The most promising breast cancer studies using bevacizumab combined with traditional cytotoxic agents in advanced breast cancer are outlined. The current indications for bevacizumab reviewed by the Oncologic Drug Advisory Committee are discussed and how benefit in patient clinical trials should be measured is proposed.
Keywords: Antiangiogenesis, Advanced breast cancer, Bevacizumab, FDA
Abstract
Significant advances in the treatment of patients with breast cancer have been made in the past 10 years. The current systemic treatment of breast cancer is characterized by the discovery of multiple cancer targets leading to treatments that are more sophisticated and specific than conventional cytotoxic chemotherapy. Two classes of compounds that have helped improve clinical outcomes are small molecules and monoclonal antibodies targeting specific tyrosine kinase receptors. Many novel targets have been discovered, and parallel multiple approaches to anticancer therapy have recently emerged from the literature. One promising strategy is targeting the proangiogenic vascular endothelial growth factors (VEGFs), either by ligand sequestration (preventing VEGF receptor binding) or inhibiting downstream receptor signaling. Bevacizumab, a monoclonal antibody directed against VEGF, has been shown to improve the efficacy of taxanes in frontline treatment of patients with metastatic breast cancer. This review outlines the most promising breast cancer studies using bevacizumab combined with traditional cytotoxic agents in advanced breast cancer. In addition, we discuss the current indications reviewed by the Oncologic Drug Advisory Committee and define our vision of how the benefit of patient clinical trials should be measured.
Introduction
Breast cancer is one of the most common malignancies in the western world. In 2009, >210,000 women were estimated to have been diagnosed with breast cancer in the U.S. and >40,000 were estimated to have died from the disease [1]. In the European Union, >330,000 new breast cancer diagnoses and 89,000 deaths from breast cancer were estimated to have occurred in 2008 [2]. Several therapeutic strategies have been proposed with the aim of improving overall survival (OS) times and quality of life (QOL). However, most metastatic disease eventually stops responding to systemic treatment. Therefore, new treatment alternatives are needed for patients with metastatic breast cancer (MBC).
Angiogenesis is an important natural process of new blood vessel formation that occurs in the body, both in health and in disease [3]. The growth and development of solid tumors are critically dependent on a functional vascular supply, which is stimulated by several proangiogenic factors. Changes in the finely balanced equilibrium between angiogenic stimulators and inhibitors that regulate angiogenesis are linked to a broad range of angiogenesis-dependent diseases, including both cancer and non-neoplastic diseases such as atherosclerosis, age-related macular degeneration, and rheumatoid arthritis [3]. Angiogenesis is now recognized as a hallmark of cancer, regulating several events required for tumor progression [4]. Additionally, a better understanding of the fundamental biology of breast cancer has led to the identification of cellular pathways that may be amenable to targeted intervention.
In 2004, the U.S. Food and Drug Administration (FDA) approved the use of bevacizumab in combination with chemotherapy for the first-line treatment of patients with metastatic colorectal cancer [5, 6] and recurrent or advanced non-small cell lung cancer (NSCLC) [7] based on longer OS times with the combination. In 2008, the FDA granted the accelerated approval of bevacizumab plus paclitaxel to treat patients with recurrent breast cancer or MBC [8] on the basis of an observed progression-free survival (PFS) benefit. Single-agent bevacizumab is active against metastatic renal cell carcinoma [9], recurrent ovarian cancer [10], and glioblastoma [11].
Role of Vascular Endothelial Growth Factor in the Tumor Microenvironment
Angiogenesis plays an essential role in breast cancer development, invasion, and metastasis [12, 13]. Moreover, vascular endothelial growth factor (VEGF) has autocrine prosurvival effects on tumor cells, protecting them from stresses such as hypoxia, chemotherapy, and radiotherapy. This fundamental mechanism in biology describes a multistep process of new blood vessel formation from existing vasculature, and it is tightly regulated by proangiogenic factors involving autocrine and paracrine signaling [14]. To grow and obtain more blood, tumors exert multiple strategies to create or stimulate the formation of blood vessels, including sprouting angiogenesis, vessel co-option, intussusception of existing vessels, and recruitment of bone marrow–derived endothelial progenitor cells into growing vessels [15].
VEGF is essential for the development of neovasculature in the early stages of tumorigenesis and is thought to play a key role in tumor metastasis. The transition of a tumor from the “avascular” or “prevascular” phase to the “vascular” phase (increased growth and metastatic potential) is termed the “angiogenic switch” [16]. This switch is considered a hallmark of the malignant process and is believed to be stimulated by an increase in expression of proangiogenic factors (such as VEGF, basic fibroblast growth factor [bFGF], and transforming growth factor β) and by a decrease in antiangiogenic factors (such as interferon-α and thrombospondin-1) [17, 18].
VEGF Family
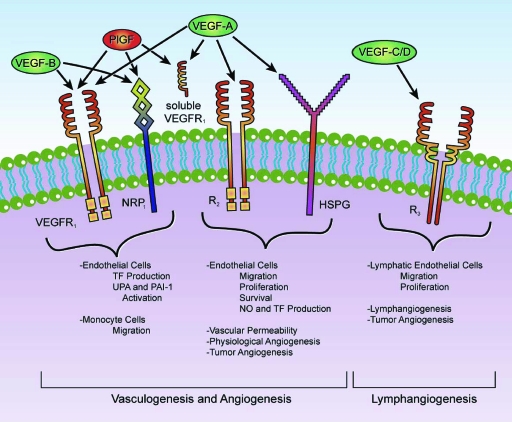
The VEGF family is comprised of seven secreted glycoproteins referred to as VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, placental growth factor (PlGF)-1, and PlGF-2 (Fig. 1) [19]. The best characterized of the VEGF family members is VEGF-A (hereafter, VEGF), a homodimeric glycoprotein that is expressed in various isoforms secondary to alternative splicing that leads to mature 121-, 165-, 189-, and 206-amino acid proteins [20]. Angiogenesis is a multistep process, and VEGF acts during several of these steps, including the following: (a) promoting endothelial cell mitogenesis and cell survival (antiapoptotic effect), (b) exerting chemotactic effects, (c) increasing expression of proteolytic enzymes (e.g., collagenases and urokinases) involved in stromal degradation, (d) mobilizing bone marrow–derived endothelial cell precursors in the promotion of vascularization, (e) increasing vascular permeability, and (f) causing vasodilation [21]. In addition to promoting division of endothelial cells, VEGF also has an important role in modulating endothelial cell migration to sites of angiogenesis.
Figure 1.
VEGF-related family. VEGF165 is the predominant isoform and is commonly overexpressed in a variety of human solid tumors. VEGF-A expression is efficiently induced by hypoxia and regulates not only physiological but also most pathological angiogenesis, such as tumor angiogenesis. Free VEGF members exert their effects by binding a variety of cell-surface receptors including VEGFR-1, a 180-kDa transmembrane protein also called macrophage-colony stimulating factor receptor (fms)-like tyrosine kinase-1, or Flt-1, and VEGFR-2, a 200-kDa transmembrane protein also called kinase insert domain-containing receptor, or KDR [19]. A third structurally related tyrosine kinase receptor is the 180-kDa VEGFR-3, which is expressed broadly on endothelial cells during early embryogenesis but becomes restrictive to endothelial cells of adult lymphatic tissues and is necessary for adult lymphangiogenesis [57]. Two additional VEGFRs, NRP-1 and NRP-2, were also recently implicated in VEGF-mediated vascularization and lymphangiogenesis [58]. These receptors have a short intracellular domain and are not capable of signal transduction but may instead function as coreceptors for VEGFR-1 and VEGFR-2 to enhance their interaction with their respective ligands. An important preclinical trial revealed that blocking anti-NRP-1 antibodies has an additive effect with anti-VEGF therapy in reducing tumor growth [59]. Both VEGFR-1 and VEGFR-2 are members of the receptor tyrosine kinase superfamily, and they belong to the same subclass as receptors for platelet derived growth factors and fibroblast growth factors. VEGFRs have an extracellular domain composed of seven immunoglobulin-like regions that bind to VEGF, a single transmembrane region, and an intracellular tyrosine kinase domain [60]. Activation of these VEGFRs triggers the phosphorylation of a multitude of proteins that are active in signal transduction cascades. Some of the signaling pathways triggered by these mechanisms include the Akt/protein kinase B, endothelial nitric oxide synthase, mitogen-activated protein kinase, focal adhesion kinase, paxillin, Ras-Raf-mitogen-activated protein kinase/extracellular signal–related kinase kinase-extracellular signal–related kinase, and phospholipase C-γ pathways [61].
Abbreviations: HSPG, heparan sulfate proteoglycans; NO, nitric oxide; NRP, neuropilin; PAI-1, plasminogen activator inhibitor-1; PlGF, placental growth factor; TF, tissue factor; UPA, urokinase plasminogen activator; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Tumors can absorb sufficient nutrients and oxygen by simple diffusion up to a size of 1–2 mm, at which point their further growth requires the generation of a vascular supply [17] (Fig. 2). Although formation of new blood vessels is considered to be the predominant mode of tumor angiogenesis, recent data indicate that some tumors may grow by co-opting existing blood vessels [22].
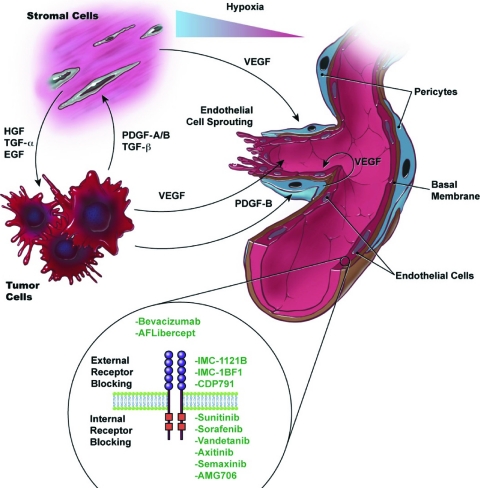
Figure 2.
Intercellular signaling among tumor cells, stroma cells, pericytes, and endothelial cells: cellular players in the tumor/microvascular microenvironment. VEGF is antiapoptotic for endothelial cells via several pathways, including induction of expression of the antiapoptotic proteins Bcl-2 and A1, activation of the phosphoinositide 3-kinase/Akt signaling pathway, stimulation of nitrous oxide and prostacyclin, and increasing focal adhesion kinase tyrosine phosphorylation [62]. The activity of VEGF receptors is primarily regulated by the availability of their respective ligands. Ligand expression levels depend on many factors, such as hypoxia, environmental stress, and glucose deficiency. VEGF-A is the most important factor whose expression is upregulated under hypoxic conditions. Hypoxia allows the stabilization of hypoxia-inducible factors that bind to specific promoter elements that are present in the promoter region of VEGF-A [63].
Abbreviations: EGF, endothelial growth factor; HGF, hepatocytic growth factor; PDGF, platelet-derived growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
Hypoxia, a known inducer of angiogenic responses in a wide variety of tumor types, involves induction of gene expression via hypoxia-inducible factor and various proangiogenic factors, including VEGF and FGFs [23]. Most human cancers express six or more angiogenic proteins [24]. Furthermore, preclinical studies have demonstrated that long-term suppression of a single proangiogenic pathway (e.g., VEGF) can result in increased expression of other proangiogenic proteins such as bFGF or PlGF [25]. This phenomenon is termed “tumor escape” to differentiate it from acquired resistance to cytotoxic chemotherapy.
Angiogenesis in Breast Cancer
Studies in patients with early-stage breast cancer showed that elevated expression of VEGF can be associated with shorter relapse-free survival and OS times in patients with both positive and negative lymph nodes [24, 26]. Of interest, human epidermal growth factor receptor (HER)-2 amplification in breast cancer induces overexpression of VEGF, suggesting that induction of angiogenesis may contribute to this cancer's lethality [26]. In a recent publication, Linderholm et al. [27] demonstrated that triple receptor-negative breast cancer possesses higher intratumoral levels of VEGF and is associated with a shorter OS duration.
Different Approaches for Targeting the VEGF Pathway
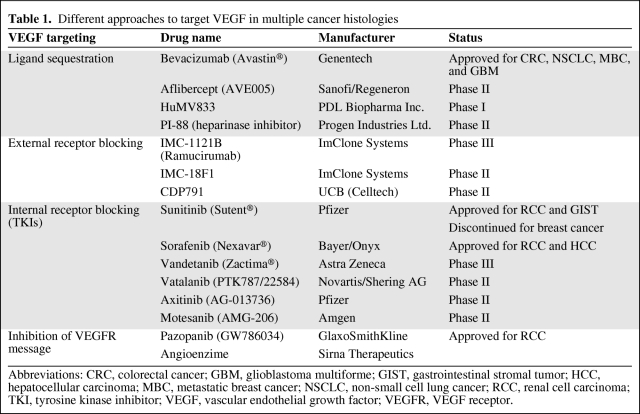
In the past decade, major advances have occurred in the development of therapeutic agents that modulate tumor angiogenesis. Agents that block the VEGF pathway have been shown to be effective at inhibiting tumor angiogenesis and growth in preclinical tumor models. How to best target VEGF has been the subject of several interesting approaches, many of which have been translated to the clinic. These approaches include: (a) ligand sequestration, (b) external receptor blocking, (c) internal receptor blocking (tyrosine kinase inhibitors), and (d) inhibiting the VEGF receptor message. Table 1 summarizes the experience in multiple cancer histologies. Bevacizumab has been the most studied agent in the field of angiogenesis in multiple cancer types.
Table 1.
Different approaches to target VEGF in multiple cancer histologies
Abbreviations: CRC, colorectal cancer; GBM, glioblastoma multiforme; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; MBC, metastatic breast cancer; NSCLC, non-small cell lung cancer; RCC, renal cell carcinoma; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
Bevacizumab Treatment for Breast Cancer Patients
Bevacizumab (Avastin®; Genentech Inc., South San Francisco, CA) is derived from the murine VEGF monoclonal antibody A4.6.1 [28] and is composed of ∼93% human and ∼7% murine protein sequences. An experimental study showed that bevacizumab neutralized all isoforms of human VEGF with a dissociation constant of 1.1 nmol/L [29]. A clinical pharmacology study of bevacizumab demonstrated a linear pharmacokinetic profile and a long terminal half-life of ∼21 days (range, 11–50 days) [30]. These same studies showed that bevacizumab inhibited VEGF-induced endothelial cell proliferation and migration, and in an in vivo model of a range of tumor types (including breast cancer), bevacizumab led to significantly slower tumor growth [31]. A 2002 study found that bevacizumab reduced microvessel density in some human breast carcinoma models [32].
Phase I and II Studies of Bevacizumab as a Single Agent and in Combination with Chemotherapy
Two phase I clinical trials using bevacizumab as a single agent in patients with solid tumors have been reported [30, 31]. In the first trial, 25 patients with refractory solid tumors received doses of bevacizumab in the range of 0.1–10 mg/kg over 8 weeks [30]. In the second trial, 12 patients with a variety of solid tumors received 3-mg/kg doses of bevacizumab every other week in combination with chemotherapy [33]. Both studies showed that bevacizumab is safe, that is, without dose-limiting toxicities, at doses up to 10 mg/kg and can be combined with chemotherapy without apparent added toxicity.
An early dose-escalation phase I/II clinical trial was conducted in 75 patients with MBC treated with bevacizumab to determine its safety, efficacy, and pharmacokinetics [34]. The majority of the patients (96%) had received prior anthracycline- or taxane-based chemotherapy for MBC, and 28% of those patients were HER-2+. The overall response rate (ORR) was 9.3% (confirmed response rate, 6.7%). The median duration of confirmed response was 5.5 months (range, 2.3–13.7 months). Four patients (5.3%) discontinued study treatment because of an adverse event. Hypertension was reported as an adverse event in 22% of patients. Thus, the optimal dose of bevacizumab in that trial was 10 mg/kg every other week, and toxicity was deemed to be acceptable.
Another phase II trial of bevacizumab with vinorelbine included patients who were refractory to one or two prior regimens and had disease progression within 1 year of adjuvant chemotherapy for MBC [35]. Fifty-six patients received treatment with bevacizumab (10 mg/kg every 2 weeks) and vinorelbine (25 mg/m2 every week) until disease progression or severe toxicity. The ORR was 34% (17 patients had a partial response and one patient had a complete response), and the median time to progression (TTP) was 5.5 months. Overall, the regimen was very well tolerated and no major episodes of bleeding or thrombotic events were encountered.
The multicenter, phase II Xeloda in Combination with Avastin as First-Line Treatment for HER2-Negative Metastatic Breast Cancer trial tested the combination of bevacizumab (15 mg/kg every 3 weeks) with capecitabine (1,000 mg/m2 twice daily for two of every 3 weeks) as first-line treatment [36]. The primary endpoint of that nonrandomized trial was TTP. The median TTP for capecitabine plus bevacizumab was 5.7 months, thus satisfying the planned 1.6-month longer TTP than the 4-month median TTP estimated with capecitabine monotherapy. An unplanned subset analysis of efficacy based on hormone receptor status suggested greater benefit in patients with estrogen receptor–positive disease.
Phase III Study of Bevacizumab in Previously Treated MBC Patients
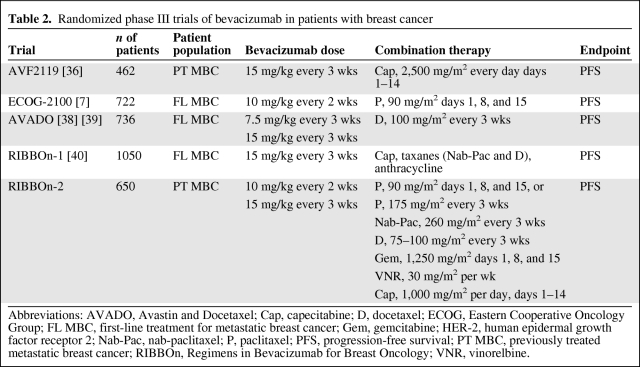
On the basis of previously collected data, a pivotal phase III randomized trial (AVF2119) was undertaken to evaluate bevacizumab in women with heavily pretreated MBC [37]. These patients' disease had been refractory to an anthracycline and a taxane and had relapsed within the first 12 months after adjuvant therapy. In total, 462 patients were randomized to receive bevacizumab (15 mg/kg every 3 weeks) plus capecitabine (2,500 mg/m2 in two divided doses for two of every 3 weeks) or capecitabine alone. The primary endpoint of the trial was the PFS interval, and this was statistically identical between the two arms: capecitabine, 4.2 months; capecitabine plus bevacizumab, 4.9 months. Likewise, the OS duration was not significantly different between arms (15.1 months versus 14.5 months). The ORR was significantly higher in the combination arm than in the single-agent capecitabine arm: 19.8% for the former versus 9.1% for the latter (p = .001). The combination arm was well tolerated. However, 17% of the patients treated with both bevacizumab and capecitabine required antihypertensive treatment, compared with 0.5% of patients in the capecitabine-only arm. A higher rate of grade 3 and 4 cardiotoxicity existed in the combination arm (3% versus 0.5%). Table 2 shows a total of five randomized, phase III studies conducted in MBC patients using bevacizumab as first- or second-line therapy [8, 37, 39–41].
Table 2.
Randomized phase III trials of bevacizumab in patients with breast cancer
Abbreviations: AVADO, Avastin and Docetaxel; Cap, capecitabine; D, docetaxel; ECOG, Eastern Cooperative Oncology Group; FL MBC, first-line treatment for metastatic breast cancer; Gem, gemcitabine; HER-2, human epidermal growth factor receptor 2; Nab-Pac, nab-paclitaxel; P, paclitaxel; PFS, progression-free survival; PT MBC, previously treated metastatic breast cancer; RIBBOn, Regimens in Bevacizumab for Breast Oncology; VNR, vinorelbine.
Phase III Studies of Bevacizumab as First-Line Treatment for MBC Patients
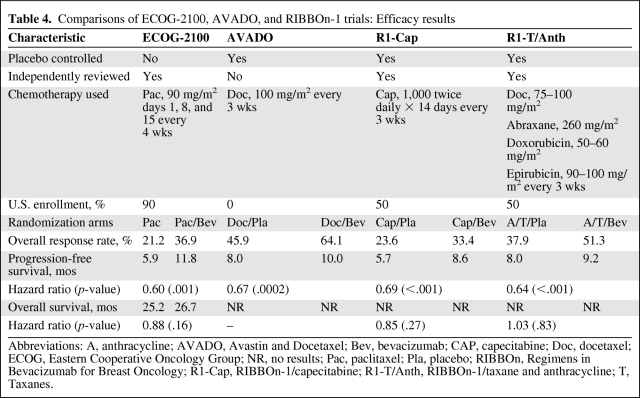
A phase III clinical trial conducted by the Eastern Cooperative Oncology Group (ECOG-2100) enrolled a total of 680 patients with previously untreated locally recurrent breast cancer or MBC [8]. Patients received 90 mg/m2 paclitaxel weekly on days 1, 8, and 15 with or without 10 mg/kg bevacizumab on days 1 and 15; medications were given in 4-week cycles until the cancer progressed. All patients with HER-2+ disease were required to have received prior trastuzumab, and the majority of the patients (96%) were HER-2−. The primary endpoint of the study was the PFS interval, which was significantly longer in patients who received the combination of bevacizumab plus paclitaxel than in those who received paclitaxel as a single agent (11.8 months versus 5.9 months; hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.43–0.62; p < .001). The PFS benefit with bevacizumab was observed across all subgroups, regardless of age, number of metastatic sites, previous adjuvant taxane use, disease-free interval after adjuvant therapy, or hormone receptor status. In terms of the ORR, patients in the combination arm had a 36.9% ORR and those in the single-agent paclitaxel arm had a 21.2% ORR (p = .001). The Kaplan–Meier curve demonstrated that the median OS duration for patients treated with the combination of paclitaxel and bevacizumab was 26.5 months, versus 24.8 months for those treated with paclitaxel, with an HR of 0.87 (p = .14). The FDA raised concerns about this trial because the PFS evaluation was investigator assessed and the study did not have an independent radiological review. Independent review facility (IRF) analysis was not included in the original ECOG-2100 study design but was implemented after the study was completed, per the FDA's request that it be included in the registration application. At least one image was submitted to the IRF evaluation for 649 (89.9%) of the 722 patients. Thirty-eight patients (10.3%) in the paclitaxel plus bevacizumab arm and 35 patients (9.9%) in the paclitaxel-alone arm have missing radiographic images. The IRF demonstrated a 52% lower risk for progression or death (HR, 0.48; p < .001) for patients treated with bevacizumab plus paclitaxel than for those in the control arm, and the rate of objective response was more than double [38].
The Avastin and Docetaxel (AVADO) trial was a phase III placebo-controlled, randomized study of two doses of bevacizumab with or without docetaxel as first-line therapy for patients with recurrent breast cancer or MBC [39]. A longer PFS interval was observed with docetaxel (100 mg/m2 every 3 weeks) plus bevacizumab (7.5 mg/kg or 12 mg/kg every week). In total, 736 patients were analyzed for treatment toxicity and efficacy. In terms of the primary objective, the HR for docetaxel plus bevacizumab at 7.5 mg/kg was 0.80 (95% CI, 0.65–1.00; p = .045) and the HR for docetaxel plus bevacizumab at 15 mg/kg was 0.67 (95% CI, 0.48–0.78; p = .0002). The ORRs were 46.4%, 55.2%, and 64.1% for docetaxel plus placebo, docetaxel plus bevacizumab at 7.5 mg/kg, and docetaxel plus bevacizumab at 15 mg/kg, respectively. In patients with measurable disease at baseline, the response rate also was higher with bevacizumab at 15 mg/kg—placebo, 46%; 7.5 mg/kg, 55% (p = .07), and 15 mg/kg, 64% (p < .001). The OS duration for patients treated with docetaxel plus bevacizumab was 31.9 months, versus 30.2 months for patients treated with docetaxel plus placebo (HR, 1.0; p = .98). The rates of grade 3 and 4 adverse events were 67.0%, 74.8%, and 74.1% for docetaxel plus placebo, docetaxel plus bevacizumab at 7.5 mg/kg, and docetaxel plus bevacizumab at 15 mg/kg, respectively. A subanalysis in a cohort of elderly patients (aged ≥65 years) showed that the magnitude of the benefit was similar to that in the overall study population but that the study was underpowered to demonstrate statistical significance [40].
The Regimens in Bevacizumab for Breast Oncology (RIBBOn)-1 trial evaluated chemotherapy agents by physician choice in combination with bevacizumab or placebo as first-line MBC treatment [41]. In that randomized, double-blinded, placebo-controlled trial, 1,237 patients in total were enrolled, with a median follow-up of 15.6 months in the capecitabine arm and 19.2 months in the taxane/anthracycline arm. Patients in that trial were randomized in a 2:1 ratio to receive bevacizumab (15 mg/kg every 3 weeks) in addition to standard first-line chemotherapy or placebo plus chemotherapy. Chemotherapy options included capecitabine (2,000 mg/m2 every 2 weeks), the taxane nab-paclitaxel (260 mg/m2), and docetaxel (75–100 mg/m2) every 3 weeks or anthracycline-based chemotherapy every 3 weeks. The ORR was superior for the group treated with bevacizumab; for patients treated with capecitabine, the ORRs were 35.4% and 23.6% for the bevacizumab and placebo arms, respectively. For patients in the taxane/anthracycline group, the ORRs were 51.3% and 37.9% for the bevacizumab and placebo arms, respectively. In the capecitabine group, patients treated with bevacizumab had a longer PFS interval (the primary endpoint) than those given placebo (8.6 months versus 5.7 months; HR, 0.69; 95% CI, 0.56–0.84; p < .001). Similarly, in the taxane/anthracycline group, the PFS interval was longer for patients who received bevacizumab than for patients who received placebo (9.2 months versus 8.0 months; HR, 0.64; 95% CI, 0.52–0.80; p < .001). OS and 1-year survival data showed no statistically significant differences. The estimated HRs for OS were 0.85 (95% CI, 0.63–1.14; p = .27) and 1.03 (95% CI, 0.77–1.38; p = .83) in the capecitabine and taxane/anthracycline cohorts, respectively.
The toxicity profiles were similar to those in other trials using bevacizumab.
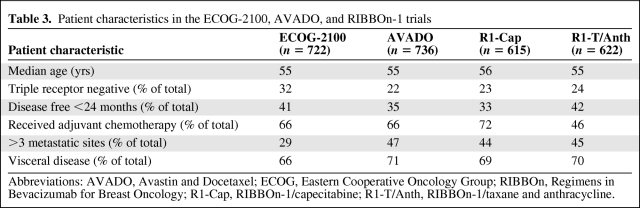
A pooled analysis of the three randomized phase III trials (ECOG-2100, AVADO, and RIBBOn-1) [42] showed a 36% lower risk for disease progression or death with the addition of bevacizumab to chemotherapy in patients with previously untreated MBC. Table 3 shows the disease characteristics of the patients in all three trials. The median age was nearly equal across the trials. Triple-negative breast cancer was present in 32% of patients in the ECOG-2100 trial and in 23% and 22% of patients in the RIBBOn-1 and AVADO trials, respectively. The efficacy results from the three randomized clinical trials demonstrated an important treatment effect, with a higher ORR (11%–28% more responses) and longer PFS interval (31%–52% lower risk) and consistent results in different subpopulations. However, for reasons not yet clear, the OS time was not longer with bevacizumab nor was there an apparent nonsignificant trend for a higher survival rate. Table 4 shows the details of the chemotherapy regimens used in each trial and describes the characteristics of each study with the corresponding ORR, PFS, and OS results. The PFS results from the three trials showed a lower risk for cancer progression or death, with PFS intervals of 9.2 months and 6.7 months for patients treated with bevacizumab and control patients, respectively (HR, 0.64; 95% CI, 0.48–0.69).
Table 3.
Patient characteristics in the ECOG-2100, AVADO, and RIBBOn-1 trials
Abbreviations: AVADO, Avastin and Docetaxel; ECOG, Eastern Cooperative Oncology Group; RIBBOn, Regimens in Bevacizumab for Breast Oncology; R1-Cap, RIBBOn-1/capecitabine; R1-T/Anth, RIBBOn-1/taxane and anthracycline.
Table 4.
Comparisons of ECOG-2100, AVADO, and RIBBOn-1 trials: Efficacy results
Abbreviations: A, anthracycline; AVADO, Avastin and Docetaxel; Bev, bevacizumab; CAP, capecitabine; Doc, docetaxel; ECOG, Eastern Cooperative Oncology Group; NR, no results; Pac, paclitaxel; Pla, placebo; RIBBOn, Regimens in Bevacizumab for Breast Oncology; R1-Cap, RIBBOn-1/capecitabine; R1-T/Anth, RIBBOn-1/taxane and anthracycline; T, Taxanes.
In preclinical animal models, withdrawal of VEGF inhibitors led to accelerated tumor growth, resulting in greater local invasion and distant metastasis [43, 44]. It has been provocatively suggested that the lack of any survival benefit with bevacizumab in face of a consistent PFS advantage might be caused by accelerated disease progression on bevacizumab cessation. However, a pooled analysis of five placebo-controlled clinical trials did not show a higher mortality rate, shorter TTP, or different disease progression pattern after bevacizumab discontinuation [45].
RIBBOn-2 is an ongoing, randomized, phase III multicenter study that is comparing second-line chemotherapy for MBC patients with and without bevacizumab. Preliminary results from RIBBOn-2 demonstrated that bevacizumab had a positive effect as second-line treatment for patients with MBC. There are ongoing trials evaluating the combination of bevacizumab with endocrine therapy. The Grupo Español de Investigación de Cáncer de Mama (GEICAM)/German Breast Group (GBG) Letrozole/Fulvestrant and Avastin trial is a randomized, first-line study in which hormone receptor–positive patients are randomized to receive letrozole or fulvestrant with or without bevacizumab (GEICAM 2006–11/GBG-51). In the Cancer and Leukemia Group B 40503 study, ∼500 patients with stage IIIB–IV disease will be randomized to receive letrozole or tamoxifen with or without bevacizumab.
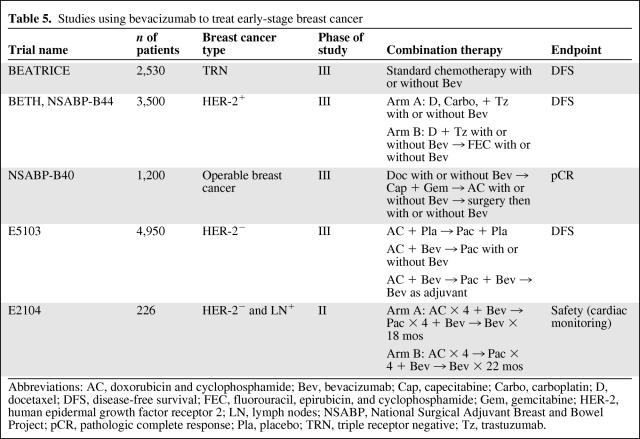
The results of the Gepar Quinto (the fifth German Preoperative Trial) neoadjuvant study were presented at the 2010 San Antonio Breast Cancer Symposium [46]. In that study, 1,948 patients with HER-2− disease were randomly assigned to receive neoadjuvant sequential epirubicin plus cyclophosphamide (EC) followed by docetaxel with or without bevacizumab. No significant differences were observed in the pathologic complete response (pCR) rate (17.5% with EC, docetaxel, and bevacizumab versus 15% with EC and docetaxel) or in the breast conservation rate (65.8% versus 66.6%, respectively) between the two arms. However, the subgroup of triple-negative patients seemed to have a significantly higher pCR rate when given bevacizumab (odds ratio, 1.42). Table 5 summarizes ongoing adjuvant and neoadjuvant bevacizumab studies. Bevacizumab as neoadjuvant therapy is currently being investigated in a large study by the National Surgical Adjuvant Breast and Bowel Project (the NSABP-B40 trial), and the use of bevacizumab as maintenance therapy is being studied in patients with triple receptor-negative breast cancer in the BEATRICE trial (Bevacizumab Adjuvant Therapy in Triple-Negative Breast Cancer, a randomized, phase III, open-label study of bevacizumab as adjuvant therapy for triple-negative disease). There are two large, ongoing, randomized, phase III trials of bevacizumab as adjuvant therapy in patients with HER-2+ breast cancer: the ECOG-E5103 trial (adjuvant doxorubicin plus cyclophosphamide followed by paclitaxel with or without bevacizumab) and the NSABP B44/BETH trial (Bevacizumab and Trastuzumab Adjuvant Therapy in HER2-Positive Breast Cancer, comparing docetaxel and carboplatin plus chemotherapy and trastuzumab with and without bevacizumab).
Table 5.
Studies using bevacizumab to treat early-stage breast cancer
Abbreviations: AC, doxorubicin and cyclophosphamide; Bev, bevacizumab; Cap, capecitabine; Carbo, carboplatin; D, docetaxel; DFS, disease-free survival; FEC, fluorouracil, epirubicin, and cyclophosphamide; Gem, gemcitabine; HER-2, human epidermal growth factor receptor 2; LN, lymph nodes; NSABP, National Surgical Adjuvant Breast and Bowel Project; pCR, pathologic complete response; Pla, placebo; TRN, triple receptor negative; Tz, trastuzumab.
FDA Approves Bevacizumab Indication for Breast Cancer
On February 22, 2008, the FDA granted bevacizumab “accelerated approval” for breast cancer treatment based on the ECOG-2100–documented longer PFS interval with this agent. The accelerated designation provides “conditional” approval for a lifesaving drug that appears effective for the treatment of fatal diseases. About 90 drugs have been approved under the accelerated approval program in the past 20 years, and none of these drugs has ever had its approval revoked. However, one drug (Mylotarg®; Wyeth Pharmaceuticals, Inc., Madison, NJ) was pulled from the market by the manufacturer after postmarketing studies showed it was not effective and that it actually increased the risk for death in patients using it.
The accelerated approval of bevacizumab was controversial at the time because the FDA went against the recommendation of its advisory panel, which voted five to four against approval. The panel objected because in the ECOG-2100 trial, bevacizumab treatment led to a 5.5-month longer PFS interval but did not result in longer life expectancy. Bevacizumab was later approved in combination with paclitaxel for first-line chemotherapy for patients with HER-2− MBC under an accelerated approval program. The FDA asked the manufacturer to conduct additional clinical studies to confirm the PFS benefit of adding bevacizumab to chemotherapy and to demonstrate safety data that included no OS detriment; a longer survival time was not required. The manufacturer completed two randomized phase III trials (AVADO and RIBBOn-1) in which the PFS duration was the primary endpoint. On July 20, 2010, the FDA's Oncologic Drug Advisory Committee (ODAC) reviewed data from these studies that demonstrated that bevacizumab-based chemotherapy resulted in a longer PFS interval, which was in the range of ∼1 month to nearly 3 months, without extending patients' OS times. The ODAC panel voted 13 to zero, indicating that the more modest PFS difference, although statistically significant, did not confirm the results of the ECOG-2100 trial. The panel also decided, with a 12 to one vote, to remove the indication of bevacizumab for advanced breast cancer because it lacked a clinically significant PFS benefit in additional studies. The ODAC panel also emphasized bevacizumab's potential for toxic events. These safety concerns surfaced after >800,000 patients with various tumor types had been treated with bevacizumab; most of these toxic events were hypertension and proteinuria, which were asymptomatic and easily controlled. A recent meta-analysis of 16 randomized clinical trials using bevacizumab in combination with chemotherapy for several cancer types revealed a greater relative risk (RR) for fatal adverse events (FAEs) (RR, 1.46; 95% CI, 1.09–1.94; p =.01; incidence, 2.5% versus 1.7%) when bevacizumab was added to chemotherapy. The most frequent FAEs were hemorrhage (23.5%), neutropenia with lethal infection (12.3%), gastrointestinal (GI) tract perforation (7.1%), pulmonary embolism (5.1%), and cerebrovascular accident (5.1%). This analysis also suggested that FAEs are dependent on tumor type (prostate and lung cancer versus renal cell carcinoma and breast cancer) and the chemotherapy partner used (taxanes and platinum compounds), and are not related to the dose of bevacizumab (low dose versus high dose) [47].
There is consensus in the scientific community that bevacizumab is very expensive, and there is concern about the increasingly expensive new drugs coming to the market. The European Medicines Agency (EMA) does not consider costs, but can grant approval; national regulatory bodies, after EMA approval, decide whether or not the drug is reimbursed by national health insurance. The FDA is not supposed to consider costs in its decisions, but if the FDA rescinds approval, insurers are likely to stop paying for this treatment. Medical oncologists are allowed to prescribe bevacizumab for off-label uses. In fact, a recent report using data from the National Cancer Institute Surveillance, Epidemiology, and End Results Program database [48] revealed that ∼35% of women aged >65 years who have breast cancer are treated with off-label chemotherapy at some point during their care. That study demonstrated that the most common off-label drugs used to treat breast cancer patients are vinorelbine and gemcitabine. However, because of the high cost of bevacizumab, insurers will rarely pay for its off-label use.
On December 17, 2010, the FDA announced its recommendation to remove the advanced breast cancer indication from the bevacizumab label. The manufacturer initiated a formal appeal process to reverse this recommendation.
At the time of this writing, the EMA had maintained the approval for bevacizumab in combination with paclitaxel as first-line therapy for patients with advanced breast cancer. The National Comprehensive Cancer Network guidelines, version 2.2011 (http://www.nccn.org), still include bevacizumab plus paclitaxel among the possible regimens for recurrent breast cancer or MBC.
Clinical Endpoints for the Next Generation of Clinical Trials
It is still debatable whether the OS time represents the most appropriate endpoint to evaluate the superiority of an experimental treatment over a standard treatment in patients with advanced cancers. For most of the current phase III clinical trials in MBC patients, the OS time was not the primary endpoint; however, a survival analysis was performed in those studies and represented a secondary objective. The OS time is an objective endpoint, whereas the evaluation of the ORR and TTP might be biased by knowledge of the therapy received (“ascertainment bias”). Nevertheless, use of the OS time as a primary endpoint is associated with some drawbacks, which can in turn lead to inappropriate conclusions. For instance, the OS time may be influenced by therapies used after a patient participates in a given trial, thus making it a less useful trial endpoint in this era of effective subsequent-line agents. Indeed, OS gains have reportedly been achieved only occasionally in the hundreds of randomized trials in patients with advanced breast cancer conducted to date [49].
Johnson et al. [50] and Sherrill et al. [51] studied the relationship between PFS and OS times in patients with metastatic colon cancer, NSCLC, and MBC. It is interesting that both these studies demonstrated a positive correlation between either disease-free survival (DFS) and OS times or PFS and OS times. Although some degree of association has been detected between PFS and OS times, in breast cancer patients this association remain inconsistent.
In patients with advanced colorectal cancer, the OS duration has been considered an insensitive efficacy criterion because potentially active subsequent therapies are not controlled in most randomized trials and the OS time may be increased or decreased by such therapies [52]. The same concern may also be valid for advanced breast cancer, given the existence of several effective lines of therapy. In addition, the contribution of the crossover designs may dilute the OS effects of an investigational agent.
A recent review of clinical outcomes from 73 phase III MBC trials, conducted over the last three decades, revealed considerable discordance between regulatory standards for outcomes in MBC and clinical trial design [53]. Interestingly, only five of 73 trials overall (7%) and four of 48 first-line trials (8%) reported the OS time as a primary endpoint. Saad and collaborators [54] reported similar findings. In a review of recent phase III, randomized trials in MBC patients, the OS time was reported in 15 of 76 trials (19.7%). The authors concluded that the OS time is a less useful trial endpoint for MBC in an era of effective subsequent-line agents.
Modena International Breast Cancer Conference
During the Sixth Annual Modena International Breast Cancer Conference, held in September 2010, an expert panel from Europe and the U.S. discussed the role of bevacizumab in the treatment of advanced breast cancer. Each member of the panel was asked to express his or her opinion on four main questions. A summary of the discussion as well as the final statement of the expert panel for each question are reported below. The panel members were: Valentina Guarneri, Fikri Icli, Stephen Johnston, David Khayat, Sibylle Loibl, Miguel Martin, Christoph Zielinski, PierFranco Conte, and Gabriel N. Hortobagyi.
Question #1: Bevacizumab Is Approved for First-Line Therapy of MBC Patients. Is This Indication Appropriate?
Data from clinical trials clearly indicate that the addition of bevacizumab to chemotherapy as a first-line treatment significantly and consistently results in a higher response rate and longer PFS interval. The almost 6-month longer PFS time observed in the ECOG-2100 study was not observed in the AVADO or the RIBBOn-1 trial. However, as pointed out by the panel members, although the differences in the median PFS times were not impressive, the HRs were very consistent across all studies. Panel members felt that, because sample sizes are calculated on the basis of a prespecified HR, judging treatment efficacy on the basis of the median PFS time is not fully appropriate from a methodologic perspective. Some panel members emphasized that, in spite of a longer PFS duration, bevacizumab does not provide any OS benefit. More recently, a meta-analysis conducted by pooling the data from >2,400 patients from three randomized trials [42] confirmed bevacizumab's benefit in terms of the ORR and PFS interval, without a difference in terms of the OS time. A longer OS time is the most desired endpoint of anticancer treatments; however, some panel members emphasized that the efficacy of subsequent lines of treatment as well as crossover to bevacizumab can dilute the impact of first-line therapy on OS results. The panel members also said that, although bevacizumab has a specific target, treatment with bevacizumab was developed in unselected populations, which may have obscured a greater potential benefit in subgroups of any breast cancer or MBC patients.
The lack of an OS advantage and the absence of a biomarker to identify patients more likely to benefit from treatment represent limitations to the widespread use of bevacizumab in daily clinical practice. According to a survey conducted by the Intercontinental Marketing Service Health Spa in the first quarter of 2010 in six countries, bevacizumab was used as part of first-line treatment for patients with HER-2− MBC in percentages in the range of 2.6% in the U.K., 34% in France and the U.S., and 21%–26% in other European countries.
In conclusion, all but one member of the panel agreed that, from a scientific perspective, bevacizumab used in combination with chemotherapy is an appropriate indication for MBC. The panel member from Turkey pointed out that, in his country, bevacizumab is considered to be not cost-effective and therefore had not been approved. Apart from discussion on the clinical value of bevacizumab, all the experts emphasized the need for uniformity and transparency from regulatory agencies in the drug-approval process.
Question #2: Do the Results of the AVADO (Docetaxel) and/or RIBBOn-1 (Taxanes, Anthracyclines, or Capecitabine) Trials Represent a Favorable Risk–Benefit Analysis for the Upfront Therapy of MBC?
There was general agreement that bevacizumab is well tolerated. In fact, the most frequent grade 3 or 4 adverse events are hypertension and proteinuria, which are asymptomatic and are therefore not relevant from a patient's perspective and are generally easily manageable.
The panel members felt that clinically relevant side effects depend mostly on the chemotherapy agent combined with bevacizumab. Serious side effects, such as major bleeding, visceral perforation, and thromboembolic events, vary in frequency according to the chemotherapy background, disease site, and, particularly for other tumor types, extent of prior surgery. Panel members emphasized that taxanes can cause bowel visceral perforation, and GI perforations are more frequently reported when bevacizumab is combined with these drugs than with capecitabine or anthracyclines. Panel members felt that cardiac toxicity is mostly observed when bevacizumab is combined with anthracyclines. The toxicity profile of bevacizumab in breast cancer patients is more favorable than that observed in lung, colorectal, and ovarian cancer patients, probably also as a consequence of different chemotherapy combinations, different surgeries, and different locations of disease spread.
Learning how to monitor these toxicities is important because they are different from those seen with typical chemotherapy. Oncologists are aware of potential toxicities with antiangiogenic agents in general and with bevacizumab in particular. Careful selection is needed when planning surgery because of the greater risk for complications if bevacizumab is not discontinued at the appropriate time. However, because very few breast cancer patients generally need major surgical procedures, this is usually a minor problem. The panel emphasized the importance of considering the individual patient's risk as well as the chemotherapy companion.
All but two panel members agreed on the good toxicity profile; those two panel members felt that the benefit–risk ratio was debatable, mostly because the benefits are uncertain. In particular, one panel member felt that, although the average risk–benefit is favorable, it is possible that this is the result of a substantial benefit for a few with no benefit or even some harm for the majority of breast cancer patients. Panel members also discussed that the preclinical data suggest a rebound in tumor growth following the discontinuation of bevacizumab. This phenomenon has not been documented in the clinical setting; however, it is not possible to discern those patients who would receive a substantial clinical benefit from chemotherapy plus bevacizumab from those patients in whom this therapy might accelerate a slow-growing tumor.
Question #3: Do the Results of the RIBBOn-1 and AVADO Trials Confirm the Clinical Benefit of Bevacizumab in Combination with Paclitaxel for the Initial Treatment of Patients with HER-2− MBC?
There was general consensus that the AVADO and RIBBOn-1 trials confirmed the results of the ECOG-2100 trial. There was discussion that the benefit in the latter studies was of lower magnitude. A head-to-head comparison of different chemotherapy agents in combination with bevacizumab was not available. However, some members of the panel said that when chemotherapy is more active, the benefit of adding bevacizumab appears to be less impressive. In fact, the response rate in the control arm of the AVADO trial was almost double that in the control arm in the ECOG-2100 trial. Panel members also discussed the lack of an independent review in the ECOG-2100 trial, with the subsequent risk for an overestimation of responses in the experimental arm. More important, however, was the fact that the three trials gave consistent results according to HRs. One panelist also noted that, for all the reasons mentioned previously, the ECOG-2100 trial was probably the outlier, with intrinsic limitations in its study design. However, the same panel member felt that it is much easier to administer prolonged therapy with weekly paclitaxel than with docetaxel administered three times a week; it is therefore possible that more patients in the AVADO trial discontinued treatment earlier than patients in the ECOG-2100 trial.
Question #4: Will the PFS Time No Longer Be Accepted as an Endpoint Without QOL Data?
There was general agreement that the PFS duration is a more sensitive endpoint to demonstrate the efficacy of a new drug, because the OS time is influenced by salvage therapies and crossover designs. In particular, the longer the OS time after first disease progression, the higher the number of patients needed to translate a PFS benefit into an OS benefit. It has been estimated that, for patients with an expected OS time of 24 months, >3,000 patients are needed to demonstrate a longer OS time [55]. Therefore, at least in the first-line setting, the PFS duration should still be considered an appropriate endpoint. Of note, when the AVADO and RIBBOn-1 trials were discussed with the FDA prior to their initiation, a planned HR of 0.75 for the PFS time was judged to be sufficient to confirm the approval of bevacizumab.
The panel felt that the PFS interval by itself is not the only parameter to be considered and that other factors play a role in the complex process of drug approval, reimbursement, and use. One panel member emphasized that, if a drug is expected to be expensive, a longer PFS time or a lower HR for progression are needed to justify the additional costs. Moreover, a longer OS time should be expected, and it is hoped that these data will translate to a DFS benefit earlier in the disease setting. All but one panel member agreed that the HR, rather than the median PFS time, is the most significant parameter of meaningful OS benefit.
The panel also discussed that the other important aspect related to the value of the PFS duration is the assessment of QOL. QOL analysis is considered an indirect way to assess the risk–benefit ratio of a treatment, because it should reflect both disease and treatment toxicity. It is unfortunate that QOL studies are sometimes conducted in subgroups of patients and that complete data are available only from a limited number of treated patients. Another limitation of QOL data comes from the fact that the majority of MBC patients in the first-line treatment setting may have oligometastatic disease, be in good clinical condition, and be without symptoms. Moreover, the majority of trials exclude patients with a poor performance status. Therefore, it is virtually impossible to show a QOL improvement for patients starting with asymptomatic disease. If the aim is to demonstrate that a given treatment is able to improve QOL, these studies should include patients with impaired QOL at the beginning of treatment. The other key point is the negative effect of treatment toxicities on QOL. Again, the panelists recognized that the majority of the toxicities observed in the bevacizumab trials were related to chemotherapy and that the more frequent bevacizumab-related toxicities were asymptomatic and did not impact patient QOL.
Future Directions and Conclusions
The field of angiogenesis continues to grow, and it is a very exciting area of research. Data consistent with those for other tumor types have demonstrated that bevacizumab is effective in patients with MBC and carries manageable toxicity.
However, new obstacles have emerged. An imperative need exists for quantitative biomarkers of response to anti-VEGF inhibitors and for understanding of the molecular mechanisms of activity and resistance. It will also be important to understand chemosensitization effects at the cellular and molecular levels. In parallel to advances in antiangiogenesis, the development of new drugs in oncology faces multiple challenges related to our better understanding of molecular events. The application of traditional response criteria to new targeted therapies may be inaccurate because neither tumor response nor drug toxicity is a useful surrogate for dose selection or efficacy. Despite modest improvements, the prognosis for patients with advanced breast cancer continues to be poor. Bevacizumab is a first step into the field of angiogenesis inhibitors and results from three large, randomized trials that showed an important PFS benefit in all patient populations with MBC. There is hope that further improvements in the survival of MBC patients will follow. Bevacizumab should still be considered as a treatment option as a first-line chemotherapy for patients with locally recurrent breast cancer or MBC that is HER-2−. Patients treated with bevacizumab should be monitored carefully for bleeding, GI tract perforation, and neutropenia. A cost decision-analytical model was recently reported by Montero et al. [56]. That model, using efficacy and adverse event data from the ECOG-2100 study, demonstrated that bevacizumab added 0.49 years of PFS time and 0.135 quality-adjusted life-years (QALY), with an incremental cost of $100,300 and therefore a cost of $204,000 per year of PFS gained and an incremental cost-effectiveness ratio of $745,000 per QALY.
In summary, we believe that the recently reported pooled analyses of MBC patients receiving bevacizumab-based therapy illustrate the following issues. First, although an OS benefit was not found in these analyses, the significantly longer PFS time with bevacizumab was seen across the three clinical trials. Second, although the PFS interval might depend on the evaluation methods and schedules used, the PFS time as a study endpoint currently represents the most sensitive parameter to assess the efficacy of an experimental drug in metastatic disease, especially when a longer PFS duration is associated with a higher objective response rate or a measurable improvement in QOL. Third, if the OS time is the main endpoint of trial interest, trials will have to recruit more patients and will therefore be more expensive than current clinical studies.
See the accompanying article on pages 1669–1671 of this issue.
Acknowledgments
We thank Brenda McNaughton from the Department of Breast Medical Oncology and Kristi M. Speights from the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for their editorial assistance.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Ricardo H. Alvarez, PierFranco Conte, Valentina Guarneri, Gabriel N. Hortobagyi
Provision of study material or patients: Ricardo H. Alvarez, Valentina Guarneri
Collection and/or assembly of data: Ricardo H. Alvarez, Valentina Guarneri
Data analysis and interpretation: Ricardo H. Alvarez, PierFranco Conte, Valentina Guarneri, Gabriel N. Hortobagyi
Manuscript writing: Ricardo H. Alvarez, Valentina Guarneri
Final approval of manuscript: Ricardo H. Alvarez, PierFranco Conte, Valentina Guarneri, Stephen Johnston, David Khayat, Fikri Icli, Sibylle Loibl, Miguel Martin, Christoph Zielinski, Gabriel N. Hortobagyi
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin H-R, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 9.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burger RA, Sill MW, Monk BJ, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2007;25:5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 11.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 12.Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. The Oncologist. 2000;5(suppl 1):37–44. doi: 10.1634/theoncologist.5-suppl_1-37. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 14.Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:1007–1021. viii. doi: 10.1016/j.hoc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6:395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 17.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 18.Dameron KM, Volpert OV, Tainsky MA, et al. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2:re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 20.Tischer E, Mitchell R, Hartman T, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 21.Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. The Oncologist. 2004;9(suppl 1):2–10. doi: 10.1634/theoncologist.9-suppl_1-2. [DOI] [PubMed] [Google Scholar]
- 22.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 23.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 24.Relf M, LeJeune S, Scott PA, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 25.Casanovas O, Hicklin DJ, Bergers G, et al. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Konecny GE, Meng YG, Untch M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10:1706–1716. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- 27.Linderholm BK, Hellborg H, Johansson U, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;20:1639–1646. doi: 10.1093/annonc/mdp062. [DOI] [PubMed] [Google Scholar]
- 28.Kim KJ, Li B, Houck K, et al. The vascular endothelial growth factor proteins: Identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors. 1992;7:53–64. doi: 10.3109/08977199209023937. [DOI] [PubMed] [Google Scholar]
- 29.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 30.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 31.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Ran S, Sambade M, et al. A monoclonal antibody that blocks VEGF binding to VEGFR2 (KDR/Flk-1) inhibits vascular expression of Flk-1 and tumor growth in an orthotopic human breast cancer model. Angiogenesis. 2002;5:35–44. doi: 10.1023/a:1021540120521. [DOI] [PubMed] [Google Scholar]
- 33.Margolin K, Gordon MS, Holmgren E, et al. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: Pharmacologic and long-term safety data. J Clin Oncol. 2001;19:851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 34.Cobleigh MA, Langmuir VK, Sledge GW, et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30(suppl 16):117–124. doi: 10.1053/j.seminoncol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Burstein HJ, Chen YH, Parker LM, et al. VEGF as a marker for outcome among advanced breast cancer patients receiving anti-VEGF therapy with bevacizumab and vinorelbine chemotherapy. Clin Cancer Res. 2008;14:7871–7877. doi: 10.1158/1078-0432.CCR-08-0593. [DOI] [PubMed] [Google Scholar]
- 36.Sledge G, Miller K, Moisa C, et al. Safety and efficacy of capecitabine (C) plus bevacizumab (B) as first-line in metastatic breast cancer. J Clin Oncol. 2007;25(18 suppl) Abstract 1013. [Google Scholar]
- 37.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 38.Gray R, Bhattacharya S, Bowden C, et al. Independent review of E2100: A phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27:4966–4972. doi: 10.1200/JCO.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 40.Pivot X, Verma S, Thomssen C, et al. Clinical benefit of bevacizumab (BV) plus first-line docetaxel (D) in elderly patients (pts) with locally recurrent (LR) or metastatic breast cancer (MBC): AVADO study. J Clin Oncol. 2009;27(15 suppl) Abstract 1094. [Google Scholar]
- 41.Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 42.O'Shaughnessy J, Miles D, Gray RJ, et al. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC) J Clin Oncol. 2010;28(15 suppl) Abstract 1005. [Google Scholar]
- 43.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Píez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miles D, Harbeck N, Escudier B, et al. Disease course patterns after discontinuation of bevacizumab: Pooled analysis of randomized phase III trials. J Clin Oncol. 2011;29:83–88. doi: 10.1200/JCO.2010.30.2794. [DOI] [PubMed] [Google Scholar]
- 46.von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy with or without bevacizumab: Primary efficacy endpoint analysis of the GEPARQUINTO study (GBG 44) [abstract S4–6]. Presented at the 2010 San Antonio Breast Cancer Symposium; December 8–11, 2010; San Antonio, Texas. [Google Scholar]
- 47.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: A meta-analysis. JAMA. 2011;505:487–494. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 48.Dean-Colomb W, Fang S, Smith W, et al. Off-label drug use in women with breast cancer. J. Clin Oncol. 2009;27(15 suppl) Abstract 1016. [Google Scholar]
- 49.Smith I. Goals of treatment for patients with metastatic breast cancer. Semin Oncol. 2006;33(suppl 2):S2–S5. doi: 10.1053/j.seminoncol.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 50.Johnson KR, Ringland C, Stokes BJ, et al. Response rate or time to progression as predictors of survival in trials of metastatic colorectal cancer or non-small-cell lung cancer: A meta-analysis. Lancet Oncol. 2006;7:741–746. doi: 10.1016/S1470-2045(06)70800-2. [DOI] [PubMed] [Google Scholar]
- 51.Sherrill B, Amonkar M, Wu Y, et al. Relationship between effects on time-to-disease progression and overall survival in studies of metastatic breast cancer. Br J Cancer. 2008;99:1572–1578. doi: 10.1038/sj.bjc.6604759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Louvet C, de Gramont A, Tournigand C, et al. Correlation between progression free survival and response rate in patients with metastatic colorectal carcinoma. Cancer. 2001;91:2033–2038. [PubMed] [Google Scholar]
- 53.Verma S, McLeod D, Batist G, et al. In the end what matters most? A review of clinical endpoints in advanced breast cancer. The Oncologist. 2011;16:25–35. doi: 10.1634/theoncologist.2010-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saad ED, Katz A, Buyse M. Overall survival and post-progression survival in advanced breast cancer: A review of recent randomized clinical trials. J Clin Oncol. 2010;28:1958–1962. doi: 10.1200/JCO.2009.25.5414. [DOI] [PubMed] [Google Scholar]
- 55.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montero AJ, Gluck S, Lopez G., Jr The cost-effectiveness of bevacizumab in combination with paclitaxel in first-line treatment of patients with metastatic breast cancer [abstract 6060] J Clin Oncol. 2011;29(suppl):398s. [Google Scholar]
- 57.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 58.Ellis LM. The role of neuropilins in cancer. Mol Cancer Ther. 2006;5:1099–1107. doi: 10.1158/1535-7163.MCT-05-0538. [DOI] [PubMed] [Google Scholar]
- 59.Pan Q, Chanthery Y, Liang WC, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 61.Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280:C1375–C1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- 62.Gerber HP, Condorelli F, Park J, et al. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 63.Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]