Background: Most primary cancer cells are resistant to TRAIL-mediated apoptosis.
Results: Wogonin and related natural flavones suppress c-FLIP but up-regulate TRAIL receptor-2 expression and thereby sensitize resistant tumor cells to TRAIL-mediated apoptosis.
Conclusion: Wogonin and related natural flavones can overcome TRAIL resistance of cancer cells.
Significance: These flavones can be developed as adjuvants for TRAIL-based anticancer therapy.
Keywords: Anticancer Drug, Apoptosis, Bcl-2 Family Proteins, Cancer, Caspase, p53, Trail, HTLV-1, Mdm2, c-FLIP
Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising anticancer agent that kills various tumor cells without damaging normal tissues. However, many cancers remain resistant to TRAIL. To overcome TRAIL resistance, combination therapies using sensitizers of the TRAIL pathway would be an efficacious approach. To investigate potential sensitizers of TRAIL-induced apoptosis, we used TRAIL-resistant human T cell leukemia virus type 1 (HTLV-1)-associated adult T cell leukemia/lymphoma (ATL) cells as a model system. So far, HTLV-1-associated ATL is incurable by presently known therapies. Here, we show that wogonin and the structurally related natural flavones apigenin and chrysin break TRAIL resistance in HTLV-1-associated ATL by transcriptional down-regulation of c-FLIP, a key inhibitor of death receptor signaling, and by up-regulation of TRAIL receptor 2 (TRAIL-R2). This effect is mediated through transcriptional inhibition of the p53 antagonist murine double minute 2 (Mdm2), leading to an increase in p53 levels and, consequently, to up-regulation of the p53 target gene TRAIL-R2. We also show that these flavones can sensitize to TNFα- and CD95-mediated cell death. Furthermore, we show that wogonin, apigenin, and chrysin also enhance TRAIL-mediated apoptosis in other human cancer cell lines including breast cancer cell line MDA-MB-231, colon cancer cell line HT-29, hepatocellular carcinoma cell line HepG2, melanoma cell line SK-MEL-37, and pancreatic carcinoma cell line Capan-1 by the same mechanism. Thus, our study suggests the potential use of these flavones as an adjuvant for TRAIL-mediated anticancer therapy.
Introduction
Due to virtually no toxicity for normal cells, the tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)2 has been considered to be a promising therapeutic agent against different types of cancer, and it is currently tested in phase I-II clinical trials (1–3). Binding of TRAIL to its death receptors (TRAIL-Rs) leads to recruitment of the adaptor molecule FADD, procaspase-8, or procaspase-10 to the death domain and, subsequently, to activation of the death signaling cascade. To date, four human TRAIL-Rs have been identified: TRAIL-R1 (also known as DR4 and TNFRSF10A), TRAIL-R2 (also known as DR5, KILLER, and TNFRSF10B), and the putative decoy receptors TRAIL-R3 (also known as DCR1, TRID, and TNFRSF10C) and TRAIL-R4 (also known as DCR2 and TNFRSF10D) (1). TRAIL-R1 and -R2 are apoptosis-inducing receptors characterized by containing a death domain. TRAIL also interacts with decoy receptors that do not have a death domain and, consequently, cannot form signaling complexes. One major negative regulator of receptor-mediated apoptosis is the cellular caspase-8 (FLICE)-inhibitory protein (c-FLIP) (4). So far, three alternatively spliced isoforms of c-FLIP have been identified: c-FLIPL, c-FLIPS, and c-FLIPR. In most cases, c-FLIPs function as antiapoptotic proteins that block processing and activation of caspase-8 at the DISC level (4). In addition, receptor-mediated apoptosis is also negatively regulated by the inhibitor of apoptosis proteins (IAPs), particularly the X-linked IAP (XIAP), which blocks caspase-3 activity (5, 6).
Although TRAIL is a promising anticancer agent, many cancer cells have been found to resist TRAIL-induced apoptosis (7). For instance, most of leukemia samples taken from patients were not sensitive to TRAIL in vitro (8). The expression levels of c-FLIP have been shown to be one of the major determinants of the resistance to death ligands (4, 7). Another well known example is the human T cell leukemia virus type 1 (HTLV-1)-associated adult T cell leukemia/lymphoma (ATL), a malignancy caused by clonal proliferation of infected mature CD4+ T cells (9). Worldwide 15–20 million people are infected by HTLV-1. Patients have a poor prognosis after disease development with a survival expectancy of less than one year. So far, HTLV-1-associated ATL is incurable by presently known therapies (9). HTLV-1-associated ATL is highly resistant to TRAIL-mediated cell death due to overexpression of c-FLIP (10, 11). Because c-FLIPs are very short lived proteins, especially c-FLIPS which contains a unique carboxyl terminus that confers its ubiquitylation and proteasome-mediated degradation (12, 13), they are a promising target of chemotherapy.
Wogonin, a naturally occurring flavone, has been shown to preferentially induce apoptotic cell death in cancer cells through the mitochondrial pathway by induction of phospholipase Cγ1- and Ca2+-mediated apoptosis and by suppression of the antiapoptotic Bcl-2 family protein Mcl-1 (14, 15). Wogonin has also been demonstrated to inhibit growth of xenografted tumor cells in vivo in different tumor models with virtually no toxicity for the animals (14, 16–18). We have shown previously that wogonin can sensitize TRAIL-mediated apoptosis in leukemic cell lines and in primary leukemic cells freshly isolated from patients but has no effect on normal peripheral blood lymphocytes (19). However, the molecular mechanisms of how wogonin sensitizes TRAIL-mediated apoptosis in malignant cells are still unknown.
Recently, we have identified wogonin and several naturally occurring anticancer flavones as inhibitors of the key transcription regulator cyclin-dependent kinase 9 (CDK9) (15). We have shown that transcriptional inhibition of the short-lived antiapoptotic Bcl-2 family protein Mcl-1 is one of the anticancer actions of these natural flavones (15). Because c-FLIPs are also short lived proteins, we asked whether the expression of c-FLIP could be inhibited by these flavones and if so, whether they could sensitize TRAIL-mediated apoptosis in resistant cancer cells. To address this question, we examined effects of wogonin, apigenin, and chrysin on c-FLIP expression in different tumor cells including the TRAIL-resistant ATL cell lines SP and MT-2 derived from HTLV-1-infected patients, the human breast cancer cell line MDA-MB-231, the human colon cancer cell line HT-29, the human hepatocellular carcinoma cell line HepG2, the human melanoma cell line SK-MEL-37, and the human pancreatic carcinoma cell line Capan-1. We show that wogonin, apigenin, and chrysin sensitize tumor cells to TRAIL-induced apoptosis by down-regulation of c-FLIP expression at the transcriptional level. In addition, we show that TRAIL-R2 expression, in contrast to c-FLIP, is up-regulated by wogonin, apigenin, and chrysin treatment due to transcriptional inhibition of the short-lived p53 antagonist murine double minute 2 (Mdm2). Our study suggests that wogonin, apigenin, and chrysin are promising adjuvants for TRAIL-based anticancer therapy.
MATERIALS AND METHODS
Cell Lines and Culture
The following human cancer cells were used in this study: the human leukemic T cell line Jurkat, the HTLV-1-associated ATL cell lines SP and MT-2 (20, 21), the human breast cancer cell line MDA-MB-231, the human colon cancer cell line HT-29, the human hepatocellular carcinoma cell line HepG2, the human melanoma cell line SK-MEL-37, and the human pancreatic carcinoma cell line Capan-1. All cell lines except SP and MT-2 were cultured in RPMI 1640 medium or DMEM (GIBCO Laboratories), respectively, supplemented with 10% FCS, 100 units/ml penicillin (GIBCO), 100 μg/ml streptomycin (GIBCO), and 2 mm l-glutamine (GIBCO) at 37 °C and 5% CO2. The SP and MT-2 cells were cultured with supplementation of IL-2 as described previously (20, 21).
Determination of Apoptosis
Cells were plated in triplicate and treated for the indicated periods of time at 37 °C with different doses of wogonin (>98% pure) (BIOTREND Chemicals AG, Wangen, Switzerland), SuperKiller(SK)-TRAIL (Alexis Biochemicals, Loerrach, Germany), TNFα (Sigma), anti-APO-1 (produced in our laboratory) or in combinations as indicated in the figure legends. Apoptotic cell death was examined by analysis of DNA fragmentation as described previously (14). Specific apoptosis in percentage was calculated as (percentage of experimental apoptosis − percentage of spontaneous apoptosis)/(100 − percentage of spontaneous apoptosis) × 100.
Western Blot Analysis
For each sample, 1 × 106 cells were lysed as described previously (14). Equal amounts of proteins were separated on 7.5–13% SDS-PAGE depending on the molecular sizes of the proteins, blotted onto a nitrocellulose membrane (Amersham Biosciences), and blocked with 5% nonfat dry milk in PBS/Tween (0.05% Tween 20 in PBS). Bad, Bak, Bax, Bcl-xL, ERK1, PUMA, Bid, p53, TRAIL-R2, -R3, -R4, caspase-2, caspase-3, XIAP, and phospho-p65 antibodies were purchased from Cell Signaling Technology. Antibodies detecting Bcl-2 (N-19), caspase-9, NF-κB subunit p65 (A, sc-109) and IκBα (C21, sc-371) were purchased from Santa Cruz Biotechnology. Mcl-1 was from BD Biosciences. Tubulin was from Sigma. TRAIL-R1 was from Prosci, Inc. Tax hybridoma clone 168B17-46-34 was from National Institutes of Health AIDS Research. Mdm2 antibody was a kind gift from Dr. Hanswalter Zentgraf (German Cancer Research Center). The caspase-8 mAb C15 (mouse IgG2b) recognizing the p18 subunit of caspase-8 and the c-FLIP mAb NF6 were generated in our laboratory as described previously (22).
Plasmid Constructs and Transient Transfection
Luciferase reporter constructs containing multiple copies of the NF-κB-binding DNA motif (Luc-4×NF-κB) (23) were transfected into MT-2 cells using Nucleofector solution (Nucleofector kit V; Amaxa Biosystems, Cologne, Germany). After overnight recovery, the cells were divided and treated either with wogonin or solvent dimethyl sulfoxide for 8 h. Luciferase activity was determined in 10 μl of cell lysates using the luciferase assay substrate (Promega) with a Duolumat LB9507 luminometer (Berthold, Bad Wildbad, Germany).
Knockdown of Mcl-1
MT-2 cells (3 × 106) were transfected in Nucleofector solution with 1 μm nonsense siRNA or Mcl-1 siRNA (HP GenomeWide siRNA; Qiagen, Hilden, Germany) using the Amaxa Nucleofector apparatus and the program X-01. Cells were split 48 h after transfection for controlling Mcl-1 protein knockdown and for further TRAIL treatment as described in the figure legend (supplemental Fig. S4).
RNA Isolation and Quantitative Real-time PCR
Extraction of total cellular RNA was performed using RNeasy Mini kit (Qiagen) with 1 × 106 cells/preparation according to the manufacturer's instructions. The primers for c-FLIPL, c-FLIPS, p53, Mdm2, 18S rRNA, TRAIL-R2, GAPDH, and fluorescent-labeled probes for TaqMan or SYBR quantitative real-time PCR were described previously (10, 24–28). Briefly, PCR was performed in a 12.5 μl of reaction mixture (PCR kit from Eurogentec, Cologne, Germany) that contained 0.08 μg of reverse transcribed cDNA and proper amounts of primers and probe. For each sample three PCRs were performed. The resulting relative increase in reporter fluorescent dye emission was monitored by the TaqMan system or the SYBR Green system (7500 Fast Real-time PCR system; Applied Biosystems). The relative mRNA levels of the target genes, relative to 18S rRNA or GAPDH, was calculated using the formula: Relative mRNA expression = 2−(Ct of target gene − Ct of control gene) where Ct is the threshold cycle value. The absolute c-FLIP mRNA level was determined using a c-FLIP cDNA standard curve by TaqMan quantitative PCR.
Cell Surface Staining of Receptors
For analysis of the surface expression levels of TRAIL receptors, cells (5 × 105) were washed with PBS and incubated with 10 μg/ml corresponding antibodies for 30 min at 4 °C, washed with PBS, and incubated for 30 min with FITC-conjugated goat anti-mouse antibody (USBiological) and analyzed by flow cytometry using a FACS Canto II (BD Biosciences). The following anti-TRAIL receptor antibodies were used for surface staining: HS101 (TRAIL-R1), HS201 (TRAIL-R2) (Alexis Biochemicals, Loerrach, Germany).
RESULTS
Wogonin, Apigenin, and Chrysin Suppress c-FLIP Expression at the Transcriptional Level in ATL Cells
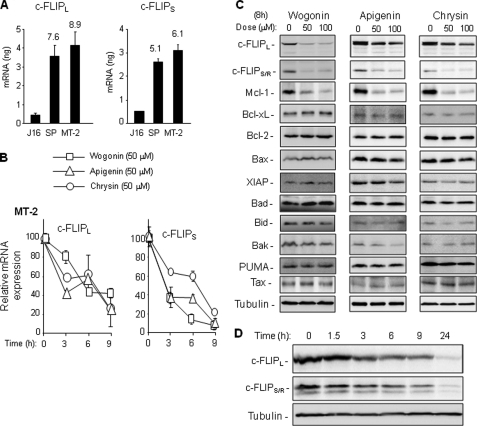
We have recently shown that the natural flavones wogonin, apigenin, and chrysin are inhibitors of CDK9, an important regulator of transcription elongation, and that they induce apoptosis in various tumor cells by transcriptional down-regulation of the short lived antiapoptotic protein Mcl-1 (15). Because cFLIPs are also short lived proteins, we asked whether these natural flavones could suppress c-FLIP expression in tumor cells. To address this question, we chose the two ATL cell lines, SP and MT-2, derived from HTLV-1-infected ATL patients as a model system because overexpression of c-FLIP has been shown to contribute to resistance to receptor-mediated apoptosis in these cells (10, 11). Comparison of the c-FLIP mRNA expression levels of SP and MT-2 cells with the non-HTLV-1-infected leukemic Jurkat T cells by quantitative PCR showed that the SP and MT-2 cells express 5–9-fold higher amounts of c-FLIP mRNA than Jurkat cells, indicating that c-FLIP expression is up-regulated at the transcriptional level in ATL cells (Fig. 1A). We treated MT-2 and SP cells with 50 μm wogonin, apigenin, and chrysin and monitored the c-FLIP mRNA expression by quantitative PCR. The concentration of flavones used has been shown to sufficiently inhibit CDK9 activity (15). These experiments showed that the c-FLIP mRNA expression levels in MT-2 and SP cells were down-regulated by wogonin, apigenin, or chrysin treatment (Fig. 1B and supplemental Fig. S1A). Inhibition of mRNA expression correlated with reduction of the protein expression levels of c-FLIPs (Fig. 1C and supplemental Fig. S1B). Consistent with the previous study (15), the level of the short lived Mcl-1 protein was also down-regulated by wogonin, apigenin, or chrysin treatment in MT-2 and SP cells. However, the protein expression levels of the long lived pro- and antiapoptotic proteins, e.g. Bax, Bad, Bid, Bak, XIAP, Bcl-2 and Bcl-xL, remained unchanged up to 24 h. The HTLV-1 Tax protein plays a key role in overexpression of c-FLIP (10, 11). Because the Tax protein expression levels were not altered by wogonin, apigenin, or chrysin treatment (Fig. 1C and supplemental Fig. S1B), the observed down-regulation of the c-FLIP level was not due to changes in Tax expression. Kinetic analysis showed that the expression levels of c-FLIPs were rapidly (∼50% down-regulation within 3 h) reduced (Fig. 1D).
FIGURE 1.
Wogonin, apigenin, and chrysin inhibit c-FLIP expression in HTLV-1-associated ATL cells. A, ATL cells expressing elevated levels of c-FLIP mRNA. HTLV-1-associated ATL cell lines SP and MT-2 and the non-HTLV-1-infected leukemic T cell line Jurkat were subjected to quantitative PCR analysis to determine the expression levels of c-FLIPL and c-FLIPS. Data are representative of three independent experiments performed in triplicate. Means ± S.D. (error bars) are shown. Numbers on columns of SP and MT-2 represent -fold expression compared with Jurkat. B, kinetic analysis of wogonin-, apigenin-, and chrysin-mediated inhibition of c-FLIP mRNA expression. MT-2 cells were treated with different flavones at 50 μm as indicated for 3 h, 6 h, and 9 h. The c-FLIP mRNA expression levels were monitored by quantitative PCR. Data are representative of two independent experiments performed in triplicate. Means ± S.D. are shown. C, c-FLIP expression levels down-regulated by wogonin, apigenin, and chrysin. MT-2 cells were treated with different concentrations of flavones for 8 h. The protein expression levels of various pro- and antiapoptotic proteins were analyzed by Western blotting. Tubulin was used to control for equal protein loading. The results are representative of two independent experiments. D, kinetic analysis of wogonin-mediated down-regulation of c-FLIPs. SP cells were treated with 50 μm wogonin for different time periods as indicated. The protein expression levels of c-FILPL and c-FLIPS were determined by Western blotting. The results are representative of two independent experiments.
c-FLIP is a NF-κB target gene (33, 34). In HTLV-1-infected cells, NF-κB has been shown to be constitutively activated by the HTLV-1 Tax protein (9). Constitutive NF-κB activation is responsible for the elevation of c-FLIP expression (11). Although wogonin, apigenin, and chrysin showed no influence on the expression levels of Tax protein (Fig. 1C), they might interfere with Tax function and thereby down-regulate c-FLIP expression. To investigate this possibility, we first examined the expression status of IκBα and the NF-κB subunit p65 after wogonin treatment. Western blot analysis showed that wogonin had no influence on the expression levels of p65 and IκBα (supplemental Fig. S2A). Wogonin treatment also did not affect the phosphorylation status of p65 (supplemental Fig. S2A). To investigate further whether wogonin interferes with NF-κB activity in HTLV-infected cells, a Luc-4×NF-κB reporter plasmid was transfected into MT-2 cells, and the NF-κB-mediated promoter activity was examined in the absence or presence of wogonin. 50 μm wogonin, which was shown to down-regulate c-FLIP mRNA expression effectively, did not influence the NF-κB-mediated expression of luciferase (supplemental Fig. S2B). These experiments demonstrate that wogonin does not suppress c-FLIP expression by inhibition of Tax-mediated NF-κB activation.
Wogonin, Apigenin, and Chrysin Sensitize ATL Cells to TRAIL-, TNFα-, and Anti-APO-1-mediated Apoptosis
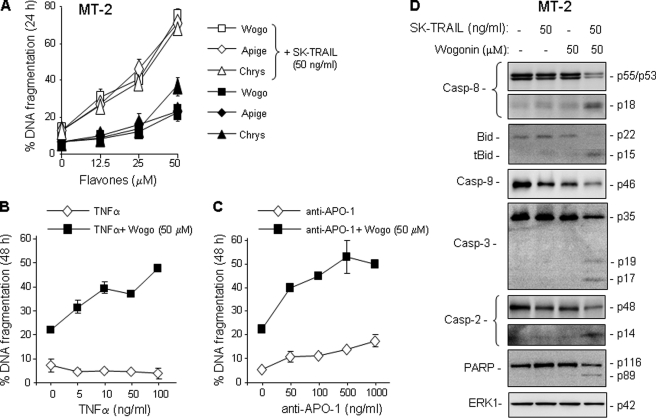
Elevation of c-FLIP expression has been shown to contribute to resistance to receptor-mediated apoptosis in HTLV-1-associated ATL cells (10, 11). Consistent with the previous studies, MT-2 and SP cells were shown to be highly resistant to TRAIL treatment (supplemental Fig. S3, A and B). Because wogonin, apigenin, and chrysin could down-regulate c-FLIP expression and because specific knockdown of c-FLIP alone can restore sensitivity of ATL cells toward receptor-mediated apoptosis (10, 11), we predicted that these flavones could break TRAIL resistance in ATL cells. As expected, inhibition of c-FLIP expression by wogonin, apigenin, and chrysin was shown to correlate with sensitization of the resistant MT-2 and SP cells to TRAIL-induced apoptosis in a dose-dependent matter (Fig. 2A and supplemental Fig. S3A). Wogonin was also shown to sensitize TNFα- and anti-APO-1-mediated apoptosis in a dose-dependent manner (Fig. 2, B and C). The effects of these flavones were further confirmed by Western blot analysis showing increased cleavage of caspase-8 and Bid and consequently enhanced cleavage of PARP in MT-2 and SP cells when treated by a combination of TRAIL and wogonin (Fig. 2D and supplemental Fig. S3D). Combination treatment also enhanced caspase-2 activation, whose activity has been indicated to participate in both receptor- and mitochondria-mediated apoptosis (29, 30).
FIGURE 2.
Wogonin, apigenin, and chrysin sensitize ATL cells to TRAIL-, TNFα-, and anti-APO-1-mediated apoptosis. A, wogonin, apigenin, and chrysin sensitize MT-2 cells to TRAIL-mediated apoptosis. MT-2 cells were treated with 50 ng/ml SK-TRAIL in combination with different concentrations of wogonin, apigenin, or chrysin for 24 h. Apoptosis was determined by DNA fragmentation. Data are representative of three independent experiments performed in duplicate. Means ± S.D. (error bars) are shown. B and C, wogonin sensitizes MT-2 cells to TNFα- and anti-APO-1-mediated apoptosis. Cells were treated with 50 μm wogonin in combination with different concentrations of TNFα- or anti-APO-1 for 48 h. Data are representative of two independent experiments performed in duplicate. D, wogonin enhances TRAIL-induced procaspase-8 processing. MT-2 cells were treated with TRAIL or wogonin either alone or in combination. Cell lysates were subjected to Western blot analysis with antibodies against caspase-8, -9, -3, -2, Bid, and PARP as indicated. For equal protein loading ERK1 protein levels are shown.
Because Mcl-1 expression was also down-regulated by flavones (Fig. 1C), we asked whether the decrease in Mcl-1 may also account for the enhancement of TRAIL-induced cell death. To address this question, we performed a siRNA knockdown experiment in MT-2 cells. The experiment showed that knockdown of Mcl-1 had no influence on TRAIL-mediated apoptosis (supplemental Fig. S4).
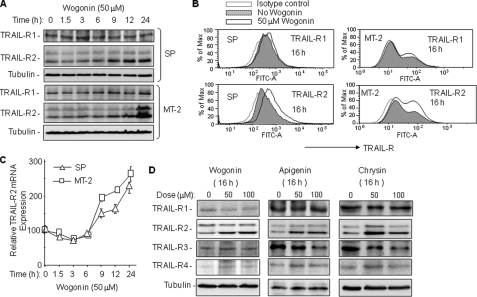
Wogonin, Apigenin, and Chrysin Enhance TRAIL-R2 Expression in ATL Cells
Sensitivity and resistance toward receptor-mediated apoptosis can be regulated at different levels, e.g. at the level of receptors, caspase-8, c-FLIP, and/or XIAP. Because the protein expression levels of XIAP and caspase-8 did not show substantial changes after treatment with wogonin, apigenin, and chrysin for 8 h (Figs. 1C and 2D), we further examined the effects of these flavones on the expression levels of TRAIL receptors in MT-2 and SP cells. A kinetic analysis using Western blotting showed a significant increase in TRAIL-R2, but not TRAIL-R1 protein expression in wogonin-treated ATL cells (Fig. 3A). The increase of TRAIL-R2 expression occurred after 6 h of wogonin treatment and increased continuously until 24 h. FACS analysis confirmed the increase in surface expression of TRAIL-R2 but not TRAIL-R1 in wogonin-treated MT-2 and SP cells (Fig. 3B). Further investigation of the kinetics of mRNA expression levels revealed that, although TRAIL-R2 mRNA was down-regulated at the early time points, it was constantly increased after 6 h of wogonin treatment (Fig. 3C). Elevated expression of TRAIL-R2 was also observed in ATL cells treated with apigenin and chrysin (Fig. 3D). In contrast to wogonin, substantial reduction in the decoy TRAIL-R3 levels were seen by treatment with apigenin and chrysin (Fig. 3D). The data indicate that TRAIL-R2 expression may be up-regulated at the transcriptional level by flavone treatment.
FIGURE 3.
Wogonin, apigenin, and chrysin enhance TRAIL-R2 expression in ATL cells. A, wogonin enhances TRAIL-R2 expression in ATL cells. SP and MT-2 cells were treated with 50 μm wogonin for different time periods as indicated. Cell lysates were subjected to Western blot analysis with antibodies against TRAIL-R1 and TRAIL-R2. Equal protein loading was controlled by tubulin. Data are representative of two independent experiments. B, wogonin increases cell surface expression of TRAIL-R2. SP and MT-2 cells were treated with 50 μm wogonin for 16 h. Cell surface expression of TRAIL-R1 and TRAIL-R2 was analyzed by FACS. Data are representative of three independent experiments. C, wogonin increases transcription of TRAIL-R2 in ATL cells. SP and MT-2 cells were treated with 50 μm wogonin for different time periods as indicated. The expression levels of TRAIL-R2 mRNA were monitored by quantitative PCR. Data are representative of three independent experiments performed in triplicate. Means ± S.D. (error bars) are shown. D, wogonin, apigenin, and chrysin enhance TRAIL-R2 protein expression in ATL cells. MT-2 cells were treated with different concentrations of flavones for 16 h as indicated. Cell lysates were subjected to Western blot analysis with antibodies against TRAIL-R1, -R2, -R3, and -R4. Equal protein loading was controlled by tubulin. Data are representative of two independent experiments.
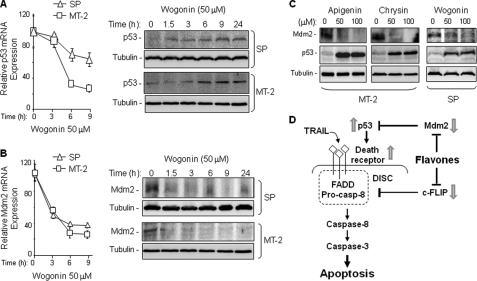
Wogonin, Apigenin, and Chrysin Enhance TRAIL-R2 Expression via Increasing p53 Expression by Transcriptional Suppression of Mdm2
TRAIL-R2 is a p53 target gene (31). Therefore, we further examined the expression levels of p53 in MT-2 and SP cells following wogonin treatment. Quantitative PCR showed that the p53 mRNA expression was reduced after treatment with wogonin. However, the protein expression levels of p53 were, surprisingly, increased (Fig. 4A). These data indicate that wogonin does not directly up-regulate p53 expression.
FIGURE 4.
Wogonin, apigenin, and chrysin enhance p53 expression in ATL cells via inhibition of Mdm2 expression. A, wogonin down-regulates p53 mRNA but up-regulates p53 protein expression in ATL cells. SP and MT-2 cells were treated with 50 μm wogonin for different time periods as indicated. The effect of wogonin on p53 expression was monitored by quantitative PCR (left) and Western blot analysis (right). Data are representative of two independent experiments. Means ± S.D. (error bars) are shown. B, wogonin suppresses Mdm2 mRNA and protein expression in ATL cells. SP and MT-2 cells were treated with 50 μm wogonin for different time periods as indicated. The effect of wogonin on Mdm2 expression was monitored by quantitative PCR (left) and Western blot analysis (right). Data are representative of two independent experiments. Means ± S.D. are shown. C, wogonin, apigenin, and chrysin inhibit Mdm2 but up-regulate p53 expression in ATL cells. MT-2 and SP cells were treated with different concentration of flavones for 16 h. Mdm2 and p53 expression levels were examined by Western blot analysis. Data are representative of two independent experiments. D, model of the mechanism by which flavones sensitize TRAIL-induced apoptosis.
One important negative regulator of p53 is the RING-finger-containing Mdm2 oncoprotein. Mdm2 plays a key role in regulation of p53 levels by an ubiquitylation-mediated degradation (32). Therefore, we examined the mRNA expression levels of Mdm2 after wogonin treatment. Quantitative PCR analysis showed a rapid down-regulation of Mdm2 mRNA expression after wogonin treatment (Fig. 4B). Wogonin-mediated suppression of Mdm2 mRNA expression resulted in a dramatic reduction in Mdm2 protein level (Fig. 4B). These data suggest that wogonin-mediated transcriptional inhibition leads to down-regulation of Mdm2 and, consequently, up-regulation of p53 expression levels. Down-regulation of Mdm2 and up-regulation of p53 expression levels were also observed in ATL cells treated with apigenin and chrysin (Fig. 4C). Taken together, these data suggest that flavone-mediated sensitization of TRAIL-induced apoptosis may also involve increased levels of TRAIL-R2 expression (Fig. 4D).
Wogonin Sensitizes TRAIL-induced Apoptosis in Several Cancer Cell Lines by Down-regulation of c-FLIP but Up-regulation of TRAIL-R2 Expression
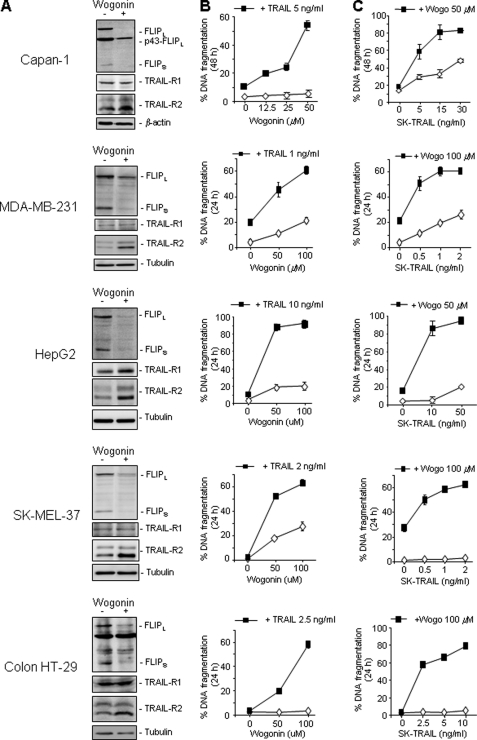
To investigate further whether wogonin could also differentially regulate expression of c-FLIP and TRAIL-R2 in other cancer types, we examined five different types of malignant cell lines including the human pancreatic carcinoma cell line Capan-1, the human breast cancer cell line MDA-MB-231, the human hepatocellular carcinoma cell line HepG2, the human melanoma cell line SK-MEL-37, and the human colon cancer cell line HT-29. For all cell lines tested, wogonin was shown to suppress c-FLIP expression but to enhance TRAIL-R2 expression (Fig. 5A). This effect correlated with enhanced TRAIL killing of malignant cells (Fig. 5, B and C). Thus, wogonin can sensitize TRAIL-mediated apoptosis in different cancers. Down-regulation of c-FLIP but up-regulation of TRAIL-R2 is an important mechanism by which wogonin and related natural flavones enhance TRAIL-induced cell death in tumor cells.
FIGURE 5.
Wogonin sensitizes TRAIL-induced apoptosis in different malignant cell lines by down-regulation of c-FLIP but up-regulation of TRAIL-R2 expression. A, wogonin inhibits c-FLIP but up-regulates TRAIL-R2 expression in different malignant cell lines. The human pancreatic carcinoma cell line Capan-1, the human breast cancer cell line MDA-MB-231, the human hepatocellular carcinoma cell line HepG2, the human melanoma cell line SK-MEL-37, and the human colon cancer cell line HT-29 were incubated with 50 μm wogonin for 16 h. The protein expression levels of c-FLIP and TRAIL-R1/-R2 were examined by Western blotting. B and C, wogonin sensitizes TRAIL-induced apoptosis in different malignant cell lines. Different tumor cell lines were treated with combinations of different concentrations of wogonin and TRAIL for 24 or 48 h as indicated. Apoptotic cells were determined by measuring DNA fragmentation. Means ± S.D. (error bars) are shown. Results are representative of two (Western blot) to three (apoptosis) independent experiments.
DISCUSSION
Overexpression of c-FLIP causes resistance to receptor-induced apoptosis and limits the therapeutic use of TRAIL in cancer treatment. This obstacle underscores the demand for combination therapies using agents that target c-FLIP expression. In this study, we demonstrate that the natural flavones wogonin, apigenin, and chrysin suppress c-FLIP expression and, consequently, overcome TRAIL resistance in various types of cancer cells. In addition, we also explored that wogonin, apigenin, and chrysin can enhance TRAIL-R2 expression in tumor cells. Increase in TRAIL-R2 expression, in addition to suppression of c-FLIP expression, may further benefit TRAIL-mediated apoptosis.
At the transcriptional level, c-FLIP expression is regulated by several transcription factors including NF-κB (33, 34). The HTLV-1 Tax protein is a well known activator of the NF-κB signaling pathway and has been shown to induce c-FLIP expression in HTLV-1-infected ATL cells through NF-κB activation (11). In this study, we show that the c-FLIP mRNA expression is significantly elevated in ATL cells compared with non-HTLV-1-infected leukemic cells (Fig. 1A). Thus, c-FLIP is up-regulated at the transcriptional level in ATL cells. We have recently demonstrated that wogonin, apigenin, and chrysin are naturally occurring inhibitors of CDK9, an important regulator of transcription elongation (15). The molecular mechanism by which wogonin, apigenin, and chrysin sensitize TRAIL-mediated apoptosis can be attributed to the transcriptional suppression of c-FLIP expression (Fig. 1B). Although CDK9 is a general regulator of transcription, recent studies indicate that CDK9 activity is rather involved in pathologic cellular processes than in normal cellular functions (35, 36). We have also demonstrated that wogonin preferentially inhibits CDK9 activity in malignant versus normal T cells (15). Therefore, wogonin, apigenin, and chrysin may be promising sensitizers of the TRAIL signaling pathway.
Treatment of tumor cells with wogonin, apigenin, and chrysin leads to rapid down-regulation of c-FLIP and Mcl-1 but not Tax and other pro- and antiapoptotic proteins such as Bcl-xL, Bcl-2, Bax, Bad, Bid, Bak, PUMA, and XIAP (Fig. 1C). c-FLIPs are known short lived proteins because the turnover of these proteins is actively regulated by ubiquitin-mediated proteasomal degradation (12, 13). In particular, c-FLIPS contains a unique carboxyl terminus that confers its preferred ubiquitylation (13). Inhibition of transcription would lead to rapid down-regulation of short lived proteins like c-FLIP but not long lived proteins. Because specific down-regulation of c-FLIP by siRNA has been shown to be sufficient to sensitize various types of cancer cells, including the HTLV-1-associated ATL, to receptor-mediated apoptosis, c-FLIP represents a promising therapeutic target, especially for TRAIL-based therapies (10, 11).
To our surprise, wogonin, apigenin, and chrysin up-regulated expression of TRAIL-R2 despite their transcriptional inhibitory activities (Fig. 3A). Kinetic analysis of the effect of flavones on TRAIL-R2 transcription showed that wogonin suppressed TRAIL-R2 mRNA expression only at early time points (1–3 h). At later time points (after 6 h), wogonin dramatically increased TRAIL-R2 mRNA expression (Fig. 3C). This observation indicates that the TRAIL-R2 promoter may be activated by a transcription factor induced by wogonin. Because only TRAIL-R2 but not the other three TRAIL receptors were up-regulated by wogonin, apigenin, and chrysin, we searched the literature and found that only TRAIL-R2 is described to be a p53 target gene (31). Indeed, the p53 protein expression level was constantly increased following flavone treatment (Fig. 4A). However, p53 transcription was not increased by wogonin treatment, suggesting that the observed increase in the p53 protein level might be due to protein stabilization. One negative regulator of p53 is the RING-finger-containing oncoprotein Mdm2. It has been shown that Mdm2 negatively regulates p53 expression by targeting it for proteasomal degradation (32, 37). Mdm2 is also a very short lived protein with a half-life of approximately 20 min (38). We found that treatment of ATL cells with wogonin resulted in down-regulation of Mdm2 mRNA expression and, consequently, complete inhibition of Mdm2 protein expression within 1.5 h (Fig. 4B). Consequently, p53 protein expression levels were up-regulated after 1.5 h of wogonin treatment (Fig. 4A). Mdm2 has also been shown to directly suppress p53 activity by binding to the amino terminus of p53 (38). Therefore, inhibition of Mdm2 expression would not only lead to increased p53 protein expression but also to increased p53 activity. This double effect may explain why flavones are able to increase TRAIL-R2 mRNA expression while CDK9-dependent transcription was inhibited (Fig. 3C). These data demonstrate that wogonin, apigenin, and chrysin may sensitize TRAIL-mediated apoptosis by two mechanisms: down-regulation of c-FLIP and up-regulation of TRAIL-R2 expression (Fig. 4D). Besides p53, the TRAIL-R2 promoter has also been shown to be activated by the transcription factors CCAAT/enhancer-binding protein homologous protein (CHOP) and Sp1 (39, 40). In our studies, neither CHOP nor Sp1 expression was up-regulated by wogonin treatment (data not shown). Therefore, we exclude the possibilities that CHOP and Sp1 are involved in flavone-mediated up-regulation of TRAIL-R2 expression.
PUMA is also a p53 target gene. However, in contrast to TRAIL-R2, no increase in the expression level of PUMA protein was seen in cells treated with wogonin, apigenin, or chrysin (Fig. 1C). This inconsistency may be explained by the short half-life of PUMA. The stability of PUMA has recently been shown to be regulated by post-translational control through phosphorylation at multiple sites leading to turnover with a half-life of about 4 h through the proteasome (41). In addition, a recent study on p53 target genes demonstrates a critical role of core promoter elements at p53 target loci, in that they dictate RNA polymerase II recruitment and activity in a p53-autonomous fashion (42). The TRAIL-R2 and PUMA promoter are shown to possess significant differences in the core promoter architectures which may lead to different kinetics of transcriptional competence (42). This difference might be another factor accounting for the different responses of TRAIL-R2 and PUMA to wogonin treatment.
So far, HTLV-1-associated ATL has been shown to resist standard anticancer therapies. We have shown previously that wogonin and its structurally related flavones apigenin, chrysin, and luteolin induce apoptosis in different types of cancer cells by transcriptional inhibition of the short lived antiapoptotic Bcl-2 family protein Mcl-1 (15). Wogonin, apigenin, and chrysin were shown to induce a low level of apoptotic cell death in HTLV-1 ATL cells (∼15–20% in 24 h) probably via inhibition of Mcl-1 expression (Fig. 1 and 2). However, a combination of these flavones with TRAIL resulted in approximately 80% apoptosis induction in TRAIL-resistant ATL cells (Fig. 2). Enhanced killing of cancer cells was also seen in other malignant human cell lines by combining TRAIL with flavones, e.g. the human pancreatic carcinoma cell line Capan-1, the human breast cancer cell line MDA-MB-231, the human hepatocellular carcinoma cell line HepG2, the human melanoma cell line SK-MEL-37, and the human colon cancer cell line HT-29 (Fig. 5). These data raise the possibility of using these flavones as adjuvants for TRAIL-mediated anticancer therapy.
Taken together, our results demonstrate that the natural flavones wogonin, apigenin, and chrysin can sensitize TRAIL-mediated apoptosis in different types of cancer cells by down-regulation of c-FLIP and up-regulation of TRAIL-R2 expression. We have shown previously that wogonin enhances TRAIL-mediated apoptosis in leukemic but not in normal healthy lymphocytes (19). In particular, toxicological studies in experimental animals (mouse and dog) showed that up to 60 mg/kg/day wogonin had no organ toxicity when intravenously administered for 90 days (43, 44). Therefore, wogonin may offer relative safety for long term therapies.
Supplementary Material
Acknowledgments
We thank Gerda Weidner and Ingrid Hengge-Rauchhaus for supporting this study.
This work was supported by the Helmholtz Alliance on Immunotherapy of Cancer in the Helmholtz Association.

This article contains supplemental Figs. S1–S4.
- TRAIL
- TNF-related apoptosis-inducing ligand
- ATL
- adult T cell leukemia/lymphoma
- CDK9
- cyclin-dependent kinase 9
- HTLV-1
- human T cell leukemia virus type 1
- IAP
- inhibitor of apoptosis proteins
- Mdm2
- murine double minute 2
- TRAIL-R
- TRAIL receptor
- XIAP
- X-linked IAP.
REFERENCES
- 1. Johnstone R. W., Frew A. J., Smyth M. J. (2008) Nat. Rev. Cancer 8, 782–798 [DOI] [PubMed] [Google Scholar]
- 2. Lawrence D., Shahrokh Z., Marsters S., Achilles K., Shih D., Mounho B., Hillan K., Totpal K., DeForge L., Schow P., Hooley J., Sherwood S., Pai R., Leung S., Khan L., Gliniak B., Bussiere J., Smith C. A., Strom S. S., Kelley S., Fox J. A., Thomas D., Ashkenazi A. (2001) Nat. Med. 7, 383–385 [DOI] [PubMed] [Google Scholar]
- 3. Walczak H., Miller R. E., Ariail K., Gliniak B., Griffith T. S., Kubin M., Chin W., Jones J., Woodward A., Le T., Smith C., Smolak P., Goodwin R. G., Rauch C. T., Schuh J. C., Lynch D. H. (1999) Nat. Med. 5, 157–163 [DOI] [PubMed] [Google Scholar]
- 4. Krammer P. H., Arnold R., Lavrik I. N. (2007) Nat. Rev. Immunol. 7, 532–542 [DOI] [PubMed] [Google Scholar]
- 5. Eckelman B. P., Salvesen G. S., Scott F. L. (2006) EMBO Rep. 7, 988–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salvesen G. S., Duckett C. S. (2002) Nat. Rev. Mol. Cell Biol. 3, 401–410 [DOI] [PubMed] [Google Scholar]
- 7. Yang J. K. (2008) Yonsei Med. J. 49, 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wuchter C., Krappmann D., Cai Z., Ruppert V., Scheidereit C., Dörken B., Ludwig W. D., Karawajew L. (2001) Leukemia 15, 921–928 [DOI] [PubMed] [Google Scholar]
- 9. Matsuoka M., Jeang K. T. (2007) Nat. Rev. Cancer 7, 270–280 [DOI] [PubMed] [Google Scholar]
- 10. Krueger A., Fas S. C., Giaisi M., Bleumink M., Merling A., Stumpf C., Baumann S., Holtkotte D., Bosch V., Krammer P. H., Li-Weber M. (2006) Blood 107, 3933–3939 [DOI] [PubMed] [Google Scholar]
- 11. Okamoto K., Fujisawa J., Reth M., Yonehara S. (2006) Genes Cells 11, 177–191 [DOI] [PubMed] [Google Scholar]
- 12. Chang L., Kamata H., Solinas G., Luo J. L., Maeda S., Venuprasad K., Liu Y. C., Karin M. (2006) Cell 124, 601–613 [DOI] [PubMed] [Google Scholar]
- 13. Poukkula M., Kaunisto A., Hietakangas V., Denessiouk K., Katajamäki T., Johnson M. S., Sistonen L., Eriksson J. E. (2005) J. Biol. Chem. 280, 27345–27355 [DOI] [PubMed] [Google Scholar]
- 14. Baumann S., Fas S. C., Giaisi M., Müller W. W., Merling A., Gülow K., Edler L., Krammer P. H., Li-Weber M. (2008) Blood 111, 2354–2363 [DOI] [PubMed] [Google Scholar]
- 15. Polier G., Ding J., Konkimalla B. V., Eick D., Ribeiro N., Köhler R., Giaisi M., Efferth T., Desaubry L., Krammer P. H., Li-Weber M. (2011) Cell Death Dis. 2, e182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chung H., Jung Y. M., Shin D. H., Lee J. Y., Oh M. Y., Kim H. J., Jang K. S., Jeon S. J., Son K. H., Kong G. (2008) Int. J. Cancer 122, 816–822 [DOI] [PubMed] [Google Scholar]
- 17. Lu N., Gao Y., Ling Y., Chen Y., Yang Y., Gu H. Y., Qi Q., Liu W., Wang X. T., You Q. D., Guo Q. L. (2008) Life Sci. 82, 956–963 [DOI] [PubMed] [Google Scholar]
- 18. Wang W., Guo Q. L., You Q. D., Zhang K., Yang Y., Yu J., Liu W., Zhao L., Gu H. Y., Hu Y., Tan Z., Wang X. T. (2006) Biol. Pharm. Bull 29, 1132–1137 [DOI] [PubMed] [Google Scholar]
- 19. Fas S. C., Baumann S., Zhu J. Y., Giaisi M., Treiber M. K., Mahlknecht U., Krammer P. H., Li-Weber M. (2006) Blood 108, 3700–3706 [DOI] [PubMed] [Google Scholar]
- 20. Miyoshi I., Kubonishi I., Yoshimoto S., Shiraishi Y. (1981) Gann 72, 978–981 [PubMed] [Google Scholar]
- 21. Rowe T., Dezzutti C., Guenthner P. C., Lam L., Hodge T., Lairmore M. D., Lal R. B., Folks T. M. (1995) Leuk. Res. 19, 621–628 [DOI] [PubMed] [Google Scholar]
- 22. Scaffidi C., Medema J. P., Krammer P. H., Peter M. E. (1997) J. Biol. Chem. 272, 26953–26958 [DOI] [PubMed] [Google Scholar]
- 23. Proksch P., Giaisi M., Treiber M. K., Palfi K., Merling A., Spring H., Krammer P. H., Li-Weber M. (2005) J. Immunol. 174, 7075–7084 [DOI] [PubMed] [Google Scholar]
- 24. Baumbusch L. O., Myhre S., Langerød A., Bergamaschi A., Geisler S. B., Lønning P. E., Deppert W., Dornreiter I., Børresen-Dale A. L. (2006) Mol. Cancer 5, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilda M., Bruch J., Harder L., Rawer D., Reiter A., Borkhardt A., Woessmann W. (2004) Leukemia 18, 584–588 [DOI] [PubMed] [Google Scholar]
- 26. McCullough K. D., Martindale J. L., Klotz L. O., Aw T. Y., Holbrook N. J. (2001) Mol. Cell. Biol. 21, 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Osman F., Rowhani A. (2008) J. Virol. Methods 154, 69–75 [DOI] [PubMed] [Google Scholar]
- 28. Sun M., Zhang J., Liu S., Liu Y., Zheng D. (2008) Oncol. Rep. 19, 177–185 [PubMed] [Google Scholar]
- 29. Enoksson M., Robertson J. D., Gogvadze V., Bu P., Kropotov A., Zhivotovsky B., Orrenius S. (2004) J. Biol. Chem. 279, 49575–49578 [DOI] [PubMed] [Google Scholar]
- 30. Robertson J. D., Gogvadze V., Kropotov A., Vakifahmetoglu H., Zhivotovsky B., Orrenius S. (2004) EMBO Rep. 5, 643–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takimoto R., El-Deiry W. S. (2000) Oncogene 19, 1735–1743 [DOI] [PubMed] [Google Scholar]
- 32. Marine J. C., Lozano G. (2010) Cell Death Differ. 17, 93–102 [DOI] [PubMed] [Google Scholar]
- 33. Kreuz S., Siegmund D., Scheurich P., Wajant H. (2001) Mol. Cell. Biol. 21, 3964–3973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Micheau O., Lens S., Gaide O., Alevizopoulos K., Tschopp J. (2001) Mol. Cell. Biol. 21, 5299–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Romano G., Giordano A. (2008) Cell Cycle 7, 3664–3668 [DOI] [PubMed] [Google Scholar]
- 36. Wang S., Fischer P. M. (2008) Trends Pharmacol. Sci. 29, 302–313 [DOI] [PubMed] [Google Scholar]
- 37. Manfredi J. J. (2010) Genes Dev. 24, 1580–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Itahana K., Mao H., Jin A., Itahana Y., Clegg H. V., Lindström M. S., Bhat K. P., Godfrey V. L., Evan G. I., Zhang Y. (2007) Cancer Cell 12, 355–366 [DOI] [PubMed] [Google Scholar]
- 39. Yoshida T., Maeda A., Tani N., Sakai T. (2001) FEBS Lett. 507, 381–385 [DOI] [PubMed] [Google Scholar]
- 40. Yamaguchi H., Wang H. G. (2004) J. Biol. Chem. 279, 45495–45502 [DOI] [PubMed] [Google Scholar]
- 41. Fricker M., O'Prey J., Tolkovsky A. M., Ryan K. M. (2010) Cell Death Dis. 1, e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gomes N. P., Espinosa J. M. (2010) Genes Dev. 24, 111–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peng J., Qi Q., You Q., Hu R., Liu W., Feng F., Wang G., Guo Q. (2009) J. Ethnopharmacol. 124, 257–262 [DOI] [PubMed] [Google Scholar]
- 44. Qi Q., Peng J., Liu W., You Q., Yang Y., Lu N., Wang G., Guo Q. (2009) Phytother. Res. 23, 417–422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.