Abstract
Mice lacking the oxalate transporter SLC26A6 develop hyperoxalemia, hyperoxaluria, and calcium-oxalate stones as a result of a defect in intestinal oxalate secretion, but what accounts for the absorptive oxalate flux remains unknown. We measured transepithelial absorption of [14C]oxalate simultaneously with the flux of [3H]mannitol, a marker of the paracellular pathway, across intestine from wild-type and Slc26a6-null mice. We used the anion transport inhibitor DIDS to investigate other members of the SLC26 family that may mediate transcellular oxalate absorption. Absorptive flux of oxalate in duodenum was similar to mannitol, insensitive to DIDS, and nonsaturable, indicating that it is predominantly passive and paracellular. In contrast, in wild-type mice, secretory flux of oxalate in duodenum exceeded that of mannitol, was sensitive to DIDS, and saturable, indicating transcellular secretion of oxalate. In Slc26a6-null mice, secretory flux of oxalate was similar to mannitol, and no net flux of oxalate occurred. Absorptive fluxes of both oxalate and mannitol varied in parallel in different segments of small and large intestine. In epithelial cell lines, modulation of the charge selectivity of the claudin-based pore pathway did not affect oxalate permeability, but knockdown of the tight-junction protein ZO-1 enhanced permeability to oxalate and mannitol in parallel. Moreover, formation of soluble complexes with cations did not affect oxalate absorption. In conclusion, absorptive oxalate flux occurs through the paracellular “leak” pathway, and net absorption of dietary oxalate depends on the relative balance between absorption and SLC26A6-dependent transcellular secretion.
Nephrolithiasis is the second most common chronic kidney condition after hypertension and demonstrates a rising prevalence and association with chronic kidney disease.1 Calcium oxalate (CaOx) is the predominant component of most stones, and the level of urinary oxalate is an important risk factor for CaOx nephrolithiasis.2,3 The amount of urinary oxalate depends on the net effect of metabolic production, intestinal absorption, and renal excretion.4 The integral role of the intestine in oxalate homeostasis becomes most evident in diseases of intestine. Inflammatory bowel disease and small-bowel resections are conditions that are strongly associated with hyperoxaluria and CaOx nephrolithiasis.5
New insight into the importance of the intestine in the pathogenesis of CaOx nephrolithiasis has come from studies of anion transporter SLC26A6. Slc26a6-null mice have a defect in intestinal secretion of oxalate, which results in enhanced net oxalate absorption in the intestine leading to hyperoxalemia and hyperoxaluria.6,7 In addition, Slc26a6-null mice have a high incidence of CaOx stones.6 The source of the excess oxalate in the urine of Slc26a6-null mice is dietary oxalate.6 Therefore, SLC26A6-mediated back secretion of oxalate appears to play a critical role in limiting net intestinal absorption of dietary oxalate, which would otherwise result in hyperoxalemia, hyperoxaluria, and CaOx stones.
Despite its importance for oxalate homeostasis, the mechanism(s) for oxalate absorption in the intestine remain incompletely defined.8 Specifically, the relative magnitude of paracellular versus transcellular oxalate absorption is unclear. We have addressed this issue by performing a systematic comparison of absorptive fluxes of oxalate and paracellular marker mannitol along mouse intestine. Moreover, we have taken advantage of the availability of Slc26a6-null mice to avoid the possibility that SLC26A6-mediated back-secretion of oxalate across the apical membrane might mask transcellular oxalate absorption. Finally, we have used recent advances in understanding of tight junction structure and function to probe the molecular pathway for paracellular oxalate absorption. Using these approaches, we find that transepithelial oxalate absorption is predominantly if not exclusively passive and paracellular. Moreover, we demonstrate that oxalate traverses the paracellular pathway via the low capacity “leak” pathway for larger solutes rather than through claudin-based pores. These findings support the concept that the role of SLC26A6 in mediating active oxalate secretion is to counteract the passive paracellular absorption of ingested oxalate.
RESULTS
Comparison of Oxalate and Mannitol Fluxes in Mouse Duodenum
We had previously demonstrated the presence of a secretory flux of oxalate in the mouse duodenum that is dependent on the expression of apical transporter SLC26A6 and that therefore must be transcellular.6 We thus focused on defining the mechanism of oxalate absorption in this segment of the intestine so that we would be able to compare and contrast characteristics of oxalate absorption and secretion.
Using mouse duodenum mounted in an Ussing chamber, we performed measurements of transepithelial absorption and secretion of [14C]oxalate simultaneously with flux of [3H]mannitol. Mannitol is an inert, nonmetabolized sugar that is water-soluble and that cannot penetrate cell membranes. It has therefore been frequently used as a marker for transepithelial transport via the paracellular pathway.9,10
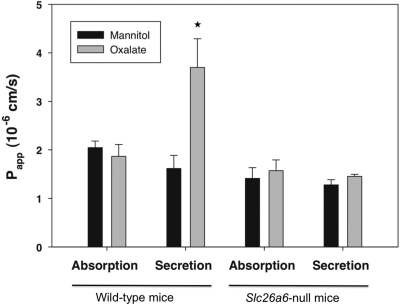
As shown in Figure 1, the apparent permeability (Papp) for unidirectional absorption of oxalate was the same as that of mannitol, suggesting that oxalate absorption is predominantly passive and paracellular. In contrast, the Papp for unidirectional secretion of oxalate exceeded that of mannitol in wild-type mice, indicating transcellular secretion of oxalate. In Slc26a6-null mice, the Papp values for absorptive fluxes of oxalate and mannitol were similar to each other and similar to their respective values in wild-type mice. However, in the absence of SLC26A6, the higher secretory Papp of oxalate compared with mannitol in wild-type mice was no longer observed. Moreover, in tissue from Slc26a6-null mice, the Papp values for absorptive and secretory fluxes of mannitol and oxalate were all virtually identical. These findings strongly suggest that in the absence of SLC26A6, transepithelial oxalate transport in both directions is largely passive and paracellular. These results therefore argue against any significant contribution of mediated transcellular transport to oxalate absorption. In addition, our findings support the concept that SLC26A6 is absolutely required for the transcellular secretion of oxalate in the mouse duodenum.6
Figure 1.
In the absence of SLC26A6, oxalate transport is largely passive and paracellular. Transepithelial absorption and secretion of [14C]oxalate were measured simultaneously with fluxes of [3H]mannitol across duodenum of wild-type and Slc26a6-null mice. The data are expressed as the means ± SEM (n = 8). *P < 0.05 versus oxalate absorptive flux.
Effect of the Anion Transport Blocker 4,4′-Diisothiocyanatostilbene-2,2′-disulfonate (DIDS) on Oxalate Fluxes
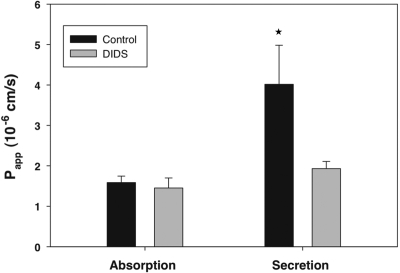
Characteristics of mediated transport include sensitivity to transport inhibitors. Recent molecular cloning and expression studies of members of the SLC26 transporter family have shown that at least two apical membrane transporters in addition to SLC26A6 are capable of transporting oxalate and are sensitive to DIDS. SLC26A3, also known as down-regulated in adenoma (DRA), has been shown to be present on the apical membrane11 in human and rat intestine12 and is capable of DIDS-sensitive oxalate transport when expressed in Xenopus oocytes.13 Similarly, SLC26A2 is expressed in the intestine of rats and humans14 and can function as a DIDS-sensitive oxalate transporter.15 Therefore, it is conceivable that these members of the SLC26 family are involved in mediating transcellular oxalate absorption. To evaluate this possibility, we examined the effect of DIDS on [14C]oxalate absorption in the duodenum. As shown in Figure 2, luminal addition of DIDS to wild-type duodenum did not affect unidirectional oxalate absorption. In contrast, as also shown in Figure 2, luminal DIDS reduced the unidirectional secretory flux of oxalate to that observed in Slc26a6-null mice (Figure 1). The latter finding again supports the concept that a component of oxalate secretion is transcellular and dependent on the activity of apical membrane SLC26A6, a DIDS-sensitive transporter.6,16 The lack of effect of apical DIDS on the absorptive oxalate flux provides additional evidence that oxalate absorption is largely passive and paracellular rather than mediated and transcellular.
Figure 2.
Lack of effect of the anion inhibitor DIDS on oxalate absorption. We examined the effect of DIDS on [14C]oxalate absorption and secretion in the duodenum. The data are expressed as the means ± SEM (n = 4). *P < 0.05 versus oxalate secretory flux in absence of DIDS.
Effect of Unlabeled Oxalate on [14C]Oxalate Fluxes
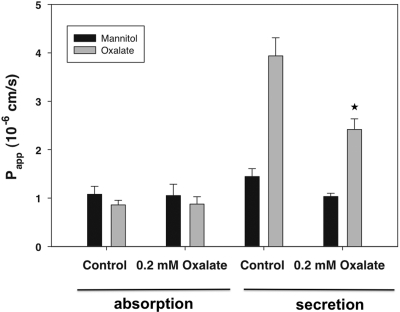
A characteristic of passive and paracellular transport is lack of substrate saturability. To evaluate this issue, we examined the effect of adding 0.2 mM unlabeled oxalate on absorption of 2 μM [14C]oxalate in the duodenum. As shown in Figure 3, the addition of a 100-fold excess of unlabeled oxalate to wild-type duodenum did not affect unidirectional absorption of [14C]oxalate. In contrast, as also shown in Figure 3, unlabeled oxalate significantly reduced the unidirectional secretory flux of [14C]oxalate. The latter finding again supports the concept that a component of oxalate secretion is mediated and transcellular. The lack of saturability of the absorptive oxalate flux provides additional evidence that oxalate absorption is largely passive and paracellular rather than mediated and transcellular.
Figure 3.
Lack of effect of unlabeled oxalate on [14C]oxalate absorption. We examined the effect of 0.2 mM unlabeled oxalate on absorption and secretion of 2 μM [14C]oxalate absorption and secretion in the duodenum. The data are expressed as the means ± SEM (n = 4). *P < 0.05 versus oxalate secretory flux in absence of unlabeled oxalate.
Effect of Transepithelial Potential Difference on Oxalate Absorption
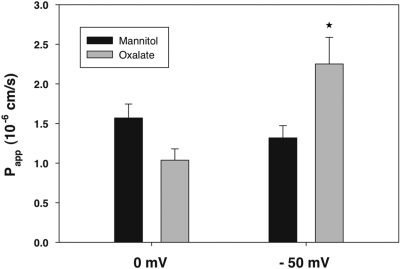
Oxalate is a divalent anion at physiologic pH. However, in physiologic solutions, a large fraction of oxalate is chelated with cations including Ca++, Mg++, and Na+. Shown in Table 1 is the calculated oxalate species in the solution used in our experiments. The average net charge of oxalate species is approximately −1. It would therefore be predicted that if oxalate absorption is predominantly passive and paracellular, it should be enhanced by imposition of a lumen-negative transepithelial potential difference. As shown in Figure 4, a lumen-negative potential significantly increased unidirectional oxalate absorption in accord with this prediction. In contrast, movement of mannitol remained unaffected, as expected for passive transport of a neutral molecule. (Of note, we observed slightly lower oxalate than mannitol flux at 0 mV as compared with our previous experiments but the difference was not statistically significant.) Again, these results are consistent with the concept that oxalate absorption in the mouse duodenum is predominantly passive and paracellular.
Table 1.
Calculated oxalate species in the solution
| Total calcium (mmol/L) | 1.20 | 1.20 |
| Ionized calcium (mmol/L) | 1.08 | 1.10 |
| Total magnesium (mmol/L) | 1.20 | 10.00 |
| Ionized magnesium (mmol/L) | 1.14 | 9.58 |
| Total oxalate (× 10−7 mol/L) | 20.00 | 20.00 |
| Ox2− (× 10−7 mol/L) | 7.90 | 3.30 |
| HOx− (× 10−7 mol/L) | 0.00 | 0.00 |
| H2Ox (× 10−7 mol/L) | 0.00 | 0.00 |
| CaOx (× 10−7 mol/L) | 2.64 | 1.01 |
| MgOx (× 10−7 mol/L) | 4.09 | 12.98 |
| NaOx− (× 10−7 mol/L) | 4.96 | 1.97 |
| KOx− (× 10−7 mol/L) | 0.18 | 0.07 |
| Ca2Ox2+ (× 10−7 mol/L) | 0.20 | 0.08 |
| Mg2Ox2+ (× 10−7 mol/L) | 0.02 | 0.59 |
| Net sum of charges of Ox species | −20.50 | −7.30 |
Figure 4.
A lumen-negative electrical gradient enhances oxalate absorption. We measured absorptive fluxes of [14C]oxalate and [3H]mannitol in the absence and presence of a lumen negative-electrical gradient. The data are expressed as the means ± SEM (n = 8). *P < 0.05 versus oxalate absorption in the absence of an electrical gradient.
Apparent Permeability Values for Absorption of Oxalate and Mannitol in Different Segments of Mouse Intestine
Having demonstrated that oxalate absorption in the duodenum is predominantly passive and paracellular, we surveyed the mechanism of oxalate absorption in distal segments of the intestine by comparing Papp for unidirectional absorption of oxalate and mannitol. Studies were performed in Slc26a6-null mice to avoid the possibility that SLC26A6-mediated back-secretion of oxalate across the apical membrane might mask transcellular oxalate absorption.
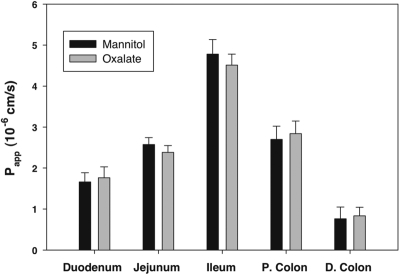
Papp for unidirectional absorption of mannitol and oxalate varied in different segments of small and large intestine as shown in Figure 5. We observed highest apparent permeability for both mannitol and oxalate in the ileum, and lowest permeability in the distal colon. Although we observed regional differences in mannitol and oxalate permeability, Papp for oxalate and mannitol varied in parallel. Moreover, in no segment did the Papp for absorptive flux of oxalate significantly exceed that of mannitol. Taken together, these findings indicate that oxalate and mannitol share a common route and mechanism of absorption in all segments of the intestine, namely the paracellular pathway.
Figure 5.
Oxalate and mannitol fluxes vary in parallel in different segments of the intestine. We measured absorptive fluxes of [14C]oxalate and [3H]mannitol along different segments of intestine of Slc26a6-null mice: duodenum, jejunum, ileum, proximal colon (P. colon), and distal colon (D. colon). The data are expressed as the means ± SEM (n = 4).
Effect of Inducing Claudin-10a Expression on Tight Junction Charge Selectivity and Oxalate Absorption
Having demonstrated that absorption of oxalate is predominantly passive and paracellular throughout the mouse intestine, we next investigated the molecular determinants of oxalate permeability across tight junctions. Recent studies of the tight junction have revealed that the paracellular barrier has at least two physiologic components. One component is a higher capacity pore-like pathway with a steep size dependence for solutes less than about 4 Å in radius.17,18 It is largely regulated by claudins, which are responsible for charge selectivity.19 The second component is a lower capacity “leak” pathway for solutes larger than ∼4 Å in radius that does not show selectivity for ionic charge and size.20 The effective radius of oxalate has been estimated to be around 3 to 4 Å on the basis of its structure and negative charge size.21 In addition, oxalate forms soluble complexes with Na+, Ca++, and Mg++ that can increase its calculated size.22 Mannitol has an estimated size of 4.2 Å18 and has been demonstrated to traverse the size-independent “leak” pathway.23
To test the hypothesis that oxalate traverses the claudin-based pores, we used Madin-Darby canine kidney (MDCK) II cells with inducible expression of claudin-10a. It has previously been shown that expression of claudin-10a markedly affects the charge selectivity of the paracellular pathway by increasing anion permeability as measured by dilution potentials.24 Because, as described above, free oxalate is a divalent anion and the average net charge of oxalate species in our solution is −1, we would predict that oxalate flux should be enhanced by induction of claudin-10a expression if transport occurs through the claudin-based pore pathway.
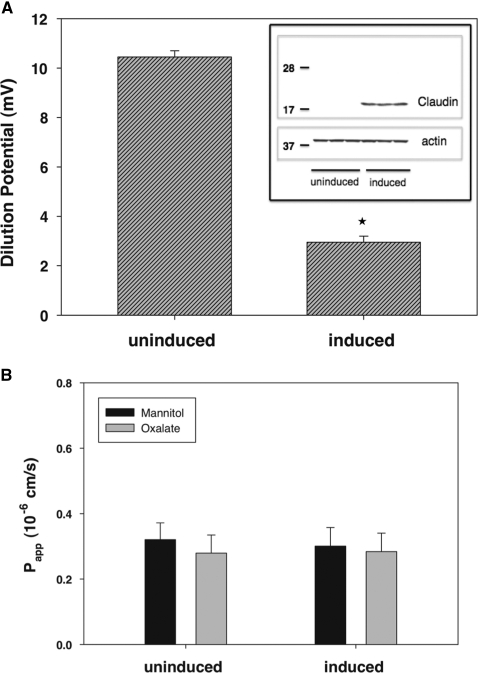
The inset of Figure 6A shows an immunoblot confirming doxycycline-regulated induction of claudin-10a expression under the conditions of our flux measurements. We next confirmed the findings of Van Itallie et al.24 that expression of claudin-10a markedly affected charge selectivity as measured by dilution potential. As shown in Figure 6A, in the absence of induced claudin-10a expression, MDCK II cells form a paracellular barrier with cation preference as revealed by the lumen-positive dilution potential when a serosal-to-basolateral NaCl gradient is imposed. Induction of claudin-10a results in a sharp decrease in this dilution potential. Transepithelial resistance was unaffected by expression of claudin-10a (data not shown), consistent with an opposing decrease in Na+ permeability and increase in Cl− permeability as reported by Van Itallie et al.24
Figure 6.
Lack of effect of claudin-10a expression on oxalate and mannitol permeability. (A) We measured dilution potentials (mV) across MDCK II monolayers following a 120 to 60 mM apical NaCl dilution without and with induction of claudin-10a expression. Immunoblot confirming claudin-10a induction is shown in the inset. The data are expressed as the means ± SEM (n = 4). *P < 0.05 versus uninduced cells. (B) Despite an effect of claudin-10a on anion permeability as measured by dilution potential, oxalate and mannitol permeability remained unaffected by induction of claudin-10a. The data are expressed as the means ± SEM (n = 4).
We next tested whether induction of claudin-10a also increases oxalate permeability. Similar to our studies in the intestine, we measured the Papp for unidirectional absorption of [14C]oxalate and [3H]mannitol. As shown in Figure 6B, induction of claudin-10a had no effect on oxalate flux, suggesting that oxalate does not traverse this claudin-dependent pathway. We similarly found a lack of effect of induced claudin-10a expression on Papp for oxalate absorption in LLC-PK1 cells (not shown), under which conditions there is a marked increase in tight junction anion selectivity.24
Effect of ZO-1 Knockdown on the Tight Junction “Leak” Pathway and Oxalate Absorption
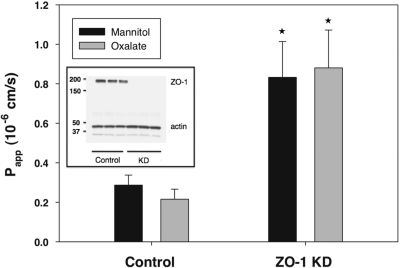
We next tested the hypothesis that oxalate traverses the tight junction through the lower-capacity, size-independent pathway. It has previously been shown that ZO-1 knockdown selectively increases the permeability of the low capacity pathway for larger molecules such as mannitol.23 The inset of Figure 7 shows an immunoblot demonstrating doxycycline-induced knockdown of ZO-1 expression under the conditions of our flux measurements. As shown in Figure 7, we confirmed that ZO-1 knockdown increases Papp for unidirectional mannitol absorption. ZO-1 knockdown caused an equivalent increase in Papp for unidirectional oxalate absorption. These findings are consistent with the concept that oxalate and mannitol share the size-independent “leak” pathway across the tight junctions of epithelial cells.
Figure 7.
Permeability of oxalate and mannitol increased in parallel with knockdown of ZO-1. Unidirectional absorptive fluxes of [14C]oxalate and [3H]mannitol were measured in control and ZO-1 knockdown cells (ZO-1 KD). Immunoblot confirming ZO-1 knockdown is shown in inset. The data are expressed as the means ± SEM (n = 4). *P < 0.05 of oxalate and mannitol permeability in ZO-1 knockdown cells versus oxalate and mannitol permeability, respectively, in control cells.
Effect of Cation Complex Formation on Oxalate Fluxes in Mouse Duodenum
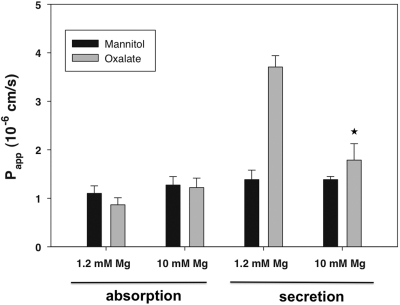
If epithelial oxalate absorption is largely through the size-independent “leak” pathway across the tight junctions, we would predict that permeability to oxalate should be independent of its forming soluble complexes with cations. As shown in Table 1, raising the medium magnesium concentration from 1.2 to 10 mM is calculated to decrease the free oxalate concentration from 0.79 to 0.33 μM as more oxalate becomes complexed with magnesium. However, as shown in Figure 8, raising the medium magnesium concentration had no effect on the apparent permeability for oxalate absorption. This lack of dependence of oxalate absorption on the free oxalate concentration provides additional support for the concept that it takes place via the size-independent “leak” pathway across the tight junction rather than by a mediated transcellular process. In contrast to the lack of effect of oxalate speciation on oxalate absorptive flux, the active component of oxalate secretion was greatly inhibited when the free oxalate concentration was decreased.
Figure 8.
Lack of effect of medium magnesium concentration on oxalate absorption. We examined the effect of increasing MgCl2 in the medium from 1.2 to 10 mM on oxalate absorption and secretion in the duodenum. The data are expressed as the means ± SEM (n = 4). *P < 0.05 versus oxalate secretory flux in presence of 1.2 mM magnesium.
DISCUSSION
We have demonstrated that the apparent permeability for unidirectional absorption of [14C]oxalate was identical to that of the paracellular marker mannitol in mouse duodenum and was inhibited neither by luminal application of the anion transport inhibitor DIDS nor by an excess of unlabeled oxalate. These findings are consistent with the concept that oxalate absorption in the mouse duodenum is passive and paracellular. In contrast, the apparent permeability for secretion of oxalate exceeded that of mannitol. The excess permeability for secretion of oxalate compared with mannitol was abolished by luminal application of DIDS and was absent in duodenum of Slc26a6-null mice. Moreover, the secretory flux of [14C]oxalate was significantly inhibited by unlabeled oxalate. These findings support the conclusion that the secretory flux of oxalate has two components: a paracellular flux with identical apparent permeability to mannitol, and a transcellular flux that is completely dependent on apical membrane SLC26A6 activity. In addition, these findings validate comparison of apparent permeability of oxalate and mannitol as a strategy to detect transcellular oxalate transport. We therefore screened for transcellular oxalate absorption in different segments of small and large intestine by comparing apparent permeability of oxalate and mannitol. Despite large regional differences in the absolute values, permeability to oxalate and mannitol varied in parallel, and in no intestinal segment did we detect a significantly higher permeability for absorption of oxalate compared with mannitol. Taken together, these studies indicate that oxalate absorption in the intestine is predominantly if not exclusively passive and paracellular. Our results are consistent with previous findings indicating an important role of passive oxalate absorption in the intestine.25,26 Nevertheless, we cannot exclude the possibility that a component of transcellular oxalate absorption was not detected because of the absence of a critical substrate or regulatory factor under the conditions of our experiments. Indeed, a component of active transcellular oxalate absorption was identified in the rabbit colon on the basis of sensitivity of oxalate absorptive flux to metabolic inhibitors and the disulfonic stilbene SITS.27 However, flux of oxalate was not compared with mannitol in that study, and it is possible that metabolic inhibitors and SITS can affect paracellular permeability.28
Given the importance of the paracellular pathway in mediating oxalate absorption, we then investigated the mechanism by which oxalate traverses the tight junction in epithelial cells. By use of epithelial cell lines with altered claudin expression, we demonstrated that oxalate permeability was unaffected by modulation of the charge selectivity of the claudin-based pore pathway. However, because we only manipulated the expression of a single claudin, namely claudin-10a, we cannot exclude the possibility that oxalate transport would be changed by alteration of other claudins affecting charge selectivity. In contrast to the lack of effect of claudin-10a, when permeability of the size-independent “leak” pathway was increased by knockdown of the tight junction protein ZO-1, permeability to oxalate and mannitol was enhanced in parallel. Consistent with the concept that oxalate absorption takes place via the size-independent “leak” pathway, it was unaffected when the free oxalate concentration was reduced in favor of increased formation of soluble complexes with magnesium. Our finding that forming soluble complexes with cations does not impede absorption of oxalate suggests that the previously observed effects of oral calcium and magnesium to inhibit oxalate absorption in vivo29 is due to another mechanism, such as promoting luminal precipitation of calcium oxalate out of solution.
Our results indicating that oxalate is mainly absorbed by the paracellular “leak” pathway may be relevant to the observation that inflammatory bowel disease has been associated with absorptive hyperoxaluria and increased risk for CaOx stones.30 The permeability of the low capacity, size-independent, paracellular “leak” pathway can be enhanced by pro-inflammatory cytokines without altering the claudin-based pore pathway.31 Therefore, inflammatory intestinal disorders may be associated with an increased permeability of the low capacity “leak” pathway to oxalate, thereby predisposing to hyperoxalemia, hyperoxaluria, and CaOx stone formation.
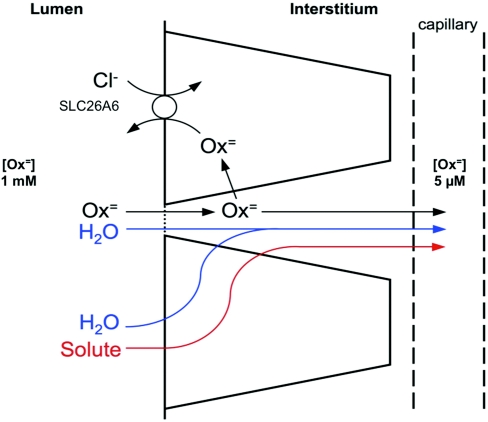
The findings of this study led us to propose the model of intestinal oxalate handling depicted in Figure 9 in which oxalate absorption is passive and paracellular across the tight junction. The main driving force for this passive absorption is the large concentration gradient of oxalate from lumen to blood (>100-fold) that has been estimated to be present when an oxalate-containing diet is ingested.32 In addition, there may be solvent drag through the tight junction coupled to water flux associated with transcellular absorption of solutes like Na+ and glucose.33 Given the unavoidable passive absorption of the potentially toxic oxalate through the “leak” pathway of the tight junction, transcellular oxalate secretion plays a pivotal role in mediating backflux of oxalate to limit its net absorption. Apical membrane SLC26A6 activity is required for transcellular oxalate secretion. The important role of oxalate secretion in counteracting passive oxalate absorption is underscored by the absorptive hyperoxaluria that occurs in Slc26a6-null mice.6 We hypothesize that in the intestine, the role of SLC26A6 in mediating active oxalate secretion is to counteract the passive paracellular absorption of ingested oxalate.
Figure 9.
Proposed model of epithelial oxalate transport. Oxalate absorption is largely passive and paracellular across the tight junction. Oxalate then is back-secreted by a transcellular route requiring apical membrane SLC26A6.
In conclusion, the results of this study indicate that net absorption of dietary oxalate depends on the relative balance between oxalate absorption through the paracellular “leak” pathway and transcellular oxalate secretion dependent on SLC26A6 activity. Accordingly, inherited or acquired defects that enhance paracellular “leak” or reduce SLC26A6 activity are potential molecular mechanisms causing enteric hyperoxaluria and increased risk for calcium oxalate kidney stones. Conversely, therapeutic interventions that reduce paracellular oxalate absorption or enhance SLC26A6 activity may lower urinary oxalate and diminish risk for calcium oxalate kidney stones.
CONCISE METHODS
Measurement of Oxalate and Mannitol Fluxes across Mouse Intestine
Intestinal segments of wild-type and Slc26a6-null mice were opened longitudinally along the mesenteric border and mounted as an intact sheet in a modified Ussing chamber that had an exposed surface area of 0.30 cm2. We bathed mucosal and serosal surfaces with 8 ml of warmed (37°C), oxygenated bicarbonate Ringer's solution (140 mM Na+, 119.8 mM Cl−, 5.2 mM K+, 1.2 mM Ca2+, 1.2 mM Mg2+, 25 mM HCO3−, 2.4 mM HPO4−, 0.4 mM H2PO42−, and 10 mM glucose at pH 7.4). We measured transepithelial short-circuit current (ISC) and total tissue conductance (GTE), as described previously.6 We added 2 μM [14C]oxalate (specific activity, 117 mCi/mmol; Amersham Biosciences) and 0.08 μM [3H]mannitol (specific activity, 20 Ci/mmol; MP Biomedicals, Santa Ana, CA) to either the mucosal or serosal bath and unlabeled 2 μM oxalate and 0.08 μM mannitol to the opposite bath. After a 150-minute equilibration period to achieve steady-state flux rates, we collected samples before and after the 60-minute flux period to calculate values for unidirectional mucosa-to-serosa flux (JMS) and serosa-to-mucosa flux (JSM). We performed all flux studies under voltage clamp conditions using a multichannel voltage/current clamp model VCC MC6 (Physiologic Instruments, San Diego, CA). The apparent permeability coefficients (Papp) for oxalate and mannitol were calculated according to the following equation,
J = Papp × A × [S]
where J is the flux (mucosa-to-serosa or serosa-to-mucosa), A is the cross-sectional tissue area of the Ussing chamber, and [S] is the substrate concentration of [14C]oxalate or [3H]mannitol. All of the animal protocols were approved by the Yale University Institutional Animal Care and Use Committee. The calculation of oxalate speciation shown in Table 1 was made using SUPERSAT, the updated version of a program originally published in 1969.34 This program calculates the complete speciation of ions important in the stone-forming process in various biologic media.
Measurement of Dilution Potential, Oxalate, and Mannitol Fluxes in MDCK II Cells
MDCK II cells with inducible expression of claudin-10a and knockdown of ZO-1 were used as described previously.23,24 The cells were cultured under standard conditions in DMEM (4.5 g/L glucose), 10% Tet-system approved fetal bovine serum (Clontech Laboratories, Mountain View, CA), and penicillin/streptomycin. The cells were maintained in the presence of 50 ng/ml doxycycline to repress transgene expression and plated for experiments on removable filters (Snapwell; Corning Life Sciences, Acton, MA) in the presence (not induced) or absence (induced) of doxycycline. Four days after transgene induction, the filters were placed in a modified Ussing chamber with exposed surface area 1.12 cm2. Transmonolayer resistance was determined in a (apical and basal) solution (buffer A) containing 120 mM NaCl, 10 mM HEPES, 5 mM KCl, 10 mM NaHCO3, 1.2 mM CaCl2, 1 mM MgSO4 and 10 mM Glucose at pH 7.4. Dilution potentials were then determined after replacing the apical solution with a solution (buffer B) containing 60 mM NaCl, 10 mM HEPES, 5 mM KCl, 10 mM NaHCO3, 1.2 mM CaCl2, 1 mM MgSO4, 10 mM glucose, and 120 mM mannitol at pH 7.4. For radioactive tracer studies, we added 2 μM [14C]oxalate and 0.08 μM [3H]mannitol to the mucosal bath and unlabeled 2 μM oxalate and 0.08 μM mannitol to the serosal side. After a 30-minute equilibration period, we collected samples before and after the 60-minute flux period to calculate values for unidirectional mucosa-to-serosa flux. Papp was calculated as described above.
Immunoblotting
Immunoblots were performed on cells grown on filters after induction of protein. The filters were excised and placed in TBS 1% Triton buffer for 30 minutes, cleared by centrifugation for 15 minutes at 21,000 × g, and stored at −80°C until used for analysis. Equal volumes of lysate were subjected to SDS-PAGE and then transferred to nitrocellulose and immunoblotted. Claudin-10a was detected using a monoclonal antibody as previously reported.24 ZO-1 was detected using an antibody from Invitrogen (catalog number 33-9100). Detection was performed using enhanced chemiluminescence (PerkinElmer Life Sciences) after incubation in horseradish peroxidase-coupled secondary antibodies (anti-mouse from Jackson Immunoresearch, West Grove, PA).
DISCLOSURES
None.
Acknowledgments
The authors would like to thank Thecla Abbiati for her assistance with animal care. The work was funded by National Institutes of Health Grant R37DK33793 (to P.S.A.), a National Kidney Foundation Postdoctoral Fellowship (to F.K.), and an American Society of Nephrology Student Scholar Grant (to N.K.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Gillen DL, Worcester EM, Coe FL: Decreased renal function among adults with a history of nephrolithiasis: A study of NHANES III. Kidney Int 67: 685–690, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Robertson WG, Peacock M: The cause of idiopathic calcium stone disease: Hypercalciuria or hyperoxaluria? Nephron 26: 105–110, 1980 [DOI] [PubMed] [Google Scholar]
- 3. Worcester EM, Coe FL: Clinical practice: Calcium kidney stones. N Engl J Med 363: 954–963, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coe FL, Parks JH, Asplin JR: The pathogenesis and treatment of kidney stones. N Engl J Med 327: 1141–1152, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Sutton RA, Walker VR: Enteric and mild hyperoxaluria. Miner Electrolyte Metab 20: 352–360, 1994 [PubMed] [Google Scholar]
- 6. Jiang Z, Asplin JR, Evan AP, Rajendran VM, Velazquez H, Nottoli TP, Binder HJ, Aronson PS: Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat Genet 38: 474–478, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Freel RW, Hatch M, Green M, Soleimani M: Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am J Physiol Gastrointest Liver Physiol 290: G719–G728, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Hatch M, Freel RW: Intestinal transport of an obdurate anion: Oxalate. Urol Res 33: 1–16, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Hollander D: Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep 1: 410–416, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Karbach U: Segmental heterogeneity of cellular and paracellular calcium transport across the rat duodenum and jejunum. Gastroenterology 100: 47–58, 1991 [DOI] [PubMed] [Google Scholar]
- 11. Walker NM, Simpson JE, Brazill JM, Gill RK, Dudeja PK, Schweinfest CW, Clarke LL: Role of down-regulated in adenoma anion exchanger in HCO3- secretion across murine duodenum. Gastroenterology 136: 893–901, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Byeon MK, Westerman MA, Maroulakou IG, Henderson KW, Suster S, Zhang XK, Papas TS, Vesely J, Willingham MC, Green JE, Schweinfest CW: The down-regulated in adenoma (DRA) gene encodes an intestine-specific membrane glycoprotein. Oncogene 12: 387–396, 1996 [PubMed] [Google Scholar]
- 13. Silberg DG, Wang W, Moseley RH, Traber PG: The down regulated in adenoma (dra) gene encodes an intestine-specific membrane sulfate transport protein. J Biol Chem 270: 11897–11902, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Haila S, Hastbacka J, Bohling T, Karjalainen-Lindsberg ML, Kere J, Saarialho-Kere U: SLC26A2 (diastrophic dysplasia sulfate transporter) is expressed in developing and mature cartilage but also in other tissues and cell types. J Histochem Cytochem 49: 973–982, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Heneghan JF, Akhavein A, Salas MJ, Shmukler BE, Karniski LP, Vandorpe DH, Alper SL: Regulated transport of sulfate and oxalate by SLC26A2/DTDST. Am J Physiol Cell Physiol 298: C1363–C1375, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS: Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci U S A 98: 9425–9430, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watson CJ, Rowland M, Warhurst G: Functional modeling of tight junctions in intestinal cell monolayers using polyethylene glycol oligomers. Am J Physiol Cell Physiol 281: C388–C397, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Anderson JM, Van Itallie CM: Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1:a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Angelow S, Ahlstrom R, Yu AS: Biology of claudins. Am J Physiol Renal Physiol 295: F867–F876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knipp GT, Ho NF, Barsuhn CL, Borchardt RT: Paracellular diffusion in caco-2 cell monolayers: Effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J Pharm Sci 86: 1105–1110, 1997 [DOI] [PubMed] [Google Scholar]
- 21. Verkoelen CF, Romijn JC: Oxalate transport and calcium oxalate renal stone disease. Urol Res 24: 183–191, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Buchner R, Samani F, May PM, Sturm P, Hefter G: Hydration and ion pairing in aqueous sodium oxalate solutions. Chemphyschem 4: 373–378, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Van Itallie CM, Fanning AS, Bridges A, Anderson JM: ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol Biol Cell 20: 3930–3940, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM: Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol 291: F1288–F1299, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Binder HJ: Intestinal oxalate absorption. Gastroenterology 67: 441–446, 1974 [PubMed] [Google Scholar]
- 26. Kathpalia SC, Favus MJ, Coe FL: Evidence for size and charge permselectivity of rat ascending colon: Effects of ricinoleate and bile salts on oxalic acid and neutral sugar transport. J Clin Invest 74: 805–811, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hatch M, Freel RW, Goldner AM, Earnest DL: Oxalate and chloride absorption by the rabbit colon: Sensitivity to metabolic and anion transport inhibitors. Gut 25: 232–237, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weinstein SW, Jones SM, Weinstein RJ: Evidence that alteration of charge modifies proximal tubular shunt pathway permselectivity. Am J Physiol 257: F1079–F1086, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Liebman M, Costa G: Effects of calcium and magnesium on urinary oxalate excretion after oxalate loads. J Urol 163: 1565–1569, 2000 [PubMed] [Google Scholar]
- 30. Parks JH, Worcester EM, O'Connor RC, Coe FL: Urine stone risk factors in nephrolithiasis patients with and without bowel disease. Kidney Int 63: 255–265, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Watson CJ, Hoare CJ, Garrod DR, Carlson GL, Warhurst G: Interferon-gamma selectively increases epithelial permeability to large molecules by activating different populations of paracellular pores. J Cell Sci 118: 5221–5230, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Jaeger P, Robertson WG: Role of dietary intake and intestinal absorption of oxalate in calcium stone formation. Nephron Physiol 98: 64–71, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Krugliak P, Hollander D, Schlaepfer CC, Nguyen H, Ma TY: Mechanisms and sites of mannitol permeability of small and large intestine in the rat. Dig Dis Sci 39: 796–801, 1994 [DOI] [PubMed] [Google Scholar]
- 34. Robertson WG: Measurement of ionized calcium in biological fluids. Clin Chim Acta 24: 149–157, 1969 [DOI] [PubMed] [Google Scholar]