Abstract
In eukaryotes, the post-translational addition of methyl groups to histone H3 lysine 4 (H3K4) plays key roles in maintenance and establishment of appropriate gene expression patterns and chromatin states. We report here that an essential locus within chromosome 3L centric heterochromatin encodes the previously uncharacterized Drosophila melanogaster ortholog (dSet1, CG40351) of the Set1 H3K4 histone methyltransferase (HMT). Our results suggest that dSet1 acts as a “global” or general H3K4 di- and trimethyl HMT in Drosophila. Levels of H3K4 di- and trimethylation are significantly reduced in dSet1 mutants during late larval and post-larval stages, but not in animals carrying mutations in genes encoding other well-characterized H3K4 HMTs such as trr, trx, and ash1. The latter results suggest that Trr, Trx, and Ash1 may play more specific roles in regulating key cellular targets and pathways and/or act as global H3K4 HMTs earlier in development. In yeast and mammalian cells, the HMT activity of Set1 proteins is mediated through an evolutionarily conserved protein complex known as Complex of Proteins Associated with Set1 (COMPASS). We present biochemical evidence that dSet1 interacts with members of a putative Drosophila COMPASS complex and genetic evidence that these members are functionally required for H3K4 methylation. Taken together, our results suggest that dSet1 is responsible for the bulk of H3K4 di- and trimethylation throughout Drosophila development, thus providing a model system for better understanding the requirements for and functions of these modifications in metazoans.
POST-TRANSLATIONAL modification of histones can alter the local chromatin environment and affect the recruitment of transcriptional regulatory machinery (Ebert et al. 2006; Vermuelen et al. 2007; Choi and Howe 2009; Bannister and Kouzarides 2011). These modifications can play diverse roles in transcriptional activation or silencing (reviewed in Gartner et al. 2011), and cross talk between different activating and silencing modifications may fine-tune levels of transcription (reviewed in Munshi et al. 2009; Lee et al. 2010; Gartner et al. 2011).
The post-translational addition of up to three methyl groups to histone H3 lysine 4 (H3K4) residues (H3K4me1, H3K4me2, and H3K4me3) correlates with active transcription (Bernstein et al. 2002; Santa-Rosa et al. 2002). H3K4 di- and trimethylation is often enriched at the promoter and 5′ coding regions of active genes, whereas H3K4 monomethylation is commonly found near the 3′ ends of active genes and within enhancer elements (Bernstein et al. 2002; Santa Rosa et al. 2002; Pokholok et al. 2005; Heintzman et al. 2007). Although the mechanisms of methyl-H3K4-mediated transcriptional activation are not fully elucidated, trimethyl-H3K4 is thought to act as a docking scaffold for the recruitment of the transcription pre-initiation complex and transcriptionally activating chromatin-remodelling complexes (Wysocka et al. 2006; Vermuelen et al. 2007).
In the budding yeast, Saccharomyces cerevisiae, all mono-, di-, and trimethylation of H3K4 is catalyzed by the Set1 enzyme, and the enzymatic activity of Set1 is modulated through a multi-subunit protein complex known as the Complex of Proteins Associated with Set1 (COMPASS) (Miller et al. 2001; Roguev et al. 2001; Nagy et al. 2002; Dehe et al. 2005). COMPASS is evolutionarily conserved, with functional orthologs of Set1 acting as major H3K4 histone methyltransferases (HMTs) in metazoans (Lee and Skalnik 2005; Lee et al. 2007; Simonet et al. 2007).
Higher metazoans possess additional H3K4 methylases, the mixed lineage leukemia (MLL) class of proteins, which act through distinct complexes similar to COMPASS (reviewed by Eissenberg and Shilatifard 2010). The MLL proteins (MLL1–5) are required at limited but important subsets of gene targets, such as homeotic and hormone response genes (J. Lee et al. 2008; S. Lee 2008; Wang et al. 2009; reviewed by Ansari and Mandal 2010; Eissenberg and Shilatifard 2010). H3K4 methylases identified in Drosophila melanogaster to date include Trx (homologous to MLL1–2), Trr (homologous to MLL3–4), and Ash1 (Beisel et al. 2002; Byrd and Shearn 2003; Sedkov et al. 2003; Smith et al. 2004). Ash1 and Trx are members of the Trithorax group of proteins that antagonize Polycomb group-mediated gene silencing (Klymenko and Muller 2004). In addition, Trx methylates H3K4 at heat-shock loci upon induction and appears to be required for mediating stress responses to heat stimuli (Smith et al. 2004). Trr is recruited to and required for H3K4 methylation at gene targets responsive to the insect nuclear hormone ecdysone (Sedkov et al. 2003). Although these HMTs are known to catalyze H3K4 methylation and are widely believed to act as the main global H3K4 methylases in Drosophila (Beisel et al. 2002; Byrd and Shearn 2003; Sedkov et al. 2003; Smith et al. 2004), the functional roles of the Drosophila ortholog of Set1 (dSet1) have remained undefined, largely because its location within centric heterochromatin makes genetic and molecular analysis particularly challenging.
In our efforts to functionally annotate essential heterochromatic genes in Drosophila (see Fitzpatrick et al. 2005; Schulze et al. 2005; Hallson et al. 2008; Sinclair et al. 2009), we have linked dSet1/CG40351, the Drosophila set1 ortholog, to an essential genetic locus previously known as lethal 5 or l(3L)h5, residing in chromosome 3L centric heterochromatin (Marchant and Holm 1988; Fitzpatrick et al. 2005). Surprisingly, we find that dSet1, and not Trx, Trr, or Ash1, acts as the main global H3K4 di- and trimethylase throughout Drosophila development. We also provide genetic and molecular evidence that Drosophila orthologs of other COMPASS members are required for H3K4 methylation and physically interact with dSet1. Our findings establish a foundation for examining transcriptional regulatory mechanisms underlying this key post-translational modification.
Materials and Methods
Drosophila cultures/crosses
Cultures were maintained on standard yeast–cornmeal–molasses media, and crosses were performed at 25° unless otherwise stated. All background strains, GAL4 drivers, RNAi lines, and stocks used for generating mosaics are publicly available [through the Bloomington, Kyoto, and Vienna Drosophila Rnai Center (VDRC) stock centers] and have been described previously (http://www.flybase.org and http://stockcenter.vdrc.at). Most mutations affecting and deficiencies removing the lethal 5 region have been described previously (Marchant and Holm 1988; Fitzpatrick et al. 2005; Hallson et al. 2008).
The following strains were generous gifts: G5 e/TM3 Sb and G12 e/TM3 Sb (D. Deitcher); Z480/TM3 Sb (C. Zuker); trxZ11/TM3 Kr-GFP and trxB11/TM3 Kr-GFP (H. Brock); ash1B1/TM3 Sb and ash1B7/TM3 Sb (unpublished alleles from J. Kennison); trr1/FM7 (A. Mazo); and UAS-ash2-3X HA/Cyo; MKRS/TM6 (M. Corominas). Unless noted, stocks listed have been described previously (Petruk et al. 2001; Sedkov et al. 2003; Koundakjian et al. 2004; Smith et al. 2004; Beltran et al. 2007; Fitzpatrick et al. 2005).
To isolate mutant animals at non-adult stages, mutants were balanced with the GFP-marked balancer chromosome, TM3 Sb Ser Twi-GAL4 UAS-GFP. Trans-heterozygous mutant animals were produced by crossing combinations of these GFP-balanced strains and were selected on the basis of the absence of GFP fluorescence. RNAi knockdown animals were generated in crosses using either VDRC RNAi lines (dSet1: VDRC #40682, #40683, and #10833; wds: VDRC #105371; dRbbp5: VDRC #106139; dWdr82: VDRC #25246; hcf: VDRC #46998; trx: VDRC #108122) or the Transgenic RNAi Resource Project (TRiP) RNAi line BL29563, which targets trr. Each of these RNAi lines was crossed to the tub-GAL4/TM3 Sb Ser Twi-Gal4 UAS-GFP driver line at 29° and RNAi knockdown animals were selected on the basis of the absence of GFP fluorescence. RNAi lines used in this study are not predicted to produce nonspecific 19-mers resulting in “off-target” effects (data not shown).
Single-embryo PCR
Homozygous embryos were selected from lethal 5 mutation or deficiency strains as described previously (Hallson et al. 2008). To detect the presence or absence of the CG40351 genomic sequence in various deficiencies, DNA from homozygous embryos was amplified using the primers SER2F (5′-AGGTTTGTTCATAATTGACACAGATGC-3′) and SER3R (5′-TCATACCTTTCCCATTACAGACTTTTG-3′). PCR conditions were as follows: 94° for 5 min, followed by 33 cycles of 94° for 30 sec, 60° for 40 sec, and 72° for 1 min. Grip84 was also amplified to ensure DNA integrity as described previously (Hallson et al. 2008). Lesions associated with lethal 5 alleles were identified as described in the supporting information, File S1.
Quantitative PCR
For details, see File S1.
Nuclear extracts
All steps were performed at 4°. Nuclei were isolated as described previously (Shaffer et al. 1994). Nuclei were sonicated for 5 min (30 sec on followed by 30 sec off) on medium power in a Diagenode Bioruptor in PIPES buffer [10 mM PIPES (pH 7.0), 300 mM sucrose, 3 mM MgCl, 1 mM EGTA, and 1× Protease Inhibitor Cocktail I (Calbiochem)] containing 400 mM NaCl and 0.5% Triton X-100 or HEPES high-salt buffer [20 mM HEPES (pH 7.5), 300 mM NaCl, 10% glycerol, 3 mM MgCl, 2mM DTT, and 0.1% Triton X-100] and then samples were centrifuged at 13,000 × g in a Heraeus Biofuge 13 table-top centrifuge for 15 min and the pellet was discarded.
Western blots
Western blots were performed following standard procedures. The following primary antibodies and antibody concentrations were used: H3K4me3 (Active Motif, 39159; lot nos.: 573636, 961715) at 1/5000; H3K4me2 (Millipore, 07-030; lot nos.: DAM147603, DAM1724042) at 1/5000, H3K4me (Abcam, ab8895; lot nos.: 573636, 961715) at 1/5000; H3 (Abcam, ab1791) at 1/10,000; hemagluttinin (HA) tag (ABM, G036) at 1/2500; anti-FLAG (ABM, G0191) at 1/200; and anti-GST (ABM, G0181) at 1/10,000. Binding specificities of each lot of methyl-H3K4 antibodies have been tested by us and others: these data can be found in Figure S1 in File S1 and at the Antibody Validation Database (http://compbio.med.harvard.edu/antibodies/about) (Egelhofer et al. 2010). Quantitative Western blotting details are provided in File S1.
Binding assays
The cDNA sequences encoding the full-length dSet1 protein or the dSet1 carboxy terminus (amino acids 1329–1641; dSet1np) were cloned into the CMV-HA vector (Promega) in frame with an N terminus HA tag (described in detail in File S1). HEK293T cells cultured in 75-cm2 flasks under standard conditions and grown to 50% confluence were transfected with 5 μg DNA and 50 μl of Polyfect (Qiagen) following the manufacturer’s instructions. Thirty-six hours post transfection, nuclear extractions were performed as described above. These extracts were diluted fourfold in PIPES buffer (with a final concentration of 150 mM NaCl and 0.25% Triton X-100) and precleared overnight with GST attached to glutathione (GSH) sepharose. A total of 600 μl of the precleared extracts were incubated with 30 μl of GST, GST-Ash2, GST-Wds (see File S1 for cloning details), or nothing (mock) attached to GSH sepharose beads (GE Life Sciences) for 3 hr with gentle rotation at 4°. The beads were washed with 1 ml of PIPES buffer (with 0.25% Triton X-100 and 150 mM NaCl) for 5 min followed by liquid aspiration three times. Samples were run on SDS-PAGE gels, followed by Western blot analysis.
Immunoprecipitation
Nuclear protein extracts were prepared from adult flies of the genotype ash2-3X HA/+; tub-Gal4/UAS-dSet1-2X FLAG as described above. These extracts were diluted twofold in PIPES buffer (with 0.25% Triton X-100 and 150 mM NaCl) and precleared overnight with 40 μl of protein G agarose (PGA) beads (Genscript) at 4°. Anti-HA antibodies (Abcam, ab9110) were added at a 1:200 dilution to precleared extracts and extracts were rotated overnight followed by the addition of 30 μl of PGA beads (Genscript) for 30 min at 4°. The PGA beads were washed a total of three times for 5 min each with PIPES buffer (containing 0.25% Triton and 150 mM NaCl) at 4°. After washing, the PGA beads were boiled in Laemmli sample buffer and run on 5% SDS-PAGE gels, followed by Western blot analysis. A mock immunoprecipitation experiment was also performed as above without antibodies added.
Glycerol gradient centrifugation
Protein extracts (500 μl) from ∼50 adult flies of the genotype ash2-3X HA/+; tub-Gal4/UAS-dSet1-2X FLAG were loaded onto a 16–40% glycerol step gradient (in HEPES high-salt buffer) with 1-ml steps in 3% increments in 16- × 102-mm polyallomer tubes (Beckman). Gradients were run at 30,000 × g for 18 hr at 4° in an SW32.1 swinging bucket rotor (Beckman). Fractions were run on SDS-PAGE gels, followed by Western blot analysis. Approximately 500-μl fractions were collected from top to bottom. Size markers (MWGF1000, Sigma-Aldrich) were run on parallel gradients and detected in fractions run on SDS-PAGE gels by Coomassie staining.
Immunostaining
Head structures from third instar larvae were isolated and fixed in 4% paraformaldehyde in PBST (1× PBS + 0.1% Triton X-100) and blocked in 1% BSA diluted in PBST, followed by incubation overnight at 4° with the primary antibodies rabbit anti-H3K4me3 (ActiveMotif) and mouse anti-phosphotyrosine-100 (Cell Signaling) diluted to 1:500 and 1:200, respectively, in PBT. Six 10-min washes with PBST were performed to remove unbound primary antibodies, followed by incubation with the secondary antibodies (goat anti-mouse DyeLight 694 1:500 (Thermo Scientific) and goat anti-rabbit Texas red 1:500 (Cell Signaling) for 2 hr and six 10-min washes with PBST. Imaginal discs were dissected and mounted on slides containing VectaShield mounting medium (Cell Signaling). Images were obtained on a Zeiss LSM140 spinning disc confocal microscope.
Results
dSet1 maps to the lethal 5/l(3L)h5 locus
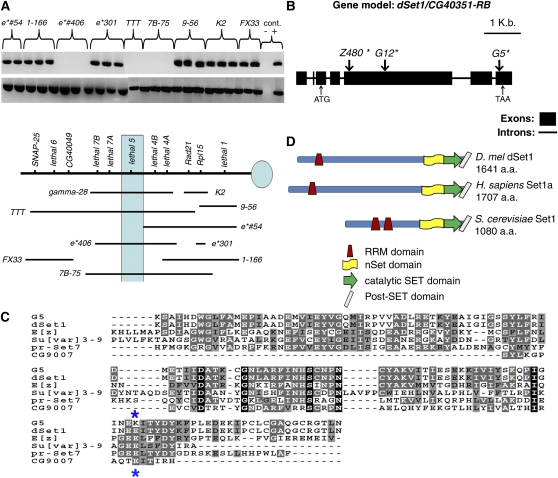
dSet1 (CG40351), the Drosophila ortholog of Set1, has been difficult to characterize because it resides in centric heterochromatin on the left arm of Drosophila chromosome 3 (3L). To determine the location of dSet1 along a genetic map of 3L centric heterochromatin (Schulze et al. 2001; Fitzpatrick et al. 2005; M. Syrzycka, unpublished data), we attempted to PCR-amplify the second and third exons of dSet1 using, as template, DNA isolated from embryos carrying deletions of 3L centric heterochromatin (Figure 1A). In this way, we determined that dSet1 resides in the vicinity of the lethal 5/l(3)h5, lethal 7A/l(3)h7A, and lethal 7B/l(3)h7B loci (Figure 1A). Further PCR mapping of dSet1 using small chromosomal aberrations that genetically complement lethal 7A and 7B alleles, but not lethal 5 alleles, narrowed the candidacy of dSet1 to lethal 5 (data not shown).
Figure 1.
The lethal 5 locus encodes the Drosophila ortholog of the Set1 H3K4 methylase (dSet1). (A) Using single-embryo PCR mapping, we demonstrate that CG40351 is absent in deficiencies that remove the lethal 5 and lethal 4B loci. (Top row gel lanes) CG40351 amplicons. (Bottom row gel lanes) Grip84 amplicons (+ve control). (B) Transcript model of dSet1/CG40351-RB. The position of the start codon (ATG) and stop codon (TAA) are indicated with small arrows. Locations of mutations are given below relative to the translational start site and are marked by large arrows along the gene model. Descriptions of mutations identified are the following: mutant G12 has a deletion of nt A1717, leading to a premature stop at amino acid 596; mutant Z480 has a T-to-A transversion at nt 548, resulting in premature termination at amino acid 183; and mutant G5 has a G-to-A transition at nt 4713, resulting in an E1613K missense mutation. (C) The alignment of diverse SET domains present in Drosophila demonstrates that the G5 missense mutation changes a conserved residue (E1613, marked with asterisks) thought to be required for methylase activity (Wilson et al. 2002). (D) Structural comparisons indicate significant homology between dSet1 (CG40351), Homo sapiens Set1a, and S. cerevisiae Set1.
lethal 5 mutations contain corresponding lesions in dSet1
To determine if EMS-induced lethal 5 mutant alleles result from mutations in dSet1, we PCR-amplified and sequenced the dSet1 genic region from homozygous lethal 5 mutant embryos. Figure 1B shows a correspondence between lethal 5 mutant alleles and molecular lesions in dSet1. The mutations identified were predicted to strongly reduce dSet1 function (described in the legend to Figure 1B).
lethal 5 mutants are rescued with a dSet1 cDNA transgene
Most lethal 5 mutants hemizygous with Df(3L)γ28 (which completely removes dSet1) are predominantly lethal at the pupal stage with low levels of late L3 larval lethality (<20%); we obtained similar patterns of lethality by driving dSet1-targeting RNAi constructs ubiquitously (data not shown) (Table 1). We were able to rescue lethality associated with complete loss of dSet1 function [amorphic G12 allele over the deficiency Df(3L)γ28] by expressing a dSet1 cDNA transgene (Table 2). This result unequivocally links phenotypic defects resulting from lethal 5 mutations to molecular defects in the dSet1 coding sequence.
Table 1. RNAi knockdown of Drosophila COMPASS members results in lethality.
| RNAi target | VDRC ID | Total flies | dsRNA + GAL4 flies expected | dsRNA + GAL4 flies observed | Predominant lethal stage |
|---|---|---|---|---|---|
| CG40351 | 40682 (ch2) | 202 | 101 | 0 | Pupala |
| CG40351 | 40683 (ch2) | 158 | 79 | 0 | Pupala |
| CG40351 | 10833 (ch3) | 189 | 63 | 0 | Pupala |
| wds | 105371 (ch2) | 343 | 172 | 0 | L1–L3 larvalb |
| CG5585/dRbbp5 | 106139 (ch2) | 247 | 148 | 0 | Pupal |
| CG17293/dWdr82 | 25246 (ch2) | 287 | 144 | 0 | Pupal |
| Ash2+Dcr2 | 7141 (ch2) | 230 | 115 | 0 | Pupalc |
| dHcf1 | 46999 (ch3) | 108 | 54 | 9 | Semilethal/pupald |
RNAi-mediated depletion of transcripts encoding Drosophila COMPASS homologs was accomplished by driving specific double-strand RNA targeting sequences with constitutively expressed GAL4 (UAS-dsRNA/UAS-dsRNA × tub-GAL4/TM3 Sb or UAS-dsRNAi/TM3 Sb × tub-GAL4/TM3 Sb performed at 29°). ch, chromosome.
dSet1 mutants display similar lethal phases.
Based on analysis of wds mutant phenotypes described in Shannon et al. (1972).
Based on analysis of ash2 mutant phenotypes described in Adamson and Shearn (1996).
Survivors display phenotypic defects of varying penetrance including held-out wings and mild wing vein and tergite defects.
Table 2. lethal 5 alleles placed over a deficiency [Df(3L)γ28].
| Genotype of rescued flies | Total flies scored | Rescued flies |
|---|---|---|
| w1118/UAS-CG40351-4F-X; Act5C-Gal4/+; G5 e/Df(3L)γ28 | 197 | 25 |
| w1118/UAS-CG40351-4F-X; Act5C-Gal4/+; G12 e/Df(3L)γ28 | 221 | 30 |
| w1118/UAS-CG40351-4F-X; Act5C-Gal4/+; Z480/Df(3L)γ28 | 128 | 10 |
lethal 5 alleles were rescued with a dSet1 cDNA transgene (UAS-CG40351-4F-X) driven by GAL4 mimicking the pattern of Act5C expression The progeny reported were generated by crossing w1118/w1118; Act5C-Gal4/Cyo; Df(3L)γ28/TM6 Hu Tb flies to flies of the genotype UAS-CG40351-4F-X/Y; +/+; G5 e/TM6 Hu Tb; UAS-CG40351-4F-X/Y; +/+; G12 e/TM6 Hu Tb or UAS-CG40351-4F-X/Y; +/+; Z480 e/TM6 Hu Tb.
Global levels of H3K4me2 and H3K4me3 are reduced in dSet1 mutants
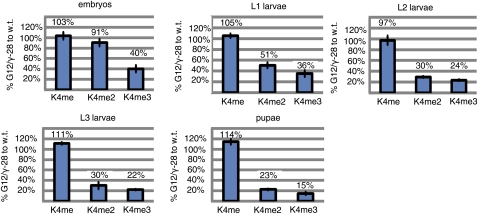
The predicted dSet1 protein appears structurally orthologous to the Set1 class of H3K4 methylases present in yeast and mammals (Figure 1D) (Roguev et al. 2001; Lee and Skalnik 2005; Lee et al. 2007). To determine if dSet1 functions similarly to Set1 in other organisms, we quantified levels of methylated H3K4 in nuclear extracts from wild-type (OreR) or amorphic dSet1 mutant animals (G12/γ28) using antibodies specific for each methylated form of H3K4 (details of our methodology and an example of this approach are provided, as supporting information and Figure S2 respectively, in File S1). Our results suggest that dSet1 acts as a major H3K4 di- and trimethyltransferase: relative to wild-type levels, we observed reductions of di- and trimethyl H3K4, but not monomethyl H3K4, in dSet1 mutant animals (Figure 2). Reductions of di- and trimethyl H3K4 in dSet1 mutants relative to wild-type levels appear to increase over developmental time (Figure 2). We have confirmed the requirement of dSet1 for H3K4 di- and trimethylation by using other dSet1 mutant allele combinations (Figure S3 in File S1) and by observing similar decreases after knocking down dSet1 via RNAi (VDRC line #40683).
Figure 2.
dSet1 mutations result in dramatic losses of di- and trimethylated H3K4 at multiple developmental time points. H3K4 methylation levels from nuclear extracts were detected on Western blots using H3K4 methyl-specific antibodies. Extracts were normalized to total histone H3 present. The H3K4 methylation levels measured from wild-type (OreR) and dSet1 mutants (G12/γ-28) were compared across a range of developmental time points: embryonic extracts (14–20 hr after egg lay), L1 extracts (34–40 hr post egg lay), L2 larval extracts (56–64 hr post egg lay), L3 larval extracts (104–110 hr post egg lay), and pupal extracts (7–8 days post egg lay).
Other Drosophila COMPASS members are required for bulk H3K4 methylation
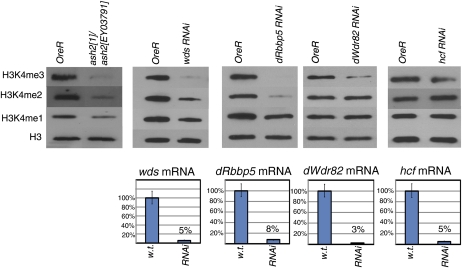
Since, in other eukaryotes, the Set1 class of proteins act through conserved multimeric protein complexes (Miller et al. 2001; Roguev et al. 2001; Nagy et al. 2002; Lee and Skalnik 2005; Lee et al. 2007), we predicted that a similar complex exists in Drosophila. RNAi knockdown of several genes encoding proteins orthologous to members of the human and yeast COMPASS complexes demonstrates that putative Drosophila COMPASS members are essential (Table 1). Loss of function via mutation or RNAi-induced depletion of several putative COMPASS members (Ash2, Wds, dRbbp5, and dWdr82) results in dramatic reductions of bulk H3K4 methylation levels (Figure 3). In addition, hcf knockdown results in slightly decreased H3K4 trimethylation—to ∼60% of wild-type levels on the basis of scanned band intensities (Figure 3, quantitation not shown). These results implicate Drosophila COMPASS members as key players required for global H3K4 methylation.
Figure 3.
The loss of function or depletion of Drosophila COMPASS members results in reduced global H3K4 methylation. A comparison of global H3K4 methylation levels in L3 larvae containing a trans-heterozygous combination of ash2 mutant alleles (ash21/ash2EY03971) or expressing double-strand RNA targeting wds, dRbbp5, dWdr82, and hcf, relative to wild-type (OreR) larvae. Levels of mRNA knockdown in RNAi experiments are represented as a percentage of wild-type levels and presented as bar graphs below.
dSet1 is a member of a Drosophila COMPASS complex
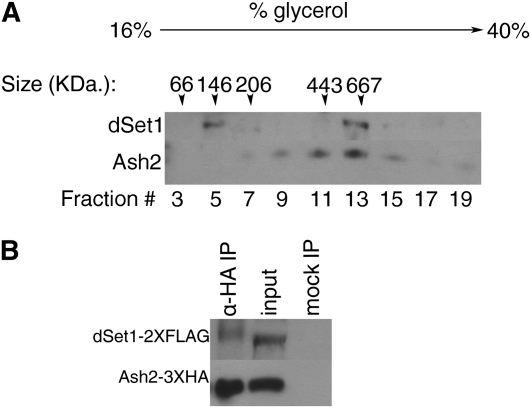
To demonstrate that dSet1 is present in a high-molecular-weight complex similar to yeast and mammalian COMPASS, we used glycerol gradients to fractionate high-molecular-weight species from nuclear extracts isolated from adult flies overexpressing tagged forms of Ash2 and dSet1. In this way, we isolated tagged dSet1 (predicted molecular weight ∼190 kDa) in a complex with a molecular mass of ∼667 kDa (fraction 13, Figure 4A), correlating closely with the additive mass of Drosophila COMPASS members (∼610 kDa). We noted a significant presence of dSet1 in a low-molecular-weight form (∼146 kDa, fraction 5), likely corresponding to unbound dSet1 fractionating slightly more slowly than expected. A large proportion of tagged Ash2 (molecular weight ∼67 kDa) copurifies in fraction 13 (∼667 kDa) with dSet1, suggesting that these two proteins are indeed present together in a complex. Ash2 also appears in other fractions, although to a lesser extent, which can be explained by the presence of multiple complexes containing Ash2 in flies, yeast, and mammals (Papoulas et al. 1998; Roguev et al. 2003; Secombe et al. 2007; Ansari and Mandal 2010; Eissenberg and Shilatifard 2010).
Figure 4.
dSet1 is a component of a macromolecular complex. (A) Size fractionation of adult nuclear extracts containing dSet1-2X FLAG and Ash2-3X HA on a 16–40% glycerol gradient. Fractions were recovered from top to bottom (left to right) and detected with anti-HA and anti-FLAG antibodies. The numbers listed above lanes indicate sizes (in kDa) of markers that were isolated at the respective fraction number when run on a parallel gradient. Note that the peak of dSet1-2X FLAG recovery occurs in a high-molecular-weight fraction (fraction 13, 667 kDa) and that significant levels of ash2-3X HA are also recovered in this fraction. (B) Anti-HA immunoprecipitation of Ash2-3X HA from adult nuclear extracts isolated from the genotype UAS-ash2-3X HA/+; tub-Gal4/UAS-dSet1-2X FLAG. dSet1 and Ash2 were detected with anti-FLAG and anti-HA antibodies, respectively. Note that dSet1 coprecipitates with Ash2.
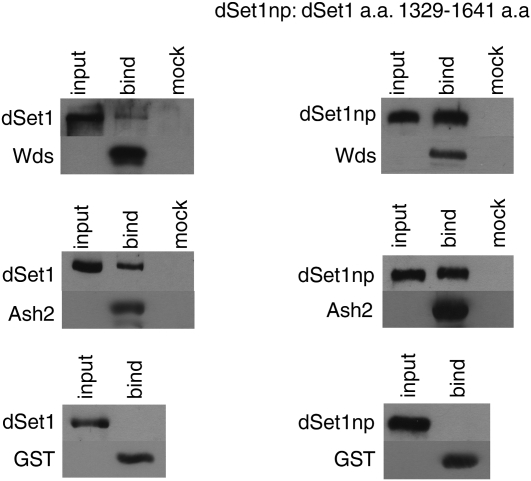
To obtain more direct evidence that dSet1 and Ash2 interact within COMPASS, we immunoprecipitated tagged Ash2 from whole-fly nuclear extracts and, by doing so, coprecipitated dSet1 (Figure 4B). We also performed in vitro binding assays: HA-tagged dSet1 or a truncated version of dSet1 containing the catalytic carboxy terminus (aa 1329–1641) were pulled down by GST-tagged Ash2 or Wds attached to glutathione-conjugated beads (Figure 5). These results indicate that dSet1 directly interacts with Ash2 and Wds and that the catalytic carboxy-terminal region of dSet1 is sufficient for facilitating these interactions (Figure 5).
Figure 5.
dSet1 physically interacts with the Drosophila COMPASS members Wds and Ash2. To identify binding partners of dSet1, in vitro binding assays were performed by incubating a solution containing HA-tagged versions of dSet1 or a fragment containing the catalytic carboxy terminus of dSet1 (HA-dSet1np amino acids 1329–1641) (“input” lanes) with GST, GST-Wds, or GST-Ash2 immobilized on glutathione sepharose beads (“bind” lanes) or unbound beads (“mock” lanes), followed by washes to remove nonspecific interactors. Bound products were detected on Western blots using anti-HA and anti-GST antibodies.
Trx, Trr, and Ash1 are not late-acting global H3K4 methylases
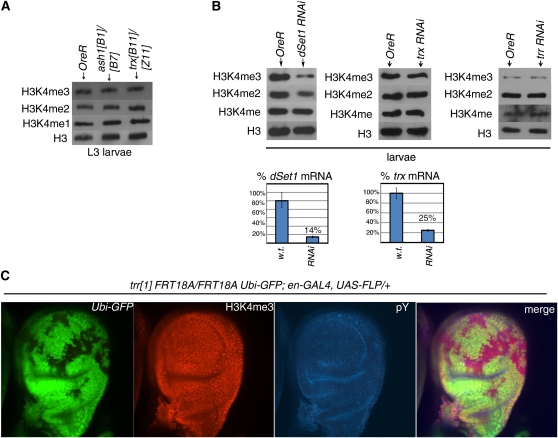
Given the surprising result that dSet1 is required for most H3K4 di- and trimethylation from the larval stages onward, we asked whether Trr, Trx, and Ash1 are also required for bulk H3K4 methylation at these same developmental stages. Levels of H3K4 methylation observed in trx mutant (trxB11/trxZ11) or RNAi knockdown larvae were similar to wild-type levels; a similar result was obtained with ash1 mutant (ash1B1/ash1B7) (Figure 6, A and B). Our findings are consistent with those of Srinivasan et al. (2008) who demonstrate that H3K4 trimethylation patterns in third instar salivary glands are not significantly altered in the presence of ash1 and trx mutations (Srinivasan et al. 2008).
Figure 6.
trr, ash1, and trx mutations do not significantly alter global H3K4 methylation levels. (A) Global H3K4 levels are unaltered in trx and ash1 mutant animals. Comparison of H3K4 methylation profiles of OreR, ash1B1/ash1B7, and trxB11/trxZ11 larvae. (B) Comparison of global H3K4 methylation levels between OreR larvae and larvae expressing double-strand RNA targeting (left to right) of dSet1, trx, and trr. Levels of dSet1 and trx mRNA knockdown in RNAi experiments are represented as a percentage of wild-type levels and presented as bar graphs below. We had technical problems in quantifying the extent of knockdown in the trr RNAi line, but Mohan et al. (2011) have verified that this line shows significantly reduced levels of Trr protein. (C) H3K4 methylation defects are not observed in trr1/trr1 mutant haltere disc clones (marked by an absence of GFP). Clones were generated in L3 larvae of the genotype trr1 FRT18A/Ubi-GFP FRT18A; UAS-FLP, en-Gal4/+. Note the similar amounts of H3K4 trimethylation present (red) in trr1/trr1 clones vs. surrounding GFP-positive tissue. (Left to right) Ubi-GFP (green); H3K4me3 staining (red); phosphotyrosine (pY) cell-surface staining (blue); and merge.
Since trr mutants arrest during embryogenesis, we were unable to compare levels of methyl-H3K4 between wild-type and trr mutant whole larvae. Instead, we generated clonal patches homozygous for the trr1 null allele in third instar larval haltere discs. We noted no differences in H3K4 trimethylation levels between trr1/trr1 haltere disc cells (marked by an absence of GFP) and cells containing functional Trr (marked by the presence of GFP) (Figure 6C), which is in stark contrast to the clear reductions of H3K4me3 that we observed in dSet1 mutant imaginal discs (File S1, Figure S4). Levels of H3K4 methylation present in trr RNAi knockdown larvae were also comparable to wild-type amounts (Figure 6B). Taken together, our results indicate Trx, Trr, or Ash1 are not required for bulk H3K4 methylation at the larval stages.
Discussion
Our results indicate that dSet1 acts as the main global H3K4 methylase throughout Drosophila development and is required for completion of late developmental stages. Although developmental roles of Set1 have been reported in Caenorhabditis elegans (Simonet et al. 2007), to our knowledge, this is the first report that a Set1 ortholog is essential for the somatic development of a multicellular organism.
We cannot rule out a persisting maternal contribution of dSet1 mRNA or protein to the catalysis of bulk H3K4me2/me3 during early developmental stages. Indeed, RNA sequencing data available on FlyBase (http://www.flybase.org) indicate that dSet1 is present in embryos aged 0–2 hr post egg lay, suggesting significant maternal loading of dSet1 transcripts (Daines et al. 2011). However, knocking down this maternal contribution during embryogenesis by expressing dSet1 RNAi in a dSet1 mutant background only slightly increases lethality at the L3 larval stage relative to dSet1 mutants (data not shown). It is possible that, in addition to dSet1, other H3K4 methylases are responsible for “early” bulk H3K4 methylation. Consistent with this, mutations in trr result in major losses of H3K4me2 and H3K4me3 levels during Drosophila embryogenesis (Sedkov et al. 2003). Moreover, bulk H3K4 dimethylation during C. elegans embryogenesis depends mostly on ASH-2, and not on SET-2 (the C. elegans dSet1 ortholog), suggesting that other H3K4 HMT players are involved (Xiao et al. 2011).
Trx and Trr, while apparently not required for bulk H3K4 methylation (Figure 6, A–C), may be important for transcriptional regulation of a subset of specific gene targets later in development. This would be consistent with their proposed role in human cells, where the Trr and Trx homologs MLL1-2 and MLL3-4 are thought to methylate H3K4 at a limited number of non-overlapping gene targets (Wang et al. 2009; Ansari and Mandal 2010; Eissenberg and Shilatifard 2010). We will not discuss the roles of Ash1 further, as recent findings suggest that the predominant function of Ash1 is the catalysis of H3K36, and not H3K4 methylation (Tanaka et al. 2007; An et al. 2011).
We have shown that dSet1 interacts within the Drosophila COMPASS and have demonstrated the requirement of other Drosophila COMPASS members for H3K4 methylation, which now places us in a position to dissect the functional roles of individual COMPASS members. Upon hcf and dWdr82 RNAi knockdown, only levels of H3K4me3 are reduced, an effect differing from that associated with loss of dSet1 function and suggesting specialized roles for Hcf and dWdr82 within the COMPASS (Figure 3). A nearly complete loss of H3K4me2 and H3K4me3 in ash2 mutants and dRbbp5 and wds RNAi knockdown animals suggests that these members are critical for COMPASS function; a loss of H3K4 monomethylation in these same animals is an effect not observed in dSet1 mutants and suggests distinct roles of dRbbp5, Wds, and Ash2 aside from their roles within the COMPASS.
Although Set1 has been reported to target H3K4me1 in Saccharomyces cerevisiae (Santa-Rosa et al. 2002), there have been no reports indicating that Set1 plays a similar role in metazoans, and we did not observe reductions in monomethyl H3K4 in dSet1 mutants. Our results also rule out the individual contributions of Ash1, Trr, or Trx to general H3K4 monomethylation. It has been reported that the human MLL/COMPASS complex subunits WDR5, Rbbp5, ASH2L, and DPY-30 form a complex (known as WRAD) that monomethylates recombinant histone H3 at lysine 4 in vitro (Patel et al. 2009, 2011). The involvement of a Drosophila form of WRAD in H3K4me1 seems plausible as ash2 mutations as well as wds and dRbbp5 RNAi knockdown (Figure 3) result in decreased levels of H3K4me1. Alternatively, bulk H3K4 monomethylation may be catalyzed or targeted by an as-yet-uncharacterized H3K4 HMT complex containing these members or by combinatorial effects of Trr- and Trx-containing complexes.
In summary, our results indicate that dSet1 acts through the COMPASS to promote global H3K4 di- and trimethylation and appears to be indispensable during Drosophila development. While we were preparing this report, Ardehali et al. (2011) reported that dSet1 is associated within a COMPASS complex and is responsible for the majority of H3K4 methylation in Drosophila S2 tissue culture cells. Mohan et al. (2011) have also recently reported on the central role of dSet1; moreover, they have comprehensively characterized all three COMPASS complexes in Drosophila containing dSet1, Trx, or Trr and associated proteins (Mohan et al. 2011). Our work provides a complementary analysis of dSet1 function in the context of whole-organism development and includes data on functional roles for other COMPASS members. These results lay the groundwork for studying mechanisms and functional roles of H3K4 methylation by the COMPASS and other HMTs in metazoans.
Supplementary Material
Acknowledgments
We thank Zhuo (Bill) Li, Jessica Tom, Sabrina Rayworth, Mary Chan, Michelle Higginson, Amanda Berscht, Rachel Chan, Sara Brown, Linda Yang, and Arthur Lee for technical assistance. We also thank Nicholas Harden for helpful discussions and Hugh Brock for critical insights prior to and during manuscript preparation, as well as two anonymous referees for valuable feedback. In addition, we thank the Bloomington Stock Center and the TRiP at Harvard Medical School (National Institutes of Health/National Institute of General Medical Sciences R01-GM084947) for providing fly stocks used in this study. This work was supported by a Discovery grant from the National Sciences and Engineering Research Council (NSERC) Canada to Barry Honda and NSERC postgraduate scholarships to Graham Hallson and Monika Syrzycka.
Literature Cited
- Adamson A. L., Shearn A., 1996. Molecular genetic analysis of Drosophila ash2, a member of the trithorax group required for imaginal disc pattern formation. Genetics 144: 633–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S., Yeo K. J., Jeon Y. H., Song J. J., 2011. Crystal structure of the human histone methyltransferase ASH1L catalytic domain and its implication for the regulatory mechanism. J. Biol. Chem. 286: 8360–8374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari K. I., Mandal S. S., 2010. Mixed lineage leukemia: roles in gene expression, hormone signalling and mRNA processing. FEBS J. 277: 1790–1804 [DOI] [PubMed] [Google Scholar]
- Ardehali M. B., Mei A., Zobeck K. L., Caron M., Lis J. T., et al. , 2011. Drosophila Set1 is the major histone H3 lysine 4 trimethyltransferase with role in transcription. EMBO J. 30: 2817–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister A. J., Kouzarides T., 2011. Regulation of chromatin by histone modifications. Cell Res. 3(2): 381–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel C., Imhof A., Greene J., Kremmer E., Sauer F., 2002. Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature 419: 857–862 [DOI] [PubMed] [Google Scholar]
- Beltran S., Angulo M., Pignatelli M., Serras F., Corominas M., 2004. Functional dissection of the ash2 and ash1 transcriptomes provides insights into the transcriptional basis of wing phenotypes and reveals conserved protein interactions. Genome Biol. 8(4): R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B. E., Humphrey E. L., Erlich R. L., Schneider R., Bouman P., et al. , 2002. Methylation of histone H3 Lys4 in coding regions of active genes. Proc. Natl. Acad. Sci. USA 99(13): 8695–8700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd K. N., Shearn A., 2003. ASH1, a Drosophila trithorax group protein, is required for methylation of lysine 4 residues on histone H3. Proc. Natl. Acad. Sci. USA 100(20): 11535–11540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. K., Howe L. J., 2009. Histone acetylation: Truth of consequences? Biochem. Cell Biol. 87(1): 139–150 [DOI] [PubMed] [Google Scholar]
- Daines B., Wang H., Wang L., Li Y., Han Y., et al. , 2011. The Drosophila melanogaster transcriptome by paired-end RNA sequencing. Genome Res. 21(2): 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehe P.-M., Pamblanco M., Luciano P., Lebrun R., Moiner D., et al. , 2005. Histone H3 lysine 4 mono-methylation does not require ubiquination of histone H2B. J. Mol. Biol. 353(3): 477–484 [DOI] [PubMed] [Google Scholar]
- Ebert A., Lein S., Schotta G., Reuter G., 2006. Histone modification and the control of heterochromatic gene silencing in Drosophila. Chromosome Res. 14(4): 377–392 [DOI] [PubMed] [Google Scholar]
- Egelhofer T. A., Minoda A., Klugman S., Lee K., Kolasinka-Zwierz P., et al. , 2010. An assessment of histone-modification antibody quality. Nat. Struct. Mol. Biol. 18: 91–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg J. C., Shilatifard A., 2010. Histone H3 lysine 4 (H3K4) methylation in development and differentiation. Dev. Biol. 339(2): 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick K. A., Sinclair D. A., Schulze S. R., Syrzycka M., Honda B. M., 2005. A genetic and molecular profile of third chromosome centric heterochromatin in Drosophila melanogaster. Genome 48(4): 571–584 [DOI] [PubMed] [Google Scholar]
- Gartner K. E., Allis C. D., Strahl B. D., 2011. OPERating ON chromatin, a colorful landscape where context matters. J. Mol. Biol. 409(1): 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallson G., Syrzycka M., Beck S. A., Kennison J. A., Dorsett D., et al. , 2008. The Drosophila cohesion subunit Rad21 is a trithorax group (trxG) protein. Proc. Natl. Acad. Sci. USA 105: 12405–12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintzman N. D., Stuart R. K., Hon G., Fu Y., Ching C. W., et al. , 2007. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat. Genet. 39(3): 311–318 [DOI] [PubMed] [Google Scholar]
- Kylmenko T., Muller J., 2004. The histone methyltransferases trithorax and Ash1 prevent transcriptional silencing by Polycomb group proteins. EMBO Rep 5(4): 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koundakjian E. J., Mulle J., Cowan D. M., Hardy R. W., Becker A. H., 2004. The Zucker Collection: a resource for the analysis of autosomal gene function in Drosophila melanogaster. Genetics 167: 203–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Saha P. K., Yang Q. H., Lee S., Park J. Y., et al. , 2008. Targeted inactivation of MLL3 histone H3-Lys4 methyltransferase activity in the mouse reveals vital roles for MLL3 in adipogenesis. Proc. Natl. Acad. Sci. USA 105(49): 19229–19234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Skalnik D. G., 2005. CpG-binding protein (CXXC finger protein 1) is a component of the mammalian Set1 histone H3-Lys4 methyltransferase complex, the analogue of the yeast Set1/COMPASS complex. J. Biol. Chem. 280(50): 41725–41731 [DOI] [PubMed] [Google Scholar]
- Lee J. H., Tate C. M., You J. S., Skalnik D. G., 2007. Identification and characterization of the human Set1B histone H3-Lys4 methyltransferase complex. J. Biol. Chem. 282(18): 13419–13428 [DOI] [PubMed] [Google Scholar]
- Lee J. S., Smith E., Schilatifard A., 2010. The language of histone crosstalk. Cell 142(5): 682–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee J., Lee S. K., Lee J. W., 2008. Activating signal cointegrator-2 is an essential adaptor to recruit histone H3 lysine 4 methyltransferases MLL3 and MLL4 to liver X receptors. Mol. Endocrinol. 22(6): 1312–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant G. E., Holm D. G., 1988. Genetic analysis of the heterochromatin of chromosome 3 in Drosophila melanogaster. II. Vital loci identified through EMS mutagenesis. Genetics 120: 519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T., Krogan N. J., Dover J., Erdjument-Bromage H., Tempst P., et al. , 2001. COMPASS: a complex of proteins associated with a trithorax-related SET domain protein. Proc. Natl. Acad. Sci. USA 98(23): 12902–12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M., Herz H. M., Smith E. R., Zhang Y., Jackson J., et al. , 2011. The COMPASS family of H3K4 methylases in Drosophila. Mol. Cell. Biol. 31: 4310–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi A., Shafi G., Aliya N., Jyothy A., 2009. Histone modifications dictate specific biological readouts. J. Genet. Genomics 36(2): 75–88 [DOI] [PubMed] [Google Scholar]
- Nagy P. L., Griesenbeck J., Kornberg R. D., Cleary M. L., 2002. A trithorax-group complex purified from Sacchromyces cerevisiae is required for methylation of histone H3. Proc. Natl. Acad. Sci. USA 99(1): 90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoulas O., Beek S. J., Moseley S. J., McCallum C. M., Sarte M., et al. , 1998. The Drosophila trithorax group proteins BRM, ASH1 and ASH2 are subunits of distinct protein complexes. Development 125: 3955–3966 [DOI] [PubMed] [Google Scholar]
- Patel A., Dharmarjan V., Vought V. E., Cosgrove M. S., 2009. On the mechanism of multiple lysine methylation by the human mixed lineage leukemia protein-(MLL1) complex. J. Biol. Chem. 284(36): 24242–24256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A., Vought V. E., Dharmarjan V., Cosgrove M. S., 2011. A novel non-SET domain multi-subunit methyltransferase required for the sequential nucleosomal histone H3 methylation by the mixed lineage leukemia protein-1 (MLL1) core complex. J. Biol. Chem. 286(5): 3359–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruk S., Sedkov Y., Smith S., Tillim S., Kraevski V., et al. , 2001. Trithorax and dCBP acting in a complex to maintain expression of a homeotic gene. Science 294: 1331–1334 [DOI] [PubMed] [Google Scholar]
- Pokholok D. K., Harbison C. T., Levine S., Cole M., Hannett N. M., et al. , 2005. Genome-wide Map of Nucleosome Acetylation and Methylation in Yeast. Cell 122(4): 2005. [DOI] [PubMed] [Google Scholar]
- Roguev A., Schaft D., Shevchenko A., Pijnappel W. W., Wilm M., et al. , 2001. The Sacchromyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone H3 lysine 4. EMBO J. 20(24): 7137–7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roguev A., Schaft D., Shevchenko A., Aasland R., Shevchenko A., et al. , 2003. High conservation of the Set1/Rad6 axis of histone 3 lysine 4 methylation in budding and fission yeasts. J. Biol. Chem. 278(10): 8487–8493 [DOI] [PubMed] [Google Scholar]
- Santa-Rosa H., Schneider R., Bannister A. J., Sherriff J., Bernstein B. F., et al. , 2002. Active genes are tri-methylated at K4 of histone H3. Nature 419(6905): 407–411 [DOI] [PubMed] [Google Scholar]
- Schulze S. R., Sinclair D. A. R., Silva E., Fitzpatrick K. A., Singh M., et al. , 2001. Essential genes in proximal 3L heterochromatin in Drosophila melanogaster. Mol. Gen. Genet. 264(6): 782–789 [DOI] [PubMed] [Google Scholar]
- Schulze S. R., Sinclair D. A., Fitzpatrick K. A., Honda B. M., 2005. A genetic and molecular characterization of two proximal heterochromatic genes on chromosome 3 of Drosophila melanogaster. Genetics 169: 2165–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secombe J., Li L., Carlos L., Eisenman R. N., 2007. The Trithorax group protein Lid is a trimethyl histone H3K4 demethylase required for dMyc-induced cell growth. Genes Dev. 21(5): 537–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedkov Y., Cho E., Petruk S., Cherba L., Smith S. T., et al. , 2003. Methylation at lysine 4 of histone H3 in ecdysone-dependent development of Drosophila. Nature 426(6962): 78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer C. D., Wuller J. M., Elgin S. C. R., 1994. Preparation of Drosophila nuclei, pp. 185–189 Drosophila melanogaster: Practical Uses in Cell and Molecular Biology, edited by Goldstein L. S. B., Fyrberg E. A. Academic Press, San Diego: [DOI] [PubMed] [Google Scholar]
- Shannon M. P., Kaufman T. C., Shen M. W., Judd B. H., 1972. Lethality patterns and morphology of selected lethal and semi-lethal mutations in the zeste-white region of Drosophila melanogaster. Genetics 72: 615–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet T., Dulermo R., Schott S., Palladino F., 2007. Antagonistic functions of SET-2/SET1 and HPL/HP1 proteins in C. elegans. Dev. Biol. 312(1): 367–383 [DOI] [PubMed] [Google Scholar]
- Sinclair D. A. R., Syrzycka M., Macauley M. S., Rastgardani T., Komljenovic I., et al. , 2009. Drosophila O-GlcNac transferase (OGT) is encoded by the Polycomb group (PcG) gene, super sex combs (sxc). Proc. Natl. Acad. Sci. USA 106: 13427–13432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. T., Petruk S., Sedkov Y., Cho E., Tillub S., et al. , 2004. Modulation of heat shock gene expression by TAC1 chromatin modifying complex. Nat. Cell Biol. 6: 162–167 [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Dorighi K. M., Tamkun J. W., 2008. Drosophila Kismet regulates histone H3 lysine 27 methylation and early elongation by RNA Polymerase II. PLoS Genet. 4(10): e1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Katagiri Z., Kawahasi K., Kioussis D., Kitajima S., 2007. Trithorax-group protein ASH1 methylates histone H3 lysine 36. Gene 396: 161–168 [DOI] [PubMed] [Google Scholar]
- Vermuelen M., Mulder K. W., Denissov S., Pijnappel W. W., Van Schaik F. M., et al. , 2007. Selective anchoring of TFIID to nucleosomes by trimethylation of histone H3 lysine 4. Cell 131(1): 58–69 [DOI] [PubMed] [Google Scholar]
- Wang P., Lin C., Smith E. R., Guo H., Anderson B. W., et al. , 2009. Global analysis of H3K4 methylation defines MLL family member targets and points to a role for MLL1-mediated H3K4 methylation in the regulation of transcriptional initiation by RNA polymerase II. Mol. Cell. Biol. 29(22): 6074–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. R., Jing C., Walker P. A., Martin S. R., Howell S. A., et al. , 2002. Crystal structure and functional analysis of the histone methyltransferase SET7/9. Cell 111(1): 105–115 [DOI] [PubMed] [Google Scholar]
- Wysocka J., Swigut T., Xiao H., Milne T. A., Kwon S. J., et al. , 2006. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature 442(7098): 86–90 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Bedet C., Robert V. J., Simonet T., Dunkelbarger S., et al. , 2011. Caenorhabditis elegans chromatin-associated proteins SET-2 and ASH-2 are differentially required for histone H3 Lys 4 methylation in embryos and adult germ cells. Proc. Natl. Acad. Sci. USA 108(20): 8305–8310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.