Abstract
Most herbaceous plants employ thermodynamically active mechanisms of phloem loading, whereas in many trees, the mechanism is passive, by diffusion. Considering the different water transport characteristics of herbs and trees, we hypothesized that water relations play a role in the adoption of phloem loading strategies. We measured whole-plant hydraulic conductance (Kp), osmolality, concentrations of polar metabolites, and key inorganic ions in recently mature leaves of 45 dicotyledonous species at midafternoon. Trees, and the few herbs that load passively, have low Kp, high osmolality, and high concentrations of transport sugars and total polar metabolites. In contrast, herbs that actively load sucrose alone have high Kp, low osmolality, and low concentrations of sugars and total polar metabolites. Solute levels are higher in sugar alcohol-transporting species, both herbs and trees, allowing them to operate at lower leaf water potentials. Polar metabolites are largely responsible for leaf osmolality above a baseline level (approximately 300 mm) contributed by ions. The results suggest that trees must offset low Kp with high concentrations of foliar transport sugars, providing the motivating force for sugar diffusion and rendering active phloem loading unnecessary. In contrast, the high Kp of most herbaceous plants allows them to lower sugar concentrations in leaves. This reduces inventory costs and significantly increases growth potential but necessitates active phloem loading. Viewed from this perspective, the elevation of hydraulic conductance marks a major milestone in the evolution of the herbaceous habit, not only by facilitating water transport but also by maximizing carbon use efficiency and growth.
Before Suc can be transported from leaves to heterotrophic regions of the plant, it must enter the phloem, a process known as phloem loading. Three phloem loading strategies have been identified for Suc, two of which are thermodynamically active and the third passive (Reidel et al., 2009; Rennie and Turgeon, 2009; Turgeon, 2010a). In the more common active mechanism, apoplastic loading, Suc enters the apoplast and is pumped into sieve element-companion cell complexes by membrane-bound transporters, using energy derived from the proton motive force (Sauer, 2007). In the second active process, polymer trapping, Suc diffuses into specialized companion cells (intermediary cells) through plasmodesmata and is converted into raffinose family oligosaccharides; it is the synthesis of raffinose family oligosaccharides that elevates the overall sugar concentration in the sieve element-companion cell complexes. In this paper, when we use the term “active loading,” we are referring to both the apoplastic and polymer trapping strategies. In the passive loading mechanism, which is not as well studied as the active strategies, diffusion alone is apparently sufficient. According to this model, the concentration of Suc in the cytosol of mesophyll cells is high enough that there is no need for any further elevation in concentration in the phloem to drive long-distance transport by Münch pressure flow (Turgeon and Medville, 1998; Reidel et al., 2009; Turgeon, 2010a).
In addition to Suc, sugar alcohols are synthesized and transported in many plants. Phloem loading of sugar alcohols is closely related to the loading of Suc because the molecules have to migrate along structurally compatible pathways. Two loading strategies have been found for sugar alcohols, apoplastic and passive; there is no polymer trapping equivalent (Reidel et al., 2009; Rennie and Turgeon, 2009).
Surveys of dicotyledonous species indicate that most herbaceous plants employ one or the other of the active loading mechanisms, whereas loading in most tree species is passive (Gamalei, 1989; Rennie and Turgeon, 2009). Indeed, a recent analysis indicates that passive loading is more highly restricted to the woody habit than previously recognized; very few herbs use this strategy (Davidson et al., 2011). Both active loading mechanisms elevate the sugar concentration, and the hydrostatic pressure, of the phloem to higher levels than passive loading (Turgeon, 2010a, 2010b). The striking difference in phloem pressure has fundamental implications with regard to the mechanism of long-distance transport, carbon partitioning, defense, and responses to elevated CO2 (Körner et al., 1995; Long et al., 2006; Turgeon, 2010b). However, to date, there is no hypothesis that explains the tight association between loading strategies and growth form.
One possibility is that active loading allows herbaceous plants to maintain low inventories of nonstructural carbohydrates in leaves to maximize the efficiency of carbon use, which has a strong positive effect on growth rate (Turgeon, 2010a). This is consistent with the fact that actively loading herbaceous plants are almost devoid of nonstructural carbohydrates in their leaves at dawn (Fondy and Geiger, 1982; Qiu and Israel, 1992; Camacho-Cristobal and González-Fontes, 1999; Niittylä et al., 2004). Indeed, Arabidopsis (Arabidopsis thaliana) leaves retain only about 10% of the daily carbon gain at the end of the night and exhibit carbon starvation responses when the dark period is extended by just 2 to 4 h (Usadel et al., 2008). However, this does not explain why most trees use passive loading instead.
Considering that trees and herbs have different water transport characteristics and that phloem loading must operate within the constraint of plant water relations, we hypothesized that loading strategies are determined, in part, by whole-plant hydraulic conductance (Kp), the ease with which water is transported from soil to the evaporation sites within leaves. Kp follows Darcy’s law (Hubbard et al., 1999), Kp = E/(ψsoil – ψleaf), where E is leaf transpiration and ψsoil and ψleaf are soil and leaf water potential, respectively. Ignoring matric potential, ψleaf = ψs + ψp + ψg, where ψs is leaf osmotic potential, ψp is leaf turgor potential, and ψg is gravitational potential. By substituting and rearranging, ψp = ψsoil – E/ Kp – ψs – ψg.
Since ψg is approximately 0.01 MPa for every 1-m increase in height, its value is relatively small, except in tall trees. Therefore, ignoring ψg, leaf turgor can be maintained by one of three strategies: (1) low transpirational water loss, (2) high hydraulic conductance, or (3) high concentrations of foliar solutes (low ψs). We reasoned that, to maintain leaf turgor at a given transpiration level, plants must have high Kp to operate at low foliar solute levels. As a consequence of having low foliar solute levels, active loading is required to drive sufficient sugar into the phloem to drive long-distance transport. Plants with low Kp, on the other hand, must accumulate high concentrations of foliar solutes, including transport sugars. High sugar levels are compatible with either active or passive loading, the latter requiring sufficiently high sugar concentrations to motivate diffusion from mesophyll cells into the phloem, and a sufficient number of plasmodesmata. We suggest that the relationship between Kp and sugar concentrations is a major factor in determining the associations between phloem loading strategies and growth form.
RESULTS
Phloem Loading Strategies
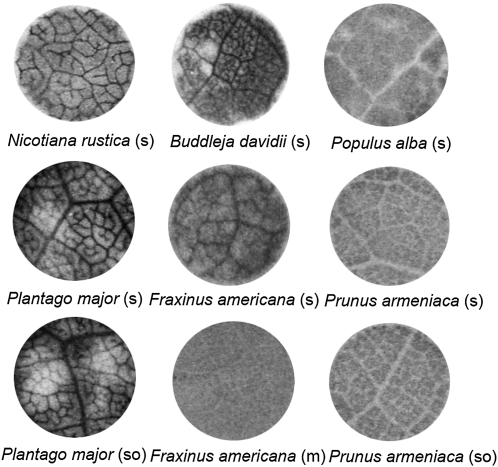
In this survey, we analyzed 17 species included in a previous study (Rennie and Turgeon, 2009) and, to extend the list of plants in our long-term program, we also studied 28 additional species, for a total of 45 (for the species list, see Table I). Loading strategies were assigned based on three parameters: transport sugars (Zimmermann and Ziegler, 1975; Rennie and Turgeon, 2009), plasmodesmatal counts from the literature (Gamalei, 1989), and autoradiographic analysis (Reidel et al., 2009; Rennie and Turgeon, 2009). In the autoradiographs (Fig. 1; Supplemental Fig. S1), radiolabel accumulates in the minor veins if phloem loading is active but not if it is passive (Reidel et al., 2009; Rennie and Turgeon, 2009). Nicotiana species load Suc actively, via the apoplast (Sauer, 2007). Plantago major loads both Suc and sorbitol apoplastically (Reidel et al., 2009), Buddleja davidii loads Suc actively, by polymer trapping (Rennie and Turgeon, 2009), as does Fraxinus americana (Zimmermann and Ziegler, 1975). However, in F. americana, as in Asarina scandens (Reidel et al., 2009), mannitol loads passively via plasmodesmata. Populus alba also loads Suc passively, consistent with the presence of abundant plasmodesmata in the minor veins (Russin and Evert, 1985), and Prunus armeniaca loads both Suc and sorbitol passively, as in apple (Malus domestica; Reidel et al., 2009). The white vein images in the autoradiographs of P. alba and P. armeniaca leaf discs are due to the presence of bundle sheath extensions (i.e. dead sclerenchyma cells above and below the veins; Reidel et al., 2009).
Table I. List of 45 species used in the survey.
Apo (s), Apoplastic loading of Suc (s); Apo (s+sa), apoplastic loading of Suc (s) and sugar alcohol (sa); GF, growth form; H, herbaceous plants; LT, loading type; Pas (s), passive loading of Suc; Pas (s+sa), passive loading of Suc and sugar alcohol; PT (s), polymer trapping of Suc; T, trees. In group PT (mix), Catalpa loads Suc alone and Fraxinus and Syringa load both Suc and mannitol.
| Species | Family | GF | LT |
| Helianthus annuus | Asteraceae | H | Apo |
| Solanum lycopersicum | Solanaceae | H | (s) |
| Nicotiana rustica | Solanaceae | H | |
| Phaseolus vulgaris | Fabaceae | H | |
| Salvia officinalis | Lamiaceae | H | |
| Solanum tuberosum | Solanaceae | H | |
| Buddleja davidii | Scrophulariaceae | H | PT |
| Coleus blumei | Lamiaceae | H | (s) |
| Cucumis sativus | Cucurbitaceae | H | |
| Cucurbita maxima | Cucurbitaceae | H | |
| Cucurbita moschata | Cucurbitaceae | H | |
| Verbascum phoeniceum | Scrophulariaceae | H | |
| Alchemilla mollis | Rosaceae | H | Pas |
| Filipendula vulgaris | Rosaceae | H | (s) |
| Fragaria ananassa | Rosaceae | H | |
| Potentilla erecta | Rosaceae | H | |
| Sanguisorba officinalis | Rosaceae | H | |
| Apium graveolens | Apiaceae | H | Apo |
| Penstemon digitalis | Scrophulariaceae | H | (s+sa) |
| Plantago major | Plantaginaceae | H | |
| Veronica spicata | Scrophulariaceae | H | |
| Alnus glutinosa | Betulaceae | T | Apo |
| Halesia tetraptera | Styracaceae | T | (s) |
| Liquidambar styraciflua | Hamamelidaceae | T | |
| Liriodendron tulipifera | Magnoliaceae | T | |
| Phellodendron lavallei | Rutaceae | T | |
| Cercidiphyllum japonicum | Cercidiphyllaceae | T | Pas |
| Corylus colurna | Betulaceae | T | (s) |
| Fagus sylvatica | Fagaceae | T | |
| Juglans ailanthifolia | Juglandaceae | T | |
| Platanus acerifolia | Platanaceae | T | |
| Populus alba | Salicaceae | T | |
| Quercus coccinea | Fagaceae | T | |
| Amelanchier laevis | Rosaceae | T | Pas |
| Malus domestica | Rosaceae | T | (s+sa) |
| Prunus armeniaca | Rosaceae | T | |
| Prunus avium | Rosaceae | T | |
| Prunus cerasus | Rosaceae | T | |
| Prunus domestica | Rosaceae | T | |
| Prunus persica | Rosaceae | T | |
| Pyrus communis | Rosaceae | T | |
| Spiraea japonica | Rosaceae | T | |
| Catalpa speciosa | Bignoniaceae | T | PT |
| Fraxinus americana | Oleaceae | T | (mix) |
| Syringa reticulata | Oleaceae | T |
Figure 1.
Representative 14C autoradiographs illustrating active loading and passive loading in the minor veins. Discs were cut from abraded leaf tissue and floated on [14C]Suc (s), [14C]mannitol (m), or [14C]sorbitol (so) for 1 h, flash frozen, lyophilized, and autoradiographed.
The 45 species in the survey represent seven combinations of growth form (herbs or trees), loading strategy (apoplastic, polymer trap, passive), and type of sugars loaded into the phloem (Suc, Suc plus sugar alcohol) that have been well characterized and are abundant enough at the species level to allow statistical comparisons. Another category in Table I, trees that load Suc by polymer trapping, with or without sugar alcohol, was not included in the statistical comparisons due to its heterogeneity and low number of species. It should be noted that we did not choose species randomly; rather, we picked those that were likely to fall into the various categories so that all categories could be compared statistically (see “Materials and Methods”). Therefore, the number of species in each category cannot be taken as representative of their relative proportions in nature, which appear to be dominated by two groups: herbs that load actively, with low foliar Suc content, and trees that load passively, with high foliar Suc content (Rennie and Turgeon, 2009; Davidson et al., 2011).
Hydraulic Conductance and Total Leaf Solutes
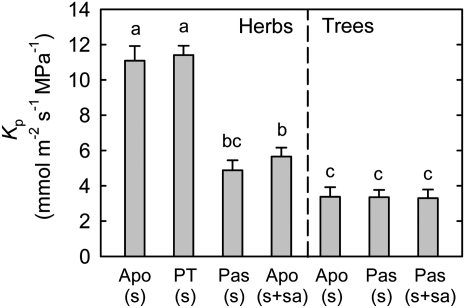
Kp values are by far highest in herbaceous species that actively load Suc alone (Fig. 2). Trees, regardless of their loading type, have the lowest Kp. Herbs that load passively have low Kp. The herbs in our survey that actively load sugar alcohol, all of which are adapted to dry or saline environments, also have low Kp.
Figure 2.
Kp in relation to phloem loading type. Leaf transpiration (E) and water potential (ψleaf) were measured at midafternoon. Predawn leaf water potential was used as an estimate of soil water potential (ψsoil) after adjusting for gravitational potential. Kp was calculated as Kp = E/(ψsoil – ψleaf). Apo, Apoplastic loading; PT, polymer trapping; Pas, passive loading; s, Suc; sa, sugar alcohols. Values are means ± se (n = 4–9 species). Different letters above the bars indicate significant differences using Duncan’s multiple range test at P < 0.05 after ANOVA.
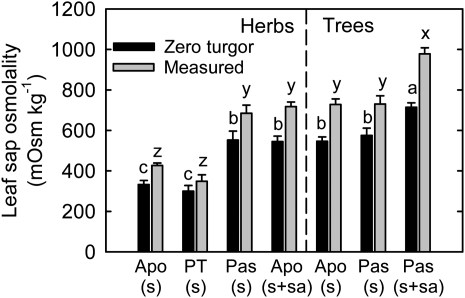
Total solute levels, measured as the osmolality of expressed leaf sap (Fig. 3), are consistent with the hydraulic conductance data. The lowest solute levels are found in herbs that actively load Suc alone. The low solute levels in these herbs (approximately 400 mm) would not be sufficient to maintain positive turgor in passive loading species (minimum of approximately 550 mm required), given their lower Kp values (Fig. 3). Consistent with these data, species that load passively, both trees and herbs, have higher solute levels (Fig. 3). Herbs that actively load sugar alcohol in addition to Suc, and trees that actively load Suc alone, also have high solute concentrations. At similar Kp values, trees that load sugar alcohol in addition to Suc have higher total solutes (approximately 1.0 m), allowing them to operate at lower leaf water potentials (Supplemental Fig. S2). Interestingly, these sugar alcohol-transporting trees have higher rates of photosynthesis than the other tree species sampled (Supplemental Table S1). A reciprocal trend was found in midafternoon leaf water potentials corresponding to leaf sap osmolality (Supplemental Fig. S2), but the measured leaf osmolality is more than enough to keep positive turgor in all loading types (Fig. 3).
Figure 3.
Calculated leaf sap osmolality at zero turgor and measured leaf sap osmolality at midafternoon in relation to phloem loading type. Leaf sap osmolality at zero turgor (C0) was calculated using the equation ψs = –C0RT, where ψs is osmotic potential, which was assumed to be equal to midafternoon leaf water potential at zero turgor, R is a universal gas constant (0.008314 L MPa−1 K mol−1), and T is the absolute temperature (K) at which leaf water potential was measured. Osmolality of the expressed sap from frozen leaf samples taken at midafternoon was measured with a freezing-point osmometer. Apo, Apoplastic loading; PT, polymer trapping; Pas, passive loading; s, Suc; sa, sugar alcohols. Values are means ± se (n = 4–9 species). Different letters above the bars of the same dependent variable indicate significant differences using Duncan’s multiple range test at P < 0.05 after ANOVA.
Leaf Polar Metabolites and Inorganic Ions
The data above indicate that high solute levels are associated with low Kp. To determine which solutes are responsible, we measured 50 polar metabolites in leaves (Supplemental Table S2), including transport sugars (defined here as sugars and sugar alcohols transported in the phloem). Stachyose, found in polymer trapping plants, is not included, as it is not resolved by our analytical procedure. However, this omission does not substantially alter the results, since stachyose is typically confined to intermediary cells and whole-leaf concentrations therefore are low (Turgeon et al., 1993).
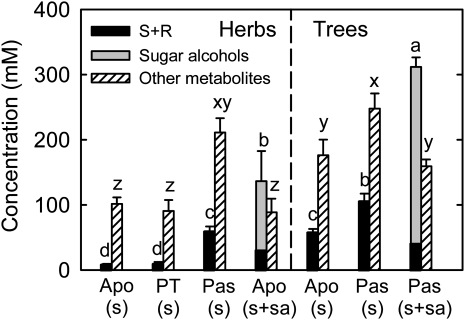
In herbaceous plants, the concentrations of transport sugars and other polar metabolites are significantly higher in passive loading species than in apoplastic loading and polymer trapping species that do not transport sugar alcohols (Fig. 4). Significantly, concentrations of transport sugars are also higher in passive loading tree species than in apoplastic loading tree species. Within the same loading type, trees have higher concentrations of transport sugars and other polar metabolites than herbaceous plants. Comparing herbs that load via the apoplast, the presence of sugar alcohols results in higher concentrations of transport sugars but not in other polar metabolites. In trees that load passively, the presence of sugar alcohol again drives up the overall concentration of transport sugars, but the concentration of other polar metabolites is lower than in species that transport Suc alone (Fig. 4).
Figure 4.
Concentrations of leaf transport sugars and other polar metabolites in relation to phloem loading type. A total of 50 polar metabolites were measured via GC-MS and HPLC. Leaf transport sugars are Suc, Suc plus raffinose (S+R) in the case of polymer trapping species, and Suc plus sugar alcohols in the case of sugar alcohol-transporting species. Other metabolites refer to polar metabolites other than transport sugars. Apo, Apoplastic loading; PT, polymer trapping; Pas, passive loading; s, Suc; sa, sugar alcohols. Values are means ± se (n = 4–9 species). Different letters above the bars of the same dependent variable indicate significant differences using Duncan’s multiple range test at P < 0.05 after ANOVA.
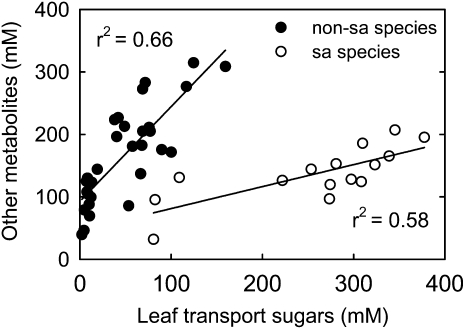
It is notable that plants with high concentrations of Suc (plus raffinose in the case of polymer trapping plants) also have high concentrations of other polar metabolites (Fig. 4). The reason for this correlation could be that the concentration of Suc is higher in the cytosol than in the vacuoles of leaf cells (Winter et al., 1993, 1994; Heineke et al., 1994; Voitsekhovskaja et al., 2006), which requires that other solutes accumulate in the vacuoles to balance the water potential in the two compartments. To explore these relationships, we plotted concentrations of other polar metabolites against transport sugars (Fig. 5), including polymer trapping trees that were not included in the statistical comparisons above. In species that do not transport sugar alcohols, a positive, linear correlation was found between concentrations of transport sugars and other polar metabolites. However, when sugar alcohol-transporting species are plotted (Fig. 5), they do not follow the same trend. The concentration of other polar metabolites increases only slightly as the concentration of transport sugars increases.
Figure 5.
Concentrations of other polar metabolites in relation to transport sugars in leaves. Leaf transport sugars are Suc, Suc plus raffinose in the case of polymer trapping species, and Suc plus sugar alcohol (sa) in the case of sugar alcohol-transporting species. Other metabolites refer to polar metabolites other than transport sugars. Non-sugar alcohol species are those that do not transport sugar alcohols. Regression equations are as follows: for non-sugar alcohol species, y = 1.517x + 92.50 (r2 = 0.66, P < 0.0001, n = 30); for sugar alcohol species, y = 0.352x + 45.89 (r2 = 0.58, P = 0.001, n = 15).
We considered the possibility that hexoses are the metabolites most responsible for balancing the osmotic effect of Suc in the cytosol. Although passive loading herbs have higher hexose concentrations than other loading types (Supplemental Fig. S3), no significant correlations are found between Suc and Glc, or the concentration of Glc and Fru combined, in leaves of different species (Supplemental Fig. S4). Among broad groups of other polar metabolites, concentrations of other sugars (soluble carbohydrates other than transport sugars) are higher in both herbs and trees that passively load Suc alone than in other loading types. Trees that load Suc alone have higher concentrations of organic acids than other loading types. Concentrations of amino acids are similar in different loading types (Supplemental Fig. S5). When metabolites are identified more specifically, the data indicate considerable species specificity. For example, more than half of the other polar metabolites in Liquidambar styraciflua and Cercidyphyllum japonicum leaves consist of shikimate and quinic acid, respectively.
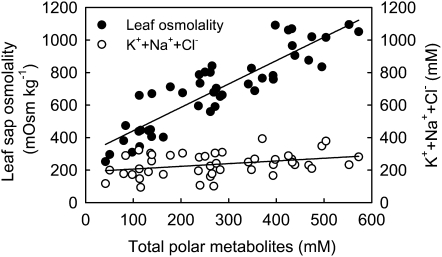
When leaf sap osmolality is plotted against the concentrations of total polar metabolites, a positive, linear relationship is apparent (Fig. 6), with an intercept of approximately 300 mOsm kg−1. To explain this intercept, we measured leaf potassium (K), sodium (Na), and chlorine (Cl) concentrations, assuming they are all in ionic forms. We found a slightly positive, linear relationship between the concentration of total polar metabolites and the concentration of K+, Na+, and Cl− combined, which accounts for about two-thirds of the intercept value of leaf sap osmolality. No significant difference was detected in the combined concentration of K+, Na+, and Cl− between loading types (Supplemental Fig. S6), whereas the concentrations of total polar metabolites (Supplemental Fig. S6) and leaf sap osmolality are very similar (Fig. 3) in relation to loading types.
Figure 6.
Leaf sap osmolality and the combined concentration of K+, Na+, and Cl– in relation to the total concentration of polar metabolites. Leaf samples were taken at midafternoon. Leaf K, Na, and Cl were measured, and the combined concentration of K+, Na+, and Cl– was calculated on the basis of leaf water content by assuming that they were all in their ionic forms. Regression equations are as follows: for leaf osmolality, y = 1.438x + 297.80 (r2 = 0.80, P < 0.0001, n = 45); for concentration of ions, y = 0.166x + 189.83 (r2 = 0.12, P = 0.021, n = 45).
Starch
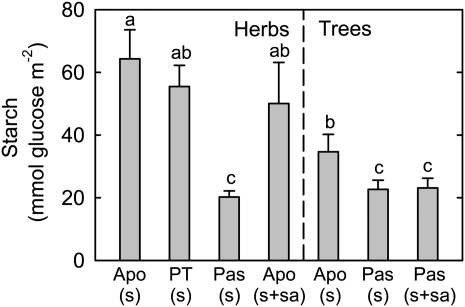
Corresponding to higher concentrations of transport sugars, leaf starch concentration is lower in passive loading species than in active loading species in both herbaceous plants and trees; apoplastic loading tree species have lower leaf starch concentrations than apoplastic loading herbaceous plants (Fig. 7).
Figure 7.
Leaf starch levels in relation to phloem loading type. Leaf starch was measured enzymatically as Glc equivalents on samples taken at midafternoon. Apo, Apoplastic loading; PT, polymer trapping; Pas, passive loading; s, Suc; sa, sugar alcohols. Values are means ± se (n = 4–9 species). Different letters above the bars indicate significant differences using Duncan’s multiple range test at P < 0.05 after ANOVA.
DISCUSSION
Our results indicate that, within the inherent limitations of a survey, passive loading is associated with low Kp, high leaf osmolality, and high concentrations of transport sugars (Figs. 2–4). This finding supports the hypothesis that passive loading species require high concentrations of foliar metabolites to maintain leaf turgor as a consequence of low hydraulic conductance. All the tree species surveyed have low Kp (Fig. 2), which is in agreement with previous work showing that, in general, hydraulic conductance is lower in trees than in herbs (Camacho-B et al., 1974; Iovi et al., 2009). Compared with herbs, trees have lower root density (Newman, 1969; Atkinson, 1980) and a much longer and more convoluted flow path (Tyree and Ewers, 1991), both of which contribute to larger whole-plant hydraulic resistance (lower Kp). The hydraulic resistance in the leaf, which accounts for about 30% of that in the whole plant, is also larger in trees than in herbaceous plants (Sack et al., 2003; Sack and Holbrook, 2006).
Considering herbs alone, those species that load Suc passively have significantly lower Kp (Fig. 2), higher leaf osmolality (Fig. 3), and higher foliar Suc concentration (Fig. 4) than the herbs that load Suc actively, indicating the need for an accumulation of high concentrations of solutes to offset low Kp. One could ask why these herbs have adopted a phloem loading mechanism more often associated with trees. Why have they not switched to active loading? It should be noted that passive loading is rare in herbs and that all five species in this study are from one family, the Rosaceae. This may be a case of phylogenetic inertia, where traits are retained for a variety of reasons in phylogenetic lineages (Blomberg and Garland, 2002). Indeed, phloem loading mechanisms are known to be conserved at the family and subfamily levels (Gamalei, 1989; Turgeon et al., 2001), and tree species are common in the Rosaceae.
Given that species with low Kp values accumulate transport sugar in their leaves, is the concentration high enough to drive long-distance transport if it simply diffuses into the phloem? Passive loading species that do not transport sugar alcohol have, on average, about 60 to 110 mm foliar Suc on a leaf water content basis (Fig. 4). Since the Suc concentration in the phloem of passive loaders must be at least marginally lower than in the mesophyll, it seems unlikely that these Suc concentrations could generate sufficient hydrostatic pressure in the phloem to drive long-distance transport. However, this reasoning neglects the effects of subcellular compartmentation in mesophyll cells. It has been consistently shown, in nonaqueous fractionation studies of leaves, that the concentration of Suc in the cytosol is much higher than in the vacuoles and can be more than 10 times higher than bulk leaf Suc concentration (Winter et al., 1993, 1994; Heineke et al., 1994; Nadwodnik and Lohaus, 2008). Therefore, with 60 to 110 mm bulk leaf Suc, the concentration in the cytosol of mesophyll cells could be very high. It is also possible that phloem Suc levels in passive loaders are relatively low. For example, willow (Salix spp.) trees are passive loaders and have the lowest phloem Suc concentration (235 mm) and lowest phloem turgor pressure value (0.79 MPa) of any species yet examined (Turgeon and Medville, 1998, and refs. therein).
As a result of the disproportionate accumulation of Suc in the cytosol of leaf mesophyll cells, other solutes must accumulate in the vacuoles and other subcellular compartments (e.g. a large proportion of amino acids are found in chloroplasts, in addition to the cytosol; Riens et al., 1991) to counterbalance the osmotic effect of Suc in the cytosol. This is most likely the reason for the correlation between leaf transport sugars and other polar metabolites in species that do not transport sugar alcohols (Fig. 5). In herbs that actively load Suc alone, inorganic ions play a major role in serving as counterbalancing solutes, as the concentrations of other polar metabolites are low (Figs. 4 and 5; Supplemental Fig. S6). In passive loading species, however, the higher Suc concentrations require additional counterbalancing solutes. The additional solutes are mainly polar metabolites, since concentrations of inorganic ions increase only slightly at higher concentrations of transport sugars (Figs. 5 and 6). Both higher Suc concentration and the correspondingly higher levels of other metabolites elevate leaf sap osmolality in passive loaders to offset lower Kp.
One of the intriguing aspects of this survey is the influence of sugar alcohols on osmolality. Sugar alcohol-transporting species have high leaf sap osmolality, due primarily to the sugar alcohol itself (Fig. 4). The shallower slope of the regression line between leaf transport sugars and other polar metabolites for sugar alcohol-transporting species (Fig. 5) suggests that a significant proportion of the sugar alcohol resides in the vacuoles and other organelles. Indeed, in peach (Prunus persica) leaves, the sorbitol concentration is similar in the cytosol and the vacuole (but higher in the chloroplasts; Nadwodnik and Lohaus, 2008). In terms of carbon cost, the number of carbon atoms in sorbitol or mannitol is half that of Suc, so it is cost effective to use sugar alcohol in the vacuole and other organelles to balance the osmolality of Suc and the sugar alcohol itself in the cytosol, as well as to elevate the hydrostatic pressure in the minor vein phloem. Not all species adhere to this scheme, however. In celery (Apium graveolens) leaves, the concentration of mannitol is much higher in the cytosol than in the vacuole of the leaf cells (Nadwodnik and Lohaus, 2008), implying that other osmotica accumulate in the vacuole to high concentration. This role is apparently played by ions: the combined concentration of K+, Na+, and Cl– in celery leaves found in this study is 392 mm.
Perhaps not surprisingly, passive loading species, with their high soluble sugar levels, tend to have less starch than active loading species (Fig. 7). Only 8% to 10% of the newly fixed 14C is partitioned to starch in poplar (Populus spp.; Dickson and Larson, 1981) and apple (Cheng et al., 2005), whereas this figure is more than 30% in garden bean (Phaseolus vulgaris; Vassey and Sharkey, 1989), potato (Solanum tuberosum; Rung et al., 2004), and Arabidopsis (Sun et al., 1999). Apparently, the synthesis of transport sugars in passive loading plants diverts carbon away from starch synthesis and provides a pool of soluble carbohydrate for use in metabolism and export at night.
In contrast to all passive loading species, the two groups of herbs that actively load Suc alone have much higher Kp values and much lower concentrations of transport sugars in their leaves (Figs. 2 and 4). These active loading herbs represent most of the herbaceous plants, which are characterized by high Kp and low foliar Suc concentration (Camacho-B et al., 1974; Iovi et al., 2009; Rennie and Turgeon, 2009). Indeed, the low foliar sugar concentration of herbs is one of their signature features. The high Kp values make it possible for these active loading herbs to have low osmolality and low foliar Suc concentrations while still maintaining turgor (Figs. 2–4). This leads to lower total nonstructural carbohydrate inventories at dawn during active growth, largely because the starch accumulated during the day is almost all used up by the end of the night (Fondy and Geiger, 1982; Qiu and Israel, 1992; Camacho-Cristobal and González-Fontes, 1999; Niittylä et al., 2004). By reducing foliar carbohydrate inventories, they free up carbon to fuel rapid growth (Smith and Stitt, 2007; Turgeon, 2010a). High relative growth rate is a critically important, adaptive characteristic of herbaceous plants (Grime and Hunt, 1975; Hunt and Cornelissen, 1997). Even within the relatively narrow ranges of specific growth forms, high Kp is associated with fast growth (Tyree et al., 1998; Pratt et al., 2010), although this is not always the case within an individual species (Fichot et al., 2011).
Although active loading is most often associated with high Kp, not all active loading species have high Kp. Two groups of plants in this survey are exceptions: herbs that actively load sugar alcohol in addition to Suc, and trees that actively load Suc, both of which have low Kp (Fig. 3). It should be pointed out that, mechanistically, active loading from the apoplast, or by polymer trapping, can function within a wide range of Suc concentrations in the cytosol of mesophyll cells and therefore can operate in species with high Kp or low Kp. The relatively small group of herbs that actively load sugar alcohols in addition to Suc have high concentrations of transport sugars and total osmolality, largely due to the presence of sugar alcohol (Figs. 3 and 4). Low Kp in these species is consistent with their high foliar solute levels. The low-Kp/high-osmolality strategy makes sense in these plants, given that they are all adapted to dry or saline conditions. Accumulation of sugar alcohols is a well-known adaptation to these harsh environments (Everard et al., 1994). Low Kp is also associated with desiccation tolerance, because smaller xylem vessel diameter reduces the risk of cavitation (Tyree and Sperry, 1989). The presence of these plants in nature only emphasizes the fact that every one of the active loading herbs that we encountered that do not transport sugar alcohol has high Kp and exceptionally low sugar.
Turning to the active loading trees, they clearly have low Kp due to their growth form; all trees have this characteristic. Significantly, they maintain lower Suc concentrations in their leaves than passive loading trees (Fig. 4), once again reinforcing the association between active loading and reduced sugar levels. Note that both Kp and sugar concentrations are similar in the active loading trees and passive loading herbs. This suggests that these sugar levels define the lowest amount of sugar that low-Kp plants can maintain. Active loading trees need only that minimal concentration because they use energy to elevate sugar levels in the phloem. Passive loading herbs are able to maintain the same low level because that is enough to motivate long-distance transport. (Passive loading trees have higher concentrations of Suc than passive loading herbs, perhaps because the transport distances are longer and they need more phloem pressure.)
Considering each of the categories of plants in this survey, the data strongly support the hypothesis that active loading is associated with efficient water conduction and low foliar sugar level, whereas the reverse is true for passive loading. In the majority of trees, high Suc concentrations apparently provide the driving force for phloem loading, whereas in the majority of herbs, low Suc concentrations in leaves necessitate active phloem loading.
CONCLUSION
Our evidence is consistent with a link between hydraulic conductance, phloem loading strategy, and growth habit. Trees must offset meager Kp values with high standing concentrations of foliar metabolites in order to maintain turgor. Given high concentrations of Suc, or Suc plus sugar alcohol, most trees do not load actively: diffusion from mesophyll cells to the phloem suffices. Sugar alcohol confers a potential advantage in this regard, since mannitol and sorbitol have twice the osmolality per unit of carbon as Suc and increase mesophyll and phloem hydrostatic pressure proportionately. High Kp values in most herbs make it possible for them to reduce foliar Suc concentrations, freeing up carbon to fuel rapid growth. However, if the Suc concentration in the mesophyll is low, energy must be expended in one of the mechanisms of active loading to elevate hydrostatic pressure in the phloem to drive long-distance transport. Viewed from this perspective, the elevation of hydraulic conductance marks a major milestone in plant evolution, not only by facilitating water transport but also by maximizing carbon use efficiency and growth, cardinal features of the herbaceous habit.
MATERIALS AND METHODS
Plant Materials
All of the 45 dicotyledonous species (Table I) grew on the Cornell University campus under natural conditions. The trees were in an open canopy with ages ranging from 4 to 25 years old, and leaves at a height of 1.5 to 3.5 m were used. Species were chosen for study based on several criteria. First, we included 17 species from a previous study (Rennie and Turgeon, 2009) for purposes of continuity and comparison. Additional species were chosen largely on the basis of availability. We selected species from different families, if possible, to avoid the problem of phylogenetic inertia, in which characters share traits due to their phylogenetic relationship rather than as evolutionary adaptations to the environment (Blomberg and Garland, 2002).
Autoradiography
14C autoradiography was performed as described (Rennie and Turgeon, 2009). Briefly, discs were cut from abraded leaf tissue and floated on [14C]Suc, [14C]mannitol, or [14C]sorbitol (20 kBq mL−1) for 1 h, flash frozen, lyophilized, pressed flat, and exposed to Kodak BioMax MR film for 24 to 72 h.
Sampling and Measurements of Kp
Recently mature, fully exposed leaves on the south-southwest side of the plant canopy were sampled at midafternoon (3:30–5:00 pm Daylight Saving Time) on clear days during active plant growth from June 20 to July 15 in both 2009 and 2010. Leaf transpiration (E) was measured with a CIRAS-1 gas-exchange system (PP Systems) at a CO2 concentration of 360 μmol mol−1, photon flux density of 1,734 ± 151 μmol m−2 s−1, air temperature of 27.8°C ± 2.3°C, and water vapor pressure of 1.31 ± 0.24 kPa. Immediately after measuring E, leaf discs and entire leaves with the major veins removed were placed into premade aluminum bags or 5-mL syringes, respectively, frozen in liquid N2, and stored at −80°C for measuring polar metabolites, starch, and osmolality. Simultaneously, leaf water potential (ψleaf) was measured with a pressure chamber (Soilmoisture Equipment). In addition, predawn leaf water potential was measured the next day and was used as an estimate of soil water potential (ψsoil) after adjusting for gravitational potential for calculating Kp = E/(ψsoil – ψleaf) (Hubbard et al., 1999). For each species, five leaf samples, each from a different plant, were taken, and the mean value was used to represent the species. The sample numbers in the figures (n = 4–9) refer to the number of species in different loading categories (Table I). We chose to sample leaves at midafternoon because (1) maximum accumulation of transport sugars and starch occurs at midafternoon instead of midday under natural conditions (Zhou et al., 2001; Rogers et al., 2004), and (2) E and ψleaf are still in steady state at midafternoon under saturating light and Kp remains stable from midday through midafternoon (Hubbard et al., 1999; Tsuda and Tyree, 2000). Prior to sampling on the same day, gas-exchange measurements were also made with a CIRAS-1 system on five fully exposed leaves per species from 10:30 am to 1:00 pm (Supplemental Table S1) to ensure that the plants to be sampled were not under water stress.
Leaf Sap Osmolality
Frozen leaf tissue stored in syringes was allowed to thaw, and the osmolality of the expressed sap was measured with a Fiske model 110 freezing-point osmometer (Advanced Instruments).
Leaf Polar Metabolites and Starch
Leaf polar metabolites and starch were extracted and measured as described (Wang et al., 2010). Briefly, soluble sugars and organic acids were extracted in 75% methanol with ribitol added as an internal standard. After fractionation of nonpolar metabolites into chloroform, 5 μL and 100 μL of the polar phase of each sample were transferred into two 2.0-mL Eppendorf vials and then dried under vacuum without heating for measuring highly abundant metabolites and less abundant metabolites, respectively. The samples were derivatized with methoxyamine hydrochloride and N-methyl-N-trimethylsilyl-trifluoroacetamide sequentially and then analyzed with an Agilent 7890A gas chromatograph/5975C mass spectrometer (Agilent Technology). Metabolites were identified by comparing fragmentation patterns with those in a mass spectral library generated on our gas chromatography-mass spectrometry (GC-MS) system and an annotated quadrupole GC-MS spectral library downloaded from the Golm Metabolome Database (http://csbdb.mpimp-golm.mpg.de/csbdb/gmd/msri/gmd_msri.html) and quantified based on standard curves generated for each metabolite and internal standards.
The tissue residue after 75% methanol extraction for GC-MS analysis was reextracted with 80% ethanol at 70°C for 30 min three times. The residue was air dried and digested with 30 units of amyloglucosidase (EC 3.2.1.3) at pH 4.5 overnight. Starch was determined enzymatically as Glc equivalents.
Free amino acids were extracted in 20 mm HCl with nor-Leu added as an internal standard. Derivatization of free amino acids was carried out using the AccQ·Fluor reagent kit (Waters). Individual amino acids were quantified using an Agilent 1100 HPLC device with a fluorescence detector. The concentration of each polar metabolite in leaves was calculated based on leaf water content.
Analysis of Leaf K, Na, and Cl
Leaf K and Na concentrations were measured with an Accuris inductively coupled plasma emission spectrometer (Fison). Tissue Cl concentration was measured with a Chloridometer (Labconco). The combined concentration of K+, Na+, and Cl– was calculated on the basis of leaf water content by assuming 100% of tissue K, Na, and Cl were in their ionic forms.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Additional 14C autoradiographs of leaf discs.
Supplemental Figure S2. Midafternoon leaf water potentials in relation to phloem loading type.
Supplemental Figure S3. Concentrations of hexoses (Glc + Fru + Gal) in relation to phloem loading type.
Supplemental Figure S4. Concentrations of Glc and Glc + Fru in relation to Suc concentration in leaves.
Supplemental Figure S5. Concentrations of other sugars, organic acids, and amino acids in relation to phloem loading type.
Supplemental Figure S6. Concentrations of total polar metabolites and the combined concentration of K+, Na+, and Cl− in relation to phloem loading type.
Supplemental Table S1. Leaf CO2 assimilation and stomatal conductance in relation to phloem loading type.
Supplemental Table S2. List of leaf polar metabolites measured via GC-MS and HPLC.
Acknowledgments
The Agilent GC-MS system used in this work was generously donated by Dr. David Zimerman. We thank Z. Liang, F. Ma, P. Li, and C. Zhang for help in sample collection and analysis and T. Björkman, J. Comstock, A. Jagendorf, A. Lakso, P. Melcher, R. Spanswick, R. Wayne, and T. Whitlow for valuable discussions of the manuscript.
References
- Atkinson D. (1980) The distribution and effectiveness of the roots of tree crops. Hortic Rev (Am Soc Hortic Sci) 2: 424–490 [Google Scholar]
- Blomberg SP, Garland T. (2002) Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods. J Evol Biol 15: 899–910 [Google Scholar]
- Camacho-B SE, Hall AE, Kaufmann MR. (1974) Efficiency and regulation of water transport in some woody and herbaceous species. Plant Physiol 54: 169–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Cristobal JJ, González-Fontes A. (1999) Boron deficiency causes a drastic decrease in nitrate content and nitrate reductase activity, and increases the content of carbohydrates in leaves from tobacco plants. Planta 209: 528–536 [DOI] [PubMed] [Google Scholar]
- Cheng L, Zhou R, Reidel EJ, Sharkey TD, Dandekar AM. (2005) Antisense inhibition of sorbitol synthesis leads to up-regulation of starch synthesis without altering CO2 assimilation in apple leaves. Planta 220: 767–776 [DOI] [PubMed] [Google Scholar]
- Davidson A, Keller F, Turgeon R. (2011) Phloem loading, plant growth form, and climate. Protoplasma 248: 153–163 [DOI] [PubMed] [Google Scholar]
- Dickson RE, Larson PR. (1981) 14C fixation, metabolic labeling patterns, and translocation profiles during leaf development in Populus deltoides. Planta 152: 461–470 [DOI] [PubMed] [Google Scholar]
- Everard JD, Gucci R, Kann SC, Flore JA, Loescher WH. (1994) Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiol 106: 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichot R, Chamaillard S, Depardieu C, Le Thiec D, Cochard H, Barigah TS, Brignolas F. (2011) Hydraulic efficiency and coordination with xylem resistance to cavitation, leaf function, and growth performance among eight unrelated Populus deltoides x Populus nigra hybrids. J Exp Bot 62: 2093–2106 [DOI] [PubMed] [Google Scholar]
- Fondy BR, Geiger DR. (1982) Diurnal pattern of translocation and carbohydrate metabolism in source leaves of Beta vulgaris L. Plant Physiol 70: 671–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamalei Y. (1989) Structure and function of leaf minor veins in trees and herbs. Trees (Berl) 3: 96–110 [Google Scholar]
- Grime JP, Hunt R. (1975) Relative growth rate: its range and adaptive significance in a local flora. J Ecol 63: 393–422 [Google Scholar]
- Heineke D, Wildenberg K, Sonnewald U, Willmitzer L, Heldt HW. (1994) Accumulation of hexoses in leaf vacuoles: studies with transgenic tobacco plants expressing yeast-derived invertase in the cytosol, vacuole or apoplasm. Planta 194: 29–33 [Google Scholar]
- Hubbard RM, Bond BJ, Ryan MG. (1999) Evidence that hydraulic conductance limits photosynthesis in old Pinus ponderosa trees. Tree Physiol 19: 165–172 [DOI] [PubMed] [Google Scholar]
- Hunt R, Cornelissen JHC. (1997) Components of relative growth rate and their interrelations in 59 temperate plant species. New Phytol 135: 395–417 [Google Scholar]
- Iovi K, Kolovou C, Kyparissis A. (2009) An ecophysiological approach of hydraulic performance for nine Mediterranean species. Tree Physiol 29: 889–900 [DOI] [PubMed] [Google Scholar]
- Körner CH, Pelaez-Riedl S, Van Bel AJE. (1995) CO2 responsiveness of plants: a possible link to phloem loading. Plant Cell Environ 18: 595–600 [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR. (2006) Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science 312: 1918–1921 [DOI] [PubMed] [Google Scholar]
- Nadwodnik J, Lohaus G. (2008) Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta 227: 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EI. (1969) Resistance to water flow in soil and plants. I. Soil resistance in relation to amounts of toot: theoretical estimates. J Appl Ecol 6: 1–12 [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. (2004) A previously unknown maltose transporter essential for starch degradation in leaves. Science 303: 87–89 [DOI] [PubMed] [Google Scholar]
- Pratt RB, North GB, Jacobsen AL, Ewers FW, Davis SD. (2010) Xylem root and shoot hydraulics is linked to life history type in chaparral seedlings. Funct Ecol 24: 70–81 [Google Scholar]
- Qiu J, Israel DW. (1992) Diurnal starch accumulation and utilization in phosphorus-deficient soybean plants. Plant Physiol 98: 316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel EJ, Rennie EA, Amiard V, Cheng L, Turgeon R. (2009) Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol 149: 1601–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Turgeon R. (2009) A comprehensive picture of phloem loading strategies. Proc Natl Acad Sci USA 106: 14162–14167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riens B, Lohaus G, Heineke D, Heldt HW. (1991) Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol 97: 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers A, Allen DJ, Davey PA, Morgan PB, Ainsworth EA, Bernacchi CJ, Cornic G, Dermody O, Dohleman FG, Heaton EA, et al. (2004) Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under free-air carbon dioxide enrichment. Plant Cell Environ 27: 449–458 [Google Scholar]
- Rung JH, Draborg HH, Jørgensen K, Nielsen TH. (2004) Carbon partitioning in leaves and tubers of transgenic potato plants with reduced activity of fructose-6-phosphate,2-kinase/fructose-2,6-bisphosphatase. Physiol Plant 121: 204–214 [DOI] [PubMed] [Google Scholar]
- Russin WA, Evert RF. (1985) Studies on the leaf of Populus deltoides (Salicaceae): ultrastructure, plasmodesmatal frequency, and solute concentrations. Am J Bot 72: 1232–1247 [Google Scholar]
- Sack L, Cowan PD, Jaikumar N, Holbrook NM. (2003) The ‘hydrology’ of leaves: coordination of structure and function in temperate woody species. Plant Cell Environ 26: 1343–1356 [Google Scholar]
- Sack L, Holbrook NM. (2006) Leaf hydraulics. Annu Rev Plant Biol 57: 361–381 [DOI] [PubMed] [Google Scholar]
- Sauer N. (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581: 2309–2317 [DOI] [PubMed] [Google Scholar]
- Smith AM, Stitt M. (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149 [DOI] [PubMed] [Google Scholar]
- Sun J, Okita TW, Edwards GE. (1999) Modification of carbon partitioning, photosynthetic capacity, and O2 sensitivity in Arabidopsis plants with low ADP-glucose pyrophosphorylase activity. Plant Physiol 119: 267–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Tyree MT. (2000) Plant hydraulic conductance measured by the high pressure flow meter in crop plants. J Exp Bot 51: 823–828 [PubMed] [Google Scholar]
- Turgeon R. (2010a) The role of phloem loading reconsidered. Plant Physiol 152: 1817–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R. (2010b) The puzzle of phloem pressure. Plant Physiol 154: 578–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Beebe DU, Gowan E. (1993) The intermediary cell: minor-vein anatomy and raffinose oligosaccharide synthesis in the Scrophulariaceae. Planta 191: 446–456 [Google Scholar]
- Turgeon R, Medville R. (1998) The absence of phloem loading in willow leaves. Proc Natl Acad Sci USA 95: 12055–12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Medville R, Nixon KC. (2001) The evolution of minor vein phloem and phloem loading. Am J Bot 88: 1331–1339 [PubMed] [Google Scholar]
- Tyree MT, Ewers FW. (1991) The hydraulic architecture of trees and other woody plants. New Phytol 119: 345–360 [Google Scholar]
- Tyree MT, Sperry JS. (1989) Vulnerability of xylem to cavitation and embolism. Annu Rev Plant Physiol Plant Mol Biol 40: 19–38 [Google Scholar]
- Tyree MT, Velez V, Dalling JW. (1998) Growth dynamics of root and shoot hydraulic conductance in seedlings of five neotropical tree species: scaling to show possible adaptation to differing light regimes. Oecologia 114: 293–298 [DOI] [PubMed] [Google Scholar]
- Usadel B, Bläsing OE, Gibon Y, Retzlaff K, Höhne M, Günther M, Stitt M. (2008) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146: 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassey TL, Sharkey TD. (1989) Mild water stress of Phaseolus vulgaris plants leads to reduced starch synthesis and extractable sucrose phosphate synthase activity. Plant Physiol 89: 1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voitsekhovskaja OV, Koroleva OA, Batashev DR, Knop C, Tomos AD, Gamalei YV, Heldt HW, Lohaus G. (2006) Phloem loading in two Scrophulariaceae species: what can drive symplastic flow via plasmodesmata? Plant Physiol 140: 383–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Ma F, Cheng L. (2010) Metabolism of organic acids, nitrogen and amino acids in chlorotic leaves of ‘Honeycrisp’ apple (Malus domestica Borkh) with excessive accumulation of carbohydrates. Planta 232: 511–522 [DOI] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW. (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191: 180–190 [Google Scholar]
- Winter H, Robinson DG, Heldt HW. (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193: 530–535 [Google Scholar]
- Zhou R, Sicher R, Quebedeaux B. (2001) Diurnal changes in carbohydrate metabolism in mature apple leaves. Aust J Plant Physiol 28: 1143–1150 [Google Scholar]
- Zimmermann MH, Ziegler H. (1975) List of sugars and sugar alcohols in sieve-tube exudates. Zimmermann MH, Milburn JA, , Encyclopedia of Plant Physiology, New Series: Transport in Plants 1. Phloem Transport. Springer, New York, pp 480–503 [Google Scholar]