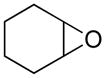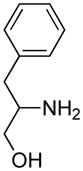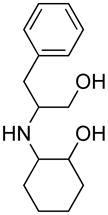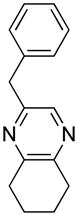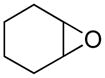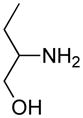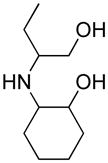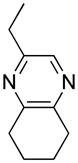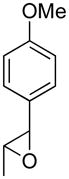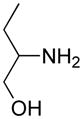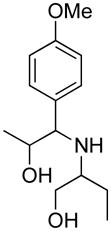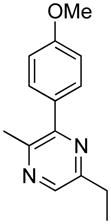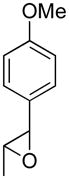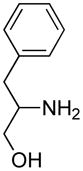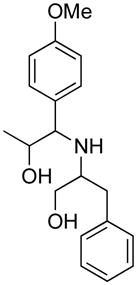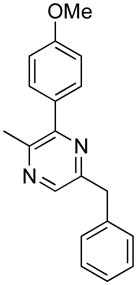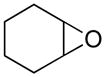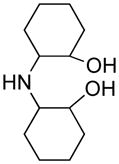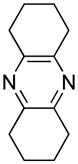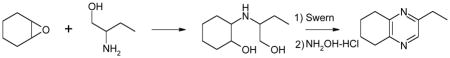
Opening of representative epoxides with 1,2-amino alcohols delivered the amino diols. The product amino diols were then oxidized under Swern conditions. The amino diketones so prepared were not isolated, but were condensed directly with hydroxylamine to give the substituted pyrazines.
Pyrazines are important as intermediates for fragrances,1 pharmaceuticals,2 and agricultural chemicals.3 Remarkably, given the importance of other aromatic heterocycles in medicinal chemistry, there are fewer than 100 trialkyl-substituted pyrazines in the SciFinder database. This is due, not to lack of interest on the part of the pharmaceutical community, but to limited methods for their preparation.4
In the course of other work, we had occasion to briefly explore the coupling of epoxides with 1,2-aminodiols. The coupled products could then be oxidized under Swern conditions and condensed with NH2OH to give pyrazines.5 Herein we report our results.
Epoxide Opening
It is important to note that the hydrogen bonded amino alcohol is much less nucleophilic, perhaps due to intramolecular hydrogen bonding, than is an isolated amine. We initially had difficulty finding conditions for the epoxide opening. We heated cyclohexene oxide and 2-amino-3-phenyl-1-propanol under solvent-free conditions, but after seven days only starting materials were visible by TLC. While LiClO4 and BF3·OEt2 failed to activate the epoxide, the addition of a catalytic amount of Yb(OTf)3 to the reaction6 facilitated an easy transformation to the amino diol. This is thought to be due to the oxophilicity of the early lanthanides. Further investigations later showed that identical loading of LiBr7 under solvent-free conditions effected an even faster transformation to the amino diol. When an activated epoxide such as 1b was used (Entries 3 and 4, Table 1), additions were carried out without catalyst. Indeed, if catalysts were added, an increased amount of the undesired regioisomer was observed.
Table 1.
Preparation of Pyrazines
Oxidation and Pyrazine Formation
We carried out our initial investigations with 2,2′-bis(cyclohexanol)amine (3e). This amino diol provided a fine platform for the elucidation of oxidation strategies. Amino diol 3e was readily prepared by Taguchi’s procedure,8 combining cyclohexene oxide and aqueous ammonia.
The Jones reagent,9a Dess-Martin periodinane,9b and the Swern reaction with trifluoroacetic anhydride9c each failed to produce the desired amino diketone. The Swern reaction utilizing oxalyl chloride10 gave some promising results, but incomplete conversion of amino diols (indicated by the presence of amino diol by TLC after workup) proved to be troublesome. When the oxidations were performed at or near the upper temperature limit of −10 °C with an excess of oxidant, the reactions went to completion.
The organic extract from the workup of the oxidation was dried over Na2SO4, then directly added to a refluxing solution of ethanolic NH2OH·HCl. The reaction flask was fitted with an air condenser which allowed the CH2Cl2 to distill out over the course of the cyclization. The brown mixture so produced could then be subjected to acid/base extraction, or evaporated directly onto silica gel for chromatography, to give the product pyrazines (Table 1).
Alternatively, it was not necessary to purify the intermediate amino diol. The amino alcohol 2a was coupled with the epoxide 1a. The crude amino diol 3a was carried directly to Swern oxidation, followed by condensation with NH2OH·HCl. The overall yield of the pyrazine 4a from 2a was slightly improved (10.1% vs. 7.8%) and the procedure was easily scaled.
Conclusion
We have developed what appears to be a versatile route to symmetrical and unsymmetrical pyrazines. It is particularly noteworthy that the Swern oxidation of the aminodiols can be carried out without protection of the basic amines.
Experimental Section
Amino diols 3b
In a 25 mL round bottom flask, (R)-(−)-2-amino-1-butanol (2b) (2.00 g, 23.5 mmol) was added to cyclohexene oxide (1a) (2.31 g, 23.6 mmol). To this was then added LiBr (102 mg, 5 mol %). The flask was sealed and the reaction was heated to 70 °C for 2 days, and then subjected to bulb-to-bulb distillation (100 °C, 2 mmHg) to remove unreacted amino alcohol and epoxide. The residue was chromatographed over basic alumina with a gradient of acetone in CH2Cl2 (0–60% in 20% increments) that had been saturated with NH4OH. The eluent was dried over Na2SO4, filtered with CH2Cl2, and concentrated to give the diastereomeric mixture of amino diols 3b (3.12 g, 70% yield) as a viscous, pale yellow oil. TLC: Rf = 0.43 (5:44:1 MeOH/CH2Cl2/NH4OH); IR (film): 3364, 2931, 2858, and 1450 cm−1; 1H NMR (CD3OD) δ 0.93 (t, J = 7.41 Hz, 3H), 1.11 (m, 1H), 1.27 (m, 3H), 1.52 (m, 2H), 1.69 (m, 2H), 1.94 (m, 2H), 2.38 (m, 1H), 2.60 (m, 1H), 3.23 (m, 1H), 3.48 (m, 2H); 13C NMR (CD3OD)13 δ d 10.12, 11.03, 58.36, 59.06, 61.88, 62.32, 74.72, 75.02; u 24.13, 25.62, 25.79, 26.38, 31.65, 32.00, 35.21, 35.30, 62.68, 64.81; HRMS calcd for C10H21NNaO2: 210.147, obsd: 210.147 [M+Na].
Pyrazine 4b
Oxalyl chloride (2.13 g, 14.0 mmol) diluted to 10 mL with CH2Cl2 was added to a 100 mL round bottom flask in a −40 °C bath. To this was added DMSO (1.31 g, 16.9 mmol, diluted to 10 mL with CH2Cl2) over the course of one min (gas evolution). Amino diols 3b (200 mg, 1.07 mmol, in 10 mL CH2Cl2) were then added. The reaction was allowed to proceed with the temperature being kept between −20 °C and −40 °C. After 2 h, 5 mL of triethylamine (35.8 mmol) was added (exotherm) to give a turbid yellow solution. The mixture was allowed to warm to 0 °C over the course of 30 min, and the mixture was then partitioned between water and CH2Cl2. The combined organic extract was dried over Na2SO4. TLC indicated the absence of amino diols 3b. The CH2Cl2 solution was decanted into a 250 mL round bottom flask, to which was added 20 mL of absolute EtOH and NH2OH·HCl (88 mg, 1.27 mmol). The round bottom flask was fitted with a distillation apparatus and the mixture was heated until the bulk of the CH2Cl2 had distilled out. The mixture was then kept at reflux for two hours with an air condenser. The brown mixture was then concentrated onto flash silica gel and chromatographed on flash silica gel with a MTBE/PE gradient to give 88 mg of crude pyrazine 4b. This was then further purified via TLC mesh chromatography (1:1 MTBE/PE) to give 25 mg of analytically pure pyrazine 4b as a pale yellow oil, 15% yield overall from 3b. TLC Rf = 0.43 (MTBE); IR: 2937, 1651, 1463, and 1386 cm−1; 1H NMR (CD3OD) δ 1.26 (t, J = 7.6 Hz, 3H), 1.88 (m, 4H), 2.73 (q, J = 7.6 Hz, 2H), 2.89 (m, 4H), 8.16 (s, 1H); 13C NMR (CD3OD) δ d 14.03, 140.44; u 22.69, 28.46, 31.55, 31.97, 149.95, 151.84, 155.29; HRMS calcd for C10H15N2: 163.124, obsd: 163.123 [M+].
Telescoped procedure: Pyrazine 4a
In a 25 mL sealed tube, S-(−)-2-amino-3-phenyl-1-propanol (2a) (3.0 g, 20 mmol) was combined with cyclohexene oxide (1a) (3.9 g, 40 mmol). LiBr (50 mg) was added and the flask was sealed. The reaction was maintained at 80 °C for 4 days, at which point the mixture was cooled to room temperature. NMR of the reaction mixture indicated complete consumption of 2a. A 2.0 g portion of the reaction mixture was dissolved into 15 mL CH2Cl2 and carried on to the oxidation/cyclization protocol.
Oxalyl chloride (6.87 mL, 80 mmol) was added to 100 mL of CH2Cl2 in a 250 mL round bottom flask at −78 °C. DMSO (7 mL, 90 mmol diluted to 20 mL with CH2Cl2) over the course of three minutes with gas evolution and apparent exotherm. The crude reaction mixture 3a (2.0 g in 15 mL CH2Cl2) was then added over the course of a minute. The reaction was allowed to proceed with the temperature being kept between −20 °C and −40 °C. After 2h, the reaction was taken back to −78 °C and triethylamine (20 mL, 143 mmol) was then added with accompanying exotherm to give a turbid yellow solution. The mixture was kept at −78 °C for 15 minutes, then allowed to warm to 0 °C over the course of 30 min, at which point the mixture was then partitioned between 75 mL of a 1:1:1 (sat NaCl, H2O, 15% NaOH) solution and CH2Cl2. The combined organic extract was dried over Na2SO4. TLC indicated the absence of amino diols 3a. The CH2Cl2 solution was decanted into a 250 mL round bottom flask, to which was added 25 mL of absolute EtOH and NH2OH · HCl (880 mg, 12.7 mmol). The round bottom flask was fitted with a distillation apparatus and the mixture was heated until the bulk of the CH2Cl2 had distilled out. The mixture was then kept at reflux overnight with an air condenser. The resulting black solution was then concentrated onto flash silica gel and filtered through a plug of flash silica gel with 100 mL of MTBE to give 240 mg of crude pyrazine 4a. This was then subjected to kugelrohr distillation (130 °C, 200 mbar) and the bottoms were chromatographed with a TLC mesh column (10–40% MTBE/PE, 50 mL/10% increment) to give 103 mg of pyrazine 4a as a pale yellow oil, 10.1% yield overall from the starting amino alcohol 2a.
TLC
Rf = 0.56 (MTBE); IR (film): 2936, 1454, 1387, and 1126 cm−1; 1H NMR (CDCl3)δ 1.73 (m, 4H), 2.75 (m, 4H), 3.93 (s, 2H), 7.10 (m, 5H), 7.99 (s, 1H); 13C NMR (CDCl3) δ d 126.39, 128.47, 128.64, 128.76, 141.08; u 22.43, 29.52, 31.34, 31.78, 41.51, 138.52, 150.17, 151.95, 152.59; HRMS calcd for C15H15N2: 223.124, obsd: 223.124 [M-H].
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM 42056). We thank John Dykins of the University of Delaware for high resolution mass spectroscopic analysis.
Footnotes
General experimental procedures, experimental details, and spectra for all new compounds. This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.Maga JA, Sizer CE. J Agr Food Chem. 1973;21:22. [Google Scholar]
- 2.(a) Nie SQ, Kwan CY, Epand RM. Eur J Pharmacol, Mol Pharm Sec. 1993;244:15. doi: 10.1016/0922-4106(93)90054-d. [DOI] [PubMed] [Google Scholar]; (b) Palacios F, Retana AMO, Munain RL. Org Lett. 2002;14:2405. doi: 10.1021/ol0261534. [DOI] [PubMed] [Google Scholar]; (c) McCullough KL. Heterocyclic Compounds. In: Sainsbury M, editor. Rodd’s Chemistry of Carbon Compounds. 2. Parts I-J. Vol. 4. Elsevier; Amsterdam: 2000. p. 99. 2nd supplement. [Google Scholar]; (d) Urban S, Hickford SJH, Blunt JW, Munro MHG. Curr Org Chem. 2000;4:765. [Google Scholar]; (e) Ohta A, Aoyagi Y. Rev Het Chem. 1998;18:141. [Google Scholar]; (f) Sato NI. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Boulton AJ, editors. Vol. 6. Elsevier; Oxfords: 1996. p. 233. [Google Scholar]
- 3.For leading references to methods for the preparation of alkyl-substituted pyrazines, see Ohta A, Itoh R, Kaneko Y, Koike H, Yuasa K. Heterocycles. 1989;29:939.Heathcock CH, Smith SC. J Org Chem. 1994;59:6828.Guo C, Bhandaru S, Fuchs PL. J Am Chem Soc. 1996;118:10672.Drogemuller M, Flessner T, Jautelat R, Scholz U, Winterfeldt E. Eur J Org Chem. 1998:2811.Elmaaty TA, Castle LW. Org Lett. 2005;7:5529. doi: 10.1021/ol0523751.Buchi G, Galindo J. J Org Chem. 1991;56:2605.
- 4.(a) Fales HM, Blum MS, Southwick EW, William DL, Roller PP, Don AW. Tetrahedron. 1988;44:5045. [Google Scholar]; (b) Tecle B, Sun CM, Borphy JJ, Toia RF. J Chem Ecol. 1987;13:1811. doi: 10.1007/BF01013230. [DOI] [PubMed] [Google Scholar]; (c) Wheeler JW, Avery J, Olubajo O, Shamim MT, Storm CB. Tetrahedron. 1982;38:1939. [Google Scholar]; (d) Brown WV, Moore BP. Insect Biochem. 1979;9:451. [Google Scholar]; (e) Cross JH, Byler RC, Ravid U, Silverstein RM, Robinson SW, Baker PM, DeOliveira JS, Jutsum AR, Cherrett JM. J Chem Ecol. 1979;5:187. [Google Scholar]; (f) Oldham NJ, Morgan ED. J Chem Soc, Perkin Trans. 1993;1:2713. [Google Scholar]
- 5.Higasio YS, Shoji T. Applied Catalysis A: General. 2001;221:197. [Google Scholar]
- 6.Chini M, Crotti P, Favero L, Macchia F, Pineschi M. Tet Lett. 1994;35:433. [Google Scholar]
- 7.Chakraborti AK, Rudrawar S, Kondaskar A. Eur J Org Chem. 2004:3597. doi: 10.1039/b400588k. [DOI] [PubMed] [Google Scholar]
- 8.Taguchi T, Hayashida K. J Am Chem Soc. 1958;80:2522. [Google Scholar]
- 9.(a) Mueller RH, DiPardo RM. J Org Chem. 1977;42:3210. [Google Scholar]; (b) Dess DB, Martin JC. J Am Chem Soc. 1991;113:7277. [Google Scholar]; (c) Omura K, Sharma AK, Swern D. J Org Chem. 1976;41:957. [Google Scholar]
- 10.Mancuso AJ, Huang S, Swern D. J Org Chem. 1978;43:2480. [Google Scholar]
- 11.Vitzthum OG, Werkhoff P. J Ag Food Chem. 1975;23:510. [Google Scholar]
- 12.Suzuki H, Kawaguchi T, Takaoka K. Bull Chem Soc Japan. 1986;59:665. [Google Scholar]
- 13.13C multiplicities were determined with the aid of a JVERT pulse sequence, differentiating the signals for methyl and methine carbons as “d”, from methylene and quaternary carbons as “u”.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.