 ± 3.23a | |
| 2-Nβ TM1 + 2 | 2.7 ± 0.6 | 90.4 ± 5.80a | 69.3 ± 2.5a | ||
| 2-(D17E) TM1 + 2 | 15.0 ± 1.6 | 9.6 ± 0.75 | 64.8 ± 3.60a | 43.2 ± 2.81a | |
| 2-(20–23) TM1 + 2 | 4.9 ± 0.8 | 95.4 ± 1.40a | 69.4 ± 2.46a | ||
| 2-(21–23) TM1 + 2 | 8.1 ± 0.8 | 99.2 ± 0.28a | 93.8 ± 2.30a | ||
| 2-(27–29) TM1 + 2 | 12.0 ± 2.5 | 2.3 ± 0.28 | 13.0 ± 2.90a | 4.3 ± 0.67 | |
| 2-TM1 + 2 Cα | 3.5 ± 0.5 | 0.2 ± 0.64 | 0.9 ± 0.49b | 1.02 ± 0.38 | |
| 2-Nβ TM1 + 2 Cα | 4.6 ± 0.4 | 80.9 ± 4.40a | 59.8 ± 1.86a | ||
| 2-TM2 Cα | 1.4 ± 0.3a | 92.3 ± 3.70a | 73.7 ± 5.88a | ||
a p < 0.001.
b p < 0.05.
c p < 0.01.
At the P2X2 receptor the rate of deactivation on washout of ATP was concentration-dependent (supplemental Fig. 1), and this reflects the limitations of solution exchange around the oocyte. However, it is clear that for the two chimeras, P2X1-2TM1 and P2X1-2TM2 (supplemental Fig. 1), the slope of this concentration dependence is much shallower and reflects that it is not the rate of solution exchange that limits the speed of current deactivation for these chimeras.
Data Analysis
Desensitization of chimeric receptor and P2X receptor mutants was assessed initially by a 3-s application of maximal ATP (100 μm). If the receptor current decayed by 50% within the ATP application, then data are presented as “time to 50% decay” (ms). For chimeras and mutants whose currents failed to decay by 50% within 3 s, a 20-s application was given, and the percentage of the peak current remaining at the end of the 20 s was calculated (% remaining at 20 s). In addition to measuring desensitization, the deactivation of chimeras and mutants after washout of a 3-s maximal ATP application was also determined. This was done by calculating the percentage of receptor current remaining 50 s after the 3-s ATP application (% current remaining at 50 s).
Individual normalized concentration response curves were fitted with the Hill equation: Y = XH/(XH + EC50)H, where Y is response, X is agonist concentration, H is the Hill coefficient, and EC50 is the concentration of agonist evoking 50% of the maximum response. pEC50 is the −log10 of the EC50 value. In the figures, concentration-response curves are fitted to the mean normalized data.
RESULTS
Replacing Either of the Transmembrane Domains of P2X1 with P2X2 Reduced Desensitization
ATP evoked currents in Xenopus oocytes expressing human P2X1 or P2X2 receptors showed marked differences in the time-course of response to prolonged application of a maximal concentration of ATP (100 μm) (Fig. 1b). For P2X1 receptors the current rapidly peaked (10–90% rise time 73.53 ± 9.59 ms, n = 19) with a mean amplitude of 10.8 ± 1.3 μA (n = 19) before declining (time to 50% decay 1.1 ± 0.12 s, n = 19) to 4.8 ± 1.0% (n = 7) of the peak response at the end of a 20-s pulse. At the P2X2 receptor the rise of the current was ∼6-fold slower (426 ± 98 ms, n = 8), and the currents had a mean peak amplitude of 14.7 ± 2.2 μA (n = 8). In contrast to P2X1 receptor-mediated responses there was little decline in the response at hP2X2 receptors, with 76.13 ± 5.4% (n = 8) remaining at the end of a 20-s pulse.
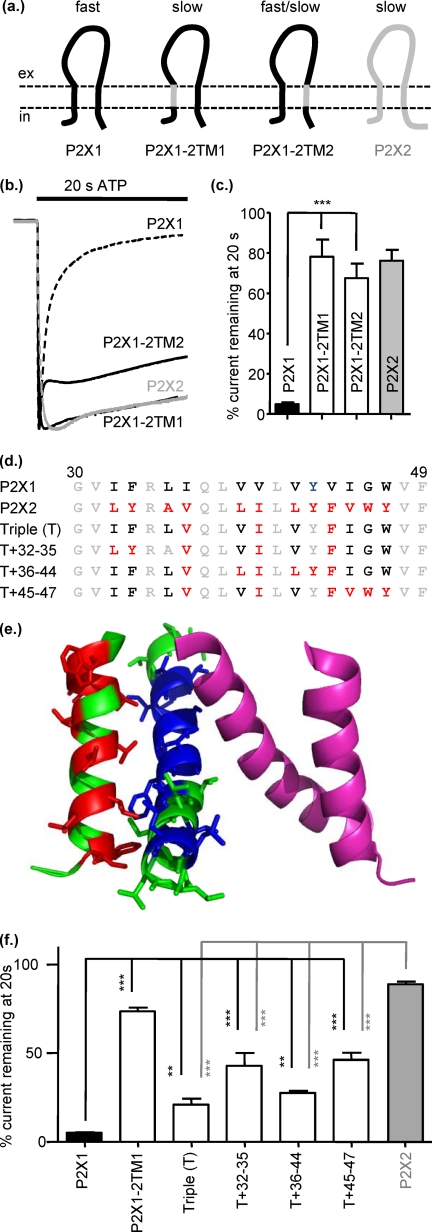
FIGURE 1.
Replacing either of the transmembrane domains of P2X1 with those from P2X2 reduced desensitization. a, schematic shows P2X1 (black) and P2X2 (gray) receptors and the chimeras P2X1-2TM1 and P2X1-2TM2. b, representative currents mediated by application of 100 μm ATP to P2X1 (dotted line), P2X2 (gray line), and chimeric receptors expressed in Xenopus oocytes. c, histogram summary shows the percentage of current remaining at the end of a 20-s ATP application. d, shown is an amino acid sequence lineup of P2X1 and P2X2 TM1. Conserved amino acids are shown in gray, variant amino acids are in black, and mutants of non-conserved amino acids are shown in red. e, a P2X1 receptor homology model shows the TM regions for two subunits (green and magenta); the green subunit non-conserved amino acids in TM1 are shown in red and in blue for TM2. f, shown is a summary of the percentage current remaining at 20s to100 μm ATP application. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 3–10).
The two transmembrane regions, TM1 and TM2, constitute the P2X receptor channel, and mutations in the TMs can have an effect on channel gating. To determine whether the TMs contribute to the regulation of the time-course of P2X receptor currents, we generated chimeras where either the first or second transmembrane segments of the human P2X1 receptor were replaced with the corresponding region from the human P2X2 receptor. Replacement of TM1 (residues 30–49) of the human P2X1 receptor (chimera P2X1-2TM1) resulted in an ∼8-fold slowing in the rise time of the response (567 ± 67.5 ms). The peak current 11.5 ± 2.2 μA and the current decline during the 20-s pulse was indistinguishable from that of the hP2X2 receptor (78.1 ± 8.5 versus 76.1 ± 5.39% for P2X2) (Fig. 1). Swapping TM2 (residues 331–351, chimera P2X1-2TM2) resulted in an intermediate phenotype with a biphasic response to ATP application. The initial peak (10–90% rise time 98.3 ± 5.4 ms and 5.5 ± 1.3 μA peak amplitude) declined rapidly (time for 50% of rapid decay 243 ± 22.6 ms) to 74.2 ± 2.2% of the peak response and then was relatively sustained for the remainder of the ATP application (67.5 ± 7.2% remaining at the end of the 20s pulse) (Fig. 1, Table 1). These chimeras indicate that the transmembrane segments can make a significant contribution to regulation of the time-course of P2X1 receptors.
The crystallization of the zebrafish P2X4 receptor provided a major advance in understanding of the structural and molecular basis of P2X receptor properties (36). The change in time-course of responses after swapping of either of the TM segments suggests that an interaction between TM1 and TM2 is important for stabilizing the P2X receptor in an agonist-bound open conformation. We generated a homology model of the human P2X1 receptor (47) and mapped the amino acids that differ in the TMs between human P2X1 and hP2X2 receptors. This indicates a number of variant amino acids in TM1 that are close to variant residues in TM2 (Fig. 1, d and e). Cysteine scanning mutagenesis studies have investigated the contribution of TM1 to P2X2 receptor properties (33, 37, 38). Interestingly, cysteine mutants of three of the variant amino acids in the middle of TM1 (Val-36, Ile-40, and Phe-44, P2X2 receptor numbering) have been shown to be modified by silver and suggested to interact with TM2 (35). To test whether these three amino acids were important for regulation of the time-course of the response, we generated a triple (T) point mutant where these residues in the P2X1 receptor were mutated to the corresponding residues from the P2X2 receptor (P2X1 Triple (T)) (Fig. 1f). In comparison to the P2X1 receptor, the triple mutation slowed the desensitization of current by 4.4-fold (to 21.08 ± 3.3% remaining at the end of 20s pulse) but did not mimic the effect of swapping the whole of TM1. There are an additional three variant amino acids at the base of TM1 that could interact with TM2. A mutant incorporating the initial triple mutation and these residues P2X1-T+32–35 produced a further reduction in the decay of the response (42.9 ± 7.18% remaining at the end of the 20s pulse); however, it did not match that of the P2X1-2TM1 chimera. There are two conservative valine to leucine substitutions (P2X1 versus P2X2 at positions 39 and 42, P2X1 receptor numbering), raising the possibility that the increased side chain length of the leucine could stabilize the open channel. We, therefore, mutated these residues on the P2X1-T background to give the T+36–44 mutant; however, this had no additional effect on time-course compared with the P2X1-T mutant. This suggests that these two variant amino acids do not contribute to the variations in time-course between P2X1 and P2X2 receptors. There are also three variant amino acids at the top of TM1 (IGW P2X1 and VWY for P2X2), and a mutant incorporating these additional differences to the triple mutant background resulted in a further decrease in current decay during the 20-s ATP pulse but not to levels recorded for the P2X1-2TM1 chimera. These results indicate that no individual amino acids can account for the change in time-course of the P2X1-2TM1 chimera and that it is the general configuration of the TM1 that is important with residues at the bottom, middle, and top of the TM that could form a contact with TM2 that can modify the time-course of the response. This is consistent with a previous study (48) where no individual residue could account for differences in α,β-methylene ATP sensitivity in a chimera-swapping TM1.
Prolongation of Recovery after ATP Washout for P2X1-2TM Chimeras
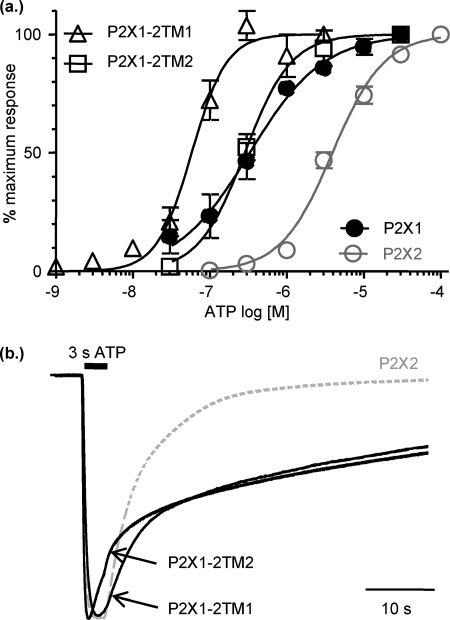
The chimeras of the P2X1 receptor with either of the TMs from the P2X2 receptor show amplitudes of current at the end of a 20-s pulse of ATP similar to those of the P2X2 receptor, suggesting that this simple swap has converted the P2X1 time-course to that of the P2X2 receptor. However, on washing out of ATP (100 μm), it is clear that the deactivation (decline in current on washout of ATP, 100 μm) is considerably prolonged for both the P2X1-2TM1 and P2X1-2TM2 chimeras (current remaining after a 50-s washout as % of response at the end of the ATP pulse, 41.8 ± 2.80, 48.3 ± 2.80, 0.3 ± 0.18 for P2X1-2TM1, P2X1-2TM2, and P2X2, respectively, p < 0.001 compared with P2X2). A previous chimera of the rat P2X1 receptor with the amino terminus and TM1 of the rat P2X2 receptor also showed prolongation of washout, and this was suggested to be due to a change in agonist affinity at the receptor (49). However, P2X2/3 receptor chimeras with equivalent ATP sensitivity had different rates of deactivation (50), and it was suggested that a change in channel gating was likely to be responsible for the variation in speed of current decline on agonist washout. We, therefore, determined whether the TM chimeras had an effect on ATP sensitivity. Swapping TM1 for that from the human P2X2 receptor (chimeras P2X1-2TM1) increased ATP potency at the receptor 12-fold (pEC50 7.3 ± 0.09, p < 0.001) compared with the parent P2X1 receptor (pEC50 6.3 ± 0.02); however, swapping the second TM had no effect (pEC50 6.0 ± 0.09) (Fig. 2a). We, therefore, repeated the washout studies comparing an EC75 concentration of ATP at P2X2 and the TM chimeras to allow direct comparisons of deactivation independent of any change in ATP sensitivity (Fig. 2b). Currents at the P2X2 receptor showed a single exponential decline on washout of ATP with a decay constant of 5.9 ± 0.6 s (n = 4). For either of the TM chimeras there was a considerable slowing in the washout of the ATP current compared with the P2X2 receptor at an EC75 concentration of agonist, but even though there was an ∼12-fold difference in agonist sensitivity, the deactivation rate was the same for both chimeras (decay time constants 54 ± 7 and 47 ± 5 s, respectively, for P2X1-2TM1 and P2X1-2TM2, respectively, n = 3 and 4, p < 0.001 compared with P2X2). These data indicate that for the TM chimeras the slowing in deactivation results predominantly from a change in the gating kinetics of the channel.
FIGURE 2.
Prolongation of current deactivation after ATP washout for P2X1-2TM chimeras. a, shown are concentration responses to ATP for P2X2 (gray line) and chimeric P2X1-2TM1 and P2X1-2TM2 receptors (n = 3–5). b, shown are time-course of currents evoked by a 3-s application of an EC75 concentration of ATP (P2X2 10 μm, P2X1-2TM1 0.1 μm, P2X1-2TM2 1 μm). ATP was applied for 3 s as shown by the black bar. Traces are normalized to the peak current to highlight the prolonged deactivation for the chimeric receptors on washout of ATP.
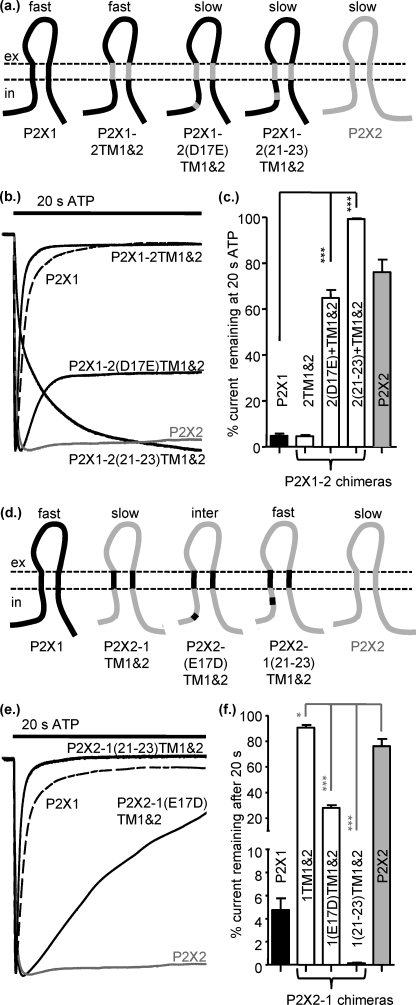
Swapping Both TMs between P2X1 and P2X2 Receptors Has No Effect on the Time-Course of Response
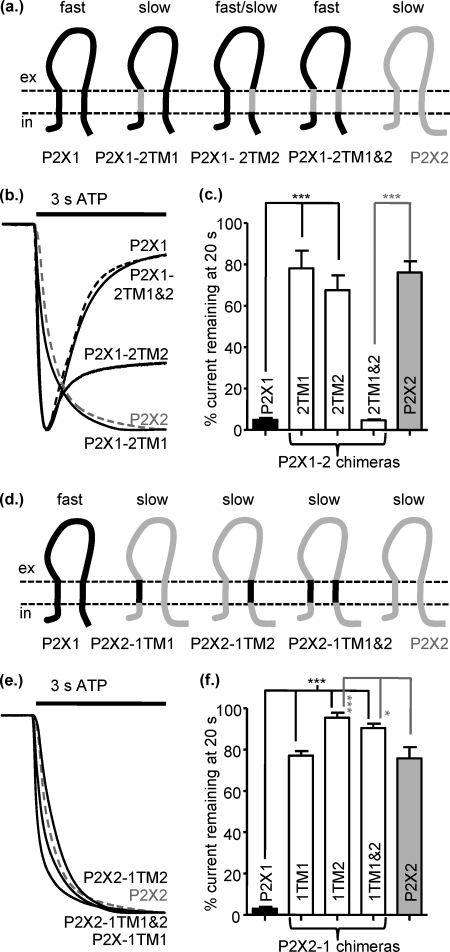
Destabilization of the interactions between the TMs in the P2X1-2TM1 and P2X1-2TM2 chimeras resulted in receptors that appeared like the P2X2 receptor during ATP application but showed delayed deactivation on agonist washout. This suggested that perhaps both TMs are required to recapitulate a P2X2-like time-course to ATP application and washout. We, therefore, generated a chimera P2X1-2TM1/-2, where both TMs were replaced. To our surprise this chimera had the transient time-course of the P2X1 receptor (Fig. 3, a–c) but no effect on ATP sensitivity (pEC50 6.5 ± 0.16). This suggests that it was the interaction between the introduced TMs and the parent P2X1 receptor that resulted in the change in time-course and not an inherent property of the P2X2 receptor TMs themselves.
FIGURE 3.
Swapping both TMs between P2X1 and P2X2 receptors has no effect on the time-course of response. a, shown is a schematic representation of P2X1 (black), P2X2 (gray), and chimeric receptors. b, representative currents mediated by a 3-s application of 100 μm ATP to P2X1 (black dotted line), P2X2 (gray dotted line), and chimeric P2X receptors expressed in Xenopus oocytes. c, a histogram summary shows the percentage of current remaining at the end of a 20-s ATP application. d, shown is a schematic of the reciprocal chimeras on a P2X2 receptor background. e, representative currents are mediated by a 3-s application of 100 μm ATP. f, a histogram summary show the percentage of current remaining at the end of a 20-s 100 μm ATP application. *, p < 0.05; ***, p < 0.001 (n = 3–10).
To test further whether it was the interaction between the TMs that was important, we generated the reciprocal chimeras on a P2X2 receptor background. The chimeras P2X2-1TM1 and P2X2-1TM2 were essentially indistinguishable from the P2X2 receptor in terms of time-course of response (both current remaining at 20 s and time for 50% deactivation on ATP washout) (Fig. 3, d–f, Table 2). These results suggest that having one TM from P2X1 and one from TM2, i.e. interaction between the TMs, does not by itself result in slowed deactivation; if this was the case one would predict that there would be an increase of the time-course of deactivation for the P2X2-1TM1 and P2X2-1TM2 chimeras. The P2X2-1TM1/-2 chimera also had essentially the same time-course as the P2X2 receptor (Fig. 3, d–f, Table 2). This demonstrates that the P2X1 TMs themselves do not independently dictate a transient desensitizing response. Taken together these results suggest that the TM regions can play a role in regulation of the time-course but that interactions with other regions of the receptor play a dominant role.
TABLE 2.
Summary of effect of chimeras and point mutations on a human P2X2 receptor background
Mean data are shown for peak current amplitude to a maximal concentration of ATP (usually 100 μm) as well as the time course for the peak current to decay by 50%. For mutants that did not decay by 50% during a 20-s application of a maximal concentration of ATP, the % of peak current remaining at 20 s is given. The decline in current amplitude on washout of ATP (deactivation) is expressed as the % of the current remaining at 50 s washout. ATP potency is given as pEC50 for some mutants. n = 3–20.
| Predominant P2X2 chimera/mutant | Peak current | pEC50 | In the presence of 100 μm ATP |
Washout of ATP % remaining at 50 s washout | |
|---|---|---|---|---|---|
| Desensitization to 50% peak | % remaining at 20 s | ||||
| μA | s | ||||
| P2X2 | 14.7 ± 2.2 | 4.9 ± 0.02 | 76.1 ± 5.39 | 0.3 ± 0.18 | |
| 1-N | 3.4 ± 0.5 | 4.8 ± 0.05 | 0.38 ± 0.15 | 8.8 ± 0.55a | 1.1 ± 0.97 |
| 1-Nα | 6.0 ± 1.5 | 93.0 ± 3.40b | 3.8 ± 0.50 | ||
| 1-Nβ | 1.2 ± 0.7 | 5.0 ± 0.05 | 0.38 ± 0.40 | 1.7 ± 0.67a | 1.3 ± 0.63 |
| 1-(E17D) | 11.3 ± 1.2 | 91.6 ± 2.58 | 0.8 ± 0.12 | ||
| 1-(20–23) | 3.8 ± 0.7 | 0.60 ± 0.05 | 0.8 ± 0.13a | 0.2 ± 0.06 | |
| 1-(21–23) | 9.3 ± 1.6 | 6.8 ± 0.90 | 21.2 ± 4.29a | 0.7 ± 0.66 | |
| 1-(27–29) | 17.0 ± 1.1 | 84.3 ± 2.47 | 8.0 ± 2.46c | ||
| 1-TM1 | 15.2 ± 1.6 | 77.0 ± 2.21 ns | 6.0 ± 1.73 | ||
| 1-ex | 4.5 ± 0.60 | 81.7 ± 3.43 | 26.2 ± 2.22a | ||
| 1-TM2 | 14.3 ± 1.6 | 95.0 ± 2.44a | 5.4 ± 2.72 | ||
| 1-TM1 and -2 | 20.0 ± 1.9 | 90.4 ± 2.08b | 4.2 ± 1.88 | ||
| 1-C | 6.4 ± 1.3 | 5.2 ± 0.04 | 86.6 ± 1.25 | 1.1 ± 0.70 | |
| 1-NC | 18.3 ± 1.2 | 7.2 ± 0.80 | 39.9 ± 5.42a | 4.0 ± 1.09 | |
| 1-(E17D) TM1 + 2 | 4.6 ± 0.9 | 9.6 ± 0.75 | 27.8 ± 2.28a | 0.005 ± 0.005 | |
| 1-(21–23) TM1 + 2 | 2.6 ± 0.5a | 0.1 ± 0.07 | 0 ± 0 | ||
| 1-TM1 + 2 Cα | 12.3 ± 0.9 | 87.8 ± 1.40 | 9.4 ± 2.33c | ||
a p < 0.001.
b p < 0.05.
c p < 0.01.
Contribution of Intracellular Regions to Regulation of P2X Receptor Time-Course
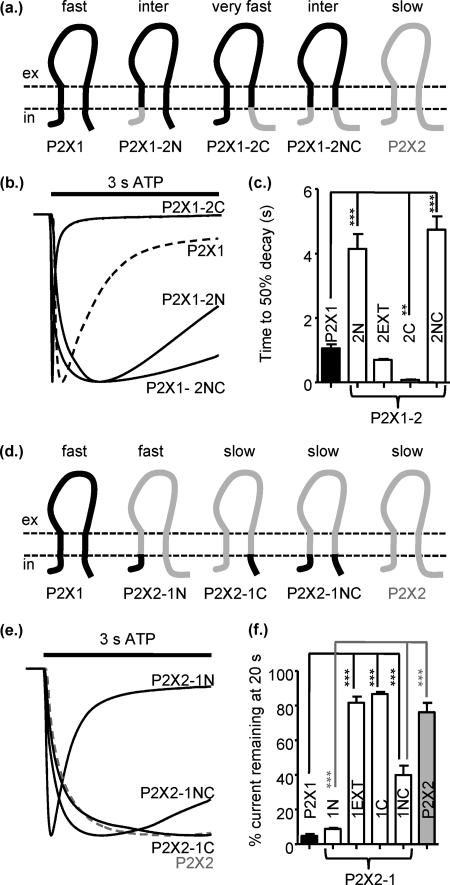
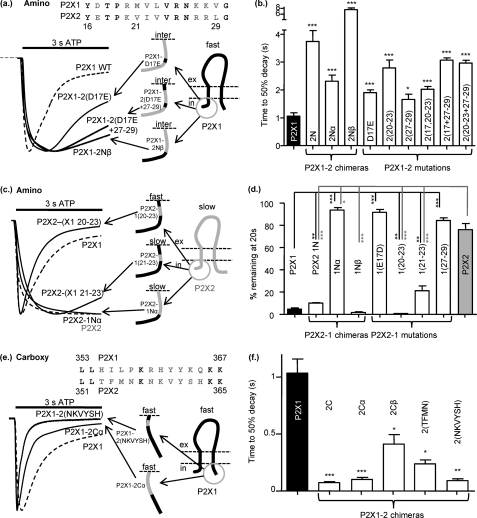
The TM chimeras indicated that additional regions of the receptor are important for the regulation of time-course. Swapping the extracellular regions had no effect on the extent of receptor desensitization (P2X1-2EXT fast desensitization and P2X2-1EXT little desensitization; Fig. 4) and suggests that this ligand binding region does not play a major role in regulation of the time-course. Previous studies had indicated that the intracellular regions could play a role in regulating time-course (39–44), so we generated chimeras swapping the intracellular amino and carboxyl termini. Swapping the intracellular amino termini resulted in a 3.4-fold slowing of desensitization in the P2X1 receptor (the P2X1-2N chimera) and for the P2X2 receptor (the P2X2-1N chimera) introduced marked desensitization similar to that seen for the P2X1 receptor (Fig. 4). These results show that the amino terminus makes a significant contribution to the time-course, and the domain swap has a dominant effect on the resultant receptor. When the carboxyl terminus of the P2X1 receptor was replaced with that from the P2X2 receptor (P2X1-2C), ATP evoked very rapid transient currents (time to 50% decay 0.07 ± 0.01, p < 0.001 compared with P2X1) with peak current amplitudes only 1% of those for P2X1 receptors. The reciprocal chimera on the P2X2 receptor background (P2X2-1C) had essentially the same time-course as the P2X2 receptor. Swapping both the intracellular termini P2X1-2NC, P2X2-1NC produced channels with intermediate rates of current in the continued presence of ATP (times to 50% decay 1.1 ± 0.1, 4.7 ± 0.4, 7.2 ± 0.8s for P2X1, P2X1-2NC, and P2X2-1NC, respectively). These results show that the intracellular amino terminus from the P2X2 receptor can slow P2X1 receptor desensitization, and likewise the P2X1 receptor amino terminus can speed P2X2 receptor time-course, demonstrating that the time-course tracks with the origin of the intracellular amino terminus.
FIGURE 4.
Contribution of the intracellular regions to regulation of P2X receptor time-course. a, shown is a schematic representation of P2X receptors and chimeras. inter, intermediate. b, representative currents were mediated by application of 100 μm ATP to chimeric receptors expressed in Xenopus oocytes. c, a histogram summary shows the time to 50% current decay during the continued presence of ATP. d, shown is a schematic representation of P2X receptors and chimeras. e, representative currents were mediated by application of 100 μm ATP to chimeric receptors expressed in Xenopus oocytes. c, a histogram summary shows the percentage of current remaining at the end of a 20-s 100 μm ATP application. **, p < 0.01; ***, p < 0.001 (n = 5–17).
What Part of the Amino Terminus Is Important for Regulation of Time-Course?
To determine which part(s) of the amino terminus was important for regulation of the time-course, we generated additional chimeras swapping either the first 1–16 amino acids (P2X1-2Nα and P2X2-1Nα) or residues 16–30 (P2X1-2Nβ and P2X2-1Nβ) (Fig. 5). Swapping the first 16 amino acids had little (time to 50% decay 2.1-fold slower for P2X1-2Nα) or no effect (P2X2-1Nα) on responses. However, in contrast-swapping residues 16–30 had a dramatic effect on the time-course, slowing desensitization for the P2X1-2Nβ (7-fold slower to decay to 50%) and for the P2X2-1Nβ (45-fold less current remaining at the end of 20 s ATP), resulting in desensitization that was even faster than for the P2X1 receptor. These results demonstrate that residues just before TM1 play an important role in regulation of time-course and can reciprocally regulate P2X1 and P2X2 receptors.
FIGURE 5.
Regions of the intracellular amino and carboxyl termini involved in regulating time-course. a, shown is a schematic representation of chimeras and representative currents (to 100 μm ATP) of P2X1-2N, P2X1-2Nβ, and P2X1-2 mutations. Mutations are based on the non-conserved amino acid residues within the Nβ region between P2X1 and P2X2 (shown in gray in the amino acid lineup, P2X1 receptor numbering). b, a histogram summary shows the time to 50% decay during continued ATP application. c, shown is a schematic representation and representative currents (to 100 μm ATP) of the reciprocal set of chimeras and P2X2-1 mutations. d, a histogram summary shows the percentage of current remaining at the end of a 20-s 100 μm ATP application. e, a schematic representation and representative currents (to 100 μm ATP) of P2X1-2Cα and P2X1-2 mutations of non-conserved amino acids within this region (non-conserved amino acids between P2X1 and P2X2 are shown in gray on the sequence lineup). f, a histogram summary shows the time to 50% decay during continued ATP application. *, p < 0.05; **, p < 0.01; ***, p < 0.001 (n = 5–17).
Analysis of the amino acid sequence of P2X1 and P2X2 receptors shows there are eight conserved and eight variant residues in the region 16–30 (Fig. 5a). Of the variant amino acids, four are considered conservative substitutions (P2X1 receptor numbering) maintaining the charge D-E (17), R-K (20), and KK-RR (27 and 28) between the two receptor subtypes. The P2X1 receptor point mutations D17E and K27R/K28R/V29L (P2X1-2-(27–29) slowed desensitization but not to the same extent as the P2X1-2Nβ chimera (Fig. 5, a and b). The reciprocal mutations in the P2X2 receptor had no effect on the time-course of the response compared with the parent receptor. At the P2X1 receptor mutation of the other four variant amino acids 20RMNL23 to the corresponding P2X2 residues (P2X1-2-(20–23) also slowed the time-course of the response. We tested combinations of residues, and D17E+27–29 or 20–23 + 27–29 further slowed the decay of the resultant mutants receptors ∼3.5-fold (3.8 ± 0.14, and 3.5 ± 0.73, respectively, n = 6) (Fig. 5). These results suggest that for the P2X1 receptor a considerable portion of the variant region before TM1 needs to be swapped with the P2X2 receptor to modify receptor desensitization and that it is the interaction of residues 17, 20–23, and 27–29 that is important. Swapping the four variant residues 20–23 in the P2X2 receptor (P2X2-1-(20–23)) produced a rapidly desensitizing response very similar to that of the P2X1 receptor (Fig. 5, c and d). When just residues 21–23 were swapped (P2X2-1-(21–23), there was a more modest speeding in the time-course of desensitization (time to 50% decay 6.8 ± 0.90); however, there was still an ∼80% decrease during a 20-s pulse (Fig. 5, c and d). These results suggest that the run of residues 20–23 plays an important role in regulation of the time-course of P2X2 receptors, and relatively conservative mutations can have a marked effect on time-course.
Mutations in the Intracellular Carboxyl Terminus That Speed P2X1 Receptor Desensitization
The marked speeding in desensitization for the P2X1-2C chimera indicated the carboxyl terminus can play a significant role in the regulation of the P2X1 receptor. Interestingly the carboxyl terminus from P2X1 did not have an effect when swapped into the P2X2 receptor. This suggests that that the carboxyl terminus of the P2X1 receptor has a stabilizing influence on the receptor. To address the role of the carboxyl terminus in the regulation of the P2X1 receptor, we generated two additional chimeras swapping either the first 12 amino acids (chimera P2X1-2Cα) or the remainder of the carboxyl terminus (residues 365–460 P2X1-2Cβ) (Fig. 5, e and f). Swapping the first 12 amino acids after TM2 produced a similar speeding in desensitization as changing the whole loop. Changing the remainder of the loop (P2X1-2Cβ) produced a more modest 2.75-fold increase in the desensitization. These results are similar to those from the amino terminus showing that the region closest to the TMs is most important in regulation of the time-course. Analysis of the amino acid sequence shows that there are two clusters of amino acids just after TM2 that are variant between the P2X1 and P2X2 receptors. Mutation of either of these clusters (P2X2 amino acids 353–356 TFMN or 358–363 NKVYSH) to change the P2X1 sequence for the corresponding cluster from the P2X2 receptor resulted in a speeding in desensitization (14- and 12-fold, respectively) (Fig. 5, e and f). This suggests that it is not any individual amino acid that is responsible for the speeding but a conformational/structural property of the carboxyl terminus.
Interactions of Intracellular and TM Regions Regulate Time-Course of P2X Receptors
The chimeras swapping the TMs and point mutants suggested that it was not individual residues/regions of the receptor but interactions between the TMs and the intracellular amino terminus that were important for determining the time-course of response. This was further supported by the finding that swapping both TMs had no effect on the time-course of responses (P2X1-2TM1/-2-fast and P2X2-1TM1/-2-slow; Fig. 3). To test the importance of the interaction, we generated reciprocal mutants in the pre-TM1 residues for the double TM chimeras. The addition of either the point mutation D17E or the triple mutation 21–23 to the P2X1-2TM1 and -2 chimera resulted in swapping the time-course from P2X1 receptor-like rapid desensitization to a P2X2 receptor-like sustained response (Fig. 6, a–c). The equivalent mutations on the P2X2-1TM1/-2 chimera changing either residue(s) 17 or 21–23 from P2X2 to P2X1 had reciprocal effects and produced a marked speeding in desensitization that in the case of the P2X2-1-(21–23)TM1/-2 mutant was indistinguishable from the P2X1 receptor (Fig. 6, d–f). These results show that variations in amino acids between the P2X1 and P2X2 receptors in the region before TM1 play a dominant role in the regulation of the time-course of desensitization of the receptor.
FIGURE 6.
Interactions of intracellular and TM regions regulate time-course of P2X receptors. a, shown is a schematic representation of P2X1-2TM1/-2 chimeric receptors with additional substitution of amino acids 17 and 21–23. Mutations are based on the non-conserved amino acid residues within the Nβ region between P2X1 and P2X2. b, representative currents mediated by application of 100 μm ATP to chimeric receptors expressed in Xenopus oocytes. c, a histogram summary shows the percentage of current remaining at the end of a 20-s ATP application. d, shown is a schematic representation of the reciprocal set of chimeras. e, representative currents mediated by application of 100 μm ATP to chimeric receptors expressed in Xenopus oocytes are shown. f, a histogram summary shows the percentage of current remaining at the end of a 20-s ATP application. *, p < 0.05; ***, p < 0.001 (n = 4–12).
DISCUSSION
In this study we have used a combination of chimeras and point mutations to address the molecular basis of the control of P2X receptor time-course. We have shown that there are significant interactions between the TM segments and the intracellular domains of the P2X receptor. However, it is the intracellular portion of the receptor, in particular the pre-TM1 region, that dominates the time-course of P2X receptor-mediated currents.
The initial chimeras replacing either TM1 or TM2 of the P2X1 receptor with the corresponding region from the P2X2 receptor resulted in chimeric receptors that had sustained responses to the continued presence of ATP. This was consistent with the results reported for similar chimeras that replaced either of the TMs and adjoining parts of the amino or carboxyl termini (45, 49). A study on chimeric rat P2X1/2 receptors suggested that desensitization leads to an underestimate of ATP affinity (by >200-fold) at the P2X1 receptor and that removal of desensitization in the chimera unmasked the true nanomolar potency of ATP at the receptor (49). However, in the current study chimeras or point mutations that changed the time-course from desensitizing to non-desensitizing or vice versa had little (P2X1-2TM1, ∼10-fold) or no effect (P2X1-2TM2 and P2X2-1Nβ) on ATP sensitivity. This is similar to that reported for previous studies on the P2X2 receptor where point mutations that introduced rapid desensitization (K365Q and K369Q) had no effect on ATP potency (42) (these mutations are close to TM2 and may result in desensitization through destabilization of interaction with the pre-TM1 region). These results suggest desensitization has little effect on agonist potency, the human P2X1 receptor has ∼micromolar ATP sensitivity, and that additional effects on channel gating at the rat P2X1-2 chimeras may account for the increase ATP sensitivity.
The structure of the zebrafish P2X4 receptor shows that three TM2s line the channel pore surrounded by three TM1s (36). Mutations in either TM1 or TM2 have effects on the time-course and agonist potency at P2X receptors and suggest that perturbations of the TMs or interactions between them are important for control/regulation of channel gating (31–36). In the present study replacing either of the TMs in the P2X1 receptor (P2X1-2TM1 and P2X1-2TM2) resulted in sustained P2X receptor currents that slowly deactivated. If these changes resulted solely from the effect of changing the TM or disruption of the interaction between the TMs, then it would be predicted that reciprocal chimeras of the P2X2 receptor (P2X2-1TM1 and P2X2-1TM2) should also be non-desensitizing and have prolonged deactivation. Although the P2X2-1TM1 and P2X2-1TM2 chimeras were non-desensitizing, they did not show prolonged deactivation. This suggests that the change in channel behavior for the single TM chimeras P2X1-2TM1 and P2X1-2TM2 (this study) and for cysteine point mutants described in previous studies (31–36) may not necessarily reflect that the TMs play a dominant role in determining the time-course of ATP-evoked responses in native receptors but rather result from structural changes or modifications in regulation by other regions of the receptor. If the TMs played an independent and dominant role in the regulation of time-course, it would be expected that when both TMs were replaced, the time-course of the resulting chimera should be determined by the origin of the TMs. This was not the case, as transferring both TMs between P2X1 and P2X2 receptors had no effect on the time-course of currents in either of the resulting chimeras (P2X1 and P2X1-2TM1/-2 desensitizing, P2X2 and P2X2-1TM1/-2 non-desensitizing). Taken together, these results suggest that it is not the TMs themselves that independently control the time-course but the regulation of the TMs by other regions of the receptor.
Swapping the extracellular domains between P2X1 and P2X2 receptors had no effect on the time-course of desensitization. This is consistent with previous reports where either the introduction of the extracellular loop from a non-desensitizing receptor to a desensitizing receptor (rat P2X1-2EXT) or vice versa (P2X2–3EXT) had no effect on the time-course of the recipient receptor (45, 50). This suggests that the extracellular ligand binding domain by itself does not play a major role in the differences in time-course between P2X receptors. This contrasts with the important role of the agonist binding extracellular amino-terminal domain of AMPA (51) and nicotinic acetylcholine (52) receptors to channel desensitization.
The intracellular amino terminus of P2X receptors is of similar length for all mammalian receptor subtypes and contains three totally conserved residues Tyr-16, Thr-18, and Gly-30 (P2X1 receptor numbering). The threonine residue is also part of a conserved consensus sequence for protein kinase C, and there is evidence that both P2X1 and P2X2 receptors are basally phosphorylated (41, 54). Mutation of the threonine residue led to speeding in desensitization for both P2X1 and P2X2 receptors, indicating the importance of this region of the receptor in the regulation of channel behavior (41, 54, 55). However, cysteine point mutations of the downstream positive charge in the protein kinase C consensus sequence TX(R/K) at P2X1 or P2X2 receptors (37, 53) that would remove phosphorylation have no effect on the time-course of the response, suggesting that it is not the phosphorylation of the threonine but the conformation of the protein that is important for channel regulation.
In this study we show that chimeras swapping the region corresponding to 16 residues before TM1 (Nβ) or the variant amino acids (17 and 20–23, P2X1 receptor numbering) had reciprocal effects on time course, slowing desensitization for P2X1-2Nβ and speeding desensitization for P2X2-1Nβ. These results show that variations in the amino acid sequence in the pre-TM1 region of the amino terminus make a significant contribution to the differences in time-course of P2X1 and P2X2 receptors. The reciprocal mutations at positions 17 and 21–23 had an even greater effect when added to chimeras swapping of both TMs (that on their own had no effect on time-course). These results suggest that the amino terminus plays a dominant role in channel gating and the control of time-course and that this is sensitive to the nature of the TMs. Previous studies on the pre-TM1 region for both P2X1 (53) and P2X2 (37) receptors have shown that ATP-evoked currents amplitudes of cysteine point mutants in this region are sensitive to cysteine-reactive methanethiosulfonate compounds, further highlighting the contribution of this region of the receptor to gating of the ion channel.
ATP binding to the extracellular loop of the P2X receptor leads to conformational changes linked to movements in TM2 and the opening of the receptor channel. The results of the current study suggest that it is the intracellular domains of the receptor, in particular the pre-TM1 amino terminus, that controls the time-course of the channel openings. TM2 forms the pore of the P2X receptor channel, so how could the pre-TM1 region have an effect on this? At present there is little structural information on the organization of the intracellular domains, as the amino and carboxyl termini of the zebrafish P2X4 receptor were truncated to aid crystallization. One possibility is that interactions between the amino and carboxyl termini regulate the movement of TM2. Alternatively coordinated movement of the amino terminus and the attached TM1 could directly regulate the conformational change in TM2.
Supplementary Material
Acknowledgment
We thank Manijeh Maleki-Dizaji for preparation of Xenopus oocytes and RNA injections.
This work was supported by the Wellcome Trust.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- TM
- transmembrane regions
- T
- triple.
REFERENCES
- 1. Burnstock G. (2006) Trends Pharmacol. Sci. 27, 166–176 [DOI] [PubMed] [Google Scholar]
- 2. Surprenant A., North R. A. (2009) Annu. Rev. Physiol. 71, 333–359 [DOI] [PubMed] [Google Scholar]
- 3. Hechler B., Lenain N., Marchese P., Vial C., Heim V., Freund M., Cazenave J.-P., Cattaneo M., Ruggeri Z. M., Evans R. J., Gachet C. (2003) J. Exp. Med. 198, 661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oury C., Kuijpers M. J., Toth-Zsamboki E., Bonnefoy A., Danloy S., Vreys I., Feijge M. A., De Vos R., Vermylen J., Heemskerk J. W., Hoylaerts M. F. (2003) Blood 101, 3969–3976 [DOI] [PubMed] [Google Scholar]
- 5. Mulryan K., Gitterman D. P., Lewis C. J., Vial C., Leckie B. J., Cobb A. L., Brown J. E., Conley E. C., Buell G., Pritchard C. A., Evans R. J. (2000) Nature 403, 86–89 [DOI] [PubMed] [Google Scholar]
- 6. Vial C., Evans R. J. (2002) Mol. Pharmacol. 62, 1438–1445 [DOI] [PubMed] [Google Scholar]
- 7. Vial C., Evans R. J. (2000) Br. J. Pharmacol. 131, 1489–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lecut C., Frederix K., Johnson D. M., Deroanne C., Thiry M., Faccinetto C., Marée R., Evans R. J., Volders P. G., Bours V., Oury C. (2009) J. Immunol. 183, 2801–2809 [DOI] [PubMed] [Google Scholar]
- 9. Vulchanova L., Arvidsson U., Riedl M., Wang J., Buell G., Surprenant A., North R. A., Elde R. P. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 8063–8067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collo G., North R. A., Kawashima E., Merlo-Pich E., Neidhart S., Surprenant A., Buell G. (1996) J. Neurosci. 16, 2495–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pankratov Y., Lalo U., Krishtal O. A., Verkhratsky A. (2009) Neuroscience 158, 137–148 [DOI] [PubMed] [Google Scholar]
- 12. Ren J., Bian X., DeVries M., Schnegelsberg B., Cockayne D. A., Ford A. P., Galligan J. J. (2003) J. Physiol. 552, 809–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finger T. E., Danilova V., Barrows J., Bartel D. L., Vigers A. J., Stone L., Hellekant G., Kinnamon S. C. (2005) Science 310, 1495–1499 [DOI] [PubMed] [Google Scholar]
- 14. North R. A., Surprenant A. (2000) Annu. Rev. Pharmacol. Toxicol. 40, 563–580 [DOI] [PubMed] [Google Scholar]
- 15. Coddou C., Yan Z., Obsil T., Huidobro-Toro J. P., Stojilkovic S. S. (2011) Pharmacol. Rev. 63, 641–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lalo U., Pankratov Y., Wichert S. P., Rossner M. J., North R. A., Kirchhoff F., Verkhratsky A. (2008) J. Neurosci. 28, 5473–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Surprenant A., Rassendren F., Kawashima E., North R. A., Buell G. (1996) Science 272, 735–738 [DOI] [PubMed] [Google Scholar]
- 18. North R. A. (2002) Physiol. Rev. 82, 1013–1067 [DOI] [PubMed] [Google Scholar]
- 19. Benham C. D. (1989) J. Physiol. 419, 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Evans R. J., Lewis C., Virginio C., Lundstrom K., Buell G., Surprenant A., North R. A. (1996) J. Physiol. 497, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Egan T. M., Khakh B. S. (2004) J. Neurosci. 24, 3413–3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewis C. J., Evans R. J. (2000) Br. J. Pharmacol. 131, 1659–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lalo U., Allsopp R. C., Mahaut-Smith M. P., Evans R. J. (2010) J. Neurochem. 113, 1177–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahaut-Smith M. P., Jones S., Evans R. J. (2011) Purinergic Signal 7, 341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robertson S. J., Ennion S. J., Evans R. J., Edwards F. A. (2001) Curr. Opin. Neurobiol. 11, 378–386 [DOI] [PubMed] [Google Scholar]
- 26. Nakazawa K. (1994) J. Neurosci. 14, 740–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khakh B. S., Zhou X., Sydes J., Galligan J. J., Lester H. A. (2000) Nature 406, 405–410 [DOI] [PubMed] [Google Scholar]
- 28. Khakh B. S., North R. A. (2006) Nature 442, 527–532 [DOI] [PubMed] [Google Scholar]
- 29. Evans R. J. (2010) Br. J. Pharmacol. 161, 961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Surprenant A., Buell G., North R. A. (1995) Trends Neurosci. 18, 224–229 [DOI] [PubMed] [Google Scholar]
- 31. Rassendren F., Buell G., Newbolt A., North R. A., Surprenant A. (1997) EMBO J. 16, 3446–3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Egan T. M., Haines W. R., Voigt M. M. (1998) J. Neurosci. 18, 2350–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li M., Chang T. H., Silberberg S. D., Swartz K. J. (2008) Nat. Neurosci. 11, 883–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kracun S., Chaptal V., Abramson J., Khakh B. S. (2010) J. Biol. Chem. 285, 10110–10121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li M., Kawate T., Silberberg S. D., Swartz K. J. (2010) Nat. Commun. 1, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kawate T., Michel J. C., Birdsong W. T., Gouaux E. (2009) Nature 460, 592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang L. H., Rassendren F., Spelta V., Surprenant A., North R. A. (2001) J. Biol. Chem. 276, 14902–14908 [DOI] [PubMed] [Google Scholar]
- 38. Haines W. R., Voigt M. M., Migita K., Torres G. E., Egan T. M. (2001) J. Neurosci. 21, 5885–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brändle U., Spielmanns P., Osteroth R., Sim J., Surprenant A., Buell G., Ruppersberg J. P., Plinkert P. K., Zenner H. P., Glowatzki E. (1997) FEBS Lett. 404, 294–298 [DOI] [PubMed] [Google Scholar]
- 40. Simon J., Kidd E. J., Smith F. M., Chessell I. P., Murrell-Lagnado R., Humphrey P. P., Barnard E. A. (1997) Mol. Pharmacol. 52, 237–248 [DOI] [PubMed] [Google Scholar]
- 41. Boué-Grabot E., Archambault V., Séguéla P. (2000) J. Biol. Chem. 275, 10190–10195 [DOI] [PubMed] [Google Scholar]
- 42. Fujiwara Y., Kubo Y. (2006) J. Physiol. 576, 135–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fountain S. J., North R. A. (2006) J. Biol. Chem. 281, 15044–15049 [DOI] [PubMed] [Google Scholar]
- 44. Bavan S., Farmer L., Singh S. K., Straub V. A., Guerrero F. D., Ennion S. J. (2011) Mol. Pharmacol. 79, 776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Werner P., Seward E. P., Buell G. N., North R. A. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 15485–15490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ennion S., Hagan S., Evans R. J. (2000) J. Biol. Chem. 275, 29361–29367 [DOI] [PubMed] [Google Scholar]
- 47. Allsopp R. C., El Ajouz S., Schmid R., Evans R. J. (2011) J. Biol. Chem. 286, 29207–29217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Haines W. R., Migita K., Cox J. A., Egan T. M., Voigt M. M. (2001) J. Biol. Chem. 276, 32793–32798 [DOI] [PubMed] [Google Scholar]
- 49. Rettinger J., Schmalzing G. (2004) J. Biol. Chem. 279, 6426–6433 [DOI] [PubMed] [Google Scholar]
- 50. Zemkova H., He M. L., Koshimizu T. A., Stojilkovic S. S. (2004) J. Neurosci. 24, 6968–6978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Armstrong N., Jasti J., Beich-Frandsen M., Gouaux E. (2006) Cell 127, 85–97 [DOI] [PubMed] [Google Scholar]
- 52. Giniatullin R., Nistri A., Yakel J. L. (2005) Trends Neurosci. 28, 371–378 [DOI] [PubMed] [Google Scholar]
- 53. Wen H., Evans R. J. (2009) J. Neurochem. 108, 331–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu G. J., Brockhausen J., Bennett M. R. (2003) Auton. Neurosci. 108, 12–16 [DOI] [PubMed] [Google Scholar]
- 55. Ennion S. J., Evans R. J. (2002) Biochem. Biophys. Res. Commun. 291, 611–616 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.