Abstract
Study Objectives:
One task that has been used to assess memory effects of prior total sleep deprivation (TSD) is the immediate free recall of word lists; however, results have been mixed. A possible explanation for this is task impurity, since recall of words from different serial positions reflects use of distinct types of memory (last words: short-term memory; first and intermediate words: episodic memory). Here we studied the effects of 2 nights of TSD on immediate free recall of semantically unrelated word lists considering the serial position curve.
Design:
Random allocation to a 2-night TSD protocol followed by one night of recovery sleep or to a control group.
Setting:
Study conducted under continuous behavioral monitoring.
Participants:
24 young, healthy male volunteers.
Intervention:
2 nights of total sleep deprivation (TSD) and one night of recovery sleep.
Measurements and Results:
Participants were shown five 15 unrelated word-lists at baseline, after one and 2 nights of TSD, and after one night of recovery sleep. We also investigated the development of recall strategies (learning) and susceptibility to interference from previous lists. No free recall impairment occurred during TSD, irrespective of serial position. Interference was unchanged. Both groups developed recall strategies, but task learning occurred earlier in controls and was evident in the TSD group only after sleep recovery.
Conclusion:
Prior TSD spared episodic memory, short-term phonological memory, and interference, allowed the development of recall strategies, but may have decreased the advantage of using these strategies, which returned to normal after recovery sleep.
Citation:
Zanini GAV; Tufik S; Andersen ML; da Silva RCM; Bueno OFA; Rodrigues CC; Pompéia S. Free recall of word lists under total sleep deprivation and after recovery sleep. SLEEP 2012;35(2):223-230.
Keywords: Total sleep deprivation, memory, primacy, recency, free recall, interference, output order
INTRODUCTION
Total sleep deprivation (TSD) negatively affects memory encoding1–4 including impairment of memory for verbal/phono-logical stimuli1,5,6 learned when people are sleep deprived. This phenomenon differs from the effects of sleep deprivation in the consolidation of memory, in which learning is followed by sleep deprivation,7 which was not the object of the present study.
Deficits in encoding of phonological stimuli that are not semantically related may involve alterations in short- and/or long-term episodic memory. Short-term or working memory holds a limited amount of verbal information for short periods of time,8–10 whereas the episodic memory system stores information or events together with the temporal and spatial relationship between them, has unlimited capacity and a duration that extends beyond the temporal limits of working memory.8,11 Recall of information stored in episodic memory occurs consciously through the reinstatement and recognition of information as part of the past, a process based on a series of strategies including the use of temporal information between events.12
Free recall of word lists is a classic test of verbal episodic memory in which the order of words in the list provides temporal cues that are used at recall, in much the same way as when people try to remember episodic events in everyday life. When recall of words occurs immediately after presentation of the lists and the order of words at encoding is taken into account (the serial position effect), both episodic and short-term memory of these words may be determined.13–17 The primacy effect reflects the tendency of respondents to remember the first item on a list and indexes episodic memory for such items, while the recency effect, or the tendency to remember the last items in a list, indicates use of short-term memory.13,18 Recall of intermediate words in a list is also a measure of episodic memory, but these items tend to be less well remembered because they have reduced temporal distinction and are unlikely to be rehearsed subvocally, both processes that generate the primacy effect.14,19 Hence, this apparently simple cognitive task reflects multiple cognitive processes, some of which may be more affected by TSD than others. Consequently, the use of single measures of the total number of words recalled, as seen in some cases found in the acute TSD literature, may lead to misleading or contradictory conclusions. In fact, some studies have shown impairment,4,5,20 while others have found that TSD does not alter performance on this type of task.21–24
The mixed results on the effects of TSD on free recall of word lists may be easily explained by the different methods used. For example, free recall of short lists5,20 leads to the assessment of both short- and declarative long-term memory, as explained above, which may be unevenly sensitive to TSD. In contrast, delayed recall of words4,22 can eliminate the use of short-term memory. Repeating the same list various times21,22 yields measures of learning of the words in the list, which can be confused with the development of organizational strategies at encoding25–27 or during retrieval.28,29 Finally, using related words21,24 involves aspects of semantic memory that may contaminate recall of words from episodic memory.30,31
Only two TSD studies in the literature have reported findings after presentation of the classic free recall paradigm or a single presentation of lists of unrelated words followed by immediate free recall.21,23 Both showed no change in performance in terms of the total number of words remembered, which does not confirm the suggestion that declarative verbal memory is impaired when people are fully sleep deprived.7 This could be explained by the lack of an analysis adjusting for serial position effect since the computation of the total number of words recalled includes retrieval of the last words in the list; this reflects use of verbal short-term memory, which seems to be unaltered or only slightly affected by acute lack of sleep.1,32 Another reason for this negative finding may have been insufficient power because both of these investigations included a single word list and a small number of participants.
In addition to failing to consider the serial position effect, most previous studies of TSD effects on recall of word lists have not examined output strategies used33 or word recall order,28,29,34–36 nor have they investigated practice or training effects arising from repeated exposure to the same task37–40 and how lack of sleep affects proactive interference41,42 (for exceptions see 43,44).
Therefore, studies of the effects of acute TSD on free recall that take into consideration the serial position effect, the strategies learned from repeated administration of the task, and possible changes in interference have the potential to further the understanding of cognitive processes affected by lack of sleep, and may show whether people can adapt or adjust to these possible deficits. This was the aim of the present study. To this end we recruited young, healthy male volunteers who carried out immediate free recall of unrelated word lists in 4 sessions: at baseline, after one and 2 nights of TSD, and after recovery sleep. We chose to investigate performance after 2 nights of total sleep deprivation having in mind that shift workers and military personnel can be subjected to these conditions. This also allows the determination of “time-dependent” increases in cognitive deficits which can be used to better substantiate the effects of total sleep deprivation. Performance was compared to that of a control group that was not sleep deprived at any time during the experiment. This population was chosen to avoid any bias in performance related to sleep or other organic disorders and cognitive deficits due to aging and/or use of medication. The tests were performed at the same time, midafternoon, to control for any possible effects of circadian variation.45–47 Based on previously published data, we hypothesized that sleep deprived individuals would exhibit impaired recall of words from the initial and intermediary serial positions (episodic memory impairment) but that recency, a measure of short-term memory use, would be unchanged. We had no predictions on the TSD effects on use of organizational strategies and susceptibility to interference, since these issued have been seldom investigated.
The order in which words were recalled was examined using the relative index of priority (RIP score).34 RIP scores are useful for lists of any size and are independent of the number of words recalled (see details in the methods section). Because people tend to recall the last words first followed by the first and lastly by the middle words, RIP curves usually have a U shape. Any changes in the strategy used to recall words will alter this pattern. Susceptibility to interference was measured by determining number of errors during recall.
METHODS
Participants
Volunteers were 24 healthy males volunteer, aged 18 to 29 with ≥ 11 years of schooling, whose first language was Portuguese, who slept on average 7-9 h/night, were on no psychotropic medication at the time of the study, and had normal (20-25 kg/m2) body mass index (BMI). We excluded individuals with self-reported neurological, psychiatric, or other organic diseases, including sleep disorders (confirmed by prior polysomnography), shift workers, drug users (confirmed by urine screening), and extreme morning and evening types with a self-assessment questionnaire for the determination of morningness-eveningness types in Brazil.48 Recruitment occurred through posters and e-mails distributed to students and staff at the university where the study was conducted.
Procedure
This study was approved by the Ethics Committee (project #1514/07) of the Universidade Federal de São Paulo, Brazil, and all volunteers signed informed consent forms. The study was conducted in a hospital setting with continuous medical supervision.
Volunteers were recruited only if prior polysomnography (see details below) confirmed absence of sleep disorders. Participants were instructed to maintain their normal sleep schedule and not to consume alcohol or caffeine for a week before (controlled by sleep logs) and during their stay in the laboratory.
The study commenced with an overnight habituation polysomnography (data not shown). The next morning participants were dismissed and instructed to return to the lab at night for baseline polysomnography, after which they were randomly distributed into 2 groups. Eleven subjects were allocated to the TSD group and 13 to the control group, which was not subjected to any kind of TSD. From baseline night, the volunteers were under constant monitoring in the laboratory throughout the experiment to avoid daytime naps and naps during the night in a sleep deprived group. Participants were allowed to read books, watch television, use the internet, and play cards or video games. Standard meals were served to all volunteers at the following times: 08:30, 12:00, 16:00, and 20:00. The deprived group received an extra snack at 00:00 h.
The immediate free recall test (see details below) was carried out in 4 sessions on consecutive days at midafternoon, starting after the baseline night on which both groups slept normally. In each session, participants were shown 5 lists balanced between subjects and groups. The control group slept on all 4 nights of the experiment while the sleep deprived group had no sleep from baseline night through the fourth and last night (recovery night), and were therefore sleep deprived when carrying out free recall at sessions 2 and 3. Note that we used the following designation of sessions and nights: session 1 (the day following baseline night – night 1); session 2 (the day after the first night of TSD in the deprived group and the second night of normal sleep for the control group – night 2); session 3 (the day after the second night of TSD in the deprived group and the third night of normal sleep in the control group – night 3); and session 4 (the day after the sleep recovery night in the deprived group and the fourth night of normal sleep in the control group – night 4). Hence, at session 2 sleep deprived individuals were in approximately 36 h of continuous vigilance and at session 3, around 60 h of continuous vigilance. The participants of the present study were in part a subset of the volunteers of another study,49 from whom various other measures were obtained.
Measures
Polysomnography
To record sleep parameters, we used an integrated amplified data collection system through the computerized polygraph Embla/A10 Digital Recording/amplifier with Somnologica software. We obtained data for electroencephalogram (EEG), electrooculogram (EOG), electromyogram (EMG), electrocardiogram (ECG), nasal and oral airflow, respiratory movements, movements of the lower limbs, and oxyhemoglobin saturation. We used Rechtschaffen and Kales standardized criteria for sleep staging. The following polysomnographic parameters were analyzed: sleep latency (min), REM sleep latency (min), sleep efficiency (%), total sleep time (min), stage 1 (%), stage 2 (%), stage 3-4 (%), REM sleep (%), and number of microarousals.
Free recall of word lists
We used 20 lists of 15 common Portuguese disyllabic and trisyllabic nouns that were not semantically related as determined by pilot studies. Briefly, in these pilot studies, lists were shown to roughly 60 university graduates when they were attending classes. Students were asked to indicate if there were any semantic or phonetic relations between words. When a relation was pointed out by > 20% of the individuals, one of the words was replaced and the list was submitted again to a new group of people using the same method. This was repeated until we obtained no more indications of relations. Words in the lists were balanced according to written frequency.50
Subjects were assessed individually. Words were presented one at a time for a period of 2 s on a computer screen, followed immediately by the subsequent stimuli. Participants were required to read each word aloud. Immediately after the end of each list they were asked to recall as many words as possible, verbally and in any order, without clues. There was no time limit. After recall, a new list was presented, and so on, totaling 5 lists per session. Scores were as follows:
-
Recall of words according to serial positions: because the use of all serial positions in the analysis with this number of individuals would tend to dampen results, the recalled words were grouped into 5 consecutive positions (see footnote following article) following Gershberg et al.26,31 That is, for each group and session we added all words recalled in the 5 lists in positions 1 to 3 (hereafter pooled position 1), 4-6 (pooled position 2), 7-9 (pooled position 3), 10 to 12 (pooled position 4), and 13 to 15 (pooled position 5). Thus, scores could vary from 0 to 15 for each of the 5 pooled positions per participant per session.
Serial position effects15 analyzed were: primacy (higher recall of words in pooled position 1 than in adjacent pooled positions 2 and/or 3), recency (higher recall of words in the pooled position 5 than of words in the pooled positions preceding it, or pooled position 4 and/or 3), and comparisons between all pooled serial positions.
To confirm that the recency effect reflected the use of short-term memory,51 we determined the number of times the first 3 remembered words belonged to the recency portion of the list (from serial position 13-15) which in this analysis we will call “true recency” when the words of the last 3 serial positions were not among the first 3 recalled, they were classified as “false recency,” which would indicate use of long-term episodic memory.
Errors: sum of intrusions (words from previous lists), inventions (words absent from previous lists), and repetitions were counted per participant per session.
Recall order: to determine output order we calculated the relative index of priority (RIP score)34 per list, per participant, per session. RIP scores have an upper and a lower limit (+1 to −1). The first word recalled is given a score of +1, the last, −1 and median items score 0. To calculate RIP scores we used the formula RIP = (T + 1 − 2Ri / T − 1), where Ri is the relative position of output for each recalled item and T is the total number of items recalled.
Statistical Analysis
StatSoft version 6.0 was used for analysis of variance (ANOVAs) followed by post hoc Tukey HSD tests for samples of different sizes per group when appropriate. For the factors in each analysis, see the results section. When there were interactions, only contrasts for the highest order interaction are described below. The level of significance adopted was 5%.
RESULTS
Groups did not differ in age (F1,22 = 0.92, P = 0.34; mean ± SD: 22.6 ± 2.4) or years of schooling (F1,22 = 1.98, P = 0.17; mean ± SD: 13.7 ± 1.07; Ps > 0.4).
Polysomnography (Table 1)
Table 1.
Mean (± SD) polysomnographic parameters per group over the four nights of the experiment
| Parameters | Control |
Sleep Deprived |
||||||
|---|---|---|---|---|---|---|---|---|
| Session 1 | Session 2 | Session 3 | Session 4 | Session 1 | Session 2 | Session 3 | Session 4 | |
| Total sleep time (min) | 409.8 ± 49.8 | 425.1 ± 30.6 | 429.2 ± 59.4 | 386.7 ± 69.8 | 446.8 ± 31.3 | — | — | 624.5 ± 33.0 |
| Sleep latency (min) | 11.5 ± 8.4 | 12.1 ± 14.9 | 11.6 ± 7.8 | 14.1 ± 24.9 | 14.5 ± 11.5 | — | — | 2.4 ± 4.0 |
| REM latency (min) | 95.0 ± 53.1 | 115.9 ± 41.8 | 78.9 ± 18.7 | 71.5 ± 33.7 | 68.7 ± 9.0 | — | — | 138.4 ± 88.9 |
| Sleep efficiency (%) | 89.8 ± 8.5 | 93.1 ± 5.4 | 93.7 ± 3.5 | 92.5 ± 7.9 | 91.7 ± 3.7 | — | — | 97.9 ± 1.3 |
| Stage 1 (%) | 3.6 ± 3.3 | 2.8 ± 1.6 | 2.2 ± 1.3 | 1.7 ± 1.4 | 3.2 ± 2.1 | — | — | 0.9 ± 0.6 |
| Stage 2 (%) | 53.8 ± 7.9 | 53.5 ± 5.9 | 52.2 ± 6.1 | 46.9 ± 6.4 | 51.8 ± 7.0 | — | — | 49.2 ± 5.5 |
| Stage 3-4 (%) | 21.5 ± 8.5 | 24.0 ± 6.9 | 22.6 ± 8.3 | 25.6 ± 6.5 | 20.7 ± 5.7 | — | — | 31.2 ± 4.7 |
| REM (%) | 21.0 ± 5.6 | 19.8 ± 4.1 | 22.9 ± 5.3 | 24.8 ± 5.3 | 24.2 ± 4.9 | — | — | 18.9 ± 4.1 |
| Microarousal (no./h) | 8.0 ± 4.1 | 7.9 ± 3.1 | 8.1 ± 3.2 | 7.6 ± 2.3 | 7.4 ± 2.8 | — | — | 3.1 ± 1.2 |
Session 1 (baseline), session 2 and 3 (total sleep deprivation in deprived group, normal sleep in the control group), session 4 (recovery sleep in the deprived group, normal sleep in controls). See text for detailed statistical analysis.
Polysomnographic data were lost for one control participant due to computational problems. We compared the polysomno-graphic parameters between groups from night 1 (baseline) and night 4 using a 2-way ANOVA with group and night as factors. There were no statistical differences between groups during the baseline night. However, we found significant interactions between group and night for REM sleep latency (F1,21 = 6.45, P < 0.01), total sleep time (F1,21 = 49.06, P < 0.0001), and microarousals (F1,21 = 9.05, P < 0.007), with the deprived group showing better sleep parameters on night 4 (recovery sleep) than the control group in the same night (Ps < 0.05) (Table 1). There was also longer total sleep time, REM sleep latency and fewer microarousals in the sleep deprived group on night 4 in relation to its baseline night (Ps < 0.05), which were expected effects since the control group was not sleep deprived.
In the analysis of REM sleep percentage, we also observed an interaction between group and night (F1,21 = 17.01, P < 0.0001). The post hoc analysis showed that on night 4, the deprived group had less REM sleep compared to their baseline night and to the control group on night 4 (P values < 0.04), which may reflect a nonsignificant increase in slow wave sleep rebound occurring during recovery sleep. There was also lower sleep efficiency (F1,21 = 6.68, P < 0.02), higher percentage of stage 1 (F1,21 = 14.39, P < 0.002) and stage 2 (F1,21 = 5.03, P < 0.04), and a lower percentage of stage 3-4 (F1,21 = 16.84, P < 0.0001) at baseline compared to night 4 irrespective of group, probably due to changes on the recovery night in the deprived group, which did not reach a significant group effect.
To check whether participants in the control group had slept normally in the 4 nights of the experiment we ran an ANOVA with night as factor for each sleep parameter investigated. We observed overall equivalent sleep in the 4 nights, except for small fluctuations which reflected the usual sleep changes associated with monitoring sleep in a laboratory setting. REM sleep latency (F3,33 = 3.46, P < 0.03) was lower on night 2 than night 4 (P < 0.03), while the percentage of stage 2 (F3,33 = 4.48, P < 0.009) was lower on night 4 compared to nights 1 and 2 (P values < 0.03). Also, the percentage of REM sleep (F3,33 = 3.10, P < 0.04) was higher on night 4 than night 2 (P < 0.04).
Free Recall of Word Lists
Recall of words by serial position
A 3-way ANOVA with the following factors was used: group, pooled positions (1, 2, 3, 4, and 5), and session (1, 2, 3, and 4). In this analysis, we observed several individual effects and interactions, including an interaction of all factors. There was, however, no significant difference between groups in different sessions and serial positions—only changes in the pattern of recall of words between groups throughout the experiment.
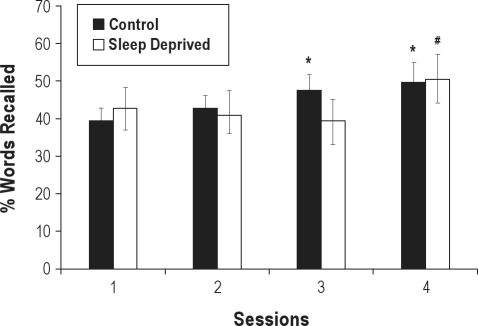
First we will describe the effect irrespective of serial position, like previous studies of sleep deprivation using free recall of words (Figure 1). There was an interaction between session and group (F3,66 = 4.30, P < 0.008). The post hoc analysis showed that the control group recalled more words in sessions 3 and 4 than baseline (Ps < 0.02), revealing a learning effect for the task that was only observed in the deprived group after recovery sleep (session 4 > 2 and 3, Ps < 0.01).
Figure 1.
Total mean percentage (± SE) of words freely recalled per group in each session (session 1: baseline; session 2: session after 1st night of deprivation; session 3: after the 2nd night of deprivation; session 4: after recovery sleep). *Different from session 1 (baseline) in the control group (P < 0.05). #Different in the total sleep deprived group at sessions 2 and 3 (P < 0.05).
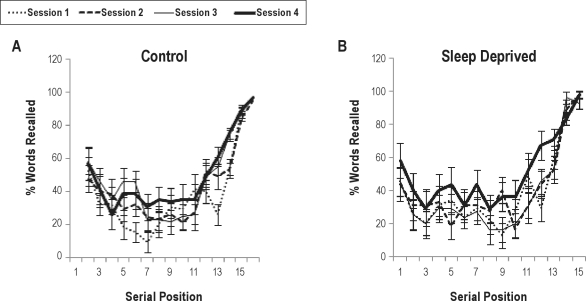
The pattern of differences between groups is clearer when we observe the interaction between group, session, and serial position (F12,264 = 1.83, P < 0.05; see Figure 2). Again there were no significant differences between groups in the same serial positions in any of the sessions. In both groups, there was an equivalent recency effect in all sessions (Ps < 0.0001), thus showing that TSD did not impair recall of words in the last serial positions. At baseline, a primacy effect was observed for both groups (Ps < 0.005) but tended to disappear from the second sessions due to increased recall of words in intermediate serial positions. Together with this finding, various other measures indicated better performance due to task training only in the control group: (a) there was an increase in recall of words in the second pooled serial position as of the third session when compared to baseline (Ps < 0.006); (b) in the third session there was also a significantly higher recall of words in the primacy portion of the list in relation to serial position 3 (P < 0.002), but this effect was no longer present at the next session; (c) an “extended recency” effect was found in session 2 and 3 (Ps < 0.05), i.e., higher recall of words in pooled position 4 relative to position 3, which disappeared in the last session, possibly due to a nonsignificant improvement in recall of words in intermediate positions.
Figure 2.
Percentage mean (± SE) free recall of words by serial position in the control group (A) and sleep deprived group (B) per session (session 1: baseline; session 2: session after 1st night of deprivation; session 3: after the 2nd night of deprivation; session 4: after recovery sleep). There were significant position effects, but no effect of group or interaction of group with others factors. For detailed statistics see text.
Sixty-seven percent of the recalled words from the last pooled serial position were among the first 3 words recalled, indicating that most of the results for this position in fact reflected use of short-term memory. We performed a 3-way ANOVA to confirm this result including group, session, and type of recency (“true” or “false”) as factors. A type of recency effect (F1,22 = 92.60, P < 0.0001), with higher recall of words in the recency portion being among the first recalled, endorsed the short-term nature of this effect, irrespective of group (Ps < 0.0002).
Errors
Errors (intrusion, inventions, and repetitions) were very few throughout the experiment; thus we added all types of error for the analyses (maximum mean per group of 4 errors). Because of the large number of zero errors, we transformed data using log of errors plus one.52 An ANOVA with group and session as factors on error showed no significant effects (Ps > 0.19).
Output order (Figure 3)
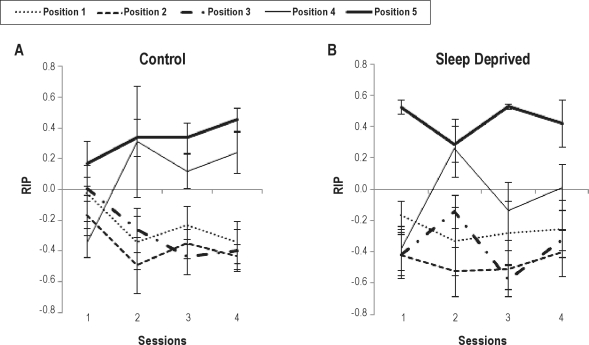
Figure 3.
Mean (± SE) output RIP score by serial position in control (A) and total sleep deprived (B) groups per session (session 1 [baseline], session 2 and 3 [total sleep deprivation in the deprived group, normal sleep in the control group], session 4 [recovery sleep in the deprived group, normal sleep in controls]). There was a significant position and session interaction, but no effect of group or interaction of group with others factors. For detailed statistics see text.
RIP scores were obtained for all subjects in all serial positions, lists, and sessions. However, in many cases there was no recall on at least one of the pooled positions in each session, precluding the inclusion of all subjects in the ANOVA on mean RIP scores of all lists in each session, which had as factors group, pooled position, and session. Therefore, this analysis was performed with only 7 control-group and 6 deprived-group subjects. Because of the small number of participants we tested for sphericity violations using the Mauchly test and found violations in 2 of the measures (5th serial position at baseline and session 3). We then re-ran the ANOVA using the Huynh-Feldt and Greenhouse-Geisser corrections which yielded similar results. We report below the ANOVA results with Greenhouse-Geisser (epsilon = 0.57) corrections of degrees of freedom and P values.
The mean RIP analysis showed no effect of group, nor of interaction of this factor with the others (Ps > 0.35), indicating no change in the output strategy developed throughout the experiment by TSD. However, there was an interaction of position and session (F6.82,150.18 = 3.47, P < 0.002). The post hoc analysis considering the same positions in different sessions showed that the RIP scores relative to pooled position 4 were lower at baseline than in sessions 2 and 4 (Ps < 0.009). In terms of differences between pooled positions in each session, we found that RIPs relative to pooled position 4 were also higher than those of pooled positions 1, 2, and 3 in session 2 (Ps < 0.009), 3 (Ps < 0.007, but 2 < 4; P = 0.06), and 4 (Ps < 0.005), showing the development of an output strategy or recency extension by both groups (see Figure 3). An analysis in which missing data were replaced by group means showed the same effects (data not shown).
Supplementary analysis of long-term memory recall
Since we showed that recall of words in the last serial positions reflected use of short-term memory (see above) and that both groups changed output strategies for the fourth pooled position in the form of an extended recency effect as the experiment progressed, one more analysis was performed considering only the first 3 pooled serial positions in order to obtain a purer measure of use of episodic long-term memory during recall. This analysis again showed no significant differences between groups in the same session and positions (Ps > 0.9) and replicated primacy changes along sessions that were shown by the above mentioned analyses.
DISCUSSION
This study showed that acute lack of sleep for one and two nights, or up to 60 hours of continuous vigilance did not impair free recall of words considering the contribution to performance of retrieval of information from both short- and episodic long-term memory. This corroborates previous null results using various list paradigms21–24 but is contrary to suggestions that verbal memory encoding is impaired by acute lack of sleep.4,5,20 An explanation for these contradictory findings is task impurity,32 which we tried to resolve here using various different measures of distinct cognitive processes in the same free recall task.
By taking the serial position of words into account, it was possible to show primacy effects at baseline, an effect that corresponds to the use of long-term episodic memory.13,15,18 In general, however, this effect was no longer evident from the second session due to an increase in the recall of intermediate items, indicating a practice effect, or “learning to learn” that has been found after repetition of this type of task.37 This was clearly shown by the improved total recall throughout the experiment that occurred in the control group from sessions 2 and 3 but in the sleep deprived group only after recovery sleep, which showed architectural changes in sleep pattern characteristic of sleep rebound.53,54 Control group sleep parameters were characteristic of normal sleep and generally comparable for the four nights of the experiment except for small fluctuations that probably reflected adaptation to the laboratory and polysomnography recoding, a procedure which decreases the quality of normal sleep.55
When considering serial positions, the increase in recall of intermediate items in relation to the baseline session reached significance only in the control group, indicating a possible impairment in the sleep deprived participants' ability to develop organizational strategies during encoding, and/or in the capacity to benefit from these strategies during recall, since there was no difference in the output strategy (RIP score) of words of the first three serial positions in both groups during the experiment. Changes in organizational strategies may also explain the fluctuation of primacy, which occurred in the control group alone.
The suggestion that TSD affects development or use of memory strategies corroborates previous work38–40,44 that investigated this issue using very different paradigms and stimulus modalities, thus such strategies may vary in sensitivity and the extent of effects due to TSD. Also, there is evidence that sleep-deprived individuals vary in this respect,40 making it difficult to establish this as a fact when considering groups of individuals. These TSD effects should come as no surprise, since organizational strategies play an important role in free recall56 and are dependent on the integrity of frontal regions,26 whose functioning is often cited as altered by sleep deprivation.5,57,58 More specifically, these changes may be triggered by decreases in attention/vigilance,1 and executive functions1,6,58 that have been shown during TSD, despite the fact that the occurrence of these executive deficits is far from resolved.32,43 Yet, the hypothesis that TSD alters the development and/or use of organizational or input strategies in free recall of word lists should be confirmed in studies designed for this purpose,59 since this cannot be answered using the present paradigm.
Here it was only possible to assess output strategies. In this respect, no change in word output relative to the last pooled serial position throughout the experiment was found in either group. Both RIP and “true” recency scores showed that the final words were recalled first, indicating that participants began the task by recalling words still present in short-term phonological memory.8,18 This is a classic pattern in immediate free recall of word lists13 and was also observed in the serial position analysis by the occurrence of the recency effect,13,15,18 present and unchanged throughout the experiment irrespective of group. This endorses data on the small effect size of acute TSD on the classical measure for determining storage of phonological material in short-term memory, the digit span.1
In contrast, an extension of recency, or an increase in the output of words from the fourth pooled serial position from the second session occurred in an equivalent way in both groups. This has been termed lag recency60 or the benefit in recalling words from serial positions near to those of the recency portion of the list. As participants repeated the task, words in the fourth pooled serial position became the most remembered subsequently to, or together with, those occupying the last pooled serial position in both groups, suggesting the development of similar output strategies for these items that tend to be coded very superficially35 and are thus more easily lost from short-term memory. In other words, on repeating the test over various sessions there was an increased probability of short-term memory use even in sleep deprived individuals, showing adaptability to increased sleep pressure.
Nevertheless, use of this strategy was more successful in the group that slept normally, because in this group alone was recall of the fourth pooled serial position higher in session 2 and 3 than at baseline. Decreased attention and/or executive functions could be to blame for this not having reached significance in the sleep deprived group. However, we suggest that use of strategies may be more impaired by these factors than the actual development of strategies because the change in output order in the sleep deprived group was equivalent to that of the control group.
There was no evidence of increased proactive interference as errors (including repetitions, inventions, and intrusions) were few and statistically equivalent in both groups, confirming the absence of changes in resistance to interference for verbal stimuli observed by Tucker et al.43 during acute TSD. This is in sharp contrast with results for visual (facial) information, for which sleep deprivation seems to negatively affect the ability to discriminate information that was in subjects' memory.44 This supports the claim of Whitney and Hinson32 that visual information is more affected by TSD than memory of verbal material.
As for the limitation of the present study, although our sample was small, we used five lists in each of the four test sessions, which increases the power of the study. We tested participants only during the afternoon, a time of day that does not show the greatest cognitive effects of TSD.46,61 It is thus possible that different effects would have been observed had we carried out the tests at a different time. Also, we opted not to use specific encoding instructions to enable the development of individual strategies for remembering information, a fact that may have increased variability in the data and thus decreased the likelihood of finding sleep deprivation effects. In addition, apparent lack of TSD-induced cognitive effects comes at a cost of increases in effort,39 and because we used young, highly educated volunteers, our findings cannot be extended to other populations that may be less able to use these compensatory mechanisms. Finally, we chose to obtain a baseline measure before depriving participants of sleep in order to ascertain that groups were cognitively equivalent. Hence, if participants had learned the task under sleep deprivation, results may have differed. However, we believe this does not invalidate our results because performance in the control group improved in relation to baseline only from the third session, so maximum learning of the task did not seem to have occurred at baseline.
In summary, our results showed that immediate free recall of words, when assessed during the afternoon in young, healthy male adults is not a sensitive task to TSD cognitive effects, even when several different measures of performance are evaluated. Acute TSD was only shown to impair the development and/or use of input strategies learned with the repetition of the free recall task, but this disadvantage disappears after recovery sleep, suggesting that despite the well-described deleterious effects of TSD on attention/vigilance, sleep deprived healthy individuals are capable of learning tasks while sleep deprived, and that any change in their performance is restored to normal levels after recovery sleep.
Therefore we did not confirm our hypothesis that TSD impairs episodic memory, but did corroborate our initial idea that lack of sleep does not affect short-term verbal memory. We also substantiated that TSD does not increase proactive interference of verbal material. As concerns output organizational strategies results were mixed, since sleep deprived participants showed indications of development of strategies but were nevertheless less efficient at benefiting from them while sleep deprived.
FOOTNOTE
Among the reasons for this choice of grouping is that primacy effects are difficult to show using larger number of words since this extends the capacity for rehearsal of words in short-term memory (while people are learning the stimuli), which is one of the mechanisms involved in this effects.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Associação Fundo de Incentivo à Pesquisa (AFIP), Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP, grant #1998/14303-3, 2006/58276-8 and 2008/08921-0), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, due to fellowships awarded to Dr. Pompéia, Dr. Tufik and Dr. Andersen). All of these are nonprofit organizations that sponsor research in Brazil. We thank also the volunteers for participating in this study.
REFERENCES
- 1.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–89. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoo SS, Hu PT, Gujar N, Jolesz FA, Walker MP. A deficit in the ability to form new human memories without sleep. Nature. 2007;10:385–92. doi: 10.1038/nn1851. [DOI] [PubMed] [Google Scholar]
- 3.Alhola P, Tallus M, Kylmälä M, Portin R, Polo-Kantola P. Sleep deprivation, cognitive performance, and hormone therapy in postmenopausal women. Menopause. 2005;12:149–55. doi: 10.1097/00042192-200512020-00008. [DOI] [PubMed] [Google Scholar]
- 4.Stricker JL, Brown GG, Wetherell LA, Drummond SP. The impact of sleep deprivation and task difficulty on networks of fMRI brain response. J Int Neuropsychol Soc. 2006;12:591–7. doi: 10.1017/S1355617706060851. [DOI] [PubMed] [Google Scholar]
- 5.Drummond SPA, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 6.Harrison Y, Horne JA. The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl. 2000;6:236–49. doi: 10.1037//1076-898x.6.3.236. [DOI] [PubMed] [Google Scholar]
- 7.Walker MP, Stickgold R. Sleep, memory and plasticity. Annu Rev Psychol. 2006;57:139–66. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 8.Atkinson RC, Shiffrin RM. Human memory: A proposed system and its control process. In: Spence KW, editor. The psychology of learning and motivation: advances in research and theory. Vol. 2. New York: Academic Press; 1968. pp. 89–195. [Google Scholar]
- 9.Salamé P, Baddeley A. Phonological factors in STM: similarity and the unattended speech effect. Bull Psychon Soc. 1986;24:263–65. [Google Scholar]
- 10.Miller GA. The magical number seven, plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956;63:81–97. [PubMed] [Google Scholar]
- 11.Tulving E. How many memory systems are there? Am Psychol. 1985;40:385–98. [Google Scholar]
- 12.Nairne JS, Riegler GL, Serra M. Dissociative effects of generation on item and order retention. J Exp Psychol Learn Mem Cogn. 1991;17:702–9. doi: 10.1037//0278-7393.17.4.702. [DOI] [PubMed] [Google Scholar]
- 13.Murdock BB. The serial position effect of free recall. J Exp Psychol Learn Mem Cogn. 1962;64:482–8. [Google Scholar]
- 14.Rundus D, Loftus GRL, Atkinson RC. Immediate free recall and three-week delayed recognition. J Verbal Learning Verbal Behavior. 1970;9:684–8. [Google Scholar]
- 15.Glanzer M, Cunitz AR. Two storage mechanisms in free recall. J Verbal Learning Verbal Behavior. 1966;5:352–60. [Google Scholar]
- 16.Postman L, Phillips LW. Short-term temporal change in free recall. Q J Exp Psychol. 1965;17:132–8. [Google Scholar]
- 17.Nogueira AML, Pompéia S, Galduroz JCF, Bueno OFA. Effects of a benzodiazepine of free recall semantically related words. Hum Psychopharmacol. 2006;21:327–36. doi: 10.1002/hup.775. [DOI] [PubMed] [Google Scholar]
- 18.Capitani E, Della Sala S, Logie RH, Spinder H. Recency, primacy and memory: reappraising and standardizing the serial position curve. Cortex. 1992;28:315–42. doi: 10.1016/s0010-9452(13)80143-8. [DOI] [PubMed] [Google Scholar]
- 19.Rouder JN, Gomez P. Modelling serial position curves with temporal distinctiveness. Memory. 2001;9:301–11. doi: 10.1080/09658210042000102. [DOI] [PubMed] [Google Scholar]
- 20.Drummond SPA, Meloy MJ, Yanagi MA, Orff HJ, Brown GG. Compensatory recruitment after sleep deprivation and the relationship with performance. Psychiatry Res. 2005;140:211–23. doi: 10.1016/j.pscychresns.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Halback MM, Spann CO, Egan G. Effect of sleep deprivation on medical resident and student cognitive function: a prospective study. Am J Obstet Gynecol. 2003;188:1198–201. doi: 10.1067/mob.2003.306. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson JP, Soderstrom M, Karlsson AU, et al. Less effective executive after one night's sleep deprivation. J Sleep Res. 2005;14:1–6. doi: 10.1111/j.1365-2869.2005.00442.x. [DOI] [PubMed] [Google Scholar]
- 23.Quigley N, Green JF, Morgan D, Idzikowski C, King DJ. The effect of sleep deprivation on memory and psychomotor function in healthy volunteers. Hum Psychopharmacol. 2000;15:171–7. doi: 10.1002/(SICI)1099-1077(200004)15:3<171::AID-HUP155>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 24.Bray CL, Cahill KS, Oshier JT, et al. Methylphenidate does not improve cognitive function in healthy sleep-deprived young adults. J Investig Med. 2004;52:192–201. doi: 10.1136/jim-52-03-34. [DOI] [PubMed] [Google Scholar]
- 25.Chapman C, Pellegrino JW, Battig WF. Input order and grouping in free-recall learning and organization. Am J Psychol. 1974;87:565–77. [Google Scholar]
- 26.Gershberg FB, Shimamura AP. Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychology. 1995;13:1305–33. doi: 10.1016/0028-3932(95)00103-a. [DOI] [PubMed] [Google Scholar]
- 27.Frankel F, Cole M. Measures of category clustering in free recall. Psychol Bull. 1971;76:39–44. [Google Scholar]
- 28.Bjork RA, Whitten WB. Recency-sensitive retrieval processes in long term free recall. Cogn Psychol. 1974;6:173–89. [Google Scholar]
- 29.Wright RE. Adult age similarities in free recall output order and strategies. J Gerontol. 1982;37:76–9. doi: 10.1093/geronj/37.1.76. [DOI] [PubMed] [Google Scholar]
- 30.Glanzer M, Koppenaal L, Nelson R. Effects of relations between words on short-term storage and long-term storage. J Verbal Learning Verbal Behavior. 1972;11:403–16. [Google Scholar]
- 31.Bueno OFA, Bertolucci PHF, Oliveira MGM, Gomez JA. Effects of semantic relations, repetition of words, and list length in word list recall of Alzheimer's patients. Arq Neuropsiquiatr. 2008;66:312–17. doi: 10.1590/s0004-282x2008000300005. [DOI] [PubMed] [Google Scholar]
- 32.Whitney P, Hinson JM. Measurement of cognition in studies of sleep deprivation Prog Brain Res. 2010;185:37–48. doi: 10.1016/B978-0-444-53702-7.00003-8. [DOI] [PubMed] [Google Scholar]
- 33.Tulving E. Subjective organization in free recall of unrelated words. Psychol Rev. 1962;69:344–54. doi: 10.1037/h0043150. [DOI] [PubMed] [Google Scholar]
- 34.Flores LM, Brown SC. Comparison of output order in free recall. Behav Res Methods Instrum Comput. 1974;6:385–88. [Google Scholar]
- 35.Maskarinec AS, Brown SC. Positive and Negative recency effects in free recall learning. J Verbal Learning Verbal Behavior. 1974;16:328–34. [Google Scholar]
- 36.Santos-Galduroz RF, Oliveira FG, Galduroz JCF, Bueno OFA. Cognitive performance of young and elderly subjects on the free word recall memory test: effect of presentation order on recall order. Braz J Med Biol Res. 2009;42:988–92. doi: 10.1590/s0100-879x2009001000019. [DOI] [PubMed] [Google Scholar]
- 37.Dallett KM. Practice effects in free and ordered recall. J Exp Psychol. 1963;66:65–71. doi: 10.1037/h0043223. [DOI] [PubMed] [Google Scholar]
- 38.Hagewoud R, Havekes R, Tiba PA, et al. Coping with sleep deprivation: shifts in regional brain activity and learning strategy. Sleep. 2010;33:1465–73. doi: 10.1093/sleep/33.11.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hockey GR, Wastell DG, Sauer J. Effects of sleep deprivation and user interface on complex performance: a multilevel analysis of compensatory control J Hum Factors. 1998;40:233–53. doi: 10.1518/001872098779480479. [DOI] [PubMed] [Google Scholar]
- 40.Maddox WT, Glass BD, Wolosin SM, Savarie ZR, Matthews MD, Schnyer DM. The effects of sleep deprivation on information-integration categorization performance. Sleep. 2009;32:1439–48. doi: 10.1093/sleep/32.11.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maddox WT, Glass BD, Zeithamova D, et al. The effects of sleep deprivation on dissociable prototype learning systems. Sleep. 2011;34:253–60. doi: 10.1093/sleep/34.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilley AJ, Brown S, Donald M, et al. Human sleep and memory process. In: Broughton RJ, Ogilvie RD, editors. Sleep, arousal and performance. Boston: Birkhäuser- Boston; 1992. pp. 117–27. [Google Scholar]
- 43.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HP. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33:47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mograss MA, Guillem F, Brazzini-Poisson V, Godbout R. The effects of total sleep deprivation on recognition memory processes: a study of event-related potential Neurobiol Learn Mem. 2009;91:343–52. doi: 10.1016/j.nlm.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Folkard S, Monk T, Bradbury R, Rosenthall J. Time of day effects in school children's immediate and delayed recall of meaningful material. Br J Psychol. 1977;68:45–50. [Google Scholar]
- 46.Folkard S, Monk T. Time of day and processing strategy in free recall. Q J Exp Psychol. 1979;31:461–75. [Google Scholar]
- 47.Campos TF, Souza DE, Pinheiro CDG, Menezes AAL. Temporal variation in memory tests performance in cerebral vascular disease patients. Psicologia: Reflexão e Crítica. 2007;20:212–9. [Google Scholar]
- 48.Benedito-Silva AA, Menna-Barreto L, Marques N, Tenreiro S. A self-assessment questionnaire for the determination of morningness-eveningness types in Brazil. Prog Clin Biol Res. 1990;341B:89–98. [PubMed] [Google Scholar]
- 49.Martins RCS, Andersen ML, Garbuio SA, et al. Dopamine transporter regulation during four nights of REM sleep deprivation followed by recovery--an in vivo molecular imaging study in humans. Sleep. 2010;33:243–51. doi: 10.1093/sleep/33.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berber TS. Paper presented at 4th Corpus Linguistics Symposium, InPLA PUC/SP. Brazil: 2001. The bank of Portuguese. A multimillion word monitor corpus of Brazilian Portuguese. [Google Scholar]
- 51.Tulving E, Patterson RD. Functional units and retrieval process in free recall. J Exp Psychol. 1968;77:239–48. doi: 10.1037/h0025788. [DOI] [PubMed] [Google Scholar]
- 52.Field AP. Discovering statistics using SPSS. Porto Alegre: ArtMed; 2009. [Google Scholar]
- 53.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- 54.Curcio G, Ferrara M, Pellicciari MC, Cristiani R, Gennaro LD. Effect of total sleep deprivation on the landmarks of stage 2 sleep. Clin Neurophysiol. 2003;114:2279–85. doi: 10.1016/s1388-2457(03)00276-1. [DOI] [PubMed] [Google Scholar]
- 55.Weiner IB, Freedheim DK, Nezu AM. Health Psychology. 2003. Handbook of psychology. [Google Scholar]
- 56.Long N, Öztekin I, Badre D. Separable prefrontal cortex contributions to free recall. J Neurosci. 2010;30:10967–76. doi: 10.1523/JNEUROSCI.2611-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drummond SPA, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial substraction. NeuroReport. 1999;10:3745–48. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- 58.Jones K, Harrison Y. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med Rev. 2001;5:463–75. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- 59.Larigauderie P, Michaud A, Vicente S. The role of semantic memory in short-term recall: effect of strategic retrieval ability in a elderly population. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2010;18:1–33. doi: 10.1080/13825585.2010.521813. [DOI] [PubMed] [Google Scholar]
- 60.Kahana MJ. Associative retrieval processes in free recall. Mem Cognit. 1996;24:103–9. doi: 10.3758/bf03197276. [DOI] [PubMed] [Google Scholar]
- 61.Dinges DF, Williams K, Gillen KA, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]