Background: Translation of the α-secretase ADAM10 is repressed by its 5′-untranslated region (5′-UTR).
Results: A G-rich region in the ADAM10 5′-UTR forms a highly stable G-quadruplex secondary structure, which inhibits translation of a luciferase reporter and ADAM10.
Conclusion: The G-quadruplex secondary structure is one inhibitory element for ADAM10 translation.
Significance: Our findings provide new insights in the translational regulation of ADAM10.
Keywords: Alzheimer Disease, Neurodegeneration, RNA Structure, Secretases, Translation Control, ADAM10, G-Quadruplex
Abstract
Anti-amyloidogenic processing of the amyloid precursor protein APP by α-secretase prevents formation of the amyloid-β peptide, which accumulates in senile plaques of Alzheimer disease patients. α-Secretase belongs to the family of a disintegrin and metalloproteases (ADAMs), and ADAM10 is the primary candidate for this anti-amyloidogenic activity. We recently demonstrated that ADAM10 translation is repressed by its 5′-UTR and that in particular the first half of ADAM10 5′-UTR is responsible for translational repression. Here, we asked whether specific sequence motifs exist in the ADAM10 5′-UTR that are able to form complex secondary structures and thus potentially inhibit ADAM10 translation. Using circular dichroism spectroscopy, we demonstrate that a G-rich region between nucleotides 66 and 94 of the ADAM10 5′-UTR forms a highly stable, intramolecular, parallel G-quadruplex secondary structure under physiological conditions. Mutation of guanines in this sequence abrogates the formation of the G-quadruplex structure. Although the G-quadruplex structure efficiently inhibits translation of a luciferase reporter in in vitro translation assays and in living cells, inhibition of G-quadruplex formation fails to do so. Moreover, expression of ADAM10 was similarly repressed by the G-quadruplex. Mutation of the G-quadruplex motif results in a significant increase of ADAM10 levels and consequently APPsα secretion. Thus, we identified a critical RNA secondary structure within the 5′-UTR, which contributes to the translational repression of ADAM10.
Introduction
The pathological hallmarks of Alzheimer disease are extracellular amyloid plaques and intracellular neurofibrillary tangles. Amyloid plaques are composed of the amyloid-β peptide, which is liberated via sequential cleavage of the amyloid precursor protein (APP)3 by β- and γ-secretase (1). Alternatively, α-secretase cleaves APP within its amyloid-β domain and therefore prevents formation of the neurotoxic amyloid-β peptide. α-Secretase cleavage liberates APPsα, which may have neuroprotective and neurotrophic properties (2, 3). The remaining C-terminal fragment of APP is further processed by γ-secretase to produce the non-amyloidogenic fragment p3 (4).
Three members of the large family of disintegrin and metalloproteinases (ADAM) apparently exert α-secretase activity: ADAM9, ADAM10, and ADAM17 (5–7). Among these, ADAM10 is the major candidate for the physiological α-secretase because moderate overexpression of ADAM10 in an Alzheimer disease mouse model resulted in increased APPsα shedding, lowering of amyloid-β peptide generation, and consequently, a reduction of the amyloid plaque load (8). In addition, it was shown recently that siRNA-mediated knockdown of ADAM10 in mammalian cells and primary neurons abolished the generation of APPsα, whereas knockdown of ADAM9 or ADAM17 still allowed robust production of APPsα (9). ADAM10 knock-out mice die at day embryonic day 9.5 of embryogenesis with multiple defects in the developing central nervous system, somite segmentation, and the cardiovascular system, emphasizing an important role of ADAM10 in development (10). To analyze the function of ADAM10 in brain development, neuron-specific ADAM10-deficient mice were generated (11). These mice die perinatally due to a down-regulation of Notch signaling in the brain. However, analysis of APP processing in primary neuronal cultures from embryonic day 14.5 of these conditional ADAM10 knock-out mice revealed a 90% reduction of APPsα generation (11). These data therefore demonstrate that ADAM10 is the predominant α-secretase activity in the brain. Apart from APP and Notch, ADAM10 cleaves other neuronal proteins, like ephrins, L1 adhesion molecule, and N-cadherin, which are important for neurite outgrowth and migration (12, 13). Finally, ADAM10 is up-regulated in several cancers and plays a role in inflammation processes (13).
Regulation of ADAM10 is achieved by multiple mechanisms. ADAM10 is expressed as an inactive prodomain containing zymogene, which is activated by the proprotein convertases furin and PC7 (14). The prodomain is important for folding of ADAM10 and acts as a potent inhibitor of ADAM10 activity (14, 15). Recently, it was demonstrated that ADAM10 transcription is stimulated by all-trans retinoic acid and by the deacetylase Sirtuin1 (16, 17). Moreover, we demonstrated previously that ADAM10 expression could be suppressed at the translational level by its unusual long GC-rich 5′-UTR (18). Therefore, we hypothesize that the 5′-UTR of ADAM10 may affect its translation via RNA binding proteins and/or stable RNA secondary structures.
Current knowledge implicates that translational regulation occurs predominantly at the level of initiation and two distinct general modes could be discriminated, global translational regulation, and mRNA-specific regulation (19–21). In general, global control of translation is mediated by phosphorylation of initiation factors (19–21). Translational regulation of specific mRNAs is often achieved by cis-acting elements within UTRs of these mRNAs such as secondary structures, upstream ORFs, internal ribosomal entry sites, and/or trans-acting elements like mRNA binding proteins and microRNAs (19–21). Interestingly, translation of the β-secretase BACE1 is suppressed by its long GC-rich 5′-UTR, which contains several upstream ORFs (22–24). Increased translation of BACE1 was observed in response to energy deprivation, due to increased translation reinitiation at the start codon of the BACE1 message (25), similar to the translation of yeast GCN4 and ATF4 (26, 27). Moreover, translational repression could be achieved by blocking the recruitment of the preinitiation complex to an mRNA. The best characterized examples for this mechanism are iron-regulatory proteins (IRPs) (28, 29). In iron-deficient cells, IRPs bind to iron-responsive elements (IREs), which form stable stem loops in 5′-UTRs of L- and H-ferritin mRNAs and inhibit translation of these mRNAs (28, 29). An increase of the cellular iron concentration results in dissociation of IRPs from the 5′-UTRs, which leads to increased ferritin mRNA translation (29). More recently, it was hypothesized that RNA G-quadruplex structures within 5′-UTRs are potent translational repressors that block formation or scanning of the preinitiation complex (30). G-quadruplexes are higher-order nucleic acid secondary structures formed by G-rich sequences. The basic structural core motif of G-quadruplexes consists of a series of G-quartet planes, each of which consists of four guanines connected by Hoogsteen-hydrogen bonds and stabilized by monovalent ions, particularly potassium ions (31–34). There is growing evidence that the formation of G-quadruplex secondary structures within 5′-UTRs results in translational repression of a number of different mRNAs, including N-ras, Zic-1, membrane-type matrix metalloproteinase 3, ESR-1, and Bcl-2 (35–42). Similar to the IRE stem loop, the stability of G-quadruplex structures could be modulated by RNA binding proteins such as FMRP or members of the hnRNP A family (43, 44).
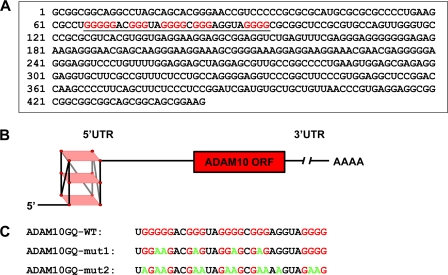
Interestingly, the 5′-UTR of ADAM10 contains a G-rich sequence between nucleotides 66–94, which, based on a prediction algorithm, may form a stable G-quadruplex secondary structure (see Fig. 1, A and B). Using CD spectroscopy, we provide evidence that this sequence indeed folds into a stable RNA G-quadruplex in vitro and that mutation of several guanines inhibits G-quadruplex formation. The G-quadruplex inhibits a luciferase reporter in cell free lysates and living cells. Moreover, we demonstrate that mutation of the G-quadruplex motif results in increased ADAM10 translation and consequently increased the anti-amyloidogenic processing of APP.
FIGURE 1.
The 5′-UTR of ADAM10 contains a G-quadruplex motif. A, representation of the human ADAM10 5′-UTR-RNA sequence. The predicted G-quadruplex sequence located between nucleotides 66 and 94 of the ADAM10 5′-UTR is underlined. The guanines predicted to be involved in the formation of the potential G-quadruplex secondary structure are highlighted in red. B, model of the parallel ADAM10 5′-UTR G-quadruplex secondary structure. Guanines (red circles) of the canonical repeats of the G-rich stretches involved in G-quadruplex formation are located at the four edges of each plane marked in light red. C, sequences of RNA oligonucleotides used for CD spectroscopy measurements in this study. Guanines potentially involved in G-quadruplex formation are marked in red, and substitutions to adenines are highlighted in green.
EXPERIMENTAL PROCEDURES
Oligonucleotides
The following RNA oligonucleotides were purchased from Thermo Scientific: ADAM10GQ-WT, UGGGGGACGGGUAGGGGCGGGAGGUAGGGG; ADAM10GQ-mut1, UGGAAGACGAGUAGGAGCGAGAGGUAGGGG; and ADAM10GQ-mut2, UAGAAGACGAAUAGAAGCGAAAAGUAGAAG.
CD Spectroscopy
For spectroscopic studies, RNA oligonucleotides were prepared at 5 μm strand concentration in RNase-free water containing 10 mm Tris/HCl, 0.1 mm EDTA, pH 7.4 in a final volume of 250 μl. The samples were annealed by heating at 90 °C for 10 min and subsequent slow cooling to 20 °C at a constant rate of 0.2 °C/min. CD measurements were performed after a 10-min equilibration at 20 °C using a Jasco J-810 spectropolarimeter equipped with a Peltier temperature cooler in a 0.1-cm cell at a scanning speed of 50 nm/min with a response time of 8 s. The spectra were averaged over 11 scans from 200–320 nm, and data were zero-corrected at 320 nm. For each sample, a buffer baseline was obtained in the same cuvette and subtracted from the average scan.
CD melting curves were recorded as described above in the presence of 1 mm KCl by monitoring ellipticity at 263 nm between 20 and 90 °C. The melting temperature (Tm) was calculated using the van't Hoff method (45).
cDNA Constructs
The ADAM10 G-quadruplex motif and mutated variants thereof in front of Renilla luciferase of the psiCHECK-2 vector (Promega) were generated by PCR using the unique NheI restriction site upstream of the Renilla luciferase start codon. pcDNA6/V5-HisA-5′-UTR-Luc with the intact ADAM10 G-quadruplex motif was described previously (18). Mutated variants of the G-quadruplex motif, 5′-UTR-GQmut1-Luc (TGGAAGACGAGTAGGAGCGAGAGGTAGGGG) and 5′-UTR-GQmut2-Luc (TAGAAGACGAATAGAAGCGAAAAGTAGAAG) were introduced by PCR using appropriate primers. In addition, plasmid pcDNA6/V5HisA-ADAM10 (18), was used to generate GQ-WT ADAM10 and the corresponding mutants GQ-mut1-ADAM10 and GQ-mut2-ADAM10 using NheI/HindIII restriction sites and appropriate primers. All cDNAs were verified by sequencing.
Cell Culture and cDNA Transfections
Human embryonic kidney 293EBNA (HEK293) cells were cultured in DMEM supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, and 2 mm glutamine. Transfections were carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
Protein Analysis
1.8 × 106 HEK293 cells were plated in 6-cm dishes and transiently transfected with 8 μg of cDNA encoding ADAM10 variants and 0.1 μg of pEGFP-N1 (Clontech). Protein analysis was performed as described previously (18). For analysis of APP processing, the cell culture medium was replaced 24 h after transfection, and the cells were incubated for 4 h in fresh medium. Equal amounts of conditioned media were analyzed for APPsα using antibody 2D8 (1 μg/ml) (46). Full-length APP was detected using the APP-C-terminal antibody (A8717) from Sigma.
Dual-Luciferase Reporter Assay
1.8 × 105 HEK293 cells were seeded in 24-well plates and transfected with 0.8 μg of psiCHECK-2-GQ-WT, psiCHECK-2-GQ-mut1, or psiCHECK-2-GQ-mut2. 24 h after transfection, cell lysates were prepared, and luciferase activity was measured with the Dual-Luciferase reporter assay system (Promega) according to the manufacturer's instructions. Quantification was performed using an LB96V luminometer (Berthold Technologies) and analyzed with WinGlow software (Berthold Technologies). Renilla luciferase activity was normalized to firefly luciferase activity.
Quantitative Real-time PCR
Total RNA was isolated from HEK293 cells 24 h after transfection with psiCHECK-2-GQ plasmids using the RNeasy mini kit (Qiagen), including an on-column DNase digest. Subsequently, the RNA was treated a second time with DNase I (DNA-free, Ambion). cDNA was synthesized from 250 ng of total RNA using MessageSensor RT (Ambion) with random hexamer primers. Quantitative real-time PCR was performed with 2× Power SYBR Green PCR Master Mix (Applied Biosystems) and 0.5 μm of each primer pair (RLuc 344, 5′-TCTTTGTGGGCCACGACTGGGG-3′ (forward primer); RLuc 603, 5′-GGCAGCGAACTCCTCAGGCTCC-3′ (reverse primer); and FLuc 976, 5′-GCCGTGGCCAAGCGCTTTCATC-3′ (forward primer); FLuc 1150, 5′-CTCCCAGGGTCTTGCCGGTGTC-3′ (reverse primer)). ADAM10 mRNA levels were determined as described previously (18). Quantification was performed with the 7500 Fast Real-time PCR system (Applied Biosystems). For each RNA sample, triplicates were analyzed with each primer set, and Renilla luciferase RNA expression was normalized to firefly luciferase as described (47).
In Vitro Transcription
Plasmids were linearized using the XhoI restriction enzyme, which cuts at the 3′ end of the coding region of the luciferase reporter gene. 5′-Capped transcripts were generated in vitro using the mMESSAGE mMACHINE T7 kit (Ambion), following the manufacturer's instructions. The RNA concentration was determined by UV spectroscopy. The integrity and the size of each transcript were confirmed by 1% agarose gel analysis.
In Vitro Translation
In vitro translation of 100 ng of in vitro-transcribed mRNAs was carried out in a cell-free translation system consisting of extracts from nuclease-treated rabbit reticulocyte lysate (Promega) as described (48). Firefly luciferase activity was measured in duplicate as described above.
RESULTS
The 5′-UTR of ADAM10 Contains a G-rich Region, which Forms a Stable G-quadruplex Secondary Structure
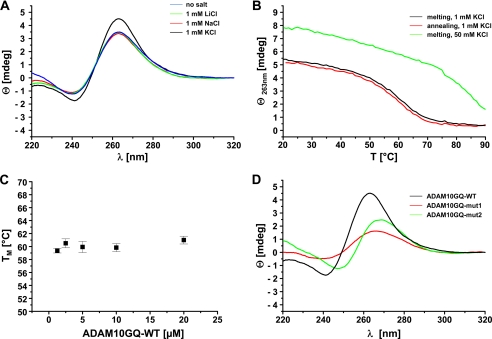
Using the G-quadruplex secondary prediction algorithm Quadfinder (49), we identified a potential G-quadruplex motif between nucleotide 66 and 94 of the human ADAM10 5′-UTR (Fig. 1, A and B). This G-quadruplex motif is evolutionary conserved between human, chimpanzee, and rhesus monkey, indicating that it might have an important physiological function. To prove that this sequence indeed forms a stable G-quadruplex secondary structure, we performed CD spectroscopy, a standard technique to investigate the formation of G-quadruplex structures by oligonucleotides (34, 50). We analyzed the CD spectrum of ADAM10GQ-WT RNA-oligonucleotide (Fig. 1C) at pH 7.4 in the absence of salt or in the presence of 1 mm LiCl, NaCl, or KCl (Fig. 2A). As a control, we determined the CD spectrum for the previously characterized G-quadruplex of N-ras in the presence of 1 mm and 100 mm KCl (supplemental Fig. 1) (35). We observed the characteristic CD signature for a parallel RNA G-quadruplex (51) with a positive peak at 263 nm and a negative peak at 241 nm for ADAM10GQ-WT in the absence of salt, suggesting an inherent propensity of the sequence to form a G-quadruplex (Fig. 2A) (35, 37). Addition of 1 mm KCl significantly increased the positive peak at 263 nm and a negative peak at 241 nm, consistent with the finding that potassium ions could stabilize the formation of G-quadruplexes (32, 34–37, 52). In the presence of 1 mm LiCl, the CD spectrum was not altered as expected because lithium ions do not support the formation of G-quadruplexes (35, 37, 52). In our experiments, addition of 1 mm NaCl also had no effect on G-quadruplex formation, possibly due to the lower propensity of sodium ions compared with potassium ions to stabilize G-quadruplex structures (32, 34, 35, 37, 42, 52).
FIGURE 2.
Biophysical analysis of the ADAM10 G-quadruplex motif. A, CD spectra of 5 μm ADAM10GQ-WT oligonucleotide in the absence (blue) or presence of different monovalent cations (green, LiCl; red, NaCl; black, KCl; 1 mm each) in 10 mm Tris/HCl (pH 7.4), 0.1 mm EDTA. Note that the formation of a stable G-quadruplex structure was strongly induced in the presence of 1 mm KCl. B, CD melting experiments of 5 μm ADAM10GQ-WT in the presence of 1 mm KCl. Melting (black) and annealing (red) curves are almost identical and show a Tm of 60 ± 1 °C. In contrast, at 50 mm KCl (green), the folded G-quadruplex could not be unfolded at higher temperatures. C, plot of Tm values for ADAM10GQ-WT at various strand concentrations. All experiments were performed in the presence of 10 mm Tris/HCl (pH 7.4), 0.1 mm EDTA, and 1 mm KCl. Results are expressed as the mean ± S.D. of at least three different measurements. D, CD spectra in the presence of 1 mm KCl of ADAM10GQ-WT (black) and mutated variants thereof (red, ADAM10GQ-mut1; green, ADAM10GQ-mut2).
To determine the thermal stability of the G-quadruplex, we performed CD melting experiments at 263 nm of ADAM10GQ-WT in the presence of 1 mm KCl (Fig. 2B). Melting and annealing curves were virtually identical and Tm was determined to be 60 ± 1 °C, assuming a single cooperative transition between the folded and unfolded state of the G-quadruplex structure (37, 45). Consistent with previous reports (35–37, 40–42, 53), at higher KCl concentrations (50 mm), the structure could not be unfolded even at 90 °C, which is indicative of a very stable G-quadruplex (Fig. 2B). Based on the van't Hoff method (45), we determined the thermodynamic parameters for the melting curves at 1 mm KCl. The Gibbs' free energy ΔGvH at 37 °C was −10.6 ± 0.8 kJ/mol, suggesting the formation of a stable G-quadruplex structure at 37 °C. Moreover, the calculated values for ΔHvH (−151.5 ± 13.2 kJ/mol) and ΔSvH (−0.45 ± 0.04 kJ/mol K) were comparable with published data of other G-quadruplexes (37, 53–55). To further investigate whether ADAM10GQ-WT forms an intermolecular or intramolecular G-quadruplex, we determined the melting temperature Tm at different concentrations of ADAM10GQ-WT in the range of 1–20 μm and in the presence of 1 mm KCl (35–37, 53, 56). As shown in Fig. 2C, Tm remains unchanged at 60 °C in this concentration range, indicating that ADAM10GQ-WT forms a unimolecular G-quadruplex. Finally, we performed CD spectroscopy with mutant variants of ADAM10GQ-WT. We substituted several guanines, which might be involved in G-quadruplex formation by adenines (Fig. 1C), to prevent the formation of an intramolecular, parallel G-quadruplex structure (35–37). Indeed, for ADAM10GQ-mut1 and ADAM10GQ-mut2, both CD spectra showed a lower ellipticity in the presence of 1 mm KCl (Fig. 2D). Moreover, compared with the CD spectrum of the wild-type sequence, the maxima and minima were shifted slightly to higher wave lengths similar to the CD spectra of unstructured, single-stranded RNA (37, 40, 57). Taken together, our biophysical analysis confirms that the ADAM10GQ-WT RNA forms a highly stable, unimolecular, parallel G-quadruplex near physiological pH and salt conditions.
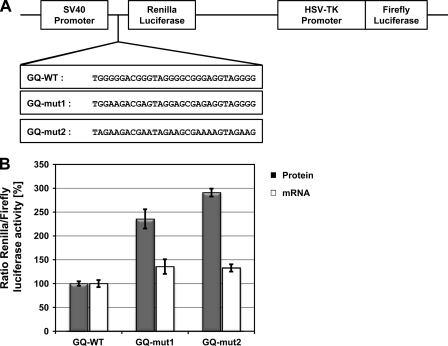
The ADAM10 G-quadruplex Motif Inhibits Translation of a Luciferase Reporter in Vivo
Recently, it was shown that G-quadruplex motifs within 5′-UTRs inhibit translation of their downstream gene in vitro and in vivo (35–42). Moreover, artificially introduced RNA-G-quadruplex motifs near the ribosomal binding site suppress bacterial gene expression (58). Therefore, we investigated whether the ADAM10 G-quadruplex motif could inhibit translation in living cells. We cloned the ADAM10 G-quadruplex motif and its two mutant variants in front of the Renilla luciferase coding region of the psiCHECK-2 vector (Fig. 3A). 24 h after transfection of the resulting plasmids in HEK293 cells, we performed Dual-Luciferase reporter assays as described (36, 37, 39). Strikingly, for both mutant variants, GQ-mut1 and GQ-mut2, we observed a significant increase in Renilla luciferase activity normalized to firefly luciferase activity ratios (Fig. 3B). Using quantitative RT-PCR, we observed only a very subtle increase in mRNA levels for both mutants (Fig. 3B). These results confirm that the ADAM10 G-quadruplex can suppress translation.
FIGURE 3.
Translational repression of a luciferase reporter by the ADAM10 G-quadruplex motif. A, Schematic representation of the plasmids used for reporter gene assays. The wild-type G-quadruplex sequence (GQ-WT) of the ADAM10 5′-UTR or mutated variants thereof were cloned directly in front of the Renilla coding region. B, 24 h after transfection of the indicated plasmids in HEK293 cells dual-luciferase assays were performed and mRNA was isolated. Renilla luciferase activity was normalized to Firefly luciferase activity and the value for GQ-WT was set to 100%. CT values for Renilla and Firefly luciferase mRNA were determined by quantitative RT-PCR and the ratio of CT Renilla/CT Firefly was calculated as described (47). Results are expressed as means ± S.D. of at least three independent experiments made in triplicates.
Inhibition of G-quadruplex Formation in the Context of the Entire ADAM10–5′-UTR Facilitates Luciferase Translation
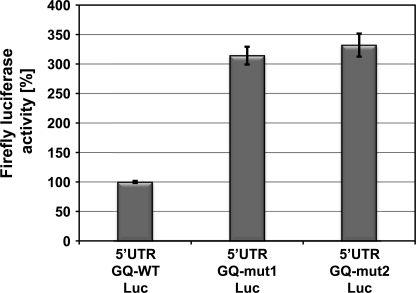
To further demonstrate that the G-quadruplex motif is an inhibitory element within the entire context of the ADAM10-5′-UTR, we performed in vitro translation assays. Equal amounts of in vitro-transcribed 5′-UTR-GQ-WT-luciferase, 5′-UTR-GQ-mut1 luciferase and 5′-UTR-GQ-mut2 luciferase mRNA were translated in nuclease-treated rabbit reticulocyte lysate. Consistent with the data presented in Fig. 3B, luciferase activity measurements revealed that both mutations of the ADAM10 G-quadruplex motif resulted in a 3-fold increase in firefly luciferase activity compared with the wild-type 5′-UTR-luciferase construct (Fig. 4). Taken together, these findings demonstrate that the ADAM10 G-quadruplex alone or within the context of the ADAM10-5′-UTR represses translation of a reporter gene and suggests that the formation of a very stable G-quadruplex secondary structure is responsible for translational repression.
FIGURE 4.
ADAM10 G-quadruplex motif in context of entire 5′-UTR inhibits translation of firefly luciferase reporter. Equal amounts of in vitro-transcribed firefly luciferase mRNAs with the full-length 5′-UTR of ADAM10 containing the wild-type G-quadruplex sequence (5′-UTR-GQ-WT-Luc) or the indicated mutations as depicted in Fig. 1C (5′-UTR-GQ-mut1-Luc, 5′-UTR-GQ-mut2-Luc) were subjected to in vitro translation using nuclease-treated rabbit reticulocyte lysates. Results are expressed as means ± S.D. of three independent experiments made in triplicate.
The G-quadruplex Efficiently Inhibits ADAM10 Translation
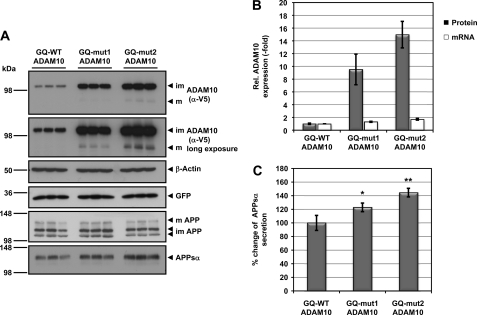
Recently, we demonstrated that the 5′-UTR of ADAM10 is involved in translational repression of ADAM10 (18). Based on the in vitro findings, we assumed that the ADAM10 G-quadruplex motif could contribute to this translational inhibition. To prove this, we cloned the ADAM10 G-quadruplex motif and the corresponding mutants directly in front of the ADAM10 coding region. After transient transfection of these cDNA constructs in HEK293 cells, we observed a 9.5- to 15-fold increase in ADAM10 protein levels when the ADAM10 G-quadruplex motif was mutated (Fig. 5, A and B). As shown previously, overexpression of ADAM10 results predominantly in the generation of immature ADAM10 (18). Nevertheless, we detected elevated levels of secreted APPsα in supernatants of cells transfected with G-quadruplex mutations (Fig. 5, A and C). Because there was only a small increase in mRNA levels (Fig. 5B), our data strongly suggest that the G-quadruplex is involved in translational repression of ADAM10.
FIGURE 5.
ADAM10 expression is repressed by the G-quadruplex motif. A, HEK293 cells were transiently transfected with the indicated ADAM10 cDNA constructs, and lysates were analyzed by immunoblotting for V5-tagged ADAM10, endogenous APP, β-actin as loading control, and GFP as transfection control. Supernatants were analyzed for APPsα secretion using antibody 2D8. Cellular APP is present in low molecular weight immature forms (im) and high molecular weight mature form (m). ADAM10 is present as a mature (m) form and predominantly as an immature (im) form. B, quantification of ADAM10 protein (black bars) and mRNA levels (white bars) from cells transfected with ADAM10 cDNA constructs shown in A. ADAM10 protein levels were normalized to GFP and actin levels. The signal for ADAM10 with the wild-type G-quadruplex GQ-WT ADAM10 was set to 1. Results are expressed as the means ± S.D. from three experiments made in triplicate. ADAM10 mRNA was normalized to glycerolaldehyde-3-phosphate-dehydrogenase mRNA levels, and the signal for GQ-WT ADAM10 was set to 1. Results are expressed as the means ± S.D. from three experiments. C, quantification of secreted APPsα from cells transfected with the indicated ADAM10 variants were shown in A. The signal for APPsα from GQ-WT ADAM10 transfected cells was set to 100%. Results are expressed as the means ± S.D. from three experiments. Asterisks indicate statistical significance (one-way analysis of variance with Dunnett's post test) relative to GQ-WT ADAM10 transfected cells (*, p < 0.05; **, p < 0.01).
Taken together, we demonstrate that the ADAM10-5′-UTR contains a G-quadruplex motif that is able to form a highly stable secondary structure in vitro. This G-quadruplex is sufficient for translational suppression of a reporter gene and ADAM10 in living cells. Hence, the G-quadruplex secondary structure is one element contributing to the translational inhibition of ADAM10 expression via its 5′-UTR.
DISCUSSION
There is growing evidence that canonical repeats of G-rich stretches in DNA or RNA can form stable G-quadruplex secondary structures, which are implicated in a variety of biological processes like telomere protection, stabilization, and replication, as well as transcription, splicing, and translation of RNA (30, 33, 59–61). Recently, it was demonstrated that besides telomeres and promoter regions, 5′- and 3′-untranslated regions of mRNAs are hotspots of potential G-quadruplex forming sequences (30). The authors suggested that G-quadruplex structures within 3′-UTRs might facilitate transcriptional termination leading to an efficient cleavage at the polyadenylation site and polyadenylation of the mRNA (30). In addition, more recently, it was demonstrated that stable G-quadruplex secondary structures within the 3′-UTR of PSD95 and CaM kinase IIa are important neurite mRNA-targeting elements (62). On the other hand, G-quadruplex signature motifs occur predominantly at the 5′ end of the 5′-UTR, which suggests that G-quadruplex secondary structures are involved in translational regulation either by inhibiting the formation of the initiation complex or by inhibition of the scanning ribosome (30). In agreement with this hypothesis, a series of recent reports demonstrate that the 5′-UTRs of N-ras, Zic-1, membrane-type matrix metalloproteinase 3, ESR-1, Bcl-2, and others contain a G-quadruplex motif that inhibits the translation of their downstream genes (35–42).
We recently demonstrated that the 444-nucleotide-long, GC-rich 5′-UTR of ADAM10 is responsible for the translational repression of ADAM10 (18). Using the G-quadruplex secondary prediction algorithm Quadfinder (49), we now identified a potential G-quadruplex motif between nucleotides 66 and 94 of the human ADAM10 5′-UTR consistent with the transcriptome-wide prediction analysis of G-quadruplex structures reported previously (30, 40). It was reported that RNA nucleosides prefer the anti-conformation of the glycosidic bond due to the C3′-endo puckering of sugar, and hence RNA G-quadruplex structures adopt a parallel topology with a characteristic CD spectrum (34, 51). Consistent with this finding and previously reported RNA-G-quadruplexes, our CD spectroscopy analyses provide evidence that the G-quadruplex sequence of the ADAM10 5′-UTR forms a parallel, intramolecular G-quadruplex secondary structure that is further stabilized by potassium ions. We demonstrate that even at 1 mm KCl, the G-quadruplex is extremely stable and has a high melting temperature of 60 ± 1 °C, whereas at 50 mm KCl, the G-quadruplex could not be unfolded even at 90 °C, arguing that under physiological conditions at 37 °C and an intracellular potassium concentration of ∼130 mm, the formation of the G-quadruplex structure is favored highly. Similar results were reported for the G-quadruplex of the N-ras 5′-UTR and TRF2 5′-UTR in the presence of 1 mm KCl (35, 41) (see also supplemental Fig. 1). Moreover, determination of the thermodynamic parameters ΔGvH = −10.6 ± 0.8 kJ/mol, ΔHvH = −151.5 ± 13.2 kJ/mol, and ΔSvH = −0.45 ± 0.04 kJ/mol K, in the presence of 1 mm KCl are comparable with known DNA and RNA G-quadruplexes (37, 54, 55). In a recent study, the dependence of the stability of RNA G-quadruplexes on the loop length between the G-quadruplex forming G-tetrads was determined (53). The authors found that the thermodynamic stability but not the structure is dependent on loop length. G-quadruplexes with long loops, e.g. oligonucleotides with two or four nucleotides between each G-tetrad, which were termed oligonucleotide library L222 or L444, have melting temperatures between 67 and 50 °C in the presence of 5 mm KCl (53). Our data are in accordance with these results as the ADAM10 G-quadruplex could be grouped in the oligonucleotide library categories L222, L331, or L322 with a total loop length of six or seven nucleotides (53).
To investigate the effect of the G-quadruplex structure on translation, we performed two different well established assays (35–42): Dual-Luciferase reporter assays and in vitro translation assays with luciferase mRNAs containing the wild-type ADAM10 5′-UTR or ADAM10 5′-UTRs with a mutated G-quadruplex motif. Using CD spectroscopy, we demonstrated that our two RNA oligonucleotides with the mutated G-quadruplex sequences do not have the same characteristic CD spectrum as the wild-type oligonucleotide. Dual-Luciferase reporter assays revealed a significant increase in luciferase activity for the two mutant variants compared with the wild-type G-quadruplex-containing reporter. This increase in reporter activity is consistent with previously published reports for G-quadruplex containing 5′-UTR mRNAs such as Zic-1 (36), membrane-type matrix metalloproteinase 3 (37), EBAG9, AASDHPPT, BARHL2, THRA, NCAM2, and FDZ2 (40). To further confirm these results, we performed in vitro translation assays using equal amounts of in vitro-transcribed luciferase mRNAs containing the full-length ADAM10 5′-UTR with the wild-type G-quadruplex motif and mutant variants thereof. Mutation of the ADAM10 G-quadruplex signature motif resulted in a 3-fold increase of luciferase activity for both mutants compared with the wild-type 5′-UTR. These findings are again in agreement with recently described results for the 5′-UTR mRNAs of N-ras (35), ESR-1 (38), Bcl-2 (42), and TRF2 (41).
We further investigated the effect of the G-quadruplex and mutations thereof on the translation of ADAM10. As expected from our reporter assays, we observed that mutation of the G-quadruplex motif resulted in higher ADAM10 levels and in an increase of APPsα secretion. We observed a 9.5- to 15-fold increase in ADAM10 expression with our mutated G-quadruplex ADAM10 constructs, which is in accordance to our recently observed 9-fold increase of ADAM10 expression after transient expression of Δ1–155-5′-UTR ADAM10, a construct lacking the first 155 nucleotides of the ADAM10 5′-UTR and hence the entire G-quadruplex motif (18). However, deletion of the first 259 nucleotides of the ADAM10 5′-UTR led to a nearly 100-fold increase in expression of ADAM10 (18), strongly suggesting that the ADAM10 5′-UTR contains several translational inhibitory elements and that the G-quadruplex motif is one of these inhibitory elements. Although we observed a significant increase in ADAM10 protein, we only detected a 1.3- and 1.7-fold increase in ADAM10 mRNA levels for GQ-mut1-ADAM10 and GQ-mut2-ADAM10, respectively. However, such a moderate increase in mRNA levels could not explain the 9.5- and 15-fold increase of protein levels. Because DNA could also form G-quadruplex secondary structures, we could not completely rule out that transcription of ADAM10 might be influenced by a DNA-G-quadruplex formed in the complementary strand (30, 59). In fact, it was reported that the transcription of several proto-oncogenes, including c-myc, K-ras, c-kit, Bcl-2, and platelet-derived growth factor receptor-β is suppressed by G-quadruplex structures in their promoters (63–67). Because the expression of ADAM10 in our cDNA constructs is under the control of the very strong CMV promoter, it is rather unlikely that increased transcription is responsible for the observed effects. Instead, it was reported that the cytoplasmic 5′-3′-exoribonuclease mXrn1, which is involved in mRNA degradation, exhibits a substrate preference for G-quadruplex containing mRNAs (68). This could explain the slight increase in mRNA levels of GQ-mut1-ADAM10 and GQ-mut2-ADAM10.
Because the G-quadruplex in the ADAM10 5′-UTR is involved in translational repression, it appears reasonable that genetic variations in the G-quadruplex motif might have an impact on translation of ADAM10. To identify SNPs in G-quadruplex motif containing 5′-UTRs, a bioinformatic analysis was recently performed using all human G-quadruplex sequences from the UTRef collection of the UTRdb database (40). Interestingly, in 5% of these sequences, SNPs occur, which might have an impact on translation of the downstream gene (40). However, no SNP was found for the ADAM10 G-quadruplex sequence, which does not rule out that SNPs in other regions of the ADAM10 5′-UTR might have an impact on translation.
Translational repression of mRNAs is often achieved via RNA secondary structures in 5′-UTRs (19, 69, 70). Interestingly, the 5′-UTRs of the mRNAs coding for BACE1, APP, and ADAM10 were found to be involved in translational regulation of their downstream gene products (18, 71, 72). Translational repression of BACE1 is mediated by its long, structured, and upstream ORFs containing 5′-UTR (22–24). Energy deprivation results in phosphorylation of eIF2α and consequently in an arrest of global translation; however, BACE1 expression is increased due to reinitiation of translation at the initiation codon of the BACE1 mRNA (25, 73). In contrast, it was reported that similar to the 5′-UTR of L- and H-ferritin the 5′-UTR of APP contains an IRE, and translation of APP was repressed by IRP1 under low cellular iron concentrations (74). Iron uptake in cells result in the dissociation of the IRE-IRP repressor complex and increased translation of ferritin and APP (72). The exact mechanism how this is accomplished is not known yet; however, a recent study suggests that Fe2+ might weaken the IRE-IRP repressor complex in vitro (75). Moreover, it was reported that binding of poly-C binding protein 1 to the acute box cis element within the 5′-UTR of the human H-ferritin mRNA facilitates H-ferritin translation when the cytosolic iron concentration is increased (76). These data demonstrate that translational inhibitory RNA elements could be modulated by RNA binding proteins. Therefore, we hypothesize that such RNA binding proteins selectively bind and modulate the ADAM10 5′-UTR G-quadruplex structure. FMRP, an RNA binding protein involved in mRNA translation, splicing, and mRNA transport in the cell, was identified as a G-quadruplex interacting protein (77–79). FMRP binds tightly to G-quadruplexes in the 5′-UTRs of protein phosphatase 2A catalytic subunit and MAP1B (microtubule-associated protein 1B), and it was suggested that this interaction represses translation of both mRNAs (77, 80). Furthermore, it was demonstrated that the FMRP-G-quadruplex repressor in the MAP1B 5′-UTR was destabilized by an increased FMRP concentration, suggesting that the variation of FMRP concentration in response to neuronal stimulation might act as a regulatory switch from translational repressor to a translational activator (43). The RGG box of FMRP was shown to be responsible for the interaction with the G-quadruplex secondary structure in the MAP1B 5′-UTR (43). Therefore, methylation of RGG motifs in FMRP by PMRT1 might regulate the interaction of FMRP with polyribosomes and G-quadruplex-containing mRNAs (81). In addition, members of the hnRNP A family were reported to destabilize G-quadruplex structures by a so-far unknown mechanism (44, 82). Apart from FMRP and hnRNP A, there are several proteins described that are able to interact and modify G-quadruplex structures in DNA and RNA substrates. For instance, specific RNA and DNA helicases are able to unwind G-quadruplex structures (83–86). Deficiencies of certain G-quadruplex resolving helicases result in abnormal mRNA deadenylation and decay and in perturbed telomere maintenance and cellular DNA replication, leading to cancer (87). In addition, it was reported recently that nucleolin stabilizes the G-quadruplex within the c-myc promoter and inhibits transcription (88). Interestingly, nucleolin interacts with G-rich sequences in UTRs or the coding region of a lot of mRNAs and was found to enhance their translation (89), suggesting that nucleolin might function as a RNA-G-quadruplex interacting factor.
ADAM10 expression and activity is reduced in platelets and neurons of Alzheimer disease patients (90, 91). Translational repression of ADAM10 might be one possible explanation for this observation. Based on the above discussed regulatory mechanisms, we are currently investigating whether ADAM10 translation is modulated by G-quadruplex interacting proteins.
Taken together, we provide evidence that a stable G-quadruplex secondary structure within the ADAM10 5′-UTR is involved in translational regulation of ADAM10. The development of selective G-quadruplex unwinding molecules, which are able to cross the blood brain barrier, might be a new therapeutic venue for the treatment of Alzheimer disease.
Acknowledgment
We thank Mehak Mumtaz, a scholar of the Amgen Summer School Programme, for technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft (Collaborative Research Center (SFB596) “Molecular Mechanisms of Neurodegeneration” (to S. L., M. W., and C. H.)), a fellowship of the Hans and Ilse Breuer Foundation (to S. Z.), the Helmholtz Alliance (Mental Health in an Aging Society, HelMA), the Bundesministerium für Bildung und Forschung (“Degenerative Dementias: Target Identification, Validation, and Translation into Treatment Strategies” (to C. H.)), and by the Center of Integrated Protein Science Munich.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- APP
- amyloid precursor protein
- IRP
- iron-regulatory protein
- FMRP
- fragile-X mental retardation protein
- IRE
- iron-responsive element
- Tm
- melting temperature
- ΔHvH
- van't Hoff enthalpy
- ΔSvH
- van't Hoff entropie
- ΔGvH
- van't Hoff free energy change
- Luc
- luciferase.
REFERENCES
- 1. Haass C., Selkoe D. J. (2007) Nat. Rev. Mol. Cell Biol. 8, 101–112 [DOI] [PubMed] [Google Scholar]
- 2. Meziane H., Dodart J. C., Mathis C., Little S., Clemens J., Paul S. M., Ungerer A. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 12683–12688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Furukawa K., Sopher B. L., Rydel R. E., Begley J. G., Pham D. G., Martin G. M., Fox M., Mattson M. P. (1996) J. Neurochem. 67, 1882–1896 [DOI] [PubMed] [Google Scholar]
- 4. Haass C., Hung A. Y., Schlossmacher M. G., Teplow D. B., Selkoe D. J. (1993) J. Biol. Chem. 268, 3021–3024 [PubMed] [Google Scholar]
- 5. Koike H., Tomioka S., Sorimachi H., Saido T. C., Maruyama K., Okuyama A., Fujisawa-Sehara A., Ohno S., Suzuki K., Ishiura S. (1999) Biochem. J. 343, 371–375 [PMC free article] [PubMed] [Google Scholar]
- 6. Buxbaum J. D., Liu K. N., Luo Y., Slack J. L., Stocking K. L., Peschon J. J., Johnson R. S., Castner B. J., Cerretti D. P., Black R. A. (1998) J. Biol. Chem. 273, 27765–27767 [DOI] [PubMed] [Google Scholar]
- 7. Lammich S., Kojro E., Postina R., Gilbert S., Pfeiffer R., Jasionowski M., Haass C., Fahrenholz F. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 3922–3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Postina R., Schroeder A., Dewachter I., Bohl J., Schmitt U., Kojro E., Prinzen C., Endres K., Hiemke C., Blessing M., Flamez P., Dequenne A., Godaux E., van Leuven F., Fahrenholz F. (2004) J. Clin. Invest. 113, 1456–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuhn P. H., Wang H., Dislich B., Colombo A., Zeitschel U., Ellwart J. W., Kremmer E., Rossner S., Lichtenthaler S. F. (2010) EMBO J. 29, 3020–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hartmann D., de Strooper B., Serneels L., Craessaerts K., Herreman A., Annaert W., Umans L., Lübke T., Lena Illert A., von Figura K., Saftig P. (2002) Hum. Mol. Gen. 11, 2615–2624 [DOI] [PubMed] [Google Scholar]
- 11. Jorissen E., Prox J., Bernreuther C., Weber S., Schwanbeck R., Serneels L., Snellinx A., Craessaerts K., Thathiah A., Tesseur I., Bartsch U., Weskamp G., Blobel C. P., Glatzel M., De Strooper B., Saftig P. (2010) J. Neurosci. 30, 4833–4844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang P., Baker K. A., Hagg T. (2006) Prog. Neurobiol. 79, 73–94 [DOI] [PubMed] [Google Scholar]
- 13. Pruessmeyer J., Ludwig A. (2009) Semin. Cell Dev. Biol. 20, 164–174 [DOI] [PubMed] [Google Scholar]
- 14. Anders A., Gilbert S., Garten W., Postina R., Fahrenholz F. (2001) FASEB J. 15, 1837–1839 [DOI] [PubMed] [Google Scholar]
- 15. Moss M. L., Bomar M., Liu Q., Sage H., Dempsey P., Lenhart P. M., Gillispie P. A., Stoeck A., Wildeboer D., Bartsch J. W., Palmisano R., Zhou P. (2007) J. Biol. Chem. 282, 35712–35721 [DOI] [PubMed] [Google Scholar]
- 16. Tippmann F., Hundt J., Schneider A., Endres K., Fahrenholz F. (2009) FASEB J. 23, 1643–1654 [DOI] [PubMed] [Google Scholar]
- 17. Donmez G., Wang D., Cohen D. E., Guarente L. (2010) Cell 142, 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Lammich S., Buell D., Zilow S., Ludwig A. K., Nuscher B., Lichtenthaler S. F., Prinzen C., Fahrenholz F., Haass C. (2010) J. Biol. Chem. 285, 15753–15760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gebauer F., Hentze M. W. (2004) Nat. Rev. Mol. Cell Biol. 5, 827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson R. J., Hellen C. U., Pestova T. V. (2010) Nat. Rev. Mol. Cell Biol. 11, 113–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sonenberg N., Hinnebusch A. G. (2009) Cell 136, 731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Pietri Tonelli D., Mihailovich M., Di Cesare A., Codazzi F., Grohovaz F., Zacchetti D. (2004) Nucleic Acids Res. 32, 1808–1817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lammich S., Schöbel S., Zimmer A. K., Lichtenthaler S. F., Haass C. (2004) EMBO Rep. 5, 620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rogers G. W., Jr., Edelman G. M., Mauro V. P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2794–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Connor T., Sadleir K. R., Maus E., Velliquette R. A., Zhao J., Cole S. L., Eimer W. A., Hitt B., Bembinster L. A., Lammich S., Lichtenthaler S. F., Hébert S. S., De Strooper B., Haass C., Bennett D. A., Vassar R. (2008) Neuron 60, 988–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hinnebusch A. G. (1997) J. Biol. Chem. 272, 21661–21664 [DOI] [PubMed] [Google Scholar]
- 27. Vattem K. M., Wek R. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muckenthaler M., Gray N. K., Hentze M. W. (1998) Mol. Cell 2, 383–388 [DOI] [PubMed] [Google Scholar]
- 29. Hentze M. W., Muckenthaler M. U., Galy B., Camaschella C. (2010) Cell 142, 24–38 [DOI] [PubMed] [Google Scholar]
- 30. Huppert J. L., Bugaut A., Kumari S., Balasubramanian S. (2008) Nucleic Acids Res. 36, 6260–6268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huppert J. L. (2010) FEBS J. 277, 3452–3458 [DOI] [PubMed] [Google Scholar]
- 32. Burge S., Parkinson G. N., Hazel P., Todd A. K., Neidle S. (2006) Nucleic Acids Res. 34, 5402–5415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel D. J., Phan A. T., Kuryavyi V. (2007) Nucleic Acids Res. 35, 7429–7455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paramasivan S., Rujan I., Bolton P. H. (2007) Methods 43, 324–331 [DOI] [PubMed] [Google Scholar]
- 35. Kumari S., Bugaut A., Huppert J. L., Balasubramanian S. (2007) Nat. Chem. Biol. 3, 218–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arora A., Dutkiewicz M., Scaria V., Hariharan M., Maiti S., Kurreck J. (2008) RNA 14, 1290–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morris M. J., Basu S. (2009) Biochemistry 48, 5313–5319 [DOI] [PubMed] [Google Scholar]
- 38. Balkwill G. D., Derecka K., Garner T. P., Hodgman C., Flint A. P., Searle M. S. (2009) Biochemistry 48, 11487–11495 [DOI] [PubMed] [Google Scholar]
- 39. Halder K., Wieland M., Hartig J. S. (2009) Nucleic Acids Res. 37, 6811–6817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beaudoin J. D., Perreault J. P. (2010) Nucleic Acids Res. 38, 7022–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gomez D., Guédin A., Mergny J. L., Salles B., Riou J. F., Teulade-Fichou M. P., Calsou P. (2010) Nucleic Acids Res. 38, 7187–7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shahid R., Bugaut A., Balasubramanian S. (2010) Biochemistry 49, 8300–8306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Menon L., Mader S. A., Mihailescu M. R. (2008) RNA 14, 1644–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khateb S., Weisman-Shomer P., Hershco-Shani I., Ludwig A. L., Fry M. (2007) Nucleic Acids Res. 35, 5775–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mergny J. L., Lacroix L. (2003) Oligonucleotides 13, 515–537 [DOI] [PubMed] [Google Scholar]
- 46. Shirotani K., Tomioka M., Kremmer E., Haass C., Steiner H. (2007) Neurobiol. Dis. 27, 102–107 [DOI] [PubMed] [Google Scholar]
- 47. Pfaffl M. W. (2001) Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kumari S., Bugaut A., Balasubramanian S. (2008) Biochemistry 47, 12664–12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scaria V., Hariharan M., Arora A., Maiti S. (2006) Nucleic Acids Res. 34, W683–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Balagurumoorthy P., Brahmachari S. K., Mohanty D., Bansal M., Sasisekharan V. (1992) Nucleic Acids Res. 20, 4061–4067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tang C. F., Shafer R. H. (2006) J. Am. Chem. Soc. 128, 5966–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hardin C. C., Watson T., Corregan M., Bailey C. (1992) Biochemistry 31, 833–841 [DOI] [PubMed] [Google Scholar]
- 53. Zhang A. Y., Bugaut A., Balasubramanian S. (2011) Biochemistry 50, 7251–7258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rachwal P. A., Brown T., Fox K. R. (2007) Biochemistry 46, 3036–3044 [DOI] [PubMed] [Google Scholar]
- 55. Rachwal P. A., Brown T., Fox K. R. (2007) FEBS Lett. 581, 1657–1660 [DOI] [PubMed] [Google Scholar]
- 56. Zhang D. H., Fujimoto T., Saxena S., Yu H. Q., Miyoshi D., Sugimoto N. (2010) Biochemistry 49, 4554–4563 [DOI] [PubMed] [Google Scholar]
- 57. Gray D. M., Liu J. J., Ratliff R. L., Allen F. S. (1981) Biopolymers 20, 1337–1382 [Google Scholar]
- 58. Wieland M., Hartig J. S. (2007) Chem. Biol. 14, 757–763 [DOI] [PubMed] [Google Scholar]
- 59. Huppert J. L., Balasubramanian S. (2007) Nucleic Acids Res. 35, 406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kostadinov R., Malhotra N., Viotti M., Shine R., D'Antonio L., Bagga P. (2006) Nucleic Acids Res. 34, D119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brooks T. A., Kendrick S., Hurley L. (2010) FEBS J. 277, 3459–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Subramanian M., Rage F., Tabet R., Flatter E., Mandel J. L., Moine H. (2011) EMBO Rep. 12, 697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siddiqui-Jain A., Grand C. L., Bearss D. J., Hurley L. H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11593–11598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cogoi S., Xodo L. E. (2006) Nucleic Acids Res. 34, 2536–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fernando H., Reszka A. P., Huppert J., Ladame S., Rankin S., Venkitaraman A. R., Neidle S., Balasubramanian S. (2006) Biochemistry 45, 7854–7860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dai J., Chen D., Jones R. A., Hurley L. H., Yang D. (2006) Nucleic Acids Res. 34, 5133–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Qin Y., Fortin J. S., Tye D., Gleason-Guzman M., Brooks T. A., Hurley L. H. (2010) Biochemistry 49, 4208–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bashkirov V. I., Scherthan H., Solinger J. A., Buerstedde J. M., Heyer W. D. (1997) J. Cell Biol. 136, 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pickering B. M., Willis A. E. (2005) Semin. Cell Dev. Biol. 16, 39–47 [DOI] [PubMed] [Google Scholar]
- 70. van der Velden A. W., Thomas A. A. (1999) Int. J. Biochem. Cell Biol. 31, 87–106 [DOI] [PubMed] [Google Scholar]
- 71. Willem M., Lammich S., Haass C. (2009) Semin. Cell Dev. Biol. 20, 175–182 [DOI] [PubMed] [Google Scholar]
- 72. Cahill C. M., Lahiri D. K., Huang X., Rogers J. T. (2009) Biochim. Biophys. Acta 1790, 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Velliquette R. A., O'Connor T., Vassar R. (2005) J. Neurosci. 25, 10874–10883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cho H. H., Cahill C. M., Vanderburg C. R., Scherzer C. R., Wang B., Huang X., Rogers J. T. (2010) J. Biol. Chem. 285, 31217–31232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Khan M. A., Walden W. E., Goss D. J., Theil E. C. (2009) J. Biol. Chem. 284, 30122–30128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thomson A. M., Cahill C. M., Cho H. H., Kassachau K. D., Epis M. R., Bridges K. R., Leedman P. J., Rogers J. T. (2005) J. Biol. Chem. 280, 30032–30045 [DOI] [PubMed] [Google Scholar]
- 77. Darnell J. C., Jensen K. B., Jin P., Brown V., Warren S. T., Darnell R. B. (2001) Cell 107, 489–499 [DOI] [PubMed] [Google Scholar]
- 78. Schaeffer C., Bardoni B., Mandel J. L., Ehresmann B., Ehresmann C., Moine H. (2001) EMBO J. 20, 4803–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Melko M., Bardoni B. (2010) Biochimie 92, 919–926 [DOI] [PubMed] [Google Scholar]
- 80. Castets M., Schaeffer C., Bechara E., Schenck A., Khandjian E. W., Luche S., Moine H., Rabilloud T., Mandel J. L., Bardoni B. (2005) Hum. Mol. Genet. 14, 835–844 [DOI] [PubMed] [Google Scholar]
- 81. Blackwell E., Zhang X., Ceman S. (2010) Hum. Mol. Genet. 19, 1314–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Khateb S., Weisman-Shomer P., Hershco I., Loeb L. A., Fry M. (2004) Nucleic Acids Res. 32, 4145–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chakraborty P., Grosse F. (2011) DNA Repair 10, 654–665 [DOI] [PubMed] [Google Scholar]
- 84. Lattmann S., Giri B., Vaughn J. P., Akman S. A., Nagamine Y. (2010) Nucleic Acids Res. 38, 6219–6233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Creacy S. D., Routh E. D., Iwamoto F., Nagamine Y., Akman S. A., Vaughn J. P. (2008) J. Biol. Chem. 283, 34626–34634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. London T. B., Barber L. J., Mosedale G., Kelly G. P., Balasubramanian S., Hickson I. D., Boulton S. J., Hiom K. (2008) J. Biol. Chem. 283, 36132–36139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu Y., Brosh R. M., Jr. (2010) FEBS J. 277, 3470–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. González V., Guo K., Hurley L., Sun D. (2009) J. Biol. Chem. 284, 23622–23635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abdelmohsen K., Tominaga K., Lee E. K., Srikantan S., Kang M. J., Kim M. M., Selimyan R., Martindale J. L., Yang X., Carrier F., Zhan M., Becker K. G., Gorospe M. (2011) Nucleic Acids Res. 39, 8513–8530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Colciaghi F., Borroni B., Pastorino L., Marcello E., Zimmermann M., Cattabeni F., Padovani A., Di Luca M. (2002) Mol. Med. 8, 67–74 [PMC free article] [PubMed] [Google Scholar]
- 91. Bernstein H. G., Bukowska A., Krell D., Bogerts B., Ansorge S., Lendeckel U. (2003) J. Neurocytol. 32, 153–160 [DOI] [PubMed] [Google Scholar]