Non-technical summary
Inter-individual differences in regional GABA as assessed by magnetic resonance spectroscopy (MRS) relate to behavioural variation in humans. However, it is not clear what the relationship is between MRS measures of the concentration of neurotransmitters in a region and synaptic activity. Transcranial magnetic stimulation (TMS) techniques provide physiological measures of cortical excitation or inhibition. Here, we investigated the relationship between MRS and TMS measures of glutamatergic and GABAergic activity within the same individuals. We demonstrated a relationship between MRS-assessed glutamate levels and a TMS measure of global cortical excitability, suggesting that MRS measures of glutamate do reflect glutamatergic activity. However, there was no clear relationship between MRS-assessed GABA levels and TMS measures of synaptic GABAA or GABAB activity. A relationship was found between MRS-assessed GABA and a TMS protocol with less clearly understood physiological underpinnings. We speculate that this protocol may therefore reflect extrasynaptic GABA tone.
Abstract
Abstract
Magnetic resonance spectroscopy (MRS) allows measurement of neurotransmitter concentrations within a region of interest in the brain. Inter-individual variation in MRS-measured GABA levels have been related to variation in task performance in a number of regions. However, it is not clear how MRS-assessed measures of GABA relate to cortical excitability or GABAergic synaptic activity. We therefore performed two studies investigating the relationship between neurotransmitter levels as assessed by MRS and transcranial magnetic stimulation (TMS) measures of cortical excitability and GABA synaptic activity in the primary motor cortex. We present uncorrected correlations, where thePvalue should therefore be considered with caution. We demonstrated a correlation between cortical excitability, as assessed by the slope of the TMS input–output curve and MRS-assessed glutamate levels (r = 0.803, P = 0.015) but no clear relationship between MRS-assessed GABA levels and TMS-assessed synaptic GABAA activity (2.5 ms inter-stimulus interval (ISI) short-interval intracortical inhibition (SICI); Experiment 1:r = 0.33, P = 0.31; Experiment 2:r = –0.23, P = 0.46) or GABAB activity (long-interval intracortical inhibition (LICI); Experiment 1:r = –0.47, P = 0.51; Experiment 2:r = 0.23, P = 0.47). We demonstrated a significant correlation between MRS-assessed GABA levels and an inhibitory TMS protocol (1 ms ISI SICI) with distinct physiological underpinnings from the 2.5 ms ISI SICI (r = –0.79, P = 0.018). Interpretation of this finding is challenging as the mechanisms of 1 ms ISI SICI are not well understood, but we speculate that our results support the possibility that 1 ms ISI SICI reflects a distinct GABAergic inhibitory process, possibly that of extrasynaptic GABA tone.
Introduction
There is a great deal of individual variability in the performance of behavioural tasks in healthy humans. Recent efforts have tried to explain some of this variability by variation in brain structure, activity, or neurochemistry (Boy et al. 2010; Sumner et al. 2010; Stagg et al. 2011; Tomassini et al. 2011). The latter can be effectively assessed using MRS, a non-invasive tool to measure neurochemical concentrationsin vivowithin localized regions of tissue (typically in the order of 8 cm3). For example, recent studies have investigated the relationship between GABA levels within specific motor cortical regions and behaviour in motor tasks known to depend on those regions, suggesting that GABA concentration may indeed be involved in behavioural performance (Boy et al. 2010; Sumner et al. 2010; Stagg et al. 2011).
However, it is not clear how the total concentration of GABA within a relatively large volume of cortical tissue relates to local synaptic activity. GABA is found at high concentrations in the vesicles within the presynaptic boutons but it is also found in the cytoplasm of the GABAergic interneurons and in the extracellular fluid, where it acts via extrasynaptic GABAA receptors to produce non-synaptic ‘GABAergic tone’ (Martin & Rimvall, 1993; Farrant & Nusser, 2005) and there is evidence from the animal literature that the MRS GABA resonance in the main reflects this extrasynaptic GABA (Mason et al. 2001).
TMS allows the measurement of various parameters of excitatory and inhibitory processes within the primary motor cortex (M1). Active and resting motor thresholds (aMT and rMT) and input–output (IO) curves give measures of global cortical excitability. The motor thresholds are defined as the minimum stimulation intensity needed to elicit a motor-evoked potential (MEP) of a predefined size, either with the muscle at rest (rMT) or at a percentage of maximum voluntary activity (aMT), and reflect focal cortical excitability at the stimulated site. The MEP IO curve is acquired by recording the MEP amplitude in response to pulses of TMS at a range of intensities. The slope of the MEP IO curve is an index of excitability within a wider region of the cortex with steeper slopes reflecting increased cortical excitability. The relationship between MEP IO curve slope and neurotransmitter concentration is not completely elucidated, but may be due, at least in part, to increased glutamatergic activity (Di Lazzaro et al. 2003).
In addition, TMS can be used in paired-pulse protocols to study aspects of cortical excitability and inhibition with greater specificity. Short-interval intracortical inhibition (SICI) is a TMS protocol where a sub-threshold conditioning stimulus (CS) is followed a few milliseconds later by a supra-threshold test stimulus (TS), which elicits an MEP. The ratio of the size of the unconditioned to the conditioned MEP is then calculated. Depending on the inter-stimulus interval (ISI) two distinct phases of inhibition can be determined, one at an ISI of 1 ms and one at an ISI of 2–4 ms. The SICI seen at an ISI of 2–4 ms (Kujirai et al. 1993) has been shown to be dependent on synaptic GABAA receptor activity (Ziemann et al. 1996; Ilić et al. 2002). Less is understood about the 1 ms SICI; like the 2–4 ms SICI, it is thought to reflect GABAergic activity (Ni et al. 2007), although via a distinct mechanism to the 2–4 ms SICI (Fisher et al. 2002; Roshan et al. 2003). Longer ISIs, in the order of 10–15 ms, result in facilitation of the MEP, known as intracortical facilitation (ICF), thought to be a reflection of both synaptic GABAA and glutamate activity.
By contrast, GABAB activity can be assessed via another paired-pulse TMS protocol – long-interval intracortical inhibition (LICI), a protocol involving suprathreshold CS and TS stimuli 100–200 ms apart (Werhahn et al. 1999; McDonnell et al. 2006). We acquired IO curves for our paired-pulse measures as these are likely to be a more sensitive measure of GABAergic activity across subjects than assessing SICI at a single CS intensity.
Here, we wanted to directly investigate the relationship between TMS and MRS measures in the primary motor cortex. In Experiment 1 we studied the relationship between the MRS measure of GABA (referred to as MRS-GABA) and TMS measures of GABAA and GABAB activity. In Experiment 2 we explored TMS and MRS relationships more widely, with the following specific questions: (1) is the concentration of GABA within M1 related to GABA synaptic activity? and (2) is the slope of the MEP IO curve related to the concentration of glutamate within M1? In addition we hoped to be able to explore the possibility of a relationship between MRS-assessed GABA concentration and 1 ms SICI.
Methods
Ethical approval
This study was approved by the Oxfordshire Regional Ethics Committee A and the University College London Hospital Trust Research Ethics Committee. Written informed consent was obtained from all subjects and all experiments conformed to the standards set by the latest revision of theDeclaration of Helsinki.
Experiment 1
Twelve subjects gave their informed consent to participate in the study (mean age 25 years, range 19 to 40 years, 3 male). All subjects participated in one testing session at the University of Oxford where they had TMS and MRS measures of GABA in an order counter-balanced across the group.
MRS data
Data acquisition
MRS data were acquired using a 3T Siemens Verio system and a 32-channel head coil (Siemens, Germany). During acquisition subjects lay at rest. First a standard T1-weighted MR scan was acquired (magnetization prepared rapid gradient echo; MPRAGE; Repetition Time (TR), 2040 ms; Echo Time (TE), 4.7 ms; inversion time (TI), 900 ms; flip angle, 8 deg; voxel size 1 mm3). MRS data were acquired using an ultra-short TE spin echo acquisition (the spin-echo full-intensity acquired localized (SPECIAL) sequence (128 averages; TR, 2000 ms; TE, 8.5 ms)) (Mlynárik et al. 2006; Mekle et al. 2009). VAPOR (variable power RF pulses with optimized relaxation delays) water suppression was used (Tkác et al. 1999), and outer volume suppression was used to eliminate signal contamination from outside the MRS voxel. A 2 cm × 2 cm × 2 cm MRS voxel of interest was then centred on the hand knob in the left hemisphere, a landmark previously described to represent the hand area of the primary motor cortex (Yousry et al. 1997) (Fig. 1B).
Figure 1. MRS details.
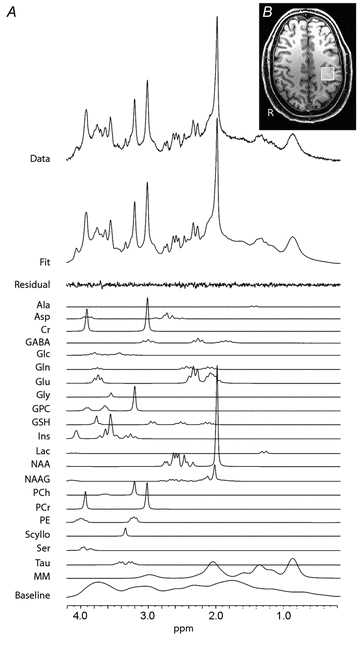
A, typical spectrum acquired using the SPECIAL sequence from the M1 voxel. The original MRS data is shown in the top row. The next row is the full model fit produced from LCModel (Provencher, 1993). The high quality of the fit is demonstrated by the small residual signal remaining after fitting; shown by the row labelled ‘residual’. Individual fits for all neurochemicals are also demonstrated – each neurochemical has multiple fitted peaks that reflect the individual protons within the molecule. GABA is found at a low concentration in the brain, as reflected by the relatively low-amplitude peaks. Despite this low concentration, the high quality of the fit for GABA is demonstrated by Cramér–Rao bands <20%.B, location of the left primary motor cortex (M1) voxel.
TMS data
Data acquisition
All TMS data were acquired using a monophasic BiStim machine, connected to a figure-of-eight coil with an outer diameter of 70 mm (Magstim Co., Whitland, Dyfield, UK). The primary motor cortex of the left hemisphere was stimulated in all subjects. All TMS was applied to the motor-hotspot for the first dorsal interosseus (FDI), which was defined as the location where a TMS pulse consistently produced the largest MEPs at 120% of motor threshold. The TMS coil was held at 45 deg to the mid-sagittal line with the handle pointing posteriorly. For the duration of the experiment the subjects were seated comfortably in an armchair with their eyes open.
EMG recording
Electromyography (EMG) was recorded via one pair of disposable neonatal ECG electrodes (Henley's Medical Ltd, Welwyn Garden City, UK) placed over the FDI of the right hand, using a belly-tendon montage. Signals were sampled at 5 kHz, amplified, filtered (10 Hz to 1 kHz) and recorded using a CED 1902 amplifier, a CED 1401 analog-to-digital converter and Spike 2 v3.2 software (Cambridge Electronic Design Ltd, Cambridge, UK).
Short-interval intracortical inhibition (SICI)
First, the active motor threshold (aMT) was defined as the intensity necessary to evoke a 200 μV MEP while subjects maintained approximately 10% contraction of the target muscle. Paired-pulse TMS was then performed with an inter-stimulus interval of 2.5 ms, using low intensities of conditioning pulse to avoid contamination with superimposed short-interval (I-wave) facilitation (Peurala et al. 2008). To reduce inter-subject variability, an IO curve was acquired with conditioning stimulus (CS) intensities of 60%, 70%, 80% and 90% of aMT and a test stimulus (TS) of the necessary intensity to evoke an MEP of approximately 1 mV peak-to-peak amplitude (SI 1 mV). For each CS intensity, 15 unconditioned and 15 conditioned MEPs were recorded.
Long-interval intracortical inhibition (LICI)
LICI was measured using an inter-stimulus interval of 150 ms and a CS and TS intensity of SI 1 mV. Fifteen unconditioned and 15 conditioned MEPs were recorded.
Experiment 2
We performed a second experiment in order to replicate the findings from Experiment 1 in a new population and to extend the range of TMS protocols tested in order to increase our understanding of the MRS-assessed measurements.
Twelve male subjects gave their informed consent to participate in the study (mean age 25 years; range 19–46 years). No subjects participated in both experiment 1 and experiment 2. All subjects participated in two testing sessions: one MRS session and one TMS session. The order of the two sessions was counterbalanced across the group and they were a mean of 13 days (range 1 to 44 days) apart.
MRS data
Data acquisition
MRS data were acquired at the University of Oxford using a 3T Siemens Trio system and a 12-channel head coil (Siemens, Germany). During acquisition subjects lay at rest and listened to a radio station of their choice. First a standard T1-weighted MR scan was acquired as in Experiment 1. MRS data were acquired using an ultra-short TE spin echo acquisition (SPECIAL) sequence (170 averages; TR, 2000 ms; TE, 8.5 ms) (Mlynárik et al. 2006; Mekle et al. 2009). Three MRS voxels of interest were acquired: (1) a 2 cm × 2 cm × 2 cm voxel centred on the hand knob in the left hemisphere; (2) a 2 cm × 2 cm × 2 cm voxel centred on the hand knob in the right hemisphere; and (3) a 2 cm × 3 cm × 2 cm voxel centred on the occipital cortex.
TMS session
All TMS data were acquired at University College London using a monophasic BiStim machine, connected to a figure-of-eight coil with an outer diameter of 70 mm (Magstim Co.). The primary motor cortex of the left hemisphere was stimulated in all subjects. All TMS was applied as in Experiment 1.
EMG recording
EMG was recorded via one pair of Ag–AgCl electrodes placed over the FDI of the right hand, using a belly-tendon montage. Signals were sampled at 5 kHz, filtered (10 Hz to 1 kHz), amplified (Digitimer 360, Digitimer Ltd, Welwyn Garden City, Herts, UK) and stored on computer via a Power 1401 data acquisition interface (Cambridge Electronic Design Ltd). All analysis was carried out using Signal Software (Cambridge Electronic Design).
MEP input–output (IO) curves
At the beginning of the experiment the stimulus intensity required to evoke an MEP of approximately 1 mV peak-to-peak amplitude was defined (SI 1 mV). Ten MEPs were then recorded with stimuli intensities of 50%, 70%, 80%, 90%, 100%, 110%, 120%, 130% and 150% of SI 1 mV. The order in which the individual intensities were acquired was randomized.
Short-interval paired-pulse TMS
The aMT was defined as in Experiment 1. Paired-pulse TMS was then performed with three inter-stimulus intervals: 1 ms (1 ms SICI), 2.5 ms (2.5 ms SICI) and 12 ms (ICF). For all ISIs an IO curve was acquired with conditioning stimulus (CS) intensities of 60%, 70%, 80% and 90% of aMT and a test stimulus (TS) of SI 1 mV. For each ISI and each CS intensity, 20 unconditioned and 20 conditioned MEPs were recorded.
LICI
LICI was measured using an inter-stimulus interval of 150 ms. Again an IO curve was acquired, with CS of 80%, 100%, 120% and 140% of SI 1 mV and a TS of SI 1 mV. Twenty unconditioned and 20 conditioned MEPs were recorded for each CS intensity.
Data analysis
MRS data
All MRS data were processed using LCModel (Provencher, 1993). Any spectra with a water line-width of ≥10 Hz were excluded from further analysis. GABA and glutamate values are given as a ratio to creatine, a simultaneously acquired reference peak, to reduce inter-subject variability, and are henceforth referred to in the text as MRS-GABA and MRS-glutamate to avoid confusion. Individual resonances with Cramér–Rao bounds >20% were excluded from further analysis.
The T1-weighted structural image was segmented using FAST (FMRIB's automated segmentation tool, part of the FMRIB software library (FSL)), (Zhang et al. 2001), and the relative grey matter, white matter and cerebrospinal fluid contributions to each of the MRS voxels were calculated and used to correct MRS values for grey matter volume within the voxel (Stagg et al. 2009a).
TMS data
EMG activity in the 50 ms period prior to the first TMS pulse was analysed, and any trace with significant pre-contraction of the FDI was excluded. The peak-to-peak amplitudes of the remaining TS were calculated. For each TMS block (i.e. each TS for the MEP IO curve, each CS for each ISI for the paired pulse TMS (ppTMS) measures), significant outliers were detected using Grubb's test (http://www.graphpad.com/quickcalcs/Grubbs1.cfm) and removed from further analysis. For the MEP IO curve, the mean peak-to-peak amplitude for the remaining MEPs was calculated for each TS intensity. For the paired-pulse measures the mean peak-to-peak amplitude for the conditioned and unconditioned stimuli were calculated for each ISI and CS intensity separately. The percentage inhibition was then calculated.
All slope fits were performed in Matlab (Mathworks, MA, USA). For the MEP IO curve, a sigmoidal curve of best fit was determined, using the error function (erf) within Matlab, and the maximum slope of this sigmoidal curve calculated. For two subjects a fit could not be performed, and these subjects were excluded from further analysis. For the ppTMS measures we performed both a second-order polynomial fit and a linear fit, as previously described (Orth et al. 2003). The goodness-of-fit was better for the linear fit over this range of CS intensities (data not shown) and therefore the slope of the linear fit was calculated to gain a measure of recruitment for each subject. For one subject a fit could not be performed for the 1 ms SICI data and therefore this subject was excluded from further analysis. To investigate the main effects of paired-pulse stimulation, repeated-measures ANOVAs were performed and in all group mean graphs data points refer to mean ± standard error.
Correlations between MRS and TMS measures
The strength of all correlations was assessed using Pearson's correlation statistic in PASW statistical package v18.0 (IBM, Chicago, IL, USA). A Bonferroni threshold correction for multiple comparisons was performed for each neurotransmitter and each experiment separately. In the case where this results in a correctedPvalue greater than 1 this is given asP = 1. For all correlations the percentage of variance explained is given and for non-significant correlations a power calculation was performed and the resulting number of subjects required to give a significant correlation is also given.
Results
Experiment 1
Group mean effects
A typical spectrum from the left M1 is shown in Fig. 1A. The SICI protocol led to a significant inhibition (repeated-measures ANOVA main effect of conditioning stimulus;F(1,11) = 25.3, P< 0.001), as did the LICI protocol (mean unconditioned MEP size 1.27 mV; mean conditioned MEP size 0.3 mV; pairedttest, P = 0.004).
Relationship between MRS-GABA and TMS measures of synaptic GABA activity
We had an a priori hypothesis that the concentration of GABA within M1 is related to GABA synaptic activity. In order to investigate the relationship between MRS-GABA within the left M1 and measures of GABAergic synaptic activity, we acquired a 2.5 ms SICI IO curve, thought to be a measure of GABAA synaptic activity, and LICI, thought to be a measure of GABAB activity. As MRS-GABA may represent a total of synaptic activity, rather than either GABAA or GABAB activity independently, we combined these measures into a linear regression model. There was no linear combination of the two measures that significantly described the GABA levels (P> 0.2). We then went on to investigate GABAA and GABAB synaptic activity separately. There was no significant correlation between either MRS-GABA and 2.5 ms SICI (r = 0.33, P = 0.62 (corrected), % variance explained = 11%, power calculation suggestsn = 50 required to demonstrate significance) or between MRS-GABA and LICI (r = –0.47, P = 1 (corrected), % variance = 22%, power calculation:n = 22; Fig. 2).
Figure 2. Experiment 1.
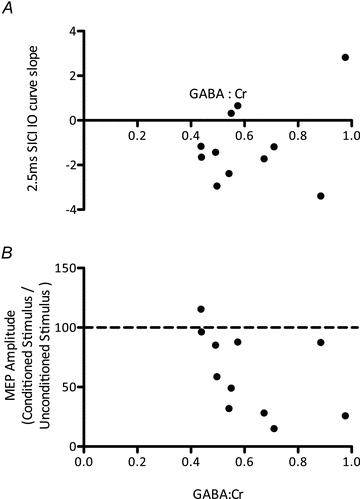
A, no significant relationship between 2.5 ms SICI (higher numbers reflect greater inhibition) and MRS-assessed GABA levels (r = 0.33, P = 0.31).B, no significant relationship between LICI (lower numbers reflect greater inhibition) and MRS-assessed GABA levels (r = –0.47, P = 0.51). Data points reflect individual subjects.
Experiment 2
We then went on to test whether there is a relationship between MRS-GABA and MRS-glutamate and other TMS measures of excitation and inhibition.
Group mean effects
An SICI of 1 ms led to a significant inhibition of MEP amplitude (repeated-measures ANOVA main effect of conditioning stimulus;F(1,11) = 36.5, P< 0.001) as did the 2.5 ms SICI (repeated-measures ANOVA main effect of conditioning stimulus;F(1,11) = 24.7, P< 0.001). The 12 ms ISI ICF led to a significant facilitation of MEP amplitude (repeated-measures ANOVA main effect of conditioning stimulus;F(1,11) = 7.78, P = 0.01). LICI led to a significant inhibition of MEP amplitude (repeated-measures ANOVA main effect of conditioning stimulus;F(1,11) = 13.39, P = 0.004).
Relationship between MRS-GABA and TMS measures of synaptic GABA activity
In order to test our first a priori hypothesis that there is a relationship between MRS-GABA within the left M1 and GABAergic synaptic activity, we acquired a 2.5 ms SICI IO curve, and a LICI IO curve (Fig. 3AandB). As in Experiment 1, as MRS-GABA may represent a total of synaptic activity, rather than either synaptic GABAA or GABAB activity independently, we combined these TMS GABAA and GABAB measures into a linear regression model. There was no linear combination of the two measures that significantly described the MRS-GABA (P> 0.9 (corrected)). We then went on to investigate the relationship between MRS-GABA and GABAA and GABAB synaptic activity independently. There was no relationship between the slope of the 2.5 ms SICI IO curve and MRS-GABA (r = –0.23, P = 1 (corrected), % variance = 5%, power calculation:n> 100; Fig. 3C) nor between the slope of the LICI IO curve and MRS-GABA (r = 0.23, P = 1 (corrected); Supplementary Fig. S1), % variance = 5%, power calculation:n> 100). By contrast, there was a significant relationship between MRS-GABA and the 1 ms SICI IO curve (Fig. 3D, see below) (r = –0.79, P = 0.03 (corrected), % variance = 63%).
Figure 3. GABA measures.
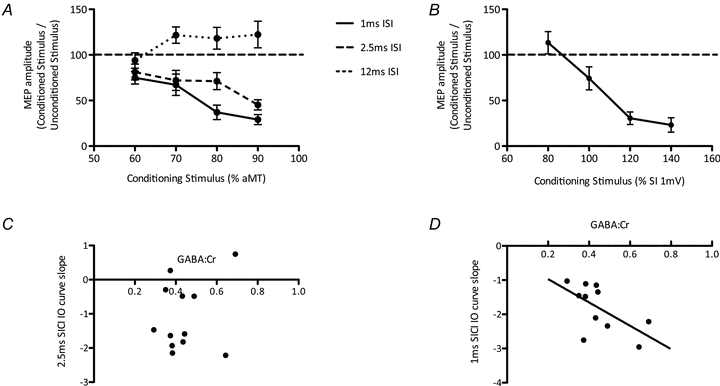
A, group mean IO curves for 1 ms SICI, 2.5 ms SICI and ICF.B, group mean IO curve for LICI. Data points are mean ± SEM.C, there was no significant relationship between 2.5 ms SICI and GABA levels (r = –0.23, P = 0.46).D, there was a significant relationship between 1 ms SICI and GABA levels (r = –0.79, P = 0.006) (lower numbers reflect greater inhibition). Data points are individual subjects.
Relationship between MRS-assessed neurotransmitters and TMS measures of global motor cortical excitability
We then tested our second a priori hypothesis that there is a relationship between the slope of the MEP IO curve, commonly held to be a measure of cortical excitability, and MRS-glutamate. The group mean MEP IO curve is shown in Fig. 4A. There was a significant positive correlation between the concentration of glutamate within the left M1 and the slope of the MEP IO curve such that subjects with higher MRS-glutamate also had steeper IO curves (r = 0.803, P = 0.025 (corrected), % variance = 65%; Fig. 4B).
Figure 4. Glutamate levels and cortical excitability.
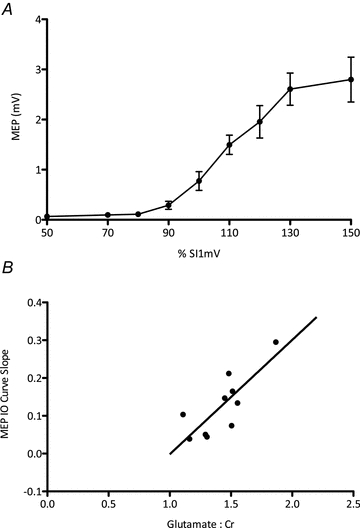
A, group mean MEP IO curve. Data points are mean ± SEM.B, there was a significant relationship between glutamate levels within M1 and the slope of the MEP IO curve, a global measure of cortical excitability (r = 0.803, P = 0.015). Higher numbers reflect greater excitability. Data points reflect individual subjects.
We then went on to explore the specificity of this relationship. As described previously (Stagg et al. 2009b, 2011), we found a significant correlation between MRS-glutamate and MRS-GABA within the left M1 (r = 0.56, P = 0.05 (uncorrected)). We therefore assessed whether left M1 MRS-GABA also correlated with MEP IO slope. There was a significant positive correlation between MRS-GABA within the left M1 and the slope of the MEP IO curve, reflecting the counter-intuitive relationship that subjects with higher MRS-GABA had steeper IO curves (r = 0.733, P = 0.05 (corrected), % variance = 53%). To test whether this relationship was driven by MRS-glutamate (given the positive correlation between MRS-glutamate and MRS-GABA), we co-varied out MRS-GABA but found that the correlation between MRS-glutamate and IO curve slope did not survive correction for MRS-GABA (r = 0.433, P = 1 (corrected), % variance = 19%, power calculation:n = 60).
Relationship between MRS-assessed neurochemicals and 1 ms SICI
Finally we tested our third a priori hypothesis that there was a relationship between 1 ms ISI and MRS-assessed neurochemical levels. We found a significant relationship between MRS-GABA and the 1 ms SICI IO curve (r = –0.79, P = 0.03 (corrected), % variance = 63%; Fig. 3D).
To investigate the specificity of this relationship between MRS-GABA and 1 ms SICI we performed exploratory tests on the relationship between MRS-glutamate and the 1 ms SICI IO curve. MRS-glutamate and 1 ms SICI were not significantly correlated (r = –0.34, P = 1 (corrected), (% variance = 12%, power calculation:n = 45), Supplementary Fig. S2), and the relationship between MRS-GABA and the 1 ms SICI IO curve slope remained significant when corrected for MRS-glutamate (r = –0.78, P< 0.05,% variance = 60%).
Neurochemical and anatomical specificity of the relationships
There were no significant correlations between MRS-GABA or between MRS-glutamate measures across any pairs of the three voxels tested (left (stimulated) M1, right M1, occipital cortex). There were no significant correlations between neurochemical measures taken from either of the control voxels (right M1 and the occipital cortex) and any TMS measures.
There was no correlation between either MRS-GABA or MRS-glutamate and the slope of the 12 ms ISI ICF protocol (Supplementary Figs S1 and S2).
Relationship between different TMS inhibitory measures
As there are few data directly investigating the relationship between the different SICI protocols, we tested the relationship between 1 ms SICI, 2.5 ms SICI and LICI. In line with previous findings (Fisher et al. 2002; Roshan et al. 2003), there was no relationship between the degree of 1 ms SICI and 2.5 ms SICI (r = 0.107, P = 1 (corrected), % variance = 1%, power calculation:n> 100); between 2.5 ms SICI and LICI (r = –0.05, P = 1 (corrected), % variance < 1%, power calculation:n> 100); or between 1 ms SICI and LICI (r = –0.285, P = 1 (corrected), % variance = 8%, power calculation:n> 70), suggesting that these three measures reflect different aspects of cortical inhibition (Supplementary Fig. S3).
Discussion
This study was performed with the aim of relating MRS and TMS measures of cortical excitability and of GABAergic and glutamatergic function. We have demonstrated three key findings: (1) the slope of the MEP IO curve, a measure of global motor cortical excitability, is related to the MRS-assessed glutamate levels within M1; (2) there is no clear relationship between MRS-assessed GABA levels in M1 and synaptic GABA activity; and (3) there is a relationship between MRS-assessed GABA concentration and the 1 ms SICI slope.
Global motor cortical excitability is best reflected by glutamate concentration
We have demonstrated a significant relationship between MEP IO curve slope and MRS-glutamate within M1, suggesting that MRS-glutamate is an important indicator of motor cortical excitability. By correcting IO slopes for motor threshold, a set increase in TS intensity will recruit approximately the same number of additional neurons across subjects. A higher MRS-glutamate presumably reflects, therefore, greater pre-synaptic glutamate stores, which can be released in response to an increasing TS intensity.
Here, as in previous studies, there is a significant correlation between MRS-glutamate and MRS-GABA within the same voxel. We found a correlation between MRS-GABA and the slope of the TMS IO curve, such that subjects with higher MRS-GABA, and therefore higher levels of inhibition, have steeper TMS IO curve slopes, reflecting greater excitability of the cortex. This relationship seems to be physiologically unlikely, and therefore, although we cannot statistically separate the two effects given the tight biochemical relationship between GABA and glutamate, we believe that the implausible relationship between MRS-GABA and TMS IO slope is driven by MRS-glutamate. More generally, the close relationship between MRS-GABA and MRS-glutamate means that detected MRS-GABA behavioural correlations could additionally be influenced by relationships between MRS-glutamate and behaviour. This possibility should be taken into consideration when relating inter-individual task performance measures to neurotransmitter concentrations as assessed by MRS.
MRS-GABA does not solely reflect synaptic activity
The lack of a relationship between MRS-GABA and TMS-assessed synaptic GABA activity that we found in both experiments is important for interpreting the results of MRS studies. We investigated both the relationship between MRS-GABA and synaptic GABAA or GABAB activity independently, and between MRS-GABA and GABAA and GABAB activity together, and saw no significant relationship in any of those cases. While we cannot rule out a contribution of synaptic GABA activity in the MRS-GABA measure, and it might be that a much larger sample size would reveal a correlation, the lack of a close relationship contributes to our interpretation of GABA MRS results. Although the amount of GABA in the presynaptic bouton is related to vesicular GABA release (Golan et al. 1996), this is only approximately 30% of the total GABA in the cortex (Petroff, 2002), and it is possible that it is less visible to MRS than other pools as it is bound by macromolecules.
Does tonic GABA activity underlie 1 ms SICI inhibition?
The significant positive relationship found between MRS-GABA and slope of 1 ms SICI is somewhat difficult to interpret as it is not yet clear exactly what mechanism underpins the 1 ms SICI. Previous work has suggested that the 1 ms ISI SICI is at least in part related to membrane refractory period (Fisher et al. 2002), although direct electrophysiological recordings do not support this hypothesis (Di Lazzaro et al. 1998). There is some evidence that there is GABAergic involvement, as the degree of 1 ms SICI decreases with the cortical silent period (CSP) which is thought to be GABA dependent (Ni et al. 2007) although 1 ms SICI is thought to be underpinned by a distinct cortical mechanism from 2.5 ms SICI (Fisher et al. 2002; Roshan et al. 2003), a hypothesis supported by the lack of a relationship between the two measures here.
The question, then, is what GABAergic inhibitory processes occur in the neocortex that could be reflected by these findings. The other GABAergic inhibitiory mechanism is GABAergic ‘tone’, which is produced via activation of extrasynaptic GABAA receptors.
Our data do not provide a clear explanation as tohowincreased activation of the extrasynaptic GABAA receptors confers greater inhibition measured by 1 ms SICI but we can speculate on possible mechanisms (see Supplementary discussion).
It should be noted, however, that this interpretation of 1 ms ISI SICI reflecting extrasynaptic GABA tone is speculative; it is also possible that the 1 ms ISI SICI reflects GABAA synaptic activity. We think this is a less likely explanation for two reasons: (1) the lack of a significant relationship between the degree of inhibition at 1 ms ISI and 2.5 ms ISI demonstrated here and elsewhere (Fisher et al. 2002; Roshan et al. 2003) and (2) the lack of effect of lorazepam, a non-selective GABAA agonist, on the 1 ms ISI SICI (Ziemann et al. 1996). However, we cannot conclusively discriminate between these potential mechanisms, and it is hoped that the data presented here will act as a starting point for further research to test this hypothesis the physiological underpinning of 1 ms ISI SICI.
General remarks
It is possible that the interval between MRS and TMS sessions in Experiment 2 reduced the possibility of finding a significant relationship between MRS-assessed GABA and TMS-assessed synaptic GABA activity. Data from previous studies have demonstrated a coefficient of variation of 10–12% across days for MRS-assessed measures of GABA (Bogner et al. 2010) and 31% for 2–4 ms ISI SICI (Orth et al. 2003). However, the TMS and MRS sessions in Experiment 1 were performed on the same day and no clear relationship between MRS-assessed GABA and TMS-assessed synaptic GABA activity was demonstrated. We included only male subjects in Experiment 2 as GABA levels in females varies significantly with the menstrual cycle (Epperson et al. 2005; Harada et al. 2011). We have not specifically addressed the question of sex differences in this study; further work could test for sex differences and test whether relationships between MRS-assessed GABA and TMS measures are consistent across the menstrual cycle.
Conclusions
This study was performed with the aim of characterizing the relationships between neurotransmitter concentrations and cortical excitability, as assessed using MRS and TMS measures. We have demonstrated a significant relationship between glutamate levels and overall cortical excitability, suggesting that MRS-assessed glutamate levels may accurately reflect glutamatergic signalling. In addition, we have demonstrated no relationship between synaptic measures of GABAA and GABAB activity and MRS-assessed GABA levels, a negative finding that is important in interpreting GABA MRS results. Further, we have demonstrated a relationship between GABA levels and 1 ms SICI, which we hypothesize may therefore reflect tonic extrasynaptic GABAA activity.
Acknowledgments
We thank Dr Ben Cox for help with curve fitting and Dr Jill O'Reilly for her technical expertise. Development of the SPECIAL sequence was supported by the CIBM of the UNIL, EPFL, IUNIGE, HUG and CHUV and the Jeantet and Leenaard foundations. The study was supported by the NIHR Oxford Biomedical Research Council (to H.J.-B. and C.J.S.); the Wellcome Trust (to H.J.-B.); the Biotechnology and Biological Sciences Research Council (to S.B.); and the European Union via ‘PLASTICISE’: an FP7 Collaborative Project (223524) (to J.C.R.). J.N. is supported by the Medical Research Council. A.O.C. was supported by an InEurope grant from the International Brain Research Organization.
Glossary
Abbreviation
- aMT
active motor threshold
- CS
conditioning stimulus
- EMG
electromyography
- FDI
first dorsal interosseus
- ICF
intracortical facilitation
- IO
input–output
- ISI
inter-stimulus interval
- LICI
long-interval intracortical inhibition
- MEP
motor-evoked potential
- M1
primary motor cortex
- MRS
magnetic resonance spectroscopy
- rMT
resting motor threshold
- SICI
short-interval intracortical inhibition
- TMS
transcranial magnetic stimulation
- TS
test stimulus
Author contributions
C.J.S., S.B., H.J.-B. & J.C.R. conceived and designed the experiments. C.J.S., S.B., L.M.M., C.A. & J.N. collected the data and C.J.S., A.O.C., R.M. & M.W. analysed the data. C.J.S., S.B., H.J.-B. & J.C.R. were involved in data interpretation. C.J.S. drafted the paper and all authors revised it and approved the final version of the manuscript.
Supplementary material
Supplementary Fig. S1
Supplementary Fig. S2
Supplementary Fig. S3
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors
References
- Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, Trattnig S, Doerfler A, Stefan H, Hammen T. In vivoquantification of intracerebral GABA by single-voxel 1H-MRS – How reproducible are the results? Eur J Radiol. 2010;73:526–531. doi: 10.1016/j.ejrad.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Boy F, Evans CJ, Edden RAE, Singh KD, Husain M, Sumner P. Individual differences in subconscious motor control predicted by GABA concentration in SMA. Curr Biol. 2010;20:1779–1785. doi: 10.1016/j.cub.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, Dileone M, Nicoletti R, Pasqualetti P, Tonali PA. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol. 2003;547:485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Epperson C, O'Malley S, Czarkowski K, Gueorguieva R, Jatlow P, Sanacora G, Rothman DL, Krystal J, Mason G. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psych. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Nakamura Y, Bestmann S, Rothwell JC, Bostock H. Two phases of intracortical inhibition revealed by transcranial magnetic threshold tracking. Exp Brain Res. 2002;143:240–248. doi: 10.1007/s00221-001-0988-2. [DOI] [PubMed] [Google Scholar]
- Golan H, Talpalar AE, Schleifstein-Attias D, Grossman Y. GABA metabolism controls inhibition efficacy in the mammalian CNS. Neurosci Lett. 1996;217:25–28. doi: 10.1016/0304-3940(96)13061-5. [DOI] [PubMed] [Google Scholar]
- Harada M, Kubo H, Nose A, Nishitani H, Matsuda T. Measurement of variation in the human cerebral GABA level byin vivoMEGA-editing proton MR spectroscopy using a clinical 3 T instrument and its dependence on brain region and the female menstrual cycle. Hum Brain Mapp. 2011;32:828–833. doi: 10.1002/hbm.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilić TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell MN, Orekhov Y, Ziemann U. The role of GABAB receptors in intracortical inhibition in the human motor cortex. Exp Brain Res. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem. 1993;60:395–407. doi: 10.1111/j.1471-4159.1993.tb03165.x. [DOI] [PubMed] [Google Scholar]
- Mason GF, Martin DL, Martin SB, Manor D, Sibson NR, Patel A, Rothman DL, Behar KL. Decrease in GABA synthesis rate in rat cortex following GABA-transaminase inhibition correlates with the decrease in GAD(67) protein. Brain Res. 2001;914:81–91. doi: 10.1016/s0006-8993(01)02778-0. [DOI] [PubMed] [Google Scholar]
- Mekle R, Mlynárik V, Gambarota G, Hergt M, Krueger G, Gruetter R. MR spectroscopy of the human brain with enhanced signal intensity at ultrashort echo times on a clinical platform at 3T and 7T. Magn Reson Med. 2009;61:1279–1285. doi: 10.1002/mrm.21961. [DOI] [PubMed] [Google Scholar]
- Mlynárik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med. 2006;56:965–970. doi: 10.1002/mrm.21043. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Chen R. Short interval intracortical inhibition and facilitation during the silent period in human. J Physiol. 2007;583:971–982. doi: 10.1113/jphysiol.2007.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth M, Snijders AH, Rothwell JC. The variability of intracortical inhibition and facilitation. Clin Neurophysiol. 2003;114:2362–2369. doi: 10.1016/s1388-2457(03)00243-8. [DOI] [PubMed] [Google Scholar]
- Petroff OA. GABA and glutamate in the human brain. Neuroscientist. 2002;8:562–573. doi: 10.1177/1073858402238515. [DOI] [PubMed] [Google Scholar]
- Peurala SH, Müller-Dahlhaus JF, Arai N, Ziemann U. Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF) Clin Neurophysiol. 2008;119:2291–2297. doi: 10.1016/j.clinph.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Provencher S. Estimation of metabolite concentrations from localizedin vivoproton NMR spectra. Magn Reson Med. 1993;30:672. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Roshan L, Paradiso GO, Chen R. Two phases of short-interval intracortical inhibition. Exp Brain Res. 2003;151:330–337. doi: 10.1007/s00221-003-1502-9. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Curr Biol. 2011;21:480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009b;29:5202–5206. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews P, Johansen-Berg H, Jezzard P, Rothwell J, Bestmann S. Neurochemical effects of theta burst stimulation as assessed by magnetic resonance spectroscopy. J Neurophysiol. 2009a;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Edden RAE, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Kincses ZT, Bosnell R, Douaud G, Pozzilli C, Matthews PM, Johansen-Berg H. Structural and functional bases for individual differences in motor learning. Hum Brain Mapp. 2011;32:494–508. doi: 10.1002/hbm.21037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry T, Schmid U, Alkadhi H, Schmidt D, Peraud A, Buettner P, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


