Abstract
Membrane proteins participate in nearly all cellular processes; however, because of experimental limitations, their characterization lags far behind that of soluble proteins. Peripheral membrane proteins are particularly challenging to study because of their inherent propensity to adopt multiple and/or transient conformations in solution and upon membrane association. In this review, we summarize useful biophysical techniques for the study of peripheral membrane proteins and their application in the characterization of the membrane interactions of the natively unfolded and Parkinson’s disease (PD) related protein, α-synuclein (α-syn). We give particular focus to studies that have led to the current understanding of membrane-bound α-syn structure and the elucidation of specific membrane properties that affect α-syn-membrane binding. Finally, we discuss biophysical evidence supporting a key role for membranes and α-syn in PD pathogenesis.
1. Introduction
Though membrane proteins are predicted to constitute 15–39% of the human proteome [1], it is striking that their structural characterization lags far behind that of their soluble counterparts [2–4]. Narrowing this gap in understanding is of upmost importance because membrane proteins participate in nearly all essential cellular processes from signaling to lipid metabolism and as such make excellent drug targets. For example, it is remarkable that while ~45% of drug targets are plasma membrane proteins, less than 1% of their three dimensional structures have been determined [2]. Moreover, membrane-mediated protein conformational changes are linked to disease pathogenesis making the study of membrane-protein interactions of particular urgency.
One of these proteins is the intrinsically disordered and amyloidogenic protein, α-synuclein (α-syn), which is involved in Parkinson’s disease (PD) etiology [5]. In this review, we provide a brief synopsis of some of the experimental techniques used to determine membrane-protein structure as well as their application in the study of α-syn-membrane interactions. Specific emphasis is given to the description and application of biophysical methods because of their unique advantages in the characterization and identification of both transient and stable membrane-bound species. We present recent work leading to the current understanding of membrane-bound α-syn structure and how specific membrane-properties mediate α-syn-membrane interactions. Finally, we discuss how α-syn-membrane interactions are linked to PD.
2. Biophysical probes of membrane protein structure
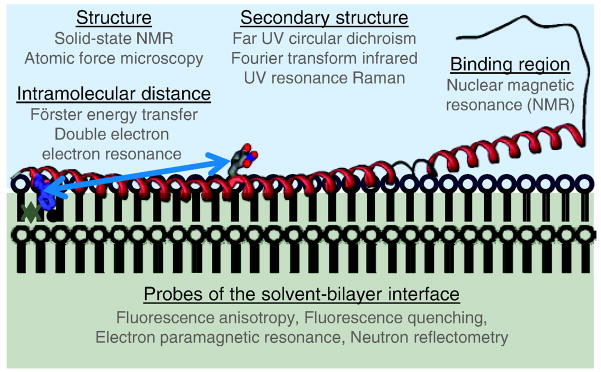
While recent advances in experimental techniques and purification procedures have facilitated the study of more membrane-protein systems than ever before [6], preparation of samples that retain functionally relevant structures after purification and/or crystallization remains a challenge in most cases. As such, various complementary biophysical approaches (Fig. 1) in addition to conventional structural techniques, such as solution-state nuclear magnetic resonance (NMR) spectroscopy [7, 8] and electron and X-ray crystallography [9, 10], have been employed to study membrane protein secondary and tertiary structure as well as the membrane-protein interface.
Fig. 1.
Selected biophysical techniques used to examine membrane binding proteins such as α-synuclein.
2.1 Secondary structure
Among the most common biophysical probes of secondary structure are circular dichroism (CD), Fourier transform infrared (FTIR), and ultraviolet resonance-Raman (UVRR) spectroscopies. CD spectroscopy, in which the difference in left- and right-handed circularly polarized light absorption is measured, allows for direct quantitation of secondary structural content because alignments of polypeptide amide bonds (i.e. specific alignments corresponding to α-helical, β-sheet, or random-coil polypeptide conformations) induce distinct UV absorption bands [11, 12].
In cases where measurements of solid and/or UV opaque samples are required, FTIR can be used since the absorption bands corresponding to the amide bond stretching frequencies are also modulated by polypeptide secondary structure [13, 14]. Though less widely applied, an emerging technique, UVRR, in which inelastic UV light scattering is measured, can also be employed to monitor secondary structural changes in proteins in presence of lipid membranes [15]. One advantage of UVRR over CD and FTIR is that by tuning into (resonance) the aromatic absorption bands, one can obtain high sensitivity to Tyr and Trp residues and hence the ability to characterize site-specific environments and structure [15, 16].
2.2 Tertiary structure
Though solution-state NMR is a widely used protein structural determination technique, high concentrations of isotopically labeled reconstituted protein is needed and the membrane bound protein must isotropically reorient on NMR relevant time scales. The latter point has particularly limited the use of solution-state NMR in the structural determination of membrane proteins because protein resonances simply disappear upon association with the comparatively large phospholipid vesicles [8]. However, this NMR signal disappearance can be useful for identifying residues that are membrane bound [17]. Alternately, by using small membrane mimics such as micelles, solution-state NMR has been quite successful in producing structural models [18, 19].
To address the problem of slow protein reorientation at the membrane interface, oriented and magic angle spinning solid-state NMR methods can be employed. In oriented solid-state NMR, membranes are aligned such that anisotropy inherent in chemical shifts, quadrupolar interactions, and dipolar couplings can be used to extract information regarding the tilt and pitch of helices interacting with membranes [7, 20]. When alignment of the membrane is not feasible, magic angle spinning solid-state NMR is particularly useful. In this technique, the sample spins at an angle with respect to the static magnetic field in such a way that signals are significantly enhanced [20, 21]. Using magic angle solid-state NMR, structural information on membrane-bound proteins can be extracted including distance and backbone dihedral angle restraints.
Distance constraints between specific residues in soluble or membrane bound proteins can also be determined using Förster resonance energy transfer (FRET) and double electron electron resonance (DEER). In FRET, the energy transfer between fluorescent donor (D) and energy acceptor (A) molecules can be determined since the energy transfer rate, or efficiency, depends on the inverse sixth power of the distance between the D and A [22–25]. By monitoring fluorescence from proteins containing D and A molecules in the presence and absence of membranes, changes in pairwise distances can be extracted [26, 27]. In DEER, the coupling between two residues tagged with electron spin labels (typically between nitroxide spin labels) can be measured and correlated to distance [28].
2.3 Probes of the membrane-protein interface
Several fluorescence methods can be utilized to probe site-specific protein interactions with the membrane. Since many intrinsic and extrinsic fluorophores are highly sensitive to local environment, changes in fluorescence features (e.g. mean wavelength and quantum yield (QY)) can indicate membrane binding. For example, upon interaction with the hydrophobic membrane core, tryptophan fluorescence can shift by as much as 25 – 28 nm [29, 30]. In addition to membrane binding, differences in QY also can reflect interactions with specific charged lipid headgroups or amino acid (aa) side chains [31]. Moreover, because QY is greatly reduced in the presence of heavy-atom quenchers such as iodide or bromine, site-specific solvent/membrane exposure can be determined [32–35]. Using fluorescence quenching, hydrophobic α-helical regions for several transmembrane peptides have been identified [36].
Similar determination of site-specific solvent or lipid exposure can be accomplished using electron paramagnetic resonance (EPR). In EPR, the interaction of unpaired electron spins in an external magnetic field is monitored. Though most systems do not contain unpaired electrons, EPR spin labels can be incorporated to determine their molecular environments. For example, EPR signals can distinguish between a spin label in the O2 rich membrane, or the aqueous phase by using an extrinsic probe such as nickel ethylenediamine-N-N′-diacetic acid (NiEDDA) [37].
Fluorescence anisotropy is another method informing on site-specific environments and membrane association. In fluorescence anisotropy, the extent of reorientation of the fluorophore between polarized excitation and emission planes is measured. Since the degree of reorientation of the fluorescence emission depends the rotational diffusion of the fluorophore, information regarding the size and shape of the diffusing molecule and the solvent viscosity can be extracted [38]. Anisotropy measurements are particularly useful in the study of membrane-associating proteins, because both solvent viscosity and size change upon membrane binding. Furthermore, membrane-induced protein oligomerization and aggregation can be monitored using this technique.
One promising emerging technique for the study of protein-membrane interactions is neutron reflectometry [39–41]. In this technique, neutrons impinge on a horizontally oriented sample at small angles (< 5°) and scattered neutrons are measured. Using neutron reflectometry, the structure of deposited materials up to several hundred nm from the surface normal can be measured with angstrom level resolution [42]. The recent development of a solid-supported sparsely-tethered bilayer lipid membrane (stBLM) for use in neutron reflectometry has enabled simultaneous measurement of both membrane properties (e.g. membrane thickness) as well membrane-bound protein structure [43]. Neutron reflectometry and a stBLM have recently been employed to study the effect of amyloid β oligomers on membrane properties [41] and the structure of functional Staphylococcus aureus α-hemolysin channels [40].
3. α-Synuclein is a membrane protein, implicated in Parkinson’s disease
The name synuclein (syn) was first given in 1988 to a novel 143 aa protein isolated from the presynaptic terminals and nuclei of the nervous tissues of the Pacific electric ray, Torpedo californica [44]. Two years later, a similar 134 aa phosphorylated protein was discovered in bovine brain neuron synapses; however, it was named phosphoneuroprotein 14 [45, 46]. In subsequent independent work, a hydrophobic 35 aa sequence (termed non-amyloid β component; NAC) was identified in Alzheimer’s disease amyloid plaques whose 140 aa precursor protein was coined NACP, or non-amyloid β component precursor protein [47]. Yet another group discovered a homolog of NACP in zebra finch and called it synelfin [48].
Finally, it became clear that synelfin, synuclein, and NACP were homologous proteins and thus, designated as α-syn. The human homolog of phosphoneuroprotein 14 was called β-syn [49]. Subsequently, yet another member of the synuclein family, γ-syn, was identified in breast cancer tissue [50].
Though the synucleins (α, β, and γ) are highly homologous [51], and likely share similar biological functions in vivo, it is α-syn that has gained widespread interest due to its involvement in PD etiology. PD is a prevalent age-related neurodegenerative disease. The pathological hallmarks include cell death in the substantia nigra and the presence of intracellular brain inclusions, or Lewy bodies, which are comprised primarily of α-syn amyloids [5]. Further implicating α-syn in PD are genetic findings that link early-onset PD to gene triplication [52] and duplication [53] and three missense mutations (A30P, E46K, and A53T) [54–56]. Other data showing that α-syn concentration increases in nigral brain regions as a function of age [57] and recent work demonstrating that triple α-, β-, and γ-syn knockout mice exhibit age-dependent neuronal dysfunction provide additional support for the tight connection between α-syn and PD [58].
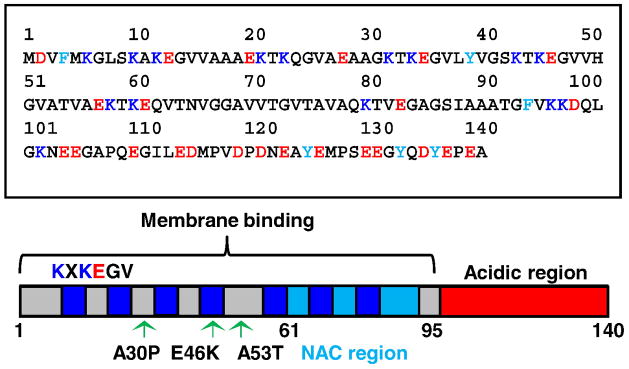
α-Syn primary structure has several distinct characteristics. One dominating feature is a series of seven imperfect amphipathic 11 aa repeats (consensus sequence KXKEGV, Fig. 2) found in the first 89 N-terminal residues. These repeats, which contain charged residues flanked by paired lysine residues at the apolar-polar interface (class A2 helix) [59] are also motifs found in the exchangeable apolipoproteins AII, CI, CII, and CIII [60, 61]. The central region (residues 61 – 95), or NAC region, is composed of nonpolar side-chains and has been identified by numerous groups to play an important role in α-syn aggregation and toxicity. Specifically, it was shown that deletion of specific residues within the NAC region abolishes α-syn aggregation [62, 63], that β-syn which does not contain residues 74 – 84 of α-syn does not aggregate [64], and that peptides derived from the NAC region can form β-sheet fibrils [65, 66]. It also has been shown that isolated NAC peptides are toxic to cells [62, 67] and that deletion of NAC residues in a transgenic Drosophila model abrogates both α-syn aggregation and cell toxicity [68]. Lastly, the C-terminal domain of α-syn is highly acidic (containing 15 carboxylates).
Fig. 2.
(Top) α-Synuclein primary amino acid sequence with aromatic (light blue), acidic (red), and lysine (blue) residues highlighted. (Bottom) Schematic representation of α-synuclein with amphipathic repeats, non-amyloid β component (NAC) region, membrane binding domain, and acidic region in blue, light blue, gray, and red, respectively. The location of the amphiphatic repeats, the disease-related mutations, and the membrane binding domain also are denoted.
In addition to evidence from primary structure, mounting work points toward either the direct interaction of α-syn with cellular membranes and/or other membrane binding proteins in vivo. The involvement of α-syn in membrane related cellular function, specifically in coordinating nuclear and synaptic events, was first predicted based on the localization of synuclein from the Torpedo californica [44]. Studies on canary α-syn further demonstrated a connection between α-syn expression and synaptic plasticity during learning [48]. Direct evidence for localization of α-syn near synaptic vesicles was later achieved via immunogold labeling of α-syn and visualization by electron microscopy [69].
Though α-syn knockout mice do not exhibit noticeable phenotypic changes as compared to wild type (WT) mice [70, 71], differences in cells cultured from knockouts [72], in the level of dopamine release [70, 71, 73], and in the quantity of the synaptic vesicle reserve pool [74, 75], point toward a role of α-syn in synaptic vesicle trafficking. More recently, it was shown that α-syn is required for the maintenance of continuous presynaptic SNARE (sensitive factor attachment protein receptor) complex assembly, a complex required for neurotransmission [76].
A correlation between mitochondrial dysfunction and PD [77] has prompted significant interest in α-syn-mitochondrial membrane interaction as well. For example, it has been proposed that intracellular acidification resulting from oxidative or metabolic stress can induce translocation of α-syn from the cytosol to the surface of the mitochondria [78]. It also has been shown using FRET-based reporters, that the conformation of α-syn is altered in the presence of mitochondrial membranes [79].
4. α-Synuclein interacts with model membranes
The finding that α-syn associates with membranous compartments in cultured cells and brain tissues [48, 80–82] has prompted substantial research aimed at achieving a molecular level understanding of α-syn interactions with model membranes. In this section, we provide an overview of commonly used membrane mimics and physiologically relevant lipids as well as a discussion of biophysical and biochemical evidence regarding the effects of specific lipid headgroups, acyl chain structure, and curvature on α-syn-membrane interactions.
4.1 Overview of membrane mimics and physiologically relevant phospholipids
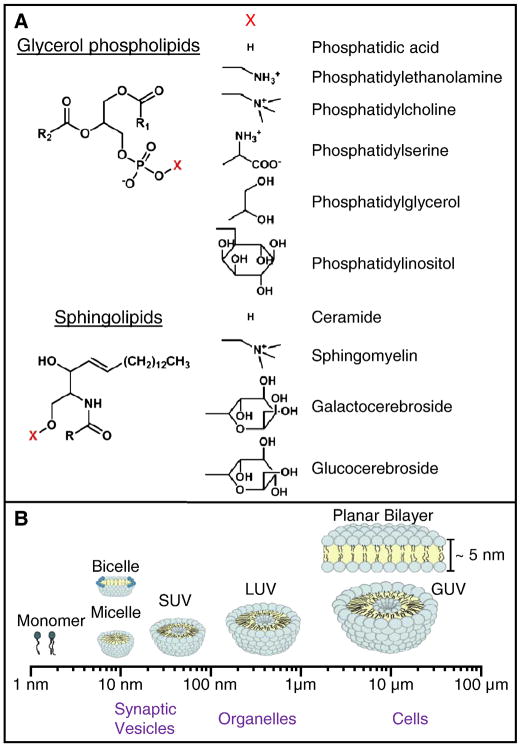
Various membrane mimics are utilized in biophysical studies of membrane proteins in vitro. Lipid molecules, composed of hydrophilic headgroups and hydrophobic, fatty acid tails, are frequently used (Fig. 3A). In an aqueous environment, the amphipathic character of lipids causes them to self assemble and sequester their hydrophobic cores. Such assemblies are predominantly dependent on the molecular shape and chemical composition. For example, lysolipids which have one acyl chain per headgroup form micelles, whereas phospholipids with two acyl chains form planar bilayers or liposome vesicles.
Fig. 3.
Lipid and membrane mimic structure. (A) Chemical structure of the glycerol phospholipid and sphingolipid backbone with different headgroups (X) and their corresponding names indicated. The R1, R2, and R designate fatty acid chains. (B) Schematic of the approximate size and organization of membrane mimics commonly used in biophysical research. Corresponding cellular compartments are provided for relative size reference.
A vesicle is a spherical structure made of two adjacent layers of lipid molecules in which the tails of each layer point toward each other and headgroups face either the water-filled interior or outside environment (Fig. 3B). Similarly, a micelle is a spherical structure in which hydrophilic headgroups are in contact with the aqueous surrounding (Fig. 3B). However, unlike a vesicle, a micelle is comprised of a single layer of molecules with all hydrophobic tails pointing toward the center of the micelle.
Typically, lipid vesicles (liposomes) are multilamellar (multilayer) until the application of external mechanical forces (e.g. sonication or vortexing) which facilitate the formation of unilamellar vesicles. These vesicles, according to their sizes, are classified into small, large, and giant unilamellar vesicles (SUVs: 10 – 100 nm, LUVs: 100 nm – 1 μm, and GUVs, > 1 μm, Fig. 3B). Unilamellar vesicles are excellent alternatives to biomembranes as they are easy to manipulate and can be made of defined chemical composition and relative sizes. They are good model systems for small trafficking vesicles (SUVs) to cellular membranes (GUVs). Additionally, since vesicle sizes determine curvature and protein accessible surface area, these properties, which are likely important in protein structure and function, can be investigated in detail.
To gain insights into how α-syn interacts with biomembranes, in vitro experiments employing membrane mimics that contain biologically relevant molecules are of great value (Fig. 3A). Among the most common membrane molecules in vivo include phospholipids, cholesterol, and sphingolipids. For example, synaptic vesicle membranes [83–86] contain phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingomyelin (SM), plasmenylethanolamine, phosphatidylserine (PS), and phosphatidylinositol (PI) (molar percent of total phospholipid: 40.9, 24.6, 12, 11.5, 7.3, and 3.7, respectively [84]) as well as phosphatidic acid (PA) (0–2%) [87]. Synaptic vesicles also contain high levels of cholesterol (38.5% of total lipid content) [84]. Notably, the inner and outer leaflets of synaptic vesicles are asymmetric in phospholipid composition with PI almost found exclusively in the inner leaflet [84]. Lipid composition asymmetry is common for biomembranes. For example, PC and SM are found primarily in the outer leaflet whereas PE and anionic phospholipids such as PA, phosphatidylglycerol (PG), and PS as well as PI and phosphoinositides (PIPs) mainly comprise the inner leaflet of the plasma membrane [88].
4.2 Effects of lipid headgroup and chain structure
The first in vitro evidence that α-syn has preferential binding affinity for different lipid headgroups was reported in 1998 when it was observed that α-syn binds to SUVs or LUVs containing anionic but not zwitterionic lipids [89]. This initial work prompted vigorous ongoing research efforts on the effects of phospholipids on α-syn membrane binding properties [90–94].
Generally, it is agreed that this binding preference for a negatively charged surface is attributable to the electrostatic attraction from multiple lysines found in the N-terminal region of α-syn (Fig. 2). However, even under high ionic strength solution conditions (up to 500 mM), substantial helical structure is maintained suggesting that while electrostatics play an important role in mediating α-syn-membrane binding, they are not the sole driving force [89]. One explanation is that the use of small headgroups may provide more space to accommodate protein insertion [91, 92, 95].
While acidic headgroups are preferred, the composition of zwitterionic lipids can also influence membrane affinity. Using a variety of techniques including fluorescence correlation spectroscopy (FCS) and CD spectroscopy, it has been shown that PE enhances α-syn membrane interaction [90] and that α-syn favors PA and PI over PS and PG [90–94]. In addition to vesicles containing anionic lipids, α-syn also can bind to lipid droplets [96] and SDS micelles [26, 97–99].
It also has been shown that α-syn interacts with gangliosides (GMs). GMs are glycosphingolipids composed of a ceramide backbone and one or more sugars as the headgroup. Using atomic force and confocal microscopies as well as CD spectroscopy and molecular simulations, a number of GMs including GM1, GM2, and GM3 have been shown to colocalize and/or interact with α-syn [100–102]. The specificity has been rationalized by the formation of a hydrogen-bonding network between the sugar alcohols and α-syn side chains [101].
The lipid chain structure can influence α-syn membrane binding as well [91, 93, 103]. Specifically, polyunsaturated chains are favorable for α-syn-membrane binding [104, 105]. A possible mechanism is that the highly disordered polyunsaturated chains cause a loose packing of membrane lipids which can lead to packing defects and thus more space for α-syn to embed into the bilayer.
Another important lipid component is cholesterol, the major fluidity modulator of biomembranes [106]. Cholesterol, along with other molecules such as GM1 [107], are proposed to be enriched in lipid rafts, liquid-ordered membrane domains that are suggested to be involved in numerous cellular processes [108–110]. Notably, the existence of lipid rafts in vivo is highly debated given that their visualization in living cells is challenging [107]. In HeLa cells, α-syn colocalized with GM1 and PI(4,5)P2 suggesting that the protein may associate specifically with lipid rafts [100]. Interestingly, this synaptic enrichment was observed only for WT and A53T but not the A30P mutant.
In contrast, a confocal laser scanning microscopic study employing GUVs showed that α-syn binds to anionic lipids in the liquid-disordered phase instead of lipid rafts [111]. This result highlights the fact that α-syn enrichment in lipid rafts remains highly controversial. Currently, it is not clear whether α-syn simply prefers the liquid-ordered raft environment or the particular raft components, such as GM1 or GPI-anchored proteins. Nevertheless, since polyunsaturated acyl chains and fatty acids of phospholipids enhance α-syn-membrane binding and alter raft packing phase [112, 113], it is possible that the association of α-syn with rafts can be involved in regulation of the subsequent cellular processes. Moreover, because PD-related, A30P mutant behaved distinctly, it is clear that further investigations are warranted and needed.
4.3 Effects of membrane curvature
Initial α-syn studies showed a binding preference for SUVs over LUVs [89], indicating that surface curvature may modulate binding affinity. Subsequent FCS, site-specific fluorescence, isothermal titration calorimetry, and gel-filtration studies show that indeed the membrane binding affinity of α-syn is dependent on vesicle size (curvature) [91, 93, 103, 114]. Nevertheless, α-syn can still bind to anionic LUVs [115, 116] and even planar, supported bilayers [117]. Elucidating the mechanism of how α-syn senses membrane curvature is likely pertinent to not only its physiology but also to its pathology.
In general, it is thought that at least three distinct steps are involved in how amphipathic helices are formed at the membrane interface [118–120]. Through long range electrostatic interactions, polypeptide binding is initiated, followed by local rearrangement and insertion of hydrophobic side chains, and finally α-helix folding. Notably, there is evidence that bilayer defects are present in small vesicles with high curvatures [121]. Thus, it is plausible that the α-syn insertion process could be enhanced due to the presence of packing defects in small highly curved vesicles [122].
Alternately, the binding features of α-syn may ultimately reflect its conformational ability to adapt to the chemistry of the membranes. For example, α-syn also appears to be sensitive to lipid geometry. Specifically, lipids with a large polar headgroup and saturated acyl chains may limit binding while lipids with a small headgroup (e.g. PA) and unsaturated acyl chains may promote interaction. Therefore, it is most likely that it is the combined effects of the packing geometry of lipid headgroups and acyl chains that contribute to the membrane binding affinity of α-syn.
5. Structural studies of membrane-bound α-synuclein
α-Syn was first hypothesized to interact with lipid membranes based on aa sequence and demonstrated by CD spectroscopy to adopt an α-helical structure upon binding to both SUVs and LUVs [89]. Soon after, NMR spectroscopy was employed by Eliezer and coworkers to investigate its lipid-associated conformation [97]. Using SDS micelle and synthetic phospholipid vesicles composed of POPC:POPA, it was determined that the first 100 residues interact with the membrane surface, whereas the C-terminal 40 residues do not. This helical conformation propensity was further realized to be inherent, albeit modest (~10%) in the N-terminal region spanning residues 6 to 37 even in its soluble, unfolded state [123]. In this section, we review subsequent biophysical studies utilizing various membrane mimics that have contributed to our current structural understanding of membrane bound α-syn.
5.1 A broken 11/3 α-helix
By comparison to apolipoproteins, a structural model consisting of five amphipathic α-helices was proposed by Davidson et al. for membrane bound α-syn [89]. Specifically, residues 1–60 form the first four helices (1–15, 17–37, 39–48, and 50–60) and are characterized by basic residues, Lys, at the bilayer interface as well as the presence of glutamates and nonpolar, hydrophobic side chains at the polar and nonpolar face, respectively. In contrast, for helix 5 (residues 61–93) a distinct charge distribution is not apparent and hence, it is classified as a class G* helix [124]. However, using SDS micelles and NMR spectroscopy, Eliezer and coworkers found contradictory evidence and concluded the presence of only two helical regions with a short linker [125].
In this micelle-bound model, α-syn adopts two helical regions from residues 1–94 with a break from residues Lys42 and Thr44 based on Cα chemical shift and sequential amide proton Nuclear Overhauser Effect data (referred to as the broken α-helix model) [125]. This folded sequence, residues 1–103, was corroborated by limited proteolysis analysis by mass spectrometry and N-terminal sequencing. Because a well-defined hydrophobic surface could not be generated using an ideal helix configuration and due to the presence of amphipathic repeats in α-syn (Fig. 2), an 11/3 α-helix conformation was proposed as in the case of apolipoprotein AI.
In an 11/3 α-helix, there are three full turns over 11 residues (3.6 6̄ residues per turn) compared to five full turns over 18 residues in an ideal helix (3.6 residues per turn) [125]. The break was hypothesized to play a role in allowing the protein to bind to a range of surface curvatures. It was suggested further that α-syn may indeed adopt a single helical conformation upon binding a less curved surface such as a vesicle.
5.2 NMR structure of a micelle-bound α-synuclein
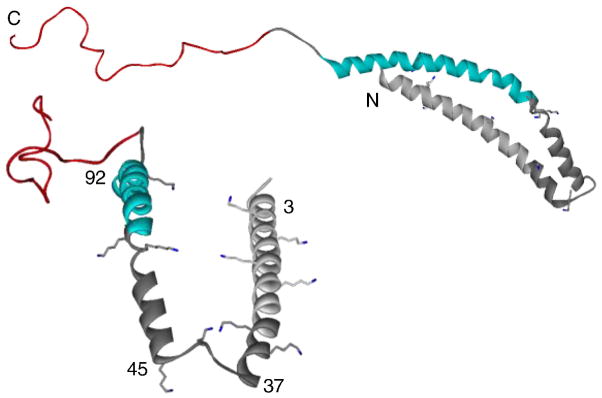
Definitive structural evidence for the formation of a broken helix upon α-syn-micelle interactions was determined by NMR spectroscopy and reported in 2005 by Ulmer et al. [126]. Although generally consistent with prior work, there were minor deviations in the helical regions; only residues 3–92 are considered to be in a helical conformation with residues 38–44 as the linker. Using residual dipolar couplings and Mn2+-induced paramagnetic relaxation enhancements labeled at residue 87, the micelle-bound structure consisted of two, anti-parallel helices, Val3-Val37 and Lys45-Thr92, with a short, 7-residue linker in between, followed by an extended region, Gly93-Lys97, and a disordered C-terminal tail, Asp98-Ala140 (Fig. 4). The interhelical distances vary and these dynamics occur on the microsecond timescale highly suggestive of global conformational flexibility on the micelle surface.
Fig. 4.
Side and top view of micelle-bound α-synuclein (PDB ID: 1XQ8). The membrane binding domain (gray), non-amyloid β component region, and acidic region are colored in gray, light blue, and red, respectively. Lysine residues found within the amphipathic repeats are shown.
In this structure [126], both the N- and C-terminal helices have structural parameters closer to an ideal helix (3.6 and 3.56 residues per turn, respectively) then the 11/3 α-helix previously proposed by Eliezer and coworkers [125]. As highlighted in Fig. 4, Lys side chains in the repeat region point sideward from the helices which allow favorable interactions with the acidic sulfate groups on the SDS micelle. Notably, the helices appear to wrap themselves around the micelle with distinct average circle diameters that substantially deviate (153 and 82 Å, respectively) from the expected value of a SDS micelle (46 Å). This observation implies that α-syn may prefer to form a less curved helix and hence, upon binding, it deforms the micelle.
The structural effects of disease-related mutations, A30P and A53T [127] as well as E46K [128] on SDS binding also have been investigated by solution NMR spectroscopy. For both E46K and A53T variants, local resonance perturbations near the mutations are observed. While the A53T-micelle structure was determined to be indistinguishable from the WT protein [127], the structural differences between micelle-bound E46K and WT remain ill-defined [128]. In contrast, the replacement of Ala for Pro at residue 30 significantly alters the micelle-polypeptide interaction with an interruption of one helix turn (Val26-Ala29), disruption of another (Thr22-Gly25), and creation of a helix register shift for at least two turns. Nevertheless, despite the substantial structural rearrangement, overall the SDS binding property is maintained; for example, the number of residues per turn is similar as well as the radii of curvature compared to the WT conformation [127].
5.3 Broken vs. extended helix
The use of micelles as a model membrane system has been particularly useful for structural determination and biophysical studies [26, 98, 125–127, 129–131]. Nevertheless, they are quite different from biological membranes. Since the aforementioned work, ongoing investigations using other membrane mimics and techniques that provide distance information such as DEER [129, 132–136] and FRET [26, 27, 116] give additional structural insights into α-syn-membrane interactions. Specifically, research from various groups provides evidence that upon membrane binding α-syn can adopt an alternative conformation, an extended [27, 116, 134, 135, 137], rather than a broken helix [129, 132]. Furthermore, given the dynamical nature of this protein to undergo large conformational rearrangements [138–140], it is even more likely that these membrane-associated structures are not mutually exclusive and can interchange [133, 136].
The first experimental evidence for the existence of an extended helix was reported by Langen and coworkers using site-directed spin labeling and continuous wave EPR spectroscopy [137]. By analyzing 47 singly labeled α-syn variants and their relative accessibilities to O2 and NiEDDA, secondary structure and topology of residues 59–90 were determined. Interestingly, the periodicity extracted yielded a value of 3.67 residues per turn, supporting an 11/3 α-helix which was favored by Eliezer and coworkers [125] and later ruled out based on the SDS-micelle bound structure [126]. Subsequent DEER experiments from 17 pairs and computational modeling [135] confirmed that this helical behavior is preserved from residues 9–89.
While there is a general consensus that membrane-bound α-syn adopts an amphipathic α-helical structure involving approximately the first 100 residues, debate remains regarding the exact nature of the interaction sites and arrangements of the helix. However, it is likely that these differences can be accounted for by the fact that various membrane mimics (micelle, bicelle, and vesicles), detergent/lipid compositions, and solution conditions (pH, salt, and temperature) were employed. Perhaps even more important than the aforementioned factors, is that experiments are performed at different lipid-to-protein ratios, a factor which has been shown to affect α-syn membrane binding modes [17]. Though some questions remain, these results highlight the likely propensity for α-syn to undergo significant structural rearrangements under different solution conditions, an inherent trait which may be related to the function of α-syn in vivo.
5.4 Multiple binding modes and conformational heterogeneity
Based on the disappearance of solution NMR signals, it was shown that multiple distinct phospholipid binding modes exist for α-syn and that these modes can be tuned by changing the lipid-to-protein ratio [17]. Specifically, two distinct binding modes were characterized, SL1 and SL2, corresponding to the association of the first 25 and 97 residues, respectively. As the lipid-to-protein ratio was increased, a smooth progression was observed in the ratio of the SL1 to SL2 binding modes [17]. Evidence for the presence of multiple-membrane bound conformations also can be found in time-resolved tryptophan fluorescence data showing increased heterogeneity near the C-terminal site, W94, compared to that of the N-terminal probe, W4 [115]. The idea that different regions of α-syn can associate with the membrane under different solution conditions is further corroborated by a recent study of the role of the N-terminus in α-syn membrane binding [141]. In this study, it was found that different isolated regions of the α-syn sequence bind to membranes to different degrees and that secondary structural formation for each of these regions is dependent on lipid composition as well as other factors such as temperature [141].
6. Involvement of α-synuclein membrane interactions in Parkinson’s disease pathogenesis
Despite a general consensus regarding the participation of α-syn in PD [5, 142], intensive research efforts are still devoted to elucidating the role of α-syn-membrane interactions in pathogenesis. One link is that the presence of disease-related mutations modulates α-syn membrane binding in vitro [111, 114, 143–147]. Moreover, as PD is classified as a protein misfolding disease [148, 149], perhaps the most compelling biophysical evidence is that the presence of membranes affects α-syn secondary structure in vitro [89]. Upon membrane binding, changes in the protein folding landscape ensue such that the energy barriers inhibiting the formation of potentially toxic, oligomeric or β-sheet structures are lowered. Another mechanism, supported by mounting biophysical data, is that α-syn membrane-binding results in membrane instability and/or permeabilization, both of which could impact various in vivo processes.
6.1 Effect of disease-related mutations on membrane binding
Disparities in the membrane binding properties of WT and PD-related mutant α-synucleins were first noted in 1998 when it was reported that WT and A53T bound to rat brain vesicles whereas A30P did not [145]. Although to varying degrees, subsequent studies revealed further differences between the membrane affinity of WT and A30P. In 2000, Perrin et al. showed using site directed mutagenesis and CD spectroscopy that the familial mutants had little (A30P) or no (A53T) effect on lipid binding or α-helicity [147]. In contrast, using ultracentrifugation, CD spectroscopy, and low-angle X-ray diffraction, Jo et al. found that A30P was defective in binding while A53T almost showed a comparable binding ability to WT α-syn [146]. Bussell et al. later confirmed that A30P, but not A53T, decreased lipid binding affinity [143].
Though some inconsistencies between the relative effects of the A30P and A53T mutation on membrane binding subsist, it is likely that these differences can be accounted for by the fact that experiments were performed using model membranes of different composition and size and under different solution conditions (vide supra). For example, in an isothermal calorimetry study, it was shown that A30P shared a similar binding affinity to WT in a gel phase (e.g. DPPC) whereas binding was weaker in the liquid crystalline phase (e.g. DOPC/DOPG) as compared to WT [114]. Moreover, it was shown using anionic GUVs that the A30P protein bound less efficiently compared to WT α-syn [111].
Though less research has been conducted on the effects of the E46K mutant due to its more recent discovery [56], unlike A30P, the presence of E46K generally increases α-syn membrane affinity [111, 144]. This increased affinity is likely due to the introduction of an additional positive charge which enhances electrostatic forces. Whether the membrane affinity is increased (E46K) or decreased (A30P), it is clear that under certain solution conditions the presence of mutations can modulate the equilibrium between solution and membrane-bound conformations.
6.2 Membrane mediated α-synuclein oligomer formation and aggregation
It is unclear whether the β-sheet structure found in the Lewy bodies of PD patients are protective or toxic to cells in the body; however, substantial evidence show that the presence of membranes or membrane mimics can modulate the formation of β-sheets in vitro [150–152]. One possibility is that the membrane surface facilitates a local increase in α-syn concentration and therefore, stimulates aggregation [18, 153]. This reasoning is attractive because it is known that increased expression of α-syn caused by gene triplication or duplication leads to early onset PD [52, 53].
Notably, α-syn aggregation kinetics are highly dependent on the ratio of protein-to-membrane mimic in solution [151, 152]. This observation is consistent with another possibility that membrane binding induces the formation of toxic or aggregation-prone oligomers. This idea first was discussed in the context of CD data showing that α-syn aggregation rate is correlated to the level of α-helicity (partial and saturating helicity correspond to accelerated or slowed aggregation, respectively) [151, 152]. This suggestion that membranes promote oligomer formation is supported by other studies [154]. For example, EPR data on SUVs bound α-syn, reveal a membrane induced dimeric structure [155]. In another recent report, a trifluoroethanol (TFE) induced partially helical monomeric species of α-syn was characterized and associated with enhanced fibril formation [156]. Interestingly, though disease-related mutants (A30P, E46K, A53T) exhibited similar TFE-induced secondary structure, oligomerization rates differed substantially as compared to WT protein, bolstering the connection between membranes and PD pathogenesis [156].
Substantial experimental data also support a likely prominent role of membranes in modulating α-syn oligomerization and/or protein aggregation in vivo. In the presence of isolated brain fractions, α-syn was found to aggregate, whereas no aggregates were detected in the cytosolic fraction [157]. The addition of membrane-bound α-syn also was found to seed aggregation in the cytosolic fraction [157]. Moreover, chemical cross-linking experiments in cells treated with high concentrations of fatty acids show that α-syn forms dimers and trimers that preferentially associate with the phospholipid monolayers surrounding triglyceride-rich lipid droplets as well as other cellular membranes [96]. Interestingly, while the PD related mutant E46K also localized to the lipid droplets and membranes, A30P remained cytosolic.
6.3 α-Synuclein induced membrane perturbation
One mechanism through which amyloidogenic proteins can cause cellular dysfunction is by inducing instability in membranes [158–161]. In 2001, it was shown that protofibrillar α-syn can cause transient permeabilization in anionic membranes and thus alter the calcium flux into the cytosol, depolarization of mitochondrial membranes, and/or leakage of intra-vesicular neurotransmitter molecules, such as dopamine [162]. Notably, later it was shown that protofibril induced membrane leakage is increased when disease-related mutations are present (A30P and A53T) [163].
Numerous other studies have since confirmed that indeed various conformations of α-syn can cause membrane instability and even pore formation. For example, using atomic force microscopy and vesicle dye leakage assays, it was shown that membrane disruption is positively correlated to α-syn membrane affinity and that fibrillar, as well as oligomeric, α-syn enhances membrane permeability [152]. The presence of WT, E46K, and A53T protein can induce formation of ion channels in anionic SUVs, whereas A30P did not under similar solution conditions [164].
Van Rooijen et al. showed that the presence of anionic lipids and lipid-disordered domains affects the ability of α-syn to perturb membranes [94, 165], offering new insight into the specific conditions promoting membrane disruption. Subsequent study revealed that disruption of vesicles is caused by non-equilibrium processes and despite rapid dye efflux from vesicles, membrane morphology is maintained in the presence of oligomeric α-syn [166].
Taking advantage of solid-state NMR and EPR methods, it has been determined that α-syn pores are comprised of β-sheet structures unique from those found in amyloid fibrils [167]. Moreover, a low resolution wreath shaped α-syn oligomer was identified by x-ray scattering methods and was shown to disrupt liposomes [168].
Though the specific mechanism of membrane disruption is yet to be determined, several recent reports have demonstrated that α-syn also can transform membrane surface topology. This phenomenon was brought to light when it was found that the addition of α-syn to SUVs induced tubular structures as well as multilamellar and branched vesicles [17, 168]. Further work showed that α-syn can increase membrane curvature causing smaller vesicles and tubules to form [169]. Accordingly, fluorescence microscopic studies on supported lipid bilayers also reveal tubule formation as well as a correlation between increased tubulation and the presence of anionic lipids and disease-related mutations [170].
7. Concluding Remarks
The finding that α-syn membrane interactions are linked not only to its biological function but also to its role in the etiology of PD points to the importance of development and application of biophysical approaches to determine protein conformation and dynamics in the presence of biomembranes. Though significant advancements in our understanding of the α-syn-membrane interface have been made, a crucial question is whether and how interactions with a phospholipid membrane surface can conformationally alter (misfold) α-syn and thus promote protein oligomerization. Furthermore, it is still debatable if and how membrane induced misfolded structures are directly involved in the pathogenic mechanism of PD. A partial answer may be found from an increased understanding of the protein-membrane interface provided us through advancements in solid-state NMR and neutron reflectometry techniques.
Numerous studies emphasize that even upon membrane binding, the helical α-syn conformers remain a dynamical polypeptide ensemble that is highly sensitive to specific solution conditions. Importantly, modifications of solution conditions, phospholipid compositions, and aa sequence do not necessarily produce unique conformational changes. These observations underscore the significance of biophysical techniques that can provide insights into the properties of dynamic and heterogeneous ensemble of α-syn polypeptides in solution, membrane-associated, and finally, in its amyloid form.
Acknowledgments
This work was supported by the Intramural Research Program at the National Institutes of Health, National Heart, Lung, and Blood Institute.
Reference List
- 1.Almen MS, Nordstrom KJV, Fredriksson R, Schioth HB. Mapping the human membrane proteome: A majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 2009;7:14. doi: 10.1186/1741-7007-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahram M, Litou ZI, Fang R, Al-Tawallbeh G. Estimation of membrane proteins in the human proteome. In Silico Biol. 2006;6:379–386. [PubMed] [Google Scholar]
- 3.Bhardwaj N, Stahelin RV, Langlois RE, Cho WW, Lu H. Structural bioinformatics prediction of membrane-binding proteins. J Mol Biol. 2006;359:486–495. doi: 10.1016/j.jmb.2006.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle DA, Shipley GG. Membranes: Editorial overview. Curr Opin Struct Biol. 2009;19:369–371. doi: 10.1016/j.sbi.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 5.Lees A, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 6.White SH. The progress of membrane protein structure determination. Protein Sci. 2004;13:1948–1949. doi: 10.1110/ps.04712004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Opella SJ, Marassi FM. Structure determination of membrane proteins by NMR spectroscopy. Chem Rev. 2004;104:3587–3606. doi: 10.1021/cr0304121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang GS. NMR of membrane-associated peptides and proteins. Curr Protein Pept Sci. 2008;9:50–69. doi: 10.2174/138920308783565714. [DOI] [PubMed] [Google Scholar]
- 9.Raunser S, Walz T. Electron crystallography as a technique to study the structure on membrane proteins in a lipidic environment. Ann Rev Biophys. 2009;38:89–105. doi: 10.1146/annurev.biophys.050708.133649. [DOI] [PubMed] [Google Scholar]
- 10.Werten PJL, Remigy HW, de Groot BL, Fotiadis D, Philippsen A, Stahlberg H, Grubmuller H, Engel A. Progress in the analysis of membrane protein structure and function. FEBS Lett. 2002;529:65–72. doi: 10.1016/s0014-5793(02)03290-8. [DOI] [PubMed] [Google Scholar]
- 11.Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1:2876–2890. doi: 10.1038/nprot.2006.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sreerama N, Woody RW. Computation and analysis of protein circular dichroism spectra. Methods Enzymol. 2004;383:318–351. doi: 10.1016/S0076-6879(04)83013-1. [DOI] [PubMed] [Google Scholar]
- 13.Byler DM, Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986;25:469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- 14.Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin. 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 15.Shafaat HS, Sanchez KM, Neary TJ, Kim JE. Ultraviolet resonance Raman spectroscopy of a β-sheet peptide: A model for membrane protein folding. J Raman Spectrosc. 2009;40:1060–1064. [Google Scholar]
- 16.Sanchez KM, Kang GP, Wu BJ, Kim JE. Tryptophan-lipid interactions in membrane protein folding probed by ultraviolet resonance Raman and fluorescence spectroscopy. Biophys J. 2011;100:2121–2130. doi: 10.1016/j.bpj.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodner C, Dobson C, Bax A. Multiple tight phospholipid-binding modes of α-synuclein revealed by solution NMR spectroscopy. J Mol Biol. 2009;390:775–790. doi: 10.1016/j.jmb.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aisenbrey C, Borowik T, Bystrom R, Bokvist M, Lindstrom F, Misiak H, Sani MA, Grobner G. How is protein aggregation in amyloidogenic diseases modulated by biological membranes? Eur Biophys J Biophys Lett. 2008;37:247–255. doi: 10.1007/s00249-007-0237-0. [DOI] [PubMed] [Google Scholar]
- 19.Beyer K. Mechanistic aspects of Parkinson’s disease: α-Synuclein and the biomembrane. Cell Biochem Biophys. 2007;47:285–299. doi: 10.1007/s12013-007-0014-9. [DOI] [PubMed] [Google Scholar]
- 20.Watts A, Straus SK, Grage SL, Kamihira M, Lam YH, Zhao X. Membrane protein structure determination using solid-state NMR. Methods Mol Biol. 2004;278:403–473. doi: 10.1385/1-59259-809-9:403. [DOI] [PubMed] [Google Scholar]
- 21.Tycko R. Solid-state nuclear magnetic resonance techniques for structural studies of amyloid fibrils. Methods Enzymol. 2001;339:390–413. doi: 10.1016/s0076-6879(01)39324-2. [DOI] [PubMed] [Google Scholar]
- 22.Beechem JM, Haas E. Simultaneous determination of intramolecular distance distributions and conformational dynamics by global analysis of energy-transfer measurements. Biophys J. 1989;55:1225–1236. doi: 10.1016/S0006-3495(89)82918-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eftink MR. Fluorescence techniques for studying protein-structure. Methods Biochem Anal. 1991;35:127–205. doi: 10.1002/9780470110560.ch3. [DOI] [PubMed] [Google Scholar]
- 24.Förster T. Zwischenmolekulare energiewanderung und fluoreszenz. Ann Phys. 1948;437:55–75. [Google Scholar]
- 25.Wu PG, Brand L. Resonance energy transfer: Methods and applications. Anal Biochem. 1994;218:1–13. doi: 10.1006/abio.1994.1134. [DOI] [PubMed] [Google Scholar]
- 26.Lee JC, Langen R, Hummel PA, Gray HB, Winkler JR. α-Synuclein structures from fluorescence energy-transfer kinetics: Implications for the role of the protein in Parkinson’s disease. Proc Natl Acad Sci U S A. 2004;101:16466–16471. doi: 10.1073/pnas.0407307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreon ACM, Gambin Y, Lemke EA, Deniz AA. Interplay of α-synuclein binding and conformational switching probed by single-molecule fluorescence. Proc Natl Acad Sci U S A. 2009;106:5645–5650. doi: 10.1073/pnas.0809232106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reginsson GW, Schiemann O. Pulsed electron-electron double resonance: Beyond nanometre distance measurements on biomacromolecules. Biochem J. 2011;434:353–363. doi: 10.1042/BJ20101871. [DOI] [PubMed] [Google Scholar]
- 29.Kim JE, Arjara G, Richards JH, Gray HB, Winkler JR. Probing folded and unfolded states of outer membrane protein A with steady-state and time-resolved tryptophan fluorescence. J Phys Chem B. 2006;110:17656–17662. doi: 10.1021/jp061991r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleinschmidt JH, den Blaauwen T, Driessen AJM, Tamm LK. Outer membrane protein A of Escherichia coli inserts and folds into lipid bilayers by a concerted mechanism. Biochemistry. 1999;38:5006–5016. doi: 10.1021/bi982465w. [DOI] [PubMed] [Google Scholar]
- 31.Callis PR, Liu TQ. Quantitative prediction of fluorescence quantum yields for tryptophan in proteins. J Phys Chem B. 2004;108:4248–4259. [Google Scholar]
- 32.Bolen EJ, Holloway PW. Quenching of tryptophan fluorescence by brominated phospholipid. Biochemistry. 1990;29:9638–9643. doi: 10.1021/bi00493a019. [DOI] [PubMed] [Google Scholar]
- 33.Kleinschmidt JH, Tamm LK. Time-resolved distance determination by tryptophan fluorescence quenching: Probing intermediates in membrane protein folding. Biochemistry. 1999;38:4996–5005. doi: 10.1021/bi9824644. [DOI] [PubMed] [Google Scholar]
- 34.London E, Feigenson GW. Fluorescence quenching in model membranes. 1. Characterization of quenching caused by a spin-labeled phospholipid. Biochemistry. 1981;20:1932–1938. doi: 10.1021/bi00510a032. [DOI] [PubMed] [Google Scholar]
- 35.Markello T, Zlotnick A, Everett J, Tennyson J, Holloway PW. Determination of the topography of cytochrome-b5 in lipid vesicles by fluorescence quenching. Biochemistry. 1985;24:2895–2901. doi: 10.1021/bi00333a012. [DOI] [PubMed] [Google Scholar]
- 36.Caputo GA, London E. Using a novel dual fluorescence quenching assay for measurement of tryptophan depth within lipid bilayers to determine hydrophobic alpha-helix locations within membranes. Biochemistry. 2003;42:3265–3274. doi: 10.1021/bi026696l. [DOI] [PubMed] [Google Scholar]
- 37.Hubbell WL, Gross A, Langen R, Lietzow MA. Recent advances in site-directed spin labeling of proteins. Curr Opin Struct Biol. 1998;8:649–656. doi: 10.1016/s0959-440x(98)80158-9. [DOI] [PubMed] [Google Scholar]
- 38.Lakowicz JR. Principles of fluorescence spectroscopy. Springer; New York: 2006. Protein fluorescence; pp. 529–575. [Google Scholar]
- 39.Krepkiy D, Mihailescu M, Freites JA, Schow EV, Worcester DL, Gawrisch K, Tobias DJ, White SH, Swartz KJ. Structure and hydration of membranes embedded with voltage-sensing domains. Nature. 2009;462:473–479. doi: 10.1038/nature08542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGillivray DJ, Valincius G, Heinrich F, Robertson JWF, Vanderah DJ, Febo-Ayala W, Ignatjev I, Losche M, Kasianowicz JJ. Structure of functional Staphylococcus aureus α-hemolysin channels in tethered bilayer lipid membranes. Biophys J. 2009;96:1547–1553. doi: 10.1016/j.bpj.2008.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valincius G, Heinrich F, Budvytyte R, Vanderah DJ, McGillivray DJ, Sokolov Y, Hall JE, Losche M. Soluble amyloid β-oligomers affect dielectric membrane properties by bilayer insertion and domain formation: Implications for cell toxicity. Biophys J. 2008;95:4845–4861. doi: 10.1529/biophysj.108.130997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lakey JH. Neutrons for biologists: A beginner’s guide, or why you should consider using neutrons. J R Soc Interface. 2009;6:S567–S573. doi: 10.1098/rsif.2009.0156.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGillivray DJ, Valincius G, Vanderah DJ, Febo-Ayala W, Woodward JT, Heinrich F, Kasianowicz JJ, Losche M. Molecular-scale structural and functional characterization of sparsely tethered bilayer lipid membranes. Biointerphases. 2007;2:21–33. doi: 10.1116/1.2709308. [DOI] [PubMed] [Google Scholar]
- 44.Maroteaux L, Campanelli JT, Scheller RH. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakajo S, Omata K, Aiuchi T, Shibayama T, Okahashi I, Ochiai H, Nakai Y, Nakaya K, Nakamura Y. Purification and characterization of a novel brain-specific 14-kDa protein. J Neurochem. 1990;55:2031–2038. doi: 10.1111/j.1471-4159.1990.tb05792.x. [DOI] [PubMed] [Google Scholar]
- 46.Nakajo S, Tsukada K, Omata K, Nakamura Y, Nakaya K. A new brain-specific 14-kDa protein is a phosphoprotein: Its complete amino acid sequence and evidence for phosphorylation. Eur J Biochem. 1993;217:1057–1063. doi: 10.1111/j.1432-1033.1993.tb18337.x. [DOI] [PubMed] [Google Scholar]
- 47.Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DAC, Kondo J, Ihara Y, Saitoh T. Molecular-cloning of cDNA-encoding an unrecognized component of amyloid in Alzheimer-disease. Proc Natl Acad Sci U S A. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 49.Jakes R, Spillantini MG, Goedert M. Identification of 2 distinct synucleins from human brain. FEBS Lett. 1994;345:27–32. doi: 10.1016/0014-5793(94)00395-5. [DOI] [PubMed] [Google Scholar]
- 50.Ji HJ, Liu YLE, Jia TL, Wang MS, Liu JW, Xiao GW, Joseph BK, Rosen C, Shi YE. Identification of a breast cancer-specific gene, BCSG1,by direct differential cDNA sequencing. Cancer Res. 1997;57:759–764. [PubMed] [Google Scholar]
- 51.George JM. The synucleins. Genome Biol. 2002;3:3002.1–3002.6. doi: 10.1186/gb-2001-3-1-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K. α-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841–841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 53.Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Levecque C, Larvor L, Andrieux J, Hulihan M, Waucquier N, Defebvre L, Amouyel P, Farrer M, Destee A. α-Synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 54.Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen JT, Schols L, Riess O. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nature Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 55.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, DiIorio G, Golbe LI, Nussbaum RL. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 56.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Tortosa EG, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 57.Chu YP, Kordower JH. Age-associated increases of α-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 58.Greten-Harrison B, Polydoro M, Morimoto-Tomita M, Diao L, Williams AM, Nie EH, Makani S, Tian N, Castillo PE, Buchman VL, Chandra SS. αβγ-Synuclein triple knockout mice reveal age-dependent neuronal dysfunction. Proc Natl Acad Sci U S A. 2010;107:19573–19578. doi: 10.1073/pnas.1005005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mishra VK, Palgunachari MN. Interaction of model class A1, class A2 and class Y amphipathic helical peptides with membranes. Biochemistry. 1996;35:11210–11220. doi: 10.1021/bi960760f. [DOI] [PubMed] [Google Scholar]
- 60.Clayton DF, George JM. The synucleins: A family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 61.Segrest JP, Jackson RL, Morriset JD, Gotto AM. Molecular theory of lipid-protein interactions in plasma lipoproteins. FEBS Lett. 1974;38:247–253. doi: 10.1016/0014-5793(74)80064-5. [DOI] [PubMed] [Google Scholar]
- 62.Bodles AM, Guthrie DJS, Greer B, Irvine GB. Identification of the region of non-Aβ component (NAC) of Alzheimer’s disease amyloid responsible for its aggregation and toxicity. J Neurochem. 2001;78:384–395. doi: 10.1046/j.1471-4159.2001.00408.x. [DOI] [PubMed] [Google Scholar]
- 63.Giasson BI, Murray IVJ, Trojanowski JQ, Lee VMY. A hydrophobic stretch of 12 amino acid residues in the middle of α-synuclein is essential for filament assembly. J Biol Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 64.Biere AL, Wood SJ, Wypych J, Steavenson S, Jiang YJ, Anafi D, Jacobsen FW, Jarosinski MA, Wu GM, Louis JC, Martin F, Narhi LO, Citron M. Parkinson’s disease-associated α-synuclein is more fibrillogenic than β- and γ-synuclein and cannot cross-seed its homologs. J Biol Chem. 2000;275:34574–34579. doi: 10.1074/jbc.M005514200. [DOI] [PubMed] [Google Scholar]
- 65.El-Agnaf OMA, Bodles AM, Guthrie DJS, Harriott P, Irvine GB. The N-terminal region of non-Aβ component of Alzheimer’s disease amyloid is responsible for its tendency to assume β-sheet and aggregate to form fibrils. Eur J Biochem. 1998;258:157–163. doi: 10.1046/j.1432-1327.1998.2580157.x. [DOI] [PubMed] [Google Scholar]
- 66.Han HY, Weinreb PH, Lansbury PT. The core Alzheimers peptide NAC forms amyloid fibrils which seed and are seeded by β-amyloid: Is NAC a common trigger or target in neurodegenerative disease. Chem Biol. 1995;2:163–169. doi: 10.1016/1074-5521(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 67.El-Agnaf OMA, Jakes R, Curran MD, Middleton D, Ingenito R, Bianchi E, Pessi A, Neill D, Wallace A. Aggregates from mutant and wild-type α-synuclein proteins and NAC peptide induce apoptotic cell death in human neuroblastoma cells by formation of β-sheet and amyloid-like filaments. FEBS Lett. 1998;440:71–75. doi: 10.1016/s0014-5793(98)01418-5. [DOI] [PubMed] [Google Scholar]
- 68.Periquet M, Fulga T, Myllykangas L, Schlossmacher MG, Feany MB. Aggregated α-synuclein mediates dopaminergic neurotoxicity in vivo. J Neurosci. 2007;27:3338–3346. doi: 10.1523/JNEUROSCI.0285-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clayton DF, George JM. Synucleins in synaptic plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:120–129. [PubMed] [Google Scholar]
- 70.Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JMG, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 71.Al-Wandi A, Ninkina N, Millership S, Williamson SJM, Jones PA, Buchman VL. Absence of α-synuclein affects dopamine metabolism and synaptic markers in the striatum of aging mice. Neurobiol Aging. 2010;31:796–804. doi: 10.1016/j.neurobiolaging.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, McIlwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J Neurosci. 2002;22:8797–8807. doi: 10.1523/JNEUROSCI.22-20-08797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yavich L, Tanila H, Vepsalainen S, Jakala P. Role of α-synuclein in presynaptic dopamine recruitment. J Neurosci. 2004;24:11165–11170. doi: 10.1523/JNEUROSCI.2559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V, Chaudhry FA, Edwards RH, Stefanis L, Sulzer D. α-Synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J Neurosci. 2006;26:11915–11922. doi: 10.1523/JNEUROSCI.3821-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH. Increased expression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron. 2010;65:66–79. doi: 10.1016/j.neuron.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC. α-Synuclein promotes SNARE-complex assembly in vivo and in vitro. Science. 2010;329:1663–1667. doi: 10.1126/science.1195227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vila M, Ramonet D, Perier C. Mitochondrial alterations in Parkinson’s disease: New clues. J Neurochem. 2008;107:317–328. doi: 10.1111/j.1471-4159.2008.05604.x. [DOI] [PubMed] [Google Scholar]
- 78.Cole NB, DiEuliis D, Leo P, Mitchell DC, Nussbaum RL. Mitochondrial translocation of α-synuclein is promoted by intracellular acidification. Exp Cell Res. 2008;314:2076–2089. doi: 10.1016/j.yexcr.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH. Optical reporters for the conformation of α-synuclein reveal a specific interaction with mitochondria. J Neurosci. 2008;28:12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Irizarry MC, Kim TW, McNamara M, Tanzi RE, George JM, Clayton DF, Hyman BT. Characterization of the precursor protein of the non-Aβ component of senile plaques (NACP) in the human central nervous system. J Neuropathol Exp Neurol. 1996;55:889–895. doi: 10.1097/00005072-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 81.McLean PJ, Kawamata H, Ribich S, Hyman BT. Membrane association and protein conformation of α-synuclein in intact neurons: Effect of Parkinson’s disease-linked mutations. J Biol Chem. 2000;275:8812–8816. doi: 10.1074/jbc.275.12.8812. [DOI] [PubMed] [Google Scholar]
- 82.Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E. Abnormal accumulation of NACP/α-synuclein in neurodegenerative disorders. Am J Pathol. 1998;152:367–372. [PMC free article] [PubMed] [Google Scholar]
- 83.Breckenridge WCMIG, Zanetta JP, Vincendon G. Adult rat brain synaptic vesicles II. Lipid composition. Biochim Biophys Acta. 1973;320:681–686. doi: 10.1016/0304-4165(73)90148-7. [DOI] [PubMed] [Google Scholar]
- 84.Daniel M, Michaelson GB, Barenholz Yechezkel. Asymmetry of lipid organization in cholinergic synaptic vesicle membranes. Biochem J. 1983;211:155–162. doi: 10.1042/bj2110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Deutsch JW, Kelly RB. Lipids of synaptic vesicles: Relevance to the mechanism of membrane-fusion. Biochemistry. 1981;20:378–385. doi: 10.1021/bi00505a024. [DOI] [PubMed] [Google Scholar]
- 86.Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, Muller SA, Rammner B, Grater F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmuller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 87.Nagy A, Baker RR, Morris SJ, Whittaker VP. Preparation and characterization of synaptic vesicles of high-purity. Brain Res. 1976;109:285–309. doi: 10.1016/0006-8993(76)90531-x. [DOI] [PubMed] [Google Scholar]
- 88.Kamp JAFOd. Lipid asymmetry in membranes. Annu Rev Biochem. 1979;48:47–71. doi: 10.1146/annurev.bi.48.070179.000403. [DOI] [PubMed] [Google Scholar]
- 89.Davidson WS, Jonas A, Clayton DF, George JM. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- 90.Jo EJ, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE. α-Synuclein membrane interactions and lipid specificity. J Biol Chem. 2000;275:34328–34334. doi: 10.1074/jbc.M004345200. [DOI] [PubMed] [Google Scholar]
- 91.Middleton ER, Rhoades E. Effects of curvature and composition on α-synuclein binding to lipid vesicles. Biophys J. 2010;99:2279–2288. doi: 10.1016/j.bpj.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rhoades E, Ramlall TF, Webb WW, Eliezer D. Quantification of α-synuclein binding to lipid vesicles using fluorescence correlation spectroscopy. Biophys J. 2006;90:4692–4700. doi: 10.1529/biophysj.105.079251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shvadchak VV, Falomir-Lockhart LJ, Yushchenko DA, Jovin TM. Specificity and kinetics of α-synuclein binding to model membranes determined with fluorescent excited state intramolecular proton transfer (ESIPT) probe. J Biol Chem. 2011;286:13023–13032. doi: 10.1074/jbc.M110.204776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Rooijen BD, Claessens M, Subramaniam V. Lipid bilayer disruption by oligomeric α-synuclein depends on bilayer charge, accessibility of the hydrophobic core. Biochim Biophys Acta, Biomembr. 2009;1788:1271–1278. doi: 10.1016/j.bbamem.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 95.Madine J, Doig AJ, Middleton DA. A study of the regional effects of α-synuclein on the organization and stability of phospholipid bilayers. Biochemistry. 2006;45:5783–5792. doi: 10.1021/bi052151q. [DOI] [PubMed] [Google Scholar]
- 96.Cole NB, Murphy DD, Grider T, Rueter S, Brasaemle D, Nussbaum RL. Lipid droplet binding and oligomerization properties of the Parkinson’s disease protein α-synuclein. J Biol Chem. 2002;277:6344–6352. doi: 10.1074/jbc.M108414200. [DOI] [PubMed] [Google Scholar]
- 97.Eliezer D, Kutluay E, Bussell R, Browne G. Conformational properties of α-synuclein in its free and lipid-associated states. J Mol Biol. 2001;307:1061–1073. doi: 10.1006/jmbi.2001.4538. [DOI] [PubMed] [Google Scholar]
- 98.Ferreon ACM, Deniz AA. α-Synuclein multistate folding thermodynamics: Implications for protein misfolding and aggregation. Biochemistry. 2007;46:4499–4509. doi: 10.1021/bi602461y. [DOI] [PubMed] [Google Scholar]
- 99.Rao JN, Kim YE, Park LS, Ulmer TS. Effect of pseudorepeat rearrangement on α-synuclein misfolding, vesicle binding, and micelle binding. J Mol Biol. 2009;390:516–529. doi: 10.1016/j.jmb.2009.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of α-synuclein. J Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Martinez Z, Zhu M, Han SB, Fink AL. GM1 specifically interacts with α-synuclein and inhibits fibrillation. Biochemistry. 2007;46:1868–1877. doi: 10.1021/bi061749a. [DOI] [PubMed] [Google Scholar]
- 102.Suzuki K, Iseki E, Katsuse O, Yamaguchi A, Katsuyama K, Aoki I, Yamanaka S, Kosaka K. Neuronal accumulation of α- and β-synucleins in the brain of a GM2 gangliosidosis mouse model. Neuroreport. 2003;14:551–554. doi: 10.1097/00001756-200303240-00004. [DOI] [PubMed] [Google Scholar]
- 103.Kjaer L, Giehm L, Heimburg T, Otzen D. The influence of vesicle size and composition on α-synuclein structure and stability. Biophys J. 2009;96:2857–2870. doi: 10.1016/j.bpj.2008.12.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kubo S, Nemani VM, Chalkley RJ, Anthony MD, Hattori N, Mizuno Y, Edwards RH, Fortin DL. A combinatorial code for the interaction of α-synuclein with membranes. J Biol Chem. 2005;280:31664–31672. doi: 10.1074/jbc.M504894200. [DOI] [PubMed] [Google Scholar]
- 105.Perrin RJ, Woods WS, Clayton DF, George JM. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J Biol Chem. 2001;276:41958–41962. doi: 10.1074/jbc.M105022200. [DOI] [PubMed] [Google Scholar]
- 106.Wood WG, Schroeder F, Avdulov NA, Chochina SV, Igbavboa U. Recent advances in brain cholesterol dynamics: Transport, domains, and Alzheimer’s disease. Lipids. 1999;34:225–234. doi: 10.1007/s11745-999-0357-9. [DOI] [PubMed] [Google Scholar]
- 107.Lagerholm BC, Weinreb GE, Jacobson K, Thompson NL. Detecting microdomains in intact cell membranes. Annu Rev Phys Chem. 2005;56:309–336. doi: 10.1146/annurev.physchem.56.092503.141211. [DOI] [PubMed] [Google Scholar]
- 108.Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 109.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 110.Simons K, Vanmeer G. Lipid sorting in epithelial-cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 111.Stockl M, Fischer P, Wanker E, Herrmann A. α-Synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J Mol Biol. 2008;375:1394–1404. doi: 10.1016/j.jmb.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 112.Michel V, Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 113.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 114.Nuscher B, Kamp F, Mehnert T, Odoy S, Haass C, Kahle PJ, Beyer K. α-Synuclein has a high affinity for packing defects in a bilayer membrane: A thermodynamics study. J Biol Chem. 2004;279:21966–21975. doi: 10.1074/jbc.M401076200. [DOI] [PubMed] [Google Scholar]
- 115.Pfefferkorn CM, Lee JC. Tryptophan probes at the α-synuclein and membrane interface. J Phys Chem B. 2010;114:4615–4622. doi: 10.1021/jp908092e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trexler AJ, Rhoades E. α-Synuclein binds large unilamellar vesicles as an extended helix. Biochemistry. 2009;48:2304–2306. doi: 10.1021/bi900114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haque F, Pandey AP, Cambrea LR, Rochet JC, Hovis JS. Adsorption of α-synuclein on lipid bilayers: Modulating the structure and stability of protein assemblies. J Phys Chem B. 2010;114:4070–4081. doi: 10.1021/jp1006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.White SH, Wimley WC. Membrane protein folding and stability: Physical principles. Annu Rev Biophys Biomolec Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 119.White SH, Wimley WC. Hydrophobic interactions of peptides with membrane interfaces. Biochim Biophys Acta-Rev Biomembr. 1998;1376:339–352. doi: 10.1016/s0304-4157(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 120.Seelig J. Thermodynamics of lipid-peptide interactions. Biochim Biophys Acta, Biomembr. 2004;1666:40–50. doi: 10.1016/j.bbamem.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Wimley WC, Thompson TE. Exchange, flip-flop of dimyristoylphosphatidylcholine in liquid-crystalline, gel, two-component, two-phase large unilamellar vesicles. Biochemistry. 1990;29:1296–1303. doi: 10.1021/bi00457a027. [DOI] [PubMed] [Google Scholar]
- 122.Drin G, Antonny B. Amphipathic helices and membrane curvature. FEBS Lett. 2010;584:1840–1847. doi: 10.1016/j.febslet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 123.Bussell R, Eliezer D. Residual structure and dynamics in Parkinson’s disease-associated mutants of α-synuclein. J Biol Chem. 2001;276:45996–46003. doi: 10.1074/jbc.M106777200. [DOI] [PubMed] [Google Scholar]
- 124.Segrest JP, Jones MK, Deloof H, Brouillette CG, Venkatachalapathi YV, Anantharamaiah GM. The amphipathic helix in the exchangeable apolipoproteins: A review of secondary structure and function. J Lipid Res. 1992;33:141–166. [PubMed] [Google Scholar]
- 125.Bussell R, Eliezer D. A structural and functional role for 11-mer repeats in α-synuclein and other exchangeable lipid binding proteins. J Mol Biol. 2003;329:763–778. doi: 10.1016/s0022-2836(03)00520-5. [DOI] [PubMed] [Google Scholar]
- 126.Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human α-synuclein. J Biol Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 127.Ulmer TS, Bax A. Comparison of structure and dynamics of micelle-bound human α-synuclein and Parkinson disease variants. J Biol Chem. 2005;280:43179–43187. doi: 10.1074/jbc.M507624200. [DOI] [PubMed] [Google Scholar]
- 128.Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, Lansbury PT. The impact of the E46K mutation on the properties of α-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46:7107–7118. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- 129.Borbat P, Ramlall TF, Freed JH, Eliezer D. Inter-helix distances in lysophospholipid micelle-bound α-synuclein from pulsed ESR measurements. J Am Chem Soc. 2006;128:10004–10005. doi: 10.1021/ja063122l. [DOI] [PubMed] [Google Scholar]
- 130.Bortolus M, Tombolato F, Tessari I, Bisaglia M, Mammi S, Bubacco L, Ferrarini A, Maniero AL. Broken helix in vesicle and micelle-bound α-synuclein: Insights from site-directed spin labeling-EPR experiments and MD simulations. J Am Chem Soc. 2008;130:6690–6691. doi: 10.1021/ja8010429. [DOI] [PubMed] [Google Scholar]
- 131.Rao JN, Jao CC, Hegde BG, Langen R, Ulmer TS. A combinatorial NMR and EPR approach for evaluating the structural ensemble of partially folded proteins. J Am Chem Soc. 2010;132:8657–8668. doi: 10.1021/ja100646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Drescher M, Veldhuis G, van Rooijen BD, Milikisyants S, Subramaniam V, Huber M. Antiparallel arrangement of the helices of vesicle-bound α-synuclein. J Am Chem Soc. 2008;130:7796–7797. doi: 10.1021/ja801594s. [DOI] [PubMed] [Google Scholar]
- 133.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. The lipid-binding domain of wild type and mutant α-synuclein: Compactness and interconversion between the broken and extended helix forms. J Biol Chem. 2010;285:28261–28274. doi: 10.1074/jbc.M110.157214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Georgieva ER, Ramlall TF, Borbat PP, Freed JH, Eliezer D. Membrane-bound α-synuclein forms an extended helix: Long-distance pulsed ESR measurements using vesicles, bicelles, and rodlike micelles. J Am Chem Soc. 2008;130:12856–12857. doi: 10.1021/ja804517m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jao CC, Hegde BG, Chen J, Haworth IS, Langen R. Structure of membrane-bound α-synuclein from site-directed spin labeling and computational refinement. Proc Natl Acad Sci U S A. 2008;105:19666–19671. doi: 10.1073/pnas.0807826105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Robotta M, Braun P, van Rooijen B, Subramaniam V, Huber M, Drescher M. Direct evidence of coexisting horseshoe and extended helix conformations of membrane-bound α-synuclein. ChemPhysChem. 2011;12:267–269. doi: 10.1002/cphc.201000815. [DOI] [PubMed] [Google Scholar]
- 137.Jao CC, Der-Sarkissian A, Chen J, Langen R. Structure of membrane-bound α-synuclein studied by site-directed spin labeling. Proc Natl Acad Sci U S A. 2004;101:8331–8336. doi: 10.1073/pnas.0400553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Allison JR, Varnai P, Dobson CM, Vendruscolo M. Determination of the free energy landscape of α-synuclein using spin label nuclear magnetic resonance measurements. J Am Chem Soc. 2009;131:18314–18326. doi: 10.1021/ja904716h. [DOI] [PubMed] [Google Scholar]
- 139.Lee JC, Gray HB, Winkler JR. Tertiary contact formation in α-synuclein probed by electron transfer. J Am Chem Soc. 2005;127:16388–16389. doi: 10.1021/ja0561901. [DOI] [PubMed] [Google Scholar]
- 140.Lee JC, Lai BT, Kozak JJ, Gray HB, Winkler JR. α-Synuclein tertiary contact dynamics. J Phys Chem B. 2007;111:2107–2112. doi: 10.1021/jp068604y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bartels T, Ahlstrom LS, Leftin A, Kamp F, Haass C, Brown MF, Beyer K. The N-terminus of the intrinsically disordered protein α-synuclein triggers membrane binding and helix folding. Biophys J. 2010;99:2116–2124. doi: 10.1016/j.bpj.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bisaglia M, Mammi S, Bubacco L. Structural insights on physiological functions and pathological effects of α-synuclein. FASEB J. 2009;23:329–340. doi: 10.1096/fj.08-119784. [DOI] [PubMed] [Google Scholar]
- 143.Bussell R, Eliezer D. Effects of Parkinson’s disease-linked mutations on the structure of lipid-associated α-synuclein. Biochemistry. 2004;43:4810–4818. doi: 10.1021/bi036135+. [DOI] [PubMed] [Google Scholar]
- 144.Choi W, Zibaee S, Jakes R, Serpell LC, Davletov B, Crowther RA, Goedert M. Mutation E46K increases phospholipid binding and assembly into filaments of human α-synuclein. FEBS Lett. 2004;576:363–368. doi: 10.1016/j.febslet.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 145.Jensen PH, Nielsen MS, Jakes R, Dotti G, Goedert M. Binding of α-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J Biol Chem. 1998;273:26292–26294. doi: 10.1074/jbc.273.41.26292. [DOI] [PubMed] [Google Scholar]
- 146.Jo E, Fuller N, Rand RP, St George-Hyslop P, Fraser PE. Defective membrane interactions of familial Parkinson’s disease mutant A30P α-synuclein. J Mol Biol. 2002;315:799–807. doi: 10.1006/jmbi.2001.5269. [DOI] [PubMed] [Google Scholar]
- 147.Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human α-synuclein and Parkinson’s disease variants with phospholipids: Structural analysis using site-directed mutagenesis. J Biol Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- 148.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 149.Dauer W, Przedborski S. Parkinson’s disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 150.Necula M, Chirita CN, Kuret J. Rapid anionic micelle-mediated α-synuclein fibrillization in vitro. J Biol Chem. 2003;278:46674–46680. doi: 10.1074/jbc.M308231200. [DOI] [PubMed] [Google Scholar]
- 151.Zhu M, Fink AL. Lipid binding inhibits α-synuclein fibril formation. J Biol Chem. 2003;278:16873–16877. doi: 10.1074/jbc.M210136200. [DOI] [PubMed] [Google Scholar]
- 152.Zhu M, Li J, Fink AL. The association of α-synuclein with membranes affects bilayer structure, stability, and fibril formation. J Biol Chem. 2003;278:40186–40197. doi: 10.1074/jbc.M305326200. [DOI] [PubMed] [Google Scholar]
- 153.Chatelier RC, Minton AP. Adsorption of globular proteins on locally planar surfaces: Models for the effect of excluded surface area and aggregation of adsorbed protein on adsorption equilibria. Biophys J. 1996;71:2367–2374. doi: 10.1016/S0006-3495(96)79430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fantini J, Yahi N. Molecular insights into amyloid regulation by membrane cholesterol and sphingolipids: Common mechanisms in neurodegenerative diseases. Expert Rev Mol Med. 2010;12:22. doi: 10.1017/S1462399410001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Drescher M, van Rooijen BD, Veldhuis G, Subramaniam V, Huber M. A stable lipid-induced aggregate of α-synuclein. J Am Chem Soc. 2010;132:4080–4082. doi: 10.1021/ja909247j. [DOI] [PubMed] [Google Scholar]
- 156.Anderson VL, Ramlall TF, Rospigliosi CC, Webb WW, Eliezer D. Identification of a helical intermediate in trifluoroethanol-induced α-synuclein aggregation. Proc Natl Acad Sci U S A. 2010;107:18850–18855. doi: 10.1073/pnas.1012336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee HJ, Choi C, Lee SJ. Membrane-bound α-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 158.Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, Glabe CG. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 159.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT. Neurodegenerative disease: Amyloid pores from pathogenic mutations. Nature. 2002;418:291–291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 160.Butterfield SM, Lashuel HA. Amyloidogenic protein membrane interactions: Mechanistic insight from model systems. Angew Chem, Int Ed. 2010;49:5628–5654. doi: 10.1002/anie.200906670. [DOI] [PubMed] [Google Scholar]
- 161.Quist A, Doudevski L, Lin H, Azimova R, Ng D, Frangione B, Kagan B, Ghiso J, Lal R. Amyloid ion channels: A common structural link for protein-misfolding disease. Proc Natl Acad Sci U S A. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, Lansbury PT. Vesicle permeabilization by protofibrillar α-synuclein: Implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 163.Volles MJ, Lansbury PT. Vesicle permeabilization by protofibrillar α-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 164.Zakharov SD, Hulleman JD, Dutseva EA, Antonenko YN, Rochet JC, Cramer WA. Helical α-synuclein forms highly conductive ion channels. Biochemistry. 2007;46:14369–14379. doi: 10.1021/bi701275p. [DOI] [PubMed] [Google Scholar]
- 165.van Rooijen BD, Claessens M, Subramaniam V. Membrane binding of oligomeric α-synuclein depends on bilayer charge and packing. FEBS Lett. 2008;582:3788–3792. doi: 10.1016/j.febslet.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 166.van Rooijen BD, Claessens M, Subramaniam V. Membrane permeabilization by oligomeric α-synuclein: In search of the mechanism. PLoS One. 2010;5:14292. doi: 10.1371/journal.pone.0014292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim HY, Cho MK, Kumar A, Maier E, Siebenhaar C, Becker S, Fernandez CO, Lashuel HA, Benz R, Lange A, Zweckstetter M. Structural properties of pore-forming oligomers of α-synuclein. J Am Chem Soc. 2009;131:17482–17489. doi: 10.1021/ja9077599. [DOI] [PubMed] [Google Scholar]
- 168.Giehm L, Svergun DI, Otzen DE, Vestergaard B. Low-resolution structure of a vesicle disrupting α-synuclein oligomer that accumulates during fibrillation. Proc Natl Acad Sci U S A. 2011;108:3246–3251. doi: 10.1073/pnas.1013225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Varkey J, Isas JM, Mizuno N, Jensen MB, Bhatia VK, Jao CC, Petrlova J, Voss JC, Stamou DG, Steven AC, Langen R. Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins. J Biol Chem. 2010;285:32486–32493. doi: 10.1074/jbc.M110.139576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pandey AP, Haque F, Rochet JC, Hovis JS. α-Synuclein-induced tubule formation in lipid bilayers. J Phys Chem B. 2011;115:5886–5893. doi: 10.1021/jp1121917. [DOI] [PMC free article] [PubMed] [Google Scholar]