Abstract
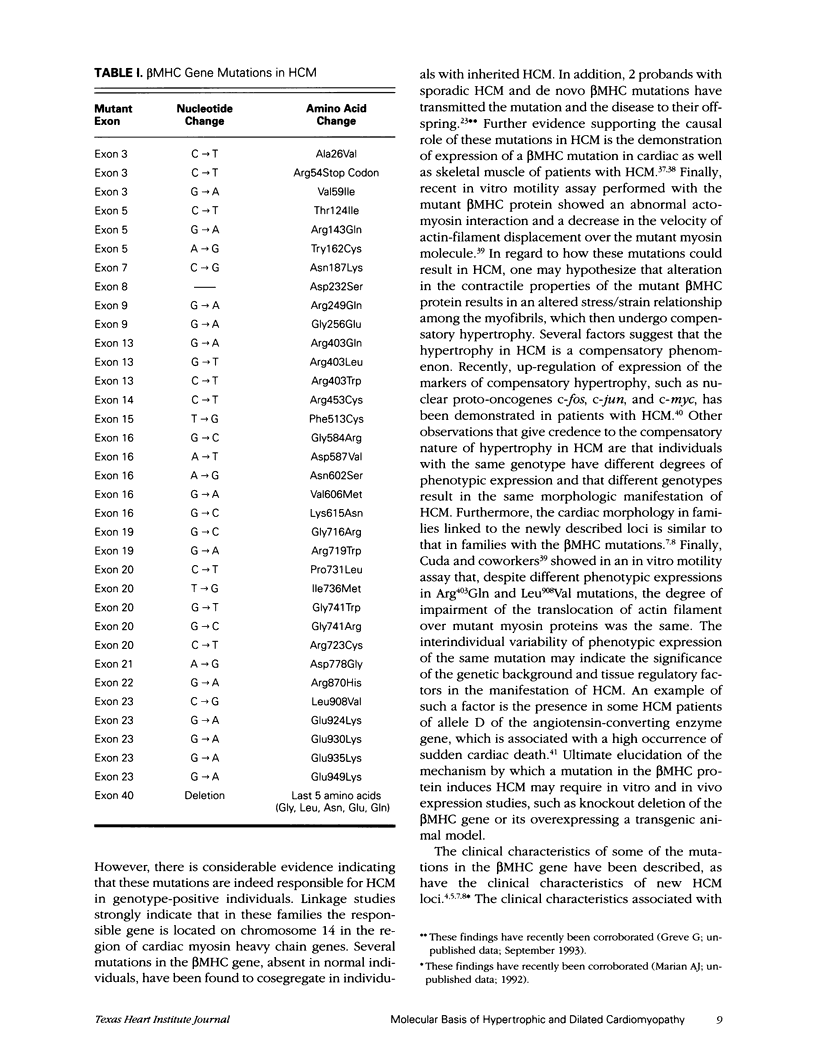
Hypertrophic cardiomyopathy is a heterogeneous disease with autosomal dominant Mendelian inheritance. In 1989, the 1st locus for hypertrophic cardiomyopathy was mapped to cardiac myosin genes located on chromosome 14q1. Soon, several mutations that cosegregated with inheritance of the disease were identified in the beta-myosin heavy chain gene, or MHY7. More than 30 missense mutations and 1 deletion mutation in the beta-myosin heavy chain gene have since been described. Recently, expression of both the mutant beta-myosin heavy chain mRNA and the mutant protein has been shown in the cardiac and skeletal muscles of individuals with hypertrophic cardiomyopathy. Characterization of the clinical features of beta-myosin heavy chain mutations has shown that certain mutations, such as Arg403Gln and Arg719Trp mutations, are associated with high rate of sudden cardiac death. In addition to the beta-myosin heavy chain gene, 3 new loci for hypertrophic cardiomyopathy have recently been described, but the candidate genes have not yet been identified. Dilated cardiomyopathy can be inherited as an autosomal dominant, autosomal recessive, and X-linked disease. The familial form of dilated cardiomyopathy comprises approximately 20% of the cases of idiopathic cardiomyopathy. Echocardiographic abnormalities such as left ventricular enlargement are present in 10% of asymptomatic relatives. No gene for familial dilated cardiomyopathy has been identified, but linkage studies using polymorphic, short-tandem repeat markers are ongoing. Dilated cardiomyopathy is a common manifestation of Duchenne/Becker muscular dystrophy. Heart failure is a common cause of death in the affected individuals. The gene responsible for this disease is the dystrophin gene located on X chromosome. There have been reports in these patients of several dystrophin-gene deletion mutations, which result in a decrease in the expression of the dystrophin protein in the cardiac and skeletal tissues. X-linked cardiomyopathy, in which the disease is restricted to the heart, has also been linked to the dystrophin gene. Myotonic dystrophy is an autosomal dominant disease that commonly involves the myocardium and the conduction tissue, resulting in conduction defects and heart failure. Sudden cardiac death is the most common cause of mortality in patients with myotonic dystrophy. Recently, the myotonin protein kinase gene located on chromosome 19 was identified as the gene responsible for this disease. Expansion of the number of trinucleotide repeats in the myotonin protein kinase gene results in myotonic dystrophy. Mutations in mitochondrial DNA have been associated with hypertrophic and dilated cardiomyopathy. The inheritance of mitochondrial cardiomyopathy is maternal and the disease is associated with certain systemic disorders.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anan R., Greve G., Thierfelder L., Watkins H., McKenna W. J., Solomon S., Vecchio C., Shono H., Nakao S., Tanaka H. Prognostic implications of novel beta cardiac myosin heavy chain gene mutations that cause familial hypertrophic cardiomyopathy. J Clin Invest. 1994 Jan;93(1):280–285. doi: 10.1172/JCI116957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATTERSBY E. J., GLENNER G. G. Familial cardiomyopathy. Am J Med. 1961 Mar;30:382–391. doi: 10.1016/0002-9343(61)90048-1. [DOI] [PubMed] [Google Scholar]
- Berko B. A., Swift M. X-linked dilated cardiomyopathy. N Engl J Med. 1987 May 7;316(19):1186–1191. doi: 10.1056/NEJM198705073161904. [DOI] [PubMed] [Google Scholar]
- Bies R. D., Friedman D., Roberts R., Perryman M. B., Caskey C. T. Expression and localization of dystrophin in human cardiac Purkinje fibers. Circulation. 1992 Jul;86(1):147–153. doi: 10.1161/01.cir.86.1.147. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Jonsson S., Hardarson T. Mode of inheritance of hypertrophic cardiomyopathy in Iceland. Echocardiographic study. Br Heart J. 1982 Feb;47(2):122–129. doi: 10.1136/hrt.47.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Brook J. D., McCurrach M. E., Harley H. G., Buckler A. J., Church D., Aburatani H., Hunter K., Stanton V. P., Thirion J. P., Hudson T. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3' end of a transcript encoding a protein kinase family member. Cell. 1992 Feb 21;68(4):799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Bulfield G., Siller W. G., Wight P. A., Moore K. J. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlquist J. F., Menlove R. L., Murray M. B., O'Connell J. B., Anderson J. L. HLA class II (DR and DQ) antigen associations in idiopathic dilated cardiomyopathy. Validation study and meta-analysis of published HLA association studies. Circulation. 1991 Feb;83(2):515–522. doi: 10.1161/01.cir.83.2.515. [DOI] [PubMed] [Google Scholar]
- Carrier L., Hengstenberg C., Beckmann J. S., Guicheney P., Dufour C., Bercovici J., Dausse E., Berebbi-Bertrand I., Wisnewsky C., Pulvenis D. Mapping of a novel gene for familial hypertrophic cardiomyopathy to chromosome 11. Nat Genet. 1993 Jul;4(3):311–313. doi: 10.1038/ng0793-311. [DOI] [PubMed] [Google Scholar]
- Chelly J., Gilgenkrantz H., Lambert M., Hamard G., Chafey P., Récan D., Katz P., de la Chapelle A., Koenig M., Ginjaar I. B. Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell. 1990 Dec 21;63(6):1239–1248. doi: 10.1016/0092-8674(90)90419-f. [DOI] [PubMed] [Google Scholar]
- Clark C. E., Henry W. L., Epstein S. E. Familial prevalence and genetic transmission of idiopathic hypertrophic subaortic stenosis. N Engl J Med. 1973 Oct 4;289(14):709–714. doi: 10.1056/NEJM197310042891402. [DOI] [PubMed] [Google Scholar]
- Codd M. B., Sugrue D. D., Gersh B. J., Melton L. J., 3rd Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975-1984. Circulation. 1989 Sep;80(3):564–572. doi: 10.1161/01.cir.80.3.564. [DOI] [PubMed] [Google Scholar]
- Cripps T. R., Counihan P. J., Frenneaux M. P., Ward D. E., Camm A. J., McKenna W. J. Signal-averaged electrocardiography in hypertrophic cardiomyopathy. J Am Coll Cardiol. 1990 Apr;15(5):956–961. doi: 10.1016/0735-1097(90)90223-c. [DOI] [PubMed] [Google Scholar]
- Cuda G., Fananapazir L., Zhu W. S., Sellers J. R., Epstein N. D. Skeletal muscle expression and abnormal function of beta-myosin in hypertrophic cardiomyopathy. J Clin Invest. 1993 Jun;91(6):2861–2865. doi: 10.1172/JCI116530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel R., Withers R., O'Brien K. Dominant and recessive modes of inheritance in idiopathic cardiomyopathy. Lancet. 1971 Nov 13;2(7733):1065–1067. doi: 10.1016/s0140-6736(71)90383-7. [DOI] [PubMed] [Google Scholar]
- Epstein N. D., Cohn G. M., Cyran F., Fananapazir L. Differences in clinical expression of hypertrophic cardiomyopathy associated with two distinct mutations in the beta-myosin heavy chain gene. A 908Leu----Val mutation and a 403Arg----Gln mutation. Circulation. 1992 Aug;86(2):345–352. doi: 10.1161/01.cir.86.2.345. [DOI] [PubMed] [Google Scholar]
- Epstein N. D., Fananapazir L., Lin H. J., Mulvihill J., White R., Lalouel J. M., Lifton R. P., Nienhuis A. W., Leppert M. Evidence of genetic heterogeneity in five kindreds with familial hypertrophic cardiomyopathy. Circulation. 1992 Feb;85(2):635–647. doi: 10.1161/01.cir.85.2.635. [DOI] [PubMed] [Google Scholar]
- Ervasti J. M., Ohlendieck K., Kahl S. D., Gaver M. G., Campbell K. P. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990 May 24;345(6273):315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- Fananapazir L., Chang A. C., Epstein S. E., McAreavey D. Prognostic determinants in hypertrophic cardiomyopathy. Prospective evaluation of a therapeutic strategy based on clinical, Holter, hemodynamic, and electrophysiological findings. Circulation. 1992 Sep;86(3):730–740. doi: 10.1161/01.cir.86.3.730. [DOI] [PubMed] [Google Scholar]
- Ferraro M., Scarton G., Ambrosini M. Cosegregation of hypertrophic cardiomyopathy and a fragile site on chromosome 16 in a large Italian family. J Med Genet. 1990 Jun;27(6):363–366. doi: 10.1136/jmg.27.6.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S., Braunwald E. Idiopathic hypertrophic subaortic stenosis. Clinical analysis of 126 patients with emphasis on the natural history. Circulation. 1968 May;37(5):759–788. doi: 10.1161/01.cir.37.5.759. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Pizzuti A., Fenwick R. G., Jr, King J., Rajnarayan S., Dunne P. W., Dubel J., Nasser G. A., Ashizawa T., de Jong P. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992 Mar 6;255(5049):1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance A. A., Kass S., Tanigawa G., Vosberg H. P., McKenna W., Seidman C. E., Seidman J. G. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990 Sep 7;62(5):999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- Graber H. L., Unverferth D. V., Baker P. B., Ryan J. M., Baba N., Wooley C. F. Evolution of a hereditary cardiac conduction and muscle disorder: a study involving a family with six generations affected. Circulation. 1986 Jul;74(1):21–35. doi: 10.1161/01.cir.74.1.21. [DOI] [PubMed] [Google Scholar]
- Greaves S. C., Roche A. H., Neutze J. M., Whitlock R. M., Veale A. M. Inheritance of hypertrophic cardiomyopathy: a cross sectional and M mode echocardiographic study of 50 families. Br Heart J. 1987 Sep;58(3):259–266. doi: 10.1136/hrt.58.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejtmancik J. F., Brink P. A., Towbin J., Hill R., Brink L., Tapscott T., Trakhtenbroit A., Roberts R. Localization of gene for familial hypertrophic cardiomyopathy to chromosome 14q1 in a diverse US population. Circulation. 1991 May;83(5):1592–1597. doi: 10.1161/01.cir.83.5.1592. [DOI] [PubMed] [Google Scholar]
- Hengstenberg C., Maisch B. Increased nuclear proto-oncogene expression in hypertrophic cardiomyopathy. Cardioscience. 1993 Mar;4(1):15–20. [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Jaenicke T., Diederich K. W., Haas W., Schleich J., Lichter P., Pfordt M., Bach A., Vosberg H. P. The complete sequence of the human beta-myosin heavy chain gene and a comparative analysis of its product. Genomics. 1990 Oct;8(2):194–206. doi: 10.1016/0888-7543(90)90272-v. [DOI] [PubMed] [Google Scholar]
- Jarcho J. A., McKenna W., Pare J. A., Solomon S. D., Holcombe R. F., Dickie S., Levi T., Donis-Keller H., Seidman J. G., Seidman C. E. Mapping a gene for familial hypertrophic cardiomyopathy to chromosome 14q1. N Engl J Med. 1989 Nov 16;321(20):1372–1378. doi: 10.1056/NEJM198911163212005. [DOI] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Liew C. C., Sole M. J., Yamauchi-Takihara K., Kellam B., Anderson D. H., Lin L. P., Liew J. C. Complete sequence and organization of the human cardiac beta-myosin heavy chain gene. Nucleic Acids Res. 1990 Jun 25;18(12):3647–3651. doi: 10.1093/nar/18.12.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limas C. J., Limas C. HLA antigens in idiopathic dilated cardiomyopathy. Br Heart J. 1989 Nov;62(5):379–383. doi: 10.1136/hrt.62.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limas C. J., Limas C. HLA-DR antigen linkage of anti-beta receptor antibodies in idiopathic dilated and ischaemic cardiomyopathy. Br Heart J. 1992 May;67(5):402–405. doi: 10.1136/hrt.67.5.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan B. A., Tsoi E. Y., Maguire C., Adgey A. A. Familial idiopathic congestive cardiomyopathy in three generations: a family study with eight affected members. Q J Med. 1987 Apr;63(240):335–347. [PubMed] [Google Scholar]
- Mahadevan M., Tsilfidis C., Sabourin L., Shutler G., Amemiya C., Jansen G., Neville C., Narang M., Barceló J., O'Hoy K. Myotonic dystrophy mutation: an unstable CTG repeat in the 3' untranslated region of the gene. Science. 1992 Mar 6;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- Malhotra S. B., Hart K. A., Klamut H. J., Thomas N. S., Bodrug S. E., Burghes A. H., Bobrow M., Harper P. S., Thompson M. W., Ray P. N. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science. 1988 Nov 4;242(4879):755–759. doi: 10.1126/science.3055295. [DOI] [PubMed] [Google Scholar]
- Marian A. J., Yu Q. T., Mares A., Jr, Hill R., Roberts R., Perryman M. B. Detection of a new mutation in the beta-myosin heavy chain gene in an individual with hypertrophic cardiomyopathy. J Clin Invest. 1992 Dec;90(6):2156–2165. doi: 10.1172/JCI116101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian A. J., Yu Q. T., Workman R., Greve G., Roberts R. Angiotensin-converting enzyme polymorphism in hypertrophic cardiomyopathy and sudden cardiac death. Lancet. 1993 Oct 30;342(8879):1085–1086. doi: 10.1016/0140-6736(93)92064-z. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Nichols P. F., 3rd, Pickle L. W., Wesley Y. E., Mulvihill J. J. Patterns of inheritance in hypertrophic cardiomyopathy: assessment by M-mode and two-dimensional echocardiography. Am J Cardiol. 1984 Apr 1;53(8):1087–1094. doi: 10.1016/0002-9149(84)90643-x. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Roberts W. C., Epstein S. E. Sudden death in hypertrophic cardiomyopathy: a profile of 78 patients. Circulation. 1982 Jun;65(7):1388–1394. doi: 10.1161/01.cir.65.7.1388. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Savage D. D., Wolfson J. K., Epstein S. E. Prognostic significance of 24 hour ambulatory electrocardiographic monitoring in patients with hypertrophic cardiomyopathy: a prospective study. Am J Cardiol. 1981 Aug;48(2):252–257. doi: 10.1016/0002-9149(81)90604-4. [DOI] [PubMed] [Google Scholar]
- Matsuoka R., Yoshida M. C., Kanda N., Kimura M., Ozasa H., Takao A. Human cardiac myosin heavy chain gene mapped within chromosome region 14q11.2----q13. Am J Med Genet. 1989 Feb;32(2):279–284. doi: 10.1002/ajmg.1320320234. [DOI] [PubMed] [Google Scholar]
- McKenna W. J., Camm A. J. Sudden death in hypertrophic cardiomyopathy. Assessment of patients at high risk. Circulation. 1989 Nov;80(5):1489–1492. doi: 10.1161/01.cir.80.5.1489. [DOI] [PubMed] [Google Scholar]
- McKenna W., Deanfield J., Faruqui A., England D., Oakley C., Goodwin J. Prognosis in hypertrophic cardiomyopathy: role of age and clinical, electrocardiographic and hemodynamic features. Am J Cardiol. 1981 Mar;47(3):532–538. doi: 10.1016/0002-9149(81)90535-x. [DOI] [PubMed] [Google Scholar]
- Mercadier J. J., Bouveret P., Gorza L., Schiaffino S., Clark W. A., Zak R., Swynghedauw B., Schwartz K. Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circ Res. 1983 Jul;53(1):52–62. doi: 10.1161/01.res.53.1.52. [DOI] [PubMed] [Google Scholar]
- Mestroni L., Miani D., Di Lenarda A., Silvestri F., Bussani R., Filippi G., Camerini F. Clinical and pathologic study of familial dilated cardiomyopathy. Am J Cardiol. 1990 Jun 15;65(22):1449–1453. doi: 10.1016/0002-9149(90)91353-8. [DOI] [PubMed] [Google Scholar]
- Michels V. V., Driscoll D. J., Miller F. A., Jr Familial aggregation of idiopathic dilated cardiomyopathy. Am J Cardiol. 1985 Apr 15;55(9):1232–1233. doi: 10.1016/0002-9149(85)90675-7. [DOI] [PubMed] [Google Scholar]
- Michels V. V., Moll P. P., Miller F. A., Tajik A. J., Chu J. S., Driscoll D. J., Burnett J. C., Rodeheffer R. J., Chesebro J. H., Tazelaar H. D. The frequency of familial dilated cardiomyopathy in a series of patients with idiopathic dilated cardiomyopathy. N Engl J Med. 1992 Jan 9;326(2):77–82. doi: 10.1056/NEJM199201093260201. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Liechti-Gallati S., Moser H., Kunkel L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Ozawa T., Tanaka M., Sugiyama S., Hattori K., Ito T., Ohno K., Takahashi A., Sato W., Takada G., Mayumi B. Multiple mitochondrial DNA deletions exist in cardiomyocytes of patients with hypertrophic or dilated cardiomyopathy. Biochem Biophys Res Commun. 1990 Jul 31;170(2):830–836. doi: 10.1016/0006-291x(90)92166-w. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Perryman M. B., Yu Q. T., Marian A. J., Mares A., Jr, Czernuszewicz G., Ifegwu J., Hill R., Roberts R. Expression of a missense mutation in the messenger RNA for beta-myosin heavy chain in myocardial tissue in hypertrophic cardiomyopathy. J Clin Invest. 1992 Jul;90(1):271–277. doi: 10.1172/JCI115848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragot T., Vincent N., Chafey P., Vigne E., Gilgenkrantz H., Couton D., Cartaud J., Briand P., Kaplan J. C., Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993 Feb 18;361(6413):647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Ray P. N., Belfall B., Duff C., Logan C., Kean V., Thompson M. W., Sylvester J. E., Gorski J. L., Schmickel R. D., Worton R. G. Cloning of the breakpoint of an X;21 translocation associated with Duchenne muscular dystrophy. Nature. 1985 Dec 19;318(6047):672–675. doi: 10.1038/318672a0. [DOI] [PubMed] [Google Scholar]
- Redman J. B., Fenwick R. G., Jr, Fu Y. H., Pizzuti A., Caskey C. T. Relationship between parental trinucleotide GCT repeat length and severity of myotonic dystrophy in offspring. JAMA. 1993 Apr 21;269(15):1960–1965. [PubMed] [Google Scholar]
- Rosenzweig A., Watkins H., Hwang D. S., Miri M., McKenna W., Traill T. A., Seidman J. G., Seidman C. E. Preclinical diagnosis of familial hypertrophic cardiomyopathy by genetic analysis of blood lymphocytes. N Engl J Med. 1991 Dec 19;325(25):1753–1760. doi: 10.1056/NEJM199112193252501. [DOI] [PubMed] [Google Scholar]
- Sasson Z., Rakowski H., Wigle E. D. Hypertrophic cardiomyopathy. Cardiol Clin. 1988 May;6(2):233–288. [PubMed] [Google Scholar]
- Schier J. J., Adelstein R. S. Structural and enzymatic comparison of human cardiac muscle myosins isolated from infants, adults, and patients with hypertrophic cardiomyopathy. J Clin Invest. 1982 Apr;69(4):816–825. doi: 10.1172/JCI110521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K., Beckmann J., Dufour C., Faure L., Fougerousse F., Carrier L., Hengstenberg C., Cohen D., Vosberg H. P., Sacrez A. Exclusion of cardiac myosin heavy chain and actin gene involvement in hypertrophic cardiomyopathy of several French families. Circ Res. 1992 Jul;71(1):3–8. doi: 10.1161/01.res.71.1.3. [DOI] [PubMed] [Google Scholar]
- Solomon S. D., Geisterfer-Lowrance A. A., Vosberg H. P., Hiller G., Jarcho J. A., Morton C. C., McBride W. O., Mitchell A. L., Bale A. E., McKenna W. J. A locus for familial hypertrophic cardiomyopathy is closely linked to the cardiac myosin heavy chain genes, CRI-L436, and CRI-L329 on chromosome 14 at q11-q12. Am J Hum Genet. 1990 Sep;47(3):389–394. [PMC free article] [PubMed] [Google Scholar]
- Solomon S. D., Jarcho J. A., McKenna W., Geisterfer-Lowrance A., Germain R., Salerni R., Seidman J. G., Seidman C. E. Familial hypertrophic cardiomyopathy is a genetically heterogeneous disease. J Clin Invest. 1990 Sep;86(3):993–999. doi: 10.1172/JCI114802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen A., Paetau A., Leinonen H., Majander A., Peltonen L., Somer H. Inherited idiopathic dilated cardiomyopathy with multiple deletions of mitochondrial DNA. Lancet. 1992 Nov 28;340(8831):1319–1320. doi: 10.1016/0140-6736(92)92496-3. [DOI] [PubMed] [Google Scholar]
- TEARE D. Asymmetrical hypertrophy of the heart in young adults. Br Heart J. 1958 Jan;20(1):1–8. doi: 10.1136/hrt.20.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniike M., Fukushima H., Yanagihara I., Tsukamoto H., Tanaka J., Fujimura H., Nagai T., Sano T., Yamaoka K., Inui K. Mitochondrial tRNA(Ile) mutation in fatal cardiomyopathy. Biochem Biophys Res Commun. 1992 Jul 15;186(1):47–53. doi: 10.1016/s0006-291x(05)80773-9. [DOI] [PubMed] [Google Scholar]
- Thierfelder L., MacRae C., Watkins H., Tomfohrde J., Williams M., McKenna W., Bohm K., Noeske G., Schlepper M., Bowcock A. A familial hypertrophic cardiomyopathy locus maps to chromosome 15q2. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6270–6274. doi: 10.1073/pnas.90.13.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin J. A., Hejtmancik J. F., Brink P., Gelb B., Zhu X. M., Chamberlain J. S., McCabe E. R., Swift M. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993 Jun;87(6):1854–1865. doi: 10.1161/01.cir.87.6.1854. [DOI] [PubMed] [Google Scholar]
- Valantine H. A., Hunt S. A., Fowler M. B., Billingham M. E., Schroeder J. S. Frequency of familial nature of dilated cardiomyopathy and usefulness of cardiac transplantation in this subset. Am J Cardiol. 1989 Apr 15;63(13):959–963. doi: 10.1016/0002-9149(89)90148-3. [DOI] [PubMed] [Google Scholar]
- Vincenzo Fragola P., Autore C., Picelli A., Sommariva L., Cannata D., Sangiorgi M. Familial idiopathic dilated cardiomyopathy. Am Heart J. 1988 Apr;115(4):912–914. doi: 10.1016/0002-8703(88)90900-3. [DOI] [PubMed] [Google Scholar]
- Vybiral T., Deitiker P. R., Roberts R., Epstein H. F. Accumulation and assembly of myosin in hypertrophic cardiomyopathy with the 403 Arg to Gln beta-myosin heavy chain mutation. Circ Res. 1992 Dec;71(6):1404–1409. doi: 10.1161/01.res.71.6.1404. [DOI] [PubMed] [Google Scholar]
- Watkins H., MacRae C., Thierfelder L., Chou Y. H., Frenneaux M., McKenna W., Seidman J. G., Seidman C. E. A disease locus for familial hypertrophic cardiomyopathy maps to chromosome 1q3. Nat Genet. 1993 Apr;3(4):333–337. doi: 10.1038/ng0493-333. [DOI] [PubMed] [Google Scholar]
- Watkins H., Rosenzweig A., Hwang D. S., Levi T., McKenna W., Seidman C. E., Seidman J. G. Characteristics and prognostic implications of myosin missense mutations in familial hypertrophic cardiomyopathy. N Engl J Med. 1992 Apr 23;326(17):1108–1114. doi: 10.1056/NEJM199204233261703. [DOI] [PubMed] [Google Scholar]
- Watkins H., Thierfelder L., Hwang D. S., McKenna W., Seidman J. G., Seidman C. E. Sporadic hypertrophic cardiomyopathy due to de novo myosin mutations. J Clin Invest. 1992 Nov;90(5):1666–1671. doi: 10.1172/JCI116038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Yamakawa K., Fukuta S., Yoshinaga T., Umemoto S., Itagaki T., Kusukawa R. Study of immunological mechanism in dilated cardiomyopathy. Jpn Circ J. 1987 Jun;51(6):665–675. doi: 10.1253/jcj.51.665. [DOI] [PubMed] [Google Scholar]
- Yu Q. T., Ifegwu J., Marian A. J., Mares A., Jr, Hill R., Perryman M. B., Bachinski L. L., Roberts R., Marlan A. J. Hypertrophic cardiomyopathy mutation is expressed in messenger RNA of skeletal as well as cardiac muscle. Circulation. 1993 Feb;87(2):406–412. doi: 10.1161/01.cir.87.2.406. [DOI] [PubMed] [Google Scholar]
- van Dorp W. G., ten Cate F. J., Vletter W. B., Dohmen H., Roelandt J. Familial prevalence of asymmetric septal hypertrophy. Eur J Cardiol. 1976 Sep;4(3):349–357. [PubMed] [Google Scholar]


