Abstract
Study Objectives:
Findings on sleep disturbances in family dementia caregivers are conflicting. We studied the longitudinal effects of dementia caregiving and major transitions in the caregiving situation on caregivers' sleep and the effect of moderating variables.
Design and Setting:
Community-based longitudinal study with assessments about once a year for up to three years.
Participants:
A sample of 109 elderly spousal Alzheimer caregivers and 48 non-caregiving age- and gender-matched controls.
Measurements and Results:
Random regression models with fixed and time-variant effects for covariates known to affect sleep were used to evaluate changes in the Pittsburgh Sleep Quality Index (PSQI) and in four actigraphy measures over time in relation to caregiving status and transitions (i.e., nursing home placement or death of the Alzheimer disease spouse). Multivariate-adjusted sleep characteristics did not significantly differ between caregivers and non-caregivers over time. Spousal death increased caregivers' nighttime wake after sleep onset (WASO) by 23 min (P = 0.002) and daytime total sleep time (TST) by 29 min (P = 0.003), while nighttime sleep percent decreased by 3.2% (P = 0.009) and nighttime TST did not change. Placement of the spouse had no significant effect on caregivers' sleep. Older age, male gender, role overload, depressive symptoms, and proinflammatory cytokines variously emerged as significant moderators of the relationships between caregiving and transitions with poor subjective and objective sleep.
Conclusions:
Alzheimer caregivers and non-caregiving controls had similar trajectories of sleep. However, there may be subgroups of caregivers who are vulnerable to develop sleep disturbances, including those whose spouses have died.
Citation:
von Känel R; Mausbach BT; Ancoli-Israel S; Dimsdale JE; Mills PJ; Patterson TL; Ziegler MG; Roepke SK; Chattillion EA; Allison M; Grant I. Sleep in spousal alzheimer caregivers: a longitudinal study with a focus on the effects of major patient transitions on sleep. SLEEP 2012;35(2):247-255.
Keywords: Actigraphy, Alzheimer disease, caregiver, cytokines, depression, longitudinal study, psychological stress, sleep
INTRODUCTION
Providing informal care to a family member with dementia compromises caregivers' mental and physical health,1–4 including sleep.5,6 Poor sleep is linked to many other health problems in dementia caregivers, such as depression, poor quality of life, and cardiovascular disease.5–10 Dementia care-giver sleep has previously been measured in subjective and objective terms, including self-rated questionnaires to assess perceived sleep quality, full-night polysomnography (PSG), and actigraphy to assess sleep-wake activity.5,6 Study results may vary depending on the sleep measures used and efforts made to consider the many demographic, medical, lifestyle, and psychological factors affecting sleep. For instance, dementia caregivers commonly report more subjective sleep complaints (e.g., greater Pittsburg Sleep Quality Index [PSQI] global scores) than non-caregiving controls,11,12 but this difference is largely explained by caregiver burden and depression.13,14 Actigraphy seems less influenced by depression than self-reported sleep,14 helping to explain in part why subjective and objective sleep measures may show little congruency in dementia caregivers.14,15
Performing in-home full-night PSG, we found shorter total sleep time (TST) and lower sleep efficiency in spousal Alzheimer disease (AD) caregivers than in their non-caregiving counterparts.16 Further analysis showed that nighttime TST was particularly short in the group of older AD caregivers (> 71 years of age) as opposed to the group of younger caregivers.12 The effect of age on caregiver sleep concurs with data showing that sleep becomes generally poorer with increasing age.17 In contrast, women in the community report more sleep complaints than men,18 but male AD caregivers showed a greater amount of wake after sleep onset (WASO) on PSG than female AD caregivers.19 Actigraphy studies have shown less sleep percent and greater amount of WASO, but similar nighttime TST, in AD caregivers compared to non-caregiving controls.15 Moreover, compared to controls, sleep percent showed an inverse association with circulating levels of the proinflammatory cytokine interleukin (IL)-6 in caregivers, even after controlling for age, body mass index (BMI), antihypertensive medication, smoking, physical exercise, and role overload,15 all of which may affect caregiver sleep.5,6 The relation of higher IL-6 levels with lower nighttime sleep percent is consistent with studies linking low-grade chronic systemic inflammation, including elevated circulating levels of IL-6 and tumor necrosis factor (TNF)-α, to poor sleep.20 Interleukin-6 and TNF-α are stress responsive,21 and IL-6 was shown to be elevated both cross-sectionally22 and longitudinally23 in AD caregivers relative to non-caregiving controls. As a whole, this research suggests that low-grade inflammation, as kindled by the chronic stress of caregiving, might impair sleep in AD caregivers.
To our knowledge, all published studies on dementia caregivers' sleep to date have utilized a cross-sectional design.6 Sleep assessment over time may also illuminate sleep effects of major transitions in the caregiving situation, such as permanent placement of the AD spouse in a nursing home or death of the spouse. Therefore, compared to non-caregiving control subjects, we examined subjective and objective sleep characteristics longitudinally using the PSQI and actigraphy in community-dwelling elderly spousal AD caregivers over a period of up to three years and adjusting for important covariates of sleep. We additionally investigated the effects of placement or death of the AD spouse on caregivers' sleep. As not all caregivers react the same way to these transitions, either psychologically or biologically,24,25 we aimed to identify potentially moderating variables of transition effects on sleep characteristics. Specifically, we hypothesized a decline in sleep quality at three months after a transition in relatively older and male caregivers, and in those caregivers with greater levels of depressive symptoms, role overload, and IL-6 and TNF-α.
MATERIALS AND METHODS
Study Participants and Design
We recruited community-dwelling spousal AD caregivers and non-caregiving married controls into the University of California, San Diego (UCSD) “Alzheimer's Caregiver Study,” which is investigating health consequences of dementia caregiving stress. Referrals came from the UCSD Alzheimer's Disease Research Center, community support groups, agencies serving caregivers, local senior citizen health fairs, and other participants. Caregivers and controls were matched in terms of age (≥ 55 years) and gender. Exclusion criteria were current major illnesses (e.g., cancer), blood pressure > 200/120 mm Hg, and certain medications (i.e., oral anticoagulants, nonselective β-blockers, and steroids). Participants underwent in-home assessments every 12 months for a period of up to 3 years (i.e., for a maximum of 4 visits). Research staff also made brief follow-up phone calls every 3 months to check for caregiver transitions and changes in health status. Participants were additionally asked to call research staff whenever there was a transition in their caregiving situation or health status. If the caregiver had placed the AD spouse in a long-term care facility or the AD spouse had died, staff set up an appointment for the post-transition assessments, which occurred at 3, 15, and 27 months after the transition. A research nurse gathered sociodemo-graphic, medical, and psychosocial data using questionnaires. For cytokine assessment, participants had their blood collected between 10:00 and 10:45 AM. Fasting state was not a requirement in order to not interfere with caregivers' daily routine.
Out of the total enrollment of 186 study participants (126 caregivers, 60 non-caregiving controls), 109 caregivers and 53 controls had complete baseline data. Because a major focus of the study was to examine the effect of spousal death on dementia caregivers' sleep, we additionally excluded 5 non-caregivers whose spouse had died during the study period, leaving a final sample of 157 subjects (109 caregivers, 48 controls). All participants provided written informed consent to the protocol approved by the UCSD Institutional Review Board.
Demographic and Health Assessment
Sociodemographic factors
We collected information on age, gender, ethnicity, years of education (reflecting socioeconomic status), and years of care-giving.
Medical data
Participants were provided a list of 17 health problems and were asked to indicate whether a doctor had informed them that they currently have or have ever had each problem. Positive items were summed to indicate a total number of health problems. Body mass index (BMI) was calculated based on subjects' self-report of weight and height by dividing weight in kilograms by the square of height in meters.
Health behaviors
Alcohol consumption during the last month was assessed by multiplying the number of days participants self-reported at least one alcoholic drink by the number of alcoholic drinks they usually drank on those days (total score 0-36). The Rapid Assessment of Physical Activity (RAPA) scale was used to assess participants' amount of physical activity at varying intensity (i.e., light, moderate, vigorous) in a typical week (total score 0-6).26 Smoking status was defined as ever smoked (i.e., former or current smoking) vs. never smoked (only 2 participants were current smokers at the baseline assessment).
Psychological distress
Role overload with life responsibilities was rated using a 4-item scale27; for example, “you work hard (as a caregiver) but never seem to make any progress.” Average responses to each item (1 = not at all, 4 = completely) were used to create a total overload score (range 1-4). The short form of the Center for Epidemiologic Studies-Depression scale (CESD-10) was used to assess the level of depressed mood.28
Cytokines
Circulating concentrations (pg/mL) of IL-6 and TNF-α were determined in duplicates from stored EDTA plasma samples using enzyme-linked immunosorbent assays per the manufacturers' instructions (Meso Scale Discovery, Gaithersburg, MD). Intra- and inter-assay coefficients of variation were < 5% and 10%, respectively.
Sleep Assessment
Subjective sleep characteristics
We assessed self-rated sleep with the interviewer-administered Pittsburg Sleep Quality Index (PSQI) comprising 19 items yielding a global PSQI score ranging from 0-21 points.29 Higher scores indicate poorer self-rated sleep quality, whereby pathological difficulties with sleep are reflected by a score > 5.
Objective sleep characteristics
Actigraphy was used to assess nighttime total sleep time (TST), nighttime wake after sleep onset (WASO), nighttime sleep percent, and daytime TST. Participants wore the Sleep-Watch-O actigraph (Ambulatory Monitoring, Inc., Ardsley, NY) on the non-dominant wrist for 3 consecutive 24-h periods (i.e., 72 h). The actigraph data represent averages of the 3 consecutive night and day periods, respectively. Missing data occurred due to battery failure, participants neglecting to remove the watch for bathing, or participants removing the watch prematurely. The device detects movement via a piezoelectric bimorph-ceramic cantilever that generates a voltage each time the actigraph is moved. Voltages are gathered continuously and summarized over 1-min intervals. We report data based on digital proportional integration (PIM) mode.30 Of the different modes to collect actigraph data, the standard PIM modality corresponds best with PSG measures in older adults.31 ActionW-2 software (Ambulatory Monitoring, Inc., Ardsley, NY) was used to analyze actigraphy data.32 Participants also completed a sleep log on which they were asked to record bed time and wake time as well as intervals during which the actigraph was removed for particular activities like showering or bathing. This information was utilized in editing the actigraph records as previously described.33
Data Analysis
Data were analyzed with PASW 18.0 statistical software package (SPSS Inc., Chicago, IL). Level of statistical significance was set at P ≤ 0.05 (2-tailed). Before performing analyses, PSQI scores, WASO, sleep percent, and daytime TST were square root transformed, and IL-6 and TNF-α values were log transformed to approximate a normal distribution as verified by the Kolmogorov-Smirnov test. Seven IL-6 values (log IL-6 > 1.182 pg/mL) and 2 TNF-α values (log TNF-α > 1.307 pg/mL) remained > 3 SDs above the log transformed sample mean and were deleted as outliers. Independent-samples t-test and χ2 test were used to compare caregivers and non-caregivers on baseline characteristics. Pearson correlation coefficients were calculated to estimate the associations between sleep characteristics at baseline. For clarity, all data are shown in original units.
We conducted a mixed (random-effects) regression analysis to examine the impact of caregiver status (i.e., caregivers vs. non-caregiving controls) and caregiver transitions (i.e., placement and death of the AD spouse) on sleep. Mixed model regression is a powerful analysis that allows one to estimate an intercept and slope for each participant based on all available data for that individual (i.e., even when some data points are missing across assessments), augmented by the data from the entire sample.34 In other words, all estimates are computed based on the total number of observations that are contributed by the 157 subjects across the entire study period. We tested 5 sleep variables, each as outcomes in a separate multivariate model without making adjustments of P-values for multiple comparisons. This approach is preferred if the data under evaluation are not random numbers but actual observations in nature, as it limits the possibility that true knowledge advancing non-null associations go undetected.35 To increase the interpretability of regression coefficients and to diminish problems associated with multicollinearity, we centered independent variables before conducting analysis,36 except for “time” (in years), which was linear in nature with the baseline assessment coded as “0.” Linear variables were centered around their grand means. Dummy coded categorical variables were centered at −0.5 (e.g., non-caregivers) and +0.5 (e.g., caregivers). The model included the following fixed effects: caregiving status, age at baseline, gender, education, number of health problems, BMI, alcohol consumption, physical activity, smoking status, number of years caregiving, role overload, depressive symptoms, IL-6 levels, TNF-α levels, placement status of the AD spouse (yes, no), and deceased status of the AD spouse (yes, no). Of these, the number of health problems, BMI, alcohol consumption, physical activity, smoking status, years caregiving, role overload, depressive symptoms, IL-6 levels, TNF-α levels, and placement and deceased status of the AD spouse were all entered as time-varying. Random intercepts were modelled for participants. A significant effect of placement and/or spousal death would mean a change in the intercept of a sleep variable as a function of the transition (i.e., from pre- to post-transition).
Because we also hypothesized that the effect of caregiving status and transitions in the caregiving situation on sleep would be moderated by demographic factors, psychological distress, and levels of proinflammatory cytokines, we probed for interactions between caregiver status and transitions on the one hand, and age, gender, role overload, depressive symptoms, IL-6, and TNF-α values on the other. For significant interactions, we applied the Holmbeck method37 to test whether high levels (+1 SD from the mean) vs. low levels (−1 SD from the mean) of a continuously scaled moderator variable would alter the association of caregiving status and caregiving transitions with the sleep measures.
The 157 subjects contributed 450 assessments. Sleep data were complete in 98.0% for PSQI globe score and in 90.7% for actigraphy measures. Per the study design, data for all of the fixed effect variables were complete in 100% of assessments. Time-variant variables were complete in 100% of cases for physical activity; in 97.6% for health problems, BMI, alcohol consumption, smoking status, role overload, and depressive symptoms; in 95.6% for TNF-α levels; and in 94.2% for IL-6 levels. In this ongoing study, of the enrolled 109 caregivers and 48 non-caregivers, 105 caregivers and 47 non-caregivers contributed data at the 12-month follow-up; 80 caregivers and 37 non-caregivers contributed data at the 24-month follow-up; and 17 caregivers and 7 non-caregivers contributed data at the 36-month follow-up. Four dropouts each occurred before the 12-month and 24-month assessments; i.e., 2 caregivers had passed away, and 3 caregivers and non-caregivers each were no longer eligible because of various reasons (i.e., had developed severe physical disease, no longer interested in participating in the study, questionable consent).
RESULTS
Baseline Characteristics of Study Participants
The mean age ± SD of all participants was 75 ± 8 years (range 55-90); 68% were women. The sample comprised 92% Caucasians and 8% other ethnicities. Caregivers had been providing care to their AD spouse for an average of 4.5 ± 3.5 years (range 0.5-17.1). Table 1 shows that caregivers and controls were similar in terms of sociodemographic factors, medical data, cytokine levels, and health behaviors, except physical activity, which was lower in caregivers. Expectedly, caregivers had greater levels of role overload and depressive symptoms than their non-caregiving counterparts. In terms of sleep measures assessed at baseline, PSQI scores were higher in caregivers than in controls. In addition, the PSQI score of the caregivers was above the cut-off of 5, suggesting that caregivers fell into the pathological sleep range, while that of the non-caregivers was in the normal range (i.e., below 5). There were no group differences in terms of actigraphy measures at baseline. PSQI scores did not significantly correlate with actigraphy measures at baseline in caregivers (nighttime TST: r = −0.15, P = 0.12; nighttime WASO: r = 0.09, P = 0.33; nighttime sleep percent: r = −0.15, P = 0.12; daytime TST: r = 0.10, P = 0.32) or in non-caregivers (nighttime TST: r = 0.07, P = 0.64; nighttime WASO: r = 0.13, P = 0.38; nighttime sleep percent: r = −0.09, P = 0.52; daytime TST: r = 0.15, P = 0.30).
Table 1.
Characteristics of 157 study participants at baseline
| Variables | Caregivers (n = 109) | Non-Caregivers (n = 48) | P-value |
|---|---|---|---|
| Age (years) | 74.1 ± 8.1 | 74.7 ± 6.0 | 0.738 |
| Gender (female) (%) | 69.7 | 62.5 | 0.373 |
| Education (years) | 15.3 ± 3.1 | 15.7 ± 3.2 | 0.426 |
| Number of health problems | 3.33 ± 1.88 | 2.81 ± 1.58 | 0.097 |
| Body mass index (kg/m2) | 26.5 ± 4.7 | 26.7 ± 6.3 | 0.865 |
| Alcohol consumption (score) | 5.76 ± 5.85 | 5.23 ± 5.08 | 0.625 |
| Physical activity (score) | 3.37 ± 1.67 | 4.04 ± 1.58 | 0.019 |
| Ever smoker (%) | 45.0 | 41.7 | 0.702 |
| Role overload (score) | 5.22 ± 3.20 | 1.40 ± 2.08 | < 0.001 |
| Depressive symptoms (score) | 8.68 ± 5.90 | 2.54 ± 4.43 | < 0.001 |
| Interleukin-6 (pg/mL) | 1.38 ± 1.20 | 1.79 ± 2.03 | 0.254 |
| Tumor necrosis factor-α (pg/mL) | 6.00 ± 2.50 | 5.75 ± 1.99 | 0.754 |
| Pittsburgh Sleep Quality Index (score) | 6.68 ± 3.57 | 4.31 ± 2.52 | < 0.001 |
| Nighttime total sleep time (min) | 437.60 ± 67.86 | 417.03 ± 56.76 | 0.068 |
| Nighttime wake after sleep onset (min) | 62.21 ± 26.73 | 59.42 ± 25.82 | 0.541 |
| Nighttime sleep percent (%) | 87.53 ± 5.19 | 87.53 ± 5.36 | 0.994 |
| Daytime total sleep time (min) | 48.64 ± 40.60 | 50.68 ± 46.89 | 0.705 |
Data are given as means ± SD or percentages.
Transitions in the Caregiving Situation
Over the course of the study, 30 (27.5%) caregivers placed their spouse in a long-term care facility and 19 (17.4%) experienced the death of their spouse. The initial post-transition assessments occurred at 3 months following placement or death of the AD spouse. The remaining assessments took place approximately 12 months (11 placements, 10 deaths) or 24 months (2 placements, 1 death) later.
Predictors of Change in Sleep over Time
Table 2 shows the multivariate model for the 5 sleep measures. Caregivers did not significantly differ from non-caregivers in any of the 5 sleep measures over time (i.e., main effect for caregiver status). Absolute values of sleep measures for caregivers vs. non-care-giving controls with the total number of contributing sleep observations over time are given in Table 3. A retrospective power analysis showed that the minimally detectable significant difference between caregivers and noncaregivers in sleep measures over time was 0.8 points for the PSQI global score, 27 min for nighttime TST, 3 min for nighttime WASO, 2% for nighttime sleep percent, and 12 min for daytime TST.
Table 2.
Multivariate linear mixed regression model for changes in sleep measures over time
| Variables entered (all in one block) | Pittsburgh Sleep Quality Index (score) |
Nighttime total sleep time (minutes) |
Nighttime wake after sleep onset (minutes) |
Nighttime sleep percent (%) |
Daytime total sleep time (minutes) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate ± SE | P | Estimate ± SE | P | Estimate ± SE | P | Estimate ± SE | P | Estimate ± SE | P | |
| Intercept | 5.96 ± 0.47 | < 0.001 | 440.53 ± 11.50 | < 0.001 | 75.85 ± 5.54 | < 0.001 | 85.40 ± 1.01 | < 0.001 | 58.47 ± 7.85 | < 0.001 |
| Caregiver | 0.29 ± 0.63 | 0.446 | 4.45 ± 13.74 | 0.746 | 0.08 ± 6.27 | 0.956 | 0.19 ± 1.21 | 0.877 | − 3.32 ± 8.62 | 0.598 |
| Age, baseline | 0.03 ± 0.03 | 0.245 | 0.86 ± 0.67 | 0.197 | 0.38 ± 0.30 | 0.268 | −0.04 ± 0.06 | 0.498 | 1.69 ± 0.41 | < 0.001 |
| Gender, female | 0.78 ± 0.47 | 0.081 | 0.04 ± 10.23 | 0.997 | −7.85 ± 4.62 | 0.073 | 1.44 ± 0.90 | 0.118 | −2.24 ± 6.31 | 0.576 |
| Education | 0.02 ± 0.07 | 0.761 | −0.05 ± 1.54 | 0.973 | −1.00 ± 0.70 | 0.143 | 0.18 ± 0.14 | 0.195 | 1.16 ± 0.95 | 0.341 |
| Health problems | 0.01 ± 0.08 | 0.898 | −2.97 ± 1.96 | 0.131 | −0.10 ± 0.94 | 0.796 | −0.01 ± 0.17 | 0.994 | 0.89 ± 1.33 | 0.814 |
| Body mass index | 0.04 ± 0.04 | 0.450 | −0.95 ± 0.82 | 0.249 | 0.71 ± 0.38 | 0.122 | −0.16 ± 0.07 | 0.025 | 1.49 ± 0.54 | 0.005 |
| Alcohol consumption | −0.03 ± 0.03 | 0.329 | −0.58 ± 0.67 | 0.383 | −0.01 ± 0.31 | 0.945 | −0.01 ± 0.06 | 0.983 | −0.60 ± 0.44 | 0.362 |
| Physical activity | 0.02 ± 0.08 | 0.336 | −3.40 ± 2.05 | 0.097 | −1.12 ± 1.00 | 0.224 | 0.12 ± 0.18 | 0.502 | 0.95 ± 1.42 | 0.859 |
| Ever smoker | −0.38 ± 0.37 | 0.354 | 9.82 ± 8.46 | 0.247 | −0.25 ± 3.94 | 0.964 | 0.25 ± 0.74 | 0.736 | −1.02 ± 5.47 | 0.418 |
| Years caregiving | −0.01 ± 0.07 | 0.598 | 1.91 ± 1.49 | 0.200 | −0.15 ± 0.67 | 0.906 | 0.07 ± 0.13 | 0.609 | 0.61 ± 0.92 | 0.462 |
| Role overload | 0.23 ± 0.05 | < 0.001 | 0.37 ± 1.32 | 0.777 | 0.40 ± 0.64 | 0.493 | −0.08 ± 0.12 | 0.474 | −1.10 ± 0.90 | 0.306 |
| Depressive symptoms | 0.18 ± 0.03 | < 0.001 | 0.61 ± 0.67 | 0.358 | −0.01 ± 0.32 | 0.974 | 0.02 ± 0.06 | 0.784 | 0.53 ± 0.46 | 0.186 |
| Interleukin-6 | 0.09 ± 0.39 | 0.652 | 6.72 ± 9.86 | 0.496 | −0.47 ± 4.81 | 0.942 | 0.41 ± 0.87 | 0.623 | −0.18 ± 6.86 | 0.682 |
| TNF-α | 1.27 ± 0.71 | 0.165 | 18.73 ± 17.46 | 0.284 | −0.22 ± 8.59 | 0.851 | 0.04 ± 1.53 | 0.974 | 6.66 ± 12.32 | 0.603 |
| Time | 0.19 ± 0.13 | 0.096 | −3.32 ± 3.18 | 0.296 | −0.76 ± 1.57 | 0.374 | 0.08 ± 0.28 | 0.808 | 2.63 ± 2.27 | 0.269 |
| Placed spouse | 0.17 ± 0.43 | 0.531 | 2.63 ± 11.16 | 0.814 | 3.34 ± 5.47 | 0.522 | −0.72 ± 0.98 | 0.466 | −9.92 ± 7.82 | 0.132 |
| Spouse deceased | 0.96 ± 0.51 | 0.058 | 11.45 ± 14.01 | 0.415 | 22.78 ± 6.89 | 0.002 | −3.20 ± 1.23 | 0.009 | 29.27 ± 9.88 | 0.003 |
All variables were centered to the mean such that the intercept shows the average value of the 5 sleep measures in the entire sample. Categorical variables were contrast coded as caregiver (+0.5) vs. non-caregiver (−0.5), female gender (+0.5) vs. male gender (−0.5), and ever smoker (+0.5) vs. never smoker (−0.5). “Placed Spouse” and “Spouse Deceased” indicate the immediate change in the sleep measures assessed 3 months after the respective transition. “Time” indicates the change in sleep measures per each assessment the participant was in the study. TNF-α, tumor necrosis factor-α. Estimates ± SE are given in original units.
Table 3.
Sleep measure values over time between caregivers and non-caregivers
| Sleep Measure | Caregivers | Observations | Non-Caregivers | Observations |
|---|---|---|---|---|
| PSQI global score | 6.10 ± 0.43 | 304 | 5.81 ± 0.67 | 137 |
| Nighttime TST (minutes) | 442.76 ± 10.50 | 280 | 438.31 ± 15.77 | 128 |
| Nighttime WASO (minutes) | 75.89 ± 5.03 | 280 | 75.81 ± 7.46 | 128 |
| Nighttime sleep percent (%) | 85.49 ± 0.92 | 280 | 85.30 ± 1.38 | 128 |
| Daytime TST (minutes) | 56.81 ± 7.12 | 280 | 60.13 ± 10.47 | 128 |
TST, total sleep time; WASO, wake after sleep onset. Values are given as estimates ± SE obtained by multivariate mixed linear regression modelling (cf. Table 2 for covariates). The total number of observations for each sleep measure across the entire study period is also shown per group.
Caregivers whose spouse had died experienced a significant increase in nighttime WASO of 23 ± 7 min and a decrease in nighttime sleep percent of 3.2% ± 1.2%, as well as an increase in daytime TST of 29 ± 10 min (main effect of “spouse deceased”). There was also an increase in the caregivers' PSQI score of almost 1 point with spousal death, however this change was not significant (P < 0.06).
The placement of the AD spouse in a long-term care facility was not significantly associated with changes in caregiver sleep (main effect of “placed spouse”). A retrospective power analysis showed that the minimally detectable significant difference between caregivers who placed their AD spouse and those who did not was 0.5 points for the PSQI global score, 22 min for nighttime TST, 10 min for nighttime WASO, 2% for nighttime sleep percent, and 13 min for daytime TST.
Table 2 also shows that greater levels of role overload (P < 0.001) and depressive symptoms (P < 0.001) predicted greater PSQI scores (i.e., poorer subjective sleep quality) but not any of the actigraphy measures. Moreover, age (P < 0.001) and BMI (P = 0.005) were both positively associated with daytime TST, and BMI was inversely associated with nighttime sleep percent (P = 0.025). Gender, education, health problems, health behaviors, years caregiving, and cytokine levels did not significantly predict sleep.
Moderating Variables of Effect of Caregiving on Sleep
Demographic factors
Caregiver status did not significantly interact with age (all P-values > 0.17) or gender (all P-values > 0.24) in determining any sleep measures.
Psychological distress
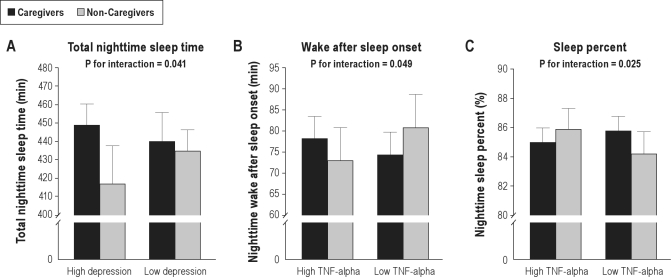
There was a significant interaction between caregiver status and depressive symptoms for nighttime TST (3.17 ± 1.54, P = 0.041), but not for the other sleep measures (all P-values > 0.38). Compared to their non-caregiving counterparts, caregivers showed longer nighttime TST if depressive symptom levels were high (32.40 ± 19.35 min, P = 0.095) but shorter nighttime TST when depressive symptom levels were low (−5.13 ± 14.54 min, P = 0.73) (Figure 1, panel A). Caregiver status showed no significant interaction with role overload for any sleep measures (all P-values > 0.18).
Figure 1.
Moderation of caregiving effects on sleep. Panels A-C illustrate the significant interactions between caregiving status and levels of depressive symptoms and circulating cytokines for sleep measures. TNF, tumor necrosis factor.
Cytokines
There were significant interactions between caregiver status and TNF-α for nighttime WASO (31.75 ± 16.53, P = 0.049) and nighttime sleep percent (−6.62 ± 2.93, P = 0.025), but not for the other sleep measures (all P-values > 0.67). Compared to non-caregivers, caregivers had longernighttime WASO if TNF-α levels were high (5.30 ± 6.84 min, P = 0.49), but shorter WASO if TNF-α levels were low (− 6.40 ± 7.11 min, P = 0.34) (Figure 1, panel B). Moreover, relative to non-caregivers, care-givers showed less nighttime sleep percent if TNF-α levels were high (−0.87 ± 1.30%, P = 0.50) but more sleep percent if TNF-α levels were low (1.57 ± 1.35%, P = 0.25) (Figure 1, panel C). Caregiver status did not significantly interact with IL-6 in determining any sleep measures (all P-values > 0.15).
Moderating Variables of Effect of Placement of Spouse on Sleep
Demographic factors
Placement status did not significantly interact with age (all P-values > 0.18) or gender (all P-values > 0.42) in determining any sleep measures.
Psychological distress
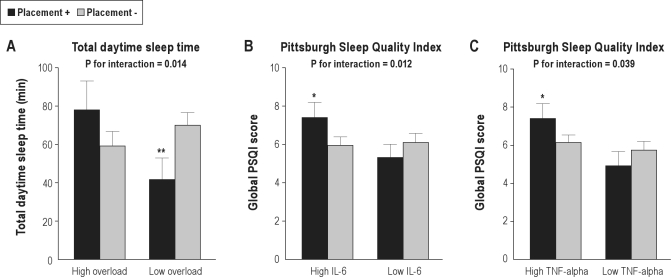
There was a significant interaction between placement and overload for daytime TST (7.55 ± 2.57, P = 0.014), but not for other sleep measures (all P-values > 0.38). Caregivers who placed their spouse showed an increase in daytime TST of 19.16 ± 12.58 min (P = 0.32) if role overload levels were high, opposed to a decrease in daytime TST of 28.31 ± 9.95 min (P = 0.007) if role overload levels were low (Figure 2, panel A). Placement did not significantly interact with depressive symptoms in determining any sleep measures (all P-values > 0.42).
Figure 2.
Moderation of placement effects on sleep. Panels A-C illustrate the significant interactions between placement of the Alzheimer spouse in a long-term care facility and levels of role overload and circulating cytokines for sleep measures. Level of significance within overload and cytokine level groups: *P ≤ 0.05, **P ≤ 0.01. IL, interleukin; TNF, tumor necrosis factor.
Cytokines
Placement significantly interacted with IL-6 (3.22 ± 1.20, P = 0.012) and with TNF-α (5.68 ± 2.44, P = 0.039) in determining PSQI scores. Caregivers who placed their spouse showed an increase in the PSQI score of 1.43 ± 0.63 (P = 0.023) if IL-6 levels were high, but a decrease in PSQI scores of 0.76 ± 0.54 (P = 0.28) if IL-6 levels were low (Figure 2, panel B). Similarly, caregivers who placed their spouse showed an increase in PSQI scores of 1.28 ± 0.64 (P = 0.050) if TNF-α levels were high, opposed to a decrease of 0.81 ± 0.60 (P = 0.32) in PSQI scores if TNF-α levels were low (Figure 2, panel C). There were no significant interactions between placement and IL-6 (all P-values > 0.18) and TNF-α (all P-values > 0.13) for any of the actigraphy measures.
Moderating Variables of Effect of Spousal Death on Sleep
Demographic factors
There were significant interactions between spousal death and age for the PSQI score (0.20 ± 0.06, P = 0.006), nighttime TST (−3.95 ± 1.53, P = 0.010), and nighttime sleep percent (−0.29 ± 0.13, P = 0.030), but not for nighttime WASO (P = 0.22) and daytime TST (P = 0.28). Spousal death was associated with an increase in PSQI scores of 2.54 ± 0.68 (P = 0.001) in older caregivers, opposed to a decrease in PSQI scores of 0.40 ± 0.65 (P = 0.83) in younger caregivers (Figure 3, panel A). Death of the AD spouse was also associated with an increase in nighttime TST of 31.75 ± 15.88 min (P = 0.046) in younger caregivers, opposed to a decreasein nighttime TST of 27.01 ± 20.41 min (P = 0.19) in older caregivers (Figure 3, panel B). Older caregivers who lost their spouse showed a greater decrease in nighttime sleep percent than younger care-givers whose spouse had died (−6.06 ± 1.80%, P = 0.001 vs. −1.73 ± 1.40%, P = 0.22) (Figure 3, panel C).
Figure 3.
Moderation of effects of spousal death on sleep. Panels A-C illustrate the significant interactions between death of the Alzheimer spouse and age for sleep measures. Level of significance within age groups: *P ≤ 0.05, ***P ≤ 0.001.
Spousal death showed a significant interaction with gender for PSQI scores (−5.00 ± 1.07, P < 0.001), but not for any of the actigraphy measures (all P-values > 0.19). Male caregivers (n = 14) whose spouse had died showed an increase in PSQI scores of 4.34 ± 1.16 (P = 0.001), opposed to female caregivers (n = 5) showing a decrease in PSQI scores of 0.14 ± 0.53 (P = 0.95) after the death of the spouse.
Psychological distress
There were no significant interactions between spousal death and role overload (all P-values > 0.05) and depressive symptoms (all P-values > 0.13) in determining any sleep measures.
Cytokines
Death of the AD spouse did not significantly interact with IL-6 (all P-values > 0.32) and TNF-α (all P-values > 0.07) to predict any sleep measures.
DISCUSSION
At baseline, elderly community-dwelling spousal AD care-givers reported significantly greater global PSQI scores but similar actigraphy measures compared to their age- and gender-matched non-caregiving counterparts. Moreover, PSQI global scores did not significantly correlate with actigraphy measures. These cross-sectional findings are consistent with previous results, supporting the notion that dementia caregivers perceive their sleep generally poorer than non-caregiving controls, but that this perception does often not correspond with objective sleep assessments.6 This discrepancy is partially explained by the high levels of caregivers' psychological distress, which may contribute to subjective sleep disturbances but contribute comparably little to objective sleep measures.13,14 In support of this notion, we found similar sleep over time in caregivers and controls with adjustments made for depressive symptoms and role overload (i.e., perceived burden of caregiving), both of which were positively related to global PSQI scores but not to the actigraphy measures. Age was positively associated with daytime TST, and BMI correlated negatively with nighttime sleep percent. An increased prevalence of napping during the day and of obesity-related sleep disordered breathing in elderly individuals might be explanations for these relationships.17
While caregiving status per se was not associated with sleep over time, we found that depressive symptoms and proinflammatory cytokine levels both moderated sleep quality in caregivers differently from controls. Concurring with a previous study,15 caregivers with high levels of depressive symptoms had longer nighttime TST than caregivers with low levels of depressive symptoms. Low-grade systemic inflammation impairs sleep20 and has also been related to the chronic stress of dementia caregiving.22,23 In agreement with this literature, we found that AD caregivers with high circulating levels of TNF-α had longer nighttime WASO and also less nighttime sleep percent than caregivers with lower levels of TNF-α.
We found that the death of the AD spouse led to a significant change in objective sleep characteristics of caregivers. At three months after their AD spouse had died, caregivers showed increased nighttime WASO and decreased nighttime sleep percent that were paralleled by unchanged nighttime TST but increased daytime TST. This suggests that caregivers whose spouse had died tried to “catch up” on impaired nighttime sleep with increased napping throughout the day. There was an additional albeit not significant trend (P < 0.06), suggesting that spousal death was associated with increased subjective sleep complaints. However, moderator analysis revealed that compared to younger caregivers and female caregivers, respectively, older caregivers and male caregivers reported poorer subjective sleep at three months after spousal death. At that time, older caregivers also had shorter nighttime TST and less nighttime sleep percent than younger caregivers. Therefore, older male caregivers may seem particularly vulnerable for the development of poor sleep after the AD spouse has died.
Although not investigated in our study, intrusive thoughts and cognitive arousal might play an important role in the development of sleep disturbances after death of the AD spouse, offering possible targets for behavioral interventions, as is purported by the bereavement literature.5 The health consequences, particularly the cardiovascular effects of alterations in caregivers' sleep behavior following the death of the AD spouse, warrant further study. Objective sleep disturbances and subjective sleep complaints, including insomnia symptoms, have all been associated with an increased cardiovascular disease risk,8,38 but whether daytime napping is good or bad for cardiovascular health is currently less clear.39,40
Placement of the AD spouse in a long-term care facility did not significantly affect sleep, likely because transition of the care recipient out of home does not uniformly relieve caregiver distress or improve caregiver health.25,26 While some burdens are lessened when a spouse is placed, others persist, or may even increase, for instance worries about the appropriateness of treatment for the loved one or financial costs. Therefore, long-term placement of the spouse should not be considered to have the same health effects as shorter term respite from caregiving that was shown to improve both subjective and objective sleep quality.41 It was further shown that, even after the institutionalization of the care recipient, caregivers' sleep problems may remain, particularly in those with grief issues and nighttime hypervigilance that had developed as a consequence of the patient's nighttime activity.5 In support of this, we found an ongoing stress response after placement of the AD spouse as a potential mechanism that might compromise caregiver sleep. That is, caregivers with high circulating levels of the two stress-responsive proinflammatory cytokines IL-6 and TNF-α perceived their sleep poorer than caregivers with low levels of these cytokines. In addition, caregivers who perceived high levels of role overload post-placement took longer nap times than caregivers with low levels of role overload.
The longitudinal design with almost three assessments per participant, the few missing follow-up data, and the adjustment for a range of sleep confounding variables were strengths of our study. Retrospective power analysis suggested that the number of total observations in our sample would have been large enough to yield clinically meaningful differences in sleep between caregivers vs. non-caregivers over time and related to placement of the AD spouse statistically significant. However, we assessed changes in caregivers' sleep at three months after a major transition in the caregiving situation. The subsequent trajectory in sleep behavior and the ultimate health consequences of the observed sleep changes remain to be determined. For instance, we would assume that cardiovascular health of caregivers would be impacted to a greater extent if sleep disturbances after the death of the AD spouse lasts comparably longer. The relationships between the moderating variables and sleep measures do not allow for causal inferences. For instance, the observed associations between inflammation and poor sleep could be bidirectional. Whereas cytokine-induced signaling of the brain may contribute to disturbed sleep, sleep deprivation may result in increased levels of proinflammatory cytokines.42
Although the results from the moderator analyses largely concurred with our hypothesis that demographic factors, psychological distress, and levels of proinflammatory cytokines affect sleep in relation to caregiving status and transitions in the care-giving situation, the validity of these findings may be limited due to the multiple comparisons performed and thus will need replication in further studies. If we had also included nonspousal caregivers in our sample, the variance in age, cytokine levels, and psychological distress likely would have increased, as, for instance, depression was shown to be higher in younger family dementia caregivers than in older ones and in the wife as opposed to the son of a male dementia patient.43 Such an increased variance might have yielded additional significant associations of caregiver status and transitions in the caregiving situation with sleep as being moderated by age, cytokine levels, and psychological distress.
Taken together, our findings suggest that Alzheimer caregiving per se does not significantly impact sleep over time, but that there might be subgroups of caregivers who are vulnerable and may develop sleep disturbances, particularly those with increased inflammation activity. Death of the AD spouse seems to impair sleep across all caregivers, whereas sleep effects of placement of the AD spouse in a long-term care facility was moderated by perceived stress and proinflammatory changes following the placement. These results suggest that treatment studies are needed to test whether improving sleep in caregivers through, for instance, cognitive behavioral therapy for insomnia, will result in improvements not only in sleep, but in the downstream symptoms of stress.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Dimsdale has received research support from Sepracor. Dr. Ancoli-Israel has served on the following Consultant/Scientific Advisory Boards (for less than $10,000 in fees): Ferring Pharmaceuticals Inc., GlaxoSmithKline, Johnson – Johnson, Merck, NeuroVigil, Inc., Pfizer, Philips, Purdue Pharma LP, Sanofi-Aventis, and Somaxon. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors are grateful to Susan Calleran and Christine Gonzaga for data collection. This study was supported by the National Institutes of Health/National Institute on Aging through award AG 15301 to Igor Grant. Additional support was provided through award AG 03090 to Brent Mausbach and AG 08415 to Sonia Ancoli-Israel.
REFERENCES
- 1.Dunkin JJ, Anderson-Hanley C. Dementia caregiver burden: a review of the literature and guidelines for assessment and intervention. Neurology. 1998;51(1 Suppl 1):S53–60. doi: 10.1212/wnl.51.1_suppl_1.s53. [DOI] [PubMed] [Google Scholar]
- 2.Grant I. Caregiving may be hazardous to your health. Psychosom Med. 1999;61:420–3. doi: 10.1097/00006842-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one's physical health? A meta-analysis. Psychol Bull. 2003;129:946–72. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 4.Schulz R, Martire LM. Family caregiving of persons with dementia: prevalence, health effects, and support strategies. Am J Geriatr Psychiatry. 2004;12:240–9. [PubMed] [Google Scholar]
- 5.McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med Rev. 2007;11:143–53. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Insomnia in caregivers of persons with dementia: who is at risk and what can be done about it? Sleep Med Clin. 2009;4:519–26. doi: 10.1016/j.jsmc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: A meta analytic evaluation of longitudinal epidemiological studies. J Affect Disord. doi: 10.1016/j.jad.2011.01.011. (in press) [DOI] [PubMed] [Google Scholar]
- 8.Quan SF. Sleep disturbances and their relationship to cardiovascular disease. Am J Lifestyle Med. 2009;3(1 Suppl):55s–59s. doi: 10.1177/1559827609331709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid KJ, Martinovich Z, Finkel S, et al. Sleep: a marker of physical and mental health in the elderly. Am J Geriatr Psychiatry. 2006;14:860–6. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 10.Neikrug AB, Ancoli-Israel S. Sleep disorders in the older adult - a mini-review. Gerontology. 2010;56:181–9. doi: 10.1159/000236900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox S, King AC. Sleep complaints in older women who are family caregivers. J Gerontol B Psychol Sci Soc Sci. 1999;54:P189–98. doi: 10.1093/geronb/54b.3.p189. [DOI] [PubMed] [Google Scholar]
- 12.McKibbin CL, Ancoli-Israel S, Dimsdale J, et al. Sleep in spousal caregivers of people with Alzheimer's disease. Sleep. 2005;28:1245–50. doi: 10.1093/sleep/28.10.1245. [DOI] [PubMed] [Google Scholar]
- 13.Kochar J, Fredman L, Stone KL, Cauley JA, Study of Osteoporotic Fractures Sleep problems in elderly women caregivers depend on the level of depressive symptoms: results of the Caregiver--Study of Osteoporotic Fractures. J Am Geriatr Soc. 2007;55:2003–9. doi: 10.1111/j.1532-5415.2007.01434.x. [DOI] [PubMed] [Google Scholar]
- 14.Rowe MA, McCrae CS, Campbell JM, Benito AP, Cheng J. Sleep pattern differences between older adult dementia caregivers and older adult noncaregivers using objective and subjective measures. J Clin Sleep Med. 2008;4:362–9. [PMC free article] [PubMed] [Google Scholar]
- 15.von Känel R, Ancoli-Israel S, Dimsdale JE, et al. Sleep and biomarkers of atherosclerosis in elderly Alzheimer caregivers and controls. Gerontology. 2010;56:41–50. doi: 10.1159/000264654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Känel R, Dimsdale JE, Ancoli-Israel S, et al. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer's disease. J Am Geriatr Soc. 2006;54:431–7. doi: 10.1111/j.1532-5415.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 17.Roepke SK, Ancoli-Israel S. Sleep disorders in the elderly. Indian J Med Res. 2010;131:302–10. [PubMed] [Google Scholar]
- 18.Middelkoop HA, Smilde-van den Doel DA, Neven AK, Kamphuisen HA, Springer CP. Subjective sleep characteristics of 1,485 males and females aged 50-93: effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol A Biol Sci Med Sci. 1996;51:M108–15. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 19.Mills PJ, Ancoli-Israel S, von Känel R, et al. Effects of gender and dementia severity on Alzheimer's disease caregivers' sleep and biomarkers of coagulation and inflammation. Brain Behav Immun. 2009;23:605–10. doi: 10.1016/j.bbi.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullington JM, Hinze-Selch D, Pollmächer T. Mediators of inflammation and their interaction with sleep: relevance for chronic fatigue syndrome and related conditions. Ann N Y Acad Sci. 2001;933:201–10. doi: 10.1111/j.1749-6632.2001.tb05825.x. [DOI] [PubMed] [Google Scholar]
- 21.Hänsel A, Hong S, Cámara RJ, von Känel R. Inflammation as a psycho-physiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–21. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 22.Lutgendorf SK, Garand L, Buckwalter KC, Reimer TT, Hong SY, Lubaroff DM. Life stress, mood disturbance, and elevated interleukin-6 in healthy older women. J Gerontol A Biol Sci Med Sci. 1999;54:M434–9. doi: 10.1093/gerona/54.9.m434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci U S A. 2003;100:9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz R, Beach SR, Lind B, et al. Involvement in caregiving and adjustment to death of a spouse: findings from the caregiver health effects study. JAMA. 2001;285:3123–9. doi: 10.1001/jama.285.24.3123. [DOI] [PubMed] [Google Scholar]
- 25.Schulz R, Belle SH, Czaja SJ, McGinnis KA, Stevens A, Zhang S. Long-term care placement of dementia patients and caregiver health and well-being. JAMA. 2004;292:961–7. doi: 10.1001/jama.292.8.961. [DOI] [PubMed] [Google Scholar]
- 26.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 27.Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30:583–94. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 28.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, 3rd, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14:331–8. [PubMed] [Google Scholar]
- 30.New York: Ardsley; Motionlogger User's Guide: Act Milennium. [Google Scholar]
- 31.Blackwell T, Redline S, Ancoli-Israel S, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–91. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.New York: Ardsley; Action-W User's Guide, Version 2.0. [Google Scholar]
- 33.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–1605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 34.Singer JD, Willett JB. Applied longitudinal data analysis: modelling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 35.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:232–8. [PubMed] [Google Scholar]
- 36.Kraemer HC, Blasey CM. Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13:141–51. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. J Pediatr Psychol. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- 38.Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Probl Cardiol. 2005;30:625–62. doi: 10.1016/j.cpcardiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Tanabe N, Iso H, Seki N, Suzuki H, Yatsuya H, Toyoshima H, Tamakoshi A, JACC Study Group. Daytime napping and mortality, with a special reference to cardiovascular disease: the JACC study. Int J Epidemiol. 2010;39:233–43. doi: 10.1093/ije/dyp327. [DOI] [PubMed] [Google Scholar]
- 40.Naska A, Oikonomou E, Trichopoulou A, Psaltopoulou T, Trichopoulos D. Siesta in healthy adults and coronary mortality in the general population. Arch Intern Med. 2007;167:296–301. doi: 10.1001/archinte.167.3.296. [DOI] [PubMed] [Google Scholar]
- 41.Lee D, Morgan K, Lindesay J. Effect of institutional respite care on the sleep of people with dementia and their primary caregivers. J Am Geriatr Soc. 2007;55:252–8. doi: 10.1111/j.1532-5415.2007.01036.x. [DOI] [PubMed] [Google Scholar]
- 42.Kapsimalis F, Basta M, Varouchakis G, Gourgoulianis K, Vgontzas A, Kryger M. Cytokines and pathological sleep. Sleep Med. 2008;9:603–14. doi: 10.1016/j.sleep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Covinsky KE, Newcomer R, Fox P, et al. Patient and caregiver characteristics associated with depression in caregivers of patients with dementia. J Gen Intern Med. 2003;18:1006–14. doi: 10.1111/j.1525-1497.2003.30103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]