Abstract
There are no established chemopreventive agents for lung cancer, the leading cause of cancer death in the United States. Prostacyclin levels are low in lung cancer and supplementation prevents lung cancer in preclinical models. We carried out a multicenter double-blind, randomized, phase II placebo-controlled trial of oral iloprost in current or former smokers with sputum cytologic atypia or endobronchial dysplasia. Bronchoscopy was performed at study entry and after completion of six months of therapy. Within each subject, the results were calculated by using the average score of all biopsies (Avg), the worst biopsy score (Max), and the dysplasia index (DI). Change in Avg was the primary end point, evaluated in all subjects, as well as in current and former smokers. The accrual goal of 152 subjects was reached and 125 completed both bronchoscopies (60/75 iloprost, 65/77 placebo). Treatment groups were well matched for age, tobacco exposure, and baseline histology. Baseline histology was significantly worse for current smokers (Avg 3.0) than former smokers (Avg 2.1). When compared with placebo, former smokers receiving oral iloprost exhibited a significantly greater improvement in Avg (0.41 units better, P = 0.010), in Max (1.10 units better, P = 0.002), and in DI (12.45%, P = 0.006). No histologic improvement occurred in current smokers. Oral iloprost significantly improves endobronchial histology in former smokers and deserves further study to determine if it can prevent the development of lung cancer.
Introduction
Lung cancer is the leading cause of cancer death globally and in the United States (1). The majority of diagnoses in the United States are made in former smokers (2). Although avoidance of tobacco abuse and smoking cessation clearly will have the greatest impact on lung cancer development, effective chemoprevention could prove to be more beneficial than treatment of established lung cancers, particularly in former smokers. Chemoprevention is defined as the use of dietary or pharmaceutical agents to reverse or inhibit the carcinogenic process and has been successfully applied to common malignancies other than lung. The World Health Organization classification for lung cancer recognizes distinct histological lesions which are precursors of lung cancer (3). For example, the development of squamous cell lung cancer starts with normal epithelium and progresses through hyperplasia, metaplasia, dysplasia (mild, moderate, and severe), and carcinoma in situ. Although no intermediate biomarker has been validated for the prevention of lung cancer, in part because of the lack of proven therapy, histology has been considered the best marker (as it is in other malignancies; ref. 4). By identifying and focusing therapy on premalignant stages of the disease, rather than the current focus on invasive disease, effective treatment and improved survival may become a more attainable goal (4).
The arachidonic acid pathway is critical in carcinogenesis. Large epidemiologic studies have shown an association between regular aspirin use and decreased rates of certain cancers, although evidence regarding lung cancer and aspirin use is not clear. There is a decrease in colon cancer rates in subjects taking aspirin [an irreversible cyclooxygenase (COX-1 and COX-2) inhibitor] and selective COX-2 inhibitors decrease the incidence of colonic polyps, a premalignant lesion (5, 6). COX inhibition, however, decreases levels of all downstream arachidonic acid metabolites, including prostacyclin, which has anti-metastastic and antiproliferative properties (7, 8). Prostacyclin and prostaglandin E2 exhibit a reciprocal relationship in normal lung and Kras mutant non–small cell lung carcinoma (NSCLC) cell lines, with the former high in normal lung and the latter elevated in tumor cell lines (9). Prostacyclin supplementation (genetic overexpression of prostacyclin synthase or administration of the oral analogue iloprost) prevents the development of lung cancer in a variety of murine models, including cigarette smoke exposure (10–12). Prostaglandin E2 has been implicated in colon carcinogenesis, but overexpression of prostaglandin E2 synthase does not increase lung carcinogenesis in murine models (13, 14). Tissue microarray studies have shown that the majority of NSCLC have decreased expression of PGIS and that PGIS expression is associated with a superior outcome, whereas prostaglandin E2 synthase expression is frequent and associated with poor survival. Therefore, multiple preclinical studies suggest that prostacyclin supplementation might be chemopreventive.
All prior lung cancer chemoprevention studies proved negative, but several studies have revealed differences between current and former smokers regarding amount and extent of central airway damage (15–17). Subjects with tobacco smoke exposure, chronic obstructive lung disease, and sputum cytologic atypia have rates of lung cancer greater than 1% yearly and are a high risk group in which chemoprevention may have utility (18). We therefore instituted a multicenter, double-blind, placebo-controlled phase II trial of the oral prostacyclin analogue iloprost in current and former smokers with sputum cytologic or endobronchial atypia by using 6-month change in average biopsy score (Avg) as the primary end point.
Materials and Methods
Study design
The iloprost lung cancer chemoprevention study was a multicenter, phase II, randomized, double-blind, placebo-controlled trial of iloprost in subjects at increased risk for lung cancer (defined as current or former smokers with 20 pack year or more smoking history, at least mild sputum cytologic atypia, or a history of biopsy proven endobronchial dysplasia). The majority of subjects were recruited from pulmonary medicine clinics. Exclusion criteria included prior history of cancer, significant comorbid disease or inability to undergo 2 bronchoscopies, hypoxemia with the required use of supplemental oxygen, use of inhaled steroids within 6 weeks of trial enrollment, and carcinoma in situ or invasive cancer on endobronchial biopsy. Sputum was collected and cytology was graded by a single cytopathologist (W. A. Franklin) as previously published (18). Autofluorescence and/or white light bronchoscopy was carried out before randomization and after 6 months of treatment, with 6 standard endobronchial sites biopsied (all were carini, identified as RUL, RML, RB6, LUL, LUDB, and LB6), along with all other visually suspicious appearing areas.
The trial sample size was 152 subjects and, after obtaining written informed consent, participants were block randomized based on smoking status (current vs. former) and study center. The randomization sequence was generated prior to trial initiation and stored in a password-protected spreadsheet accessible only to the trial biostatistician and study administrator. Subjects were randomized only after confirmation of eligibility, and blinding was maintained throughout the trial. Following randomization, subjects were started on either iloprost or placebo at an initial dose of 1 tablet BID (50 μg iloprost clathrate per tablet). The subjects had a monthly clinical evaluation and if well tolerated, iloprost or placebo was dose escalated by 1 tablet monthly to a maximum dose of 3 tablets BID. Following 6 months of treatment, a second bronchoscopy was carried out with repeat biopsies at all of the baseline sites. Adverse events were monitored and reported twice yearly to an independent data and safety monitoring board (DSMB). A final clinical visit occurred 1 month after completing the trial and subjects are currently undergoing passive follow-up (i.e., yearly questionnaires). The trial involved 7 clinical centers (listed in the Appendix) funded by the National Cancer Institute as the Lung Cancer Biomarkers and Chemoprevention Consortium and individual site SPORE grants. The institutional review boards at each study center approved the study protocol. This trial was listed and registered on ClinicalTrials.gov (Identifier: NCT00084409). Bayer-Schering Pharma AG (Berlin) provided the study medication and placebo tablets.
Biopsy analysis
All endobronchial biopsies were formalin fixed, paraffin embedded, and stained with hematoxylin and eosin for subsequent morphologic evaluation and classification. Biopsies were classified into 1 of 8 categories as defined by the WHO classification (3) and assigned a score according to the following system: 1, normal; 2, reserve cell hyperplasia; 3, squamous metaplasia without atypia; 4, mild dysplasia; 5, moderate dysplasia; 6, severe dysplasia; 7, carcinoma in situ; and 8, invasive carcinoma. All biopsies were graded by the study pathologist (WAF) in a blinded fashion as to treatment group and were read after the completion of each bronchoscopy. Blinded reproducibility studies in which 191 bronchial biopsy specimens were scored twice by the main study pathologist and a third time independently by an additional pathologist (D. T. Merrick) were completed. For intraobserver reproducibility, 86% (164/191) were given a diagnosis within 1 unit on the WHO scale (weighted κ test statistic = 0.71; 95% CI: 0.65–0.77). For the interobserver reliability, 84% (160/191) of the biopsies were scored within 1 unit (weighted κ test statistic = 0.66 (95% CI: 0.59–0.73)).
In addition to WHO histology scoring, epithelial proliferation, measured by Ki-67 immunostaining, was conducted on biopsies from former smokers carried out by techniques previously described and implemented in our laboratory. In brief, the most dysplastic region of a biopsy was selected, at least 400 cells were graded for Ki-67 positivity throughout the entire epithelium and the percentage of positive cells recorded as the Ki-67 proliferative index. The primary antibody used for Ki-67 was Dako clone mib-1 BM28 mouse monoclonal (#B58720) at a dilution of 1:100.
Statistical design and analysis
Endobronchial histology was summarized within each bronchoscopy by using 3 separate measures: Avg of all biopsy scores; worst biopsy score (Max); dysplasia index (DI; defined as the percentage of biopsies with a score of 4 or worse). The prespecified primary end point was Avg histology calculated by using all biopsies. All other end points and biopsy site groupings were predefined to be secondary, but are necessary to evaluate whether the results are sensitive to the approach used to summarize histology. The 3 summary end points were analyzed within 4 different biopsy site groupings: all biopsies (1,883 biopsies), biopsies from the 6 standard endobronchial sites only (1,450 biopsies), site-matched biopsies from both bronchoscopies (1,708 biopsies), and site-matched biopsies where the baseline biopsy was non-normal (1,122 biopsies).
The iloprost trial was designed to enroll 152 subjects with 76 in each treatment group and an equal number of current and former smokers (38 in each treatment group). On the basis of the standard deviation (SD = 0.9) from a previous chemoprevention trial in a similar population (15), the planned sample size provided 90% power for a difference in the 6-month change in Avg histology scores of 0.67 with 38 subjects per group (i.e., for comparisons within the current or former subgroups) and a difference of 0.47 with 76 subjects per group (i.e., for comparisons in the total population). As the trial neared completion, the decision was made to enroll slightly more current smokers to achieve the target sample size of 152 subjects. The trial was monitored by an independent DSMB, and no interim analyses of treatment effects on histology were planned or conducted.
All analyses were prespecified in a written statistical analysis plan (SAP) that followed from the trial protocol. The primary end point (and statistical analysis) measured the effects of iloprost by a test of the difference in Avg change between the 2 treatment groups (adjusted for baseline Avg histology by using linear regression; i.e., ANCOVA). The interaction between treatment effect and smoking status was also tested to determine if the effect of iloprost differed between former and current smokers. In accordance with the SAP, the primary conclusion of this trial was to be based on the combined results unless the interaction test was significant (P < 0.05). Ultimately, this test was borderline significant (P = 0.051), and because there are known differences between current and former smokers from previous trials, the primary conclusions reported here are based on the separated results. Secondary analyses were conducted to evaluate the consistency of the primary results across other measures of histologic change. Subject response, defined as an improvement in histology (measured separately by Avg, Max, or DI), was also analyzed as a secondary end point. Results are reported as point estimates, 95% CI, and 2-sided P-values without adjustment for multiple comparisons.
Results
Study population
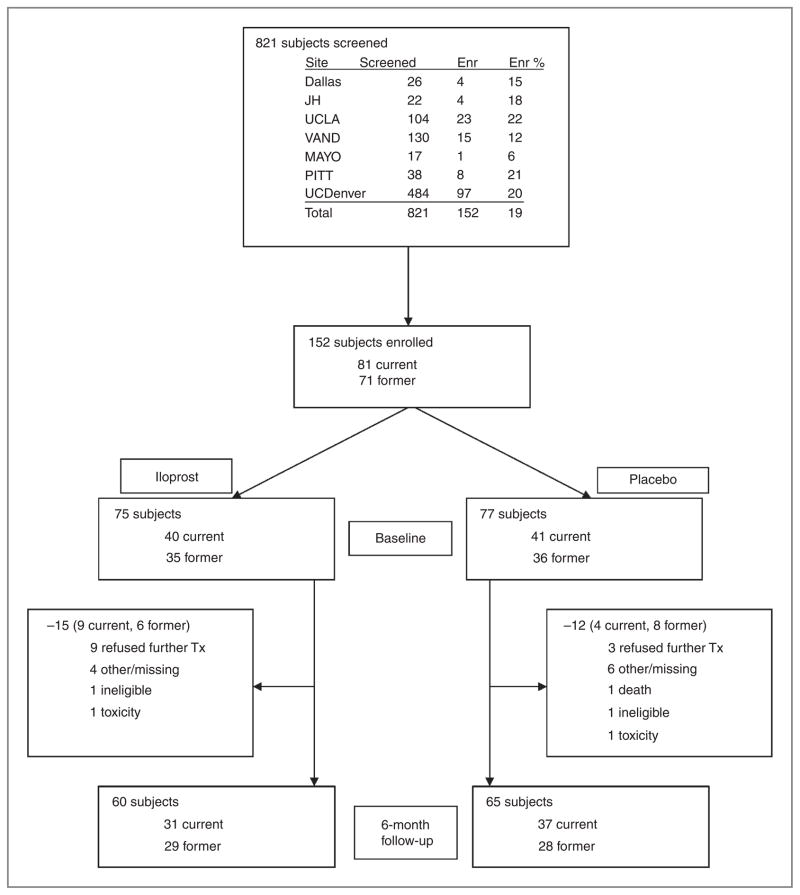
A total of 821 subjects were screened from 2002 until 2008, and 152 subjects were enrolled (Fig. 1). The main reasons for not entering the trial included lack of sputum cytologic atypia; reluctance to undergo multiple bronchoscopies; and travel issues. The study group consisted of 81 current smokers and 71 former smokers, with 75 subjects randomized to oral iloprost and 77 to placebo. There were no significant differences in baseline demographic or clinical characteristics, including tobacco exposure, time since smoking cessation, age, sputum cytology, and endobronchial histology, between the 2 treatment groups (Table 1). Seventy-two percent of the study subjects were male. Baseline bronchoscopy was completed in all 152 subjects, and both bronchoscopies were completed in 125 subjects. Similar dropout rates were observed between the treatment groups (20% versus 16%, P = 0.18), with the main reason given as “refusing further treatment.” The complete list of reasons for failing to complete the study is contained in Figure 1. No subjects successfully quit smoking during the trial.
Figure 1.
Trial flow diagram.
Table 1.
Baseline characteristics of trial subjects
| Characteristic | Iloprost (n = 75) | Placebo (n = 77) | Iloprost: former smoker (n = 35) | Placebo: former smoker (n = 36) | Iloprost: current smoker (n = 40) | Placebo: current smoker (n = 41) |
|---|---|---|---|---|---|---|
| Sex of subject–No. (%) | ||||||
| Female | 23 (30.7) | 19 (24.7) | 8 (22.9) | 9 (25.0) | 15 (37.5) | 10 (24.4) |
| Male | 52 (69.3) | 58 (75.3) | 27 (77.1) | 27 (75.0) | 25 (62.5) | 31 (75.6) |
| Race of subject–No. (%) | ||||||
| Black | 4 (5.3) | 8 (10.4) | 0 (0.0) | 3 (8.3) | 4 (10.0) | 5 (12.2) |
| Other | 2 (2.7) | 3 (3.9) | 1 (2.9) | 0 (0.0) | 1 (2.5) | 3 (7.3) |
| White | 69 (92.0) | 66 (85.7) | 34 (97.1) | 33 (91.7) | 35 (87.5) | 33 (80.5) |
| Age, y | 57.6 ± 10.6 | 58.3 ± 8.6 | 61.5 ± 10.7 | 61.2 ± 9.6 | 54.2 ± 9.2 | 55.7 ± 6.8 |
| Smoker years | 34.6 ± 10.5 | 36.0 ± 10.0 | 32.3 ± 10.1 | 33.0 ± 10.2 | 36.6 ± 10.5 | 38.7 ± 9.0 |
| Packs per day | 1.5 ± 0.6 | 1.4 ± 0.5 | 1.8 ± 0.7 | 1.4 ± 0.5 | 1.3 ± 0.4 | 1.3 ± 0.6 |
| Pack years | 51.8 ± 26.8 | 49.9 ± 26.3 | 56.4 ± 29.6 | 48.7 ± 27.0 | 47.8 ± 23.7 | 50.9 ± 26.0 |
| Smoker quit years | 12.0 ± 8.4 | 12.0 ± 10.0 | ||||
| FEV1 | 2.4 ± 0.9 | 2.3 ± 1.0 | 2.4 ± 0.9 | 2.3 ± 1.0 | 2.5 ± 0.9 | 2.4 ± 0.9 |
| FEV/FVC ratio | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| Sputum cytology | 4.4 ± 0.7 | 4.4 ± 0.6 | 4.4 ± 0.6 | 4.3 ± 0.5 | 4.4 ± 0.8 | 4.5 ± 0.7 |
| Average histology | 2.6 ± 1.1 | 2.5 ± 1.1 | 2.1 ± 1.0 | 2.0 ± 1.1 | 3.0 ± 1.1 | 2.9 ± 0.9 |
| Maximum histology | 4.3 ± 1.5 | 3.8 ± 1.6 | 3.6 ± 1.8 | 3.1 ± 1.8 | 4.8 ± 0.9 | 4.5 ± 1.1 |
| Dysplasia index | 33.3 ± 28.7 | 32.8 ± 31.1 | 20.4 ± 22.2 | 21.3 ± 28.5 | 44.6 ± 29.2 | 42.8 ± 30.1 |
| Maximum histology–No. (%) | ||||||
| No dysplasia (grades 1–3) | 16 (21.3) | 22 (28.6) | 13 (37.1) | 18 (50.0) | 3 (7.5) | 5 (12.2) |
| Mild (grade 4) | 14 (18.7) | 15 (19.5) | 7 (20.0) | 5 (13.9) | 7 (17.5) | 10 (24.4) |
| Moderate (grade 5) | 33 (44.0) | 35 (45.5) | 10 (28.6) | 12 (33.3) | 23 (57.5) | 23 (56.1) |
| Severe (grade 6) | 12 (16.0) | 4 (5.2) | 5 (14.3) | 1 (2.8) | 7 (17.5) | 3 (7.3) |
Plus-minus values are means ± SD.
Histologic analysis of baseline and follow-up bronchoscopy specimens
At baseline, there were no significant differences between the iloprost and placebo groups in any of the summary measures (Avg, Max, and DI; Table 1). At baseline, current smokers had more endobronchial dysplasia than former smokers as evidenced by significantly higher Avg score (3.0 vs. 2.1, P < 0.0001), Max (4.6 vs. 3.4, P < 0.0001), and DI (44% vs. 21%, P < 0.0001). Follow-up bronchoscopy was carried out on 60 iloprost subjects (29 former smokers, 31 current smokers) and 65 placebo subjects (28 former smokers, 37 current smokers).
Overall results combining current and former smokers (Table 2) showed no significant difference between treatment groups by 6-month change in the primary end point (Avg histology adjusted difference: −0.15; 95% CI: −0.39–0.09; P = 0.21; Fig. 2A). There was a statistically significant difference among former smokers: with iloprost treatment Avg scores improved by −0.39, and with placebo treatment they showed minor progression of 0.04 (adjusted difference = −0.41; 95% CI: −0.71–−0.11, P = 0.01; Fig. 2B). In contrast, among current smokers, there was no change in Avg score in either treatment group (iloprost change: −0.07; placebo change: −0.06; adjusted difference; 0.06; 95% CI: −0.30–0.42; P = 0.74; Fig. 2C). Formally, the test for interaction between smoking status and treatment effects on Avg histology was not statistically significant (P = 0.051). Although this interaction test did not reach our prespecified threshold (P < 0.05), the magnitude of the effect in former smokers was large and consistent across all primary and secondary end points (Table 2, Supplementary Table S1, Supplementary Fig. S2). Conversely, among current smokers there were no remarkable effects on any end point.
Table 2.
Iloprost treatment effects on bronchial histology
| IloProst
|
Placebo
|
Treatment Effect1 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6-Month | Change | Baseline | 6-Month | Change | Difference | 95% CI | P-Value | |
| All Completers2 | |||||||||
| All Biopsies | |||||||||
| Average | 2.64 | 2.41 | −0.23 | 2.56 | 2.54 | −0.02 | −0.15 | (−0.39, 0.09) | 0.210 |
| Worst | 4.25 | 3.85 | −0.40 | 3.91 | 4.14 | 0.23 | −0.43 | (−0.84, −0.03) | 0.038 |
| Dysp. Index | 34.97 | 27.27 | −7.70 | 34.23 | 33.32 | −0.92 | −5.97 | (−13.28, 1.33) | 0.112 |
| Former Smokers3 | |||||||||
| All Biopsies | |||||||||
| Average | 2.12 | 1.73 | −0.39 | 2.07 | 2.11 | 0.04 | −0.41 | (−0.71, −0.11) | 0.010 |
| Worst | 3.59 | 2.83 | −0.76 | 3.11 | 3.68 | 0.57 | −1.10 | (−1.76, −0.45) | 0.002 |
| Dysp. Index | 20.81 | 10.90 | −9.91 | 22.90 | 24.60 | 1.70 | −12.45 | (−20.98, −3.92) | 0.006 |
| Current Smokers4 | |||||||||
| All Biopsies | |||||||||
| Average | 3.13 | 3.05 | −0.07 | 2.93 | 2.87 | −0.06 | 0.06 | (−0.30, 0.42) | 0.743 |
| Worst | 4.87 | 4.81 | −0.06 | 4.51 | 4.49 | −0.03 | 0.12 | (−0.36, 0.60) | 0.621 |
| Dysp. Index | 48.22 | 42.58 | −5.64 | 42.81 | 39.91 | −2.90 | −0.33 | (−11.70, 11.05) | 0.955 |
Adjusted for baseline level of the outcome variable plus smoking status.
N = 60 (iloprost), N = 65 (placebo).
N = 29 (iloprost), N = 28 (placebo).
N = 31 (iloprost), N = 37 (placebo).
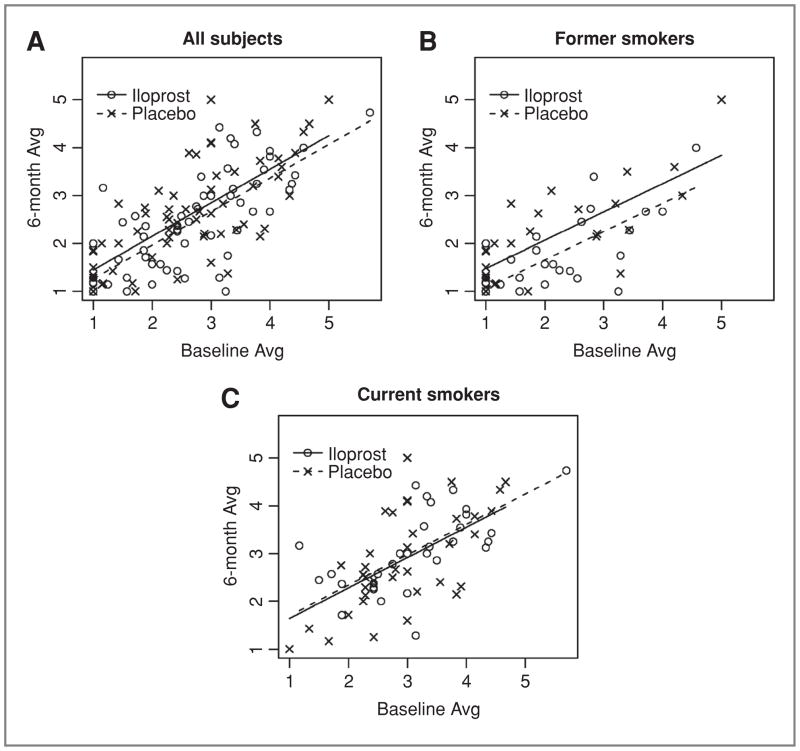
Figure 2.
Six-month histology as a function of baseline average histology in all subjects (A), former smokers (B), and current smokers (C) comparison of average histology measures on initial and follow-up bronchoscopy of subjects completing the trial (60 iloprost subjects and 65 placebo subjects). Illustrates consistent improvement across the entire histologic spectrum in former smokers receiving iloprost (P = 0.010) and a lack of effect in all subjects (P = 0.210) and current smokers (P = 0.743).
Prespecified secondary analyses show that the results based on all biopsy specimens are similar in biopsy subsets (Supplementary Fig. S2). Restricting the analysis to the 6 prespecified sites (n = 1,450; 77% of the data), “matched” biopsy sites sampled at both baseline and follow-up bronchoscopy (n = 1,708; 91% of the data), or sites that were nonnormal (histology score ≥1) at baseline (n = 1,222; 60% of the data; 107 subjects) gives the same pattern of highly significant treatment effects among former smokers receiving iloprost, no effects among current smokers, and smaller (nonsignificant) effects when the groups are combined (Supplementary Table S1).
Histologic response
Among former smokers, the iloprost group showed a significantly higher response rate when compared with placebo [Avg histology improved in 58.6% (17/29) with iloprost vs. 28.6% (8/28) with placebo, P = 0.025]. Response rates were also significantly better with iloprost if response was defined by using Max (48.3% (14/29) improved with iloprost versus 14.3% (4/28) placebo, P = 0.009). If dropouts are counted as nonresponders, the response rate with iloprost remains significantly greater than placebo for both Avg histology [48.6% (17/35) vs. 22.2% (8/36); P = 0.023] and Max histology [40.0% (14/35) vs. 11.1% (4/36); P = 0.008]. Among all study participants, the response rates were significantly different between treatment groups when response was based on Max histology. There were no differences in the response rates among current smokers (data contained in Supplementary material, Table S1).
On a per subject analysis, there were 95 subjects who completed the trial and had at least 1 site of dysplasia at baseline (48 iloprost, 47 placebo). Within this group, 36 (37.9%) of the subjects had their maximum histology regress (defined as improving by at least 1 grade; 23 iloprost, 13 placebo, P = 0.042); 39 subjects (41.1%) had their maximum histology remaining stable (17 iloprost, 22 placebo); and 20 subjects (21.1%) had their maximum histology progress (defined as worsening by at least 1 grade; 8 iloprost, 12 placebo). The maximum histology analysis by smoking status showed the following distributions of subjects (iloprost versus placebo): In former smokers, regression (13/18 versus 4/14), stable (4/18 versus 6/14), and progression (1/18 versus 4/14; P = 0.038); in current smokers, regression (10/30 versus 9/33), stable (13/30 versus 16/33), and progression (7/30 versus 8/33; P = 0.87).
Ki-67 analysis
Ki-67 proliferative index was determined by staining all endobronchial biopsies taken from former smokers receiving either iloprost (n = 29) or placebo (n = 28). Former smokers who received iloprost had slightly greater declines in Ki67 labeling index (13.0% vs. 11.8%, a decline of 1.2%) compared with placebo (13.7% vs. 13.3%, a decline of 0.4%), but the differences were not significant (P = 0.63).
Adverse events
Study subjects were evaluated before the initial bronchoscopy and then had monthly clinic visits to evaluate for dose escalation and adverse events of treatment. Dose escalation was successful in 82% of subjects who completed the trial (n = 102). In the iloprost group, 44 subjects (73%) reached complete dose escalation (total dose of 3 tablets BID), as compared with 58 (89%) in the placebo group, a statistically significant difference (P = 0.022). There were no differences in dose escalation between former (81%) and current smokers (82%; P = 0.81). For our primary end point of average biopsy score, both iloprost groups showed similar improvement (−0.20 in those fully dose escalating and −0.29 in the group not reaching the maximum dose). For the placebo group, improvements in average histology were not observed in those reaching maximum dose and those failing to dose escalate. The 3 most common adverse events were headache (52% in the iloprost group vs. 23% in the placebo group, P < 0.001), flushing (23% in the iloprost group vs. 8% in the placebo group, P = 0.006), and nausea (16% in the iloprost group vs. 8% in the placebo group, P = 0.117). This is consistent with previously published trials of the oral vasodilator iloprost (19). There were no serious adverse events (grade 4 or 5) in the treatment group, and no significant differences were observed between the 2 treatment groups for grade 3 adverse events (Supplementary Table S2 contains the most common adverse events by treatment group).
Discussion
In this randomized, double-blind, placebo-controlled trial of oral iloprost for the reduction of endobronchial histologic changes we found that 6 months of oral iloprost significantly improved the prespecified primary histologic end point Avg in former smokers, but had no effect in current smokers. This chemopreventive intervention for lung cancer showed a treatment effect similar in magnitude to the baseline difference between current and former smokers in our trial population.
Multiple preclinical studies by using both carcinogen and tobacco smoke-induced murine models have shown that manipulation of prostacyclin production and oral supplementation can prevent lung cancer, even when iloprost chow is introduced 5 weeks after carcinogen administration (10–12). Human lung cancer studies have shown that prostacyclin synthase expression is low in most lung cancers and low expression is associated with a worse outcome (9, 20). Prostacyclin analogues, such as iloprost, bind the single IP receptor and increase cAMP levels (21). Prostacyclin also is a ligand for peroxisomal proliferator-activated receptors (PPARs), which are members of the nuclear receptor superfamily of regulated transcription factors (22). Studies in IP receptor null mice show that the chemopreventive effect of prostacyclin synthase overexpression is independent of the IP receptor (12). Furthermore, mice overexpressing PPARγ exhibit chemoprevention compared with wild type littermates (12). Epidemiologic studies also support the role of PPARγ in human lung tumorigenesis as reported in a study of diabetics treated with PPARγ agonist thiazolidinedione medications. Lung cancer incidence in those treated with PPARγ agonists was decreased 33% when compared with diabetics on non-PPARγ modulating therapy (23). The combination of pre-clinical and epidemiologic data strongly supported a phase II trial of oral iloprost.
Several large placebo-controlled phase III chemoprevention trials assessing promising agents, including retinoids and combinations of vitamins and minerals, have been completed in the past. These include CARET (β-carotene and retinol), ATBC (β-carotene and α-tocopherol), Euroscan (β-carotene and N-acetyl cysteine), Intergroup (13 cis-retinoic acid), and a trial conducted in a vitamin deficient population in Linxiang, China (24–27). All proved negative and some showed adverse effects, particularly among current smokers (25, 28, 29). More recently, a phase III trial evaluating selenium for subjects with resected NSCLC has been reported in abstract form as negative (30).
Phase II trials with intermediate end points such as histologic dysplasia, metaplasia, or epithelial proliferation measured by Ki-67 immunostaining have been undertaken to more rapidly assess potential chemopreventive interventions. These have included anethole dithiolethione (a green tea component,), 13 cis-retinoic acid, fenretinide, celecoxib, and inhaled budesonide (15, 31–34). Our group conducted a phase II study of 13 cis-retinoic acid by using a similar SAP and we observed no statistically significant effect on histology in either current or former smokers (15). This agent proved ineffective in a phase III chemoprevention trial (26). In a post hoc analysis, anethole dithiolethione decreased the rate of progression of dysplasia (32). Two placebo-controlled trials assessing 13 cis-retinoic acid were in agreement on lack of histologic response, but differed in regard to an effect on Ki-67 index; the lack of efficacy of this agent in a phase III trial suggests that only agents with greater efficacy in phase II trials should be considered for phase III (15, 35) A single arm trial reported a decrease in Ki-67 index in heavy smokers after receiving celecoxib for 6 months (36). A subsequent placebo-controlled trial of celecoxib showed small but statistically significant reductions in Ki-67 index in current (−1.10%) and former (−3.75%) smokers (34). A single arm phase I trial of myoinositol showed promising results compared with historical controls (37). Iloprost is the first agent to show improvement of endobronchial dysplasia.
The choice of intermediate end point biomarkers for phase II chemoprevention trials has proven problematic. Sustained smoking cessation is the only known intervention to impact lung cancer death rates (38). In the absence of proven effective chemoprevention agents, intermediate end points such as endobronchial dysplasia and Ki-67 index cannot be validated by the Prentice criteria (39), but still represent a reasonable approach to assessment for further study in phase III trials (4). Most recent phase II studies have chosen to evaluate the effects of intervention on premalignant lesions or inhibition of the carcinogenic progression. The current study confirms the finding that histology is less severe in former smokers [as is Ki-67 index (17)] and supports its feasibility for use as a biomarker. Endobronchial histology can be assessed by using a variety of measures, including Avg, Max, and DI, all of which improved in the current trial. Although we chose Avg as the primary end point in this trial because it estimates the within-subject mean condition of the sampled bronchial epithelium, one could argue that Max or DI might be equally or more biologically plausible.
The natural history of dysplastic endobronchial lesions is difficult to predict, with published reports failing to reach consensus on the risk of progression of severely dysplastic or carcinoma in situ lesions to invasive cancer (40, 41). Molecular or immunohistochemical analysis may improve prediction of lesions that will progress or which are associated with invasive lung cancer as a manifestation of a field effect (42–44). The placebo group in our trial helps to further define this natural history and illustrates that merely obtaining an endobronchial biopsy does not produce a significant therapeutic effect, as the histology scores did not improve between the first and second bronchoscopies. Most importantly, this report shows that dysplastic lesions can be targets of chemoprevention trials and, because they typically contain fewer genetic derangements and signaling abnormalities, they may be amenable to treatment aimed at blocking progression.
For effective chemoprevention to impact outcome, high-risk populations must be clinically identifiable. Several groups have published models based on age, gender, smoking history, airflow obstruction, family history, and radiographic emphysema which can risk stratify current or former smokers for lung cancer risk (40, 41, 45). The development of COPD (as evidenced by airflow obstruction on spirometry or CT detected emphysema) also significantly increases lung cancer incidence (46, 47). Our group reported that a cohort of high-risk current and exsmokers with airflow obstruction exhibited an overall rate of incident lung cancer of 1.85 per 100 person-years on longitudinal follow-up (18). Survivors of a previous tobacco-induced cancer are an additional high-risk population appropriate for phase II or III chemoprevention trials. Therefore, groups with a significant risk for the development of lung cancer can be identified for prevention trials, although further refinement of risk assessment would be desirable.
The current study has similar limitations to other phase II trials in that it was not powered to provide information about the clinically important end point of lung cancer incidence (no participants developed lung cancer during this trial). The potential for confounding was controlled through full concealment of the treatment allocation/randomization system. Information bias was controlled through careful blinding, in particular, a blinded central pathology review of all biopsy specimens. The potential for false positive conclusions (type I statistical errors) was controlled through prespecification and prioritization of all trial end points and analyses. In addition, we required that the results be reproducible regardless of the particular method chosen to summarize histologic response. Dropout rates were similar between treatment groups, although the iloprost group may have had an increased tendency for treatment termination because of an increased risk of grades 1 and 2 adverse events (summarized in Supplementary Table S2).
Our recruitment model for this trial showed that we were successful in identifying subjects with endobronchial dysplasia. Of the 152 enrolled subjects, 74% (113/152) had at least 1 mildly dysplastic or worse biopsy, and 54% (1,120/2,088) of our matched sites were from histologically non-normal areas. Ki-67 did not improve significantly in any of the treatment groups, consistent with iloprost’s prodifferentiation properties and lack of effect on NSCLC cell line proliferation (48).
Previous phase III chemoprevention trials were undertaken with agents without prior positive results in phase II trials, and some of these agents had not shown strong chemopreventive efficacy in animal models (49, 50). Iloprost is therefore an attractive agent for additional studies. Follow-up studies are warranted to better evaluate the duration of treatment, durability of response, optimal route of delivery (inhaled compared with oral) and reproducibility of findings in different populations of former smokers (such as survivors of early stage lung and head and neck cancer who are at extremely high risk for second primary lung tumors).
Although avoidance of tobacco use and smoking cessation clearly will have the greatest impact on lung cancer reduction, effective chemoprevention could have a major effect, particularly in the large population of former smokers. Manipulation of prostaglandin production distal to the COX enzymes may represent an attractive lung cancer chemopreventive strategy, and the results of our randomized phase II trial are supportive of continued studies of iloprost in former smokers.
Supplementary Material
Acknowledgments
Grant Support
This work was supported by the NIH SPOREs in Lung Cancer (P50 CA58187, P50 CA909949, P50 CA090388, P50 CA090440) and Department of Veterans Affairs Merit Review program (RLK and YEM).
Appendix
The Lung Cancer Biomarkers and Chemoprevention Consortium includes the following: Investigators: University of Colorado Cancer Center –Mary Jackson, Brandi Bagwell, and Vicky Meisinger; Vanderbilt University –David Carbone and Lynne Fenner; UCLA – Steven Dubinett, Brad Adams, Vivian Nguyen, Pratima Solanki, and Steve Kye; Dallas VA Medical Center – Jonathon Dowell, Laura Crittenden, and Stephanie Pearson; Mayo Clinic –Rochester – James Jett, David Midthun, and Cindy Fitting; University of Pittsburgh – Jill Siegfried, Chandra Belani, Joel Weissfeld, and Lois Knipling; Johns Hopkins University – Steven Baylin, Rex Yung, and Anti Gramatikova; Data Safety and Monitoring Board: A. Prochazka (Denver Veterans Affairs Medical Center); R. Greenberg (Fred Hutchinson Cancer Research Center); P. Raich (Denver Health Medical Center); and J. Murphy (National Jewish Health).
Footnotes
For the SPORE in Lung Cancer Iloprost study group and the Lung Cancer Biomarkers and Chemoprevention Consortium*
Note: Supplementary data for this article are available at Cancer Prevention Research Online (http://cancerprevres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
Honoraria from Speakers Bureau (Pfizer), and ownership interests (coinventor on a patent regarding the use of prostacycin agaonists).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Tong L, Spitz MR, Fueger JJ, Amos CA. Lung carcinoma in former smokers. Cancer. 1996;78:1004–10. doi: 10.1002/(SICI)1097-0142(19960901)78:5<1004::AID-CNCR10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Nicholson AG, Perry LJ, Cury PM, Jackson P, McCormick CM, Corrin B, et al. Reproducibility of the WHO/IASLC grading system for preinvasive squamous lesions of the bronchus: a study of interobserver and intraobserver variation. Histopathology. 2001;38:202–8. doi: 10.1046/j.1365-2559.2001.01078.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Shaughnessy JA, Kelloff GJ, Gordon GB, Dannenberg AJ, Hong WK, Fabian CJ, et al. Treatment and prevention of intraepithelial neoplasia: an important target for accelerated new agent development. Clin Cancer Res. 2002;8:314–46. [PubMed] [Google Scholar]
- 5.Dube C, Rostom A, Lewin G, Tsertsvadze A, Barrowman N, Code C, et al. The use of aspirin for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:365–75. doi: 10.7326/0003-4819-146-5-200703060-00009. [DOI] [PubMed] [Google Scholar]
- 6.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 7.Honn KV, Cicone B, Skoff A. Prostacyclin: a potent antimetastatic agent. Science. 1981;212:1270–2. doi: 10.1126/science.7015512. [DOI] [PubMed] [Google Scholar]
- 8.Schirner M, Schneider MR. Inhibition of metastasis by cicaprost in rats with established SMT2A mammary carcinoma growth. Cancer Detect Prev. 1997;21:44–50. [PubMed] [Google Scholar]
- 9.Heasley LE, Thaler S, Nicks M, Price B, Skorecki K, Nemenoff RA. Induction of cytosolic phospholipase A2 by oncogenic Ras in human non-small cell lung cancer. J Biol Chem. 1997;272:14501–4. doi: 10.1074/jbc.272.23.14501. [DOI] [PubMed] [Google Scholar]
- 10.Keith RL, Miller YE, Hoshikawa Y, Moore MD, Gesell TL, Gao B, et al. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 2002;62:734–40. [PubMed] [Google Scholar]
- 11.Keith RL, Miller YE, Hudish TM, Girod CE, Sotto-Santiago S, Franklin WA, et al. Pulmonary prostacyclin synthase overexpression chemoprevents tobacco smoke lung carcinogenesis in mice. Cancer Res. 2004;64:5897–904. doi: 10.1158/0008-5472.CAN-04-1070. [DOI] [PubMed] [Google Scholar]
- 12.Nemenoff R, Meyer AM, Hudish TM, Mozer AB, Snee A, Narumiya S, et al. Prostacyclin prevents murine lung cancer independent of the membrane receptor by activation of peroxisomal proliferator–activated receptor gamma. Cancer Prev Res. 2008;1:349–56. doi: 10.1158/1940-6207.CAPR-08-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaine SA, Meyer AM, Hurteau G, Wick M, Hankin JA, Murphy RC, et al. Targeted overexpression of mPGES-1 and elevated PGE2 production is not sufficient for lung tumorigenesis in mice. Carcinogenesis. 2005;26:209–17. doi: 10.1093/carcin/bgh302. [DOI] [PubMed] [Google Scholar]
- 14.Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med. 2002;8:289–93. doi: 10.1038/nm0302-289. [DOI] [PubMed] [Google Scholar]
- 15.Kelly K, Kittelson J, Franklin WA, Kennedy TC, Klein CE, Keith RL, et al. A randomized phase II chemoprevention trial of 13-CIS retinoic acid with or without alpha tocopherol or observation in subjects at high risk for lung cancer. Cancer Prev Res. 2009;2:440–9. doi: 10.1158/1940-6207.CAPR-08-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spira A, Beane J, Shah V, Liu G, Schembri F, Yang X, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci U S A. 2004;101:10143–8. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller YE, Blatchford P, Hyun DS, Keith RL, Kennedy TC, Wolf H, et al. Bronchial epithelial Ki-67 index is related to histology, smoking, and gender, but not lung cancer or chronic obstructive pulmonary disease. Cancer Epidemiol Biomarkers Prev. 2007;16:2425–31. doi: 10.1158/1055-9965.EPI-07-0220. [DOI] [PubMed] [Google Scholar]
- 18.Prindiville SA, Byers T, Hirsch FR, Franklin WA, Miller YE, Vu KO, et al. Sputum cytological atypia as a predictor of incident lung cancer in a cohort of heavy smokers with airflow obstruction. Cancer Epidemiol Biomarkers Prev. 2003;12:987–93. [PubMed] [Google Scholar]
- 19.Black CM, Halkier-Sorensen L, Belch JJ, Ullman S, Madhok R, Smit AJ, et al. Oral iloprost in Raynaud’s phenomenon secondary to systemic sclerosis: a multicentre, placebo-controlled, dose-comparison study. Br J Rheumatol. 1998;37:952–60. doi: 10.1093/rheumatology/37.9.952. [DOI] [PubMed] [Google Scholar]
- 20.Stearman RS, Dwyer-Nield L, Zerbe L, Blaine SA, Chan Z, Bunn PA, Jr, et al. Analysis of orthologous gene expression between human pulmonary adenocarcinoma and a carcinogen-induced murine model. Am J Pathol. 2005;167:1763–75. doi: 10.1016/S0002-9440(10)61257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997;94:4312–7. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta RA, Tan J, Krause WF, Geraci MW, Willson TM, Dey SK, et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci U S A. 2000;97:13275–80. doi: 10.1073/pnas.97.24.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, et al. Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 2007;25:1476–81. doi: 10.1200/JCO.2006.07.2777. [DOI] [PubMed] [Google Scholar]
- 24.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334:1150–5. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 25.van Zandwijk N, Dalesio O, Pastorino U, de Vries N, van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–86. doi: 10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 26.Lippman SM, Lee JJ, Karp DD, Vokes EE, Benner SE, Goodman GE, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001;93:605–18. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]
- 27.Kamangar F, Qiao YL, Yu B, Sun XD, Abnet CC, Fan JH, et al. Lung cancer chemoprevention: a randomized, double-blind trial in Linxian, China. Cancer Epidemiol Biomarkers Prev. 2006;15:1562–4. doi: 10.1158/1055-9965.EPI-06-0316. [DOI] [PubMed] [Google Scholar]
- 28.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: effects of base-line characteristics and study compliance. J Natl Cancer Inst. 1996;88:1560–70. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 29.The effect of vitamin E beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–35. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 30.Karp DD, Lee S, Wright GS, Johnson D, Johnston M, Goodman GE, et al. E5597: phase III trial of selenium supplementation in persons with resected stage I non-small cell lung cancer. ASCO 2010 Annual Meeting. 2010:Abstract CRA7004. [Google Scholar]
- 31.Lam S, LeRiche JC, McWilliams A, Macaulay C, Dyachkova Y, Szabo E, et al. A randomized phase IIb trial of pulmicort turbuhaler (budesonide) in people with dysplasia of the bronchial epithelium. Clin Cancer Res. 2004;10:6502–11. doi: 10.1158/1078-0432.CCR-04-0686. [DOI] [PubMed] [Google Scholar]
- 32.Lam S, Macaulay C, Le Riche JC, Dyachkova Y, Coldman A, Guillaud M, et al. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J Natl Cancer Inst. 2002;94:1001–9. doi: 10.1093/jnci/94.13.1001. [DOI] [PubMed] [Google Scholar]
- 33.Kurie JM, Lee JS, Khuri FR, Mao L, Morice RC, Lee JJ, et al. N-(4-hydroxyphenyl)retinamide in the chemoprevention of squamous metaplasia and dysplasia of the bronchial epithelium. Clin Cancer Res. 2000;6:2973–9. [PubMed] [Google Scholar]
- 34.Kim ES, Hong WK, Lee JJ, Mao L, Morice RC, Liu DD, et al. Biological activity of celecoxib in the bronchial epithelium of current and former smokers. Cancer Prev Res. 2010;3:148–59. doi: 10.1158/1940-6207.CAPR-09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hittelman WN, Liu DD, Kurie JM, Lotan R, Lee JS, Khuri F, et al. Proliferative changes in the bronchial epithelium of former smokers treated with retinoids. J Natl Cancer Inst. 2007;99:1603–12. doi: 10.1093/jnci/djm205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao JT, Fishbein MC, Adams B, Roth MD, Goodglick L, Hong L, et al. Celecoxib decreases Ki-67 proliferative index in active smokers. Clin Cancer Res. 2006;12:314–20. doi: 10.1158/1078-0432.CCR-05-1440. [DOI] [PubMed] [Google Scholar]
- 37.Lam S, McWilliams A, LeRiche J, Macaulay C, Wattenberg L, Szabo E. A phase I study of myoinositol for lung cancer chemoprevention. Cancer Epidemiol Biomarkers Prev. 2006;15:1526–31. doi: 10.1158/1055-9965.EPI-06-0128. [DOI] [PubMed] [Google Scholar]
- 38.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14. 5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 39.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–40. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee AK. Preinvasive lesions of the bronchus. J Thorac Oncol. 2009;4:545–51. doi: 10.1097/JTO.0b013e31819667bd. [DOI] [PubMed] [Google Scholar]
- 41.Breuer RH, Pasic A, Smit EF, van VE, Vonk NA, Risse EJ, et al. The natural course of preneoplastic lesions in bronchial epithelium. Clin Cancer Res. 2005;11:537–43. [PubMed] [Google Scholar]
- 42.Jonsson S, Varella-Garcia M, Miller YE, Wolf HJ, Byers T, Braudrick S, et al. Chromosomal aneusomy in bronchial high-grade lesions is associated with invasive lung cancer. Am J Respir Crit Care Med. 2008;177:342–7. doi: 10.1164/rccm.200708-1142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salaun M, Sesboue R, Moreno-Swirc S, Metayer J, Bota S, Bourguignon J, et al. Molecular predictive factors for progression of high-grade preinvasive bronchial lesions. Am J Respir Crit Care Med. 2008;177:880–6. doi: 10.1164/rccm.200704-598OC. [DOI] [PubMed] [Google Scholar]
- 44.Ponticiello A, Barra E, Giani U, Bocchino M, Sanduzzi A. P53 immunohistochemistry can identify bronchial dysplastic lesions proceeding to lung cancer: a prospective study. Eur Respir J. 2000;15:547–52. doi: 10.1034/j.1399-3003.2000.15.20.x. [DOI] [PubMed] [Google Scholar]
- 45.Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95:470–8. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 46.Tockman MS, Anthonisen NR, Wright EC, Donithan MG. Airways obstruction and the risk for lung cancer. Ann Intern Med. 1987;106:512–8. doi: 10.7326/0003-4819-106-4-512. [DOI] [PubMed] [Google Scholar]
- 47.Islam SS, Schottenfeld D. Declining FEV1 and chronic productive cough in cigarette smokers: a 25-year prospective study of lung cancer incidence in Tecumseh, Michigan. Cancer Epidemiol Biomarkers Prev. 1994;3:289–98. [PubMed] [Google Scholar]
- 48.Bren-Mattison Y, Van PV, Chan D, Winn R, Geraci MW, Nemenoff RA. Peroxisome proliferator-activated receptor-gamma (PPAR(gamma)) inhibits tumorigenesis by reversing the undifferentiated phenotype of metastatic non-small-cell lung cancer cells (NSCLC) Oncogene. 2005;24:1412–22. doi: 10.1038/sj.onc.1208333. [DOI] [PubMed] [Google Scholar]
- 49.Frasca JM, Garfinkel L. 13-cis retinoic acid and murine pulmonary adenomas: a preliminary report. Nutr Cancer. 1981;3:72–4. doi: 10.1080/01635588109513704. [DOI] [PubMed] [Google Scholar]
- 50.Mian TA, Theiss JC, Gesell TF. Effect of vitamin A on lung tumorigenesis in irradiated and unirradiated strain A mice. Cancer Lett. 1984;22:103–12. doi: 10.1016/0304-3835(84)90051-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.