Abstract
Obesity-related diseases are associated with vascular dysfunction and impaired revascularization. Omentin is a fat-derived secreted protein, which is down-regulated in association with obese complications. Here, we investigated whether omentin modulates endothelial cell function and revascularization processes in vitro and in vivo. Systemic delivery of an adenoviral vector expressing omentin (Ad-omentin) enhanced blood flow recovery and capillary density in ischemic limbs of wild-type mice in vivo, which were accompanied by increased phosphorylation of Akt and endothelial nitric oxide synthase (eNOS). In cultured human umbilical vein endothelial cells (HUVECs), a physiological concentration of recombinant omentin protein increased differentiation into vascular-like structures and decreased apoptotic activity under conditions of serum starvation. Treatment with omentin protein stimulated the phosphorylation of Akt and eNOS in HUVECs. Inhibition of Akt signaling by treatment with dominant-negative Akt or LY294002 blocked the stimulatory effects of omentin on differentiation and survival of HUVECs and reversed omentin-stimulated eNOS phosphorylation. Pretreatment with the NOS inhibitor also reduced the omentin-induced increase in HUVEC differentiation and survival. Omentin protein also stimulated the phosphorylation of AMP-activated protein kinase in HUVECs. Transduction with dominant-negative AMP-activated protein kinase diminished omentin-induced phosphorylation of Akt and omentin-stimulated increase in HUVEC differentiation and survival. Of importance, in contrast to wild-type mice, systemic administration of Ad-omentin did not affect blood flow in ischemic muscle in eNOS-deficient mice in vivo. These data indicate that omentin promotes endothelial cell function and revascularization in response to ischemia through its ability to stimulate an Akt-eNOS signaling pathway.
Keywords: Adipokines, Akt PKB, Angiogenesis, Apoptosis, Endothelial Cell, Nitric Oxide Synthase, Omentin, Revascularization
Introduction
Obesity, in particular, visceral fat accumulation, is associated strongly with the development of type 2 diabetes, hypertension, and dyslipidemia (1, 2). Obesity-related disorders such as type 2 diabetes are accompanied frequently by microvascular rarefaction and reduced collateralization in ischemic tissues, ultimately resulting in the progression of cardiovascular diseases (3–5). Conversely, therapeutic strategies that enhance blood vessel growth are believed to have favorable effects on coronary and peripheral ischemic diseases. However, the mechanisms of how obesity affects the process of ischemic vascular disorders are understood incompletely at a molecular level.
Accumulating evidence suggests that adipose tissue is not simply an energy storage organ, but it also functions as a secretory tissue producing a variety of bioactive molecules, referred to as adipokines or adipocytokines, which directly or indirectly affect the pathogenesis of obesity-linked diseases (6, 7). Omentin, also referred to as intelectin-1, is an adipocytokine that exists abundantly in human visceral fat tissue (8–10). Omentin is detectable in human blood stream (100 to 800 ng/ml) (11, 12), and circulating omentin levels are decreased in obesity (11). Furthermore, circulating omentin levels are down-regulated in association with obesity-linked disorders, including type 2 diabetes, metabolic syndrome, endothelial dysfunction, carotid atherosclerosis, and coronary artery disease (12–16). It has been shown that treatment of adipocytes with the omentin protein results in enhancement of glucose uptake (8). Treatment with the omentin protein stimulates vasodilation in isolated blood vessels and suppresses cytokine-stimulated inflammation in cultured endothelial cells (17, 18). These data suggest that omentin may modulate obesity-related metabolic and vascular complications. However, nothing is known about the role of omentin in ischemic vascular diseases.
In the present study, we investigated whether omentin participates in blood vessel growth under ischemic conditions in vivo and tested whether it modulates endothelial cell function in vitro. We first investigated the effects of systemic delivery of omentin on revascularization in a mouse model of vascular insufficiency using an adenoviral vector expression system. We also examined the effects of omentin protein on cellular behavior and intracellular signaling in cultured endothelial cells. Our findings indicate that omentin promotes blood vessel recruitment in response to ischemia through activation of eNOS-dependent3 signaling within endothelial cells.
EXPERIMENTAL PROCEDURES
Materials
The following primary antibodies were purchased from Cell Signaling Technology: phospho-Akt (Ser-473) antibody, Akt antibody, phospho-eNOS (Ser-1177) antibody, eNOS antibody, phospho-AMPK (Thr-172) antibody, pan-α-AMPK antibody, ACC antibody, α-tubulin antibody, and HA antibody. Phospho-ACC (Ser-79) and c-Myc tag antibodies were purchased from Upstate Biotechnology. Recombinant human omentin protein produced from mammalian cell expression system was purchased from Alexis Biochemicals. LY294002 was purchased from Calbiochem. l-NAME was purchased from Sigma.
Construction of Adenoviral Vector Expressing Human Omentin
Full-length human omentin cDNA was subcloned into an adenovirus shuttle vector. After linearization, shuttle vector was cotransformed into Escherichia coli with the adenoviral backbone plasmid pAdEasy-1. The resultant recombinant pAdEasy-1 containing omentin cDNA was transfected into HEK 293 cells to generate the recombinant adenoviral vector expressing omentin (Ad-omentin). An adenoviral vector expressing β-galactosidase (Ad-β-gal) was used as a control. Adenoviral vectors were purified by Adeno-XTM Maxi purification kit (Clontech Laboratories).
Mouse Model of Revascularization
WT and eNOS-deficient (eNOS-KO) mice in a C57/BL6 background were used for this study. Study protocols were approved by the Institutional Animal Care and Use Committee in Nagoya University. Mice, at the ages of 10 weeks, were subjected to unilateral hind limb surgery under anesthesia with sodium pentobarbital (50 mg/kg intraperitoneally) (19, 20). In this model, the entire left femoral artery and vein were excised surgically. The 2 × 107 pfu of Ad-omentin or Ad-β-gal were injected into the jugular vein 3 days prior to surgery.
Laser Doppler Blood Flow (LDBF) Analysis
Hind limb blood flow was measured using a LDBF analyzer (Moor LDI, Moor Instruments) immediately before surgery, after surgery, and on postoperative days 3, 7, and 14 (19, 20). LDBF analysis was performed on legs and feet. Blood flow was displayed as changes in the laser frequency using different color pixels. After scanning, stored images were analyzed to quantify blood flow. To avoid data variations due to ambient light and temperature, hind limb blood flow was expressed as the ratio of left (ischemic) to right (nonischemic) LDBF.
Capillary Density Analysis
Capillary density within thigh adductor muscle was quantified by histological analysis as described previously (19, 20). Muscle samples were imbedded in OCT compound (Sakura) and snap-frozen in liquid nitrogen. Tissue slices (5 μm in thickness) were prepared and stained with CD31 (PECAM-1: Becton Dickinson) followed by treatment with Alexa Fluor® 488-conjugated secondary antibody (Invitrogen) to detect CD31. The signals were detected and analyzed by a fluorescence microscopy. Fifteen randomly chosen microscopic fields from three different sections in each tissue block were examined for the presence of CD31-positive capillary endothelial cells. Capillary density was expressed as the number of CD31-positive cells per muscle fiber or per high power field.
Measurement of Plasma Parameters
Total cholesterol, high density lipoprotein cholesterol, triglyceride, and plasma glucose levels were measured with enzymatic kits (Wako Chemicals). Plasma omentin and VEGF levels were determined by ELISA (human omentin ELISA kit (BioVendor) and mouse VEGF ELISA kit (R&D Systems)). Blood was collected by heart puncture from mice that were fasted for 6 h at 7 or 14 days post-surgery.
Cell Culture
Human umbilical vein endothelial cells (HUVECs) were cultured in endothelial cell growth medium-2. Before each experiment, cells were placed in endothelial cell basal medium-2 (San Diego, CA) with 0.5% FBS for 16 h for serum starvation. Experiments were performed by the addition of the indicated amount of human recombinant omentin or vehicle for the indicated lengths of time. In some experiments, HUVECs were infected with an adenoviral vector encoding a HA-tagged dominant-negative mutant form of AKT1 (Ad-dnAkt) (21), c-Myc-tagged dominant-negative AMPKα2 (Ad-dnAMPK) (19, 22) or Ad-β-gal as a control at a multiplicity of infection of 50 for 24 h. In some experiments, HUVECs were treated with LY294002 (50 μmol/liter), l-NAME (1 mmol/liter), or vehicle (dimethyl sulfoxide) for 60 min.
Differentiation Assay
The formation of vascular-like structures by HUVECs on growth factor-reduced Matrigel (BD Biosciences) was assessed according to the manufacturer's instructions as described previously (22). HUVECs were seeded on coated plates at 5 × 104 cells/cm2 in endothelial cell basal medium-2 and incubated at 37 °C for 18 h. Network formation was assessed using an inverted phase contrast microscope (Nikon, Tokyo, Japan) and photomicrographs were taken at ×40 magnification. The degree of tube formation was quantified by measuring the length of tubes in three randomly chosen fields from each well using the ImageJ program. Each experiment was repeated three times.
Analysis of Apoptotic Activity
Cells were treated with recombinant omentin protein or vehicle followed by 48 h of incubation in serum-free endothelial cell basal medium-2. Nucleosome fragmentation was assessed by enzyme-linked immunosorbent assay using a cell death detection kit as described previously (Roche Diagnostics) (20, 23). TUNEL staining was performed using the in situ cell death detection kit as described previously (Roche Applied Science) (20). TUNEL-positive cells were counted in five randomly selected microscopic fields. Each experiment was repeated three times.
Western Blot Analysis
Tissue samples obtained on post-operative day 7 were homogenized in lysis buffer containing 20 mm Tris-HCl (pH 8.0), 1% Nonidet P-40, 150 mm NaCl, 0.5% deoxycholic acid, 1 mm sodium orthovanadate, and protease inhibitor mixture (Roche Applied Science). Cell lysates or culture media were resolved by SDS-PAGE. The membranes were immunoblotted with the indicated antibodies at a 1:1000 dilution followed by the secondary antibody conjugated with horseradish peroxidase at a 1:1000 dilution. ECL and ECL plus Western blotting detection kit (GE Healthcare) were used for detection. Relative phosphorylation or protein levels were quantified by using the ImageJ program. Immunoblots were normalized to α-tubulin.
Statistical Analysis
Data are presented as mean ± S.D. as indicated in the figure legends. Statistical analysis was performed by ANOVA followed by Turkey's HSD test or Student's unpaired t test. A value of p < 0.05 was accepted as statistically significant.
RESULTS
Omentin Enhances Ischemia-induced Revascularization in Vivo
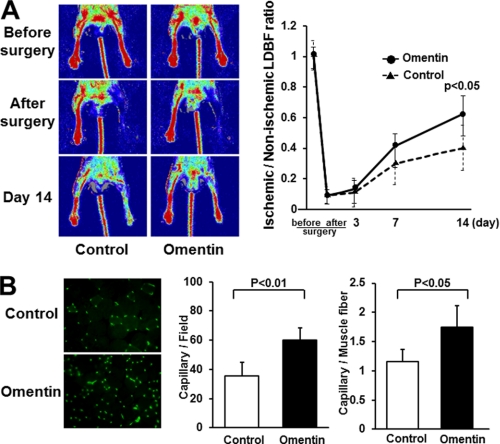
We first examined whether omentin can modulate revascularization under conditions of ischemia in vivo using WT mice that underwent unilateral femoral artery resection. We systemically injected Ad-omentin or Ad-β-gal as a control through a jugular vein into WT mice at 3 days before operation. Although human omentin protein could not be detected in plasma in control WT mice, plasma human omentin levels increased to 1051.2 ± 207.8 ng/ml in Ad-omentin-treated WT mice on the sixth day after injection. No significant differences were observed in body weight and plasma concentrations of total cholesterol, high density lipoprotein cholesterol, triglyceride, or glucose between Ad-omentin-treated and control mice (Table 1). Fig. 1A shows representative LDBF images of hind limb blood flow before surgery, after surgery, and at day 14 after surgery in the Ad-omentin-treated and control mice. In control mice, hind limb perfusion fell precipitously after surgery, increased to 30% of the nonischemic limb by day 7, and returned to 40% of the nonischemic limb by day 14 (Fig. 1A). Ad-omentin-treated mice showed a significant increase in blood flow recovery at 14 days after hind limb surgery (Fig. 1A).
TABLE 1.
Characteristics of WT mice treated with Ad-omentin or Ad-β-gal
Measurements were made on day 14 after surgery in Ad-omentin-treated or Ad-β-gal-treated WT mice (control) that were fasted for 6 h (n = 5). Each value is mean ± S.D. BW, body weight (g); T-Chol, total cholesterol (mg/dl); HDL-C, high density lipoprotein cholesterol (mg/dl); TG, triglyceride (mg/dl); PG, plasma glucose (mg/dl).
| BW | T-Chol | HDL-C | TG | PG | |
|---|---|---|---|---|---|
| Control | 24.1 ± 0.7 | 106.5 ± 4.2 | 53.7 ± 3.4 | 93.4 ± 3.8 | 100.3 ± 10.6 |
| Ad-omentin | 24.7 ± 0.8 | 105.4 ± 5.1 | 56.1 ± 3.3 | 95.6 ± 4.2 | 102.7 ± 7.1 |
FIGURE 1.
Omentin promotes perfusion recovery and capillary vessel formation of ischemic limbs in mice in vivo. Ad-omentin or Ad-β-gal (control) was injected into the jugular vein of WT mice (2 × 107 pfu in each group) 3 days prior to surgery. A, representative LDBF images in ischemic limb of Ad-omentin-treated or control mice (left panels). Quantitative analysis of the ischemic/non-ischemic LDBF ratio in Ad-omentin-treated and control WT mice before surgery, after surgery, and on postoperative days 3, 7, and 14 (right panel). Results are shown as the mean ± S.D. (n = 7). B, fluorescence staining of ischemic tissues with anti-CD31 monoclonal antibody (green; left panel). Quantitative analysis of capillary density in Ad-omentin-treated and control mice on postoperative day 14. Capillary density was expressed as the number of capillaries per high power field (×400, middle panel) and capillaries per muscle fiber (right panel). Results are shown as the mean ± S.D. (n = 5).
To investigate the extent of revascularization at the microcirculatory level, capillary density was measured in ischemic tissues by staining with anti-CD31 antibody. Fig. 1B shows representative photomicrographs of tissue immunostained with CD31 on postoperative day 14. Quantitative analysis of CD31-posotive cells revealed that the capillary density was enhanced significantly in Ad-omentin-treated mice compared with control mice (Fig. 1B). Thus, systemic delivery of omentin could promote revascularization in ischemic tissue in WT mice.
Omentin Promotes Endothelial Cell Differentiation and Survival in Vitro
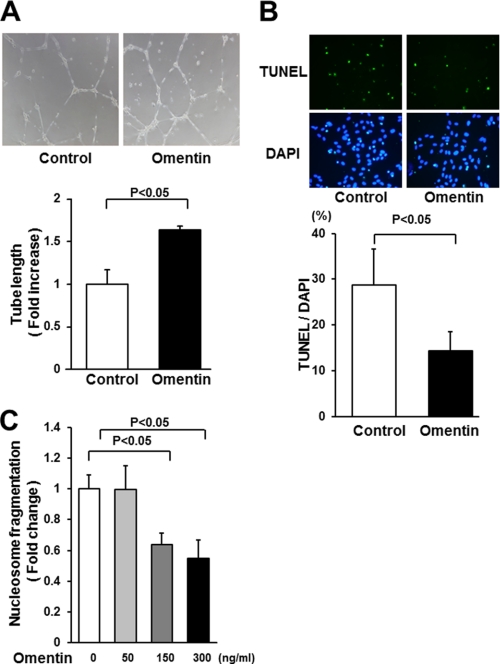
To examine whether omentin modulates endothelial cell differentiation into vascular-like structures when HUVECs were plated on a Matrigel matrix, HUVECs were treated with recombinant human omentin protein or vehicle. Treatment with a physiological concentration of omentin (300 ng/ml) promoted the formation of vascular-like tubes (Fig. 2A). Quantitative analyses of endothelial cell network revealed that treatment with omentin significantly increased the tube length relative to control cultures (Fig. 2A).
FIGURE 2.
Omentin promotes endothelial cell differentiation and survival in vitro. A, endothelial cell network formation following stimulation with omentin. After 18 h of serum deprivation, HUVECs were treated with recombinant omentin protein (300 ng/ml) or vehicle followed by culture on Matrigel-coated dishes. Representative photomicrographs of HUVEC differentiation into network structures are shown (upper panel). Quantitative analyses of network tube length are shown (bottom panel) (n = 3). B, omentin reduces the frequency of TUNEL-positive HUVECs. HUVECs were treated with omentin protein (300 ng/ml) or vehicle followed by 48 h of incubation with serum-free media. Apoptotic nuclei were identified by TUNEL staining (green), and total nuclei were identified by 4′,6-diamidino-2-phenylindole counterstaining (blue). Representative photomicrographs of TUNEL-positive HUVECs are shown (upper panels). Quantitative analyses of the frequency of TUNEL-positive HUVECs are shown (bottom panel) (n = 5). C, omentin inhibits the degree of nucleosome fragmentation of HUVECs. Nucleosome fragmentation was assessed by enzyme-linked immunosorbent assay. Results are expressed relative to the values compared with control. Results are shown as the mean ± S.D. (n = 5).
To test whether omentin affects endothelial apoptosis, HUVECs were treated with omentin or vehicle followed by 48 h of incubation in serum-free media. Analysis of apoptotic activity by TUNEL staining demonstrated that treatment with omentin significantly reduced the frequency of TUNEL-positive cells (Fig. 2B). Omentin treatment also attenuated the extent of nucleosome fragmentation of HUVECs in a dose-dependent manner under serum-deprived conditions as measured by enzyme-linked immunosorbent assay (Fig. 2C).
Omentin Stimulates Phosphorylation of Akt and eNOS
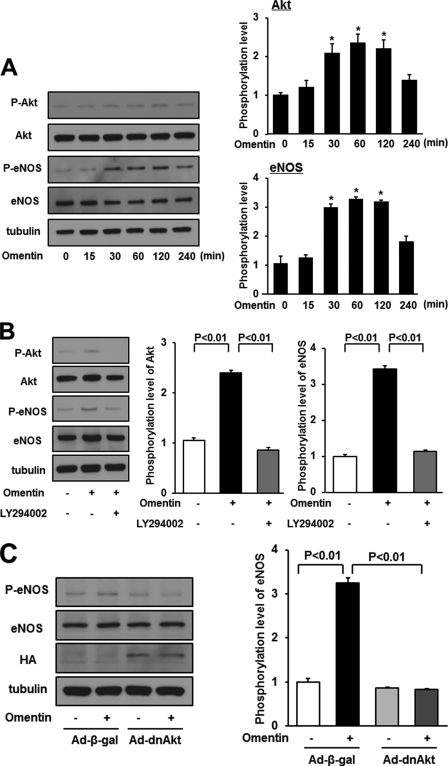
Akt plays a key role in angiogenic response to several growth factors and survival activity in endothelial cells (24). Therefore, the effect of omentin on the activating phosphorylation of Akt at Ser-473 in endothelial cells was assessed by Western blot analysis. Treatment with omentin stimulated the phosphorylation of Akt in a time-dependent manner with maximal Akt phosphorylation occurring at 60 min (Fig. 3A). Because Akt can phosphorylate and activate eNOS at Ser-1177 (25, 26), the eNOS phosphorylation level was also assessed. Omentin stimulated the phosphorylation of eNOS with maximal levels occurring at 60 min (Fig. 3A) in agreement with a previous report showing that omentin increased eNOS phosphorylation and cyclic GMP levels in HUVECs (18). Omentin had no effect on Akt and eNOS protein levels.
FIGURE 3.
Omentin stimulates the phosphorylation of eNOS in endothelial cells via activation of Akt. A, time-dependent changes in the phosphorylation of Akt and eNOS signaling in endothelial cells following stimulation with omentitn (left). Changes in the phosphorylation of Akt (P-Akt) and eNOS (P-eNOS) following omentin treatment were determined by Western blot analysis. Representative blots are shown. Relative phosphorylation levels of Akt and eNOS were quantified by using ImageJ software. Immunoblots were normalized to an α-tubulin signal. Results are shown as the mean ± S.D. (n = 3). B, effect of LY294002 on omentin-stimulated eNOS phosphorylation. HUVECs were pretreated with LY294002 (50 μmol/L) or vehicle (dimethyl sulfoxide) for 60 min and treated with omentin (300 ng/ml) or vehicle for 60 min. P-Akt and P-eNOS were determined by Western blot analysis. Relative phosphorylation levels of Akt and eNOS were quantified by using ImageJ software. Immunoblots were normalized to α-tubulin signal. Results are shown as the mean ± S.D. (n = 3). C, role of Akt in regulation of omentin-induced eNOS signaling. HUVECs were transduced with Ad-dnAkt or Ad-β-gal 24 h before serum starvation. After 16 h serum starvation, cells were treated with omentin (300 ng/ml) for 60 min. P-eNOS was determined by Western blot analysis.
PI3K functions upstream from the Akt-eNOS regulatory pathways following stimulation with many growth factors and cytokines (24). To examine the potential involvement of PI3K-Akt signaling in the omentin-induced eNOS phosphorylation, HUVECs were treated with PI3K inhibitor LY294002. Pretreatment with LY294002 reduced omentin-stimulated phosphorylation of Akt and eNOS (Fig. 3B). HUVECs were also transduced with HA-tagged dominant-negative mutant Akt (Ad-dnAkt) or control vector. Transduction with Ad-dnAkt suppressed omentin-induced eNOS phosphorylation (Fig. 3C). These data suggest that omentin stimulates eNOS phosphorylation via a PI3K-Akt-dependent signaling.
Role of Akt Signaling in Omentin-stimulated Endothelial Cell Function and Survival in Vitro
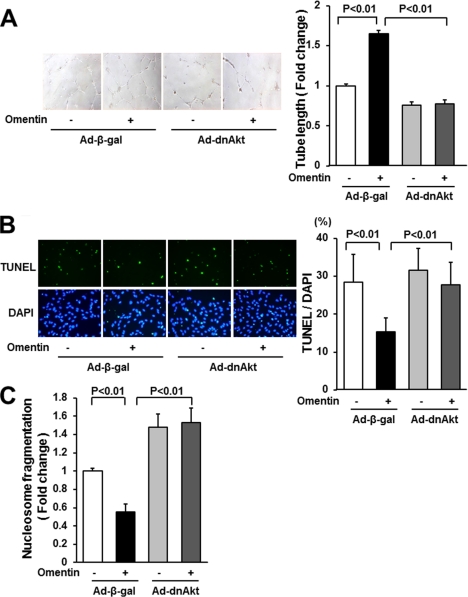
To test whether Akt signaling is involved in omentin-stimulated endothelial cell differentiation and survival, HUVECs were transduced with Ad-dnAkt or control vector, and network formation and survival of HUVECs were assessed. Transduction with Ad-dnAkt cancelled omentin-stimulated formation of vascular-like tubes of HUVECs (Fig. 4A). Transduction with Ad-dnAkt also reversed the reduced frequency of TUNEL-positive cells caused by omentin (Fig. 4B). Similarly, the suppressive effects of omentin on the degree of nucleosome fragmentation were reversed by transduction with Ad-dnAkt (Fig. 4C).
FIGURE 4.
Akt signaling participates in endothelial cell responses to omentin. A, Akt signaling is essential for omentin-mediated endothelial cell differentiation. HUVECs were transduced with Ad-dnAkt or Ad-β-gal. After 18 h of serum starvation, a Matrigel assay was performed. Cells were treated with omentin (300 ng/ml) or vehicle. Representative photomicrographs of vascular-like tube formation are shown (left panels). Quantitative analyses of the network tube length are shown (right panel). Results are shown as the mean ± S.D. (n = 5). B and C, involvement of Akt in omentin-induced endothelial cell survival. After transduction with Ad-dnAkt or Ad-β-gal, cells were incubated in serum-free media for 48 h. Cells were treated with omentin (300 ng/ml) or vehicle. Apoptotic nuclei were identified by TUNEL staining (green), and total nuclei were identified by 4′,6-diamidino-2-phenylindole counterstaining (blue; B). Representative photomicrographs of TUNEL-positive HUVECs are shown (B, left panels). Quantitative analyses of the frequency of TUNEL-positive HUVECs are shown (B, right panel). Nucleosome fragmentation was assessed by enzyme-linked immunosorbent assay (C). Results are shown as the mean ± S.D. (n = 5).
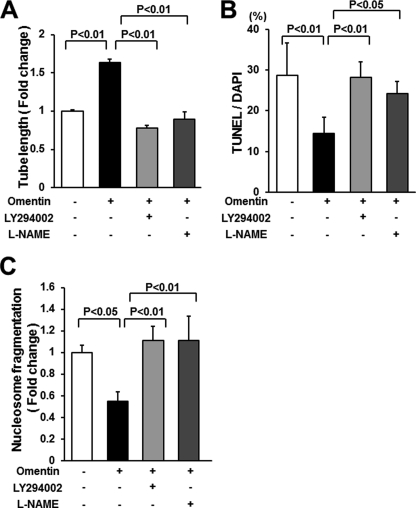
To investigate whether PI3K participates in omentin-induced endothelial cell function, HUVECs were treated with LY294002, and endothelial cell differentiation and apoptotic activity were assessed. Treatment with LY294002 abolished the stimulatory actions of omentin on HUVEC differentiation into the vascular-like structure (Fig. 5A). Treatment with LY294002 also reversed the inhibitory effects of omentin on the frequency of TUNEL-positive cells and the degree of nucleosome fragmentation (Fig. 5, B and C).
FIGURE 5.
PI3K and eNOS contribute to omentin-induced endothelial cell responses. A, contribution of PI3K and eNOS to omentin-mediated endothelial cell differentiation. HUVECs were pretreated with LY294002 (50 μmol/liter), l-NAME (1 mmol/liter), or vehicle (dimethyl sulfoxide) and treated with omentin (300 ng/ml) or vehicle for 18 h. A Matrigel assay was performed. Quantitative analyses of the network tube length are shown. Results are shown as the mean ± S.D. (n = 5). B and C, PI3K and eNOS participate in omentin-induced endothelial cell survival. After treatment with LY294002 (50 μmol/liter), l-NAME (1 mmol/liter) or vehicle (dimethyl sulfoxide), cells were treated with omentin (300 ng/ml) or vehicle followed by 48 h of incubation with serum-free media. Apoptotic nuclei were identified by TUNEL staining, and total nuclei were identified by 4′,6-diamidino-2-phenylindole counterstaining (B). Quantitative analyses of the frequency of TUNEL-positive HUVECs are shown. Nucleosome fragmentation was assessed by enzyme-linked immunosorbent assay (C). Results are shown as the mean ± S.D. (n = 5).
To test the potential involvement of eNOS in the cellular responses to omentin, HUVECs were treated with the NOS inhibitor l-NAME. Treatment with l-NAME significantly reduced omentin-stimulated formation of vascular-like structures (Fig. 5A). Treatment with l-NAME also blocked the suppressive effects of omentin on apoptotic activities of HUVECs (Fig. 5, B and C). These results indicate that Akt-eNOS signaling is required for endothelial cell responses to omentin.
Involvement of AMPK Signaling in Endothelial Cell Response to Omentin
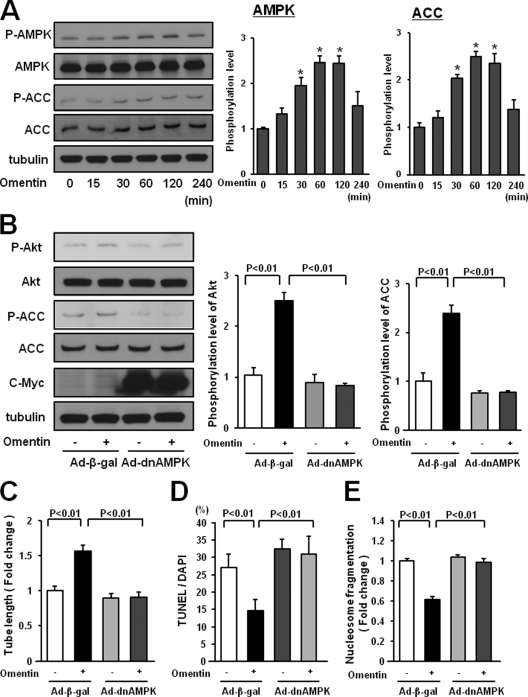
AMPK functions upstream of PI3K-Akt signaling in several types of cells, including endothelial cells (22, 27, 28). To investigate whether omentin affects AMPK signaling in endothelial cells, the activating phosphorylation of AMPK at Thr-172 was assessed by Western blotting. Treatment of HUVECs with omentin protein enhanced the phosphorylation of AMPK and ACC, a downstream target of AMPK, in a time-dependent manner with maximal AMPK phosphorylation occurring at 60 min (Fig. 6A).
FIGURE 6.
AMPK activation is essential for endothelial cell responses to omentin. A, time-dependent changes in the phosphorylation of AMPK and ACC in endothelial cells following stimulation with omentin (left panels). Changes in the phosphorylation of AMPK (P-AMPK) and ACC (P-ACC) following omentin treatment were determined by Western blot analysis. Representative blots are shown. Relative phosphorylation levels of AMPK and ACC were quantified by using ImageJ software. Immunoblots were normalized to α-tubulin signal. Results are shown as the mean ± S.D. (n = 3). B, role of AMPK in regulation of omentin-induced Akt signaling. HUVECs were transduced with Ad-dnAMPK or Ad-β-gal 24 h before serum-starvation. After a 16-h serum starvation, cells were treated with omentin (300 ng/ml) for 60 min. P-Akt was determined by Western blot analysis. C, AMPK signaling is required for omentin-induced endothelial cell differentiation. HUVECs were transduced with Ad-dnAMPK or Ad-β-gal. After 18 h of serum starvation, a Matrigel assay was performed. Cells were treated with omentin (300 ng/ml) or vehicle. Quantitative analyses of the network tube length are shown. Results are shown as the mean ± S.D. (n = 5). D and E, Involvement of AMPK in omentin-induced endothelial cell survival. After transduction with Ad-dnAMPK or Ad-β-gal, cells were incubated in serum-free media for 48 h. Cells were treated with omentin (300 ng/ml) or vehicle. Apoptotic nuclei were identified by TUNEL staining, and total nuclei were identified by 4′,6-diamidino-2-phenylindole counterstaining. Quantitative analyses of the frequency of TUNEL-positive HUVECs are shown (D). Nucleosome fragmentation was determined by enzyme-linked immunosorbent assay (E). Results are shown as the mean ± S.D. (n = 5).
To examine the possible participation of AMPK in omentin-induced signaling pathways and cellular responses in vitro, HUVECs were transduced with c-Myc-tagged dominant-negative mutant AMPK (Ad-dnAMPK) or control vector. Transduction with Ad-dnAMPK effectively suppressed omentin-stimulated phosphorylation of ACC and Akt (Fig. 6B). Transduction with Ad-dnAMPK blocked omentin-stimulated formation of vascular-like tubes of HUVECs (Fig. 6C). The inhibitory actions of omentin on the frequency of TUNEL-positive cells and the degree of nucleosome fragmentation were also reversed by transduction with Ad-dnAMPK (Fig. 6, D and E). Thus, omentin promotes Akt signaling in endothelial cells via activation of AMPK.
eNOS Activation Is Essential for Omentin-induced Revascularization in Vivo
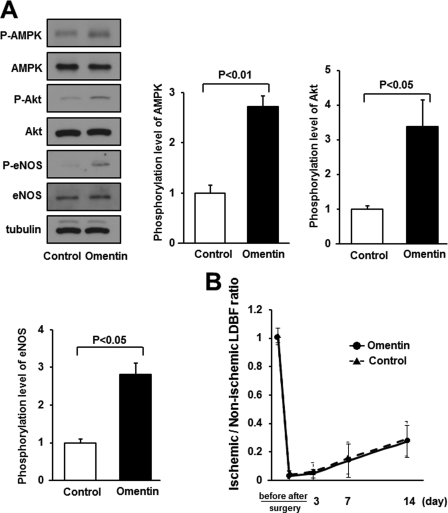
To investigate the role of eNOS signaling in omentin-induced revascularization in vivo, the expression and phosphorylation of AMPK, Akt, and eNOS in ischemic adductor muscle of WT mice were assessed by Western blot analysis on postoperative day 7. Systemic administration of Ad-omentin to WT mice increased the phosphorylation of AMPK at Thr-172, Akt at Ser-473, and eNOS at Ser-1177 in ischemic muscle (Fig. 7A). Treatment with Ad-omentin had no effects on the expression levels of total Akt and eNOS protein in ischemic muscles.
FIGURE 7.
eNOS signaling is required for omentin-stimulated revascularization in ischemic muscle. A, the phosphorylation of AMPK, Akt, and eNOS in ischemic adductor muscle of WT mice at 7 days after surgery. Ad-omentin or Ad-β-gal (control) was injected into the jugular vein of WT mice (2 × 107 pfu in each group) 3 days prior to surgery. Phosphorylation of AMPK, Akt, and eNOS, total AMPK, total Akt, total eNOS, and tubulin levels were determined by Western blot analysis. Representative blots are shown from three independent experiments (left panels). Relative phosphorylation levels of AMPK, Akt, and eNOS were quantified by using ImageJ software. Immunoblots were normalized to the tubulin signal. Results are shown as the mean ± S.D. (n = 3). B, quantitative analysis of ischemic/nonischemic LDBF ratio in Ad-omentin-treated or Ad-β-gal-treated eNOS-KO mice before surgery, after surgery, and on postoperative days 3, 7, and 14 (n = 4). Ad-omentin or Ad-β-gal (control) was injected into the jugular vein of eNOS-KO mice (2 × 107 pfu in each group) 3 days before operation.
We also assessed whether omentin treatment affects plasma VEGF levels in WT mice on postoperative day 7 and 14. Ad-omentin-treated mice had significantly higher levels of plasma VEGF compared with control mice (day 7, 62.6 ± 5.4 pg/ml in control mice and 79.3 ± 8.2 pg/ml in Ad-omentin-treated mice, p < 0.05; day 14, 15.5 ± 4.9 pg/ml in control mice and 43.0 ± 16.4 pg/ml in Ad-omentin-treated mice, p < 0.05).
Furthermore, to examine whether eNOS signaling is required for the actions of omentin on revascularization, we systemically injected Ad-omentin or control Ad-β-gal into eNOS-KO mice 3 days before surgery. On the sixth day after injection, Ad-omentin increased plasma omentin levels of eNOS-KO mice to 1114.8 ± 266.9 ng/ml, which was similar to those of Ad-omentin-treated WT mice (1051.2 ± 207.8 ng/ml). In contrast to WT mice (Fig. 1A), systemic delivery of Ad-omentin did not affect the blood flow recovery in ischemic limbs of eNOS-KO mice throughout the experimental period (Fig. 7B). Therefore, the in vivo effect of omentin on ischemia-induced revascularization is dependent on eNOS.
DISCUSSION
The present work provides the first evidence that omentin promotes endothelial cell function and revascularization process under conditions of ischemic stress. Treatment with a physiological concentration of omentin led to enhancement of endothelial cell differentiation and reduction of endothelial cell apoptosis. Systemic administration of omentin to wild-type mice resulted in a significant increase in blood flow recovery and capillary density in ischemic limbs.
It is well established that Akt and its downstream target eNOS are crucial regulators of blood vessel growth and vascular cell function (24, 25). Here, we show that omentin promoted endothelial cell differentiation and survival and stimulated the phosphorylation of Akt and eNOS in endothelial cells. Blockade of Akt signaling by transduction with dominant-negative mutant Akt cancelled the stimulatory effects of omentin on endothelial cell function and eNOS phosphorylation. In addition, inhibition of PI3K or eNOS attenuated omentin-stimulated endothelial cell differentiation and survival. Thus, the stimulation of endothelial cell function by omentin may depend on its ability to activate Akt-eNOS signaling. Furthermore, omentin promoted revascularization in ischemic muscle of WT mice in vivo, which were associated with increased phosphorylation of Akt and eNOS. Of note, the impact of omentin on blood vessel recruitment was abolished in eNOS-KO mice. Collectively, these data suggest that the omentin-Akt-eNOS regulatory axis can promote endothelial cell function under conditions of ischemia, thereby accelerating the revascularization process.
Our present data show that omentin promoted AMPK signaling pathways in endothelial cells and ischemic tissue. Inhibition of AMPK activation abolished omentin-induced stimulation of Akt activation and endothelial cell differentiation and survival in vitro. Thus, omentin can promote Akt signaling and subsequent endothelial cell responses via activation of AMPK. Our hypothesis that AMPK functions upstream of Akt signaling in endothelial cells is supported by a number of previous studies (22, 28, 29).
It has been shown that eNOS is protective against various vascular diseases, including atherosclerosis and peripheral artery diseases (30, 31). Omentin promotes vasodilation in rat isolated aorta, which is prevented by NOS inhibition (17). A recent report showed that omentin reduces cytokine-induced inflammatory responses in cultured endothelial cells, which is cancelled by a NOS inhibitor (18). Consistent with these findings, this study shows that omentin increased the activating phosphorylation of eNOS in both ischemic muscles and cultured endothelial cells. Our data also indicate that the actions of omentin on endothelial cell behavior and vessel growth are mediated, at least in part, through its ability to activate eNOS. These observations suggest that eNOS acts as a crucial mediator of the protective actions of omentin on the vasculature.
VEGF is a crucial angiogenic growth factor that stimulates Akt-eNOS signaling pathway in endothelial cells, thereby promoting revascularization in vivo (25). The present study demonstrates that omentin increased circulating VEGF levels after ischemic surgery. Thus, in addition to the direct actions on endothelial cells, omentin may stimulate ischemia-induced revascularization partly by increasing VEGF production. It has been shown that lack of TNF-α receptor (TNFR2/p75) causes impaired blood flow recovery and marked reductions in VEGF expression in ischemic muscle (32). Although the ability of omentin to increase VEGF production may be mediated, at least in part, through TNFR2/p75, future research is required to elucidate the possible link between omentin and TNF receptor signaling.
We utilized an adenoviral vector expression system to assess whether systemic delivery of omentin affects revascularization in an endocrine manner. Although the liver is the major site of adenovirus infection following an intravenous administration, we and other groups (19, 33–35) have demonstrated that adenovirus-mediated overexpression of adipocytokines, including leptin and adiponectin, is valuable to assess their biological functions. However, further study will be needed to clarify whether fat-derived omentin can modulate revascularization in response to ischemia in vivo.
Obesity-linked diseases, including type 2 diabetes, cause increased mortality and morbidity of coronary and peripheral artery diseases partly due to microvascular rarefaction and impaired collateral vessel development under ischemic conditions (3–5). Plasma omentin levels are decreased in patients with obese complications such as type 2 diabetes and coronary artery disease (13, 16). Taken together with our present findings, these results suggest that low plasma omentin levels may be causally linked with endothelial dysfunction and reduced collateral vessel growth under obesity-related conditions. Conversely, the therapeutic approaches aimed at increasing circulating omentin levels could be beneficial for treatment of ischemic heart and limb diseases.
In conclusion, our data document that omentin can function as a novel regulator of vascular response to ischemia through activation of eNOS in endothelial cells. Because omentin is an adipocytokine that is expressed mainly in human visceral adipose tissue, it is conceivable that omentin modulates vascular function by directing acting on the endothelial cells in an endocrine or paracrine manner. Therefore, the omentin-eNOS signaling axis may serve as a functional link between adipose tissue and the vasculature, thereby affecting the development of vascular disease in obese states.
Acknowledgments
We gratefully acknowledge the technical assistance of Yuya Terakura, Megumi Kondo, and Yoko Inoue.
This work was supported by a grant-in-aid for Scientific Research and grants from the Takeda Science Foundation, Suzuken Memorial Foundation, Japan Research Foundation for Clinical Pharmacology, and the Uehara Memorial Foundation (to N. O.).
- eNOS
- endothelial nitric oxide synthase
- HUVECs
- human umbilical vein endothelial cells
- Ad
- adenovirus
- LDBF
- laser Doppler blood flow
- dn
- dominant-negative
- ACC
- acetyl CoA carboxylase.
REFERENCES
- 1. Reilly M. P., Rader D. J. (2003) Circulation 108, 1546–1551 [DOI] [PubMed] [Google Scholar]
- 2. Friedman J. M. (2003) Science 299, 856–858 [DOI] [PubMed] [Google Scholar]
- 3. Yilmaz M. B., Biyikoglu S. F., Akin Y., Guray U., Kisacik H. L., Korkmaz S. (2003) Int. J. Obes. Relat. Metab. Disord. 27, 1541–1545 [DOI] [PubMed] [Google Scholar]
- 4. Warley A., Powell J. M., Skepper J. N. (1995) Diabetologia 38, 413–421 [DOI] [PubMed] [Google Scholar]
- 5. Lind L., Lithell H. (1993) Am. Heart J. 125, 1494–1497 [DOI] [PubMed] [Google Scholar]
- 6. Ouchi N., Parker J. L., Lugus J. J., Walsh K. (2011) Nat. Rev. Immunol. 11, 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ouchi N., Kihara S., Funahashi T., Matsuzawa Y., Walsh K. (2003) Curr. Opin. Lipidol. 14, 561–566 [DOI] [PubMed] [Google Scholar]
- 8. Yang R. Z., Lee M. J., Hu H., Pray J., Wu H. B., Hansen B. C., Shuldiner A. R., Fried S. K., McLenithan J. C., Gong D. W. (2006) Am. J. Physiol. Endocrinol. Metab. 290, E1253–1261 [DOI] [PubMed] [Google Scholar]
- 9. Schäffler A., Neumeier M., Herfarth H., Fürst A., Schölmerich J., Büchler C. (2005) Biochim. Biophys. Acta. 1732, 96–102 [DOI] [PubMed] [Google Scholar]
- 10. Tsuji S., Uehori J., Matsumoto M., Suzuki Y., Matsuhisa A., Toyoshima K., Seya T. (2001) J. Biol. Chem. 276, 23456–23463 [DOI] [PubMed] [Google Scholar]
- 11. de Souza Batista C. M., Yang R. Z., Lee M. J., Glynn N. M., Yu D. Z., Pray J., Ndubuizu K., Patil S., Schwartz A., Kligman M., Fried S. K., Gong D. W., Shuldiner A. R., Pollin T. I., McLenithan J. C. (2007) Diabetes 56, 1655–1661 [DOI] [PubMed] [Google Scholar]
- 12. Shibata R., Takahashi R., Kataoka Y., Ohashi K., Ikeda N., Kihara S., Murohara T., Ouchi N. (2011) Hypertens. Res. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13. Pan H. Y., Guo L., Li Q. (2010) Diabetes Res. Clin. Pract. 88, 29–33 [DOI] [PubMed] [Google Scholar]
- 14. Moreno-Navarrete J. M., Ortega F., Castro A., Sabater M., Ricart W., Fernandez-Real J. M. (2011) Obesity 19, 1552–1559 [DOI] [PubMed] [Google Scholar]
- 15. Liu R., Wang X., Bu P. (2011) Diabetes Res. Clin. Pract. 93, 21–25 [DOI] [PubMed] [Google Scholar]
- 16. Zhong X., Zhang H. Y., Tan H., Zhou Y., Liu F. L., Chen F. Q., Shang D. Y. (2011) Acta. Pharmacol. Sin. 32, 873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yamawaki H., Tsubaki N., Mukohda M., Okada M., Hara Y. (2010) Biochem. Biophys. Res. Commun. 393, 668–672 [DOI] [PubMed] [Google Scholar]
- 18. Yamawaki H., Kuramoto J., Kameshima S., Usui T., Okada M., Hara Y. (2011) Biochem. Biophys. Res. Commun. 408, 339–343 [DOI] [PubMed] [Google Scholar]
- 19. Shibata R., Ouchi N., Kihara S., Sato K., Funahashi T., Walsh K. (2004) J. Biol. Chem. 279, 28670–28674 [DOI] [PubMed] [Google Scholar]
- 20. Ouchi N., Oshima Y., Ohashi K., Higuchi A., Ikegami C., Izumiya Y., Walsh K. (2008) J. Biol. Chem. 283, 32802–32811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shiojima I., Yefremashvili M., Luo Z., Kureishi Y., Takahashi A., Tao J., Rosenzweig A., Kahn C. R., Abel E. D., Walsh K. (2002) J. Biol. Chem. 277, 37670–37677 [DOI] [PubMed] [Google Scholar]
- 22. Ouchi N., Kobayashi H., Kihara S., Kumada M., Sato K., Inoue T., Funahashi T., Walsh K. (2004) J. Biol. Chem. 279, 1304–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oshima Y., Ouchi N., Shimano M., Pimentel D. R., Papanicolaou K. N., Panse K. D., Tsuchida K., Lara-Pezzi E., Lee S. J., Walsh K. (2009) Circulation 120, 1606–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shiojima I., Walsh K. (2002) Circ. Res. 90, 1243–1250 [DOI] [PubMed] [Google Scholar]
- 25. Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999) Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo Z., Fujio Y., Kureishi Y., Rudic R. D., Daumerie G., Fulton D., Sessa W. C., Walsh K. (2000) J. Clin. Invest. 106, 493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Horike N., Sakoda H., Kushiyama A., Ono H., Fujishiro M., Kamata H., Nishiyama K., Uchijima Y., Kurihara Y., Kurihara H., Asano T. (2008) J. Biol. Chem. 283, 33902–33910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levine Y. C., Li G. K., Michel T. (2007) J. Biol. Chem. 282, 20351–20364 [DOI] [PubMed] [Google Scholar]
- 29. Kimura T., Tomura H., Sato K., Ito M., Matsuoka I., Im D. S., Kuwabara A., Mogi C., Itoh H., Kurose H., Murakami M., Okajima F. (2010) J. Biol. Chem. 285, 4387–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murohara T., Asahara T., Silver M., Bauters C., Masuda H., Kalka C., Kearney M., Chen D., Symes J. F., Fishman M. C., Huang P. L., Isner J. M. (1998) J. Clin. Invest. 101, 2567–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li H., Wallerath T., Münzel T., Förstermann U. (2002) Nitric Oxide 7, 149–164 [DOI] [PubMed] [Google Scholar]
- 32. Goukassian D. A., Qin G., Dolan C., Murayama T., Silver M., Curry C., Eaton E., Luedemann C., Ma H., Asahara T., Zak V., Mehta S., Burg A., Thorne T., Kishore R., Losordo D. W. (2007) Circulation 115, 752–762 [DOI] [PubMed] [Google Scholar]
- 33. Chen G., Koyama K., Yuan X., Lee Y., Zhou Y. T., O'Doherty R., Newgard C. B., Unger R. H. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 14795–14799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang W., Telemaque S., Augustyniak R. A., Anderson P., Thomas G. D., An J., Wang Z., Newgard C. B., Victor R. G. (2010) J. Neuroendocrinol. 22, 175–180 [DOI] [PubMed] [Google Scholar]
- 35. Enomoto T., Ohashi K., Shibata R., Higuchi A., Maruyama S., Izumiya Y., Walsh K., Murohara T., Ouchi N. (2011) J. Biol. Chem. 286, 34552–34558 [DOI] [PMC free article] [PubMed] [Google Scholar]