Abstract
Blockade of the CD40/CD154 pathway potently attenuates T-cell responses in models of autoimmunity, inflammation, and transplantation. Indeed, CD40 pathway blockade remains one of the most powerful methods of prolonging graft survival in models of transplantation. But despite this effectiveness, the cellular and molecular mechanisms underlying the protective effects of CD40 pathway blockade are incompletely understood. Furthermore, the relative contributions of deletion, anergy, and regulation have not been measured in a model in which donor-reactive CD4+ and CD8+ T-cell responses can be assessed simultaneously. To investigate the impact of CD40/CD154 pathway blockade on graft-specific T-cell responses, a transgenic mouse model was used in which recipients containing ovalbumin-specific CD4+ and CD8+ TCR transgenic T cells were grafted with skin expressing ovalbumin in the presence or absence of anti-CD154 and donor-specific transfusion. The results indicated that CD154 blockade altered the kinetics of donor-reactive CD8+ T-cell expansion, delaying differentiation into IFN-γ+ TNF+ multifunctional cytokine producers. The eventual differentiation of cytokine-producing effectors in tolerant animals coincided with the emergence of an antigen-specific CD4+ CD25hi Foxp3+ T-cell population, which did not arise from endogenous natural Treg but rather were peripherally generated from naïve Foxp3− precursors.
Keywords: costimulation blockade
Blockade of the CD40/CD154 pathway has long been appreciated as a potent means of inhibiting alloreactive T-cell responses and prolonging graft survival. But despite this pronounced effect on allograft survival in both murine and nonhuman primate models (1–3), clinical trials using anti-CD154 mAbs in transplantation were halted due to thromboembolic complications, likely related to the expression of CD154 on platelets (4, 5). However, recent studies using anti-CD40 mAbs in both murine and nonhuman primate models demonstrated comparable efficacy to CD154 blockade, and thus significant interest in the pathway remains (6–9).
Early studies by Parker et al. (3) described the treatment combination of anti-CD154 and donor-specific transfusion (DST) for the induction of immune tolerance in transplantation. Several groups have since demonstrated the potent effects of this combined therapy in the prolongation of islet (3, 10, 11), cardiac (12), skin (10, 13), and kidney (14, 15) allograft survival in murine and nonhuman primate models. Although costimulation blockade has been associated with T-cell anergy (16) or deletion (17, 18), elucidation of the precise independent and synergistic effects of DST and anti-CD154 on the kinetics of donor-reactive CD4+ and CD8+ effector T-cell expansion has been limited because of the inability to identify and track graft-specific T cells in fully allogeneic models.
Importantly, studies by Taylor et al. (19) demonstrated that CD4+ CD25+ regulatory T cells (Treg) are required for tolerance induced via CD40/CD154 pathway blockade in a graft-versus-host model. Although those studies revealed a requirement for Treg during tolerance induction, whether the Treg required are preexisting, thymically derived Treg or whether CD40/CD154 blockade induces the generation of peripherally elicited Treg is not known. Furthermore, the temporal relationship between the in vivo accumulation of Treg and deletion of graft-specific effectors after CD40/CD154 blockade has not been investigated.
To address these questions, we used an ovalbumin (OVA)-expressing transgenic mouse model (20), which allowed us to directly track both donor-reactive CD4+ and CD8+ T-cell responses simultaneously over time. Although previous studies have used transgenic models to assess the degree of T-cell deletion after CD154/CD40 blockade (16, 21), none has systematically interrogated the impact of anti-CD154 on the kinetics of both CD4+ and CD8+ T-cell deletion and acquisition of effector function. Our results indicated that although CD40/CD154 blockade alone delayed donor-reactive CD4+ and CD8+ T-cell expansion and differentiation, the addition of DST was required for antigen-specific T-cell deletion. Importantly, anti-CD154 combined with DST also led to peripheral conversion of Foxp3− donor-reactive CD4+ T cells to Foxp3+CD25+ donor-specific Treg.
Results
Combined DST/CD154 Blockade Prolongs Skin Graft Survival and Prevents Cellular Infiltration.
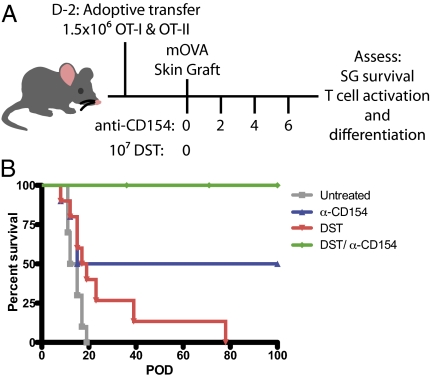
Although many groups have studied the impact of CD40 pathway blockade on graft survival and T-cell responses (16, 19, 21, 22), the precise mechanism by which inhibition of CD40-mediated signals attenuates donor-reactive T-cell responses remains incompletely understood. In fully allogeneic models, anti-CD154 mAbs combined with DST has been shown to significantly delay rejection, but the ability to track alloreactive T cells is dependent on cytokine production. This limits the ability to study donor-specific cells that might have become nonresponsive due to the absence of CD40/CD154-mediated signals. Therefore, to assess the contribution of T-cell nonresponsiveness to the protective effect of CD154 blockade on skin graft (SG) survival, naïve donor-reactive OT-I and OT-II T cells were adoptively transferred into naïve recipients that were then grafted with OVA-expressing skin (Fig. 1A). Resulting SG survival in this transgenic model reflected that previously observed in allogeneic systems (13). Untreated mice rapidly rejected SGs with a median survival time (MST) of 13.5 d, whereas treatment with anti-CD154/DST significantly prolonged survival to more than 100 d (Fig. 1B).
Fig. 1.
CD154 blockade and DST prolongs SG survival. (A) Mice were adoptively transferred with 1.5 × 106 of each OT-I and OT-II T cells 2 d before transplantation. On day 0, mice were transplanted with mOVA SG and were treated with 107 mOVA DST and/or 500 μg of MR-1 where indicated. (B) Untreated mice rejected SGs with an MST of 13.5 d. Anti-CD154 monotherapy led to bimodal survival of SGs, with 50% of mice rejecting grafts with an MST of 15 d and 50% demonstrating indefinite survival (P = 0.0027). DST monotherapy resulted in to an MST of 18 d (P = 0.031), whereas combined anti-CD154/DST led to an indefinite survival of SGs (P < 0.0001). Data are cumulative of two independent experiments with five mice per group.
CD154 Blockade/DST Inhibits Antigen-Specific CD8+ T-Cell Expansion.
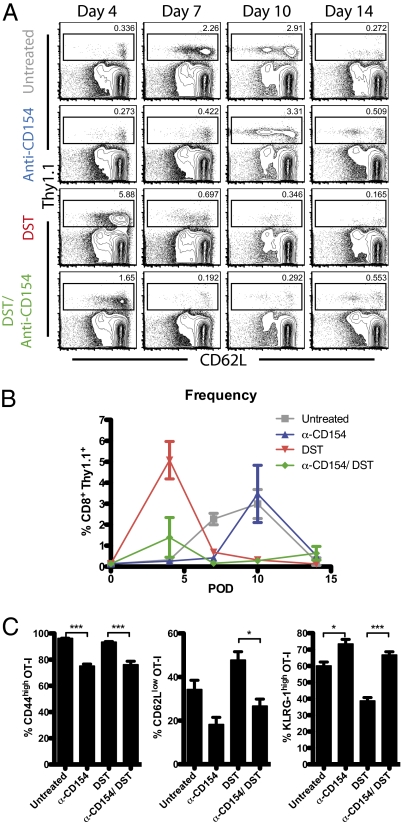
To identify the relative contributions of T-cell deletion and anergy/exhaustion, the kinetics of donor-reactive CD8+ T-cell expansion and contraction were measured over time after transplantation and treatment with anti-CD154/DST on days 0, 2, 4, and 6 posttransplantation. Spleens of untreated mice developed a donor-reactive T-cell response that peaked at days 7–10. CD154 blockade led to a significant delay (day 10) in the donor-reactive T-cell response, whereas this response was significantly accelerated (day 4) in DST-treated animals. Finally, combined anti-CD154/DST treatment led to minimal expansion of donor-reactive T cells over background at day 4 (Fig. 2 A and B). These effects of CD154 blockade on donor-reactive T-cell expansion kinetics were further confirmed in the axillary and brachial draining lymph nodes (LNs) (Fig S1).
Fig. 2.
CD154 blockade delays expansion of antigen-specific CD8+ T cells but does not alter activation status at peak of response. Mice were treated as described in Fig. 1 and killed at the indicated time points. (A) Concatenated flow plots of CD8+ splenocytes. Gates shown are on donor-reactive CD8+ (Thy1.1+) T cells. (B) Frequencies of donor-reactive CD8+ splenocytes are shown. OT-I T-cell populations in untreated mice peaked at days 7–10 with 2.27% ± 0.27% and 2.98% ± 0.69%, respectively. Compared with untreated controls, anti-CD154 treatment delayed expansion of T cells (D10: 3.47% ± 1.37%), whereas DST accelerated expansion of OT-I T cells (D4: 0.32% ± 0.04% vs. 5.08% ± 0.98%; P = 0.006). Combined treatment minimally expanded T cells (D4: 1.39% ± 0.95%). (C) Activation markers on OT-I T cells on day 7 in the spleen. CD40/CD154 blockade reduced CD44 and CD62L up-regulation, while promoting an increase in KLRG-1 expression. Data are summarized from three experiments with three mice per group. Values are mean ± SEM. *P < 0.05; ***P < 0.001.
To determine whether CD154/CD40 pathway blockade limits antigen-specific T-cell differentiation, we analyzed markers of activation (CD44 and CD62L) and a marker of short-lived effector lineage (KLRG-1) (23) in each treatment group on day 7 posttransplantation. We observed that CD154 blockade, in both the presence and absence of DST, decreased the frequencies of CD44high and CD62Llow antigen-specific CD8+ T cells and increased the frequency of KLRG-1high antigen-specific CD8+ T cells compared with untreated controls (Fig. 2C).
CD154 Blockade/DST Prevents Antigen-Specific CD8+ T-Cell Cytokine Production and Cytolytic Potential.
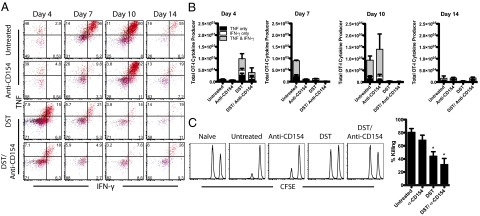
Although increased donor-reactive T-cell expansion is an important indicator of potential allograft rejection, it does not speak to the functionality of these cells after transplantation. Thus, we interrogated the role of the CD40/CD154 pathway in inducing the differentiation of competent TNF and IFN-γ cytokine-producing effectors after transplantation (Fig. 3A). We observed a minimum threshold of 105 graft-specific cytokine-secreting cells after transplantation in all of the groups that experienced rejection. This threshold number of cells was observed on days 7 and 10 in untreated animals (Fig. 3B). DST accelerated the emergence of dual cytokine-producing cells to day 4, whereas anti-CD154 delayed the differentiation of these cells until day 10 posttransplantation (Fig. 3B). Only the DST/anti-CD154 group (which did not experience rejection) failed to achieve this threshold number of cytokine-producing cells at any time point (Fig. 3B).
Fig. 3.
CD154 blockade delays donor-reactive CD8+ T cells differentiation into multifunctional cytokine-producing cells. Mice were treated as described in Fig. 1 and killed at the indicated time points. (A) Concatenated flow plots of intracellular cytokine staining in splenic OT-I T cells after stimulation for 4 h in vitro with SIINFEKL peptide. (B) Cytokine production by splenic OT-I T cells, summarized from three experiments with three mice per group. Untreated mice developed into dual cytokine producers at day 7 (6.22 × 104 ± 0.29 × 104). DST accelerated T-cell production of TNF and IFN-γ (D4: 4.90 × 104 ± 2.14 × 104). Anti-CD154 delayed T-cell differentiation (D10: 1.15 × 105 ± 0.65 × 105). Anti-CD154/DST inhibited differentiation (D4: 2.08 × 104 ± 1.98 × 104). (C) Day 10 in vivo cytotoxicity assay. Peptide-coated targets and unpulsed control targets were labeled with different concentrations of carboxyfluorescein succinimidyl ester and adoptively transferred into recipients. After 18 h, the ratio of unpulsed targets to peptide-pulsed targets remaining was assessed by flow cytometry. Relative to untreated controls, anti-CD154 treatment did not alter killing of target cells (91.01% ± 0.82% vs. 66.59% ± 11.03%; P = 0.379). DST treatment significantly impaired killing (55.52% ± 8.28%; P = 0.003). Anti-CD154/DST significantly impaired cytolysis (28.26% ± 12.78%; P = 0.001). Data are summarized from two experiments with five mice per group. Values are mean ± SEM.
To further investigate the impact of CD154 blockade on T-cell differentiation, in vivo cytotoxicity assays were performed. Anti-CD154 treatment did not attenuate the antigen-specific cytolysis of target cells relative to untreated controls. However, DST monotherapy significantly reduced the cytolysis of target cells on day 7, perhaps related to the rapid contraction of antigen-specific T cells at this time point. Consistent with the reduced expansion and cytokine production by donor-reactive T cells, anti-CD154/DST treatment significantly impaired cytolysis of target cells (Fig. 3C). Taken together, these data indicate that although CD154/CD40 pathway blockade delayed the accumulation of donor-reactive T cells, it did not prevent the killing of donor-derived targets.
CD154 Blockade Reduces Antigen-Specific CD4+ T-Cell Accumulation and Promotes Migration of Foxp3+ Treg to the Graft.
To assess the availability of CD4+ T-cell help in this model, we analyzed the effect of CD154 blockade on donor-specific helper T-cell activation. Donor-reactive CD4+ T cells expanded with similar kinetics as CD8+ T cells over time. Treatment with either anti-CD154 or DST led to a substantially reduced accumulation of donor-reactive CD4+ T cells on day 7 compared with untreated controls, but anti-CD154/DST treatment significantly impaired donor-reactive helper T-cell responses (Fig. 4 A and B).
Fig. 4.
Anti-CD154 and DST treatment reduces antigen-specific CD4+ T-cell accumulation and promotes Foxp3+ Treg cell migration to the graft. Mice were treated as described in Fig. 1 and killed at the indicated time points. (A) Day 7 concatenated flow plots of CD4+ T cells in draining LNs, with gates identifying OT-II (Thy1.1+) T cells. (B) Donor-reactive CD4+ T cells in the draining LNs. Anti-CD154 monotherapy reduced OT-II T-cell levels compared with untreated controls (0.54% ± 0.17% vs. 2.10% ± 0.55%; P = 0.054). DST monotherapy reduced OT-II T-cell levels (0.57 ± 0.07%; P = 0.051). Anti-CD154/DST significantly reduced OT-II levels (0.20% ± 0.02%; P = 0.026). (C) Frequency of total Treg (both transgenic and endogenous) cells in the draining LNs over time. (D) Day 7 Foxp3+ cells in SGs. Foxp3+ cells were counted in 10 high-power fields (HPFs; 400× magnification). Anti-CD154/DST treatment significantly increased Foxp3+ cell infiltration compared with untreated controls (14.2 ± 4.78/HPF vs. 4.95 ± 0.22/HPF; P < 0.0005). Data are summarized from three experiments with three mice per group. Values are mean ± SEM.
Because Treg have been shown to promote graft survival in several models (24), we assessed whether CD154 blockade resulted in the expansion of Treg in vivo. We found no gross change in the frequency of CD4+ CD25+ Foxp3+ T cells in mice treated with anti-CD154 (Fig. 4C). We next asked whether anti-CD154 altered the ability of Treg to accumulate in the graft (Fig. 4D). Immunohistochemical analysis of Foxp3+ cells in SGs at day 7 revealed that anti-CD154/DST treatment led to a dramatic increase in Treg infiltration compared with untreated controls.
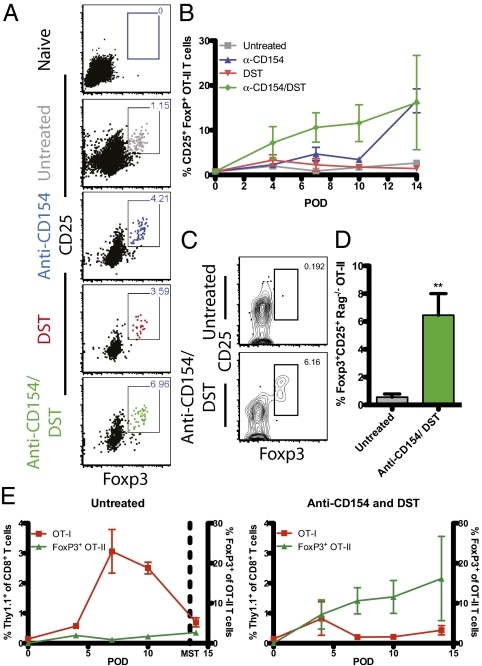
Anti-CD154/DST Promotes Conversion of Donor-Reactive CD4+ T Cells Into CD25+ Foxp3+ Induced Treg.
Although we observed no increase in total Treg in the draining LNs after CD40/CD154 blockade, the increased Treg infiltration in SGs of these animals led us to hypothesize that a graft-specific Treg population may be emerging in the context of CD40/CD154 blockade, but might be undetectable within the larger pool of non–graft-reactive Treg. To test this hypothesis, we analyzed donor-reactive Thy1.1+ CD4+ T cells in the LNs for Foxp3 expression. Before transplantation, adoptively transferred naïve OT-II T cells, did not express Foxp3 (Fig. 5A, Top), whereas donor-reactive CD4+ T cells in LNs of untreated mice developed a small population of Foxp3-expressing cells compared with naïve controls. At early time points, neither anti-CD154 nor DST alone significantly increased the frequency of Foxp3+ cells among donor-reactive CD4+ T cells compared with untreated controls. However, CD154 blockade resulted in a modest increase in the frequency of donor-specific Foxp3+ CD25+ CD4+ Treg at day 14. Interestingly, the combination of anti-CD154/DST treatment resulted in a significant increase in the frequency of graft-specific Foxp3+ CD25+ CD4+ Treg in vivo by day 7 (Fig. 5 A and B).
Fig. 5.
Anti-CD154 and DST treatment promote conversion of donor-reactive CD4+ T cells into CD25+ Foxp3+ iTreg. (A) Day 7 concatenated flow plots of OT-II (Thy1.1+) T cells in LNs, with gates on CD25+ Foxp3+ OT-II cells. (B) Longitudinal analysis of Foxp3+ OT-II T cells. On day 7, the frequency of Treg in untreated mice was 0.88% ± 0.44% that of OT-II T cells. Anti-CD154 (4.68% ± 1.49%; P = 0.071) and DST (2.27% ± 1.37%; P = 0.389) monotherapies slightly increased the frequency of Foxp3-expressing T cells within the OT-II T-cell compartment. Anti-CD154/DST significantly increased Foxp3 expression in OT-II T cells compared with untreated controls (10.61% ± 3.28%; P = 0.042). On day 14, both anti-CD154 and anti-CD154/DST reached similarly high levels of iTreg conversion (16.57% ± 2.68% and 16.17% ± 10.53%, respectively; P = 0.973). Data are summarized from three experiments with three mice per group. (C and D) B6.SJL (CD45.1+) were adoptively transferred with OT-I and RAG−/− OT-II T cells and treated with anti-CD154/DST. On day 7, draining LNs were analyzed for Foxp3-expressing OT-II T cells. (C) Representative flow plots of CD45.2+ RAG−/− OT-II T cells; gate represents CD25+ Foxp3+ iTreg cells. (D) Combined CD154 blockade and DST significantly increased the conversion of OT-II T cells into Foxp3+ iTreg compared with untreated controls (6.452% ± 1.552% vs. 0.559% ± 0.225%; P = 0.0035). Data are summarized from two experiments with between three and five mice per group. (E) Relative frequencies of Thy1.1+ OT-I T cells compared with peripherally converted Thy1.1+ OT-II iTreg from untreated LNs (Left) and anti-CD154/DST–treated LNs (Right). OT-I T cells are measured on the left y-axis, and the OT-II Treg are measured on the right y-axis. Data are summarized from three experiments with three mice per group. Values are mean ± SEM.
Because low frequencies of endogenous Treg (nTreg) have been reported in recombinant activating gene (RAG)-sufficient OT-II T cells (25), we next asked whether the observed increase in accumulated graft-specific Foxp3+ CD25+ CD4+ T cells was due to expansion of a small population of Foxp3+ OT-II nTreg that existed in the transferred cell preparation, or due to peripheral conversion of antigen-specific CD4+ T cells into induced Treg (iTreg) after CD40/CD154 blockade. Therefore, we adoptively transferred 1.5 × 106 Thy1.1+ OT-I and 1.5 × 106 RAG-deficient CD45.2+ OT-II T cells, which lack Foxp3-expressing cells (25), into CD45.1+ recipients. Mice received a mOVA SG and either remained untreated or were treated with combined anti-CD154/DST. On day 7 posttransplantation, draining LNs were analyzed for the presence of antigen-specific Foxp3+ CD4+ T cells. Results indicated that anti-CD154/DST significantly induced the conversion of donor-reactive CD4+ T cells into graft-specific Foxp3+ CD25+ iTreg compared with untreated controls (Fig. 5 C and D). Taken together, these data indicate that blockade of the CD40 pathway in the presence of DST resulted in the peripheral conversion of antigen-specific CD4+ T cells into iTreg.
The ratio of Treg to antigen-specific effector T cells has been previously identified as a predictor of the potential protective effects of Treg (26). Thus, we compared the relative levels of accumulated donor-reactive CD8+ T cells and donor-specific Foxp3+ CD25+ CD4+ T cells. As described earlier, untreated recipients substantially expanded donor-reactive CD8+ T cells, while generating negligible levels of antigen-specific Foxp3+ CD4+ T cells (Fig. 5E, Left). Conversely, anti-CD154/DST treatment dramatically increased the ratio of graft-specific Foxp3+ CD4+ T cells to donor-reactive CD8+ effectors in the draining LNs over time (Fig. 5E, Right).
Discussion
In this study, we have elucidated the effects of CD40/CD154 pathway blockade on donor-reactive CD4+ and CD8+ T-cell responses. Based on these data, we conclude that treatment with either DST or anti-CD154 resulted in mechanistically distinct modes of graft protection. Anti-CD154 treatment delayed the expansion and differentiation of donor-reactive CD8+ T cells into multifunctional cytokine-producing cells. Furthermore, CD154 blockade led to late conversion of donor-reactive Foxp3− CD4+ T cells into Foxp3+ iTreg. This effect was observed in both RAG-sufficient and RAG-deficient antigen-specific T cells, which are known to contain no FoxP3+ nTreg (25).
Although previous studies have shown a role for regulation in the tolerance induced via DST/anti-CD154 (19, 27, 28), here we show that the mechanism underlying the observed increase in Foxp3+ Treg after exposure to DST/anti-CD154 is conversion of antigen-specific naïve T-cell precursors into Foxp3+ cells. We speculate that the conversion of naïve/effector CD4+ T cells into iTreg requires the presence of antigen, which is provided much earlier in the setting of DST than in anti-CD154 monotherapy. Conversely, DST led to early expansion but abortive activation of donor-reactive CD8+ T cells, with rapid contraction that likely contributed to the decreased ability to lyse target cells by day 10. However, antigen-specific Foxp3+ CD25+ iTreg were not induced after DST treatment in the absence of CD154 blockade. Thus, we conclude that this degree of abortive activation alone was insufficient to protect grafts from rejection. Only the combination of abortive activation and the early emergence of peripherally induced iTreg was able to sufficiently attenuate donor-reactive effector T-cell responses and prolong graft survival. These data suggest that an early increase in the ratio of Treg to effector T cells may underlie the potent protective effects of anti-CD154/DST combined therapy.
Through what mechanism does interruption of CD40/CD154-mediated signals induce the expression of Foxp3? Our favored hypothesis is that inhibition of CD40 signaling conditions antigen-presenting cells (APCs) or subsets of APCs such that synaptic contact with antigen-specific T cells instructs them to become regulatory cells rather than activated effectors. This hypothesis is based on work demonstrating peripheral generation of Foxp3+ Treg after exposure to tolerogenic plasmacytoid dendritic cells (DCs) (27). The specific cell surface or soluble mediators that function to regulate iTreg conversion is an important area of future research; however, we predict that DCs in which CD40 signaling is inhibited will fail to present costimulatory molecules or secrete inflammatory cytokines to instruct CD4+ T cells to differentiate into Treg. For example, IL-6 has been shown to potently inhibit TGF-β–mediated Treg differentiation (29), and a recent study demonstrated that loss of CD40 signaling rendered DCs deficient in IL-6 production (30).
Alternatively, it is possible that anti-CD154 functions to inhibit CD40 signaling on another cell type. In particular, CD8+ T cells have been shown to express CD40 and are capable of secreting inflammatory cytokines that also might inhibit iTreg conversion (31, 32). Furthermore, peripheral conversion of naïve CD4+ T cells into Foxp3+ Treg has been observed after interruption or attenuation of TCR-mediated signals. In particular, von Boehmer's group observed the conversion of Foxp3− CD4+ T cells into Foxp3+ T cells after exposure to low-dose antigen (33), and, conversely, iTreg generation was inhibited by high TCR stimulation (34). Peripheral generation of Foxp3+ T cells also has been demonstrated after resolution of an acute systemic autoimmune disease (35). In addition, extrathymic development of CD25hi Treg from CD25− precursors was observed after in vivo treatment of anti-CD4 mAb during transplantation (36). Thus, there likely are many mechanisms by which naïve CD4+ T cells may be instructed to become Foxp3+ regulatory cells in the periphery. Here we demonstrate that in vivo blockade of the CD40/CD154 pathway is one of those mechanisms, and show that early emergence of these iTreg correlates with graft survival.
Our data also indicate that a primary effect of CD154/CD40 blockade is to delay CD8+ T-cell expansion and differentiation. Previous reports have examined donor-reactive T cells at a single time point posttransplantation and have concluded that anti-CD154 mAb leads to deletion of CD4+ and CD8+ T-cell responses (16, 21). However, our analysis of the kinetics of the T-cell response revealed that CD154/CD40 pathway blockade alone delayed, rather than deleted, antigen-specific CD4+ and CD8+ T-cell responses, with both responses in anti-CD154 treated animals eventually reaching the same magnitude as in untreated controls. Given that the half-life of anti-CD154 mAb (MR-1) is 1.5 wk in vivo, and that the last dose was given on day 6 posttransplantation, we would not expect to see a significant drop in circulating blocking antibody during the time course studied (days 0–14). This indicates that our findings are not the result of an emerging response as the CD154 blockade wanes, but rather represent a “breakthrough” response in which graft-reactive CD8+ T cells have delayed differentiation but eventually develop into fully competent effectors even in the presence of blocking antibody. Conversely, our analysis revealed that DST accelerated the kinetics of donor-reactive CD4+ and CD8+ T-cell expansion, a finding corroborated by previous studies showing that at a single time point, donor-reactive T cells isolated from mice treated with DST exhibited increased CD44 expression, indicative of a more activated phenotype (21). Taken together, our analysis of the kinetics and magnitude of donor-reactive T-cell responses suggest a mechanism in which T cells are strongly driven into division in the presence of DST, but are induced to delay differentiation in the presence of anti-CD154. In the presence of both reagents, the resulting “push and pull” results in donor-reactive T-cell deletion or conversion to Treg. A similar finding has been reported in the setting of low donor-reactive T-cell precursor frequency, in which minimal competition for antigen may provide increased access to APCs, and thus cells are driven to divide earlier and more robustly (37–40). Through the provision of an increased source of antigen, DST may similarly decrease the T-cell:APC ratio, thereby stimulating increased division. In both systems, robust division in the absence of costimulation leads to eventual deletion (39).
Our use of congenically marked TCR transgenic cells of a defined specificity in this study was advantageous, in that we were able to both identify populations of graft-specific T cells without relying on effector function for detection and definitively address the issue of iTreg conversion vs. expansion of Treg from a preexisting small population of FoxP3+ Treg. However, a potential limitation of this study is that it focused on the behavior of a monoclonal T-cell population responding to a surrogate minor antigen, and thus it is possible that this system might not be fully comparable to a polyclonal T-cell response to allogeneic tissues. Identifying polyclonal graft-specific T cells during transplantation remains an important area of future investigation.
We conclude that the mechanism by which DST and anti-CD154 blockade synergize to protect the graft involves both the abortive response of donor-reactive CD8+ T cells that have been driven into unsupported division and the conversion of antigen-specific CD4+ T cells into iTreg after CD154 blockade. Although the use of intact Fc receptor-binding anti-CD154 is not a clinically viable strategy given the potential for thromboembolic complications, blockade of this pathway remains the single most effective method of inducing transplantation tolerance in experimental models. Thus, studying the mechanisms by which this mechanism induces robust tolerance remains an important question for the field, with the aim of developing alternative methods of inhibiting CD154. Recent studies have used an RNAi approach to inhibit CD40 expression (41, 42), and a similar approach could be envisaged for CD154 inhibition. Alternatively, non–cross-linking mAbs could be developed to antagonize CD154, much like nonactivating single-chain FV-based reagents have been developed in lieu of cross-linking anti-CD28 mAbs (43), which has resulted in a much more severe side effect profile than that seen in anti-CD154 mAbs in pilot studies in humans (44). Thus, further elucidation of the mechanisms underlying the potency of CD154 blockade in inducing transplantation tolerance may allow for the identification of critical cellular and molecular pathways required for the establishment and maintenance of transplantation tolerance.
Materials and Methods
Mice.
B6-Ly5.2/Cr (H2-Kb, CD45.1) and C57BL/6 (H2-Kb, CD45.2) mice were obtained from the National Cancer Institute. OT-I and OT-II transgenic mice (purchased from Taconic Farms) were bred to a Thy1.1+ background at Emory University. Membrane bound-OVA (mOVA) mice (20) were a gift from Dr. Marc Jenkins (University of Minnesota, Minneapolis, MN) and were maintained in accordance with Emory University's Institutional Animal Case and Use Committee guidelines. OT-II × RAG−/− transgenic mice were purchased from Taconic Farms. All animals were housed in pathogen-free animal facilities at Emory University.
DST and Adoptive Transfers.
For DST administration, mOVA spleens were processed into single cell suspensions, and 107 splenocytes were given before transplantation. For adoptive transfers of donor-reactive T cells, spleen and mesenteric LNs of OT-I and OT-II mice were processed and stained with mAbs antibodies for CD4, CD8 (both from Invitrogen), Thy1.1, and Vα2 (BD Pharmingen) for flow cytometry analysis. Cells were resuspended in PBS, and then 1.5 × 106 of each Thy1.1+ OT-I and OT-II were injected i.v. In some experiments, CD45.2+ OT-II × RAG−/− cells were adoptively transferred into CD45.1+ recipients.
Skin Transplantation and Antibody Treatment.
Full thickness tail and ear skins were transplanted onto dorsal thorax of recipient mice and secured with adhesive bandages. Where indicated, mice were treated with 500 μg of hamster monoclonal anti-mouse CD154 (MR-1; BioExpress) on days 0, 2, 4, and 6 posttransplantation.
Surface Stains and Flow Cytometry.
Spleens or draining axillary and brachial LNs were stained for CD4 and CD8 (both from Invitrogen); Thy1.1, CD44, and CD62L (all from BD Pharmingen); and KLRG-1 (Southern Biotech). Samples were analyzed using a multicolor LSRII FACS machine (BD Biosciences). Data were analyzed using FlowJo software (Treestar).
Intracellular Cytokine Staining.
Responder splenocytes were stimulated with 10 nM OVA257–264 (SIINFEKL, Emory University Microchemical Core Facility) and 10 μM OVA323–339 peptides in the presence of 10 μg/mL Brefeldin A for 4 h. Intracellular staining kits were used to detect TNF and IFN-γ (BD Pharmingen), according to the manufacturer's instructions.
In Vivo Cytotoxicity Assay.
C57BL/6 (CD45.2) splenocytes were divided into two populations and stained with either 2.5 μM or 0.25 μM carboxyfluorescein succinimidyl ester. The cells either remained unpulsed or were pulsed with 1 μM SIINFEKL peptide, respectively. Once mixed at equal ratios (5 × 105 cells of each), cells were injected i.v. into experimental mice (CD45.1). At 18 h postinjection, lysis of target cells was assessed, as described previously (45).
Histology.
Skin grafts were removed and frozen in cryomolds with OCT Embedding Compound (Tissue-Tek) on day 7 posttransplantation. Longitudinal sections of grafts were cut into 5-μm-thick sections with a cryostat (CM 1850; Leica Microsystems) and mounted on Superfrost Plus microscope slides and fixed with 100% acetone. Anti-mouse Foxp3 (eBiosciences) was used for Foxp3 immunohistochemical detection by 3,3 diaminobenzidine peroxidation and counterstained with hematoxylin. A Zeiss LSM 510 META point scanning laser confocal microscope was used for immunofluorescent staining visualization. Foxp3+ cells were quantified by counting positive cells in 10 HPFs (400× magnification).
Treg Staining.
Splenocytes and draining LNs were processed and stained with antibodies to CD4 and CD8 (both from Invitrogen), Thy1.1 or CD45.2, Vα2, and CD25 (all from BD Pharmingen). Intracellular staining with anti-Foxp3 was performed using an eBiosciences intranuclear staining kit in accordance with the manufacturer's instructions.
Statistical Analysis.
Survival data were plotted on Kaplan–Meier curves, and log-rank tests were performed. Longitudinal analysis of T cells was performed using two-way ANOVA, followed by a Bonferroni posttest on significant results. One-way ANOVA was used for single time point analysis of T-cell accumulation and phenotypes, followed by Tukey posttest on significant results. All analyses were performed using GraphPad Prism software.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Heath/National Institute of Allergy and Infectious Diseases Grants AI40519 (to C.P.L.), AI073707 (to M.L.F.), and AI079409 (to M.L.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105500108/-/DCSupplemental.
References
- 1.Kirk AD, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 2.Larsen CP, et al. CD40-gp39 interactions play a critical role during allograft rejection: Suppression of allograft rejection by blockade of the CD40-gp39 pathway. Transplantation. 1996;61:4–9. doi: 10.1097/00007890-199601150-00002. [DOI] [PubMed] [Google Scholar]
- 3.Parker DC, et al. Survival of mouse pancreatic islet allografts in recipients treated with allogeneic small lymphocytes and antibody to CD40 ligand. Proc Natl Acad Sci USA. 1995;92:9560–9564. doi: 10.1073/pnas.92.21.9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 5.Henn V, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 6.Haanstra KG, et al. Prevention of kidney allograft rejection using anti-CD40 and anti-CD86 in primates. Transplantation. 2003;75:637–643. doi: 10.1097/01.TP.0000054835.58014.C2. [DOI] [PubMed] [Google Scholar]
- 7.Haanstra KG, et al. Costimulation blockade followed by a 12-week period of cyclosporine A facilitates prolonged drug-free survival of rhesus monkey kidney allografts. Transplantation. 2005;79:1623–1626. doi: 10.1097/01.tp.0000158426.64631.ed. [DOI] [PubMed] [Google Scholar]
- 8.Masunaga T, et al. Dimeric but not monomeric soluble CD40 prolongs allograft survival and generates regulatory T cells that inhibit CTL function. Transplantation. 2005;80:1614–1622. doi: 10.1097/01.tp.0000181093.50141.6c. [DOI] [PubMed] [Google Scholar]
- 9.Adams AB, et al. Development of a chimeric anti-CD40 monoclonal antibody that synergizes with LEA29Y to prolong islet allograft survival. J Immunol. 2005;174:542–550. doi: 10.4049/jimmunol.174.1.542. [DOI] [PubMed] [Google Scholar]
- 10.Zheng XX, et al. CTLA4 signals are required to optimally induce allograft tolerance with combined donor-specific transfusion and anti-CD154 monoclonal antibody treatment. J Immunol. 1999;162:4983–4990. [PubMed] [Google Scholar]
- 11.Gordon EJ, et al. Prolonged survival of rat islet and skin xenografts in mice treated with donor splenocytes and anti-CD154 monoclonal antibody. Diabetes. 1998;47:1199–1206. doi: 10.2337/diab.47.8.1199. [DOI] [PubMed] [Google Scholar]
- 12.Hancock WW, et al. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci USA. 1996;93:13967–13972. doi: 10.1073/pnas.93.24.13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markees TG, et al. Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4(+) T cells, interferon-gamma, and CTLA4. J Clin Invest. 1998;101:2446–2455. doi: 10.1172/JCI2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Preston EH, et al. IDEC-131 (anti-CD154), sirolimus and donor-specific transfusion facilitate operational tolerance in non-human primates. Am J Transplant. 2005;5:1032–1041. doi: 10.1111/j.1600-6143.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 15.Pearl JP, Xu H, Leopardi F, Preston E, Kirk AD. CD154 blockade, sirolimus, and donor-specific transfusion prevents renal allograft rejection in cynomolgus monkeys despite homeostatic T-cell activation. Transplantation. 2007;83:1219–1225. doi: 10.1097/01.tp.0000259929.04596.d5. [DOI] [PubMed] [Google Scholar]
- 16.Quezada SA, et al. Mechanisms of donor-specific transfusion tolerance: Preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood. 2003;102:1920–1926. doi: 10.1182/blood-2003-02-0586. [DOI] [PubMed] [Google Scholar]
- 17.Margenthaler JA, Kataoka M, Flye MW. Donor-specific antigen transfusion-mediated skin-graft tolerance results from the peripheral deletion of donor-reactive CD8+ T cells. Transplantation. 2003;75:2119–2127. doi: 10.1097/01.TP.0000069043.57679.85. [DOI] [PubMed] [Google Scholar]
- 18.van Maurik A, Fazekas de St Groth B, Wood KJ, Jones ND. Dependency of direct pathway CD4+ T cells on CD40-CD154 costimulation is determined by nature and microenvironment of primary contact with alloantigen. J Immunol. 2004;172:2163–2170. doi: 10.4049/jimmunol.172.4.2163. [DOI] [PubMed] [Google Scholar]
- 19.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 21.Iwakoshi NN, et al. Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J Immunol. 2000;164:512–521. doi: 10.4049/jimmunol.164.1.512. [DOI] [PubMed] [Google Scholar]
- 22.Quezada SA, et al. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: The interplay of clonal anergy and immune regulation. J Immunol. 2005;175:771–779. doi: 10.4049/jimmunol.175.2.771. [DOI] [PubMed] [Google Scholar]
- 23.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3:199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 25.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warnecke G, Bushell A, Nadig SN, Wood KJ. Regulation of transplant arteriosclerosis by CD25+CD4+ T cells generated to alloantigen in vivo. Transplantation. 2007;83:1459–1465. doi: 10.1097/01.tp.0000265446.61754.d2. [DOI] [PubMed] [Google Scholar]
- 27.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010;10:1524–1533. doi: 10.1111/j.1600-6143.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 30.Perona-Wright G, et al. A pivotal role for CD40-mediated IL-6 production by dendritic cells during IL-17 induction in vivo. J Immunol. 2009;182:2808–2815. doi: 10.4049/jimmunol.0803553. [DOI] [PubMed] [Google Scholar]
- 31.Sun JC, Bevan MJ. Cutting edge: Long-lived CD8 memory and protective immunity in the absence of CD40 expression on CD8 T cells. J Immunol. 2004;172:3385–3389. doi: 10.4049/jimmunol.172.6.3385. [DOI] [PubMed] [Google Scholar]
- 32.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002;297:2060–2063. doi: 10.1126/science.1072615. [DOI] [PubMed] [Google Scholar]
- 33.Kretschmer K, et al. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 34.Molinero LL, Miller ML, Evaristo C, Alegre ML. High TCR stimuli prevent induced regulatory T cell differentiation in a NF-κB–dependent manner. J Immunol. 2011;186:4609–4617. doi: 10.4049/jimmunol.1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–2791. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karim M, Kingsley CI, Bushell AR, Sawitzki BS, Wood KJ. Alloantigen-induced CD25+CD4+ regulatory T cells can develop in vivo from CD25−CD4+ precursors in a thymus-independent process. J Immunol. 2004;172:923–928. doi: 10.4049/jimmunol.172.2.923. [DOI] [PubMed] [Google Scholar]
- 37.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blair DA, Lefrançois L. Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci USA. 2007;104:15045–15050. doi: 10.1073/pnas.0703767104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford ML, et al. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ford ML, et al. A critical precursor frequency of donor-reactive CD4+ T cell help is required for CD8+ T cell-mediated CD28/CD154-independent rejection. J Immunol. 2008;180:7203–7211. doi: 10.4049/jimmunol.180.11.7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pluvinet R, et al. RNAi-mediated silencing of CD40 prevents leukocyte adhesion on CD154-activated endothelial cells. Blood. 2004;104:3642–3646. doi: 10.1182/blood-2004-03-0817. [DOI] [PubMed] [Google Scholar]
- 42.Ripoll E, et al. In vivo therapeutic efficacy of intra-renal CD40 silencing in a model of humoral acute rejection. Gene Ther. 2011;18:945–952. doi: 10.1038/gt.2011.39. [DOI] [PubMed] [Google Scholar]
- 43.Zhang T, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4–dependent manner. Am J Transplant. 2011;11:1599–1609. doi: 10.1111/j.1600-6143.2011.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 45.Barber DL, Wherry EJ, Ahmed R. Cutting edge: Rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.