Abstract
A key requirement for encoding the auditory environment is the ability to dynamically alter cochlear sensitivity. However, merely attaining a steady state of maximal sensitivity is not a viable solution since the sensory cells and ganglion cells of the cochlea are prone to damage following exposure to loud sound. Most often, such damage is via initial metabolic insult that can lead to cellular death. Thus, establishing the highest sensitivity must be balanced with protection against cellular metabolic damage that can lead to loss of hair cells and ganglion cells, resulting in loss of frequency representation. While feedback mechanisms are known to exist in the cochlea that alter sensitivity, they respond only after stimulus encoding, allowing potentially damaging sounds to impact the inner ear at times coincident with increased sensitivity. Thus, questions remain concerning the endogenous signaling systems involved in dynamic modulation of cochlear sensitivity and protection against metabolic stress. Understanding endogenous signaling systems involved in cochlear protection may lead to new strategies and therapies for prevention of cochlear damage and consequent hearing loss. We have recently discovered a novel cochlear signaling system that is molecularly equivalent to the classic hypothalamic-pituitary-adrenal (HPA) axis. This cochlear HPA-equivalent system functions to balance auditory sensitivity and susceptibility to noise-induced hearing loss, and also protects against cellular metabolic insults resulting from exposures to ototoxic drugs. We review the anatomy, physiology, and cellular signaling of this system, and compare it to similar signaling in other organs/tissues of the body.
Keywords: cochlea, corticotropin releasing factor (CRF), hypothalamic-pituitary-adrenal axis (HPA axis), noise-induced hearing loss (NIHL), hair cell, homeostatic control
INTRODUCTION
The cochlea is the mammalian peripheral organ responsible for detecting auditory stimuli. It is not new to think of the brain as a computational system, a dynamical system that encodes and stores information representing the past and present external world and anticipates future states. However, it is less often the case that peripheral sensory structures are considered as computational systems. Nonetheless, one may argue that peripheral processing of auditory stimuli is a complex, active computational process that depends not only on the function of numerous classes of cells, but also on feedback originating from various levels. In general, these classes of cells must be able to establish a specific ionic environment conducive to encoding auditory stimuli, alter the mechanical compliance of the inner ear, and transform encoded stimuli into a neural code recognizable by the brain. Broadly classified, these cells fall into two types: the sensory cells, known as hair cells; and support cells, responsible for numerous aspects of cochlear function and homeostasis. Feedback is used for “anti-masking”— the extraction of salient signals in a noisy background— as well as for controlling sensitivity. While the cochlea is clearly a computational system that must integrate past and present experience to prepare for future challenges, knowledge of the various elements important for cochlear-based computation, especially concerning cell-to-cell communication, remains incomplete.
Sensitive hearing is critical for evolutionary success because of its roles in general communication, hunting prey, escaping predators, and mating. Thus, the ability to establish the most sensitive hearing possible, either by controlling the computational elements of the inner ear, or generating filtering strategies useful for extracting information from a noisy background, should be under heavy genetic selection through evolution. Yet, the cochlea faces a significant biological problem when establishing very sensitive hearing that effectively limits the duration of maximal sensitivity. Hair cells are prone to damage and loss following exposure to constant moderate level sounds via metabolic insults (free radical formation, etc.), impact-like loud sounds, and numerous ototoxic compounds. Additionally problematic for mammals is that, unlike the case for birds and reptiles, hair cells do not regenerate, meaning that damage to or loss of hair cells translates to a permanent loss of frequency representation. The mammalian system must manage a balance between a need for the greatest sensitivity while at the same time not crossing into the realm of metabolic insult that could result in loss of hair cells. By establishing dynamic control over its own sensitivity, the cochlea ensures that loud sounds are encoded at an operating point that is less than its peak of sensitivity, thereby minimizing metabolic and physical insults, and preserving cochlear structure and function.
When sensitivity cannot be adequately controlled, or when the environment simply exposes the cochlea to high levels of sound, noise-induced hearing loss (NIHL) occurs. In the case of humans, the modern industrial environment represents a significant threat to the maintenance of normal hearing. Our environment constantly bombards us with high intensity, potentially damaging, sound. Noise pollution is growing, and becoming more “personal” as in canal earphones become ever more popular. Not surprisingly, the numbers of cases of NIHL also continues to grow (Niskar et al., 2001).
Because mammalian hair cells do not regenerate, there must be an endogenous (local cochlear) mechanism(s) that helps establish and adjust cochlear sensitivity as a mechanism to combat NIHL and general metabolic stress. While a neural feedback mechanism between the lower brainstem and the hair cells and ganglion cells (the olivocochlear system) has been recognized since the 1940’s (Rasmussen, 1942; Rasmussen, 1955), this system is a reactive servitor, and probably functions over a relatively short time frame. Indeed, protection against noise trauma may not even be the evolved function for the olivocochlear system (Kirk and Smith, 2003), although there is ample experimental evidence to support its protective role under experimental conditions (Maison et al., 2002). While the olivocochlear system may be sufficient in protection against NIHL if one can escape the overly loud environment, or when the loud sound is transient, it may not function optimally under prolonged exposures or at certain frequencies of auditory stimulation. Other mechanisms involving release of small active compounds, such as ATP, within the cochlea are also known to alter cochlear sensitivity (Housley et al., 1999; Housley et al., 2002; Housley et al., 2006), and likely represent a faster solution to adjusting sensitivity, but knowledge regarding regulation of this system is incomplete. Thus, open questions remain pertaining to how the cochlea dynamically modulates its own sensitivity: what are the complete molecular signaling systems involved in generating and modulating cochlear sensitivity, and are there other signaling systems expressed in the cochlea that respond to auditory environments to protect against hearing loss? Though progress has been made in identifying various manipulations and treatments that confer at least partial protection against NIHL (Pirvola et al., 2000; McFadden et al., 2001; Darrat et al., 2007; Monge Naldi et al., 2009), mechanisms protecting the cochlea against its daily metabolic and physical stress remain less clear. Since small metabolic insults accumulate over time to culminate in greater damage and hearing loss (Kujawa and Liberman, 2006, 2009), it is possible that the cochlea expresses an endogenous protective signaling system within its own cellular ensemble. An investigation into cochlear signaling systems involved in maintaining homeostasis and combating cellular stress seems warranted. Understanding such endogenous sources of auditory protection may also lead to novel targeted therapeutic strategies for prevention of cochlear damage and consequent hearing loss.
In this review, we summarize our recent work identifying and characterizing a cochlear peptidergic signaling system involved at various dynamic check-points of cochlear processing, serving to modulate cochlear sensitivity, and thereby its computational state. Beginning with our work demonstrating the expression of urocortin (Ucn1), a corticotropin releasing factor (CRF) family peptide, and the CRF receptors CRFR1 and CRFR2, in the rodent cochlea (Vetter et al., 2002), we have gone on to show that not only is CRF also expressed in the cochlea, but the cochlea possesses an entire CRF-associated signaling system molecularly equivalent to the hypothalamic-pituitary-adrenal (HPA) axis (Graham and Vetter, 2011) as well. Since activity of the classic CRF-induced HPA axis maintains system-wide homeostasis in response to stress, we have investigated whether this system plays a role in modulating the cochlea via an influence over various factors, including afferent function (i.e. setting sensitivity) (Graham et al., 2010; Graham and Vetter, 2011) and cellular response to metabolic insult (Basappa et al., 2010), thereby protecting the cochlea from damaging exposures to loud sounds. This review will cover the cochlear HPA-equivalent signaling system, and suggest possible functions and mechanisms of action of this system based on what is known of similar systems expressed by other organs/tissues of the body. In general, we propose that the cochlear HPA-equivalent signaling system functions within the cochlea as an independent homeostatic protective signaling system capable of regulating sensitivity and susceptibility to noise induced hearing loss. Cochlear CRF-based signaling systems act via the two CRF receptors. Activation of CRF receptors serve multiple functions: 1) developmentally, they dictate hair cell growth/maturation, afferent fiber targeting, and synapse formation at the inner hair cell; 2) are involved in modulating neural signaling, for example by regulation of glutamatergic signaling between hair cells and ganglion cells; 3) are involved in support cell-hair cell interactions, for example via modulation of purinergic communication that ultimately impacts the driving potential of the endolymph, thereby also modulating receptor potential generation in hair cells; and 4) promote cell survival/anti-apoptotic signaling in the face of metabolic insult. We will cover these major points and how they may be similar to mechanisms of action of CRF signaling in other tissues of the body.
THE COCHLEA AND EFFECTS OF NOISE STRESS
The mammalian cochlea- cells
The mammalian cochlea lies within the inner ear and contains hair cells, which are responsible for encoding auditory stimuli into the neural signals ultimately sent to the brain. In addition to the sensory hair cells, the cochlea is composed of numerous populations of “support” cells. The role of the support cells appears to be broad, and may include nutritive and metabolic support as well as simple structural support. Data suggest that a number of different support cell types located lateral to the sensory hair cells function to recycle ions back into the specialized compartment (scala media) above the hair cells, thus controlling the ionic composition of the fluid (endolymph) bathing the apical portions of the hair cells. The endolymph carries the electrical potential that allows sensory transduction to occur in the hair cells. The exact roles of various support cell populations are only recently being more vigorously investigated. While specific cell types will be described as required, a comprehensive review of all cell types of the cochlea is beyond the scope of this review. Excellent reviews on these subjects have been previously published (Lim, 1986; Slepecky, 1996; Raphael and Altschuler, 2003; Patuzzi, 2011), to which the reader is directed for more extensive information.
The mammalian cochlea- functional aspects
In the mammalian cochlea, sound detection is mediated by vibration of the basilar membrane, upon which sits the organ of Corti. Within the organ of Corti lie two populations of hair cells, the inner hair cells and outer hair cells, in addition to numerous populations of support cells immediately juxtaposed or in close association with the hair cells. The inner hair cells are connected to approximately 95% of the spiral ganglion cells, and therefore represent the primary afferent transducers converting mechanical sound stimuli to the neural code that will travel to the brain. The outer hair cells are electromotile and their motion can amplify vibration of the underlying basilar membrane in response to sound. Consequently, the outer hair cells collectively comprise the cochlear amplifier (Dallos, 1985, 1992; Liberman et al., 2002). The process of cellular-based amplification enhances the sensitivity of the cochlea and makes it an excellent sound detector, endowing it with the ability to detect very low intensity events. This directly leads to its ability to detect salient signals in a noisy background. In order to encode ambient sound, hair cells must be able to detect absence as well as presence of sound. This is generally accomplished by allowing the hair cells to remain spontaneously active. Thus, absence of sound will be encoded by the basal activity state of the hair cells rather than the complete absence of neurotransmitter release. The spontaneous activity of the hair cells demands that these cells be well equipped for handling potential metabolic insult, since any lapse in such protective signaling systems may leave the hair cell population at risk for damage and ultimate loss of frequency representation by the cochlea.
Noise-induced hearing loss (NIHL)
The cochlea is constantly bombarded with sound in modern industrial societies due to ever-growing levels of noise pollution. The result is an increasing prevalence of noise induced hearing loss (NIHL). The National Institute of Deafness and Communication Disorders estimates that 15 percent or 26 million Americans between the ages of 20 and 69 have some degree of high-frequency hearing loss due to exposure to loud noise at work or in recreation (www.nidcd.nih.gov). According to a report published by the National Institute of Occupational Safety and Health in 1998, thirty million Americans are exposed to hazardous noise levels at work (DHHS NIOSH pub. 96–115). While efforts have advanced to reduce noise in the work place, and NIOSH has tightened the criteria of acceptable workplace exposure from 90 dB to 85 dB, a new population of at-risk individuals has begun garnering more attention. A growing concern is the prevalence of hearing loss in children and young adults seemingly resulting from recreational activity, particularly use of portable music players. Results from the third National Health and Nutrition Examination Survey (NHANES) conducted by the Center for Disease Control between 1988 and 1994 revealed that 12.5% of the children investigated, representing roughly 5.2 million children between 6 and 19, displayed some degree of NIHL (Niskar et al., 2001). The motivation for preventing hearing loss stems from research demonstrating that individuals with hearing loss experience reduced social interaction, feelings of isolation and exclusion, depression, and even cognitive impairment (Daniel, 2007).
Exposure to loud sound can induce either a temporary or a permanent hearing threshold shift (TTS or PTS, respectively). Temporary threshold shifts are commonly experienced after exposure to excessive noise such as that experienced at a rock concert or at a nightclub. In these circumstances, hearing sensitivity decreases following the exposure but then recovers to normal levels within days or even hours. The mechanisms underlying TTS remain unclear but one theory proposes that TTS involves a temporary lapse in stability of the organ of Corti, which supports both the inner and outer hair cells. Support cells surrounding the sensory hair cells impart structural support to the organ of Corti. In response to loud sound, the stiff, microtubule-filled pillar cells buckle, causing the height of the organ of Corti to decrease and the stereocilia of the outer hair cells to uncouple from the overlying tectorial membrane (Nordmann et al., 2000). Because stereocilia deflection is required to stimulate the outer hair cells, the result is a relatively short-lived hearing impairment due to dysfunction of the cochlear amplifier. Despite the recovery in hearing thresholds following TTS, it is agreed that repeated exposures to sounds causing TTS can eventually lead to PTS. Intriguingly, new evidence suggests that even a single exposure to sound eliciting TTS can set the stage for permanent damage later in life. In one study, mice were exposed to traumatizing sound and then allowed to recover. Even after full recovery of hearing thresholds, loss of post-synaptic afferent terminals was observed over the short term followed by loss of afferent spiral ganglion neurons over the long term. Though this cell loss did not elicit a permanent change in hearing threshold, it likely entails functional consequences including difficulties with hearing in noisy environments and tinnitus (Kujawa and Liberman, 2009). Therefore, even a seemingly harmless noise producing only a temporary decrease in auditory sensitivity has the potential for long-term functional consequences.
Unlike TTS that results from reversible changes in organ of Corti structure, PTS results from overt loss of sensory hair cells or significant, irrecoverable stereocilia damage. The outer hair cells are most susceptible followed by inner hair cells and their innervation (Fredelius et al., 1988). Typically, hair cell loss does not result from exposure to a single loud sound, but from a cumulative effect of repeated exposures. However, extremely intense sounds such as the impulse noise generated from a gunshot or firecracker can elicit hair cell loss and PTS with a single exposure. The permanence of this type of hearing loss arises from the fact that mammalian hair cells do not regenerate.
Biochemical changes in the cochlea lead to hair cell destruction
Repeated exposure to loud sounds causes both apoptosis and necrosis of hair cells in the cochlea (Hu et al., 2002; Le Prell et al., 2007). Accumulating evidence implicates generation of reactive oxygen species (ROS), and associated free radicals, as a major event leading to cochlear cell loss. These ROS are produced as a result of intense metabolic activity, and their levels increase as much as four times within hours of noise exposure (Ohlemiller et al., 1999). Excess ROS production can activate caspase signaling cascades and initiate apoptosis, thereby leading to cell loss. Lipid peroxidation represents one process contributing to ROS formation and studies show that the lipid peroxidation byproduct, 8-isoprostane, increases as much as ten-fold in cochlear tissues following noise exposure (Ohinata et al., 2000). This isoprostane byproduct causes vasoconstriction and is thought to contribute to, if not cause, reduced cochlear blood flow in response to noise (Miller et al., 2003). Reduced blood flow can in turn cause ischemia and enhance the production of more free radicals, thereby perpetuating the destructive processes initiated by noise. Importantly, ROS formation can continue to increase for sustained periods following the insult. For example, ROS formation in the cochlea peaks seven to ten days following noise exposure (Yamashita et al., 2004), a finding that correlates well with the extended time frame over which hair cell loss and neural damage occurs after insult.
Although the best method of preventing NIHL is to avoid exposure to loud sounds, this is often difficult given the level of noise pollution in work and recreation environments. Sustained noise-induced biochemical changes such as increased ROS production and lipid peroxidation, suggest that intervention is possible even after exposure occurs. Furthermore, recent work suggests that similar biochemical changes may underlie age-related hearing loss, a significant problem affecting the vast majority of elderly people. Therapies designed to prevent NIHL may also prove useful for preventing age related hearing loss. Based on the evidence demonstrating that free radical production contributes to noise damage, investigators have sought to prevent cochlear damage and NIHL in animal models using a variety of anti-oxidative/trophic treatments individually or in combination. Treatments have included administration of glutathione precursors such as glutathione monoethyl ester (GSHE), superoxide dismutase-polyethylene glycol, and U74389F, a lipid peroxidation inhibitor. Also, increased dietary intake of antioxidant vitamins such as vitamin A and vitamin E has been shown to reduce NIHL (Le Prell et al., 2007). However, these treatments have achieved limited success, most likely due to the fact that noise injury is a complex process in which ROS production comprises only one part. Identifying an endogenous stress response system within the cochlea that mounts a more coordinated, multifaceted defense against noise injury may be crucial for developing more effective strategies to prevent NIHL and similar cochlear dysfunction.
ENDOGENOUS PROTECTION AGAINST NIHL
Susceptibility to noise damage varies from one individual to another, with some individuals showing greater resistance to NIHL than others. Therefore, endogenous factors must contribute to auditory protection and confer resistance to damage. Few sources of endogenous protection have been described, but several studies implicate the olivocochlear efferent system in prevention of NIHL.
The olivocochlear (auditory efferent) system
The olivocochlear efferent system is one of the most well characterized sources of auditory protection. It is comprised of two fiber systems, the medial olivocochlear system (MOCS) and the lateral olivocochlear system (LOCS), named according to their point of origin in the brainstem. The synaptic targets of these fiber systems are distinct, with the LOCS synapsing on afferent dendrites below the inner hair cells and the MOCS synapsing directly on the outer hair cells. The MOCS fibers contain γ-aminobutyric acid (GABA) and acetylcholine (ACh), and a clear link has been drawn between the level of cholinergic activity at the MOC synapse and the amount of auditory protection conveyed. Studies demonstrate increased resistance to noise damage in mice that either over-express the alpha 9 subunit of the nicotinic acetylcholine receptor (nAChR) or express a point mutation in the subunit that renders the nAChR more active due to hypersensitivity to acetylcholine and slow desensitization (Maison et al., 2002; Taranda et al., 2009). Thus individual variation in the activity of the MOC system could contribute to the observed variability in noise susceptibility, as indicated by a study in guinea pigs where the strength of the MOC reflex to incoming sound was a good indicator of resistance to NIHL (Maison and Liberman, 2000). Nonetheless, despite the ability of this system to convey noise protection, it is unlikely that it evolved for this purpose. The MOC system is present in several mammalian species and yet, for many of these species, the ambient noise encountered in their natural environment rarely reaches levels comparable to those shown to elicit MOC protective effects in the lab (Kirk and Smith, 2003). Therefore, it is more likely that protection from intense noise is a fortuitous side effect of the MOC reflex, and its actual evolutionary role in hearing more likely involves detection of salient signals (including speech) in a noisy background (Dewson, 1967; Dewson, 1968; Micheyl and Collet, 1996; de Boer and Thornton, 2008) and facilitates sound localization in noise (Andeol et al., 2011). Clear identification of a system that has evolved to maintain cochlear homeostasis in the face of everyday physical and metabolic demands is still lacking. Such a defense system should work constitutively to modulate acoustic sensitivity based on past and present experience rather than functioning as a reactive feedback mechanism that only engages upon intense stimulation. Indeed, conditioning experiments that demonstrate toughening of the cochlea against noise insult following previous exposure to more moderate sound stimuli reveal that such an integrative defense system exists and, intriguingly, that systemic stress hormones appear to play an important role.
The systemic HPA axis-induced stress response and auditory protection
The impact of the systemic stress response on auditory function and protection has been appreciated for decades. The systemic stress response involves a chain of events collectively coordinated via the hypothalamic-pituitary-adrenal (HPA) axis. In response to stress perceived across a variety of sensory systems, the hypothalamus releases corticotropin-releasing factor (CRF). CRF travels through the portal blood circulation of the hypophyseal stalk to the pituitary where it binds to its receptor, CRFR1, and initiates production of adrenocorticotropic hormone (ACTH) via proteolytic cleavage of its precursor pro-opiomelanocortin (POMC). ACTH is released into the systemic circulation and travels to the adrenal cortex where it binds to its receptor, the melanocortin 2 receptor (MC2R), and stimulates synthesis and release of glucocorticoids– cortisol in humans, corticosterone in rodents (Stevens and White, 2010). Glucocorticoids exert three major effects: 1) they stimulate release of adrenaline from adrenal chromaffin cells; 2) they stimulate gluconeogenesis to supply cellular fuel for a ‘fight or flight’ response; and 3) they suppress immune response and inflammation.
A number of controlled experiments and clinical investigations have demonstrated roles for glucocorticoids in auditory function and protection. As early as the 1960s, clinical studies revealed that patients with adrenocorticosteroid deficiency presented with greater auditory sensitivity compared to normal volunteers (Henkin et al., 1967). Moreover, treatment with prednisone brought hearing thresholds up to normal levels, demonstrating that the observed hypersensitivity was related to levels of circulating corticosteroids. Similarly other studies revealed that patients with Meniere’s disease, an inner ear disorder affecting both cochlear and vestibular function, exhibited low levels of circulating corticosteroids. Administration of adrenal cortex extract improved auditory function in these patients (Powers, 1972). One sphere in which steroids have an accepted and widespread use is in treatment of idiopathic sudden sensorineural hearing loss (Kuhn et al., 2011; Rauch et al., 2011). Exogenously administered synthetic glucocorticoids also protect the cochlea against damage induced by ototoxic drugs, acoustic trauma, and ischemia/reperfusion injury (Himeno et al., 2002; Takemura et al., 2004; Tabuchi et al., 2006). Given the transcriptional role of glucocorticoid receptors, several molecular changes likely underlie the observed protection. In particular, experiments point to enhanced biosynthesis of glutathione, reduced secretion of tumor necrosis factor induced cytokines, and altered expression of apoptotic genes as some of the changes likely to combat the free radical damage and apoptosis associated with noise- and chemically-induced cochlear damage (Maeda et al., 2005; Nagashima and Ogita, 2006; Hoang Dinh et al., 2009).
Evidence that endogenous glucocorticoid activity confers auditory protection came from studies investigating the role of the systemic stress axis in sound conditioning. Sound conditioning refers to a phenomenon whereby pre-exposure to sound stimuli toughens ears against subsequent noise trauma. Initial experiments used high-intensity sound stimuli to evoke protection against further trauma. These experiments produced variable results, largely due to differences in protocol. However, other experiments demonstrated that high-intensity conditioning stimuli were not required for auditory toughening (Canlon et al., 1988; Canlon and Fransson, 1995; Yoshida and Liberman, 2000). Instead, exposure to moderate level or low level sound stimuli, even of short duration, could confer protection against acoustic insult. These studies suggested that toughening did not result from exposure to multiple insults, but rather, from adaptive processes set in motion by a more basic response to sound.
That sound activates the systemic stress response has been acknowledged for years (Henkin and Knigge, 1963). In fact, even when not consciously perceived, as in sleep, sound exposure increases circulating stress hormones (Spreng, 2004). Studies suggest that sound-induced systemic stress may underlie some of the maladaptive consequences of constant noise exposure in the workplace such as elevated blood pressure and heart rate (Lusk et al.). Thus, it is possible that activation of the systemic stress axis contributes to sound conditioning-mediated protection. The first experiments to indicate that non-auditory induction of the stress axis can induce auditory protection revealed that mice subjected to a fifteen minute heat stress exhibited a greater resistance to threshold shifts following acoustic insult than did non-stressed mice (Yoshida et al., 1999). Restraint stress also produced auditory protection that directly correlated to levels of circulating corticosterone (Wang and Liberman, 2002). If the traumatizing stimulus was presented after corticosterone levels returned to normal, protection was no longer achieved. Thus, systemic corticosterone appeared to be an important component of acquired resistance to NIHL. A causal link was established by experiments that showed sound conditioning no longer yielded protection if HPA activation was disrupted via adrenalectomy or administration of glucocorticoid synthesis inhibitors and receptor antagonists (Tahera et al., 2007). Most recently, a corticosteroid-responsive transcription factor, promyelocytic leukemia zinc-finger protein (PLZF), was shown to mediate cochlear protection induced by acoustic conditioning stimuli and restraint stress (Peppi et al., 2011). In PLZF null mice, auditory protection was not generated by typical cochlear conditioning paradigms. Finally, an investigation into the role of the β2 nicotinic receptor subunit in auditory processing revealed that older β2 null mice, but not younger null mice, expressed higher than normal corticosterone. The increased level of corticosterone in the older null mice was found to contribute to a significant protection against noise-induced hearing loss (Shen et al., 2011). Thus, these studies all implicated HPA activation, and more specifically, circulating glucocorticoids, as an endogenous source of cochlear protection, particularly the adaptations leading to acquired resistance against NIHL.
Despite the clear contribution of the systemic stress axis to auditory protection, findings from other experiments challenged the role of systemic HPA activation as the sole mechanism involved in acquired (condition-induced) resistance. In particular, a study designed to dissect out systemic versus local contributions revealed that animals undergoing sound conditioning with one ear plugged and the other left open to the sound stimuli produced unilateral protection- only the ear left open to the preconditioning stimuli presented with resistance to auditory threshold elevation (Yamasoba et al., 1999). This finding suggested that systemic responses could not account for conditioning-mediated protection - if systemic responses were involved, both ears should have been protected even if acoustic exposure was limited to one ear. Instead, local adaptations must be responsible for acquired resistance. Could local adaptations within the cochlea share aspects of cell:cell signaling with classic HPA activation? One question in particular arises: are the same hormones involved in systemic stress response expressed within the cochlea to provide a local stress response system?
BEYOND THE CLASSIC HPA AXIS: CRF PEPTIDE SIGNALING THROUGHOUT THE BODY
Corticotropin releasing factor (CRF), the quintessential stress hormone initiating the systemic stress response, is a 41 amino acid peptide that shares homology with three other peptides in mammals: Ucn1, Ucn2 (stresscopin-related peptide), and Ucn3 (stresscopin). All ligands of the CRF family bind to the same two G- protein-coupled receptors, CRFR1 and CRFR2, albeit with varying affinities. CRF has a high affinity for CRFR1 and a lower affinity for CRFR2, Ucn1 has an equal affinity for both receptors, and Ucn2 and Ucn3 bind CRFR2 exclusively (Grammatopoulos and Chrousos, 2002). Despite the well-characterized role of CRF signaling in HPA activation, CRF, the urocortins, and the CRF receptors are abundantly expressed outside of the HPA axis both centrally and peripherally. CRF receptors have been reported centrally in the amygdala, hippocampus, hypothalamus, lateral septum, bed nucleus of the stria terminalis, and the cerebellum (Hauger et al., 2006), and peripherally in the cardiovascular system, the gastro-intestinal tract, the reproductive organs, the kidneys, the liver, and the skin (Zmijewski and Slominski, 2010). The CRF signaling system has been implicated in dendritic development in the cerebellum and hippocampus (Chen et al., 2004), ischemia/reperfusion injury of cardiac tissue (Kuizon et al., 2009), and psychiatric conditions including addiction, depression, anxiety, and post-traumatic stress disorder (Rainnie et al., 2004; Koob, 2010). Abnormalities in expression levels of CRF have also been implicated in neurological developmental disorders such as Rett Syndrome (McGill et al., 2006).
Evidence that extra-hypothalamic sources of CRF signaling can mediate local stress response comes from numerous studies of the skin demonstrating expression of a fully functional HPA equivalent signaling system that responds to local stress independent of systemic HPA activation. Initial experiments revealed local synthesis of POMC (the pituitary hormone in the hypothalamic-pituitary-adrenal axis) in skin melanocytes and modulation of POMC expression by UVB radiation (Slominski, 1991; Slominski et al., 1995). CRF and the CRF receptors are also expressed in skin cells, and their expression can also be induced by UVB exposure, suggesting that together these peptides may be involved in a stress response signaling pathway similar to the systemic HPA axis (Slominski et al., 1995; Slominski et al., 1996; Slominski et al., 1999; Slominski et al., 2005). Finally experiments in cultured human hair follicles revealed that this local stress axis is fully functional beginning with CRF stimulation, induction of POMC expression, production of ACTH, and release of cortisol (Ito et al., 2005). Because these experiments were conducted in vitro, they showed that the HPA equivalent functions autonomously, independent from systemic neural, vascular or endocrine influence. This cutaneous HPA equivalent regulates several normal and pathological processes in skin cells. It has been shown to play a role in keratinocyte proliferation and differentiation, hair growth, mast cell activation (important in immune responses and inflammation), and melanin production (Ziegler et al., 2007). Dysregulation of this local stress axis has been implicated in several inflammatory conditions such as psoriasis, dermatitis, and acne (Ziegler et al., 2007).
In addition to its protective role in the skin, CRF signaling counteracts oxidative stress in several tissues, an effect particularly relevant to the cochlea and noise damage. Experiments in neuronal cell culture and cardiac myocytes reveal that application of CRF, Ucn1, or their receptor agonists prior to oxidative insult promotes cell survival (Pedersen et al., 2001; Pedersen et al., 2002; Barry et al., 2010). The widespread expression of CRF family peptides combined with their protective actions against numerous insults, suggests that local CRF signaling outside the systemic HPA axis is an important promoter of tissue homeostasis and protection. Like the skin, the cochlea is an organ under frequent stress due to direct exposure to external stimuli. Perhaps the cochlea also employs local stress hormone signaling to combat tissue damage caused by acoustic insult.
UROCORTIN IS EXPRESSED IN THE COCHLEA
The first demonstration of CRF-like signaling in the cochlea came from a report revealing Ucn1 expression in the lateral efferent system terminals under the inner hair cells (Vetter et al., 2002), suggesting a role in modulating activity of the postsynaptic afferent cells. The lateral efferent fibers originate from cells in the lateral superior olive that have been shown to express Ucn1 (Kaiser et al., 2011). Assessment of auditory function in Ucn1 null mice revealed that Ucn1 plays an important role in sound processing. Ucn1 null mice exhibited poor hearing sensitivity compared to wild type mice (Vetter et al., 2002). Interestingly, Ucn1 null mice also exhibited defects in cochlear mechanics and cochlear amplification, suggesting effects beyond the afferent synapse. In addition to the baseline effects on auditory sensitivity, it was suggested that Ucn1 null mice were more susceptible to hearing loss than wild type mice, although this was not directly tested. However, Ucn1 null mice exhibited a greater age-related decline in auditory sensitivity than did the wild type controls, suggesting a greater susceptibility to hearing loss. Whether this more pronounced hearing loss resulted from an accelerated age-related decline or from a greater vulnerability to noise-induced damage was not examined. In either case, however, Ucn1 appears important not only for baseline auditory function but also for maintaining auditory sensitivity and cochlear homeostasis over time. Using in situ hybridization techniques, a widespread expression of both CRFR1 and CRFR2, receptors capable of binding Ucn1, was detected throughout the cochlea. The widespread expression of the CRF receptors compared to the restricted expression of Ucn1 within the inner spiral bundle implied that other members of the CRF system might be expressed and functional in the cochlea. Furthermore, the apparent impact of Ucn1 on auditory function suggested that CRF-like signaling represents an important regulator of acoustic sensitivity and susceptibility to hearing loss. These observations, combined with the well-established role of CRF-like peptide signaling in maintaining systemic and local homeostasis, make this system an excellent candidate for signaling systems involved in basal cochlear function and maintenance of cochlear homeostatic adaptation. Further elucidation of the role of CRF-like peptide signaling within the cochlea may provide insight for new therapies to prevent hearing loss based on the cochlea’s endogenous stress response.
THE COCHLEA EXPRESSES AN HPA-EQUIVALENT SIGNALING SYSTEM
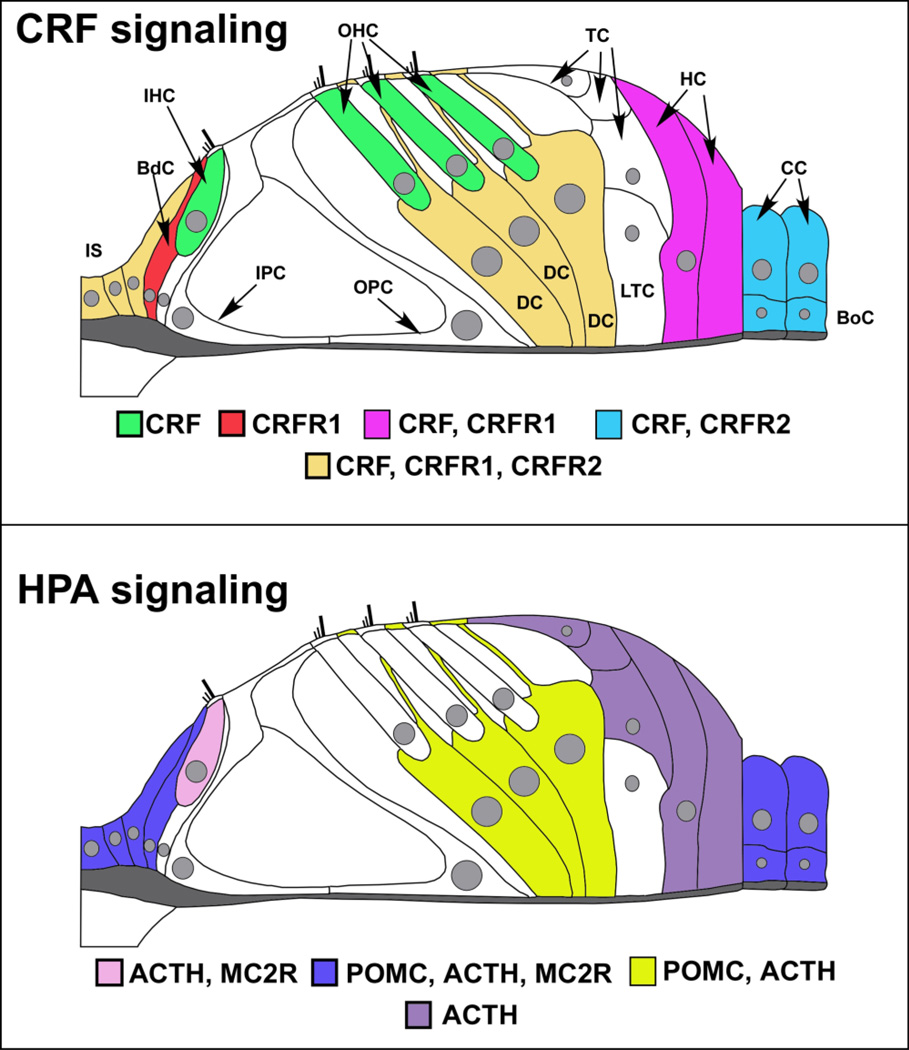
It is well recognized that the cochlea expresses glucocorticoid receptors (Shimazaki et al., 2002) and that systemic HPA activation can influence hearing (Wang and Liberman, 2002; Canlon et al., 2007), presumably via delivery of systemic glucocorticoids through the circulation. Additionally, CRF expression has recently been described in the cochlea (Graham and Vetter, 2011), indicating that both the start point (CRF) and end point (glucocorticoid receptors) of the systemic HPA axis signaling are expressed in the cochlea. Classic systemic HPA axis involves CRF release from the hypothalamus. CRF binds to CRFR1 expressed by pituitary corticotropes and stimulates cleavage of pro-opiomelanocortin (POMC) to produce adrenocorticotropic hormone (ACTH). ACTH then travels via blood circulation to the adrenal cortex, where it binds melanocortin 2 receptor (MC2R, also known as the ACTH receptor), inducing production and release of glucocorticoids. Immunofluorescence was used to ascertain whether and where POMC, ACTH, and MC2R expression occurs in the cochlea (Graham and Vetter, 2011). All of these key HPA-related molecules were detected and anatomically mapped (Fig. 1). A common site of expression for CRF, POMC, ACTH, and MC2R was the spiral ganglion cells. Otherwise, both POMC and ACTH were observed in support cells lining the inner sulcus and the lateral support cells (Claudius cells, Boettcher cells). Support cells immediately surrounding the organ of Corti also express CRF, CRFR1, and CRFR2 (Fig. 1). However, despite this overlap, mismatches were also observed in the localization of POMC, ACTH, and CRFR1. For instance, the inner hair cell contained ACTH but little to no POMC and no CRFR1. The Deiter’s cells were highly CRFR1-positive and also expressed abundant POMC and ACTH, but MC2R, the ACTH receptor was only expressed in cells situated at a distance from this source. ACTH was abundantly expressed in Tectal cells and Hensen’s cells, with little expression of POMC. Finally, while the spiral ganglion cells expressed POMC, ACTH, and MC2R, they did not express CRFR1. Such mismatches in expression of HPA components suggest that HPA-like signaling within the cochlea is not as straightforward as CRF binding to CRFR1 on a cell to stimulate breakdown of POMC and production of ACTH in that cell. Instead, the cochlear HPA equivalent signaling system likely represents a dynamic signaling system in which the components interact across the various cell types of the cochlea to orchestrate a concerted response between cell populations to organ-level stress. However, it should be stressed that the physiological and biochemical function(s) of various elements of the anatomically described system have yet to be revealed.
Figure 1. Expression of CRF, CRFR1, and HPA components in the cochlea.
Top – Molecules of the CRF signaling system are expressed in the cochlea. Cells of the inner sulcus (IS) express CRF, CRFR1, and CRFR2. CRF alone is expressed in the inner and outer hair cells (IHC, OHC respectively) of the cochlea, shown in green. These CRF-positive sensory cells are juxtaposed by support cells such as the border cell (BdC, shown in red), which expresses CRFR1, and the Deiter’s cells (DC, cells directly below the outer hair cells and shown in dark yellow) that express CRF, CRFR1, and CRFR2. In addition to the Deiter’s cells, Tectal cells (TC) and Lateral Tunnel Cells (LTC) are directly apposed to the outer hair cells and Deiter’s cells, but do not express any CRF signaling components. Support cells located more laterally include the Hensen’s cells (HC), which flank the Tectal cells and Lateral Tunnel Cells laterally and express CRF, CRFR1, and the Claudius cells (CC) and Boettcher cells (BoC), which express CRF and CRFR2. Thus there is a potential for juxtacrine interaction between hair cells and support cells in their immediate vicinity. The inner sulcus cells medial to the border cell and support cells lateral to the organ of Corti express CRF, CRFR1, and CRFR2, suggesting autocrine and paracrine communications in these peripheral support cells that could also involve the hair cell populations. Bottom – Molecules of the classic HPA signaling system are expressed in the cochlea. POMC, ACTH, and to a lesser extent, MC2R are expressed in inner sulcus cells, border cell near the IHC, and the lateral-most support cells which include Claudius cells and Boettcher cells (blue). Deiter’s cells express POMC and ACTH (depicted in yellow), but with little to no expression of MC2R. ACTH and its receptor, MC2R, are expressed in the IHC (pink), suggesting a convergence of HPA signaling on the afferent auditory transducer. ACTH alone seems to be expressed in the Hensen’s and Tectal cells (purple), with no discernable POMC expression found to date. (figure re-drawn and annotated with permission from an original provided by Dr. M. Charles Liberman, Mass Eye and Ear Infirmary, Boston, MA)
A major question that stems from these findings is why ACTH and POMC are sometimes not co-localized, such as in the case of Hensen’s cells, given that ACTH is a breakdown product of POMC. One possible explanation involves different levels of POMC cleavage in different cells, yielding different levels of ACTH. It is possible that low levels of POMC reflect more POMC cleavage and high levels reflect less. Thus, cells expressing ACTH and little to no POMC may contain more of the enzyme PC1 and its cofactors that convert POMC to ACTH. Cells containing POMC with little to no ACTH may simply exhibit less POMC proteolytic activity or they may express the enzyme PC2 that converts ACTH to alpha-melanocyte stimulating hormone (α-MSH) as occurs in the brain and skin (reviewed in (Stevens and White, 2010)). Perhaps, ACTH is quickly converted to alpha-MSH and therefore goes undetected by immunofluorescence in those cells. POMC is also constitutively secreted by cells of the pituitary, medial hypothalamus and skin (Stevens and White, 2010). Since POMC has been shown to directly stimulate melanocortin receptors, one cannot yet rule out directed secretion and physiological activity of POMC in the cochlea. Finally, experiments indicate that POMC can be processed extracellularly to produce its cleavage products, including ACTH-like peptides (Konig et al., 2006). Direct signaling activity of POMC coupled with its constitutive transcription and secretion suggest that this molecule can act independently and eliminates the necessity for co-localization with either CRFR1 or ACTH. Together, the possibilities outlined above may explain mismatches in expression of CRFR1, POMC and ACTH.
Classic HPA signaling involves release of glucocorticoids, among other bioactive compounds, that allow the organism to survive stressful events. For example, release of glucocorticoids is important for utilization of glucose by numerous organs. It is currently unknown whether the cochlear HPA-equivalent signaling system results in local production of glucocorticoids and related molecules. The question of local glucocorticoid synthesis has been investigated previously, but glucocorticoid-synthesizing enzymes were not identified in the cochlea (Lecain et al., 2003). However, other data indicate that local glucocorticoid production may take place in the cochlea. The initial step in steroid biosynthesis involves conversion of cholesterol to pregnenolone via cholesterol side chain cleavage enzyme. Pregnenolone is then converted to progesterone, which can be processed in two separate pathways, one producing sex steroids and one producing glucocorticoids. Corticosterone is created from its precursor 11-deoxycorticosterone via activity of steroid 11-beta-hydroxyalse (cytochrome P450 11B1, mitochondrial). Aldosterone, a mineralocorticoid, has been identified in the cochlea, and is created from the same precursor in a two-step reaction that produces corticosterone as an intermediate. The enzyme aldosterone synthase accomplishes this two-step conversion. Interestingly, the prior experiments investigating steroidogenic enzymes in the cochlea detected presence of the enzymes responsible for the early phases of steroid synthesis as well as aldosterone synthase, but an absence of 11-β-hydroxylase. From this, the authors conclude that sex steroids and mineralocorticoids are produced in the cochlea but not glucocorticoids. However, it has been demonstrated that 11-beta-hydroxysteriod dehydrogenase (11-HSD) isoforms are expressed in the cochlea (ten Cate et al., 1994; Terakado et al., 2011). 11-HSD is responsible for converting cortisol (with activity at glucocorticoid receptors) to cortisone (with little to no activity at the glucocorticoid receptors). While it remains an open question whether corticosterone is produced locally, it must be acknowledged that the likelihood of local production is high. This is suggested given that corticosterone is a necessary intermediate for mineralocorticoid synthesis, that molecules involved in earlier steps in the synthesis process for glucocorticoids are present in the cochlea, and that molecules involved in glucocorticoid degradation are located in the cochlea. Nonetheless, it is possible that aldosterone synthase does not act on local precursors but on systemic corticosterone delivered through the blood supply.
Whether or not glucocorticoids are produced locally within the cochlea, the cochlear HPA-equivalent system could be involved in other processes as well. Although classic HPA signaling involves ACTH binding to the MC2 receptor and the subsequent production of glucocorticoids, MC2R activity is not limited to promoting glucocorticoid synthesis. Indeed, MC2R is a G-protein-coupled receptor that couples to the cAMP-PKA pathway. Working through this pathway, ACTH activation of MC2R could potentially exert several effects on auditory processing beyond glucocorticoid production. In fact, the major site of MC2R expression, the inner hair cell, also hosts a potential target of ACTH-induced PKA signaling. Inner hair cells express the large-conductance potassium channel known as the BK channel (Brunton et al., 2007). BK activity is directly and intimately involved in hair cell physiological responses (Skinner et al., 2003; Beurg et al., 2005). ACTH has been shown to alter splicing of the BK channel and inhibit BK activity via cAMP-PKA signaling cascades (Shipston et al., 1996; Tian et al., 2001; Lai and McCobb, 2002) in other tissues (also see further discussion below). Thus HPA-like signaling within the cochlea can have effects on cochlear function that may be unrelated to glucocorticoid synthesis.
THE ROLE OF CRF RECEPTOR SIGNALING IN THE COCHLEA
In mammals, the CRFR1 and CRFR2 genes encode the CRF receptors. The CRF receptors are classic G-protein coupled receptors. CRF signaling activates a plethora of second messenger signaling cascades (including Protein Kinase A (PKA), Protein Kinase C (PKC), Protein Kinase B (PKB, also known as Akt), the mitogen-activated protein kinases (MAPK) p42/44 and p38) via activation of numerous G-proteins and can control other signaling molecules such as calcium, nitric oxide, etc. (Grammatopoulos and Chrousos, 2002). Current evidence suggests that the pattern of G-protein activation, and therefore of specific signaling cascades stimulated by CRFR activation, may be highly divergent between tissues (Grammatopoulos and Chrousos, 2002). CRF and both CRF receptors (Fig. 1, 3, and Table 1) are expressed within the mouse cochlea (Graham et al., 2010; Graham and Vetter, 2011). The role of cochlear CRF signaling (and by extension HPA-equivalent signaling within the cochlea) has begun to be revealed using transgenic mice lacking CRFR1 or CRFR2 (CRFR1 and CRFR2 null respectively). These mouse models were used to examine in vivo the structural and functional consequences induced by constitutive loss of each CRF receptor gene (Graham et al., 2010; Graham and Vetter, 2011), as well as through pharmacological activation of CRFR2 in an in vitro model (Basappa et al., 2010).
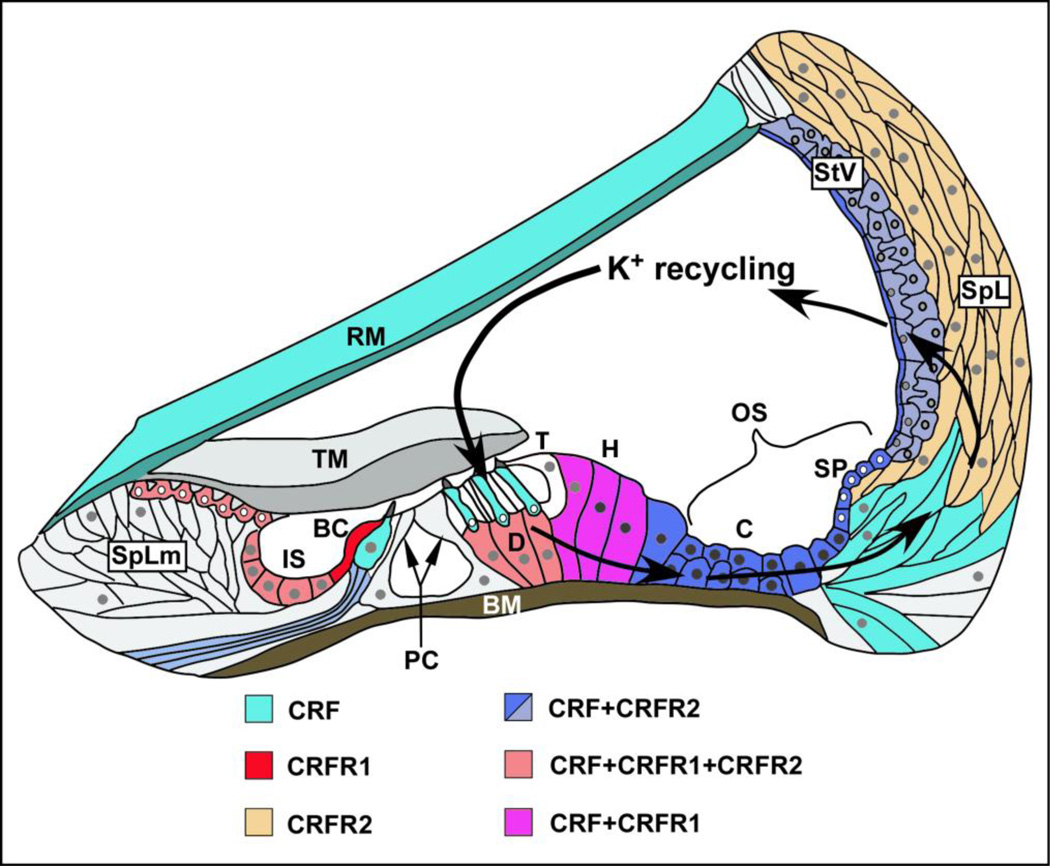
Figure 3. Schematic CRF, CRFR1 and CRFR2 of expression in the cochlea in relation to routes of potassium recycling.
CRF (teal) is expressed in inner hair cells (IHC) and outer hair cells (OHC). CRF is co-expressed with CRFR2 in afferent spiral ganglion neurons (only fibers shown here) and in the stria vascularis (StV, shown in shades of blue, reflecting level of expression; dark blue- high expression, lighter blue- lower expression). CRF is co-expressed with CRFR1 and CRFR2 in Deiter’s cells (D) below outer hair cells (orange), while in Hensen’s cells (H), lateral to the Deiter’s cells, CRF is co-expressed with CRFR1 (pink). In the support cell populations not immediately adjacent to hair cells, CRF is co-expressed with CRFR1 and CRFR2 in the inner sulcus (IS) and in interdental cells (orange) of the Spiral Limbus (SpLm), while in support cells lateral to the organ of Corti (blue), such as Claudius cells (C) Boettcher cells (below Claudius cells), and cells of the outer sulcus such as those lining the spiral prominence, CRF is co-expressed only with CRFR2. CRFR1 (red) is expressed alone (without CRF) in the border cell (BC) adjacent to the medial surface of the IHC. (BC-Border cell; BM- basilar membrane; C- Claudius cells; D- Deiter’s cells; H- Hensen’s cells; IS- inner sulcus; OS- outer sulcus; PC- Pillar cells; RM- Reissner’s membrane; SP- spiral prominence; SpL- spiral ligament (containing fibrocytes populations); SpLm- spiral limbus; StV- stria vascularis; T- Tectal cell; TM- tectorial membrane; Figure adapted from Jentsch, 2000.)
Table 1.
CRF signaling molecule expression patterns within the cochlea
| CRF alone |
CRFR1 alone |
CRFR2 alone |
CRF+ CRFR2 |
CRF+CRFR1+CRF R2 |
|
|---|---|---|---|---|---|
| Organ of Corti | |||||
| Inner phlangeal cell | |||||
| IHCs | |||||
| Border cell | ? | ||||
| Pillar cells | |||||
| OHCs | |||||
| Deiter’s cells | |||||
| Other support cells | |||||
| Interdental cells | |||||
| Inner sulcus cells | |||||
| Tectal cells | |||||
| Hensen’s cells | |||||
| Lateral support cells (Claudius and Boettcher’s cells) | |||||
| Other cells lining scala media | |||||
| Reissner’s membrane | |||||
| Spiral prominence | |||||
| Lateral Wall | |||||
| Type I fibrocytes | |||||
| Type II fibrocytes | |||||
| Type III fibrocytes | |||||
| Type IV fibrocytes | |||||
| Stria Vascularis | |||||
| Marginal cells | |||||
| Intermediate cells | |||||
| Basal cells | |||||
| Spiral Ganglion neurons |
CRFR1
Loss of CRFR1 depresses auditory sensitivity
An evaluation of auditory function using auditory brainstem response (ABR) measures in CRFR1 null mice revealed a 20–30dB deficit in auditory sensitivity across all frequencies along with slightly impaired cochlear mechanics indicated by a 5–10 dB elevation of distortion product otoacoustic emissions thresholds (Graham and Vetter, 2011). These results suggest a mixed, but predominantly inner hair cell based etiology.
Decreased levels of glutamine synthetase in the absence of CRFR1
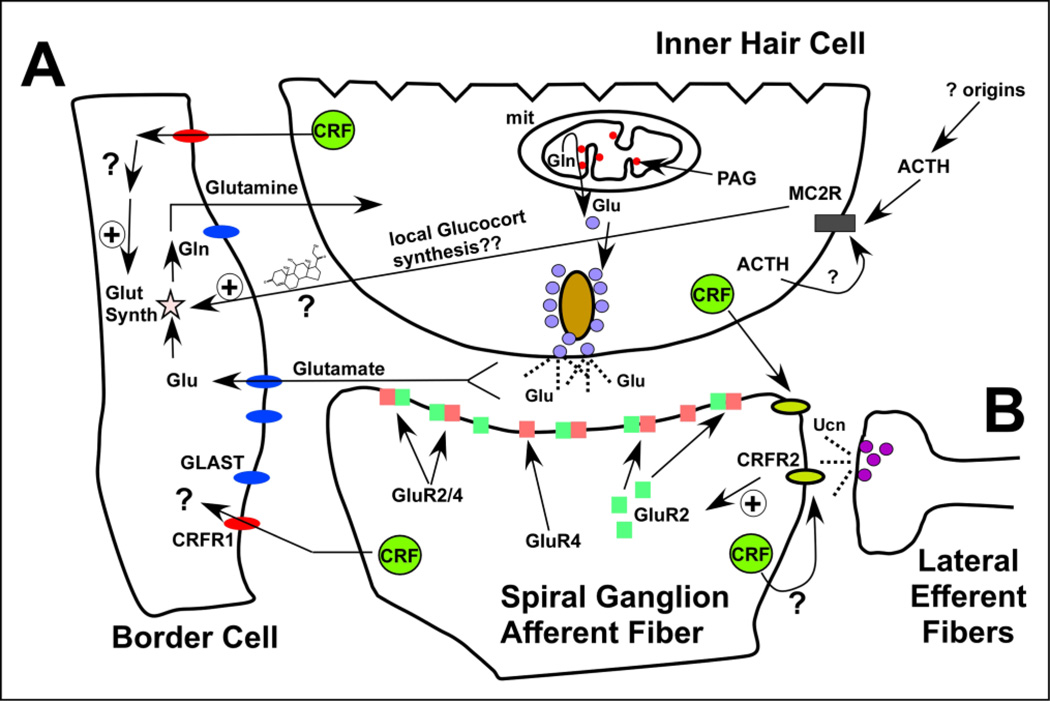
CRFR1 is not expressed in either the presynaptic inner hair cell or the postsynaptic spiral ganglion cell. Instead it is expressed in the border cell, a support cell population that sits immediately adjacent to the medial aspect of the inner hair cells (Fig. 1). Thus, CRFR1 is well positioned to regulate communication between these support cells and the inner hair cells, potentially exerting an indirect influence over auditory afferent transduction (Fig. 2). It has been proposed that various cochlear support cells interact with hair cells in a manner similar to astrocyte interactions with neurons in the central nervous system, and one potential interaction involves glutamate recycling via the glutamate-glutamine cycle (Ottersen et al., 1998; Rio et al., 2002). In the cochlear version of this cycle, glutamate is cleared from the synaptic cleft via the glutamate transporter GLAST, which is expressed by the border cell. Glutamate is then broken down to glutamine via the enzyme glutamine synthetase (GS). Glutamine is shuttled back to the inner hair cell where it is used to synthesize glutamate. In this manner, excitotoxicity is avoided by sending the precursor of glutamate back to the IHC to ultimately replenish the glutamate neurotransmitter pool. There is a 50% reduction of cochlear GS levels in CRFR1 null mice compared to wild type mice, suggesting a deficiency of glutamate-glutamine cycling and potentially reflecting a reduced ability to convert glutamate taken up from the synapse into glutamine. In turn this deficiency may lead to decreased glutamate recycling in the inner hair cell and thus a rundown of neurotransmitter supply in the face of frequent stimulation. It is possible that such a rundown contributes the hearing deficiency observed in CRFR1 null mice. It should be noted, however, that GS expression was examined in whole cochlear lysates, and therefore may not reflect processes occurring specifically at the afferent synapse. Recent work demonstrated activity of the excitatory amino acid transporter, GLAST, in fibrocytes lining the lateral wall and suggests a role for these cells in regulating glutamate homeostasis (Furness et al., 2009). It follows that glutamine synthetase may also be expressed in these cells as well to regulate glutamate recycling. If so, the GS deficiency observed in CRFR1 null mice might reflect problems that extend beyond the local confines of the inner hair cell- ganglion cell afferent synapse, representing a more global role in cochlear glutamate homeostasis.
Figure 2. CRF signaling and HPA effects on the cochlear afferent synapse.
A) CRFR1 expressed on the border cell adjacent to the inner hair cell regulates glutamate-glutamine cycling by promoting expression of Glutamine Synthetase (GS). In the absence of CRFR1, GS levels are significantly reduced suggesting impaired glutamate-glutamine cycling and, by extension, impaired afferent function. The specific ACTH receptor melanocortin receptor 2 (MC2R) is expressed specifically in the inner hair cell, suggesting an important convergence of HPA signaling here. ACTH may modify the activity of the large-conductance potassium channel (BK) by signaling through MC2R and the cAMP-PKA cascade as has been shown in other tissue. Potential CRF signaling-based modulation over BK expression/function afferent activity could establish this signaling system as an important modulator of cochlear afferent function. Additionally, stimulation of MC2R at the IHC may lead to production and release of glucocorticoids, which could have an impact on glutamine synthetase expression and thereby impact the glutamate-glutamine cycle. B) Release of Ucn1 from the lateral efferent terminals and/or release of CRF from the inner hair cells alters the molecular composition of the AMPA-class glutamate receptors. CRF-related signal modification of the degree to which GluRs include GluR2 subunits may help determine the basal cochlear sensitivity and stimulus encoding.
CRFR1 null mice are deficient in circulating corticosterone levels due to atrophy of their adrenal cortex (Smith et al., 1998). Therefore any glucocorticoid-dependent processes altered in the cochlea of CRFR1 null mice could result from either local or systemic glucocorticoid depletion. A glucocorticoid-response element is present on the promoter of the glutamine synthetase gene, suggesting that the reduced levels of GS observed in the CRFR1 null mice could result from deficient glucocorticoid activity (Vardimon et al., 1999). To distinguish whether or not the decrease in GS was a glucocorticoid-dependent effect, GS levels were evaluated in wild type and CRFR1 null mice administered corticosterone in their drinking water beginning at embryonic stages. Glucocorticoid treatment rescued GS expression in CRFR1 null mice (Graham and Vetter, 2011), indicating that CRFR1-mediated changes in cochlear GS expression depend on signaling through glucocorticoids. However, the endogenous source of the glucocorticoids (local or systemic) acting on the cochlear GS gene remains elusive. Nonetheless, this finding is particularly interesting since it implicates a novel target in glucocorticoid-mediated effects on audition. Though synthetic glucocorticoids have been administered in the clinic for various auditory deficits, their mechanisms of action have remained largely unknown. The work presented here reveals one possible signaling system mediating glucocorticoid effects on auditory function and protection.
Elimination of CRFR1 yields defects in afferent fiber targeting, highlighting a possible role for CRF signaling in dendritic development of spiral ganglion cells
Evidence suggests a developmental role for CRFR1 signaling in shaping afferent innervation to inner hair cells (Graham and Vetter, 2011). CRFR1 null mice exhibit an abnormal distribution of afferent synapses along the inner hair cell, with presynaptic ribbons and postsynaptic ganglion cell dendrites tending to cluster on the modiolar side of the inner hair cell. This clustering could have important ramifications for afferent function and may represent the major cause of auditory impairment in the CRFR1 null mice. Synaptic placement along the modiolar-pillar axis of the inner hair cell has been shown to correlate with different postsynaptic activity of the ganglion cell fibers. In the cat cochlea, synapses localized on the modiolar side of the hair cell exhibit low spontaneous rates and high response thresholds, while synapses localized on the pillar side exhibit high spontaneous rates and low response thresholds (Liberman, 1980; Liberman, 1982). Similar properties have been found relating fiber innervation of pillar/modiolar inner hair cell face and their physiological responses in the mouse cochlea (Taberner and Liberman, 2005). Therefore, the modiolar bias of synaptic distribution observed in CRFR1 null mice might indicate loss of low threshold (pillar side) fibers with sparing of the high threshold (modiolar) fibers, potentially explaining their high ABR thresholds.
In addition to modiolar-pillar localization defects in the CRFR1 null mice, inner hair cells were significantly smaller than those from homologous regions along the cochlear spiral of wild type mice. While the number of synaptic ribbons was not altered in the CRFR1 nulls, the smaller inner hair cell soma induced a tighter packing of the ribbons. Though currently untested, it is highly probable that calcium microdomains normally surrounding the ribbons (Brandt et al., 2003; Moser et al., 2006a; Moser et al., 2006b) may be distributed so close to each other in the CRFR1 null inner hair cells that they interfere with function of the more closely spaced neighboring ribbons, perturbing the tight coupling between activity at a single ribbon and firing in a single postsynaptic cell. Since the ABR measured and used to assess auditory threshold is the sum of synchronized responses of numerous ganglion cells synapsing with an individual inner hair cell, lack of sufficient spatial segregation of ribbons may result in abnormal recruitment of ribbon activity in response to inner hair cell depolarization, loss of postsynaptic response synchrony (Buran et al., 2010; Frank et al., 2010), and therefore decreased thresholds measured with ABR techniques.
CRFR2
Loss of CRFR2 enhances auditory sensitivity while also generating a greater susceptibility to noise-induced hearing loss
In contrast to the lower auditory sensitivity of the CRFR1 null mice, CRFR2 null mice exhibit ABR thresholds that are 20–25dB lower than wild type mice and distortion product otoacoustic emission thresholds that are 10–15dB lower than wild types (Graham et al., 2010). The lower distortion product thresholds suggest that enhanced cochlear amplification (via increased outer hair cell activity) contributes to some, but not all, of the hypersensitivity observed in these mice (since the increase in distortion product emissions is about half of the ABR threshold change). At least some portion of the hypersensitivity may also originate from changes in afferent transduction and/or neurotransmission. In addition to the greater sensitivity induced by loss of CRFR2, noise exposure induces approximately twice the permanent threshold shift compared to wild type mice (Graham et al., 2010). In CRFR2 null mice, the ABR threshold shift that occurs following noise exposure is not accompanied by a significant change in distortion product threshold, suggesting a predominantly afferent mechanism underlying the noise-induced hearing loss. Remarkably, noise-induced threshold shifts (i.e. loss of sensitivity) in CRFR2 null mice occurred with sound intensities as low as 50dB (Graham et al., 2010), the intensity of quiet human speech.
Glutamate receptor 2 subunit expression changes in CRFR2 null mice
Using immunofluorescence, CRFR2 was localized to areas where it can impact post-synaptic responses to inner hair cell afferent transmission (Graham et al., 2010). Afferent transmission in the cochlea is mediated by glutamate released from the inner hair cells binding to AMPA type glutamate receptors expressed on the postsynaptic surface of spiral ganglion cell dendrites (Puel, 1995). With respect to AMPA class glutamate receptors, the mature cochlea expresses the GluR2, 3, and 4 receptor subunits (Eybalin et al., 2004). Examination of GluR expression in CRFR2 null mice surprisingly revealed that when reared under quiet conditions (sound chamber isolated environments), CRFR2 null mice express 50% less GluR2/3 than wild type mice but similar levels of GluR4. When raised in an environment exposed to constant low to moderate level noise, CRFR2 null mice express similar levels of GluR2/3 as wild type mice but 80% more GluR4 (Graham et al., 2010).
Little is known regarding the contribution of each type of AMPA receptor subunit to overall receptor function and afferent transmission in the cochlea. Nonetheless, some hypotheses (Fig. 2) can be formulated to account for how the observed changes in GluR expression following loss of CRFR2 expression could lead to the afferent physiological phenotype in CRFR2 null mice. Previous studies reveal that the majority of GluR2 subunits expressed in the mature rat cochlea are in the edited form and therefore, when incorporated into AMPA receptors, render the GluR complex calcium impermeable (Eybalin et al., 2004). Additionally, incorporation of edited GluR2 confers other properties to the receptor including reduced conductance and slower kinetics (Swanson et al., 1997; Isaac et al., 2007). Reduced levels of GluR2/3 observed in CRFR2 null mice under quiet conditions could reflect deficient expression of the GluR2 subunit (but this has yet to be directly tested), and possibly an increased pool of calcium-permeable AMPA receptors at the spiral ganglion cell surface. An enrichment of calcium permeable receptors can lead to potentiation of glutamatergic transmission and thus the increased afferent sensitivity observed in the CRFR2 null mice under quiet conditions. Intracellular signaling cascades, particularly PKC-mediated signaling, have been demonstrated to play an integral role in activity-dependent recruitment of calcium-impermeable GluR2-containing AMPA receptors to the postsynaptic surface (reviewed in (Isaac et al., 2007)). Thus a decrease in such signaling in the absence of CRFR2, a G-protein coupled receptor known to stimulate PKC signaling via the Gαq protein, could lead to deficient GluR2 recruitment.
It is possible that the GluR expression changes observed in CRFR2 null mice do not result from activity of CRFR2 within the ganglion cells themselves. Instead, changes in GluR2/3 expression may represent a compensatory reaction driven by some other phenomenon. For instance, given the similarities in GluR4 expression between CRFR2 null mice and wild type mice under quiet conditions, it is possible that the decrease in GluR2/3 represents a generalized loss of AMPA receptors at the cell surface in compensation for increased presynaptic activity. Previous work has demonstrated a decrease in cell surface GluR2 expression on the spiral ganglion cells in response to excess sound in vivo or excess glutamate in vitro (Chen et al., 2007). This decrease is assumed to reflect a general decrease in surface AMPA receptors, and it has been shown that if it is prevented through genetic hindrance of endocytosis, spiral ganglion cells are more susceptible to excitotoxic stress (Chen et al., 2009). Thus, the observed decrease in GluRs may represent a compensatory adjustment to over-activity occurring elsewhere in the afferent transduction chain. Alternatively, if decreased GluR2/3 levels really do represent decreases in GluR2, it is possible that this reduction results from an excitotoxic response. Although paradoxical, it has been demonstrated in the hippocampus that ischemic or excitotoxic insult activates REST, a repressor protein, that represses expression of GluR2, leading to production of more calcium-permeable AMPA receptors (reviewed in Isaac et al., 2007). If a similar phenomenon is occurring in the cochlea, then the down regulation of GluR2/3 under quiet conditions may represent an early sign of excitotoxic stress in CRFR2 null mice. Spiral ganglion cell counts in CRFR2 null mouse cochleae revealed no overt loss of postsynaptic cells following ten days noise exposure. However, afferent cell damage may initially be subtle, involving only loss of synaptic contacts that is followed by loss of ganglion cells over a period of months to years (Kujawa and Liberman, 2009).
Glutamate receptor 4 subunit expression changes in CRFR2 null mice
Under noise conditions, GluR4 expression increases 80% in CRFR2 null mice, and this increase may reflect an exaggeration of a normal process. Wild type mice also exhibit a slight increase in GluR4 expression under noise conditions concurrent with a dramatic drop in GluR2/3 levels. This suggests that the majority of AMPA receptors expressed contain GluR4, and suggests the presence of GluR4 homomeric complexes. Experiments examining the tonically active synapses between photoreceptors and second order cells in the salamander retina demonstrate that these synapses avoid desensitization via use of presynaptic ribbons and postsynaptic AMPA receptors composed only of GluR4 (Pang et al., 2008). Synaptic ribbons provide multiquantal release, enabling longer durations between release events. GluR4 homomeric receptors are quick to desensitize to glutamatergic stimulation, but also are quick to recover, enabling full recovery of receptor activity between release events. Thus, enrichment of GluR4 subunits endows the AMPA receptor with a full operational range in the presence of tonic stimulation. Like the retinal synapses, the afferent synapse in the cochlea is spontaneously active at all times, and employs presynaptic ribbons for glutamate release. Perhaps the fast and sensitive desensitization kinetics of GluR4-enriched AMPA receptors limits postsynaptic current in the face of constant sound and allows the cochlea to maintain a dynamic signaling range with tonic stimulation. By extension, the exaggerated increase in GluR4 observed in CRFR2 null mice under noise conditions could account for the observed hearing loss compared to wild type mice. However this proposal contradicts work from other systems suggesting that GluR4 enhances postsynaptic currents and is therefore recruited for synaptic strengthening. In the early postnatal hippocampus, recruitment of GluR4 to silent synapses precedes recruitment of the other GluR subtypes and leads to a sustained increase in neuronal activity (Zhu et al., 2000). Similarly, studies comparing response properties of corticothalamic targets reveal that the neurons in the reticular nucleus exhibit a 2.6 fold larger post synaptic response to corticothalamic stimulation than relay neurons and this increase in activity correlates with enriched GluR4 subunit expression in the reticular nucleus (Golshani et al., 2001). These findings would suggest that the exaggerated elevation of GluR4 levels in CRFR2 null mice should lead to increased afferent activity under noise conditions, unless there was acute excitotoxic damage to the afferent synapses rendering them nonfunctional. Spiral ganglion cell counts suggest that there is no overt loss of afferent neurons. However, the synaptic microstructure, including ribbons and postsynaptic contacts, was not examined. Thus, the exact consequence of CRFR2 control over GluR4 expression remains to be more completely described.
CRFR2 influences connexin expression in the adult cochlea: potential ramifications for purinergic signaling and impacts on endocochlear potential control
Support cells in the cochlea release ATP through connexin hemi-channels on their surface (Zhao et al., 2005). This ATP release increases following even brief sound exposure (Munoz et al., 2001). Though a direct link between ATP release and auditory sensitivity has not been demonstrated, several studies implicate purinergic signaling as a mechanism modulating sensitivity to sound. When ATP is released from support cells into the endolymph, it exerts two main effects. The first is a reduction of the potassium concentration in the endolymph, reducing the endocochlear potential that drives hair cell mechanotransduction. The endocochlear potential (approximately +80 mV in mice) reflects a potential difference between the endolymphatic space within the scala media and surrounding compartments (Hibino et al., 2010). This potential difference creates a steep electrical gradient important for auditory mechanotransduction. Positive charge will readily flow out of the endolymph into surrounding tissue such as the sensory hair cells where it elicits a depolarization. Reduction of endolymphatic potassium concentration is accomplished through activation of P2X2 channels along the cochlear duct. These channels are ligand-gated cation permeable ion channels that provide a potassium sink, allowing potassium to flow out of the endolymph (Lee et al., 2001) to enter the potassium-recycling stream that normally brings potassium back to the endolymph (Fig 3). Also, activation of the G-protein-coupled receptor, P2Y4, triggers PKC-induced phosphorylation of the IsK (KCNE) auxiliary subunit, thereby inhibiting activity of the KCNQ1/ISK complex that enables potassium entry into the endolymph (Marcus et al., 1998). In addition to reducing endolymph potassium, the second effect of ATP release is to decrease outer hair cell motility, thereby disrupting cochlear amplification and decreasing auditory thresholds (sensitivity). This effect relies on both P2X and P2Y type receptors expressed in the outer hair cells (Zhao et al., 2005).
Connexin expression is decreased in CRFR2 null mice under quiet and noise conditions
Connexins 26 and 30 (Cx26 and Cx30) are decreased in CRFR2 null mice compared to wild type mice. Cx26 and Cx30 are the major connexins expressed in support cells lining the cochlear duct and they form the connexin hemi-channels through which ATP is released (Zhao et al., 2005; Zhao and Yu, 2006). While still speculative, a decrease in Cx26 and Cx30 expression should lead to fewer connexin hemi-channels at the support cell surface, resulting in deficient ATP release in CRFR2 null mice.
CRFR2 null mice exhibit increased expression of purinergic receptors under quiet conditions
Related to CRFR2 null induced changes in connexin hemi-channel expression, an evaluation of purinergic receptor expression revealed a slight increase in P2X2 and a significant increase in P2Y4 under quiet conditions (Graham et al., 2010). While seemingly paradoxical in light of the suppressive effect of purinergic signaling on endocochlear potential and the auditory hypersensitivity observed in CRFR2 null mice, the up-regulation of purinergic receptors may represent compensatory reactions to impaired ATP release. Importantly, this suggests a modulatory action of CRFR2 activity on purinergic receptor expression. Interestingly, P2X2 and P2Y4 expression in CRFR2 null mice returns to wild type levels under noise conditions, coinciding with a significant increase in CRFR2 null connexin expression from quiet to noise (toward the levels observed in wild type mice). If changes in connexin expression correlate with changes in ATP output, then perhaps the increase in connexin expression in CRFR2 null mice under noise conditions is enough to drive ATP release toward normal wild type levels. As a result, compensatory changes in P2X2 and P2Y4 expression are no longer necessary to make up for deficient ATP release, and the expression of these receptors falls back to normal, wild type levels.
Potential links between deficient purinergic signaling and function
The auditory physiology in CRFR2 null mice, showing both decreased DPOAE thresholds and decreased ABR thresholds, is suggestive of a change in endocochlear potential. An increase in endocochlear potential would increase electrochemical drive through both the inner hair cells and outer hair cells, thereby enhancing the sensitivity of both afferent transduction and cochlear amplification. In particular, enhancement of afferent transduction (reflected in ABR thresholds) would be more pronounced than enhancement of cochlear amplification (reflected in the DPOAE thresholds) given the compound effect of potentiating cochlear amplification and electrochemical drive on the afferent inner hair cell. Therefore, in response to an increase in endocochlear potential, one would expect to see changes similar to those observed in CRFR2 null mice – a slight decrease in DPOAE thresholds accompanied by a more substantial decrease in ABR thresholds. Although it is possible for CRFR2 to influence endocochlear potential through a variety of mechanisms (Fig. 3), its expression is most abundant in support cells lining the cochlear duct, suggesting involvement of these cells in producing the observed physiological phenotype. These cells are thought to influence endocochlear potential via ATP release (described above), and therefore it is probable that CRFR2 activity influences endocochlear potential by modulating ATP release from support cells lining the cochlear duct, linking the observed changes in connexin expression to the auditory physiology.
Emerging data thus suggest that CRF signaling modulates connexin expression in the cochlea. While the connexin/purinergic receptor signaling system can control auditory sensitivity, and may be involved in protection of the cochlea from noise-induced hearing loss, the importance of connexins for “hearing” (i.e. the summed activity of the cochlea as a whole and the transmission of neural code to the brain) has also been well appreciated. For example, connexin mutations account for over 50% of inherited non-syndromic deafness cases (Hoang Dinh et al., 2009).
Evidence from other systems supports a role for CRF-induced changes in connexin expression in health and disease
The interaction between CRF signaling and connexin expression described in the cochlea correlates with work in other tissue/organs including uterine smooth muscle and the brain. CRF is a key factor regulating the onset of labor and an important event at the onset of labor is initiation of uterine contractions. These contractions require cell-cell communication via gap junctions composed of connexin 43 (Cx43). Recent work demonstrates that CRF increases Cx43 in uterine muscle through an Activator Protein 1 (AP1)- dependent mechanism (Wu et al., 2007). Other experiments demonstrate CRF-induced increase of Cx43 in the IMR32 neuroblastoma cell line and in astrocytes within the hippocampus. This increase also occurs via an AP1/cFOS dependent mechanism and mediates CRF-induced protection of both neuroblastoma cells and hippocampal neurons against oxidative stress (Hanstein et al., 2009). Of note, experiments performed in astrocyte/ neuron co-cultures reveal that CRF protects neurons by up-regulating Cx43 in astrocytes, suggesting a supportive interplay between astrocytes and neurons that is regulated, in part, by CRF signaling.
CRFR2 SIGNALING IS A SIGNIFICANT COMPONENT OF THE ENDOGENOUS CELL STRESS-RESPONSE PROTECTIVE MECHANISM OF THE COCHLEA
As described above, data suggest that CRF (or possibly another CRF peptide) acts on support cells expressing CRFR2 to influence connexin expression, which, similar to astrocytes in hippocampal cultures, may have important implications for the survival of the cochlea’s sensory hair cells in the face of oxidative stress. Other data have indicated that abnormal expression of CRFR1 and CRFR2 lead to altered auditory sensitivity and, following loss of CRFR2 activity, significantly greater susceptibility to noise-induced hearing loss. Noise-induced damage to the cochlea that results in hearing loss is thought to result from oxidative stress caused by accumulation of reactive oxygen species within cochlear tissue. Recent data has revealed an important anti-oxidative role of CRFR2 in OCK-3 cells, an immortalized cochlear cell line. Cells treated with Ucn2, a CRFR2-specific ligand, prior to incubation with H2O2 or gentamicin (an ototoxic aminoglycoside class antibiotic still in use in the clinic) showed significantly reduced ROS production compared to control cells, and this effect was blocked by CRFR2 antagonists (Basappa et al., 2010). Therefore, CRFR2 appears to prevent oxidative stress in cochlear cells and, as described in other systems, modulation of connexin expression may contribute to this antioxidant effect. In this light, connexin deficiency in CRFR2 null mice may indicate an impaired ability to mount a survival response in the face of oxidative stress, leaving hair cells more susceptible to damage, suggesting one mechanism by which the greater susceptibility to noise-induced hearing loss, even at very mild noise levels, occurs. Also, connexins are the building blocks of gap junctions and the avascular organ of Corti depends on exchange of nutrients and biochemical messengers through these gap junctions for normal function (Zhang et al., 2005; Chang et al., 2008). Specifically, Cx30 null mice exhibit impaired glucose transfer between support cells and this leads to an increase in production of free radicals within the organ of Corti (Chang et al., 2008). Thus, deficient connexin expression in CRFR2 null mice may cause free radical production due to insufficient nutrient exchange and it may also impair the ability to cope with these free radicals.
In addition to the role CRFR2 signaling seems to play in handling ROS accumulation in response to ototoxic drug challenge, results have also shown that CRFR2 activity prevents aminoglycoside-induced caspase-3 activity (Basappa et al., 2010). Thus, activity of typical cell death pathways seems to be inhibited by CRFR2 activity. The significance of CRFR2 activation in OCK3 cochlear cell line was further revealed using a quantitative differential mass spectrometry approach (Basappa et al., 2010). Protein expression was compared between gentamicin stimulated cells and CRFR2 pre-stimulated cells that were then challenged with a gentamicin application. In general, apoptosis-related proteins were up-regulated following exposure to gentamicin, but were down-regulated in cells that were first pre-treated to activate CRFR2 and which were then treated with the same dose of gentamicin. Proteins involved in cell survival (for example, growth factors and proteins involved in cell:cell adhesion) and proteins involved in protein degradation (presumably clearing misfolded proteins resulting from cellular stress) were up-regulated under CRFR2 pre-treatment followed by gentamicin challenge, while these proteins were down-regulated under gentamicin only exposure.
A more directed examination of a number of cell signaling pathways revealed that the AKT/PKB signaling pathway was altered following loss of CRFR2 expression (Graham et al., 2010). Basal AKT1 expression is approximately 60% lower in CRFR2 null mice in both quiet and noise conditions compared to wild type mice. AKT/PKB signaling is involved in numerous cellular functions, including control over apoptotic events and cellular protein synthesis pathways. Thus, CRFR2 signaling is likely to modulate very basic cellular functions that seem to have an impact on survival in the face of environmental and ototoxic drug challenges. CRF signaling in general may therefore represent a physiologically significant endogenous cellular stress-response system for cochlear homeostasis.
A WORKING MODEL OF CRF SIGNALING IN THE COCHLEA
Neural signaling
The evidence for CRF-mediated neuromodulation builds upon a robust literature describing CRF as a neuromodulator in the central nervous system. In particular, CRF signaling exerts powerful affects on glutamatergic potentiation in the amygdala and hippocampus, leading to behaviors such as drug addiction, anxiety, and fear-associated learning and memory. There are three major avenues by which CRF signaling can influence afferent transduction and neural processing (schematically represented in Fig. 2) in the cochlea. First, CRFR2 expressed in the postsynaptic afferent dendrites could be stimulated by input from CRF expressed in and released from the inner hair cell as well as via autocrine signaling from the spiral ganglion cell fibers, and finally from Ucn1 expressed in and released from the lateral efferent terminals synapsing on the afferent dendrites. The net effect of CRFR2 activation, as demonstrated by results from the CRFR2 null mice, is to modulate GluR expression and/or membrane insertion in the postsynaptic ganglion cell. Under normal conditions, this ultimately leads to a decrease in acoustic sensitivity and a resistance to noise-induced hearing loss due to blunting of excitotoxicity. Second, CRFR1 expressed in the border cell next to the presynaptic inner hair cell is in position to receive input from CRF expressed in and released from the inner hair cell and the spiral ganglion cells (and possibly Ucn1 from the lateral efferents). CRFR1 activation in the border cells can lead to increased expression of glutamine synthetase, thereby sustaining the glutamate-glutamine cycle between inner hair cells and their neighboring support cells. Based on the phenotype resulting from loss of CRFR1 expression, the net effect of normal CRFR1 activation is an increase in acoustic sensitivity. Third, MC2 receptors expressed by the inner hair cell could receive autocrine input from ACTH expressed in the inner hair cell as well as paracrine input from ACTH expressed in more distant support cells. ACTH can exert a variety of effects on the inner hair cell by signaling through MC2R and the cAMP pathway to which it is linked. Since CRFR1 is not expressed by inner hair cells (Graham and Vetter, 2011), ACTH activation of cAMP signaling (via the MC2R) may represent a major avenue by which to activate this system. One potential effect of ACTH-induced MC2R activity is modulation of BK potassium channel activity. BK channels are a major regulator of presynaptic afferent transduction by the inner hair cells. This potassium channel is important for inducing membrane potential hyperpolarization in response to calcium and voltage increases, and therefore helps to repolarize cells following excitation. In the cochlea, BK activity is crucial for enabling repetitive firing in response to sustained stimuli (Fettiplace and Fuchs, 1999). Intriguingly, experiments on cells isolated from other tissues demonstrate a reciprocal activity between BK and HPA signaling involving both transcriptional and post-translational modifications in BK channel activity in other tissues and suggest potential mechanisms by which ACTH may influence BK activity and, by extension, cochlear function. BK null mice demonstrate a significant stress-induced hypo-responsiveness that is associated with a significant decrease in plasma levels of ACTH and glucocorticoids. This was also associated with decreased activation of the hypothalamic paraventricular neurons and basal CRF expression (Brunton et al., 2007). Since primary corticotrope cultures generated from BK null mice were able to release ACTH (Brunton et al., 2007), BK activity seems most intimately involved with ACTH release via its effects on basal CRF expression. In pituitary corticotropes, CRF signals through the PKA second messenger system to inhibit BK activity. Iberiotoxin, a selective BK channel blocker, abolishes the glucocorticoid inhibition of CRF-induced ACTH secretion, suggesting a role for BK activity in controlling consequent ACTH release (Shipston et al., 1996; Brunton et al., 2007). Conversely, data suggests that glucocorticoids counter this inhibition via fast, non-genomic mechanisms to hold cells in a hyperpolarized state and prevent ACTH secretion. Finally, ACTH, via its stimulation of glucocorticoid release, has been shown to promote the inclusion of the stress-related exon (STREX) in the BK channel transcript in chromaffin cells (Xie, 1998; Lai and McCobb, 2002). This is of special interest for further understanding the role of CRF signaling in the cochlea because BK channels possessing the STREX component activate faster and at more negative voltages, and deactivate more slowly than BK channels without STREX inclusion (Xie, 1998). This could have profound effects on inner hair cell function by way of regulating intrinsic excitability of these cells. In theory, activation of MC2R also leads to glucocorticoid synthesis, and the resulting glucocorticoids can then stimulate numerous targets within the cochlea, particularly the spiral ganglion cells and the inner border cell. Synergistically, ACTH released from the inner hair cell could bind MC2R in spiral ganglion cells and exert effects there including local glucocorticoid activity.
Support cell signaling
The evidence for CRF signaling in support cells adds to a limited knowledge of the roles in auditory function and protection of this large, heterogeneous cell population. In particular, it suggests a molecular mechanism for maintenance of cochlear homeostasis via support cell function, and their role in protecting against cellular stress. This finding compliments earlier descriptions of calcium waves that extend through support cell networks in response to hair cell damage. It has been suggested that these calcium waves represent a cell-stress signal that is communicated across the region of injury (Gale et al., 2004; Piazza et al., 2007). Given that CRF represents the quintessential stress signal of the body, the demonstration of a CRF based HPA-equivalent signaling system in cochlear support cells helps confirm a major role for these cells in responding to stress. Understanding the nature of this stress response may provide useful knowledge of endogenous mechanisms conferring resistance to NIHL, and may therefore lead to novel methods and therapeutic strategies for prevention of hearing loss.
There are two major ways in which CRF signaling can influence support cell activity. First, CRF signaling modulates connexin expression, potentially leading to changes in ATP release through connexin hemi-channels, and in potassium recycling from perilymphatic compartments back to the scala media, which contains the potassium rich endolymph (Fig. 3). In the adult cochlea, CRFR2-induced changes in Cx26 and Cx30 expression may be important for suppressing endocochlear potential, thereby dulling sensitivity to sound, and also in mounting a response against oxidative stress. Second, CRF signaling may regulate HPA components expressed in support cells. In particular, POMC and ACTH co-localize with CRFR1 in both the inner sulcus cells and the lateral support cells (Fig. 1). The complete role(s) of this HPA-equivalent signaling system in the cochlea remain to be fully elucidated; however we propose that it represents a local stress response system similar to that observed in the skin (Slominski et al., 1999; Slominski et al., 2000). The localization of the ACTH receptor, MC2R, to the inner hair cell and spiral ganglion cell suggests that the majority of support cell HPA signaling converges on the afferent system to influence activity there.
RELEVANCE OF COCHLEAR HPA-EQUIVALENT SIGNALING TO HUMAN DISORDERS
Prior investigations have revealed altered acoustic sensitivity accompanying dysfunctional stress-response system function. For example, early clinical studies demonstrated hypersensitive hearing in patients with Addison’s disease, a condition in which ACTH and cortisol are under-produced (Henkin et al., 1967). Interestingly, studies have also demonstrated abnormal auditory processing in depressed patients, and a recent study reveals hypersensitive hearing in patients with post-traumatic stress disorder (PTSD), a condition associated with hyperactivity of the stress system (Aubert-Khalfa et al.). The fact that disorders causing opposing effects on the stress axis yield similar auditory phenotypes most likely reflects the complicated nature of the interaction between systemic stress response and auditory function. Of particular interest for understanding the role of the HPA-equivalent system expressed locally within the cochlea are disorders that could cause local changes in stress hormone signaling within the cochlea separate from global effects on the systemic stress system. Most of the genetic alterations reported for CRF or the CRF receptors in humans are single nucleotide polymorphisms, and these have not yet been correlated with auditory dysfunction. However, some disorders are associated with overt changes in expression of CRF or its receptors. Rett syndrome, for instance, is a sex-linked developmental disorder causing mental retardation and autistic symptoms. It results from a genetic mutation in a transcriptional repressor, MeCP2. CRF is a target of MeCP2 repression, and therefore Rett syndrome leads to increased CRF expression (McGill et al., 2006). Mouse studies suggest that this increase in CRF contributes to the anxiety associated with Rett syndrome. Hearing deficits have also been described in patients with Rett syndrome (Nicholas et al., 1999; Pillion and Naidu, 2000; Pillion et al., 2003). Loss of MeCP2 activity affects several genes associated with auditory development and function, such as brain-derived neurotrophic factor (BDNF) (Kline et al., 2010). However, it will be critical to determine to what extent the elevated CRF expression is causal to the auditory phenotype. The opposing and balancing effects of the CRF receptors on auditory function make it difficult to precisely determine how excess CRF could lead to hearing impairments. One mechanism may involve a compensatory down regulation of CRFR1 (as has been demonstrated in the brain during exposure to exogenous CRF). This would shunt CRF signaling toward CRFR2, which typically acts to dull auditory sensitivity as indicated by the hypersensitivity exhibited by the CRFR2 null mice. Alternatively, elevated CRF may lead to changes in cochlear development, a possibility suggested by the morphological findings with CRFR1 null mice.
A chromosomal deletion of the region containing the CRFR1 gene has also been described. The resulting disorder is referred to as 17q21.31 microdeletion syndrome (Tan et al., 2009). Some patients with this disorder experience auditory impairment. However, any potential sensory-neural deficits are confounded by conductive hearing loss due to chronic middle ear infection (otitis media) experienced by these patients. Given the auditory phenotype observed in the CRFR1 null mice, it may seem strange that this chromosomal deletion syndrome does not lead to a more substantial and more pervasive auditory impairment among patients. However, it should be noted that this disorder results from a copy number variation – patients lose one copy of the genes in that chromosomal region, but not from the other chromosome. Therefore, with one functional copy of CRFR1 remaining, auditory defects may not be as robust in these patients as expected based on studies in CRFR1 homozygous null mice, which clearly possess no functional copies of the CRFR1 gene. Nonetheless, it would be interesting to observe how auditory thresholds across a population of 17q21.31 deletion patients compare to normal population thresholds. More subtle auditory deficits may emerge in such a study, even if auditory sensitivity does not fall outside the range of what is considered clinically normal.
Similarly, CRFR1 and CRFR2 polymorphisms have been implicated in a variety of conditions including, asthma, obesity, depression, and alcoholism (Tantisira et al., 2004; Jiang et al., 2006; De Luca et al., 2007; Nelson et al., 2010). A clinical study evaluating the genotype of patients presenting with auditory deficits has yet to be performed to determine if CRFR1 and CRFR2 polymorphisms show any correlation with abnormal auditory function. Linked to such a clinical investigation, a longitudinal study following populations of people with such polymorphisms designed to evaluate whether they show increased susceptibilities to noise or age-related hearing loss would help round out the role of the cochlear HPA-equivalent signaling system in human audition.
Finally, as humans age, hearing function generally decreases. This condition, known as presbycusis or simply age-related hearing loss, has been estimated to affect 30–35% of the population aged 65–75 years, and 40–50% of those individuals aged 75 years and greater. The causes of presbycusis are multi-factorial, and include both genetic and environmental factors (Van Eyken et al., 2007). Hearing dysfunction finally arises from either loss of the sensory hair cells of the cochlea (sensory presbycusis) or altered blood flow to the cochlea (strial presbycusis) (Ohlemiller, 2009; Fetoni et al., 2011). Interestingly, T-type calcium channel blockers that are used as anticonvulsant drugs seem to offer therapeutic advantage in delaying age-related loss of spiral ganglion cells and protecting against noise-induced hearing loss (Shen et al., 2007; Lei et al., 2011) Because presbycusis is considered to be the endpoint of cumulative insults over the lifetime of the individual, a system such as CRF signaling and the cochlear HPA-equivalent system may play a role in delaying or, in the case of dysfunction of the system, hastening the occurrence of presbycusis. Because the revelation of CRF signaling, and more globally the existence of a cochlear HPA-equivalent system is so recent, there is currently no known link between dysfunction of the cochlear CRF signaling system and presbycusis.
PUTTING LOCAL HPA-LIKE, CRF-BASED SIGNALING INTO A LARGER CONTEXT
The inner ear develops from the otic placode, which is a thickening of head ectoderm. The head ectoderm itself is a simple cuboidal epithelial structure situated above a basal lamina. As the otic placode develops, it ultimately invaginates, first forming the otic pit, and finally, as it continues to sink and become pinched off from the surface, it develops into the otic vesicle (also termed the otocyst). Thus, the otic vesicle is essentially an epithelial cyst. All of the cells that will develop and differentiate to generate both early and adult inner ear structures are derived from the epithelial wall of the otocyst (reviewed in (Goodyear et al., 2006).
It can be generally agreed that evolution tends to find solutions to problems and then adapt such solutions for other problems. Thus, the cochlea is considered to be an offshoot of the vestibular apparatus, and to have emerged with the transition of life from water to land environments (for review, see (Manley and Koppl, 1998). In a similar manner, cell-signaling systems can be traced from early origins to sophisticated modern examples. Of special interest is that the cochlea and the skin have been shown to share what at first inspection might be viewed as some very unusual signaling systems. Thus, hair cells express an evolutionarily ancient acetylcholine nicotinic class of receptor subunit genes: the chrna9 and chrna10, which encode the α9 and α10 proteins. These genes are expressed in peripheral tissues, but to date have not been demonstrated in the brain. However, they are expressed in some peripheral neurons, such as those of the dorsal root ganglia (Lips et al., 2002; Haberberger et al., 2004). Evidence suggests that these genes are involved in modulating cell:cell adhesion in both the inner ear and keratinocytes, and loss of function results in abnormal synapse formation in the cochlea (Murthy et al., 2009) and potentially greater susceptibility to blistering diseases (acantholysis) of the skin, such as pemphigus vulgaris (Nguyen et al., 2000; Grando et al., 2001; Nguyen et al., 2003; Nguyen et al., 2004; Chernyavsky et al., 2007).
In a similar manner, CRF and associated signaling molecules have been localized in the skin (reviewed in (Slominski et al., 1999; Slominski et al., 2000; Slominski et al., 2001)), and it has been suggested that HPA-like signaling may have originated there and been co-opted and refined with the evolution of the nervous system (Slominski and Mihm, 1996). From a functional perspective, activation of the cutaneous CRF system can be exerted by pro-inflammatory cytokines (Turnbull and Rivier, 1995; Turnbull and Rivier, 1999; Turnbull et al., 1999), as well as by POMC derivatives (Slominski et al., 2006b). While it is tempting to immediately assign a protective role against inflammation to CRF signaling, regulation, and therefore function, of the system is highly dependent on the cellular context within which the local system exists. For example, in keratinocytes, CRF signaling is pro-inflammatory due to weak coupling between CRFR1 and cAMP and POMC activity (Slominski et al., 2006a; Slominski et al., 2006b). However, in melanocytes, CRF signaling is anti-inflammatory, effectively inhibiting NF-κB via POMC-induced activity (Zbytek et al., 2006), among other mechanisms. Therefore, critical attention must be paid to downstream protein:protein interactions when assessing the role of CRF signaling in any local context.
With respect to a comparison of skin with inner ear, one can recognize basic similarities between the two systems. Most importantly, both are open to and constantly stimulated by the environment. Both are evolutionarily quite old, and play important roles in survival of the species. Thus, there must be mechanisms that ensure homeostatic balance can be maintained. From a cellular perspective, both are epithelial structures, and the inner ear has its early origins in the epithelium. Not surprisingly, therefore, it is probable that the inner ear brought with it many of the refined signaling systems of the skin while it was evolving, and maintained these systems due to the similar nature of physical challenges faced by the inner ear compared to skin. Investigation into the cutaneous HPA-like signaling system is more mature than the recently described cochlear HPA-equivalent system, and more similarities will almost surely be uncovered between these systems in the future. Given that an HPA-like signaling system has also recently been described in the retina (Zmijewski et al., 2007), repeated expression of signaling systems among different organs may be much more common than previously envisioned. Complete understanding of the exact role for such local HPA-equivalent signaling, however, will be dependent on further evaluations of downstream signaling systems set into motion by CRFR activation.
Finally, it must be recognized that any local HPA-like system, such as that described here in the cochlea, is enveloped by the systemic (classic) stress-response system. Indeed, the evidence is good for both systems being able to alter cochlear processing. The exact interplay that occurs between the local and systemic systems awaits further investigation. It is highly likely that the systemic system can impact the local system in most cases, but the level to which systemic stress-response system activity needs to rise to induce biological change in the local system versus the level of activity needed by the local stress-response system to affect change in the local environment is a topic requiring further exploration. These thresholds may be the key to understanding the function of these local systems.
acknowledgements
supported by NIH R01DC006258 (DEV) and research funds from UMMC (DEV).
REFERENCES
- Andeol G, Guillaume A, Micheyl C, Savel S, Pellieux L, Moulin A. Auditory efferents facilitate sound localization in noise in humans. J Neurosci. 2011;31:6759–6763. doi: 10.1523/JNEUROSCI.0248-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert-Khalfa S, Granier J-P, Reynaud E, El Khoury M, Grosse E-M, Samuelian J-C, Blin O. Pure-tone auditory thresholds are decreased in depressed people with post-traumatic stress disorder. J AffecDis. 2010;127:169–176. doi: 10.1016/j.jad.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Barry SP, Lawrence KM, McCormick J, Soond SM, Hubank M, Eaton S, Sivarajah A, Scarabelli TM, Knight RA, Thiemermann C, Latchman DS, Townsend PA, Stephanou A. New targets of urocortin-mediated cardioprotection. J Mol Endocrinol. 2010;45:69–85. doi: 10.1677/JME-09-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basappa J, Turcan S, Vetter DE. Corticotropin-releasing factor-2 activation prevents gentamicin-induced oxidative stress in cells derived from the inner ear. J Neurosci Res. 2010;88:2976–2990. doi: 10.1002/jnr.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Hafidi A, Skinner L, Ruel J, Nouvian R, Henaff M, Puel J, Aran J, Dulon D. Ryanodine receptors and BK channels act as a presynaptic depressor of neurotransmission in cochlear inner hair cells. Eur J Neurosci. 2005;22:1109–1119. doi: 10.1111/j.1460-9568.2005.04310.x. [DOI] [PubMed] [Google Scholar]
- Brandt A, Striessnig J, Moser T. CaV1.3 channels are essential for development and presynaptic activity of cochlear inner hair cells. J Neurosci. 2003;23:10832–10840. doi: 10.1523/JNEUROSCI.23-34-10832.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Sausbier M, Wietzorrek G, Sausbier U, Knaus HG, Russell JA, Ruth P, Shipston MJ. Hypothalamic-Pituitary-Adrenal Axis Hyporesponsiveness to Restraint Stress in Mice Deficient for Large-Conductance Calcium- and Voltage-Activated Potassium (BK) Channels. Endocrinology. 2007;148:5496–5506. doi: 10.1210/en.2007-0319. [DOI] [PubMed] [Google Scholar]
- Buran BN, Strenzke N, Neef A, Gundelfinger ED, Moser T, Liberman MC. Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. J Neurosci. 2010;30:7587–7597. doi: 10.1523/JNEUROSCI.0389-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canlon B, Fransson A. Morphological and functional preservation of the outer hair cells from noise trauma by sound conditioning. Hear Res. 1995;84:112–124. doi: 10.1016/0378-5955(95)00020-5. [DOI] [PubMed] [Google Scholar]
- Canlon B, Borg E, Flock A. Protection against noise trauma by pre-exposure to a low level acoustic stimulus. Hear Res. 1988;34:197–200. doi: 10.1016/0378-5955(88)90107-4. [DOI] [PubMed] [Google Scholar]
- Canlon B, Meltser I, Johansson P, Tahera Y. Glucocorticoid receptors modulate auditory sensitivity to acoustic trauma. Hear Res. 2007;226:61–69. doi: 10.1016/j.heares.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Chang Q, Tang W, Ahmad S, Zhou B, Lin X. Gap junction mediated intercellular metabolite transfer in the cochlea is compromised in connexin30 null mice. PLoS One. 2008;3:e4088. doi: 10.1371/journal.pone.0004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci (USA) 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kujawa SG, Sewell WF. Auditory sensitivity regulation via rapid changes in expression of surface AMPA receptors. Nat Neurosci. 2007;10:1238–1240. doi: 10.1038/nn1974. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peppi M, Kujawa SG, Sewell WF. Regulated expression of surface AMPA receptors reduces excitotoxicity in auditory neurons. J Neurophysiol. 2009;102:1152–1159. doi: 10.1152/jn.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyavsky AI, Arredondo J, Vetter DE, Grando SA. Central role of alpha9 acetylcholine receptor in coordinating keratinocyte adhesion and motility at the initiation of epithelialization. Exp Cell Res. 2007;313:3542–3555. doi: 10.1016/j.yexcr.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P. The role of outer hair cells in cochlear function. Prog Clin Biol Res. 1985;176:207–230. [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel E. Noise and hearing loss: a review. J Sch Health. 2007;77:225–231. doi: 10.1111/j.1746-1561.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Darrat I, Ahmad N, Seidman K, Seidman MD. Auditory research involving antioxidants. Curr Opin Otolaryngol Head Neck Surg. 2007;15:358–363. doi: 10.1097/MOO.0b013e3282efa641. [DOI] [PubMed] [Google Scholar]
- de Boer J, Thornton AR. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. J Neurosci. 2008;28:4929–4937. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca V, Tharmalingam S, Kennedy JL. Association study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. European psychiatry : the journal of the Association of European Psychiatrists. 2007;22:282–287. doi: 10.1016/j.eurpsy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Dewson JH., 3rd Efferent, Olivocochlear Bundle: some relationships to stimulus discrimination in noise. J Neurophysiol. 1968;31:122–130. doi: 10.1152/jn.1968.31.1.122. [DOI] [PubMed] [Google Scholar]
- Dewson JHd. Efferent olivocochlear bundle: some relationships to noise masking and to stimulus attenuation. J Neurophysiol. 1967;30:817–832. doi: 10.1152/jn.1967.30.4.817. [DOI] [PubMed] [Google Scholar]
- Eybalin M, Caicedo A, Renard N, Ruel J, Puel JL. Transient Ca2+-permeable AMPA receptors in postnatal rat primary auditory neurons. Eur J Neurosci. 2004;20:2981–2989. doi: 10.1111/j.1460-9568.2004.03772.x. [DOI] [PubMed] [Google Scholar]
- Fetoni AR, Picciotti PM, Paludetti G, Troiani D. Pathogenesis of presbycusis in animal models: a review. Experimental gerontology. 2011;46:413–425. doi: 10.1016/j.exger.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Fettiplace R, Fuchs PA. Mechanisms of hair cell tuning. Annu Rev Physiol. 1999;61:809–834. doi: 10.1146/annurev.physiol.61.1.809. [DOI] [PubMed] [Google Scholar]
- Frank T, Rutherford MA, Strenzke N, Neef A, Pangrsic T, Khimich D, Fejtova A, Gundelfinger ED, Liberman MC, Harke B, Bryan KE, Lee A, Egner A, Riedel D, Moser T. Bassoon and the synaptic ribbon organize Ca(2)+ channels and vesicles to add release sites and promote refilling. Neuron. 2010;68:724–738. doi: 10.1016/j.neuron.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredelius L, Rask-Andersen H, Johansson B, Urquiza R, Bagger-Sjoback D, Wersall J. Time sequence of degeneration pattern of the organ of Corti after acoustic overstimulation. A light microscopical and electrophysiological investigation in the guinea pig. Acta Otolaryngol. 1988;106:81–93. doi: 10.3109/00016488809107374. [DOI] [PubMed] [Google Scholar]
- Furness DN, Lawton DM, Mahendrasingam S, Hodierne L, Jagger DJ. Quantitative analysis of the expression of the glutamate-aspartate transporter and identification of functional glutamate uptake reveal a role for cochlear fibrocytes in glutamate homeostasis. Neuroscience. 2009;162:1307–1321. doi: 10.1016/j.neuroscience.2009.05.036. [DOI] [PubMed] [Google Scholar]
- Gale JE, Piazza V, Ciubotaru CD, Mammano F. A mechanism for sensing noise damage in the inner ear. Curr Biol. 2004;14:526–529. doi: 10.1016/j.cub.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Golshani P, Liu XB, Jones EG. Differences in quantal amplitude reflect GluR4- subunit number at corticothalamic synapses on two populations of thalamic neurons. Proc Natl Acad Sci (USA) 2001;98:4172–4177. doi: 10.1073/pnas.061013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Kros CJ, Richardson GP. The development of hair cells in the inner ear. In: Eatock RA, Fay RR, Popper AN, editors. Vertebrate Hair Cells. New York: Springer; 2006. pp. 20–94. [Google Scholar]
- Graham CE, Vetter DE. The mouse cochlea expresses a local hypothalamic-pituitary-adrenal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity. J Neurosci. 2011;31:1267–1278. doi: 10.1523/JNEUROSCI.4545-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CE, Basappa J, Vetter DE. A corticotropin-releasing factor system expressed in the cochlea modulates hearing sensitivity and protects against noise-induced hearing loss. Neurobiol Dis. 2010;38:246–258. doi: 10.1016/j.nbd.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos DK, Chrousos GP. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends in endocrinology and metabolism: TEM. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- Grando SA, Pittelkow MR, Shultz LD, Dmochowski M, Nguyen VT. Pemphigus: an unfolding story. J Invest Dermatol. 2001;117:990–995. doi: 10.1046/j.0022-202x.2001.01489.x. [DOI] [PubMed] [Google Scholar]
- Haberberger RV, Bernardini N, Kress M, Hartmann P, Lips KS, Kummer W. Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Autonomic neuroscience : basic & clinical. 2004;113:32–42. doi: 10.1016/j.autneu.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Hanstein R, Trotter J, Behl C, Clement AB. Increased connexin 43 expression as a potential mediator of the neuroprotective activity of the corticotropin-releasing hormone. Molecular endocrinology. 2009;23:1479–1493. doi: 10.1210/me.2009-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin RI, Knigge KM. Effect of sound on the hypothalamic-pituitary-adrenal axis. Am J Physiol. 1963;204:701–704. doi: 10.1152/ajplegacy.1963.204.4.710. [DOI] [PubMed] [Google Scholar]
- Henkin RI, McGlone RE, Daly R, Bartter FC. Studies on auditory thresholds in normal man and in patients with adrenal cortical insufficiency: the role of adrenal cortical steroids. J Clin Invest. 1967;46:429–435. doi: 10.1172/JCI105544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Nin F, Tsuzuki C, Kurachi Y. How is the highly positive endocochlear potential formed? The specific architecture of the stria vascularis and the roles of the ion-transport apparatus. Pflugers Arch. 2010;459:521–533. doi: 10.1007/s00424-009-0754-z. [DOI] [PubMed] [Google Scholar]
- Himeno C, Komeda M, Izumikawa M, Takemura K, Yagi M, Weiping Y, Doi T, Kuriyama H, Miller JM, Yamashita T. Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res. 2002;167:61–70. doi: 10.1016/s0378-5955(02)00345-3. [DOI] [PubMed] [Google Scholar]
- Hoang Dinh E, Ahmad S, Chang Q, Tang W, Stong B, Lin X. Diverse deafness mechanisms of connexin mutations revealed by studies using in vitro approaches and mouse models. Brain Res. 2009;1277:52–69. doi: 10.1016/j.brainres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Marcotti W, Navaratnam D, Yamoah EN. Hair cells--beyond the transducer. J Membr Biol. 2006;209:89–118. doi: 10.1007/s00232-005-0835-7. [DOI] [PubMed] [Google Scholar]
- Housley GD, Jagger DJ, Greenwood D, Raybould NP, Salih SG, Jarlebark LE, Vlajkovic SM, Kanjhan R, Nikolic P, Munoz DJ, Thorne PR. Purinergic regulation of sound transduction and auditory neurotransmission. Audiol Neurootol. 2002;7:55–61. doi: 10.1159/000046865. [DOI] [PubMed] [Google Scholar]
- Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res. 2002;166:62–71. doi: 10.1016/s0378-5955(02)00286-1. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005;19:1332–1334. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: Physiology and role in disease. Nat. Rev. Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Michal JJ, Williams GA, Daniels TF, Kunej T. Cross species association examination of UCN3 and CRHR2 as potential pharmacological targets for antiobesity drugs. PLoS One. 2006;1:e80. doi: 10.1371/journal.pone.0000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A, Alexandrova O, Grothe B. Urocortin-expressing olivocochlear neurons exhibit tonotopic and developmental changes in the auditory brainstem and in the innervation of the cochlea. J Comp Neurol. 2011 doi: 10.1002/cne.22650. [DOI] [PubMed] [Google Scholar]
- Kirk EC, Smith DW. Protection from acoustic trauma is not a primary function of the medial olivocochlear efferent system. J Assoc Res Otolaryngol. 2003;4:445–465. doi: 10.1007/s10162-002-3013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci. 2010;30:5303–5310. doi: 10.1523/JNEUROSCI.5503-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig S, Luger TA, Scholzen TE. Monitoring neuropeptide-specific proteases: processing of the proopiomelanocortin peptides adrenocorticotropin and alpha-melanocyte-stimulating hormone in the skin. Exp Dermatol. 2006;15:751–761. doi: 10.1111/j.1600-0625.2006.00472.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC. Sudden Sensorineural Hearing Loss: A Review of Diagnosis, Treatment, and Prognosis. Trends in amplification. 2011 doi: 10.1177/1084713811408349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuizon E, Pearce EG, Bailey SG, Chen-Scarabelli C, Yuan Z, Abounit K, McCauley RB, Saravolatz L, Faggian G, Mazzucco A, Townsend PA, Scarabelli TM. Mechanisms of action and clinical implications of cardiac urocortin: a journey from the heart to the systemic circulation, with a stopover in the mitochondria. International journal of cardiology. 2009;137:189–194. doi: 10.1016/j.ijcard.2009.03.112. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai GJ, McCobb DP. Opposing actions of adrenal androgens and glucocorticoids on alternative splicing of Slo potassium channels in bovine chromaffin cells. Proc Natl Acad Sci (USA) 2002;99:7722–7727. doi: 10.1073/pnas.112619799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2007;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecain E, Yang TH, Tran Ba Huy P. Steroidogenic enzyme expression in the rat cochlea. Acta Otolaryngol. 2003;123:187–191. doi: 10.1080/0036554021000028106. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chiba T, Marcus DC. P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. J Neurosci. 2001;21:9168–9174. doi: 10.1523/JNEUROSCI.21-23-09168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei D, Gao X, Perez P, Ohlemiller KK, Chen CC, Campbell KP, Hood AY, Bao J. Anti-epileptic drugs delay age-related loss of spiral ganglion neurons via T-type calcium channel. Hear Res. 2011;278:106–112. doi: 10.1016/j.heares.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman M. Single-neuron labeling in the cat auditory nerve. Science. 1982;216:1239–1241. doi: 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections. Hear Res. 1980;3:45–63. doi: 10.1016/0378-5955(80)90007-6. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Lim DJ. Functional structure of the organ of Corti: a review. Hear Res. 1986;22:117–146. doi: 10.1016/0378-5955(86)90089-4. [DOI] [PubMed] [Google Scholar]
- Lips K, Pfeil U, Kummer W. Coexpression of alpha 9 and alpha 10 nicotinic acetylcholine receptors in rat dorsal root ganglion neurons. Neuroscience. 2002;115:1–5. doi: 10.1016/s0306-4522(02)00274-9. [DOI] [PubMed] [Google Scholar]
- Lusk SL, Hagerty BM, Gillespie B, Caruso CC. Chronic effects of workplace noise on blood pressure and heart rate. Archives of environmental health. 2002;57:273–281. doi: 10.1080/00039890209601410. [DOI] [PubMed] [Google Scholar]
- Maeda K, Yoshida K, Ichimiya I, Suzuki M. Dexamethasone inhibits tumor necrosis factor-alpha-induced cytokine secretion from spiral ligament fibrocytes. Hear Res. 2005;202:154–160. doi: 10.1016/j.heares.2004.08.022. [DOI] [PubMed] [Google Scholar]
- Maison SF, Liberman MC. Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength. J Neurosci. 2000;20:4701–4707. doi: 10.1523/JNEUROSCI.20-12-04701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maison SF, Luebke AE, Liberman MC, Zuo J. Efferent protection from acoustic injury is mediated via alpha9 nicotinic acetylcholine receptors on outer hair cells. J Neurosci. 2002;22:10838–10846. doi: 10.1523/JNEUROSCI.22-24-10838.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Koppl C. Phylogenetic development of the cochlea and its innervation. Curr Opin Neurobiol. 1998;8:468–474. doi: 10.1016/s0959-4388(98)80033-0. [DOI] [PubMed] [Google Scholar]
- Marcus DC, Sunose H, Liu J, Bennett T, Shen Z, Scofield MA, Ryan AF. Protein kinase C mediates P2U purinergic receptor inhibition of K+ channel in apical membrane of strial marginal cells. Hear Res. 1998;115:82–92. doi: 10.1016/s0378-5955(97)00180-9. [DOI] [PubMed] [Google Scholar]
- McFadden S, Ohlemiller K, Ding D, Shero M, Salvi R. The Influence of Superoxide Dismutase and Glutathione Peroxidase Deficiencies on Noise-Induced Hearing Loss in Mice. Noise Health. 2001;3:49–64. [PubMed] [Google Scholar]
- McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci (USA) 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheyl C, Collet L. Involvement of the olivocochlear bundle in the detection of tones in noise. J Acoust Soc Am. 1996;99:1604–1610. doi: 10.1121/1.414734. [DOI] [PubMed] [Google Scholar]
- Miller JM, Brown JN, Schacht J. 8-iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol Neurootol. 2003;8:207–221. doi: 10.1159/000071061. [DOI] [PubMed] [Google Scholar]
- Monge Naldi A, Gassmann M, Bodmer D. Erythropoietin but not VEGF has a protective effect on auditory hair cells in the inner ear. Cell Mol Life Sci. 2009;66:3595–3599. doi: 10.1007/s00018-009-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Brandt A, Lysakowski A. Hair cell ribbon synapses. Cell Tissue Res. 2006a;326:347–359. doi: 10.1007/s00441-006-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser T, Neef A, Khimich D. Mechanisms underlying the temporal precision of sound coding at the inner hair cell ribbon synapse. J Physiol. 2006b;576:55–62. doi: 10.1113/jphysiol.2006.114835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. 2001;121:10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- Murthy V, Taranda J, Elgoyhen AB, Vetter DE. Activity of nAChRs containing alpha9 subunits modulates synapse stabilization via bidirectional signaling programs. Dev Neurobiol. 2009;69:931–949. doi: 10.1002/dneu.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima R, Ogita K. Enhanced biosynthesis of glutathione in the spiral ganglion of the cochlea after in vivo treatment with dexamethasone in mice. Brain Res. 2006;1117:101–108. doi: 10.1016/j.brainres.2006.07.113. [DOI] [PubMed] [Google Scholar]
- Nelson EC, Agrawal A, Pergadia ML, Wang JC, Whitfield JB, Saccone FS, Kern J, Grant JD, Schrage AJ, Rice JP, Montgomery GW, Heath AC, Goate AM, Martin NG, Madden PA. H2 haplotype at chromosome 17q21.31 protects against childhood sexual abuse-associated risk for alcohol consumption and dependence. Addiction biology. 2010;15:1–11. doi: 10.1111/j.1369-1600.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V, Ndoye A, Grando S. Novel human alpha9 acetylcholine receptor regulating keratinocyte adhesion is targeted by Pemphigus vulgaris autoimmunity. Am J Pathol. 2000;157:1377–1391. doi: 10.1016/s0002-9440(10)64651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Arredondo J, Chernyavsky AI, Kitajima Y, Grando SA. Keratinocyte acetylcholine receptors regulate cell adhesion. Life Sci. 2003;72:2081–2085. doi: 10.1016/s0024-3205(03)00087-0. [DOI] [PubMed] [Google Scholar]
- Nguyen VT, Arredondo J, Chernyavsky AI, Pittelkow MR, Kitajima Y, Grando SA. Pemphigus vulgaris acantholysis ameliorated by cholinergic agonists. Arch Dermatol. 2004;140:327–334. doi: 10.1001/archderm.140.3.327. [DOI] [PubMed] [Google Scholar]
- Nicholas S, Kei J, Woodyatt G, McPherson B. Otoacoustic emission findings in Rett syndrome. J Am Acad Audiol. 1999;10:436–444. [PubMed] [Google Scholar]
- Niskar AS, Kieszak SM, Holmes AE, Esteban E, Rubin C, Brody DJ. Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey, 1988–1994, United States. Pediatrics. 2001;108:40–43. doi: 10.1542/peds.108.1.40. [DOI] [PubMed] [Google Scholar]
- Nordmann AS, Bohne BA, Harding GW. Histopathological differences between temporary and permanent threshold shift. Hear Res. 2000;139:13–30. doi: 10.1016/s0378-5955(99)00163-x. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163–173. doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Mechanisms and genes in human strial presbycusis from animal models. Brain research. 2009;1277:70–83. doi: 10.1016/j.brainres.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Takumi Y, Matsubara A, Landsend AS, Laake JH, Usami S. Molecular organization of a type of peripheral glutamate synapse: the afferent synapses of hair cells in the inner ear. Prog Neurobiol. 1998;54:127–148. doi: 10.1016/s0301-0082(97)00054-3. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Barrow A, Jacoby RA, Wu SM. How do tonic glutamatergic synapses evade receptor desensitization? J Physiol. 2008;586:2889–2902. doi: 10.1113/jphysiol.2008.151050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patuzzi R. Ion flow in stria vascularis and the production and regulation of cochlear endolymph and the endolymphatic potential. Hear Res. 2011;277:4–19. doi: 10.1016/j.heares.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Wan R, Zhang P, Mattson MP. Urocortin, but not urocortin II, protects cultured hippocampal neurons from oxidative and excitotoxic cell death via corticotropin-releasing hormone receptor type I. J Neurosci. 2002;22:404–412. doi: 10.1523/JNEUROSCI.22-02-00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WA, McCullers D, Culmsee C, Haughey NJ, Herman JP, Mattson MP. Corticotropin-releasing hormone protects neurons against insults relevant to the pathogenesis of Alzheimer's disease. Neurobiol Dis. 2001;8:492–503. doi: 10.1006/nbdi.2001.0395. [DOI] [PubMed] [Google Scholar]
- Peppi M, Kujawa SG, Sewell WF. A corticosteroid-responsive transcription factor, promyelocytic leukemia zinc finger protein, mediates protection of the cochlea from acoustic trauma. J Neurosci. 2011;31:735–741. doi: 10.1523/JNEUROSCI.3955-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza V, Ciubotaru CD, Gale JE, Mammano F. Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti. Cell calcium. 2007;41:77–86. doi: 10.1016/j.ceca.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Pillion JP, Naidu S. Auditory brainstem response findings in Rett syndrome: stability over time. The Journal of pediatrics. 2000;137:393–396. doi: 10.1067/mpd.2000.107952. [DOI] [PubMed] [Google Scholar]
- Pillion JP, Rawool VW, Bibat G, Naidu S. Prevalence of hearing loss in Rett syndrome. Dev Med Child Neurol. 2003;45:338–343. doi: 10.1017/s0012162203000628. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WH. Metabolic aspects of Meniere's disease. Laryngoscope. 1972;82:1716–1725. doi: 10.1288/00005537-197209000-00012. [DOI] [PubMed] [Google Scholar]
- Puel JL. Chemical synaptic transmission in the cochlea. Prog Neurobiol. 1995;47:449–476. doi: 10.1016/0301-0082(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Rainnie DG, Bergeron R, Sajdyk TJ, Patil M, Gehlert DR, Shekhar A. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004;24:3471–3479. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael Y, Altschuler R. Structure and innervation of the cochlea. Brain Res Bull. 2003;60:397–422. doi: 10.1016/s0361-9230(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen GL. An efferent cochlear bundle. Anat Rec. 1942;82:441. [Google Scholar]
- Rasmussen GL. Descending, or "feed-back" connections of the auditory system of the cat. Am J Physiol. 1955;183:653. [Google Scholar]
- Rauch SD, Halpin CF, Antonelli PJ, Babu S, Carey JP, Gantz BJ, Goebel JA, Hammerschlag PE, Harris JP, Isaacson B, Lee D, Linstrom CJ, Parnes LS, Shi H, Slattery WH, Telian SA, Vrabec JT, Reda DJ. Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA : the journal of the American Medical Association. 2011;305:2071–2079. doi: 10.1001/jama.2011.679. [DOI] [PubMed] [Google Scholar]
- Rio C, Dikkes P, Liberman MC, Corfas G. Glial fibrillary acidic protein expression and promoter activity in the inner ear of developing and adult mice. J Comp Neurol. 2002;442:156–162. doi: 10.1002/cne.10085. [DOI] [PubMed] [Google Scholar]
- Shen H, Lin Z, Lei D, Han J, Ohlemiller KK, Bao J. Old mice lacking high-affinity nicotine receptors resist acoustic trauma. Hear Res. 2011;277:184–191. doi: 10.1016/j.heares.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Zhang B, Shin JH, Lei D, Du Y, Gao X, Wang Q, Ohlemiller KK, Piccirillo J, Bao J. Prophylactic and therapeutic functions of T-type calcium blockers against noise-induced hearing loss. Hear Res. 2007;226:52–60. doi: 10.1016/j.heares.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki T, Ichimiya I, Suzuki M, Mogi G. Localization of glucocorticoid receptors in the murine inner ear. Ann Otol Rhinol Laryngol. 2002;111:1133–1138. doi: 10.1177/000348940211101213. [DOI] [PubMed] [Google Scholar]
- Shipston MJ, Kelly JS, Antoni FA. Glucocorticoids block protein kinase A inhibition of calcium-activated potassium channels. J Biol Chem. 1996;271:9197–9200. doi: 10.1074/jbc.271.16.9197. [DOI] [PubMed] [Google Scholar]
- Skinner L, Enée V, Beurg M, Jung H, Ryan A, Hafidi A, Aran J, Dulon D. Contribution of BK Ca2+-activated K+ channels to auditory neurotransmission in the Guinea pig cochlea. J Neurophysiol. 2003;90:320–332. doi: 10.1152/jn.01155.2002. [DOI] [PubMed] [Google Scholar]
- Slepecky NB. Structure of the mammalian cochlea. In: Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer; 1996. pp. 44–129. [Google Scholar]
- Slominski A. POMC gene expression in mouse and hamster melanoma cells. FEBS letters. 1991;291:165–168. doi: 10.1016/0014-5793(91)81274-c. [DOI] [PubMed] [Google Scholar]
- Slominski A, Mihm MC. Potential mechanism of skin response to stress. Int J Dermatol. 1996;35:849–851. doi: 10.1111/j.1365-4362.1996.tb05049.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Baker J, Ermak G, Chakraborty A, Pawelek J. Ultraviolet B stimulates production of corticotropin releasing factor (CRF) by human melanocytes. FEBS letters. 1996;399:175–176. doi: 10.1016/s0014-5793(96)01315-4. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiol Rev. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Hwang J, Chakraborty A, Mazurkiewicz JE, Mihm M. Proopiomelanocortin, corticotropin releasing hormone and corticotropin releasing hormone receptor genes are expressed in human skin. FEBS letters. 1995;374:113–116. doi: 10.1016/0014-5793(95)01090-2. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Pisarchik A, Slominski RM, Zmijewski MA, Wortsman J. CRH functions as a growth factor/cytokine in the skin. J Cell Physiol. 2006a;206:780–791. doi: 10.1002/jcp.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton E, Mazurkiewicz J, Wei E. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am J Physiol Endocrinol Metab. 2005;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Zmijewski M, Slominski RM, Kauser S, Wortsman J, Tobin DJ. Corticotropin releasing hormone and the skin. Frontiers in bioscience : a journal and virtual library. 2006b;11:2230–2248. doi: 10.2741/1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J, Krause E, Roloff B, Sayeed M, Wei E, Zbytek B, Zipper J, Wortsman J, Paus R. Cutaneous expression of CRH and CRH-R. Is there a "skin stress response system?". Ann N Y Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee K-F. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Spreng M. Noise induced nocturnal cortisol secretion and tolerable overhead flights. Noise Health. 2004;6:35–47. [PubMed] [Google Scholar]
- Stevens A, White A. ACTH: cellular peptide hormone synthesis and secretory pathways. Results Probl Cell Differ. 2010;50:63–84. doi: 10.1007/400_2009_30. [DOI] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci. 1997;17:58–69. doi: 10.1523/JNEUROSCI.17-01-00058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol. 2005;93:557–569. doi: 10.1152/jn.00574.2004. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Oikawa K, Murashita H, Hoshino T, Tsuji S, Hara A. Protective effects of glucocorticoids on ischemia-reperfusion injury of outer hair cells. Laryngoscope. 2006;116:627–629. doi: 10.1097/01.mlg.0000200963.69342.d7. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Salman H, Canlon B. Sound conditioning protects hearing by activating the hypothalamic-pituitary-adrenal axis. Neurobiol Dis. 2007;25:189–197. doi: 10.1016/j.nbd.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Takemura K, Komeda M, Yagi M, Himeno C, Izumikawa M, Doi T, Kuriyama H, Miller JM, Yamashita T. Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig. Hear Res. 2004;196:58–68. doi: 10.1016/j.heares.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Tan TY, Aftimos S, Worgan L, Susman R, Wilson M, Ghedia S, Kirk EP, Love D, Ronan A, Darmanian A, Slavotinek A, Hogue J, Moeschler JB, Ozmore J, Widmer R, Bruno D, Savarirayan R, Peters G. Phenotypic expansion and further characterisation of the 17q21.31 microdeletion syndrome. Journal of medical genetics. 2009;46:480–489. doi: 10.1136/jmg.2008.065391. [DOI] [PubMed] [Google Scholar]
- Tantisira KG, Lake S, Silverman ES, Palmer LJ, Lazarus R, Silverman EK, Liggett SB, Gelfand EW, Rosenwasser LJ, Richter B, Israel E, Wechsler M, Gabriel S, Altshuler D, Lander E, Drazen J, Weiss ST. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004;13:1353–1359. doi: 10.1093/hmg/ddh149. [DOI] [PubMed] [Google Scholar]
- Taranda J, Maison SF, Ballestero JA, Katz E, Savino J, Vetter DE, Boulter J, Liberman MC, Fuchs PA, Elgoyhen AB. A point mutation in the hair cell nicotinic cholinergic receptor prolongs cochlear inhibition and enhances noise protection. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Cate WJ, Monder C, Marandici A, Rarey KE. 11 beta-Hydroxysteroid dehydrogenase in the rat inner ear. Am J Physiol. 1994;266:E269–E273. doi: 10.1152/ajpendo.1994.266.2.E269. [DOI] [PubMed] [Google Scholar]
- Terakado M, Kumagami H, Takahashi H. Distribution of glucocorticoid receptors and 11 beta-hydroxysteroid dehydrogenase isoforms in the rat inner ear. Hear Res. 2011 doi: 10.1016/j.heares.2011.05.006. in press. [DOI] [PubMed] [Google Scholar]
- Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier C. Regulation of the HPA axis by cytokines. Brain, behavior, and immunity. 1995;9:253–275. doi: 10.1006/brbi.1995.1026. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Smith GW, Lee S, Vale WW, Lee KF, Rivier C. CRF type I receptor-deficient mice exhibit a pronounced pituitary-adrenal response to local inflammation. Endocrinology. 1999;140:1013–1017. doi: 10.1210/endo.140.2.6675. [DOI] [PubMed] [Google Scholar]
- Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiology & neuro-otology. 2007;12:345–358. doi: 10.1159/000106478. [DOI] [PubMed] [Google Scholar]
- Vardimon L, Ben-Dror I, Avisar N, Oren A, Shiftan L. Glucocorticoid control of glial gene expression. J Neurobiol. 1999;40:513–527. doi: 10.1002/(sici)1097-4695(19990915)40:4<513::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Li C, Zhao L, Contarino A, Liberman MC, Smith GW, Marchuk Y, Koob GF, Heinemann SF, Vale W, Lee K-F. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liberman MC. Restraint stress and protection from acoustic injury in mice. Hear Res. 2002;165:96–102. doi: 10.1016/s0378-5955(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Wu X, Shen H, Yu L, Peng M, Lai WS, Ding YL. Corticotropin-releasing hormone activates connexin 43 via activator protein-1 transcription factor in human myometrial smooth muscle cells. Am J Physiol Endocrinol Metab. 2007;293:E1789–E1794. doi: 10.1152/ajpendo.00249.2007. [DOI] [PubMed] [Google Scholar]
- Xie J. Control of Alternative Splicing of Potassium Channels by Stress Hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–209. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Dolan DF, Miller JM. Acquired resistance to acoustic trauma by sound conditioning is primarily mediated by changes restricted to the cochlea, not by systemic responses. Hear Res. 1999;127:31–40. doi: 10.1016/s0378-5955(98)00178-6. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Liberman MC. Sound conditioning reduces noise-induced permanent threshold shift in mice. Hear Res. 2000;148:213–219. doi: 10.1016/s0378-5955(00)00161-1. [DOI] [PubMed] [Google Scholar]
- Yoshida N, Kristiansen A, Liberman MC. Heat stress and protection from permanent acoustic injury in mice. J Neurosci. 1999;19:10116–10124. doi: 10.1523/JNEUROSCI.19-22-10116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbytek B, Pfeffer LM, Slominski AT. CRH inhibits NF-kappa B signaling in human melanocytes. Peptides. 2006;27:3276–3283. doi: 10.1016/j.peptides.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tang W, Ahmad S, Sipp JA, Chen P, Lin X. Gap junction-mediated intercellular biochemical coupling in cochlear supporting cells is required for normal cochlear functions. Proc Natl Acad Sci (USA) 2005;102:15201–15206. doi: 10.1073/pnas.0501859102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, Yu N. Distinct and gradient distributions of connexin26 and connexin30 in the cochlear sensory epithelium of guinea pigs. J Comp Neurol. 2006;499:506–518. doi: 10.1002/cne.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci (USA) 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, Malinow R. Postnatal synaptic potentiation: delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci. 2000;3:1098–1106. doi: 10.1038/80614. [DOI] [PubMed] [Google Scholar]
- Ziegler CG, Krug AW, Zouboulis CC, Bornstein SR. Corticotropin releasing hormone and its function in the skin. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2007;39:106–109. doi: 10.1055/s-2007-961809. [DOI] [PubMed] [Google Scholar]
- Zmijewski MA, Slominski AT. Emerging role of alternative splicing of CRF1 receptor in CRF signaling. Acta Biochim Pol. 2010;57:1–13. [PMC free article] [PubMed] [Google Scholar]
- Zmijewski MA, Sharma RK, Slominski AT. Expression of molecular equivalent of hypothalamic-pituitary-adrenal axis in adult retinal pigment epithelium. J Endocrinol. 2007;193:157–169. doi: 10.1677/joe.1.06927. [DOI] [PMC free article] [PubMed] [Google Scholar]