Abstract
One of the central regulators of cellular and organismal metabolism in eukaryotes is the AMP-activated protein kinase (AMPK), which is activated when intracellular ATP levels lower. AMPK plays critical roles in regulating growth and reprogramming metabolism, and recently has been connected to cellular processes including autophagy and cell polarity. We review here a number of recent breakthroughs in the mechanistic understanding of AMPK function, focusing on a number of new identified downstream effectors of AMPK.
Core AMPK complex Components and Upstream Activators
One of the fundamental requirements of all cells is to balance ATP consumption and ATP generation. AMPK is a highly conserved sensor of intracellular adenosine nucleotide levels that is activated when even modest decreases in ATP production result in relative increases in AMP or ADP. In response, AMPK promotes catabolic pathways to generate more ATP, and inhibits anabolic pathways. Genetic analysis of AMPK orthologs in Arabidopsis1, Saccharomyces cerevisiae2, Dictyostelium3, C. elegans4, Drosophila5, and even the moss Physcomitrella patens6 has revealed a conserved function of AMPK as a metabolic sensor, allowing for adaptive changes in growth, differentiation, and metabolism under conditions of low energy. In higher eukaryotes like mammals, AMPK plays a general role in coordinating growth and metabolism, and specialized roles in metabolic control in dedicated tissues such as the liver, muscle and fat7.
In most species, AMPK exists as an obligate heterotrimer, containing a catalytic subunit (a), and two regulatory subunits (β and γ). AMPK is hypothesized to be activated by a two-pronged mechanism (for a full review, see8). Under lowered intracellular ATP levels, AMP or ADP can directly bind to the γ regulatory subunits, leading to a conformational change that protects the activating phosphorylation of AMPK9,10. Recent studies discovering that ADP can also bind the nucleotide binding pockets in the AMPK γ suggest it may be the physiological nucleotide for AMPK activation under a variety of cellular stresses18-11. In addition to nucleotide binding, phosphorylation of Thr172 in the activation loop of AMPK is required for its activation, and several groups have demonstrated that the serine/threonine kinase LKB1 directly mediates this event12-14. Interestingly, LKB1 is a tumor suppressor gene mutated in the inherited cancer disorder Peutz-Jeghers syndrome and in a significant fraction of lung and cervical cancers, suggesting that AMPK could play a role in tumor suppression15. Importantly, AMPK can also be phosphorylated on Thr172 in response to calcium flux, independently of LKB1, via CAMKK2 (CAMKKβ) kinase, which is the closest mammalian kinase to LKB1 by sequence homology16-19. Additional studies have suggested the MAPKKK family member TAK1/MAP3K7 may also phosphorylate Thr172 but the contexts in which TAK1 might regulate AMPK in vivo, and whether that involves LKB1 still requires further investigation20, 21.
In mammals, there are two genes encoding the AMPK α catalytic subunit (α1 and α2), two β genes (β1 and β2) and three γ subunit genes (γ1, γ2 and γ3)22. The expression of some of these isoforms is tissue restricted, and functional distinctions are reported for the two catalytic α subunits, particularly of AMP- and LKB1-responsiveness and nuclear localization of AMPKα2 compared to the α123. However, the α1 subunit has been shown to localize to the nucleus under some conditions24, and the myristoylation of the (β isoforms has been shown to be required for proper activation of AMPK and its localization to membranes25. Additional control via regulation of the localization of AMPK26-28 or LKB129, 30 remains an critical underexplored area for future research.
Genetic studies of tissue-specific deletion of LKB1 have revealed that LKB1 mediates the majority of AMPK activation in nearly every tissue type examined to date, though CAMKK2 appears to be particularly involved in AMPK activation in neurons and T cells31, 32. In addition to regulating AMPKα1 and AMPKα2 phosphorylation, LKB1 phosphorylates and activates another twelve kinases related to AMPK33. This family of kinases includes the MARKs (1-4), SIKs (1-3), BRSK/SADs (1-2) and NUAKs (1-2) sub-families of kinases34. Although only AMPKα1 and AMPKα2 are activated in response to energy stress, there is a significant amount of crosstalk and shared substrates between AMPK and the AMPK related kinases15.
Many types of cellular stresses can lead to AMPK activation. In addition to physiological AMP/ADP elevation from stresses such as low nutrients or prolonged exercise, AMPK can be activated in response to several pharmacological agents (see Figure 1). Metformin, the most widely prescribed Type 2 diabetes drug, has been shown to activate AMPK35 and to do so in an LKB1 dependent manner36. Metformin and other biguanides, such as the more potent analog phenformin37, are thought to activate AMPK by acting as mild inhibitors of Complex I of the respiratory chain, which leads to a drop of intracellular ATP levels38, 39. Another AMPK agonist, 5-aminoimidazole-4-carboxamide-1-b-d-ribofuranoside (AICAR) is a cell-permeable precursor to ZMP, which mimics AMP, and binds to the AMPKγ subunits40. Interestingly, the chemotherapeutic pemetrexed, which is an inhibitor of thymidylate synthase, also inhibits aminoimidazolecarboxamide ribonucleotide formyltransferase (AICART), the second folate-dependent enzyme of purine biosynthesis, resulting in increased intracellular ZMP and activation of AMPK, similar to AICAR treatment41. Finally, a number of naturally occurring compounds including Resveratrol, a polyphenol found in the skin of red grapes, have been shown to activate AMPK and yield similar beneficial effects on metabolic disease as AICAR and metformin42, 43. Resveratrol can rapidly activate AMPK via inhibition of the F1F0 mitochondrial ATPase38 and the original studies suggesting that resveratrol directly binds and activates sirtuins have come into question44, 45. Indeed, the activation of SIRT1 by resveratrol in cells and mice appears to require increased NAD+ levels by AMPK activity46, 47.
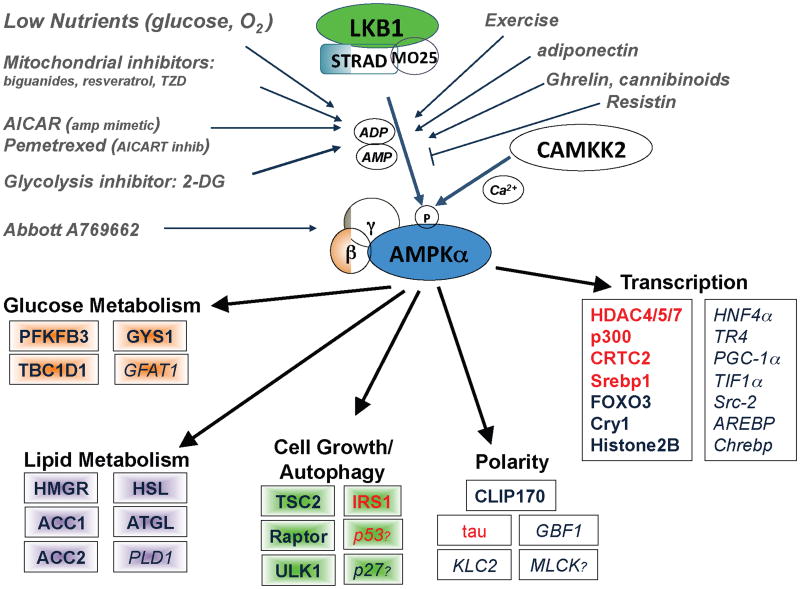
Figure 1. The AMPK signaling pathway.
AMPK is activated when AMP and ADP levels in the cells rise due to variety of physiological stresses, as well as pharmacological inducers. LKB1 is the upstream kinase activating it in response to AMP increase, whereas CAMKK2 activates AMPK in response to calcium increase. Activated AMPK directly phosphorylates a number of subtrates to acutely impact metabolism and growth, as well as phosphorylating a number of transcriptional regulators that mediate long term metabolic reprogramming. Shown are all the best-established substrates to date-those needing further in vivo examination are italicized. Question marks denote candidate substrates whose identified phosphorylation sites diverge from the established optimal substrate motif (which all the others conform to). A full lineup of the identified AMPK phosphorylation sites in these substrates in Supplemental Table 1. Substrates in red have been reported to serve as substrates of other AMPK family members (SIK1, SIK2, MARKs, SADs) in vivo in addition to being substrates of AMPK.
The principle therapeutic mode of action of metformin in diabetes is via suppression of hepatic gluconeogenesis7, 48, 49, though it remains controversial whether AMPK is absolutely required for the glucose lowering effects of metformin50. Since metformin acts as a mitochondrial inhibitor, it should be expected to activate a variety of stress sensing pathways which could redundantly serve to inhibit hepatic gluconeogenesis, of which currently AMPK is just one of the best appreciated. Critical for future studies will be defining the relative contribution of AMPK and other stress-sensing pathways impacted by metformin and the aforementioned energy stress agents in accurate in vivo models of metabolic dysfunction and insulin resistance in which these agents show therapeutic benefit. Nonetheless, metformin, AICAR51, the direct small molecule AMPK activator A76966252, and genetic expression of activated AMPK in liver53 all lower blood glucose levels, leaving AMPK activation a primary goal for future diabetes therapeutics54. As a result of the diverse beneficial effects of this endogenous metabolic checkpoint in other pathological conditions, including several types of human cancer, there is an increasing interest in identifying novel AMPK agonists to be exploited for therapeutic benefits.
AMPK coordinates Control of Cell Growth and Autophagy
In conditions where nutrients are scarce, AMPK acts as a metabolic checkpoint inhibiting cellular growth. The most thoroughly described mechanism by which AMPK regulates cell growth is via suppression of the mammalian target of rapamycin complex 1 (mTORC1) pathway. One mechanism by which AMPK controls the mTORC1 is by direct phosphorylation of the tumor suppressor TSC2 on serine 1387 (Ser1345 in rat TSC2). However, in lower eukaryotes, which lack TSC2 and in TSC2-/- mouse embryonic fibroblasts (MEFs) AMPK activation still suppresses mTORC1 55, 56. This led to the discovery that AMPK also directly phosphorylates Raptor (regulatory associated protein of mTOR), on two conserved serines, which blocks the ability of the mTORC1 kinase complex to phosphorylate its substrates42.
In addition to regulating cell growth, mTORC1 also controls autophagy, a cellular process of “self engulfment” in which the cell breaks down its own organelles (macroautophagy) and cytosolic components (microautophagy) to ensure sufficient metabolites when nutrients run low. The core components of the autophagy pathway were first defined in genetic screens in budding yeast and the most upstream components of the pathway include the serine/threonine kinase Atg1 and its associated regulatory subunits Atg13 and Atg1757, 58. In budding yeast, the Atg1 complex is inhibited by the Tor-raptor (TORC1) complex59-61. The recent cloning of the mammalian orthologs of the Atg1 complex revealed that its activity is also suppressed by mTORC1 through a poorly defined mechanism likely to involve phosphorylation of the Atg1 homologs ULK1 and ULK2, as well as their regulatory subunits (reviewed in62). In contrast to inhibitory phosphorylations from mTORC1, studies from a number of laboratories in the past year have revealed that the ULK1 complex is activated via direct phosphorylation by AMPK, which is critical for its function in autophagy and mitochondrial homeostasis (reviewed in63).
In addition to unbiased mass spectrometry studies discovering endogenous AMPK subunits as ULK1 interactors64, 65, two recent studies reported AMPK can directly phosphorylate several sites in ULK166, 67. Our laboratory found that hepatocytes and mouse embryonic fibroblasts devoid of either AMPK or ULK1 had defective mitophagy and elevated levels of p62 (Sequestrosome-1), a protein involved in aggregate turnover which itself is selectively degraded by autophagy66. As observed for other core autophagy proteins, ULK1 was required for cell survival following nutrient deprivation and this also requires the phosphorylation of the AMPK sites in ULK1. Similarly, genetic studies in budding yeast68 and in C. elegans66 demonstrate that Atg1 is needed for the effect of AMPK on autophagy. Interestingly, Kim and colleagues found distinct sites in ULK1 targeted by AMPK, though they also found that AMPK regulation of ULK1 was needed for ULK1 function67. These authors also mapped a direct mTOR phosphorylation site in ULK1 which appears to dictate AMPK binding to ULK1, a finding corroborated by another recent study, though the details differ69. Collectively, these studies show that AMPK can trigger autophagy in a double-pronged mechanism of directly activating ULK1 and inhibiting the suppressive effect of mTORC1 complex1 on ULK1 (see Fig. 2). Many of the temporal and spatial details of the regulation of these three ancient interlocking nutrient-sensitive kinases (AMPK, ULK1, mTOR) remains to be decoded.
Figure 2. The Ras/ PI3K/ mTOR pathways intersect the LKB1/AMPK pathway at multiple points.
LKB1, the upstream kinase for AMPK, is the tumor suppressor gene mutated in Peutz–Jeghers syndrome (PJS), as well a significant fraction of sporadic lung cancers and cervical cancers. PJS patients share a number of clinical features with patients inheriting defective PTEN or TSC tumor suppressors, perhaps due to their control of common biochemical pathways, best understood currently being the mammalian target of rapamycin complex 1 (mTORC1) pathway. Extensive cross-regulation of the LKB1/AMPK pathway by the oncogenic Ras and PI3K pathways has been discovered, which may explain how these commonly mutated oncogenes also try to circumvent this endogenous tumor suppressor pathway. The ULK1/hATG1 kinase complex has emerged recently as a central node receiving inputs from both AMPK and mTORC1. A number of kinases that can phosphorylate specific residues in LKB1 or AMPK have been identified (upper inset), though the contexts in which most of these regulatory events occur is poorly defined at present, as is the functional impact of these phosphorylation events on AMPK signaling. The BHD tumor suppressor and its partner FNIP1, as well as the sestrin family of proteins, have also been implicated as being upstream or downstream of AMPK and mTOR depending on the context.
Interestingly, AMPK both triggers the acute destruction of defective mitochondria through a ULK1-dependent stimulation of mitophagy, as well as stimulating de novo mitochondrial biogenesis through PGC-1α dependent transcription (see below). Thus AMPK controls mitochondrial homeostasis in a situation resembling “Cash for Clunkers” in which existing defective mitochondria are replaced by new fuel-efficient mitochondria (Fig. 3). One context where AMPK control of mitochondrial homeostasis may be particularly critical is in the context of adult stem cell populations. In a recent study on haematopoetic stem cells, genetic deletion of LKB1 or both of the AMPK catalytic subunits phenocopied fibroblasts lacking ULK1 or the AMPK sites in ULK1 in terms of the marked accumulation of defective mitochondria70.
Figure 3. AMPK acts as a mitochondrial “Cash for Clunkers”.

Activated AMPK acutely triggers the destruction of existing defective mitochondria via ULK1-dependent mitophagy and simultaneously triggers the biogenesis of new mitochondria via effects on PGC-1a dependent transcription. These dual processes controlled by AMPK have the net effect of replacing existing defective mitochondria with new functional mitochondria. This two-pronged control of mitochondria homeostasis by AMPK will have a number of physiological and pathological conditions where it plays a critical role, and a few are illustrated here.
Beyond effects on mTOR and ULK1, two other reported targets of AMPK in growth control are the tumor suppressor p5371 and the CDK inhibitor p2772, 73, though the reported sites of phosphorylation do not conform well to the AMPK substrate sequence found in other substrates. The recent discovery of AMPK family members controlling phosphatases74 presents another mechanism by which AMPK might control phosphorylation of proteins, without being the kinase to directly phosphorylate the site.
Control of Metabolism via Transcription and Direct effects on Metabolic Enzymes
AMPK was originally defined as the upstream kinase for the critical metabolic enzymes Acetyl-CoA carboxylase (ACC1 & ACC2) and HMG-CoA reductase, which serve as the rate-limiting steps for fatty-acid and sterol synthesis in wide-variety of eukaryotes75, 76. In specialized tissues such as muscle and fat, AMPK regulates glucose uptake via effects on the RabGAP TBC1D1, which along with its homolog TBC1D4 (AS160), play key roles in GLUT4 trafficking following exercise and insulin77. In fat, AMPK also directly phosphorylates lipases, including both hormone sensitive lipase (HSL)78 and adipocyte triglyceride lipase (ATGL)79, an AMPK substrate also functionally conserved in C. elegans4. Interestingly, mammalian ATGL and its liberation of fatty acids has recently been shown to be important in rodent models of cancer-associated cachexia80. Whether AMPK is important in this context remains to be seen.
In addition to acutely regulation of these metabolic enzymes, AMPK is also involved in a adaptive reprogramming of metabolism through transcriptional changes. Breakthroughs in this area have come through distinct lines of investigation. AMPK has been reported to phosphorylate and regulate a number of transcription factors, coactivators, the acetyltransferase p300, a subfamily of histone deacetylases, and even histones themselves. In 2010, Bungard et al., reported that AMPK can target transcriptional regulation through phosphorylation of histone H2B on Serine3681. Cells expressing a mutant H2B S36A blunted the induction of stress genes upregulated by AMPK including p21 and cpt1c81, 82. In addition, AMPK was chromatin immunoprecipitated at the promoters of these genes making this one of the first studies to detect AMPK at specific chromatin loci in mammalian cells81. Notably, Serine36 in H2B does not conform well to the AMPK consensus83; further studies will reveal whether this substrate is an exception or whether this phosphorylation is indirectly controlled.
AMPK activation has also recently been linked to circadian clock regulation, which couples daily light and dark cycles to control of physiology in a wide variety of tissues through tightly coordinated transcriptional programs84. Several master transcription factors are involved in orchestrating this oscillating network. AMPK was shown to regulate the stability of the core clock component Cry1 though phosphorylation of Cry1 Ser71, which stimulates the direct binding of the Fbox protein Fbxl3 to Cry1, targeting it for ubiquitin-mediated degradation24. Importantly, this is the first example of AMPK-dependent phosphorylation inducing protein turnover, although this is a common mechanism utilized by other kinases. One would expect additional substrates in which AMPK-phosphorylation triggers degradation will be discovered. Another study linked AMPK to the circadian clock via effects on Casein kinase85, though the precise mechanism requires further investigation. A recent genetic study in AMPK-deficient mice also indicates that AMPK modulates the circadian clock to different extents in different tissues86.
As the role of transcriptional programs in the physiology of metabolic tissues is well-studied, many connections between AMPK and transcriptional control have been found in these systems. Importantly, many of the transcriptional regulators phosphorylated by AMPK in metabolic tissues are expressed more ubiquitously than initially appreciated and may be playing more central roles tying metabolism to growth. One example that was recently discovered is the lipogenic transcriptional factor Srebp187. Srebp1 induces a gene program including targets ACC1 and FASN that stimulate fatty acid synthesis in cells. In addition to being a critical modulator of lipids in liver and other metabolic tissues, Srebp1 mediated control of lipogenesis is needed in all dividing cells as illustrated in a recent study identifying Srebp1 as a major cell growth regulator in Drosophila and mammalian cells88. AMPK was recently found to phosphorylate a conserved serine near the cleavage site within Srebp1, suppressing its activation87. This further illustrates the acute and prolonged nature of AMPK control of biology. AMPK acutely controls lipid metabolism via phosphorylation of ACC1 and ACC2, while mediating long-term adaptive effects via phosphorylation of Srebp1 and loss of expression of lipogenic enzymes. AMPK has also been suggested to phosphorylate the glucose-sensitive transcription factor ChREBP89 which dictates expression of an overlapping lipogenic gene signature with Srebp190. Adding an extra complexity here is the observation that phosphorylation of the histone acetyltransferase p300 by AMPK and its related kinases impacts the acetylation and activity of ChREBP as well91. Interestingly, like Srebp1, ChREBP has also been shown to be broadly expressed and involved in growth control in some tumor cell settings, at least in cell culture92.
Similarly, while best appreciated for roles in metabolic tissues, the CRTC family of transcriptional co-activators for CREB and its related family members may also play roles in epithelial cells and cancer93. Recent studies in C. elegans revealed that phosphorylation of the CRTC ortholog by AMPK is needed for AMPK to promote lifespan extension94, reinforcing the potentially broad biological functions of these coactivators. In addition to these highly conserved targets of AMPK and its related kinases, AMPK has also been reported to phosphorylate the nuclear receptors HNF4α (NR2A1)95 and TR4 (NR2C2)96, the coactivator PGC-1α97 and the zinc-finger protein AREBP (ZNF692)98, though development of phospho-specific antibodies and additional functional studies are needed to further define the functional roles of these events.
Another recently described set of transcriptional regulators targeted by AMPK and its related family members across a range of eukaryotes are the class IIa family of histone deacetylases (HDACs)99-105. In mammals the class IIa HDACs comprise a family of four functionally overlapping members: HDAC4, HDAC5, HDAC7, and HDAC9106 Like CRTCs, class IIa HDACs are inhibited by phosphorylation by AMPK and its family members, resulting in 14-3-3 binding and cytoplasmic sequestration. Recently, we discovered that similar to CRTCs, in liver the class IIa HDACs are dephosphorylated in response to the fasting hormone glucagon, resulting in transcriptional increases that are normally opposed by AMPK. Once nuclear, class IIa HDACs bind FOXO family transcription factors, stimulating their de-acetylation and activation,104 increasing expression of gluconeogenesis genes including G6Pase and PEPCK. Collectively, these findings suggest AMPK suppresses glucose production through two transcriptional effects: reduced expression of CREB targets via CRTC inactivation and reduced expression of FOXO target genes via class IIa HDAC inactivation (Figure 4). It is worth noting that while AMPK activation inhibits expression of FOXO gluconeogenic targets in the liver, in other cell types AMPK is reported to stimulate a set of FOXO-dependent target genes in stress resistance via direct phosphorylation of novel sites in FOXO3 and FOXO4 (though not FOXO1)107, an effect which appears conserved in C. elegans108. Ultimately, defining the tissues, isoforms, and conditions where the AMPK pathway controls FOXO via phosphorylation or acetylation is an important goal for understanding how these two ancient metabolic regulators are coordinated.
Figure 4. AMPK control of transcription.
AMPK regulates several physiological processes through phosphorylation of transcription factors and co-activators. It shares substrates with its AMPK family related kinases to negatively regulate gluconeogenesis in the liver by phosphorylation and inhibition of the CRCT2 and Class IIa HDACs. These phosphorylation events induce binding to 14-3-3 scaffold proteins and sequestration of these transcription regulators into the cytoplasm. AMPK also regulates transcription factors via inducing their degradation (Cry1), preventing their proteolytic activation and translocation to nucleus (Srebp1), and by disrupting protein-protein (p300) or protein-DNA interactions (Arebp, HNF4a). AMPK has also been shown to directly control phosphorylation of Histone 2B on Serine 36 as well as indirectly controlling SIRT1 activity via increasing NAD+ levels.
In addition to phosphorylating transcription regulators, AMPK has also been shown to regulate the activity of the deacetylase SIRT1 in some tissues via effects on NAD+ levels109, 110. As SIRT1 targets a number of transcriptional regulators for deacetylation, this adds yet another layer of temporal and tissue specific control of metabolic transcription by AMPK. This has been studied best in the context of exercise and skeletal muscle physiology, where depletion of ATP activates AMPK and through SIRT1 promotes fatty acid oxidation and mitochondrial gene expression. Interestingly, AMPK was also implicated in skeletal muscle reprogramming in a study where sedentary mice were treated with AICAR for 4 weeks and able to perform 44% better than control vehicle receiving counterparts111. This metabolic reprogramming was shown to require PPARβ/δ111 and likely involves PGC-1α as well97, though the AMPK substrates critical in this process have not yet been rigorously defined. Interestingly, the only other single agent ever reported to have such endurance reprogramming properties besides AICAR is Resveratrol112, whose action in regulating metabolism is now known to be critical dependent on AMPK47.
Control of Cell Polarity, Migration, & Cytoskeletal Dynamics
In addition to the ample data for AMPK in cell growth and metabolism, recent studies suggest that AMPK may control cell polarity and cytoskeletal dynamics in some settings113. It has been known for some time that LKB1 plays a critical role in cell polarity from simpler to complex eukaryotes. In C. elegans114 and Drosophila115, LKB1 orthologs establish cellular polarity during critical asymmetric cell divisions and in mammalian cell culture, activation of LKB1 was sufficient to promote polarization of certain epithelial cell lines116. Initially, it was assumed that the AMPK-related MARKs (Microtubule Affinity Regulating Kinases), which are homologs of C. elegans par-1 and play well-established roles in polarity, were the principal targets of LKB1 in polarity117. However, recent studies also support a role for AMPK in cell polarity.
In Drosophila, loss of AMPK results in altered polarity118, 119 and in mammalian MDCK cells, AMPK was activated and needed for proper re-polarization and tight junction formation following calcium switch120, 121. Moreover, LKB1 was shown to localize to adherens junctions in MDCK cells and E-cadherin RNAi led to specific loss of this localization and AMPK activation at these sites30. The adherens junctions protein Afadin122 and a Golgi-specific nucleotide exchange factor for Arf5 (GBF1)123 have been reported to be regulated by AMPK and may be involved in this polarity122, though more studies are needed to define these events and their functional consequences. In Drosophila AMPK deficiency altered multiple polarity markers, including loss of myosin light chain (MLC) phosphorylation118. While it was suggested in this paper that MLC may be a direct substrate of AMPK, this seems unlikely as the sites do not conform to the optimal AMPK substrate motif. However, AMPK and its related family members have been reported to modulate the activity of kinases and phosphatases that regulate MLC (MLCK, MYPT1), so MLC phosphorylation may be indirectly controlled via one of these potential mechanisms.
Another recent study discovered the microtubule plus end protein CLIP-170 (CLIP1) as a direct AMPK substrate124. Mutation of the AMPK site in CLIP-170 caused slower microtubule assembly, suggesting a role in the dynamic of CLIP-170 dissociation from the growing end of microtubules. It is noteworthy that mTORC1 was also previously suggested as a kinase for CLIP-170125, introducing the possibility that like ULK1, CLIP-170 may be a convergence point in the cell for AMPK and mTOR signaling. Consistent with this, besides effects on cell growth, LKB1/AMPK control of mTOR was recently reported to control cilia126 and neuronal polarization under conditions of energy stress127. In addition, the regulation of CLIP-170 by AMPK is reminiscent of the regulation of MAPs (microtubule associated proteins) by the AMPK related MARK kinases, which are critical in Tau hyperphosphorylation in Alzheimer's models128, 129. Indeed AMPK itself has been shown to target the same sites in Tau under some conditions as well130.
Finally, an independent study suggested a role for AMPK in polarizing neurons via control of PI3K localization131. Here, AMPK was shown to directly phosphorylate Kinesin Light Chain 2 (KLC2) and inhibit axonal growth via preventing PI3K localization to the axonal tip. Interestingly, a previous study examined the related protein KLC1 as a target of AMPK and determined it was not a real substrate in vivo132. Further experiments are needed to clarify whether AMPK is a bona fide kinase for KLC1 or KLC2 in vivo and in which tissues.
Emerging themes and future directions
An explosion of studies in the past 5 years has begun decoding substrates of AMPK playing roles in a variety of growth, metabolism, autophagy, and cell polarity processes. An emergent theme in the field is that AMPK and its related family members often redundantly phosphorylate a common set of substrates on the same residues, though the tissue expression and condition under which AMPK or its related family members are active vary. For example, CRTCs, Class IIa HDACs, p300, Srebp1, IRS1, and tau are reported to be regulated by AMPK and/or its SIK and MARK family members depending on the cell type or conditions. As a example of the complexity to be expected, SIK1 itself is transcriptionally regulated and its kinase activity is modulated by Akt and PKA so the conditions under which it is expressed and active will be a narrow range in specific cell types only, and usually distinct from conditions where AMPK is active. Delineating the tissues and conditions in which the 12 AMPK related kinases are active remains a critical goal for dissecting the growth and metabolic roles of their shared downstream substrates. A much more comprehensive analysis of AMPK and its family members using genetic loss of function and RNAi is needed to decode the relative importance of each AMPK family kinase on a given substrate for each cell type.
Now with a more complete list of AMPK substrates, it is also becoming clear that there is a convergence of AMPK signaling with PI3K and Erk signaling in growth control pathways, and with insulin and cAMP-dependent pathways in metabolic control. The convergence of these pathways reinforces the concept that there is a small core of rate-limiting regulators that control distinct aspects of biology and act as master coordinators of cell growth, metabolism, and ultimately cell fate. As more targets of AMPK are decoded, the challenge will be in defining more precisely which targets are essential and relevant for the beneficial effects of AMPK activation seen in pathological states ranging from diabetes to cancer to neurological disorders. The identification of these downstream effectors will provide new targets for therapeutically treating these diseases by unlocking this endogenous mechanism that evolution has developed to restore cellular and organismal homeostasis.
Supplementary Material
Acknowledgments
The authors want to apologize for the many primary studies in the AMPK field that could be not covered due to space limitations. Work in the authors' laboratory is, or has been, funded by the NIH grants R01 DK080425 and 1P01CA120964, an American Cancer Society Research Scholar Award, the American Diabetes Association Junior Faculty Award, and a Howard Hughes Medical Institute Early Career Scientist Award. We also thank the Adler Family Foundation and the Leona M. and Harry B. Helmsley Charitable Trust for their generous support.
Footnotes
The authors declare they have no competing financial interests.
References
- 1.Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J. A central integrator of transcription networks in plant stress and energy signalling. Nature. 2007;448:938–942. doi: 10.1038/nature06069. [DOI] [PubMed] [Google Scholar]
- 2.Hedbacker K, Carlson M. SNF1/AMPK pathways in yeast. Front Biosci. 2008;13:2408–2420. doi: 10.2741/2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bokko PB, et al. Diverse cytopathologies in mitochondrial disease are caused by AMP-activated protein kinase signaling. Mol Biol Cell. 2007;18:1874–1886. doi: 10.1091/mbc.E06-09-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature. 2009;457:210–214. doi: 10.1038/nature07536. [DOI] [PubMed] [Google Scholar]
- 5.Johnson EC, et al. Altered metabolism and persistent starvation behaviors caused by reduced AMPK function in Drosophila. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thelander M, Olsson T, Ronne H. Snf1-related protein kinase 1 is needed for growth in a normal day-night light cycle. Embo J. 2004;23:1900–1910. doi: 10.1038/sj.emboj.7600182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Hardie DG, Carling D, Gamblin SJ. AMP-activated protein kinase: also regulated by ADP? Trends Biochem Sci. 2011 doi: 10.1016/j.tibs.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Xiao B, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakhill JS, et al. AMPK is a direct adenylate charge-regulated protein kinase. Science. 2011;332:1433–1435. doi: 10.1126/science.1200094. [DOI] [PubMed] [Google Scholar]
- 11.Bland ML, Birnbaum MJ. Cell biology. ADaPting to energetic stress. Science. 2011;332:1387–1388. doi: 10.1126/science.1208444. [DOI] [PubMed] [Google Scholar]
- 12.Hawley SA, et al. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woods A, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 14.Shaw RJ, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawley SA, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Hurley RL, et al. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 18.Woods A, et al. C(Ca2+)/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Fogarty S, et al. Calmodulin-dependent protein kinase kinase-beta activates AMPK without forming a stable complex: synergistic effects of Ca2+ and AMP. Biochem J. 2010;426:109–118. doi: 10.1042/BJ20091372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie M, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci U S A. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrero-Martin G, et al. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 23.Salt I, et al. AMP-activated protein kinase: greater AMP dependence, and preferential nuclear localization, of complexes containing the alpha2 isoform. Biochem J. 1998;334(Pt 1):177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamia KA, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakhill JS, et al. beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK) Proc Natl Acad Sci U S A. 2010;107:19237–19241. doi: 10.1073/pnas.1009705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki A, et al. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol. 2007;27:4317–4327. doi: 10.1128/MCB.02222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kodiha M, Rassi JG, Brown CM, Stochaj U. Localization of AMP kinase is regulated by stress, cell density, and signaling through the MEK-->ERK1/2 pathway. Am J Physiol Cell Physiol. 2007;293:C1427–1436. doi: 10.1152/ajpcell.00176.2007. [DOI] [PubMed] [Google Scholar]
- 28.Kazgan N, Williams T, Forsberg LJ, Brenman JE. Identification of a nuclear export signal in the catalytic subunit of AMP-activated protein kinase. Mol Biol Cell. 2011;21:3433–3442. doi: 10.1091/mbc.E10-04-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorfman J, Macara IG. STRADalpha regulates LKB1 localization by blocking access to importin-alpha, and by association with Crm1 and exportin-7. Mol Biol Cell. 2008;19:1614–1626. doi: 10.1091/mbc.E07-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebbagh M, Santoni MJ, Hall B, Borg JP, Schwartz MA. Regulation of LKB1/STRAD localization and function by E-cadherin. Curr Biol. 2009;19:37–42. doi: 10.1016/j.cub.2008.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson KA, et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab. 2008;7:377–388. doi: 10.1016/j.cmet.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Tamas P, et al. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J Exp Med. 2006;203:1665–1670. doi: 10.1084/jem.20052469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lizcano JM, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. Embo J. 2004;23:833–843. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alessi DR, Sakamoto K, Bayascas JR. Lkb1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 35.Zhou G, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaw RJ, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dykens JA, et al. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicol Appl Pharmacol. 2008;233:203–210. doi: 10.1016/j.taap.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Hawley SA, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardie DG. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology. 2006;131:973. doi: 10.1053/j.gastro.2006.07.032. author reply 974-975. [DOI] [PubMed] [Google Scholar]
- 40.Hardie DG. AMP-Activated Protein Kinase as a Drug Target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 41.Rothbart SB, Racanelli AC, Moran RG. Pemetrexed indirectly activates the metabolic kinase AMPK in human carcinomas. Cancer Res. 2010;70:10299–10309. doi: 10.1158/0008-5472.CAN-10-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zang M, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 43.Baur JA, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacholec M, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt C. GSK/Sirtris compounds dogged by assay artifacts. Nat Biotechnol. 2010;28:185–186. doi: 10.1038/nbt0310-185. [DOI] [PubMed] [Google Scholar]
- 46.Canto C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0454-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Um JH, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hundal RS, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zang M, et al. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- 50.Foretz M, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–2369. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halseth AE, Ensor NJ, White TA, Ross SA, Gulve EA. Acute and chronic treatment of ob/ob and db/db mice with AICAR decreases blood glucose concentrations. Biochem Biophys Res Commun. 2002;294:798–805. doi: 10.1016/S0006-291X(02)00557-0. [DOI] [PubMed] [Google Scholar]
- 52.Cool B, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Foretz M, et al. Short-term overexpression of a constitutively active form of AMP-activated protein kinase in the liver leads to mild hypoglycemia and fatty liver. Diabetes. 2005;54:1331–1339. doi: 10.2337/diabetes.54.5.1331. [DOI] [PubMed] [Google Scholar]
- 54.Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9:407–416. doi: 10.1016/j.cmet.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalender A, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamada Y, et al. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol. 2000;150:1507–1513. doi: 10.1083/jcb.150.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamada Y, et al. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol Cell Biol. 2010;30:1049–1058. doi: 10.1128/MCB.01344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, Herman PK. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc Natl Acad Sci U S A. 2009;106:17049–17054. doi: 10.1073/pnas.0903316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–139. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 63.Hardie DG. AMPK and autophagy get connected. EMBO J. 2011;30:634–635. doi: 10.1038/emboj.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76. doi: 10.1038/nature09204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Egan DF, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z, Wilson WA, Fujino MA, Roach PJ. Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol Cell Biol. 2001;21:5742–5752. doi: 10.1128/MCB.21.17.5742-5752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shang L, et al. Nutrient starvation elicits an acute autophagic response mediated by Ulk1 dephosphorylation and its subsequent dissociation from AMPK. Proc Natl Acad Sci U S A. 2011;108:4788–4793. doi: 10.1073/pnas.1100844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468:653–658. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones RG, et al. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 72.Liang J, et al. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 73.Bjorklund MA, et al. Non-CDK-bound p27 (p27(NCDK)) is a marker for cell stress and is regulated through the Akt/PKB and AMPK-kinase pathways. Exp Cell Res. 2010;316:762–774. doi: 10.1016/j.yexcr.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 74.Zagorska A, et al. New roles for the LKB1-NUAK pathway in controlling myosin phosphatase complexes and cell adhesion. Sci Signal. 2010;3:ra25. doi: 10.1126/scisignal.2000616. [DOI] [PubMed] [Google Scholar]
- 75.Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223:217–222. doi: 10.1016/0014-5793(87)80292-2. [DOI] [PubMed] [Google Scholar]
- 76.Sato R, Goldstein JL, Brown MS. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc Natl Acad Sci U S A. 1993;90:9261–9265. doi: 10.1073/pnas.90.20.9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J Physiol Endocrinol Metab. 2008;295:E29–37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watt MJ, et al. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab. 2006;290:E500–508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- 79.Ahmadian M, et al. Desnutrin/ATGL Is Regulated by AMPK and Is Required for a Brown Adipose Phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Das SK, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. 2011;333:233–238. doi: 10.1126/science.1198973. [DOI] [PubMed] [Google Scholar]
- 81.Bungard D, et al. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science. 2010;329:1201–1205. doi: 10.1126/science.1191241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaugg K, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes Dev. 2011;25:1041–1051. doi: 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hardie DG. Transcription. Targeting the core of transcription. Science. 2010;329:1158–1159. doi: 10.1126/science.1195447. [DOI] [PubMed] [Google Scholar]
- 84.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Um JH, et al. Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J Biol Chem. 2007;282:20794–20798. doi: 10.1074/jbc.C700070200. [DOI] [PubMed] [Google Scholar]
- 86.Um JH, et al. AMPK Regulates Circadian Rhythms in a Tissue- and Isoform-Specific Manner. PLoS One. 2011;6:e18450. doi: 10.1371/journal.pone.0018450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Porstmann T, et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid “sparing” effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 90.Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81–86. doi: 10.1016/j.biochi.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 91.Bricambert J, et al. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 2010;120:4316–4331. doi: 10.1172/JCI41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tong X, Zhao F, Mancuso A, Gruber JJ, Thompson CB. The glucose-responsive transcription factor ChREBP contributes to glucose-dependent anabolic synthesis and cell proliferation. Proc Natl Acad Sci U S A. 2009;106:21660–21665. doi: 10.1073/pnas.0911316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mair W, et al. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278:27495–27501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- 96.Kim E, et al. Metformin Inhibits Nuclear Receptor TR4-Mediated Hepatic Stearoyl-Coenzyme A Desaturase 1 Gene Expression With Altered Insulin Sensitivity. Diabetes. 2011 doi: 10.2337/db10-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Inoue E, Yamauchi J. AMP-activated protein kinase regulates PEPCK gene expression by direct phosphorylation of a novel zinc finger transcription factor. Biochem Biophys Res Commun. 2006;351:793–799. doi: 10.1016/j.bbrc.2006.10.124. [DOI] [PubMed] [Google Scholar]
- 99.van der Linden AM, Nolan KM, Sengupta P. KIN-29 SIK regulates chemoreceptor gene expression via an MEF2 transcription factor and a class II HDAC. EMBO J. 2007;26:358–370. doi: 10.1038/sj.emboj.7601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang S, Bezprozvannaya S, Li S, Olson EN. An expression screen reveals modulators of class II histone deacetylase phosphorylation. Proc Natl Acad Sci U S A. 2005;102:8120–8125. doi: 10.1073/pnas.0503275102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dequiedt F, et al. New role for hPar-1 kinases EMK and C-TAK1 in regulating localization and activity of class IIa histone deacetylases. Mol Cell Biol. 2006;26:7086–7102. doi: 10.1128/MCB.00231-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Berdeaux R, et al. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med. 2007;13:597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- 103.McGee SL, et al. AMP-activated protein kinase regulates GLUT4 transcription by phosphorylating histone deacetylase 5. Diabetes. 2008;57:860–867. doi: 10.2337/db07-0843. [DOI] [PubMed] [Google Scholar]
- 104.Mihaylova MM, et al. Class IIa Histone Deacetylases Are Hormone-Activated Regulators of FOXO and Mammalian Glucose Homeostasis. Cell. 2011;145:607–621. doi: 10.1016/j.cell.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang B, et al. A hormone-dependent module regulating energy balance. Cell. 2011;145:596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Greer EL, et al. The Energy Sensor AMP-activated Protein Kinase Directly Regulates the Mammalian FOXO3 Transcription Factor. J Biol Chem. 2007;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- 108.Greer EL, et al. An AMPK-FOXO Pathway Mediates Longevity Induced by a Novel Method of Dietary Restriction in C. elegans. Curr Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Canto C, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Canto C, et al. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Narkar VA, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lagouge M, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 113.Mirouse V, Billaud M. The LKB1/AMPK polarity pathway. FEBS Lett. 2011;585:981–985. doi: 10.1016/j.febslet.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 114.Watts JL, Morton DG, Bestman J, Kemphues KJ. The C. elegans par-4 gene encodes a putative serine-threonine kinase required for establishing embryonic asymmetry. Development. 2000;127:1467–1475. doi: 10.1242/dev.127.7.1467. [DOI] [PubMed] [Google Scholar]
- 115.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003;421:379–384. doi: 10.1038/nature01296. [DOI] [PubMed] [Google Scholar]
- 116.Baas AF, et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004;116:457–466. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 117.Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev. 2009;89:777–798. doi: 10.1152/physrev.00026.2008. [DOI] [PubMed] [Google Scholar]
- 118.Lee JH, et al. Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature. 2007;447:1017–1020. doi: 10.1038/nature05828. [DOI] [PubMed] [Google Scholar]
- 119.Mirouse V, Swick LL, Kazgan N, St Johnston D, Brenman JE. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. J Cell Biol. 2007;177:387–392. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci U S A. 2006;103:17272–17277. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2007;104:819–822. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang L, et al. AMPK activation and GSK-3{beta} inhibition induce Ca2+ independent deposition of tight junction components at the plasma membrane. J Biol Chem. 2011 doi: 10.1074/jbc.M110.186932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miyamoto T, et al. AMP-activated protein kinase phosphorylates Golgi-specific brefeldin A resistance factor 1 at Thr1337 to induce disassembly of Golgi apparatus. J Biol Chem. 2008;283:4430–4438. doi: 10.1074/jbc.M708296200. [DOI] [PubMed] [Google Scholar]
- 124.Nakano A, et al. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12:583–590. doi: 10.1038/ncb2060. [DOI] [PubMed] [Google Scholar]
- 125.Choi JH, et al. The FKBP12-rapamycin-associated protein (FRAP) is a CLIP-170 kinase. EMBO Rep. 2002;3:988–994. doi: 10.1093/embo-reports/kvf197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Boehlke C, et al. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12:1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Williams T, Courchet J, Viollet B, Brenman JE, Polleux F. AMP-activated protein kinase (AMPK) activity is not required for neuronal development but regulates axogenesis during metabolic stress. Proc Natl Acad Sci U S A. 2011;108:5849–5854. doi: 10.1073/pnas.1013660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drewes G, Ebneth A, Preuss U, Mandelkow EM, Mandelkow E. MARK, a novel family of protein kinases that phosphorylate microtubule-associated proteins and trigger microtubule disruption. Cell. 1997;89:297–308. doi: 10.1016/s0092-8674(00)80208-1. [DOI] [PubMed] [Google Scholar]
- 129.Nishimura I, Yang Y, Lu B. PAR-1 kinase plays an initiator role in a temporally ordered phosphorylation process that confers tau toxicity in Drosophila. Cell. 2004;116:671–682. doi: 10.1016/s0092-8674(04)00170-9. [DOI] [PubMed] [Google Scholar]
- 130.Thornton C, Bright NJ, Sastre M, Muckett PJ, Carling D. AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem J. 2011;434:503–512. doi: 10.1042/BJ20101485. [DOI] [PubMed] [Google Scholar]
- 131.Amato S, et al. AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization. Science. 2011;332:247–251. doi: 10.1126/science.1201678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McDonald A, et al. Control of insulin granule dynamics by AMPK dependent KLC1 phosphorylation. Islets. 2009;1:198–209. doi: 10.4161/isl.1.3.9608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.