In cancer, despite morphological and phenotypic similarities, G-MDSCs are functionally distinct from mature neutrophils and are comprised of pathologically activated precursors of neutrophils.
Keywords: cancer, granulocytes
Abstract
MDSCs are a group of cells with potent immune-suppressive activity. These cells accumulate in many pathologic conditions and play a major role in the regulation of immune responses. The nature of MDSC remains highly debatable. In cancer, most MDSCs are represented by cells with granulocytic phenotype and morphology, G-MDSC. The relationship between G-MDSCs and Neu remains unclear. In this study, we have found that G-MDSCs, from tumor-bearing, and Neu, from tumor-free, mice share a common morphology and phenotype. However, in contrast to Neu, a substantial proportion of G-MDSCs expressed M-CSFR and a CD244 molecule. Neu had significantly higher phagocytic activity, expression of lysosomal proteins, and TNF-α than corresponding G-MDSCs, which had significantly higher activity of arginase, MPO, and ROS. In contrast to G-MDSC, neither rested nor mobilized Neu suppressed T cells. G-MDSC survived 2 days in culture in the presence of GM-CSF and within 24 h, became phenotypic and functionally similar to Neu. Tumor-associated G-MDSC shared most characteristics of splenic G-MDSC, rather then Neu. These data suggest that in cancer, despite morphological and phenotypic similarities, G-MDSCs are functionally distinct from Neu and are comprised of pathologically activated precursors of Neu.
Introduction
In recent years, MDSC emerged as an important regulator of immune responses in many pathologic conditions, including cancer, chronic infectious diseases, trauma, sepsis, and others [1–3]. In cancer, their role extended beyond the immune system, as these cells were implicated in promoting tumor angiogenesis, tumor cell invasion, and metastases [4–6]. MDSC is a heterogeneous group of myeloid cells characterized by potent immune-suppressive activity. In mice, they are generally characterized as Gr-1+CD11b+ cells. Under physiological conditions, immature myeloid cells with this phenotype are present in bone marrow and at a much lesser extent, in the spleen. They lack immune-suppressive activity. In contrast, in a tumor-bearing host, there is a dramatic expansion of cells with the same phenotype and immune-suppressive activity in various tissues [1]. In recent years, two major groups of cells that comprise MDSCs were identified: cells with morphology and phenotype (CD11b+Ly6ChighLy6G–) typical for monocytes—M-MDSCs—and cells with morphology and phenotype (CD11b+Ly6ClowLy6G+) typical for granulocytes—G-MDSCs [7–9]. M-MDSCs consist of immature myeloid cells with the ability to differentiate to MΦ and DCs, whereas the nature of G-MDSCs remains largely obscure. These cells do not survive in culture and do not differentiate to MΦ and DCs [8]. Their relationship with mature Neu remained unclear. G-MDSCs are the largest population of MDSCs in tumor-bearing mice, representing >75% of all MDSCs. They suppress antigen-specific T cell responses, primarily via release of ROS [7, 8]. G-MDSCs have also been described in cancer patients [10, 11].
Neu are terminally differentiated cells with a rather controversial role in cancer. Evidence pointed to their ability to promote tumor growth as well as kill tumor cells (reviewed in ref. [12]). Because of their morphology, G-MDSCs are considered by some investigators as tumor-associated, activated Neu [13]. Whether this is indeed the case, the possible relationship between Neu and G-MDSC remains unclear. Neu and G-MDSC share similar granulocytic morphology and CD11b+Ly6G+Ly6Clow phenotype, which makes the separation of these cells within the same host very difficult. Elucidation of the nature of G-MDSCs in cancer, vis-à-vis Neu, would be very important, not only for better understanding the biology of these cells in cancer but also for identifying the specific role of different cellular components that comprise the tumor microenvironment in regulating tumor growth.
G-MDSCs accumulate in spleens of tumor-bearing mice, considered a “classic” source of these cells, as well as in the tumor site and bone marrow. The main challenge was to determine the best source of Neu. The question we tried to address was whether G-MDSCs in tumor-bearing mice were different from mature Neu. Specific markers allowing distinction between Neu and G-MDSCs did not exist; consequently, tumor-bearing mice could not be used as a source of Neu. Therefore, we compared CD11b+Ly6G+Ly6Clow granulocytic cells isolated from spleen, blood, bone marrow, and cells, mobilized to peritoneum from tumor-bearing and naïve tumor-free mice. Here, we report that G-MDSCs and Neu, although sharing similar morphology and phenotype, have several important differences in the expression of surface receptors and functional activity, which allowed us to determine the nature of the relationship between G-MDSCs and Neu in cancer.
MATERIALS AND METHODS
Mice and tumor models
Research was approved by the University of South Florida Institutional Animal Care and Use Committee (Tampa, FL, USA). Female C57BL/6 mice were obtained from the NCI (Frederick, MD, USA). Mice were kept in pathogen-free conditions and handled in accordance with the requirements of the guidelines for animal experiments. The following s.c. tumor models in C57BL/6 mice were used: EL-4 thymoma, B16F10 melanoma, LLC [obtained from American Type Culture Collection (Manassas, VA, USA)], and MC38 colon carcinoma, provided by Irina Turkova (University of Pittsburgh, Pittsburgh, PA, USA).
Reagents
RPMI 1640, DMEM, FBS, mrGM-CSF, and antibiotics were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). The following antibodies were used for flow cytometry: CD11b (M1/70; 552850, 557657; BD Biosciences, San Jose, CA, USA), Gr-1 (RB6-8C5; BD Biosciences), Ly-6G (1A8; BD Biosciences), Ly-6C (AL-21; BD Biosciences), CD124 (mIL4Rα-M1; BD Biosciences), CD184 (2B11/CXCR4; BD Biosciences), CD115 (AFS98; eBioscience, San Diego, CA, USA), CD244 (eBio244F4; eBioscience), and F4/80 (CI:A3-1; Serotec, UK). The following antibodies were used for immunofluorescence microscopy: rabbit anti-LAMP2 (ab81479; Abcam, Cambridge, MA, USA), rabbit anti-PSMA5 (2457S; Cell Signaling Technology, Beverly, MA, USA), rabbit anti-EEA1 (ab2900, Abcam), and anti-rabbit IgG (Invitrogen Life Technologies). The following antibodies and reagents were used for cell isolations: F4/80-biotin (CI:A3-1; Serotec), Ly-6G-biotin (1A8; Miltenyi Biotec, Auburn, CA, USA), and Streptavidin MicroBeads (Miltenyi Biotec). Antipentraxin antibody was provided by Dr. Alberto Mantovani (Instituto Clinico Humanitas, Rozzano, Italy).
Neu isolation
Peritoneal mouse Neu were isolated, as described previously, with some modification [14]. Briefly, mice received two i.p. injections of 12% casein (1.5 ml) within 16-h intervals and were killed 3 h later. Cells were collected by peritoneal lavage with PBS. The red cells were removed using ACK lysis buffer. To remove MΦ, F4/80-positive cells were depleted using magnetic beads. Ly6G-positive cells were then isolated using magnetic beads and MidiMACS, according to the manufacturer′s protocol (Miltenyi Biotec). The purity of Neu (CD11b+Ly6ClowLy6G+F4/80– cells) was ∼90%, as determined by flow cytometry, and the viability was >95%, as determined by trypan blue exclusion.
G-MDSC isolation
Ly6G-positive cells were isolated from spleens of tumor-bearing mice using magnetic beads and MidiMACS, according to the manufacturer′s protocol. The purity of G-MDSC (CD11b+Ly6G+Ly6Clow) populations was >90%, as determined by flow cytometry, and the viability was >95%.
Flow cytometry
FACS data were acquired using a FACSCalibur or LSR II cytometer (BD Biosciences) and were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Immunofluorescence microscopy
Cells were placed on cytospin slides (Cytoslide, Thermo Fisher Scientific, Pittsburgh, PA, USA), fixed with Cytofix/Cytoperm solution (Becton Dickinson, Franklin Lakes, NJ, USA) for 20 min, washed with PBS, and labeled with rabbit antibodies specific for LAMP2, PSMA5, or EEA1 at a 1:100, 1:33, or 1:200 dilution, respectively, for 1 h. Secondary antibody (anti-rabbit IgG, Alexa Fluor 647 or 594) was used to label the cells. The slides were washed, dried, and mounted with antifade mount containing DAPI and observed under a Zeiss fluorescence microscope. Fluorescence intensity was quantified using Image-Pro Plus (Media Cybernetics, Bethesda, MD, USA). Briefly, image pixels were ascribed values ranging from 0 (black) to 255 (white) on a 256-value gray scale, and the intensity of red fluorescence/cell was obtained as total gray-scale value over all pixels contained in the area of a single cell. The average value of fluorescence intensity from 50 cells was presented.
Phagocytosis assay
Cells, suspended in RPMI medium (5×105 cells in 100 μl), were added into the wells of a 96-well plate. Fluorescent red latex beads (100 μl, 8%, 2 μm diameter, L3030, Sigma-Aldrich, St. Louis, MO, USA) were added, and the cells were incubated at 37°C for 1 h. The cells were washed with PBS and labeled with CD11b, Ly6G antibodies, and DAPI. Fluorescence, derived from latex beads, ingested by cells, was measured using an LSR II cytometer.
ROS production
Oxidation-sensitive dye DCFDA (C6827, Molecular Probes/Invitrogen Life Technologies) was used for the measurement of ROS production. Cells were labeled with surface markers, washed, and incubated at 37°C in the serum-free RPMI media in the presence of 3 μM DCFDA, with or without 1 mg/ml opsonized zymosan (Z4250, Sigma-Aldrich). After incubation for 30 min, the cells were analyzed using flow cytometry.
NO production
Equal volumes of culture supernatants (100 μl) were mixed with Greiss reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% N-1-naphthylethylenediamine dihydrochloride in double-distilled water). After incubation at room temperature for 10 min, the absorbance at 550 nm was measured using a microplate plate reader (Bio-Rad, Hercules, CA, USA). Nitrite concentrations were determined by comparing the absorbance values for the test samples with a standard curve generated by a serial dilution of 0.25 mM sodium nitrite.
Arginase activity
Arginase activity was measured in cell lysates, as described previously [15]. Briefly, cells were lysed for 30 min with 100 μl 0.1% Triton X-100. Subsequently, 100 μl 25 mM Tris-HCl and 10 μl 10 mM MnCl2 were added, and the enzyme was activated by heating for 10 min at 56°C. Arginine hydrolysis was conducted by incubating the lysate with 100 μl 0.5 M l-arginine (pH 9.7) at 37°C for 120 min. The reaction was stopped with 900 μl H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v). Urea concentration was measured at 540 nm after addition of 40 μl α-isonitrosopropiophenone (dissolved in 100% ethanol).
MPO activity
The activity of MPO was determined using a fluorescent MPO detection kit (FLMPO 100-3, Cell Technology, Mountain View, CA, USA), according to the manufacturer′s protocol. Fluorescent signal was measured by an Envision 2102 multilabel plate reader (Perkin Elmer, Waltham, MA, USA).
IFN-γ ELISPOT
As responder cells, total splenocytes from OT-I or HA-TCR transgenic mice were used. The number of IFN-γ-producing cells, in response to peptide-specific stimulation (1 μg/ml OVA-derived peptide SIINFEKL for OT-I transgenic mice or control peptides), was evaluated in an ELISPOT. The number of spots was counted in triplicate and calculated, using an automatic ELISPOT counter (Cell Technology).
Phosphoprotein analysis
Protein lysates were prepared in a standard RIPA buffer, as described previously [16]. An aliquot of the lysate adjusted to a concentration of 200 μg in 50 μl/sample was used for subsequent assay. The presence of phosphorylated IκBα (Ser32/36), ERK1/2 (Thr202/Tyr204, Thr185/Tyr187), p38 MAPK (Thr180/Tyr182), JNK (Thr183/Tyr185), and c-Jun (Ser63) was detected by the Bio-Plex phosphoprotein assay (Bio-Rad), according to the manufacturer′s protocol. Data were collected and analyzed using the luminex-based LiquiChip system (Qiagen, Valencia, CA USA). The phosphorylation level for each signaling molecule is shown as the MFI.
Sample processing for microarray analysis
Total RNA (2 μg) served as the mRNA source for microarray analysis. The poly(A) RNA was converted to cDNA, amplified, and labeled with biotin, following the procedure described initially by Van Gelder et al. [17]. Hybridization with the biotin-labeled RNA, staining, and scanning of the chips followed the prescribed procedure, as outlined in the Affymetrix technical manual [18]. Scanned output files were analyzed using the Affymetrix GCOS. Signal intensity was scaled to an average intensity of 500, prior to comparison analysis. Using the default settings, GCOS identifies increases and decreases in gene copy number between any two samples with a statistical algorithm that assesses the behavior of 11 different oligonucleotide probes designed to detect the same gene [19]. Probe sets that yielded a change with a P value <0.002 were identified as changed (increased or decreased), and those that yielded a P value between 0.002 and 0.002667 were identified as marginally changed. A gene was identified as consistently changed if it was identified as changed in all replicate experiments by the software. A log-base 2 transformation was applied to the data. Each array was normalized (centered) by extracting the median over the entire array. Large intensities (values that were >10,000) were truncated to be equal to 10,000. Microarray data were deposited in the GEO (Accession Number GSE24102).
Quantitative real-time PCR
Total RNA was extracted from cells with Trizol (Invitrogen Life Technologies). Traces of DNA were removed by treatment with DNase I. The cDNA was synthesized from 1 μg total RNA using random hexamers and Superscript II RT (Invitrogen Life Technologies). PCR was performed using Taqman Universal PCR master mix (Applied Biosystems, Foster City, CA, USA) and target gene assay mix containing sequence-specific primers for Arg1 and 6-carboxyfluorescein dye-labeled Taqman minor groove binder probe (assay ID Mm00500821_m1; Applied Biosystems). To detect expression of TNF and MPO, PCR was performed with SYBR master mixture (Applied Biosystems) and the following primers: mouse TNF forward primer: 5′-TAGCCCACGTCGTAGCAAAC-3′ and reverse primer: 5′-GTGGGTGAGGAGCACGTAGT-3′; mouse MPO forward primer: 5′-CCATGGTCCAGATCATCACA-3′ and reverse primer: 5′-GCCGGTACTGATTGTTCAGG-3′. Amplification with 18S endogenous control assay mix was used for the controls. Data quantitation was done using the ΔΔ comparative threshold method. Expression levels of the genes were normalized to that of 18S rRNA.
Statistical analysis
Statistical analysis was performed using a two-tailed Student′s t test and GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA), with significance determined at P < 0.05.
RESULTS
Despite similar phenotype and morphology, G-MDSC and Neu demonstrated differences in the expression of several surface markers
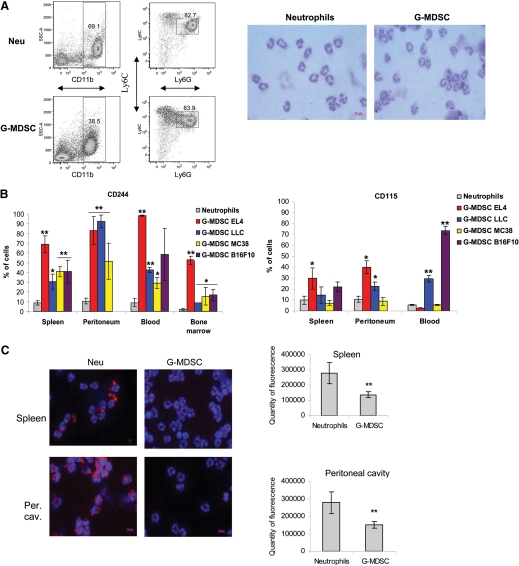
In initial experiments, we compared cells isolated from spleens of EL-4 tumor-bearing mice and from peritoneum of tumor-free mice, using casein-induced mobilization. For simplicity in referring to these cells, the term G-MDSC is provisionally used in reference to CD11b+Ly6G+Ly6Clow cells isolated from tumor-bearing mice and Neu for cells with the same phenotype isolated from naïve tumor-free mice. G-MDSC and Neu were gated as CD11b+Ly6G+Ly6Clow cells. All cells were negative for the F4/80 MΦ marker (data not shown). For the analysis of morphology, CD11b+Ly6G+Ly6Clow cells were sorted and stained with Wright-Giemsa stain. Neu and G-MDSC had very similar PMN morphology (Fig. 1A). Neu and G-MDSC had similar expression of markers used to identify these cell populations: CD11b, Gr-1, and Ly6C, although expression of Ly6G and CD11b was slightly lower in G-MDSC than in Neu (Supplemental Fig. 1). Also, no differences were found in the expression of several markers associated with MDSC: CD124 (IL-4Rα), S100A9, and S100A8 [20, 21], and the putative receptor for those proteins recognized by the GB3-1 antibody [21], as well as markers associated with Neu: pentraxines [22] and the chemokine receptors CCR5 and CXCR4 (Supplemental Fig. 1, and data not shown). However, G-MDSC had substantially higher expression than Neu of CD115 (M-CSFR) and CD244 (molecule expressed on many NK, NKT, and CD8+ T cells, as well as nonself-renewing hematopoietic progenitors; Supplemental Fig. 1) [23].
Figure 1. The morphologic features and phenotype of Neu and G-MDSC.
(A) Typical example of flow cytometric analysis of Neu and G-MDSC. Casein-induced peritoneal exudates from C57BL/6 naïve mice and splenocytes from EL-4 tumor-bearing mice were stained with anti-CD11b, anti-Ly6G, and anti-Ly6C antibodies, and CD11b+Ly6G+Ly6Chigh cells were gated as Neu and G-MDSC. Neu and G-MDSC from EL-4 tumor-bearing mice were purified as described in Materials and Methods and stained with Wright-Giemsa stain. Original magnification ×630 was used. Original scale bar = 10 μm. SSC-A=Side-scatter area. (B) Percentages of CD244- or CD115-positive cells were calculated within the populations of CD11b+Ly6G+Ly6Chigh Neu or G-MDSC. Each tumor model for G-MDSC analysis and tumor-free mice for Neu evaluation included a minimum of three mice. In all figures, mean and sd are shown. **P < 0.01; *P < 0.05, statistically significant differences between G-MDSC and Neu. Horizontal lines were used when several groups of mice had significant difference from the control. (C) Neu or G-MDSC from spleen or peritoneal cavity (Per. cav.) after mobilization with casein was purified using microbeads isolation as described in Materials and Methods. The purified cells were stained for LAMP2 (red) with counterstaining of DAPI (blue). The fluorescence intensity of LAMP2 was quantified in 50 cells. Original magnification ×630 was used. Original scale bars = 10 μm. Typical example of staining and cumulative results of four performed experiments are shown. **P < 0.01, statistically significant differences between G-MDSC and Neu.
To further evaluate the differences between G-MDSC and Neu, we performed cDNA array analyses of sorted CD11b+Ly6G+Ly6Clow G-MDSC and Neu. A large number of genes were differentially expressed in these cells (Tables 1 and 2). More than a twofold difference was found in 2261 transcripts, more than threefold in 840, and more than fourfold in 411. The pathway analysis revealed that G-MDSC had a higher expression of the genes associated with the cell cycle, autophagy, G-protein signaling, and CREB pathway (Table 3). Neu had a higher expression of the genes associated with NF-κB signaling via CD40, IL-1, IL-6, TLR, and TNF pathways, as well as lymphotoxin-β receptor signaling (Table 3). Several cytokine genes were also differentially expressed in Neu and G-MDSC. CD244 expression was 4.21 higher in G-MDSC than in Neu.
Table 1. Transcripts That Are Highly (More Than Seven-Fold) Overexpressed in G-MDSC Versus Neu.
| GenBank ID | Gene symbol | Gene description | Fold change |
|---|---|---|---|
| NM_010824.1 | Mpo | MPO | 57.35 |
| NM_152839.1 | Igj | Ig joining chain | 28.75 |
| NM_009921.1 | Camp | cathelicidin antimicrobial peptide | 23.85 |
| AF502286.1 | Igh-VJ558 | Ig heavy chain (J558 family) | 23.49 |
| NM_133246.2 | Ms4a3 | membrane-spanning 4 domains, subfamily A, member 3 | 21.72 |
| AK157885.1 | Reck | reversion-inducing cysteine-rich protein with kazal motifs | 19.77 |
| NM_010190.1 | Fcnb | ficolin B | 17.45 |
| NM_008036 | Fosb | FBJ osteosarcoma oncogene B | 17.10 |
| NM_011178.2 | Prtn3 | proteinase 3 | 17.00 |
| M26895.1 | Hba-a1 | hemoglobin α, adult chain 1 | 16.57 |
| NM_007398.2 | Ada | adenosine deaminase | 16.34 |
| AK035459.1 | Slco4c1 | solute carrier organic anion transporter family, member 4C1 | 16.05 |
| NM_008220.2 | Hbb-b1 | hemoglobin, β adult major chain | 16.04 |
| XM_911572.2 | Abca13 | ATP-binding cassette, subfamily A (ABC1), member 13 | 15.43 |
| NM_178683.2 | Depdc1b | DEP domain containing 1B | 14.18 |
| NM_001025251.2 | Mbp | myelin basic protein | 13.17 |
| NM_026785.1 | Ube2c | ubiquitin-conjugating enzyme E2C | 12.97 |
| NM_008522.2 | Ltf | lactotransferrin | 12.26 |
| NM_146151.2 | Tesk2 | testis-specific kinase 2 | 12.07 |
| XM_487151.4 | Abca13 | ATP-binding cassette, subfamily A (ABC1), member 13 | 12.07 |
| AF426024.1 | Serpinb1a | serine (or cysteine) peptidase inhibitor, clade B, member 1a | 11.34 |
| NM_008022.1 | Foxd4 | forkhead box D4 | 10.91 |
| NM_207668.2 | Acpp | acid phosphatase, prostate | 10.33 |
| NM_001005864.1 | Mtus1 | mitochondrial tumor suppressor 1 | 10.31 |
| NM_012006.1 | Acot1 | acyl-CoA thioesterase 1 | 10.29 |
| NM_001005864.1 | Mtus1 | mitochondrial tumor suppressor 1 | 10.10 |
| NM_177068.3 | Olfml2b | olfactomedin-like 2B | 9.96 |
| NM_026560.2 | Cdca8 | cell division cycle-associated 8 | 9.82 |
| NM_172658.1 | Slco4c1 | solute carrier organic anion transporter family, member 4C1 | 9.50 |
| NM_001005863.1 | Mtus1 | mitochondrial tumor suppressor 1 | 9.14 |
| NM_018769.1 | Dfna5h | deafness, autosomal-dominant 5 homolog (human) | 8.89 |
| NM_008489.1 | Lbp | LPS-binding protein | 8.87 |
| AK035772.1 | Asb7 | ankyrin repeat and SOCS box-containing protein 7 | 8.63 |
| NM_001001999.1 | Gp1bb | glycoprotein Ib, β polypeptide | 8.48 |
| NM_008517.1 | Lta4h | leukotriene A4 hydrolase | 8.43 |
| NM_175193.4 | Golph4 | golgi phosphoprotein 4 | 8.17 |
| NM_008405.1 | Itgb2l | integrin β 2-like | 8.09 |
| NM_008768.1 | Orm1 | orosomucoid 1 | 8.05 |
| AF479672.1 | Purg | purine-rich element-binding protein G | 7.95 |
| AK161616.1 | Pcyox1 | prenylcysteine oxidase 1 | 7.91 |
| NM_134163.4 | Mbnl3 | muscleblind-like 3 (Drosophila) | 7.75 |
| AK075854.1 | Edg6 | endothelial differentiation, G-protein-coupled receptor 6 | 7.72 |
| NM_145150.1 | Prc1 | protein regulator of cytokinesis 1 | 7.67 |
| NM_010232.3 | Fmo5 | flavin containing monooxygenase 5 | 7.60 |
| NM_007630.2 | Ccnb2 | cyclin B2 | 7.56 |
The complete list of transcripts is submitted to GEO (Accession Number GSE24102). SOCS, suppressor of cytokine signaling; FBJ, Finkel-Biskis-Jinkins; DEP, dishevelled, Egl-10 and Pleckstrin.
Table 2. Transcripts Highly (More Than 17-Fold) Overexpressed in Neut Versus G-MDSC.
| GenBank ID | Gene symbol | Gene description | Fold change |
|---|---|---|---|
| NM_011580.2 | Thbs1 | thrombospondin 1 | 129.47 |
| NM_008176.1 | Cxcl1 | chemokine (C-X-C motif) ligand 1 | 99.08 |
| NM_013654.2 | Ccl7 | chemokine (C-C motif) ligand 7 | 90.52 |
| NM_011580.2 | Thbs1 | thrombospondin 1 | 85.15 |
| NM_008176.1 | Cxcl1 | chemokine (C-X-C motif) ligand 1 | 81.31 |
| NM_008608.2 | Mmp14 | matrix metallopeptidase 14 (membrane-inserted) | 71.26 |
| NM_008509.1 | Lpl | lipoprotein lipase | 54.85 |
| NM_008630.1 | Mt2 | metallothionein 2 | 53.48 |
| AK036786.1 | Slc7a11 | solute carrier family 7 member 11 | 52.18 |
| NM_203320.1 | Gm1960 | gene model 1960 (NCBI) | 49.69 |
| NM_019932.1 | Cxcl4 | chemokine (C-X-C motif) ligand 4 | 46.19 |
| NM_007534.1 | Bcl2a1b | B cell leukemia/lymphoma 2-related protein A1b | 42.42 |
| NM_026644.1 | Agpat4 | 1-acylglycerol-3-phosphate O-acyltransferase 1 | 39.92 |
| NM_010493.2 | Icam1 | ICAM | 39.86 |
| NM_001033450.1 | Mnda | myeloid cell nuclear differentiation antigen | 37.60 |
| AB022345.1 | Slc7a11 | solute carrier family 7 member 11 | 37.51 |
| NM_013693.1 | Tnf | TNF | 37.38 |
| NM_009421 | Traf1 | TRAF1 | 36.02 |
| NM_011521.1 | Sdc4 | syndecan 4 | 34.15 |
| NM_031168.1 | II6 | IL-6 | 33.41 |
| NM_001039701.1 | II1m | IL-1R antagonist | 29.83 |
| NM_009263.1 | Spp1 | secreted phosphoprotein 1 | 28.38 |
| NM_009112.1 | S100a10 | S100 calcium-binding protein A10 (calpactin) | 27.97 |
| NM_013671.3 | Sod2 | SOD 2, mitochondrial | 25.96 |
| AY628210.1 | Wdt2 | whn-dependent transcript 2 | 24.91 |
| NM_011338.2 | Ccl9 | chemokine (C-C motif) ligand 9 | 24.65 |
| NM_021274.1 | Cxcl10 | chemokine (C-X-C motif) ligand 10 | 24.31 |
| NM_011311.1 | S100a4 | S100 calcium-binding protein A4 | 23.35 |
| XM_488510.4 | Cspg2 | chondroitin sulfate proteoglycan 2 | 22.55 |
| NM_011580.2 | Thbs1 | thrombospondin 1 | 22.38 |
| NM_011521.1 | Sdc4 | syndecan 4 | 22.12 |
| NM_019777.2 | Ikbke | IKKε | 21.76 |
| XM_488510.4 | Cspg2 | chondroitin sulfate proteoglycan 2 | 21.73 |
| XM_132051.7 | Rhoh | ras homolog gene family, member H | 21.38 |
| NM_009917.2 | Ccr5 | chemokine (C-C motif) receptor 5 | 20.25 |
| NM_175155.3 | Sash1 | SAM and SH3 domain-containing 1 | 19.95 |
| NM_009058.1 | Ralgds | ral guanine nucleotide dissociation stimulator | 19.80 |
| NM_009829.3 | Ccnd2 | cyclin D2 | 19.65 |
| NM_026835.1 | Ms4a6d | membrane-spanning 4 domains, subfamily A, member 6D | 19.37 |
| NM_009895.2 | Cish | cytokine-inducible SH2-containing protein | 18.73 |
| NM_013671.3 | Sod2 | SOD 2 mitochondrial | 18.71 |
| NM_008690.2 | Nfkbie | NF-κB light polypeptide gene enhancer inhibitor, ε | 18.35 |
| NM_001039701.1 | Il1rn | IL-1R antagonist | 18.26 |
| NM_009829.3 | Ccnd2 | cyclin D2 | 17.16 |
NCBI, National Center for Biotechnology Information; SAM, sterile α motif; SH 3/2, Src homology 3/2.
Table 3. Pathways Differentially Expressed in G-MDSC and Neutrophils.
| Pathways | Up-regulation |
|---|---|
| Cell cycle_Cell cycle (generic schema) | G-MDSC |
| Cell cycle_Role of APC in cell-cycle regulation | G-MDSC |
| Development_Endothelin-1/EDNRA transactivation of EGFR | G-MDSC |
| Immune response_IL-3 activation and signaling pathway | G-MDSC |
| Reproduction_GnRH signaling | G-MDSC |
| Autophagy_Autophagy | G-MDSC |
| Transcription_CREB pathway | G-MDSC |
| Development_Role of HDAC and calcium/calmodulin-dependent kinase (CaMK) in control of skeletal myogenesis | G-MDSC |
| Cell cycle_Initiation of mitosis | G-MDSC |
| Protein folding_Membrane trafficking and signal transduction of G-α (i) heterotrimeric G-protein | G-MDSC |
| Dopamine D2 receptor transactivation of EGFR | G-MDSC |
| NOTCH1-mediated pathway for NF-κB activity modulation | Neu |
| Immune response_CD40 signaling | Neu |
| Immune response_Signaling pathway mediated by IL-6 and IL-1 | Neu |
| Transcription_NF-κB signaling pathway | Neu |
| Apoptosis and survival_Antiapoptotic TNFs/NF-κB/Bcl-2 pathway | Neu |
| Apoptosis and survival_APRIL and BAFF signaling | Neu |
| Immune response_Innate immunity response to RNA viral infection | Neu |
| Immune response_MIF in innate immunity response | Neu |
| Development_Role of IL-8 in angiogenesis | Neu |
| Apoptosis and survival_Lymphotoxin-β receptor signaling | Neu |
| Immune response_TLR signaling pathways | Neu |
| Cytokine production by Th17 cells in CF | Neu |
| Bacterial infections in CF airways | Neu |
| Cytokine production by Th17 cells in CF (mouse model) | Neu |
| Immune response_Bacterial infections in normal airways | Neu |
| Transport_Macropinocytosis | Neu |
| Immune response_Antigen presentation by MHC class II | Neu |
| Cell adhesion_Role of tetraspanins in the integrin-mediated cell adhesion | Neu |
| Normal wtCFTR traffic/Sorting endosome formation | Neu |
| Transport_RAN regulation pathway | Neu |
| Immune response_IL-1 signaling pathway | Neu |
EDNRA, Endothelin receptor type A; GnRH, gonadotropin-releasing hormone; HDAC, histone deacetylase; APRIL, a proliferation-inducing ligand; BAFF, B cell-activating factor; MIF, macrophage migration inhibitors factor; CF, cystic fibrosis; CFTR, cystic fibrosis transmembrane regulator; RAN, Ras-related nuclear protein.
Based on this preliminary analysis, we evaluated the phenotype and function of Neu and G-MDSC isolated side-by-side from different tissue compartments. As the initial analysis suggested that CD244 and CD115 could be differentially expressed on Neu and G-MDSC, we focused on more detailed analyses of these two molecules. CD11b+Ly6G+Ly6Clow Neu were analyzed in spleens, blood, bone marrow, and peritoneum cavities (after casein-induced mobilization) of naive C57BL/6 mice. CD11b+Ly6G+Ly6Clow G-MDSCs were analyzed in the same organs of mice bearing 3-week tumors: EL-4 lymphoma, LLC lung carcinoma, B16F10 melanoma, or MC38 colon carcinoma. Neu from all compartments had very little expression of both molecules (Fig. 1B and Supplemental Fig. 2). In all four tumor models, >30% of spleen and blood G-MDSC expressed the CD244 receptor (P<0.01). In the peritoneal cavity, the proportion of G-MDSC positive for CD244 was even higher (>70%, P<0.01). In the bone marrow of EL-4 tumor-bearing mice, more than one-half of G-MDSC expressed CD244. In other tumor models, that proportion was slightly lower but still significantly (P<0.05) higher than in Neu from naïve mice (Fig. 1B). A significant proportion of G-MDSC from spleen or mobilized to peritoneum in EL-4 tumor-bearing mice was positive for the CD115 marker. CD115+ G-MDSCs were detected in blood and peritoneal exudates of LLC tumor-bearing mice but were not found in MC38 tumor-bearing mice, suggesting a large variability of M-CSFR expression on G-MDSC in different tumor models.
G-MDSCs and Neu have substantial differences in functional activity
To compare functional activity on naïve Neu and G-MDSC from EL-4 tumor-bearing mice, we have selected two compartments: spleen and peritoneal exudates after casein injection. In naïve, tumor-free mice spleens, Neu are mostly “rested” cells, whereas Neu, mobilized to the peritoneal cavity, are largely activated cells. Thus, these two compartments will allow us to evaluate cells at different states of activation. Neu are characterized by highly active lysosomes and proteasomes. To assess the presence of these cellular compartments, specific antibodies recognizing LAMP2, EEA1, and PSMA5 were used. As expected, Neu, isolated from spleen or mobilized to the peritoneum in naïve mice, had a high amount of these enzymes (Fig. 1C and Supplemental Fig. 3). G-MDSC from both compartments had significantly (P<0.01) lower levels of LAMP2 expression (Fig. 1C). The presence of proteosomal and endosomal enzymes in G-MDSC was also significantly (P<0.05) lower than in Neu (Supplemental Fig. 3).
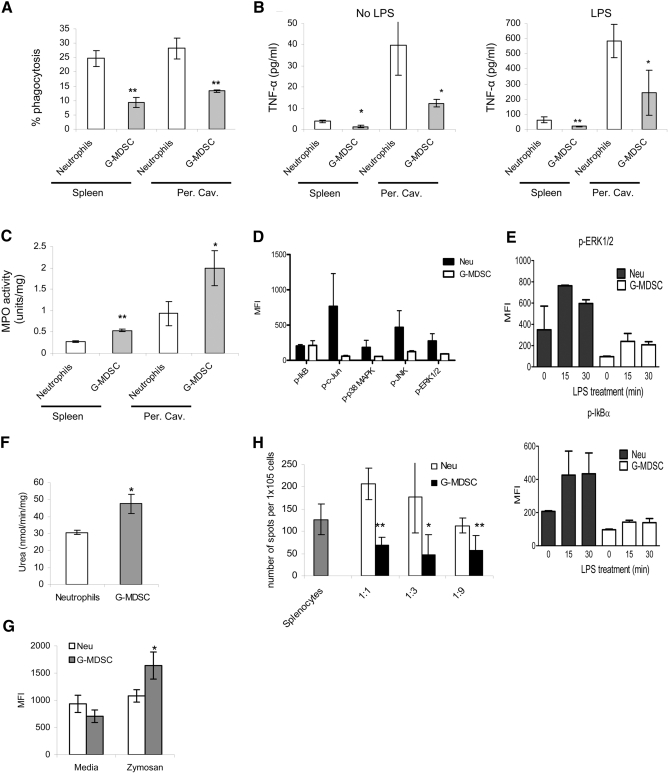
Another classical characteristic of Neu is their phagocytic activity. We compared, side-by-side, the ability of Neu and G-MDSC to take up latex beads. G-MDSC, isolated from spleen or from the peritoneum after casein-induced mobilization in EL-4 tumor-bearing mice, had significantly (P<0.01) lower phagocytic activity than Neu from the same compartments (Fig. 2A).
Figure 2. Functional activity of G-MDSC and Neu.
(A) Percentage of phagocytosis of latex beads in Neu and G-MDSC. Cumulative results of four performed experiments are shown. **P < 0.01, statistically significant differences between G-MDSC and Neu. (B) TNF-α production by Neu or G-MDSC. Cells were cultured overnight with or without LPS (100 ng/ml). Each culture supernatant was assayed for TNF-α by ELISA. *P < 0.05; **P < 0.01, statistically significant differences between G-MDSC and Neu. (C) MPO activity of Neu and G-MDSC measured in triplicates and repeated twice. *P < 0.05; **P < 0.01, statistically significant differences between G-MDSC and Neu. (D and E) Phospho (p)-protein analysis of Neu and G-MDSC. Four samples were tested in each group. (F) Arginase activity of Neu and G-MDSC from spleen was measured in triplicates. Cumulative results of three experiments are shown in G. The level of ROS was measured using DCFDA staining of opsonized, zymosan-stimulated cells by flow cytometry. Each experiment was performed in triplicates and repeated twice. *P < 0.05, statistically significant differences between G-MDSC and Neu. (H) Splenocytes from OT-1 C57BL/6 transgenic mice were stimulated in triplicates with specific or control peptides in the presence of different amounts of purified Neu or G-MDSC. IFN-γ production was measured using ELISPOT assay. The values of T cell activity in the presence of control peptides were subtracted from the value obtained in the presence of specific peptides. *P < 0.05; **P < 0.01, statistically significant differences between G-MDSC and Neu.
Two molecules (MPO and TNF-α), associated with Neu activity, which demonstrated a substantial difference in the initial gene array screen, were evaluated further. Neu, from spleen or mobilized to the peritoneal cavity, produced significantly more TNF-α spontaneously and in response to LPS stimulation than corresponding G-MDSC from EL-4 tumor-bearing mice (Fig. 2B). In contrast, G-MDSC, isolated from spleen or mobilized to the peritoneum of EL-4 tumor-bearing mice, showed a significantly higher level of MPO activity than their Neu counterparts in naïve mice (Fig. 2C). Furthermore, in splenic G-MDSC, we analyzed the activity of several signaling proteins involved in inflammatory responses. Consistent with gene array data, Neu had substantially higher basal levels of phosphorylated c-Jun, p38, JNK, and ERK1/2 MAPKs than G-MDSC (Fig. 2D). After activation with LPS, Neu also displayed higher levels of phosphorylated IκB (indicative of NF-κB activation) and MAPK than G-MDSC (Fig. 2E).
We then evaluated several factors implicated in suppression mediated by MDSC: arginase, NO, and ROS. Splenic Neu and G-MDSC had the same level of NO production (spontaneous and stimulated by IFN-γ; data not shown). Arginase activity and ROS production, in response to stimulation with opsonized zymosan, were higher (P<0.05) in G-MDSC than in Neu (Fig. 2F and G).
Consistent with previous reports [7, 8], spleen G-MDSC inhibited antigen-specific T cell responses (Fig. 2H). Spleen Neu, in contrast, did not suppress antigen-specific T cell function. In fact, at high ratios (1:1), splenic Neu actually promoted IFN-γ production by antigen-specific T cells (Fig. 2H). Thus, Neu and G-MDSC had very different functional activity. Neu were mature, activated cells, demonstrating much higher levels of lyososmal and proteosomal enzymes, as well as phagocytosis, TNF-α production, and activity of MAPK and NF-κB pathways, than G-MDSC. In contrast, G-MDSC had higher ROS production and arginase activity, which correlate with their immune-suppressive activity.
Possible role of CD244 in defining functional subpopulations of G-MDSC
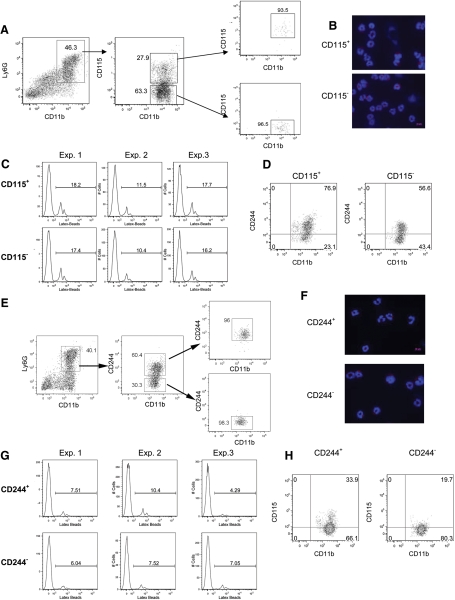
Our data demonstrated that less than one-half of G-MDSC expressed CD115 or CD244, which might suggest that these receptors may define different subpopulations of G-MDSC. To address this question, G-MDSCs from spleens of EL-4 tumor-bearing mice were sorted based on their expression of CD115 (Fig. 3A) or CD244 (Fig. 3E). Our experiments revealed no differences in the level of LAMP2 expression and phagocytic activity between CD115+ and CD115– G-MDSC (Fig. 3B and C), as well as between CD244+ and CD244– cells G-MDSC (Fig. 3F and G). The expression of CD115 and CD244 molecules on G-MDSC did not correlate with each other (Fig. 3D and H).
Figure 3. Analysis of G-MDSC populations.
Four cell populations were sorted from spleens of EL-4 tumor-bearing mice: Ly6G+CD11b+CD115+, Ly6G+CD11b+CD115–, Ly6G+CD11b+CD244+, Ly6G+CD11b+CD244–. (A and E) Typical example of sorting gates. Analysis of LAMP2 expression (B and F), phagocytosis (C and G), and CD244 or CD115 expression (D and H) in sorted cells. Each experiment was performed in triplicates. Typical examples are shown. In microphotographs, original magnification ×630 was used. Original scale bars = 10 μm.
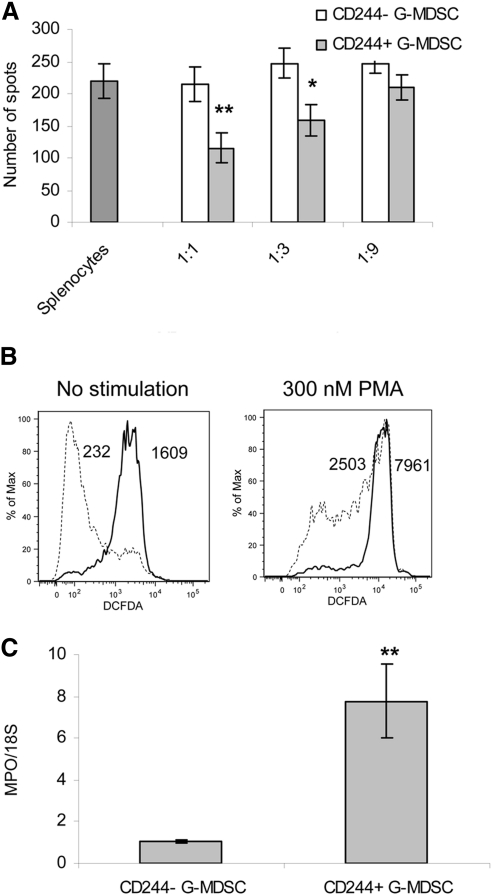
In contrast, substantial differences in functional activity were observed between CD244+ and CD244– G-MDSC. CD244– G-MDSC did not suppress an antigen-specific CD8+ T cell response at any tested ratios, whereas CD244+ G-MDSC demonstrated significant suppressive activity (Fig. 4A). This effect was associated with significant differences between these two groups of cells in the level of ROS production (Fig. 4B) and MPO expression (Fig. 4C). Thus, expression of CD244 on G-MDSC correlated with a high level of ROS and immune-suppressive activity of these cells.
Figure 4. Association between CD244 expression and functional activity of G-MDSC.
(A) Ly6G+CD11b+CD244+ and Ly6G+CD11b+CD244– were sorted from spleens of EL-4 tumor-bearing mice and incubated in triplicates at indicated ratios with OT-1 splenocytes in the presence of control or OT-1-specific peptide. IFN-γ production was measured using ELISPOT assay. The values of T cell activity in the presence of control peptides were subtracted from the value obtained in the presence of specific peptides. *P < 0.05; **P < 0.01, statistically significant differences between G-MDSC and Neu. (B) ROS level was measured by flow cytometry using DCFDA staining in gated CD244+ and CD244– G-MDSC isolated from spleens of EL-4 tumor-bearing mice. Numbers in the histograms show geometric MFI. Three experiments with similar results were performed. (C) MPO expression was measured by RT-PCR in sorted G-MDSC in triplicates. Two experiments with similar results were performed; **P < 0.01.
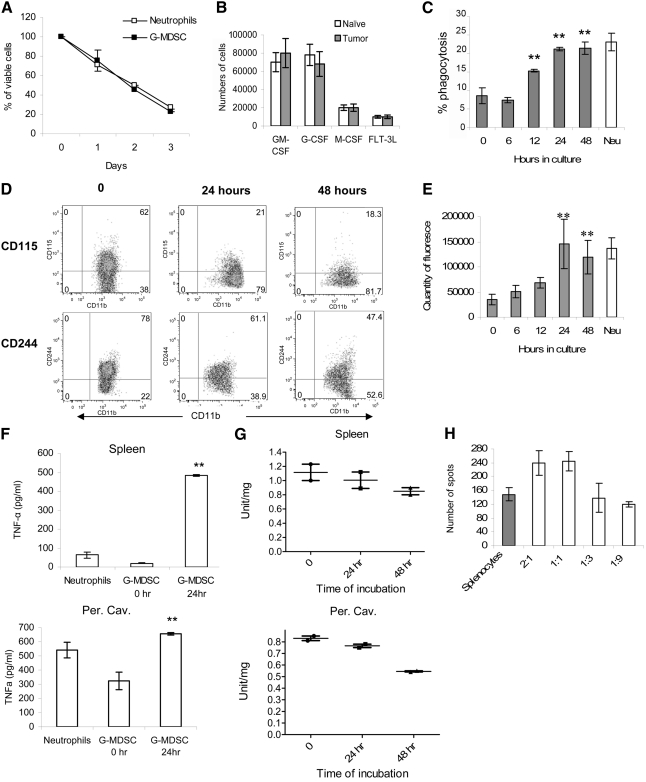
The fate of G-MDSC in culture
Previous studies have demonstrated that G-MDSC, in contrast to M-MDSC, failed to differentiate into MΦ or DCs [8]. As our experiments have identified several parameters that distinguish Neu from naïve mice and G-MDSC from tumor-bearing mice, we examined the fate of G-MDSC in vitro. G-MDSC did not survive overnight in culture without the presence of growth factors (data not shown). GM-CSF supported G-MDSC survival with 60% viable cells detected after 24 h in culture. However, by Day 3, only 20% of cells remained in culture (Fig. 5A). Neu and G-MDSC showed similar viability in the presence of GM-CSF (Fig. 5A). Several other growth factors that could potentially support differentiation of these cells were also tested. CD11b+Ly6ClowLy6G+ cells, from naïve or tumor-bearing mice, did not survive more than 24 h in the presence of M-CSF or FLT3L (Fig. 5B). G-CSF only marginally improved the survival of these cells.
Figure 5. G-MDSC differentiated into Neu in culture.
(A) CD11b+Ly6G+Ly6Chigh cells from bone marrow of naïve or EL-4 tumor-bearing mice were cultured with 10 ng/ml GM-CSF, and their survival was determined at the indicated time. (B) The same cells were cultured for 3 days with 10 ng/ml GM-CSF, 10 ng/ml G-CSF, 10 ng/ml M-CSF, or 100 ng/ml FLT3L. The number of viable cells was assessed by trypan blue exclusion. (C–H) G-MDSCs from spleen of EL-4 tumor-bearing mice were cultured with GM-CSF, and phagocytosis (C), expression of surface markers (D), and LAMP2 (E) were evaluated at the indicated time. Amount of TNF-α (F) and MPO (G) was measured in G-MDSC after cultured with GM-CSF at the indicated time-points. (H) Immune-suppressive activity was measured after 24 h incubation in complete medium. Each experiment was performed at least three times, and cumulative results are shown. **P < 0.01, statistically significant differences from freshly isolated G-MDSC (0 h). Experiments measuring MPO activity were performed twice, and individual results are presented.
G-MDSCs were isolated from spleens of EL-4 tumor-bearing mice and cultured with GM-CSF. After 24 h in culture, phagocytic activity of G-MDSC increased threefold and reached the level seen in Neu from naïve mice (Fig. 5C). Within 24 h, G-MDSC lost most of the CD115 expression and reduced the level of CD244 expression. Within 48 h, there was further decrease in the expression of CD115 and CD244 molecules (Fig. 5D). Expression of LAMP2 in G-MDSC increased within 12 h of culture and reached the level seen in Neu from naive mice by 24 h (Fig. 5E and Supplemental Fig. 4). This correlates with a dramatic up-regulation of LPS-inducible TNF-α production by G-MDSC (Fig. 5F). Similar results were obtained with G-MDSC after casein-induced mobilization to the peritoneal cavity (Fig. 5F). These changes were associated with decreased MPO activity (Fig. 5G). Arginase activity remained unchanged (data not shown). Incubation of spleen G-MDSC with GM-CSF for 24 h completely abrogated their suppressive activity against antigen-specific T cells (Fig. 5H; compare with Fig. 2H). Thus, within 24 h, in culture with GM-CSF, G-MDSC became phenotypic and functionally undistinguishable from mature Neu.
Subsequently, we asked whether Neu, cultured in the presence of tumor-derived factors, could acquire characteristics of G-MDSC. Neu were isolated from bone marrow of naïve C57BL/6 mice and cultured for 48 h with GM-CSF in the presence of TES obtained from EL-4 cells. Only 40% of the cells survived 48 h cultured with TES. The remaining viable cells retained a high level of LAMP2 expression, did not decrease TNF-α expression or phagocytosis, and did not acquire expression of CD244 (data not shown). These data argue against the possibility that Neu may be converted into G-MDSC.
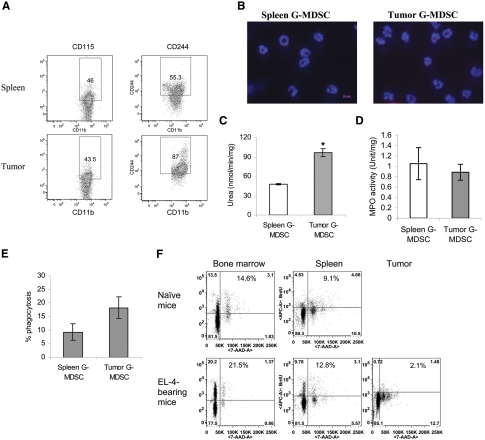
G-MDSC in the tumor site
We then wanted to examine whether G-MDSCs, present in the tumor site, share phenotypic and functional characteristics of spleen G-MDSC. To address this question, we isolated G-MDSC from tumor tissue and compared it, side-by-side, with G-MDSC from spleens of the same EL-4 tumor-bearing mice. To isolate cells in these experiments, tumor tissues and spleens were subjected to enzymatic digestion. Spleen and tumor G-MDSCs had similar levels of CD115 expression, whereas expression of CD244 was higher in tumor than in spleen G-MDSC (Fig. 6A). Spleen and tumor G-MDSCs had similar low expression of LAMP2 (Fig. 6B) and a low level of TNF-α production (data not shown). Arginase activity, observed in spleen G-MDSC, was even higher in tumor G-MDSC (Fig. 6C). Spleen and tumor G-MDSCs had the same level of MPO activity (Fig. 6D). Tumor G-MDSC had slightly higher phagocytic activity than spleen G-MDSC (Fig. 6E). We asked whether accumulation of G-MDSC in tumor tissues was the result of their proliferation in situ or as a result of migration from bone marrow or spleen. EL-4 tumor-bearing mice were injected i.p. with BrdU, and its incorporation into the CD11b+Ly6ClowLy6G+ population was evaluated 24 h later. In tumor-bearing mice, bone marrow G-MDSC demonstrated a substantially higher level of BrdU than in their control counterparts (Fig. 6F). The level of BrdU uptake by spleen G-MDSC was substantially lower than in bone marrow. In contrast, in the tumor site, CD11b+Ly6G+Ly6Clow cells showed a low level of BrdU incorporation (Fig. 6F), suggesting that the main source of these cells in the tumor site is G-MDSC, which migrated from bone marrow or spleen.
Figure 6. Characteristics of G-MDSC in tumor site.
(A) Single-cell suspension from spleen or tumor tissue of EL-4 tumor-bearing mice was obtained after collagenase treatment and stained with antibodies specific for various surface markers. CD11b+Ly6G+Ly6Chigh cells were gated as G-MDSC, and the expression of CD115 and CD124 was analyzed. A typical example of three experiments is shown. (B) Typical example of LAMP2 staining. Original magnification ×630 was used. Original scale bar = 10 μm. (C) Arginase activity. *P < 0.05, statistically significant differences between G-MDSC and tumor G-MDSC. (D) MPO activity. (E) Phagocytosis of latex beads by G-MDSC. (C–E) Each group included three mice. (F) Naïve or EL-4 tumor-bearing mice were treated with BrdU for 24 h, and the BrdU uptake by CD11b+Ly6G+Ly6Chigh cells from bone marrow, spleen, and tumor was analyzed by flow cytometry. A typical example of three performed experiments is shown. 7-AAD-A, 7-Amino-actinomycin D-area.
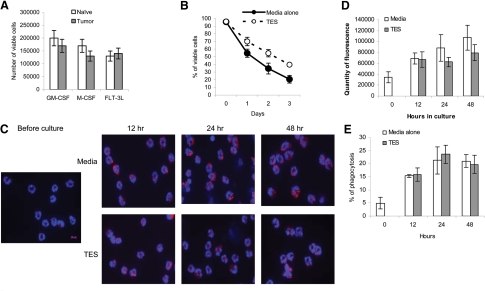
We then asked whether soluble tumor-derived factors can promote G-MDSC survival and delay their differentiation. As a source of tumor-derived factors, we used TES, obtained from EL-4 tumors. Incubation with TES substantially improved the survival of bone marrow CD11b+Ly6ClowLy6G+ cells from naïve and tumor-bearing mice. Almost 80% of cells survived a 3-day culture in the presence of GM-CSF and TES (Fig. 7A), as compared with <20% of cells incubated without TES (Fig. 5A). TES also significantly (P<0.05) increased the survival of G-MDSC from spleens of tumor-bearing mice (Fig. 7B). There were no significant differences in the expression level of LAMP2 or phagocytic activity or the expression of surface markers between the cells incubated with TES or medium alone at any time-point tested (12–48 h; Fig. 7C–E, and data not shown). Thus, it appears that soluble tumor-derived factors prolonged the survival of G-MDSCs but did not substantially affect the ability of G-MDSCs to acquire the features of Neu.
Figure 7. Effect of tumor-derived factors on G-MDSC differentiation.
(A) CD11b+Ly6G+Ly6Chigh cells (2×105) from bone marrow of naïve or EL-4 tumor-bearing mice were cultured with 10 ng/ml GM-CSF, 10 ng/ml M-CSF, or 100 ng/ml FLT3L in the presence of TES for 3 days. Viable cells were assessed using trypan blue. (B–E) G-MDSC from splenocytes of EL-4 tumor-bearing mice were cultured with 10 ng/ml GM-CSF in the presence of TES or control medium, and the viability (B), LAMP2 level (original magnification, ×630; original scale bar=10 μm; C), quantity of fluorescence intensity of LAMP2 (D), and phagocytosis (E) were assessed at indicated time-points. Each experiment was performed at least three times.
DISCUSSION
G-MDSCs represent the majority of MDSCs that accumulate in cancer and many other pathologic conditions. However, in contrast to M-MDSC, the nature of these cells remained rather obscure. These cells don't survive in culture with cytokines and don't differentiate to MΦ and DCs. They have much higher production of ROS and substantially lower production of NO than M-MDSCs. Transcriptional regulation of signaling in these cells is also different [7, 8]. These cells are morphologically similar to Neu, which raises the question of whether G-MDSCs are in fact Neu, probably in an activated state. This creates difficulties in interpreting data from different experiments, when similar cells in tumor-bearing mice and cancer patients are called “MDSC” and “neutrophils” by different investigators [8, 10, 11, 13, 24]. This question is not simply an issue of semantics or nomenclature. It is important for understanding the biology of MDSCs and their role in pathological processes. In this study, we tried to clarify the nature of the relationship between these cells in mice. This task is complicated by the fact that the phenotypic criteria used to identify G-MDSC (CD11b+Ly6G+Ly6Clow) are identical to that of Neu. Therefore, we compared G-MDSCs from tumor-bearing mice with Neu from naïve mice. To assess the possible effect of the activation state of the cells on the differences between G-MDSC and Neu, we performed a comparison between cells from different compartments: spleen, which in naïve mice, contains largely nonactivated Neu; and peritoneal exudate after casein injection, which in naïve mice, is comprised primarily of mobilized, activated Neu. In some experiments, we also evaluated cells in bone marrow and blood. Our data demonstrated that despite the morphologic and phenotypic similarity between these cells, several parameters were different. The M-CSFR (CD115) and CD244 molecules were expressed on a substantial proportion of G-MDSC from the spleen or mobilized to peritoneal cavities. In contrast, their expression was practically undetectable on Neu. Expression of CD115 and CD244 may reflect the immature, transitory nature of G-MDSC. Our results also challenge the point of view that CD115 is a marker specifically associated only with M-MDSC. We hypothesized that M-CSFR and CD244 can be used to distinguish immature myeloid cells and mature Neu, which may comprise the population of G-MDSC. However, our experiments, when sorting G-MDSC based on CD115 or CD244 expression, did not support this conclusion. It is possible that other markers may be used to identify subpopulations of G-MDSC.
Regardless of tissue localization, G-MDSC displayed characteristics of less-mature cells than Neu. This conclusion is based on our observations of the significantly lower levels of lysosomal and proteosomal protein expression, phagocytosis, TNF-α production, activity of NF-κB, and MAPK in G-MDSC than in Neu. These differences most likely represent intrinsic characteristics of the cells, as they were observed in rested and mobilized cells. In addition, when cultured overnight, in medium supplemented only with GM-CSF, G-MDSCs exhibited a dramatic up-regulation of LAMP2 expression, TNF-α production, and phagocytic activity, suggesting that G-MDSCs acquire features typical for Neu during short-term culture. Finally, G-MDSCs in tumor tissues were similar to G-MDSCs from spleen of the tumor-bearing mice, suggesting that tissue microenvironment did not change the main functional characteristics of G-MDSC.
Activation of myeloid precursors, with LPS and other TLR agonists, can cause the development of immune-suppressive MDSC [25]. If activation were the main feature distinguishing Neu from G-MDSC, then this would suggest that Neu could acquire immune-suppressive activity. However, Neu, in contrast to MDSC, not only did not suppress T cell function but also promoted their response. This was observed with Neu from spleen and mobilized to peritoneal cavity (data not shown). G-MDSCs from spleen and mobilized to the peritoneum of tumor-bearing mice had substantially higher levels of ROS production and MPO and arginase activity than Neu. Arginase and ROS are widely considered as the major factors in inhibition of T cell function by MDSC [8, 26, 27]. The differences in ROS and arginase, between Neu and G-MDSC, may explain why Neu, in contrast to G-MDSC, lack immune-suppressive activity.
G-MDSC in tumor tissues retained features that separate them from Neu. Moreover, some of these differences became even stronger. For instance, tumor G-MDSCs have higher arginase activity than spleen G-MDSCs, which is consistent with a previous report demonstrating that the tumor microenvironment up-regulates the arginase level in MDSC [28]. Our data suggest that in the presence of growth factors, G-MDSCs acquire all characteristics of Neu within 24 h, and these cells became undistinguishable. If this is the case, the fact that CD11b+Ly6G+Ly6Clow cells isolated from tumor tissues had features similar to those in spleen G-MDSC may suggest that the tumor microenvironment prevents differentiation of G-MDSC to Neu, or there is a constant influx of G-MDSC into the tumor site. Our data indicate that soluble tumor-derived factors, although increasing the survival of G-MDSCs, did not prevent their differentiation to Neu in vitro. Data with BrdU labeling suggest that G-MDSCs migrate to the tumor site from spleen and (or) bone marrow. We suggest it is more likely that G-MDSCs constantly migrate to the tumor site from bone marrow and spleen in response to stromal cell-derived factor 1α (ligand for CXCR4) and other chemokines produced in the tumor microenvironment [29]. TGF-β could play an important role in this process [13]. There, they contribute to immune suppression and die quickly without acquiring features of mature Neu. Although expression of CD115 and CD244 was not specific for all G-MDSCs, in the tumor site, these two markers identified more than three-fourths of G-MDSC. This opens the possibility to study the function of G-MDSC and Neu separately in tumor-bearing mice.
Thus, our data suggest G-MDSCs, from tumor-bearing mice, and Neu, from naïve mice, although representing the same lineage of cells and morphologically and phenotypically similar, have distinct, functional activity. It appears that G-MDSCs are relatively immature and pathologically activated precursors of Neu. In tumor-bearing, their transition to Neu is halted. Instead, they acquire features that determine their immune-suppressive activity. The exact mechanism of this phenomenon remains to be clarified.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants CA100062 and CD84488 to D.I.G. and in part by the flow cytometry, microarray, and microscopy core facilities at H. Lee Moffitt Cancer Center. S.K.B. is supported by funding from Biomedical Research Council (A*STAR), Singapore. We thank Dr. A. Mantovani for providing anti-PTX3 antibody.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- DCFDA
- 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate
- EEA1
- early endosome antigen 1 protein
- FLT3L
- fetal liver tyrosine kinase 3 ligand
- G-MDSC
- granulocytic myeloid-derived suppressor cell
- GCOS
- GeneChip Operating Software
- GEO
- Gene Expression Omnibus
- LAMP2
- lysosomal-associated membrane protein 2
- LLC
- Lewis lung carcinoma
- m
- murine
- MΦ
- macrophage(s)
- M-MDSC
- monocytic myeloid-derived suppressor cell
- MDSC
- myeloid-derived suppressor cell
- MFI
- mean fluorescence intensity
- Neu
- neutrophil(s)
- PSMA5
- proteasome subunit α type-5
- TES
- tumor-explant supernatant(s)
AUTHORSHIP
J-I.Y. and D.I.G. performed research, analyzed and interpreted data, and wrote the manuscript. M.C., I.N.S., and S.K.B. performed research.
REFERENCES
- 1. Gabrilovich D. I., Nagaraj S. (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peranzoni E., Zilio S., Marigo I., Dolcetti L., Zanovello P., Mandruzzato S., Bronte V. (2010) Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 22, 238–244 [DOI] [PubMed] [Google Scholar]
- 3. Ostrand-Rosenberg S., Sinha P. (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J. Immunol. 182, 4499–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shojaei F., Wu X., Malik A. K., Zhong C., Baldwin M. E., Schanz S., Fuh G., Gerber H. P., Ferrara N. (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b(+)Gr1(+) myeloid cells. Nat. Biotechnol. 25, 911–920 [DOI] [PubMed] [Google Scholar]
- 5. Yang L., Huang J., Ren X., Gorska A. E., Chytil A., Aakre M., Carbone D. P., Matrisian L. M., Richmond A., Lin P. C., Moses H. L. (2008) Abrogation of TGF β signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13, 23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang L., DeBusk L., Fukuda K., Fingleton B., Green-Jarvis B., Shyr Y., Matrisian L., Carbone D., Lin P. (2004) Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6, 409–421 [DOI] [PubMed] [Google Scholar]
- 7. Movahedi K., Guilliams M., Van den Bossche J., Van den Bergh R., Gysemans C., Beschin A., De Baetselier P., Van Ginderachter J. A. (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T-cell suppressive activity. Blood 111, 4233–4244 [DOI] [PubMed] [Google Scholar]
- 8. Youn J. I., Nagaraj S., Collazo M., Gabrilovich D. I. (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou Z., French D. L., Ma G., Eisenstein S., Chen Y., Divino C. M., Keller G., Chen S. H., Pan P. Y. (2010) Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells 28, 620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ko J. S., Zea A. H., Rini B. I., Ireland J. L., Elson P., Cohen P., Golshayan A., Rayman P. A., Wood L., Garcia J., Dreicer R., Bukowski R., Finke J. H. (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez P. C., Ernstoff M. S., Hernandez C., Atkins M., Zabaleta J., Sierra R., Ochoa A. C. (2009) Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 69, 1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cherdyntseva N. V., Kusmartsev S., Gabrilovich D. I. (2005) Polymorphonuclear neutrophils and cancer: ambivalent role in host defense against tumor. In The Neutrophils. New Outlook for Old Cells (Gabrilovich D. I., ed.), Imperial College Press, London, 275–300 [Google Scholar]
- 13. Fridlender Z. G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., Worthen G. S., Albelda S. M. (2009) Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 16, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo Y., Dorf M. E. (1997) Isolation of mouse neutrophils. In Current Protocols in Immunology, Vol. 1 (Coligan J. E., Kruisbeek A., Margulies D. H., Shevach E. M., Strober W., eds.), Wiley, Hoboken, NJ, USA, 3.20.1.–3.20.6 [Google Scholar]
- 15. Corraliza I. M., Campo M. L., Soler G., Modolell M. (1994) Determination of arginase activity in macrophages: a micromethod. J. Immunol. Methods 174, 231–235 [DOI] [PubMed] [Google Scholar]
- 16. Biswas S. K., Gangi L., Paul S., Schioppa T., Saccani A., Sironi M., Bottazzi B., Doni A., Vincenzo B., Pasqualini F., Vago L., Nebuloni M., Mantovani A., Sica A. (2006) A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation). Blood 107, 2112–2122 [DOI] [PubMed] [Google Scholar]
- 17. Van Gelder R. N., von Zastrow M. E., Yool A., Dement W. C., Barchas J. D., Eberwine J. H. (1990) Amplified RNA synthesized from limited quantities of heterogeneous cDNA. Proc. Natl. Acad. Sci. USA 87, 1663–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Warrington J. A., Nair A., Mahadevappa M., Tsyganskaya M. (2000) Comparison of human adult and fetal expression and identification of 535 housekeeping/maintenance genes. Physiol. Genomics 2, 143–147 [DOI] [PubMed] [Google Scholar]
- 19. Liu W. M., Mei R., Di X., Ryder T. B., Hubbell E., Dee S., Webster T. A., Harrington C. A., Ho M. H., Baid J., Smeekens S. P. (2002) Analysis of high density expression microarrays with signed-rank call algorithms. Bioinformatics 18, 1593–1599 [DOI] [PubMed] [Google Scholar]
- 20. Cheng P., Corzo C. A., Luetteke N., Yu B., Nagaraj S., Bui M. M., Ortiz M., Nacken W., Sorg C., Vogl T., Roth J., Gabrilovich D. I. (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 205, 2235–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinha P., Okoro C., Foell D., Freeze H. H., Ostrand-Rosenberg S., Srikrishna G. (2008) Proinflammatory s100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 181, 4666–4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bottazzi B., Doni A., Garlanda C., Mantovani A. (2010) An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu. Rev. Immunol. 28, 157–183 [DOI] [PubMed] [Google Scholar]
- 23. Bhat R., Eissmann P., Endt J., Hoffmann S., Watzl C. (2006) Fine-tuning of immune responses by SLAM-related receptors. J. Leukoc. Biol. 79, 417–424 [DOI] [PubMed] [Google Scholar]
- 24. Schmielau J., Finn O. J. (2001) Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 61, 4756–4760 [PubMed] [Google Scholar]
- 25. Arora M., Poe S. L., Oriss T. B., Krishnamoorthy N., Yarlagadda M., Wenzel S. E., Billiar T. R., Ray A., Ray P. (2010) TLR4/MyD88-induced CD11b(+)Gr-1(int)F4/80(+) non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol. 3, 578–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kusmartsev S., Nefedova Y., Yoder D., Gabrilovich D. I. (2004) Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172, 989–999 [DOI] [PubMed] [Google Scholar]
- 27. Corzo C. A., Cotter M. J., Cheng P., Cheng F., Kusmartsev S., Sotomayor E., Padhya T., McCaffrey T. V., McCaffrey J. C., Gabrilovich D. I. (2009) Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J. Immunol. 182, 5693–5701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corzo C. A., Condamine T., Lu L., Cotter M. J., Youn J. I., Cheng P., Cho H. I., Celis E., Quiceno D. G., Padhya T., McCaffrey T. V., McCaffrey J. C., Gabrilovich D. I. (2010) HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 207, 2439–2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ali S., Lazennec G. (2007) Chemokines: novel targets for breast cancer metastasis. Cancer Metastasis Rev. 26, 401–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.