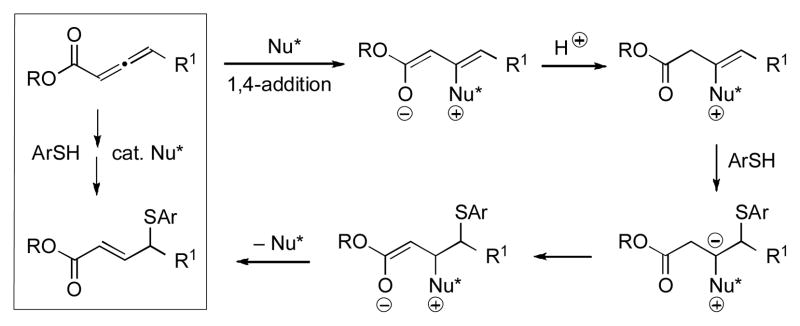
Abstract
An effective phosphine-catalyzed method has been developed for the enantioselective addition of aryl thiols to the γ position of allenoates, thereby furnishing ready access to aryl alkyl sulfides in very good ee. An array of mechanistic data are consistent with addition of the chiral phosphine to the allenoate being the turnover-limiting step of the catalytic cycle. The optimized reaction conditions, as well as the mechanistic observations, differ markedly from an earlier report on asymmetric additions of alkyl thiols to allenoates, which highlights the potential for divergent behavior between alkyl and aryl thiols when serving as nucleophiles.
As phosphines have emerged as versatile nucleophilic catalysts, the use of chiral phosphines to control the enantioselectivity of various processes has begun to be pursued with substantial vigor.1 One example of a phosphine-catalyzed transformation with significant potential utility in organic synthesis is the γ functionalization of electron-poor allenes and alkynes with carbon, nitrogen, oxygen, and sulfur nucleophiles.2 Recently, the first asymmetric variants of such processes have been described.3,4
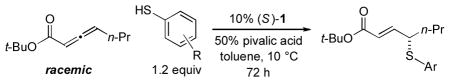
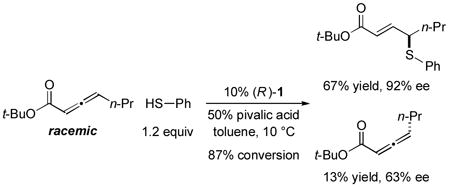
In 2010, we reported the only example of a phosphine-catalyzed γ functionalization of a carbonyl compound with a sulfur nucleophile (the addition of alkyl thiols to allenoates), and we demonstrated that this process can be achieved with high enantioselectivity in the presence of a chiral bisphosphine [Eq. (1)].4c This method complements previous catalytic asymmetric routes to α- and β-thio-substituted carbonyl compounds.5 Unfortunately, under the same conditions, aryl thiols do not add in high ee or good yield (Eq. (1): 70% ee and 9% yield with PhSH and R = n-Pr).6 Due to the significance of aryl alkyl sulfides,7 addressing this deficiency is an important objective. In this report, we establish that the use of a chiral monophosphine enables a catalytic asymmetric route to aryl alkyl sulfides through the γ addition of aryl thiols to allenoates [Eq. (2)].8
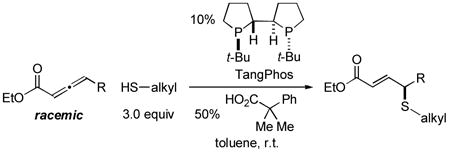 |
(1) |
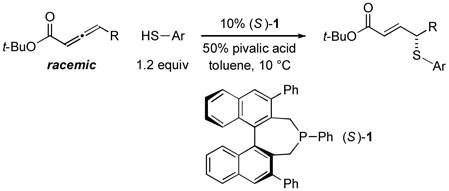 |
(2) |
In preliminary studies, we investigated the utility of a range of chiral phosphines, including those that have proved useful in other γ additions, as catalysts for the enantioselective γ addition of an aryl thiol to an allenoate (Table 1, entries 1–5). From this initial survey, a monophosphine, phosphepine 2,9 emerged as the most promising catalyst, although the ee and the yield of the aryl alkyl sulfide were only moderate (entry 2). In contrast, for the asymmetric addition of alkyl thiols to allenoates, a bisphosphine was determined to be the catalyst of choice (TangPhos; Eq. (1)).
Table 1.
Catalytic asymmetric γ addition of an aryl thiol to an allenoate: Effect of reaction parametersa
 | |||
|---|---|---|---|
| entry | change from the “standard conditions” | ee (%) | yield (%)b |
| 1 | TangPhos, instead of (S )-1 | 31 | 11 |
| 2 | (S )-2, instead of (S )-1 | 52 | 66 |
| 3 | (S )-3, instead of (S )-1 | 30 | 24 |
| 4 | (S )-4, instead of (S )-1 | 50 | 8 |
| 5 | (S )-5, instead of (S )-1 | 71 | 46 |
| 6 | none | 90 | 81 |
| 7 | no (S )-1 and no pivalic acid | – | <2c |
| 8 | no (S )-1 | – | <2c |
| 9 | no pivalic acid | 72 | 28 |
| 10 | R = Et | 88 | 75 |
| 11 | r.t. | 88 | 78 |

All data are the average of two experiments.
The yield was determined by 1H NMR analysis with the aid of an internal standard.
Major product: β addition of the thiol.
Incorporating groups into the 3,3′ positions of 1,1′-binaphthyl derivatives is an effective strategy for increasing the effectiveness of a diverse set of chiral catalysts.10 Very recently, we established for the first time that this approach is also useful in the context of asymmetric nucleophilic catalysis with chiral phosphepines, specifically, for enantioselective formal [3+2] cycloadditions catalyzed by 1 vs. 2.11 We have been pleased to determine that for asymmetric γ additions of aryl thiols, an entirely different process, this strategy also leads to a substantial enhancement in both ee and yield (entry 2 vs. entry 6).
Additional information on the impact of various parameters on the course of this catalytic asymmetric synthesis of aryl alkyl sulfides is provided in Table 1. In contrast to the phosphine-catalyzed enantioselective additions of oxygen4a and carbon4b,d nucleophiles that have been reported, in the case of thiol nucleophiles, the chiral catalyst must out-compete a significant background reaction--uncatalyzed conjugate addition of the thiol to the β carbon of the allenoate.4c In the absence of phosphepine 1, no γ addition is observed (entries 7 and 8), whereas, if the reaction is conducted in the presence of catalyst 1 but in the absence of pivalic acid, then a moderate ee and a low yield are obtained (entry 9). The reaction can be performed with a smaller substituent on the ester (R; entry 10) or at room temperature (entry 11) with only a slight erosion in efficiency.
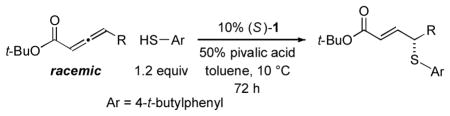
Phosphepine 1 catalyzes asymmetric C–S bond formation between aryl thiols and a variety of allenoates (Table 2).12 Thus, the γ substituent of the allenoate can range in size from methyl to isopropyl, and the allene can bear functional groups such as an olefin, a silyl ether, an ester, or a halogen.
Table 2.
Catalytic asymmetric γ additions of aryl thiols to allenoates: Variation of the allenoatea
 | |||
|---|---|---|---|
| entry | R | ee (%) | yield (%)b |
| 1 | Me | 81 | 58 |
| 2 | n-Pr | 91 | 81 |
| 3 |
|
91 | 68 |
| 4c | i-Pr | 94 | 61 |
| 5 |
|
91 | 70 |
| 6 |
|
89 | 71 |
| 7 |
|
95 | 68 |
| 8 |
|
91 | 75 |
| 9 |
|
90 | 66 |
All data are the average of two experiments.
Yield of purified product.
Run at r.t.
The scope of this method for the catalytic enantioselective synthesis of aryl alkyl sulfides is also broad with respect to the aryl thiol (Table 3). Thus, ortho-, meta-, and para-substituted, as well as electron-rich and electron-poor, aryl thiols add to allenoates in good ee and yield. An unprotected amino group is compatible with the reaction conditions (entry 7).
Table 3.
Catalytic asymmetric γ additions of aryl thiols to allenoates: Variation of the aryl thiola
 | |||
|---|---|---|---|
| entry | R | ee (%) | yield (%)b |
| 1 | H | 90 | 73 |
| 2 | 2-OMe | 90 | 67 |
| 3 | 3,5-dimethyl | 92 | 81 |
| 4 | 4-F | 91 | 64 |
| 5 | 4-Cl | 91 | 59 |
| 6 | 4-OMe | 91 | 76 |
| 7 | 4-NH2 | 86 | 64 |
All data are the average of two experiments.
Yield of purified product.
During the course of a phosphepine-catalyzed enantioselective γ addition of an aryl thiol to an allenoate, the resting state of the catalyst is the phosphine itself, not the protonated catalyst or a phosphepine–allenoate adduct (31P NMR spectroscopy). The rate law for the process is first order in the catalyst and the allenoate and zero order in the thiol and pivalic acid. During the reaction, the ee of the product is constant, but a modest kinetic resolution of the unreacted allene is observed [Eq. (3)].13 Collectively, these data are consistent with a rate-determining irreversible 1,4-addition of the phosphepine catalyst to the allenoate (Figure 1).
Fig. 1.
A possible pathway for the nucleophile-catalyzed enantioselective γ addition of an aryl thiol to an allenoate (for simplicity, the elementary steps are drawn as irreversible, and one E/Z isomer of the intermediates is illustrated).
 |
(3) |
Some of these mechanistic observations stand in intriguing contrast to those made for the asymmetric γ addition of alkyl thiols to allenoates catalyzed by TangPhos.4c For those reactions, a catalyst-substrate adduct is the resting state of the catalytic cycle, and no kinetic resolution of the starting allenoate is observed. These differences point to a delicate balance among the rates of the various steps of the catalytic cycles for phosphine-catalyzed γ additions.14
In summary, we have developed an effective method for the catalytic asymmetric addition of aryl thiols to the γ position of allenoates, overcoming the usual propensity of these two partners to bond β to the ester. This process provides ready access to aryl alkyl sulfides in good ee, and it complements earlier catalytic enantioselective processes in which sulfur substituents are introduced at the α and β positions of carbonyl compounds and in which alkyl thiols are incorporated at the γ position. A wide array of mechanistic data are consistent with the addition of the chiral phosphepine to the allene as the rate-determining step of the catalytic cycle. The difference in the optimized reaction conditions (eq 1 vs. eq 2), as well as in the mechanistic observations, attest to the potential for divergent behavior when alkyl thiols and aryl thiols serve as nucleophiles.
Supplementary Material
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, grant R01–GM57034) and Dainippon Sumitomo Pharma Co., Ltd. (fellowship for Y.F.). We thank Dr. Jonathan E. Wilson for a preliminary study and John S. Anderson for assistance with X-ray crystallography.
Footnotes
Electronic Supplementary Information (ESI) available: Synthetic methods. Specral data. Crystallographic data. See DOI: 10.1039/b000000x/
Notes and references
- 1.For leading references, see: Gröger H, Burda E. In: Phosphorus Ligands in Asymmetric Catalysis. Börner A, editor. Vol. 3. Wiley–VCH; New York: 2008. pp. 1175–1197.Methot JL, Roush WR. Science of Synthesis. 2008;42:469–501.Marinetti A, Voituriez A. Synlett. 2010:174–194.
- 2.For some of the pioneering studies, see: Trost BM, Li CJ. J Am Chem Soc. 1994;116:3167–3168.Zhang C, Lu X. Synlett. 1995:645–646.Trost BM, Dake GR. J Org Chem. 1997;62:5670–5671.Trost BM, Li CJ. J Am Chem Soc. 1994;116:10819–10820.
- 3.For a process that produces a stereocenter in the δ position, see: Chen Z, Zhu G, Jiang Q, Xiao D, Cao P, Zhang X. J Org Chem. 1998;63:5631–5635.
- 4.For processes that generate a stereocenter in the γ position, see: Chung YK, Fu GC. Angew Chem, Int Ed. 2009;48:2225–2227. doi: 10.1002/anie.200805377.Smith SW, Fu GC. J Am Chem Soc. 2009;131:14231–14233. doi: 10.1021/ja9061823.Sun J, Fu GC. J Am Chem Soc. 2010;132:4568–4569. doi: 10.1021/ja101251d.Sinisi R, Sun J, Fu GC. Proc Natl Acad Sci USA. 2010;107:20652–20654. doi: 10.1073/pnas.1003597107.
- 5.For a seminal investigation of the catalytic asymmetric synthesis of α-thio-substituted carbonyl compounds, see: Marigo M, Wabnitz TC, Fielenbach D, Jørgensen KA. Angew Chem Int Ed. 2005;44:794–797. doi: 10.1002/anie.200462101.For leading references to the catalytic enantioselective synthesis of β-thio-substituted carbonyl compounds, see: Enders D, Lüttgen K, Narine AA. Synthesis. 2007:959–980.Liu Y, Sun B, Wang B, Wakem M, Deng L. J Am Chem Soc. 2009;131:418–419. doi: 10.1021/ja8085092.
- 6.See footnote 17f of Reference 4c.
- 7.For some leading references, see: Horwood E. In: Sulphur-Containing Drugs and Related Organic Compounds. Damani LA, editor. Chichester, UK: 1989. Toru T, Bolm C, editors. Organosulfur Chemistry in Asymmetric Synthesis. Wiley–VCH; Weinheim, Germany: 2008. Pellissier H. Chiral Sulfur Ligands: Asymmetric Catalysis. Royal Society of Chemistry; Cambridge, UK: 2009. b) Diltiazem, nelfinavir, and zofenopril are examples of pharmaceuticals that include an aryl alkyl sulfide
- 8.Allenoates can be synthesized in one step through the treatment of an acid chloride with a commercially available olefinating reagent such as (carbethoxymethylene)triphenylphosphorane.
- 9.Phosphepines were originally developed as chiral ligands for transition metals. For a review, see: Gladiali S, Alberico E. In: Phosphorus Ligands in Asymmetric Catalysis. Börner A, editor. Vol. 1. Wiley–VCH; New York: 2008. pp. 177–206.For the first application of a phosphepine as a chiral nucleophilic catalyst, see: Wurz RP, Fu GC. J Am Chem Soc. 2005;127:12234–12235. doi: 10.1021/ja053277d.
- 10.For example, see: Akiyama T, Itoh J, Yokota K, Fuchibe K. Angew Chem Int Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240.Uraguchi D, Terada M. J Am Chem Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533.
- 11.Fujiwara Y, Fu GC. J Am Chem Soc. doi: 10.1021/ja2049012. ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Notes: a) The phosphine oxide of phosphepine 1 does not serve as a catalyst for the γ addition of aryl thiols to allenoates; b) On a gram scale, the reaction illustrated in entry 6 of Table 3 proceeded in 92% ee and 72% yield; c) The rate of the γ addition is not highly sensitive to water, e.g., the addition of five equivalents of water to the reaction depicted in entry 6 of Table 3 led to formation of the desired product in 87% ee and 76% yield; d) Under our standard conditions, a hydroxyl-, a nitro-, a carboxyl-, and an acetylamino-substituted aryl thiol, as well as a heteroaryl thiol, were not very effective reaction partners; e) In the presence of 5% phosphepine 1 and 25% pivalic acid (one-half of the standard catalyst loading), the γ addition illustrated in entry 6 of Table 3 proceeded in 88% ee and 67% yield; f) Additives other than pivalic acid were also examined. Use of any of an array of carboxylic acids (RCO2H, where R is an unfunctionalized alkyl group or aryl group) led to slightly diminished ee’s (within ~5%) and modest-to-substantial erosion in yields. Attempts to employ a hydroxamic acid, a sulfonic acid, or a phenol as the additive resulted in significantly lower ee’s and yields.
- 13.We are aware of only one other example of a kinetic resolution during a phosphine-catalyzed γ-addition reaction (Ref. 4d).
- 14.The divergent behavior between the two processes may be attributable in part to the bidentate (TangPhos) versus monodentate (phosphepine 1) nature of the two phosphine catalysts. Indeed, we have determined that truncated monophosphines that are structurally related to TangPhos furnish very different results as compared with TangPhos itself.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.