Abstract
Bacterial two-component systems (TCSs) sense stimuli and transduce signals intracellularly through phosphotransfer between cognate histidine kinases (HKs) and response regulators (RRs) to alter gene expression or behavioral responses. Without high phosphotransfer specificity between cognate HKs and RRs, cross-phosphorylation or cross-talk between different TCSs may occur and diminish responses to appropriate stimuli. Some mechanisms to reduce cross-talk involve HKs controlling levels of cognate RR phosphorylation. Conceivably, some RRs may have evolved HK-independent strategies to insulate themselves from cross-talk with acetyl phosphate (AcP) or other small phosphodonor metabolites. Initial steps in flagellar biosynthesis in Campylobacter jejuni stimulate phosphotransfer from the FlgS HK to the FlgR RR to promote σ54-dependent flagellar gene expression. We discovered that the FlgR C-terminal domain (CTD), which commonly functions as a DNA-binding domain in the NtrC RR family, is a specificity determinant to limit in vivo cross-talk from AcP. FlgR lacking the CTD (FlgRΔCTD) used FlgS or AcP as an in vivo phosphodonor and could be reprogrammed in ΔflgS mutants to respond to cellular nutritional status via AcP levels. Even though exclusive AcP-mediated activation of FlgRΔCTD promoted WT flagellar gene expression, proper flagellar biosynthesis was impaired. We propose that the FlgR CTD prevents phosphotransfer from AcP so that FlgR is solely responsive to FlgS to promote proper flagellar gene expression and flagellation. In addition to mechanisms limiting cross-talk between noncognate HKs and RRs, our work suggests that RRs can possess domains that prevent in vivo cross-talk between RRs and the endogenous metabolite AcP to ensure signaling specificity.
Cellular signal transduction systems link extracellular or intracellular stimuli to appropriate output responses. Prokaryotic organisms often are useful models for understanding aspects of signal transduction that may be applicable to higher organisms. Bacteria commonly use two-component systems (TCSs) to mediate responses to specific conditions. A basic TCS consists of a sensor histidine kinase (HK) that autophosphorylates upon detection of a specific signal (1). The phosphorylated histidine of the HK serves as a phosphodonor for autophosphorylation by the cognate response regulator (RR). The phosphorylated RR can then alter gene expression or a behavioral response. TCSs generally do not exist in isolation in a bacterial cell, but among a signaling network of up to as many as 200 different TCSs, depending on the species. Despite structural similarity between many TCSs, phosphotransfer specificity between cognate HK and RR pairs is high (2). In vivo cross-phosphorylation, or cross-talk, between noncognate HK and RRs is usually maintained at a minimum. If cross-talk between two TCSs occurs, correct responses to specific signals may be diminished or inhibited.
Mechanisms exist to insulate a TCS from cross-talk and ensure that intrasystem signal transduction fidelity is preserved. In many TCSs, specific amino acids mediate molecular recognition between cognate HK and RR pairs (3–6). In addition, some HKs are bifunctional with a phosphatase activity to reduce levels of phosphorylated cognate RRs. These bifunctional HKs control activity of the RR by reducing phosphorylation that may occur via cognate HKs, noncognate HKs, or low molecular-weight phosphodonors such as acetyl phosphate (AcP) (1, 7–10). Together, these mechanisms contribute to phosphotransfer specificity in some TCSs.
The FlgSR TCS of Campylobacter jejuni is required for expression of the σ54 regulon, which mainly includes flagellar rod and hook genes (11, 12). Initiation of signal transduction through FlgSR is dependent upon components of the flagellar type III secretion system (T3SS) (13, 14). After autophosphorylation of H141 of the cytoplasmic FlgS HK, FlgR autophosphorylates on D51 of the receiver domain, using the phosphohistidine of FlgS as a substrate. Phosphorylation of FlgR is required for σ54-dependent gene expression. Because the flagellar T3SS exports rod and hook proteins out of the cytoplasm, FlgSR links T3SS formation to expression of genes encoding substrates secreted by the T3SS to synthesize the organelle (14).
Most NtrC-like RRs possess an essential C-terminal DNA-binding domain (CTD) that interacts with target promoters to activate gene expression. Mutant RRs lacking the CTD fail to stimulate WT levels of σ54-dependent gene expression under normal conditions, even in the presence of the cognate HK (15–17). In contrast, FlgR lacking its CTD (FlgRΔCTD) activated WT levels of σ54-dependent gene expression in the presence of FlgS (13). However, the activities of WT FlgR and FlgRΔCTD in ΔflgS mutants differed. Whereas WT FlgR without FlgS did not stimulate σ54-dependent gene expression, FlgRΔCTD in a ΔflgS mutant activated expression of the σ54 regulon. However, the level of gene expression in ΔflgS flgRΔCTD was ∼20% of that of C. jejuni with a WT FlgSR TCS. Because FlgRΔCTD in the ΔflgS mutant required the phosphorylated D51 residue to activate gene expression (13), FlgRΔCTD must have autophosphorylated via a noncognate phosphodonor in this mutant. Thus, these results question the role of the FlgR CTD in DNA binding and suggest that the CTD may serve an alternative function to limit phosphotransfer specificity or cross-talk to FlgR.
We explored the role of the CTD of FlgR in activation of σ54-dependent flagellar gene expression. By conducting genetic, biochemical, and physiological studies, we discovered that unlike most NtrC-like RRs, the CTD of FlgR has a DNA-binding activity that is not essential for expression of target genes under physiological conditions. Instead, we show that the CTD is a specificity determinant for phosphorylation that expressly limits the ability of FlgR to autophosphorylate in vivo using the small molecular-weight phosphodonor AcP. Without the CTD, metabolic processes that alter AcP levels influence FlgR activation, but complete reliance of FlgR on AcP for WT levels of gene expression hindered flagellation. As a result of the CTD limiting cross-talk of FlgR with AcP, FlgR activation is coupled to FlgS and a step in flagellar biosynthesis to promote proper gene expression and flagellation for optimal fitness. Whereas previously known mechanisms to eliminate intersystem cross-talk mostly involved activities of HKs, our work identified a domain within a RR that specifically prevents cross-talk with the endogenous central metabolite AcP.
Results
DNA Binding by FlgR Is Not Required for σ54-Dependent Gene Expression.
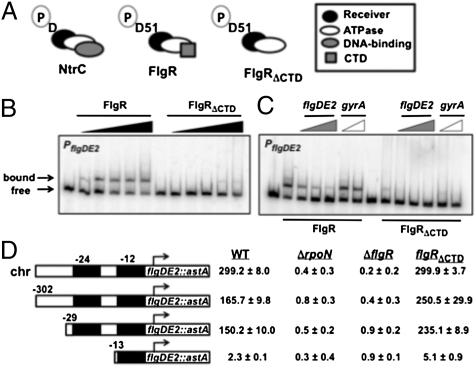
C. jejuni FlgR is a member of the NtrC family of RRs. As such, FlgR contains an N-terminal receiver domain modified by phosphorylation on D51 and a central ATPase domain necessary for oligomerization and interactions with σ54 (Fig. 1A). Within the CTD of most NtrC-like RRs is a helix-turn-helix (HTH) motif essential for DNA binding and function as a transcriptional regulator (15, 16). This HTH binds enhancer-like sites upstream of target promoters to promote WT levels of gene expression under normal conditions. Unlike most other NtrC-like RRs, the FlgR CTD lacks a strongly predicted HTH motif. Compared with WT C. jejuni with an intact FlgSR, a flgRΔCTD mutant (which produces WT FlgS) promoted similar or slightly higher levels of expression of σ54-dependent genes (Table S1) (13). These results suggested either that the FlgR CTD does not bind DNA, or that any DNA-binding activity of the CTD is not essential for FlgR-dependent gene expression. Therefore, we explored the role of the CTD in the function of FlgR as a transcriptional regulator.
Fig. 1.
Analysis of the requirement of FlgR/DNA interactions for σ54-dependent flagellar gene expression. (A) Domain organization of E. coli NtrC, C. jejuni WT FlgR, and C. jejuni FlgRΔCTD. The phosphorylated aspartic acids (D or D51; P denotes phosphoryl group) in receiver domains are indicated. (B and C) EMSAs to analyze PflgDE2 DNA binding by FlgR proteins. (B) WT FlgR and FlgRΔCTD were used (from left to right) at 0, 0.1, 0.25, 0.5, 0.75, and 1 μM. (C) Increasing ratios of unlabeled PflgDE2 DNA (1:1, 5:1, or 10:1) or PgyrA DNA (1:1 or 10:1) to labeled PflgDE2 DNA were incubated with 1 μM of FlgR proteins. (D) Expression of flgDE2::astA with 5′ promoter truncations in WT C. jejuni or flgRΔCTD, ΔrpoN (Δσ54), or ΔflgR mutants. flgDE2::astA expression levels are reported as arylsulfatase units ± SD. All transcriptional fusions were on plasmids, except for the fusion at the native chromosomal flgDE2 locus (chr). The 5′ ends of fusions on plasmids are indicated relative to the transcriptional start site (arrow). Black boxes indicate the −24 and −12 σ54 binding sites essential for σ54-dependent gene expression.
We analyzed FlgR interactions with the σ54-dependent flgDE2 promoter (PflgDE2) by EMSAs. Purified WT FlgR bound promoter DNA encompassing –302 to +79 bases relative to the flgDE2 transcriptional start site in a dose-dependent manner (Fig. 1B) (18). Binding by WT FlgR was specific, because excess unlabeled PflgDE2 DNA, but not PgyrA DNA (a FlgR-independent promoter), competed for binding (Fig. 1C). In contrast, FlgRΔCTD only bound PflgDE2 at the highest protein concentration examined, but binding was nonspecific, because both unlabeled PflgDE2 and PgyrA DNA reduced residual binding of FlgRΔCTD to labeled PflgDE2 DNA (Fig. 1C).
Because our results suggested that the FlgR CTD binds DNA but is not essential for expression of the σ54 regulon, we hypothesized that the 5′ end of a C. jejuni FlgR- and σ54-dependent promoter may only need to begin with the −24 and −12 σ54 binding sites. Thus, we tested if upstream promoter DNA, which usually contains binding sites for a NtrC-like RR, may be removed and not alter expression of C. jejuni σ54-dependent genes. Therefore, we analyzed the ability of C. jejuni strains producing WT FlgS and either WT FlgR or FlgRΔCTD to express flgDE2::astA transcriptional fusions with 5′ truncations of PflgDE2. Because the flagellar gene fliK overlaps PflgDE2, and chromosomal deletions upstream of PflgDE2 would create fliK mutants that alter expression of the σ54 regulon in C. jejuni (19), we analyzed expression of flgDE2::astA with 5′ promoter truncations on plasmids in C. jejuni strains.
Because WT FlgR, but not FlgRΔCTD, specifically bound PflgDE2 DNA from −302 to +79 (Fig. 1 B and C), we considered this promoter fragment potentially sufficient for flgDE2 expression. For all analyses in this work, WT flgR and flgRΔCTD were expressed from the native chromosomal flgR locus. As shown in Fig. 1D, WT C. jejuni and the flgRΔCTD mutant, which both produced WT FlgS, expressed chromosomal- or plasmid-borne flgDE2::astA with base −302 as the 5′ end of PflgDE2. More expression was noted in the flgRΔCTD mutant than in WT C. jejuni for plasmid-borne flgDE2::astA, which may indicate some DNA conformational changes that artificially promote slightly more expression with FlgRΔCTD. Regardless, expression of PflgDE2 was dependent on both a FlgR protein and σ54 (encoded by rpoN; Fig. 1D). Removal of DNA up to base −29 (six bases before the essential σ54 binding sites) did not significantly reduce expression of flgDE2::astA in WT C. jejuni or the flgRΔCTD mutant. However, deletion of DNA up to base −13 (which removes the essential −24 site for σ54 binding) eliminated expression of flgDE2::astA in all strains regardless of the FlgR protein produced (Fig. 1D). Therefore, we concluded that a minimal σ54-dependent promoter in C. jejuni likely includes only σ54 binding sites at the 5′ end. Furthermore, the FlgR CTD bound DNA in σ54-dependent promoters, but DNA binding by FlgR was not essential for gene expression.
The Acetogenesis Pathway Influences in Vivo Activation of FlgRΔCTD in the Absence of FlgS.
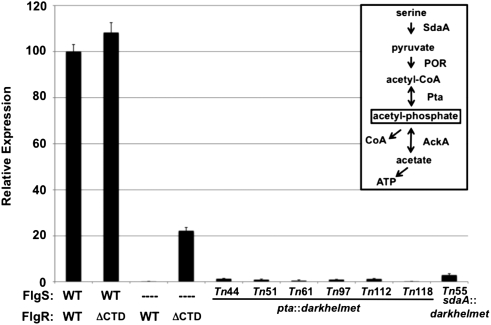
In strains producing FlgS, FlgRΔCTD promoted equal to modestly higher expression of most σ54-dependent flagellar genes relative to WT FlgR (Figs. 1D and 2 and Table S1). In a ΔflgS mutant, WT FlgR did not activate σ54-dependent flagellar gene expression (Fig. 2) (11). In contrast, FlgRΔCTD activated expression of σ54-dependent genes without FlgS (Fig. 2) (13). However, the level of gene expression promoted by FlgRΔCTD without FlgS was ∼20% of the level of C. jejuni with a WT FlgSR TCS (Fig. 2). Furthermore, FlgRΔCTD required phosphorylation of D51 in the receiver domain to activate gene expression in a ΔflgS mutant (13). These results indicated that the FlgR CTD may limit in vivo phosphotransfer to FlgR by noncognate HKs or other phosphodonors, in addition to a nonessential DNA-binding activity.
Fig. 2.
Identification of darkhelmet Tn mutants with reduced FlgRΔCTD activity in ΔflgS mutants. Arylsulfatase assay examining expression of the σ54-dependent flaB::astA transcriptional fusion in C. jejuni strains grown on MH agar. The level of flaB::astA expression in each strain is relative to WT C. jejuni producing the WT FlgSR TCS, which was set to 100 units. The FlgS and FlgR proteins produced in each strain are indicated. ΔCTD indicates FlgRΔCTD. Dashes indicate deletions of respective genes. Tn mutants are in the C. jejuni ΔastA ΔflgS flgRΔCTD flaB::astA background. Error bars indicate SDs. (Inset) Acetogenesis pathway as outlined in E. coli with minor modifications (20), and predicted to be intact in C. jejuni (24, 25).
To search for in vivo phosphodonors that activate FlgRΔCTD in the absence of FlgS, we performed transposon (Tn) mutagenesis with the darkhelmet Tn in C. jejuni ΔflgS flgRΔCTD flaB::astA and identified seven of ∼3,750 Tn mutants with seven- to 180-fold reductions in expression of the σ54-dependent flaB::astA transcriptional reporter (Fig. 2). All mutants contained a Tn in genes likely affecting the acetogenesis pathway, a multistep process that converts pyruvate to ATP and acetate (reviewed in ref. 20). An intermediate in this pathway is AcP, a low molecular-weight phosphodonor often used to autophosphorylate many RRs in vitro, and a few RRs in vivo (8, 21–23). Six independent Tn insertions were within pta, encoding phosphotransacetylase, which reversibly converts acetyl-CoA (Ac-CoA) and inorganic phosphate to CoA and AcP (Fig. 2). One mutant contained a Tn in sdaA, encoding serine dehydratase, which converts serine to pyruvate upstream of the pathway. Immediately downstream of pta on the C. jejuni chromosome is ackA, encoding acetate kinase, which reversibly converts AcP and ADP to acetate and ATP in the acetogenesis pathway. Thus, Tn insertions in pta may have had polar effects on ackA expression and eliminated AcP production altogether. Mutants with Tn insertions in genes encoding HKs were not identified in this screen. These results suggested that fluctuations in AcP biosynthesis from the acetogenesis pathway may have directly influenced in vivo phosphorylation of FlgRΔCTD and σ54-dependent flagellar gene expression.
Initial studies suggested that the acetogenesis pathway is intact in C. jejuni as in Escherichia coli (20, 24, 25). To analyze possible in vivo AcP-mediated activation of FlgRΔCTD, C. jejuni mutants predicted to produce different intracellular levels of AcP when grown on Mueller–Hinton (MH) agar were made: ΔackA, high AcP; Δpta, low AcP; and Δpta ΔackA, negligible AcP. Although we could not directly measure AcP levels in C. jejuni, our data described below suggested that the C. jejuni mutants produced similar trends in AcP levels as respective E. coli mutants (22, 26, 27). When ackA was deleted in the ΔflgS flgRΔCTD mutant, flaB::astA expression increased 4.5-fold and was 70% of that observed in WT C. jejuni with an intact FlgSR TCS (Table 1). Stepwise decreases in AcP levels by mutating pta alone and both pta and ackA reduced flaB::astA expression to negligible levels in the ΔflgS flgRΔCTD mutant (Table 1). Mutation of the phosphorylated D51 residue in FlgRΔCTD abolished all effects of the acetogenesis pathway on flaB::astA expression in the absence of FlgS (Table 1). In contrast to the ΔflgS flgRΔCTD mutant, flaB::astA expression in C. jejuni producing WT FlgR in a ΔflgS mutant was only slightly increased by deleting ackA (Table 1). We next examined if the acetogenesis pathway could influence FlgR or FlgRΔCTD activity in the presence of FlgS. Deletion of ackA did not increase flaB::astA expression in WT C. jejuni (Table 1). However, FlgRΔCTD-stimulated flaB::astA expression in the presence of FlgS increased 18% when ackA was deleted (Table 1). These results verified that the acetogenesis pathway significantly modulated FlgRΔCTD activity for promoting σ54-dependent gene expression, both in the presence and absence of FlgS.
Table 1.
Effect of the acetogenesis pathway on activation of FlgR proteins and σ54-dependent gene expression
| FlgS/FlgR protein produced |
pta ackA genotype |
|||||
| flgS flgR genotype | FlgS | FlgR | WT | ΔackA | Δpta | ΔptaA ΔackA |
| WT | WT | WT | 100 ± 6.9* | 96.4 ± 3.8 | ND† | ND |
| ΔflgR | WT | – | 0.3 ± 0.1 | ND | ND | ND |
| flgRΔCTD | WT | FlgRΔCTD | 98.3 ± 3.2 | 116.0 ± 5.9 | ND | ND |
| ΔflgS | – | WT | 0.3 ± 0.0 | 1.8 ± 0.6 | 0.3 ± 0.1 | 0.1 ± 0.0 |
| ΔflgS flgRΔCTD | – | FlgRΔCTD | 15.3 ± 2.3 | 70.6 ± 14.4 | 4.3 ± 0.5 | 0.3 ± 0.1 |
| ΔflgS flgRD51AΔCTD | – | FlgR D51AΔCTD | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.2 |
*Arylsulfatase assay examining flaB::astA expression after growth on MH agar. The level of flaB::astA expression (±SD) in each strain is relative to WT C. jejuni producing the WT FlgSR TCS, which was set to 100 units.
†Not determined.
The CTD Is a Specificity Determinant That Limits FlgR Autophosphorylation via AcP.
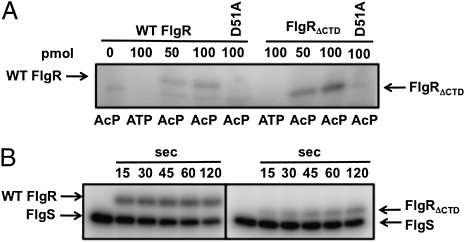
Our results suggested that in vivo FlgRΔCTD autophosphorylation via AcP is likely enhanced relative to WT FlgR. Thus, we monitored the in vitro ability of FlgR proteins to autophosphorylate with AcP as the sole phosphodonor. In our assays, we observed modification of both proteins with Ac[32P], with at least a two- to threefold greater level of autophosphorylation of FlgRΔCTD compared with WT FlgR (Fig. 3A and Fig. S1). Autophosphorylation of both RRs via AcP was increased as protein levels increased and was inhibited by mutation of the phosphorylated D51 residue. Enhanced modification of FlgRΔCTD was specific for AcP, because phosphotransfer from FlgS to FlgRΔCTD was ∼25–70% less than WT FlgR over time (Fig. 3B). Therefore, the CTD specifically limited cross-talk and phosphotransfer from AcP to FlgR.
Fig. 3.
In vitro phosphorylation of FlgR proteins. (A) Autophosphorylation of FlgR proteins with Ac[32P] or [γ32P]ATP. WT FlgR or FlgRΔCTD (50 and 100 pmol) or respective D51A mutants (100 pmol) are indicated. (B) Phosphotransfer from FlgS to FlgR proteins. Six picomoles of WT FlgR or FlgRΔCTD were mixed with autophosphorylated 32P-FlgS and removed after 15–120 s.
Reprogramming Activation of FlgRΔCTD by AcP Through Metabolism.
AcP-mediated activation of FlgRΔCTD suggested that deletion of the CTD allowed FlgR to directly respond to the nutritional status of the cell through the acetogenesis pathway, in addition to responding to steps in flagellar biosynthesis via signal transduction through FlgS. Therefore, we tested if altering physiology of C. jejuni ΔflgS mutants would stimulate FlgRΔCTD more than WT FlgR to augment both flagellar gene expression and flagellar biosynthesis. In addition, we analyzed whether FlgR or FlgRΔCTD activity could be modulated by altering physiology even in the presence of FlgS.
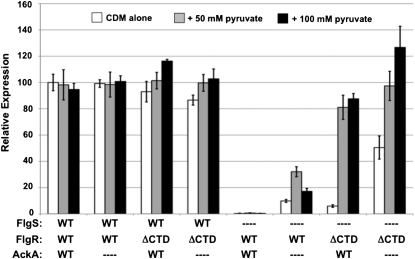
To determine if FlgR could be reprogrammed to respond to metabolic cues, we grew C. jejuni strains on Campylobacter defined media (CDM) with increasing pyruvate concentrations to increase intracellular AcP levels via the acetogenesis pathway. No changes in flaB::astA expression were observed in WT C. jejuni with an intact FlgSR TCS by deleting ackA or by increasing pyruvate levels in the media (Fig. 4). However, we observed a 9–25% increase in flaB::astA expression when strains producing WT FlgS and FlgRΔCTD were grown in increasing concentrations of pyruvate to stimulate AcP biosynthesis (Fig. 4). These results suggested that even in the presence of WT FlgS, FlgRΔCTD, but not WT FlgR, is responsive to AcP, which results in augmented levels of gene expression.
Fig. 4.
FlgS-independent activation of FlgRΔCTD by altering C. jejuni physiology. Arylsulfatase assay examining flaB::astA expression after growth on CDM alone (white bars), CDM with 50 mM (gray bars), or 100 mM (black bars) excess sodium pyruvate. The level of flaB::astA expression in each strain is relative to WT C. jejuni producing the WT FlgSR TCS grown on CDM alone, which was set to 100 units. The FlgS, FlgR, and AckA proteins produced in each strain are indicated. ΔCTD indicates FlgRΔCTD. Dashes indicate deletions of respective genes. Error bars indicate SDs.
We next examined flaB::astA expression upon modulating the physiology of C. jejuni strains lacking FlgS. As a result of deletion of flgS, AcP is presumably the only possible phosphodonor for FlgR proteins. flaB::astA expression in ΔflgS flgRΔCTD after growth on CDM alone was only 6% of that of WT C. jejuni with an intact FlgSR TCS grown on the same media (Fig. 4). However, upon increasing pyruvate concentrations, flaB::astA expression increased ∼14-fold (Fig. 4); this enhanced FlgS-independent, pyruvate-stimulated flaB::astA expression was 81–88% of that of WT C. jejuni grown in similar levels of pyruvate. When ackA was deleted, the baseline level of flaB::astA expression in C. jejuni ΔflgS flgRΔCTD grown in CDM increased (Fig. 4). In higher pyruvate concentrations, flaB::astA expression in the ΔflgS flgRΔCTD ΔackA mutant equaled and even surpassed WT C. jejuni with an intact FlgSR TCS by 26% (Fig. 4). Deletion of both pta and ackA prevented activation of FlgRΔCTD and flaB::astA expression regardless of pyruvate concentrations, presumably due to low AcP levels in this mutant (Fig. S2).
In contrast, C. jejuni producing WT FlgR without FlgS was not responsive to increasing pyruvate levels as measured by flaB::astA expression (Fig. 4). However, by presumably increasing intracellular AcP levels through deletion of ackA, an appreciable increase in FlgS-independent gene expression by WT FlgR was observed, which was further augmented two- to threefold by increasing pyruvate levels (Fig. 4). However, flaB::astA expression levels were dramatically lower with AcP-dependent activation of WT FlgR than FlgRΔCTD.
We next determined if levels of flagellation could be changed via AcP-mediated activation of WT FlgR or FlgRΔCTD by altering C. jejuni physiology. We monitored the number of flagella on individual bacteria after growth on CDM alone or CDM with excess pyruvate. Approximately 93–97% of WT C. jejuni cells produced a single flagellum at one or both poles when grown on CDM or CDM with pyruvate or with deletion of ackA (64–75% with a flagellum at each pole, 21–29% with a flagellum only at one pole; Table 2 and Table S2). Similar levels of flagellation were observed in the flgRΔCTD mutant producing WT FlgS, regardless of pyruvate levels in media or the presence of ackA (Table S2). Considering that pyruvate augmented FlgRΔCTD-dependent flagellar gene expression but not flagellation in the presence of FlgS (Fig. 4 and Table S2), these findings suggested that other factors besides expression of flagellar rod and hook proteins are rate-limiting steps in flagellation. We next analyzed if growth on pyruvate could stimulate WT FlgR or FlgRΔCTD in ΔflgS mutants to result in maximal flagellation, despite lacking the ability to use FlgS to link expression of the σ54 regulon to flagellar T3SS formation. Even though we observed pyruvate-stimulated activation of WT FlgR in the absence of FlgS that slightly increased flagellar gene expression (Fig. 4), this strain was aflagellated under all growth conditions, with or without ackA mutation. For the ΔflgS flgRΔCTD mutant, most cells were aflagellated after growth on MH or CDM agar. However, growth on CDM with excess pyruvate resulted in ∼30% of cells producing flagella (Table 2). Upon deletion of ackA, the population of flagellated ΔflgS flgRΔCTD cells increased to 56% with pyruvate supplementation (Table 2). Despite increases in flagellation, the number of ΔflgS flgRΔCTD cells producing two flagella (the predominant WT phenotype) was always lower than WT C. jejuni. Thus, removal of the CTD reprogrammed FlgR to respond to the nutritional status of C. jejuni via AcP levels and resulted in similar levels of gene expression as WT C. jejuni with an intact FlgSR TCS (Fig. 4). However, coupling FlgR activation to the flagellar T3SS through the cognate FlgS HK promoted optimal flagellation.
Table 2.
Flagellation due to AcP-dependent activation of FlgR proteins by altering C. jejuni physiology
| No. of flagella per bacterium* |
||||
| Strain | Media | 2 | 1 | 0 |
| WT | CDM | 64 ± 7 | 29 ± 7 | 6 ± 0 |
| WT | CDM + pyruvate | 67 ± 1 | 27 ± 6 | 6 ± 5 |
| ΔflgS flgRΔCTD | CDM | 0 ± 0 | 1 ± 0 | 99 ± 0 |
| ΔflgS flgRΔCTD | CDM + pyruvate | 4 ± 1 | 26 ± 4 | 71 ± 3 |
| ΔflgS flgRΔCTD ΔackA | CDM | 0 ± 0 | 5 ± 3 | 95 ± 3 |
| ΔflgS flgRΔCTD ΔackA | CDM + pyruvate | 8 ± 3 | 48 ± 8 | 44 ± 11 |
*The percentage of the population producing a single flagellum at both poles (two flagella), a single flagellum at one pole (one flagellum), or no flagella (zero) after growth on CDM or CDM with 100 mM excess sodium pyruvate are indicated. Data are the average of two experiments ± SD.
In Vivo Activation of FlgRΔCTD by AcP Is Not Reduced by a Potential FlgS Phosphatase Activity.
In addition to functioning as kinases, many HKs are bifunctional with a phosphatase activity for their cognate RRs. If conditions favor the HK possessing a net phosphatase activity, the level of the phosphorylated cognate RR decreases. The HK can dephosphorylate the RR regardless of the original phosphodonor used to autophosphorylate the RR. To date, a potential FlgS phosphatase activity has not directly been examined. If one exists, then in vivo AcP-mediated activation of FlgR may be prevented both by the CTD and FlgS.
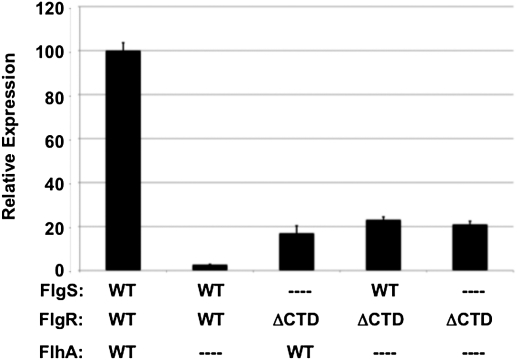
Flagellar T3SS components such as FlhA are required to stimulate FlgSR signal transduction and transcription of σ54-dependent flagellar genes (11, 14). A flgRΔCTD mutant was able to express flaB::astA in the absence of FlhA and/or FlgS, due to AcP-mediated activation of FlgRΔCTD (Fig. 5) (14). More importantly, the level of flaB::astA expression in the ΔflhA flgRΔCTD mutant (which produces FlgS with low autokinase activity) was at least the same as the ΔflgS flgRΔCTD mutant. Thus, FlgS when inactive as a kinase failed to protect FlgRΔCTD from cross-talk with AcP and reduce gene expression. Furthermore, these findings demonstrate the importance of the CTD in eliminating cross-talk of FlgR with AcP and influences from metabolism. As such, the CTD prevents AcP-mediated activation of FlgR and unnecessary flagellar gene expression and protein production in a bacterium unable to synthesize flagella due to a deficiency in producing a flagellar T3SS. Thus, the CTD is a key specificity determinant in the FlgSR TCS to prevent cross-talk between FlgR and endogenous metabolic phosphodonors.
Fig. 5.
Examination of an in vivo FlgS phosphatase activity for AcP-activated FlgRΔCTD. Arylsulfatase assay examining flaB::astA expression after growth on MH agar. The level of flaB::astA expression in each strain is relative to WT C. jejuni producing the WT FlgSR TCS, which was set to 100 units. The FlgS, FlgR, and FlhA proteins produced in each strain are indicated. ΔCTD indicates FlgRΔCTD. Dashes indicate deletions of respective genes. Error bars indicate SDs.
Discussion
Maintaining signaling fidelity within a TCS is essential for promoting the correct type and degree of response to particular stimuli. In many TCSs, HKs serve as phosphodonors for cognate RRs to result in proper output responses. If mechanisms do not exist to limit specificity of communication of each HK to a cognate RR, intersystem cross-talk between two TCSs may cause signaling interference and obstruct responses to activating stimuli. Mechanisms identified so far that promote intrasystem signaling fidelity and limit intersystem cross-talk include molecular recognition between cognate HK and RR pairs and phosphatase activities of some HKs that control levels of phosphorylation of cognate RRs (1, 3–6).
We identified a domain in a RR that limits in vivo cross-talk specifically from the metabolite AcP, rather than from a noncognate HK. This specificity determinant in C. jejuni FlgR is located in the CTD, which in most other NtrC-like RRs functions in an essential DNA-binding activity (15–17). Without the CTD, FlgR used AcP as an in vivo phosphodonor for autophosphorylation to stimulate gene expression, even in the presence of the cognate FlgS HK. Furthermore, FlgRΔCTD could be reprogrammed to respond to the nutritional status of the cell by altering the acetogenesis pathway and presumably AcP levels to result in levels of expression of the σ54 regulon that equaled and exceeded WT C. jejuni with an intact FlgSR TCS. AcP serves as an in vivo phosphodonor for autophosphorylation of many RRs (9), but AcP-dependent activation of RRs is often negated by phosphatase activities of some cognate HKs. However, AcP has been shown to be the sole in vivo phosphodonor of a few RRs that appear to lack cognate HKs, including Borrelia burgdorferi Rrp2, which is also an NtrC-like RR (22, 23). Though our data strongly suggests that AcP directly serves as a phosphodonor for FlgRΔCTD, an alternative, yet remote, possibility is that high AcP levels stimulate a noncognate HK that weakly promotes phosphotransfer to FlgR. However, we have not identified another HK that influences FlgR activity in various genetic screens we have conducted in C. jejuni.
A major question arising from this work is how the CTD limits in vivo cross-talk between AcP and FlgR. Barbieri et al. (28) recently suggested a possible explanation for how phosphotransfer from small phosphodonors to some RRs may be influenced by interdomain interactions within a RR. By examining members of the OmpR/PhoB RR family, correlations between the in vitro ability of a protein to autophosphorylate and the degree of interdomain interactions were found. More extensive contacts between the receiver and effector domains of a RR were proposed to stabilize an inactivate state that is a barrier to autophosphorylation using small phosphodonors. Thus, interdomain interactions may naturally reduce substantial phosphotransfer from small phosphodonors to many RRs in TCS.
In light of this study, we attempted to determine structural differences between WT FlgR and FlgRΔCTD that may explain how the CTD reduces or prevents AcP-dependent FlgRΔCTD autophosphorylation. However, like many NtrC family members, the FlgR proteins were refractory to crystallization for structural analyses. Docking of atomic models of individual domains of Salmonella typhimurium NtrC have provided the most complete structure possible for this family of RRs (29). In this model, the CTD does not appear to be near to or interact with the N-terminal domain. Therefore, considerable doubt exists that the CTD of FlgR may directly obstruct the receiver domain, thereby limiting its ability to use AcP as a phosphodonor. It is possible that deletion of the CTD of FlgR may cause small structural changes in the protein, which results in a receiver domain with a conformation that more readily accepts AcP as a phosphodonor.
Even though we created physiological conditions that promoted AcP-dependent activation of FlgRΔCTD to result in levels of flagellar gene expression equivalent to WT C. jejuni with an intact FlgSR TCS, linking gene expression to the nutritional status of the cell did not result in WT flagellation. An ordered expression of subsets of flagellar genes is required for proper flagellar biosynthesis. Linking FlgR activation via FlgS to the flagellar T3SS appears to allow for correct temporal expression of σ54-dependent genes for flagellation. Because the σ54 regulon encodes rod and hook proteins, expression of these genes is necessary only after the T3SS has formed so that ordered protein secretion occurs for efficient flagellar biosynthesis. AcP-dependent activation of FlgR does not allow for precise temporal gene regulation relative to T3SS formation, and consequently reduced flagellation in C. jejuni. Whereas C. jejuni would rely on environmental nutrient composition for AcP-mediated activation of FlgR for motility, FlgS activation of FlgR allows the bacterium to produce flagella independently of exogenous factors, which likely enhances in vivo fitness of C. jejuni in hosts. Furthermore, the CTD allows FlgR to remain insensitive to AcP and influences from metabolism, preventing unnecessary flagellar gene expression and protein synthesis when flagella cannot form due to incomplete initial stages of flagellar biosynthesis.
We also found that FlgR activated WT levels of gene expression in the absence of DNA binding by the CTD or without DNA upstream of a σ54-dependent promoter. These results support the hypothesis that FlgR may initiate transcription of some σ54-dependent genes without binding DNA. We propose that FlgR may interact in a soluble state with σ54 in a RNA polymerase holoenzyme that is bound to target promoters, negating the need for FlgR to be tethered directly to DNA. A similar mechanism has been proposed for a FlgR homolog in Helicobacter pylori, which naturally lacks a CTD (30). These features of FlgR proteins in different bacteria may be significant in expanding mechanisms of transcriptional initiation by the NtrC family of RRs, because many of these proteins promote only limited transcription in the absence of DNA binding under physiological conditions (16, 17).
Some HKs are bifunctional, with net autokinase and phosphatase activities that control the level of cognate RR phosphorylation, depending upon different conditions. In a flagellar T3SS mutant, the autokinase activity of FlgS is low, but AcP-mediated activation of FlgRΔCTD was not reduced. In the absence of a significant in vivo phosphatase activity of FlgS, these data demonstrate the importance of the CTD as a major specificity determinant to limit cross-talk between FlgR and AcP when conditions are not conducive for flagellation. Because this specificity determinant resides in the common DNA-binding domain of other NtrC family members, the possibility exists that this domain reduces AcP-mediated activation in these RRs to maintain intrasystem signaling fidelity. Furthermore, our findings suggest that phosphorylated regulators in other bacteria and more complex eukaryotic systems may possess domains with similar activities in providing insulation from cross-talk with small phosphodonor metabolites.
Materials and Methods
Information regarding growth of C. jejuni 81–176 strains is provided in SI Materials and Methods. Methods for generating chromosomal mutants and transposon mutants, plus a list of strains and plasmids used in this study, are located in SI Materials and Methods and Tables S3 and S4. A detailed description of experimental methods is also provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 AI065539; National Research Initiative Grant 2009-35201-05039 from the US Department of Agriculture Cooperative State Research, Education, and Extension Service Food Safety Program; and National Institutes of Health Training Grant T32 AI007520 (to J.M.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1113013108/-/DCSupplemental.
References
- 1.Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laub MT, Goulian M. Specificity in two-component signal transduction pathways. Annu Rev Genet. 2007;41:121–145. doi: 10.1146/annurev.genet.41.042007.170548. [DOI] [PubMed] [Google Scholar]
- 3.Skerker JM, et al. Rewiring the specificity of two-component signal transduction systems. Cell. 2008;133:1043–1054. doi: 10.1016/j.cell.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capra EJ, et al. Systematic dissection and trajectory-scanning mutagenesis of the molecular interface that ensures specificity of two-component signaling pathways. PLoS Genet. 2010;6:e1001220. doi: 10.1371/journal.pgen.1001220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell CH, Porter SL, Strawson A, Stuart DI, Armitage JP. Using structural information to change the phosphotransfer specificity of a two-component chemotaxis signalling complex. PLoS Biol. 2010;8:e1000306. doi: 10.1371/journal.pbio.1000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casino P, Rubio V, Marina A. Structural insight into partner specificity and phosphoryl transfer in two-component signal transduction. Cell. 2009;139:325–336. doi: 10.1016/j.cell.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Huynh TN, Noriega CE, Stewart V. Conserved mechanism for sensor phosphatase control of two-component signaling revealed in the nitrate sensor NarX. Proc Natl Acad Sci USA. 2010;107:21140–21145. doi: 10.1073/pnas.1013081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lukat GS, McCleary WR, Stock AM, Stock JB. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc Natl Acad Sci USA. 1992;89:718–722. doi: 10.1073/pnas.89.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolfe AJ. Physiologically relevant small phosphodonors link metabolism to signal transduction. Curr Opin Microbiol. 2010;13:204–209. doi: 10.1016/j.mib.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCleary WR, Stock JB, Ninfa AJ. Is acetyl phosphate a global signal in Escherichia coli? J Bacteriol. 1993;175:2793–2798. doi: 10.1128/jb.175.10.2793-2798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrixson DR, DiRita VJ. Transcription of σ54-dependent but not σ28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol Microbiol. 2003;50:687–702. doi: 10.1046/j.1365-2958.2003.03731.x. [DOI] [PubMed] [Google Scholar]
- 12.Wösten MMSM, Wagenaar JA, van Putten JPM. The FlgS/FlgR two-component signal transduction system regulates the fla regulon in Campylobacter jejuni. J Biol Chem. 2004;279:16214–16222. doi: 10.1074/jbc.M400357200. [DOI] [PubMed] [Google Scholar]
- 13.Joslin SN, Hendrixson DR. Analysis of the Campylobacter jejuni FlgR response regulator suggests integration of diverse mechanisms to activate an NtrC-like protein. J Bacteriol. 2008;190:2422–2433. doi: 10.1128/JB.01827-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joslin SN, Hendrixson DR. Activation of the Campylobacter jejuni FlgSR two-component system is linked to the flagellar export apparatus. J Bacteriol. 2009;191:2656–2667. doi: 10.1128/JB.01689-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiau SP, Chen P, Reitzer LJ. Effects of insertions and deletions in glnG (ntrC) of Escherichia coli on nitrogen regulator I-dependent DNA binding and transcriptional activation. J Bacteriol. 1993;175(1):190–199. doi: 10.1128/jb.175.1.190-199.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.North AK, Kustu S. Mutant forms of the enhancer-binding protein NtrC can activate transcription from solution. J Mol Biol. 1997;267:17–36. doi: 10.1006/jmbi.1996.0838. [DOI] [PubMed] [Google Scholar]
- 17.Huala E, Ausubel FM. The central domain of Rhizobium meliloti NifA is sufficient to activate transcription from the R. meliloti nifH promoter. J Bacteriol. 1989;171:3354–3365. doi: 10.1128/jb.171.6.3354-3365.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrixson DR, Akerley BJ, DiRita VJ. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol Microbiol. 2001;40:214–224. doi: 10.1046/j.1365-2958.2001.02376.x. [DOI] [PubMed] [Google Scholar]
- 19.Kamal N, et al. Deletion of a previously uncharacterized flagellar-hook-length control gene fliK modulates the σ54-dependent regulon in Campylobacter jejuni. Microbiology. 2007;153:3099–3111. doi: 10.1099/mic.0.2007/007401-0. [DOI] [PubMed] [Google Scholar]
- 20.Wolfe AJ. The acetate switch. Microbiol Mol Biol Rev. 2005;69(1):12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouché S, et al. Regulation of RssB-dependent proteolysis in Escherichia coli: A role for acetyl phosphate in a response regulator-controlled process. Mol Microbiol. 1998;27:787–795. doi: 10.1046/j.1365-2958.1998.00725.x. [DOI] [PubMed] [Google Scholar]
- 22.Fredericks CE, Shibata S, Aizawa S, Reimann SA, Wolfe AJ. Acetyl phosphate-sensitive regulation of flagellar biogenesis and capsular biosynthesis depends on the Rcs phosphorelay. Mol Microbiol. 2006;61:734–747. doi: 10.1111/j.1365-2958.2006.05260.x. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, et al. Role of acetyl-phosphate in activation of the Rrp2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 2010;6:e1001104. doi: 10.1371/journal.ppat.1001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright JA, et al. Metabolite and transcriptome analysis of Campylobacter jejuni in vitro growth reveals a stationary-phase physiological switch. Microbiology. 2009;155(1):80–94. doi: 10.1099/mic.0.021790-0. [DOI] [PubMed] [Google Scholar]
- 25.Kelly DJ. Complexity and versatility in the physiology and metabolism of Campylobacter jejuni. In: Nachamkin I, Szymanski CM, Blaser MJ, editors. Campylobacter. 3rd Ed. Washington, DC: Amer Soc Microbiol; 2008. pp. 41–62. [Google Scholar]
- 26.Wolfe AJ, Parikh N, Lima BP, Zemaitaitis B. Signal integration by the two-component signal transduction response regulator CpxR. J Bacteriol. 2008;190:2314–2322. doi: 10.1128/JB.01906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klein AH, Shulla A, Reimann SA, Keating DH, Wolfe AJ. The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J Bacteriol. 2007;189:5574–5581. doi: 10.1128/JB.00564-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbieri CM, Mack TR, Robinson VL, Miller MT, Stock AM. Regulation of response regulator autophosphorylation through interdomain contacts. J Biol Chem. 2010;285:32325–32335. doi: 10.1074/jbc.M110.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Carlo S, et al. The structural basis for regulated assembly and function of the transcriptional activator NtrC. Genes Dev. 2006;20:1485–1495. doi: 10.1101/gad.1418306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brahmachary P, Dashti MG, Olson JW, Hoover TR. Helicobacter pylori FlgR is an enhancer-independent activator of σ54-RNA polymerase holoenzyme. J Bacteriol. 2004;186:4535–4542. doi: 10.1128/JB.186.14.4535-4542.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.