Abstract
The endothelium is a source of molecules that either stimulate or inhibit the proliferation of the underlying smooth muscle cells. In the normal, healthy vessel wall the smooth muscle cells are quiescent, but they proliferate when damage to the endothelium occurs. The implication of such observations is that although the endothelium provides a source of growth factors, their stimulatory activity on smooth muscle cells is countered by endothelium-derived growth inhibitors. The inhibitors appear to comprise at least 3 distinct types of molecules: heparin/heparan sulfate; transforming growth factor beta; and nitric oxide. Each molecule inhibits growth of cultured smooth muscle cells by mechanisms that remain to be elucidated and are discussed in this communication. Heparin/heparan sulfate is the most thoroughly characterized of the 3, and has been used for clinical intervention to prevent restenosis. Transforming growth factor beta exhibits bimodal activity on growth, acting as a stimulant at low levels and as an inhibitor at elevated concentrations. Nitric oxide mediated vasorelaxation is dependent upon activation of soluble guanylate cyclase. Because elevation of cyclic guanosine monophosphate in smooth muscle cells depresses their proliferation, nitric oxide would appear to possess the properties necessary to inhibit vascular smooth muscle cell proliferation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assoian R. K., Sporn M. B. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986 Apr;102(4):1217–1223. doi: 10.1083/jcb.102.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battegay E. J., Raines E. W., Seifert R. A., Bowen-Pope D. F., Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990 Nov 2;63(3):515–524. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
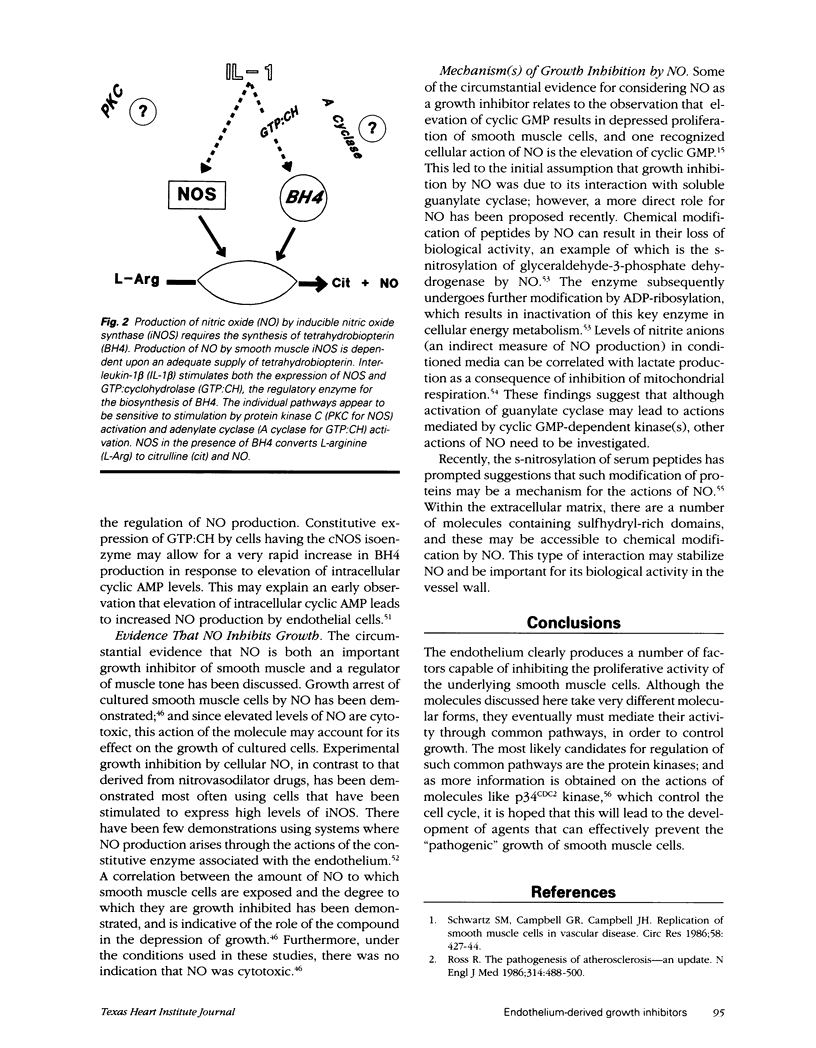
- Beasley D., Schwartz J. H., Brenner B. M. Interleukin 1 induces prolonged L-arginine-dependent cyclic guanosine monophosphate and nitrite production in rat vascular smooth muscle cells. J Clin Invest. 1991 Feb;87(2):602–608. doi: 10.1172/JCI115036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger C. M., Vanhoutte P. M. Cholera toxin augments the release of endothelium-derived relaxing factor evoked by bradykinin and the calcium ionophore A23187. Gen Pharmacol. 1992 Jan;23(1):27–31. doi: 10.1016/0306-3623(92)90042-i. [DOI] [PubMed] [Google Scholar]
- Brüne B., Lapetina E. G. Activation of a cytosolic ADP-ribosyltransferase by nitric oxide-generating agents. J Biol Chem. 1989 May 25;264(15):8455–8458. [PubMed] [Google Scholar]
- Campbell J. H., Campbell G. R. Endothelial cell influences on vascular smooth muscle phenotype. Annu Rev Physiol. 1986;48:295–306. doi: 10.1146/annurev.ph.48.030186.001455. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Wong K., Herman B., Hoover R. L., Albertini D. F., Wright T. C., Caleb B. L., Karnovsky M. J. Binding and internalization of heparin by vascular smooth muscle cells. J Cell Physiol. 1985 Jul;124(1):13–20. doi: 10.1002/jcp.1041240104. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Wright T. C., Karnovsky M. J. Regulation of vascular smooth muscle cell growth by heparin and heparan sulfates. Semin Thromb Hemost. 1987 Oct;13(4):489–503. doi: 10.1055/s-2007-1003525. [DOI] [PubMed] [Google Scholar]
- Chambard J. C., Pouysségur J. TGF-beta inhibits growth factor-induced DNA synthesis in hamster fibroblasts without affecting the early mitogenic events. J Cell Physiol. 1988 Apr;135(1):101–107. doi: 10.1002/jcp.1041350114. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Clowes M. M., Au Y. P., Reidy M. A., Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue-type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990 Jul;67(1):61–67. doi: 10.1161/01.res.67.1.61. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Karnowsky M. J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977 Feb 17;265(5595):625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- Fava R. A., McClure D. B. Fibronectin-associated transforming growth factor. J Cell Physiol. 1987 May;131(2):184–189. doi: 10.1002/jcp.1041310207. [DOI] [PubMed] [Google Scholar]
- Fritze L. M., Reilly C. F., Rosenberg R. D. An antiproliferative heparan sulfate species produced by postconfluent smooth muscle cells. J Cell Biol. 1985 Apr;100(4):1041–1049. doi: 10.1083/jcb.100.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y., Hansson G. K., Holme E. Interferon-gamma and tumor necrosis factor synergize to induce nitric oxide production and inhibit mitochondrial respiration in vascular smooth muscle cells. Circ Res. 1992 Nov;71(5):1268–1276. doi: 10.1161/01.res.71.5.1268. [DOI] [PubMed] [Google Scholar]
- Goodman L. V., Majack R. A. Vascular smooth muscle cells express distinct transforming growth factor-beta receptor phenotypes as a function of cell density in culture. J Biol Chem. 1989 Mar 25;264(9):5241–5244. [PubMed] [Google Scholar]
- Gross S. S., Levi R. Tetrahydrobiopterin synthesis. An absolute requirement for cytokine-induced nitric oxide generation by vascular smooth muscle. J Biol Chem. 1992 Dec 25;267(36):25722–25729. [PubMed] [Google Scholar]
- Herman I. M., Castellot J. J., Jr Regulation of vascular smooth muscle cell growth by endothelial-synthesized extracellular matrices. Arteriosclerosis. 1987 Sep-Oct;7(5):463–469. doi: 10.1161/01.atv.7.5.463. [DOI] [PubMed] [Google Scholar]
- Howe P. H., Cunningham M. R., Leof E. B. Distinct pathways regulate transforming growth factor beta 1-stimulated proto-oncogene and extracellular matrix gene expression. J Cell Physiol. 1990 Jan;142(1):39–45. doi: 10.1002/jcp.1041420106. [DOI] [PubMed] [Google Scholar]
- Joly G. A., Schini V. B., Vanhoutte P. M. Balloon injury and interleukin-1 beta induce nitric oxide synthase activity in rat carotid arteries. Circ Res. 1992 Aug;71(2):331–338. doi: 10.1161/01.res.71.2.331. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Somlyo A. V., Somlyo A. P. Heparin inhibits the inositol 1,4,5-trisphosphate-dependent, but not the independent, calcium release induced by guanine nucleotide in vascular smooth muscle. Biochem Biophys Res Commun. 1988 Jun 16;153(2):625–631. doi: 10.1016/s0006-291x(88)81141-0. [DOI] [PubMed] [Google Scholar]
- Laiho M., Weis F. M., Boyd F. T., Ignotz R. A., Massagué J. Responsiveness to transforming growth factor-beta (TGF-beta) restored by genetic complementation between cells defective in TGF-beta receptors I and II. J Biol Chem. 1991 May 15;266(14):9108–9112. [PubMed] [Google Scholar]
- Lindner V., Olson N. E., Clowes A. W., Reidy M. A. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest. 1992 Nov;90(5):2044–2049. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons R. M., Moses H. L. Transforming growth factors and the regulation of cell proliferation. Eur J Biochem. 1990 Feb 14;187(3):467–473. doi: 10.1111/j.1432-1033.1990.tb15327.x. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Du B. Transforming growth factor-beta 1 is a heparin-binding protein: identification of putative heparin-binding regions and isolation of heparins with varying affinity for TGF-beta 1. J Cell Physiol. 1992 Aug;152(2):430–440. doi: 10.1002/jcp.1041520226. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Schultz-Cherry S., Hök M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992 Feb;3(2):181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Geisterfer A. A., Yang Y. W., Komoriya A. Transforming growth factor-beta-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol. 1988 Aug;107(2):771–780. doi: 10.1083/jcb.107.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R. Proteoglycans in health and disease: structures and functions. Biochem J. 1986 May 15;236(1):1–14. doi: 10.1042/bj2360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Reilly C. F., Fritze L. M., Rosenberg R. D. Antiproliferative effects of heparin on vascular smooth muscle cells are reversed by epidermal growth factor. J Cell Physiol. 1987 May;131(2):149–157. doi: 10.1002/jcp.1041310203. [DOI] [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Baur U., Bürgin M., Bühler F. R. Decreased susceptibility of cultured smooth muscle cells from SHR rats to growth inhibition by heparin. J Cell Physiol. 1989 Jan;138(1):137–144. doi: 10.1002/jcp.1041380119. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Schini V. B., Junquero D. C., Scott-Burden T., Vanhoutte P. M. Interleukin-1 beta induces the production of an L-arginine-derived relaxing factor from cultured smooth muscle cells from rat aorta. Biochem Biophys Res Commun. 1991 Apr 15;176(1):114–121. doi: 10.1016/0006-291x(91)90897-g. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Buddecke E. Changes in heparan sulfate structure during transition from the proliferating to the non-dividing state of cultured arterial smooth muscle cells. Eur J Cell Biol. 1990 Aug;52(2):229–235. [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Heimark R. L., Majesky M. W. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990 Oct;70(4):1177–1209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Bühler F. R. Regulation of smooth muscle proliferative phenotype by heparinoid--matrix interactions. Trends Pharmacol Sci. 1988 Mar;9(3):94–98. doi: 10.1016/0165-6147(88)90175-7. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Bürgin M., Bühler F. R. Extracellular matrix: differential influence on growth and biosynthesis patterns of vascular smooth muscle cells from SHR and WKY rats. J Cell Physiol. 1989 Nov;141(2):267–274. doi: 10.1002/jcp.1041410206. [DOI] [PubMed] [Google Scholar]
- Scott-Burden T., Resink T. J., Hahn A. W., Bühler F. R. Induction of thrombospondin expression in vascular smooth muscle cells by angiotensin II. J Cardiovasc Pharmacol. 1990;16 (Suppl 7):S17–S20. [PubMed] [Google Scholar]
- Scott-Burden T., Schini V. B., Elizondo E., Junquero D. C., Vanhoutte P. M. Platelet-derived growth factor suppresses and fibroblast growth factor enhances cytokine-induced production of nitric oxide by cultured smooth muscle cells. Effects on cell proliferation. Circ Res. 1992 Nov;71(5):1088–1100. doi: 10.1161/01.res.71.5.1088. [DOI] [PubMed] [Google Scholar]
- Shen R. S., Zhang Y. X., Perez-Polo J. R. Regulation of GTP cyclohydrolase I and dihydropteridine reductase in rat pheochromocytoma PC 12 cells. J Enzyme Inhib. 1989;3(2):119–126. doi: 10.3109/14756368909030370. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Yamashita U., Chedid M., Mizel S. B. Cyclic AMP--an intracellular second messenger for interleukin 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8201–8205. doi: 10.1073/pnas.85.21.8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Jaraki O., Osborne J., Simon D. I., Keaney J., Vita J., Singel D., Valeri C. R., Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffer G. A., Owens G. K. Angiotensin II-induced mitogenesis of spontaneously hypertensive rat-derived cultured smooth muscle cells is dependent on autocrine production of transforming growth factor-beta. Circ Res. 1992 Apr;70(4):820–828. doi: 10.1161/01.res.70.4.820. [DOI] [PubMed] [Google Scholar]


