SUMMARY
A study was conducted of the biological, morphological and molecular characters of 3 strains of Trypanosoma cruzi (SI5, SI8 and SIGR3) isolated from specimens of Triatoma sordida collected in Santo Inácio and a domestic cat. In order to carry out the study, the following parameters were evaluated: pre-patent period, parasitaemia curves, morphology of the parasites, mortality rates, histopathological lesions and molecular typing. The strains presented variable pre-patent periods, low parasitaemia and no animal mortality. The morphological study of trypomastigotes showed a predominance of intermediate-width and short-length forms, as well as low nuclear index. Epimastigotes presented a low nuclear index, intermediate-width forms in strains SI5 and SI8, and large-width forms in SIGR3. A shorter length could be noted in strains SI8 and SIGR3, whereas SI5 displayed an intermediate length. The histopathological study did not detect amastigote nests in tissues. The amplification of the divergent domain of 24Sα rRNA, HSP60 and GPI genes of strains SI5, SI8 and SIGR3 classified the 3 strains into Group II. Biological parameters made it possible to classify the strains isolated in Santo Inácio (BA) into Biodeme III, Zymodeme 1 and Group II of T. cruzi.
Key words: Trypanosoma cruzi, Chagas’ disease, characterization, biology, lineage, DNA, DTUs, histopathological, experimental infection, strains
INTRODUCTION
Populations of Trypanosoma cruzi, which is the Chagas disease aetiological agent, have vast intraspecific variability, including differences related to morphology, virulence, pathogenicity, evasion ability in the case of an immune response from the host, sensitivity to medication, antigenic composition and biochemical properties (Fernandes et al. 1998; Tibayrenc and Ayala, 2002). It is possible that such diversity is associated with their adaptation and survival in different hosts (Zingales et al. 1997).
Data provided by the World Health Organization (WHO) indicate that 10 million people in Latin America have Chagas disease and 25 million live in risk areas (WHO, 2010). Different clinical manifestations of the disease in humans can be associated either with specific strains or with genetic markers of the host, although both can influence the course of the infection (Sturm et al. 2003).
Around 140 species of triatomines are potential vectors of T. cruzi (Rocha et al. 2009). In Brazil, there are 52 species, but only 5 of them – Panstrongylus megistus, Triatoma brasiliensis, T. infestans, T. pseudomaculata and T. sordida – have considerable epidemiological importance, as they colonize both in and around houses (Coura and Dias, 2009). In 1950, the vector-borne transmission of Chagas disease started to be controlled in the state of São Paulo, Brazil, by activities directed towards its main vector, T. infestans. That species, which is strictly domestic, was controlled in Brazil, Chile and Uruguay, and progress has been made to eradicate it in other countries of South America (Coura and Dias, 2009). On the other hand, in 2 Brazilian states, Bahia and Rio Grande do Sul, T. infestans can still be found in residences, although at low density (Moncayo and Silveira, 2009).
As soon as satisfactory results were achieved regarding the control of T. infestans, triatomines of secondary importance, collected in peridomestic areas, started gaining relevance. Among the triatomine species with higher epidemiological importance, T. sordida has been found within the state of São Paulo, in cities such as Ribeirão Preto, São José do Rio Preto and Araçatuba (Silva et al. 2003). Some authors have also reported the presence of T. sordida in areas within the state of Bahia, the city of Santo Inácio has being an example (Cerqueira et al. 1998). The presence of vector colonies in peridomestic areas can indicate an imminent colonization inside the houses if control measures are not continuously taken. There is no doubt that a careless attitude from residents and the lack of notification about the presence of such insects in the area are factors that can contribute to their proliferation (Silva et al. 2003).
This investigation was conducted to provide a biological, morphological and molecular characterization of T. cruzi isolated from specimens of T. sordida and a domestic cat in an urban community in the municipality of Santo Inácio (BA).
MATERIALS AND METHODS
Isolation of three strains of Trypanosoma cruzi
The strains SI8 and SI5 of T. cruzi were isolated from specimens of T. sordida collected in the urban community in Santo Inácio, Bahia by João Aristeu da Rosa, 2004 (personal communication). Strain SIGR3 was isolated by xenodiagnosis on a cat from the same place by João Aristeu da Rosa, 2007 (personal communication). Since then the strains have been kept by successive passaging in Swiss mice and LIT (liver infusion tryptose) culture medium, showing exponential growth.
Trypanosoma cruzi control samples used
The strain Tm of T. cruzi was isolated from Triatoma melanocephala collected in the rural community in Poções, Bahia, 2009. The strain Tl was isolated from Triatoma lenti collected in the rural community in Macaúbas, Bahia, 2009. Both strains were isolated by abdominal compression of triatomines and have been kept in LIT culture medium.
Animals
For each strain, 5 isogenic male BALB/c mice aged around 30 days and weighing approximately 20 g were inoculated with blood trypomastigote forms by intraperitoneal injection. The procedure was performed using tuberculin syringes with a BD needle of 10×5 and a 0·3 ml dosage of blood collected from an infected mouse by cardiac puncture. The concentration of 5×103 blood trypomastigote forms per 0·3 ml of blood was adopted as standard.
Biological characterization
To study the parasitaemia curve, 15 animals were intraperitoneally inoculated with 5×103 trypomastigote forms of T. cruzi. In order to establish the infection pattern, 5 μl of blood obtained from the mice's tails were examined microscopically and the number of forms was established according to Brener's technique (1962).
Counts were performed in all the strains studied (SI5, SI8 and SIGR3) on alternate days starting from the second day after the initial inoculation until the 60th day of the infection. The number of animals that could die during the course of the infection was observed in a daily basis.
The study of the dynamics of growth of epimastigote forms in all strains studied was carried out inoculating 5×106 parasites/ml in 5 ml of LIT medium. Counts were performed in triplicate for 10 days in a Neubauer chamber under an optical microscope.
Morphological characterization
The observation of blood trypomastigote forms required the use of blood smears obtained from the animals’ tails during the acute phase. The methodology defined by Dias and Freitas Filho (1943) and Barreto (1965) was adopted as the morphological parameter.
The epimastigote forms studied were collected from the LIT culture on the 7th day after the initial inoculation, when the plateau phase of the growth curve was reached (Rossi, 2007). The smears were fixed with methanol, stained by the Giemsa method and then observed under an optical microscope at 1000× magnification. Thirty epimastigotes were studied and measured per strain, according to criteria described by Brener and Chiari (1963) to characterize slender, large and intermediate forms. The mean values obtained underwent Variance Analysis to verify if there were statistically significant differences among them within a significance level of 5%.
Histopathological study
For each strain, 21 infected animals were euthanized, 3 in each of the following days of the course of the infection: 7th, 10th, 14th, 20th and 30th, for the study of the acute stage, and 150th and 180th, for the study of the chronic phase. Fragments of the following organs were taken from the animals: heart, spleen, liver and skeletal muscle (thigh). Such fragments were kept in 10% formalin for 24 h. After being washed in running water, the pieces underwent dehydration and clearing so that they could be embedded in paraffin. Then 5-μm sections of each piece were cut with a microtome, placed on slides and stained with haematoxylin and eosin, according to the Ramos technique modified by Nai et al. (2004). The sections were examined under an optical microscope.
Molecular typing of T. cruzi isolates
DNA from culture trypanosomes (∼1×106 parasites) was extracted using the phenol/chloroform method (Lewis et al. 2009). Genotyping the T. cruzi was done using PCRs based on LSU 24 Sα-rDNA (Souto et al. 1996), HSP60 and GPI (Westenberger et al. 2005) gene sequences. The genotyping assay was performed as published by Lewis et al. (2009).
PCR amplification of LSU 24 Sα-rDNA, HSP60 and GPI genes
The amplification process was performed in a thermal cycler (Gene Amp PCR System 9700 Applied BiosystemsTM) with the following thermal profile: 24 Sα rRNA gene, 94°C for 5 min for enzyme activation and 30 cycles, including denaturation at 94°C (30 sec), annealing at 55°C (30 sec) and extension at 72°C (30 sec), the final extension taking 7 min at 72°C. The amplification process of the HSP60 and GPI genes was performed with the following thermal profile: 94°C for 3 min for enzyme activation and 4 cycles, including denaturation at 94 °C (30 sec), annealing at 64°C (30 sec) and extension at 72°C (1 min). The process was repeated at 94°C (30 sec) and for 28 cycles, including denaturation at 60°C (30 sec), annealing at 72°C (1 min) and extension at 72°C (10 min).
PCR-RFLP analysis of HSP60 and GPI genes from T. cruzi
The primers and PCR conditions employed for amplification of HSP60 and GPI gene have been described previously (Westenberger et al. 2005; Lewis et al. 2009). Amplified HSP60 and GPI genes were digested with several restriction enzymes. The enzyme EcoRV was selected using the PCR-RFLP for the HSP60 gene and the enzyme HhaI was selected for the GPI gene. Length and restriction profiles of amplified HSP60 and GPI genes were analysed by electrophoresis in 1–3% agarose gels stained with ethidium bromide.
Ethics
All the procedures were reported to and approved by the Ethical Committee for Animal Experimentation at UNESP – Araraquara – SP (Protocol n. 12/2008).
RESULTS
Biological characterization
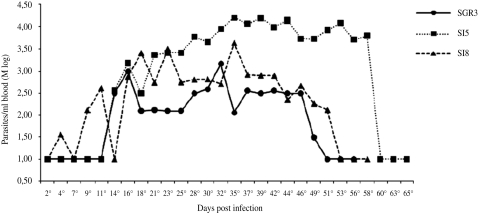
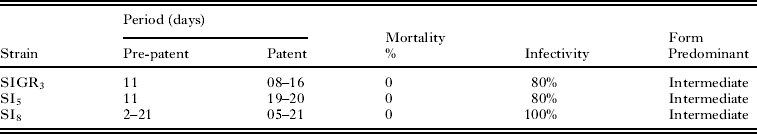
The T. cruzi isolates found in the Santo Inácio district showed low parasitaemia and infectivity, as can be seen in the average parasitaemia curve (Fig. 1). The pre-patent periods were relatively short: while SI5 and SIGR3 presented an 11-day period, the period for strain SI8 varied between 2 and 21 days (Table 1).
Fig. 1.
Parasitaemia curves from three Trypanosoma cruzi populations in isogenic BALB/c mice, expressed as logarithmic mean (Mlog).
Table 1.
Period pre-patent and patent, mortality, infectivity and prevalence in the blood of infected BALB/c mice in three Trypanosoma cruzi populations
The maximum parasitaemia for strain SI5 was reached on the 35th day (139·95×103 forms), whereas for strains SI8 and SIGR3 it was reached on the 37th (37·32×103 forms) and 32nd (30·11× 103 forms) days, respectively. After that period, the number of blood trypomastigotes starts to decrease, disappearing from the bloodstream around the 60th day of the infection.
The behaviour of each of the strains in mice showed a slow but regular development of parasitaemia until reaching a peak, followed by a regular decrease until the parasites disappeared from the peripheral blood. Animals inoculated with 5×103 trypomastigote forms of strains SI8, SI5 and SIGR3 of T. cruzi showed an infection level of 80% for strains SI5 and SIGR3 and 100% for strain SI8. All the mice infected with the 3 strains survived and developed to the chronic stage. By 180 days after the mice had been inoculated, the infection with the 3 strains (SI5, SI8 and SIGR3) had resulted in no deaths.
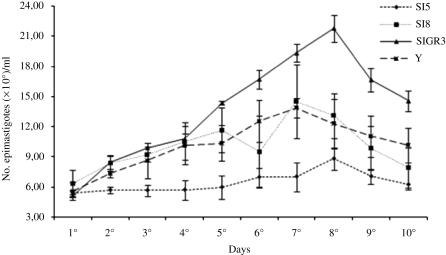
However, when their growth in LIT medium was represented in curves, some differences among the strains could be noted (Fig. 5). Strain SI5 showed a growth phase on the 8th day (8·81×106 parasites), as well as strain SIGR3 (21·79×106 parasites), whereas strain SI8 showed a growth phase on the 7th day (16·07×106 parasites), as well as control strain Y (13·90×106 parasites). Together, these results suggest that the maintenance of populations of T. cruzi II may be related to intrinsic characteristics of the parasite, such as its infection ability.
Fig. 5.
Kinetics of growth of strains of Trypanosoma cruzi. Epimastigote forms from strains SI5, SI8, SIGR3 and Y were grown in LIT medium for 10 days. The number of parasites was measured by counting in a Neubauer chamber under an optical microscope. The values represent the average of 1 experiment performed in triplicate.
Morphological characterization
By observing the blood trypomastigote forms of strains SI5, SI8 and SIGR3 during the parasitaemia peak of the experimental infection by T. cruzi, it was possible to characterize them as follows: intermediate width (P=0·0626), short length (P<0·0001) and low nuclear index (P=0·0577) (Table 2).
Table 2.
Mean morphometric parameters of blood trypomastigote forms and epimastigote forms of three Trypanosoma cruzi populations

Epimastigote forms showed: low nuclear index (P<0·0001) in all 3 strains; intermediate width in strains SI5 and SI8, and large width (P=0·0014) in strain SIGR3; short length in strains SI8 and SIGR3, and intermediate length in strain SI5 (P<0·0001) (Table 2).
Histopathological study
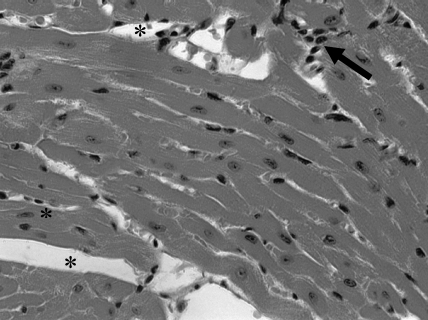
The sections prepared from heart, spleen, liver and skeletal muscle were examined under an optical microscope, but no tissue parasitism was observed, only mild inflammation was present (Fig. 6).
Fig. 6.
Myocardium of mice necropsied 180 days after inoculation of Trypanosoma cruzi obtained from strain SI5, in the presence of mononuclear cells (arrow) and intercellular spaces (*). Stain: Haematoxylin-Eosin. Magnification 20×.
Molecular characterization
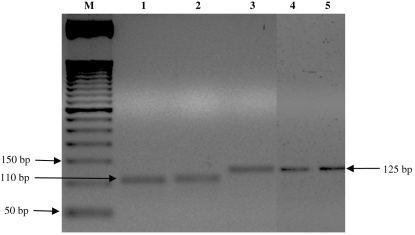
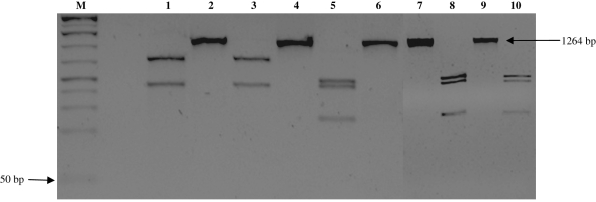
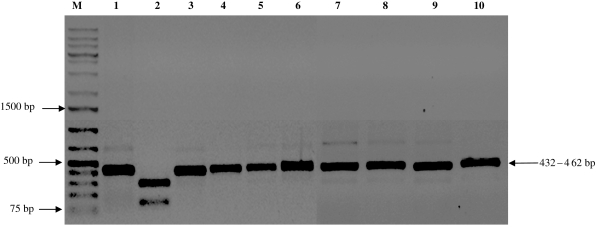
According to the DNA analysis by PCR amplification reaction, the strains isolated from specimens of T. sordida and a domestic cat in the urban community in Santo Inácio can be classified as belonging to T. cruzi group II. The amplified products presented 125 bp for the 24 Sα rRNA gene, 432–462 pb for the HSP60 gene, and 1264 bp for the GPI gene (Figs 2–4).
Fig. 2.
DNA profiles generated by genotyping of isolates of Trypanosoma cruzi using PCR assays based on 24 Sα rRNA. M, 50-bp molecular weight marker. Lane 1, Tm (control sample); lane 2, T lenti (control sample); lanes 3–5; SI5; SI8; SIGR3 strains, respectively.
Fig. 3.
DNA profiles generated by genotyping of isolates of Trypanosoma cruzi using PCR assays based on GPI fraction. M, 50-bp molecular weight marker. Lane 1, strain Tm (control sample/GPI gene digested); lane 2, strain Tm (GPI gene not digested); lane 3, T lenti, (control sample/GPI gene digested); lane 4, T lenti (GPI not digested); lane 5, SI5 strain (GPI gene digested); lane 6, SI5 strain (GPI gene not digested); lane 7, SI8 strain (GPI gene not digested); lane 9, SIGR3 strain (GPI gene not digested); lane 8, SI8 strain (GPI gene digested); lane 10, SIGR3 strain (GPI gene digested).
Fig. 4.
DNA profiles generated by genotyping of isolates of Trypanosoma cruzi using PCR assays based on HSP60 gene. M, 1-Kb Plus molecular weight marker. Lanes 1 and 2, Tm strain (control sample, HSP60 gene not digested and digested); lanes 3 and 4, T lenti strain (control sample, HSP60 gene not digested and digested); lane 5, SI5 strain (HSP60 gene not digested); lane 6, SI5 strain (HSP60 gene digested); lanes, 7 and 8: SI8 strain (HSP60 gene not digested); lanes, 9 and 10, SIGR3 strain (HSP60 gene digested).
DISCUSSION
A distinctive character of T. cruzi is its pathogenicity towards vertebrate hosts, but the level of aggressiveness varies according to the strain under study. Therefore, some strains can be highly pathogenic while others are completely unaggressive (Andrade, 1974).
Many authors, including Barreto (1965), Belda Neto (1973) and Andrade (1974), mention the variation of parasitaemia in animals inoculated with T. cruzi strains, either for samples collected from humans, wild animals or the vector itself.
The 3 strains examined in this study showed relatively short pre-patent periods, a pattern that was also observed by Martins et al. (2008) while studying strains isolated from specimens of Triatoma rubrovaria collected in the Quaraí town, Rio Grande do Sul. That variation is frequently seen when a new strain of the parasite is isolated, and it is directly related to the stabilization of the relationship between the parasite and the vertebrate host (Albuquerque, 2001).
Inoculation of laboratory animals with T. cruzi strains has resulted in a variable mortality rate (Andrade, 1974; Magalhães et al. 1985; Barata et al. 1988). In this study, such rate was equal to zero for all strains. Mice were euthanized after 180 days of the infection and no trypomastigote forms were identified in their peripheral blood. The data obtained suggest that the characteristics found for a strain in the laboratory along the years would be the result of the behaviour interaction of many clones that, being favoured by certain handling conditions, had their expression conserved in a distinctive way in comparison to other subpopulations, as already pointed out by de Araújo and Chiari (1988).
The biological study (biodeme) revealed that the strains collected in Santo Inácio (SI5, SI8 e SIGR3) belong to Biological Type III (Andrade, 1974) and Zimodeme 1 (Miles et al. 1980). The characteristics of that group include large and intermediate forms in the peripheral blood along the course of the infection, a parasitaemia peak around the 25th–30th days or after that period, and the low mortality rate of animals.
The trypomastigote form of T. cruzi can present morphological variations, as reported by Chagas back in 1909. The results obtained in this study agree with the literature, since large and intermediate blood trypomastigote forms have prevailed in most of the strains isolated from humans, triatomines and wild mammals (Brener and Chiari, 1963; Andrade, 1974; Pinto, 2000).
According to the hypothesis proposed by Zeledon and Vieto (1958), there would be a correlation between the nuclear index value and the virulence of a strain, whereupon the adaptation of a strain to a particular host would be followed by a decrease in its aggressiveness and by a migration of the nucleum to the posterior part of the parasite, causing the decrease of the nuclear index. The results obtained in this study do not support such an hypothesis because strain SI8 presented a lower nuclear index and a 100% infectivity rate for mice. The general meaning of that polymorphism has not been sufficiently investigated yet, whereupon it is unknown whether it expresses a different biological behaviour from the strains or just reflects the existence of a morphological ‘complex’, as in the case of what occurs with other trypanosomes (Brener and Chiari, 1963).
Brener and Chiari (1965) found differences in the ‘in vitro’ behaviour of 4 strains isolated from human cases and 3 isolated from Triatoma infestans in LIT medium. Chiari (1974), studying the behaviour of strain Y in LIT medium, found that there was a growth phase which corresponded to a period of 4 days. The number of epimastigotes was developing in the late exponential phase and reached its growth in the stationary phase. Our results support the authors mentioned above, as there was a growth phase on the 7th day for strain SI8 (16·07×106 parasites) and Y (13·90×106 parasites) and on the 8th day for strains SI5 (8·81×106 parasites) and SIGR3 (21·79×106 parasites).
The existence of T. cruzi strains with tropism for different tissues was reported by Melo and Brener in 1978. The study of the density of parasites in the organs of vertebrates may help to understand the pathogenesis of the disease because neuronal destruction and inflammatory processes may be directly influenced by local parasitism (Koeberle, 1968). However, tissue parasitism also depends on the intrinsic characteristics of the parasite, as well as the standardization of initial inoculation (Melo and Brener, 1978; Andrade and Magalhães, 1997; Martins et al. 2003). These authors used higher initial inoculation of 10 000 parasites/ml for histopathological study. In our study, we standardized the initial inoculum – 5×103/m (5000) – because the strains had low parasitaemia. Thus, it was not possible to view the parasite in tissues, which may have been caused by the reduced number of parasites, inherent characteristics of the strains, or the control of the experimental infection of BALB/c mice.
The presence of inflammatory changes is consistent with the observations of Andrade (1985), who noted that the parasites were often not located in tissues, particularly not in organs of animals or individuals with Chagas disease in the chronic phase. Observations by Carlos Chagas in the early twentieth century (1909) remain in effect and relate the presence of T. cruzi in tissue with the pathophysiological development of the disease. On the other hand, such observations reveal that it is common for a large number of individuals in the chronic phase to show abnormalities in tissues without the presence of the aetiological agent (Chagas and Villela, 1922).
Recently, based on the analysis of portions that codify the 24Sα rRNA, GPI and HSP60 genes by means of PCR reaction, T. cruzi strains were divided into 6 groups (Lewis et al. 2009; Zingales et al. 2009). The DNA analyses of strains collected in Santo Inácio put them into T. cruzi group II, the same group of strain Y, even though they show notable behavioural differences during the experimental infection of mice, and thus belong to different Biodemes. Regardless of the fact that the strains were isolated from hosts at different times (SI5 and SI8 were isolated from specimens of T. sordida in 2003, whereas the isolation of SIGR3 was performed by xenodiagnosis on a cat in 2006), the DNA analysis indicates that in that place there are strains belonging to the same T. cruzi group II, according to their molecular classification.
The characterization of strains SI5 and SI8 of T. cruzi – which were isolated together with another 10 from 18 specimens of T. sordida collected in the urban community in Santo Inácio, Bahia – showed no pathogenicity towards BALB/c mice regarding the morphometric parameters. Moreover, they were classified into Biodeme III (Andrade, 1974) and Zimodeme 1 (Miles et al. 1980), Group II (Zingales et al. 2009 and Lewis et al. 2009) and T. cruzi I (Anonymous, Satellite Meeting, 1999). All these characteristics are shared by strain SIGR3, which was isolated in the same place by xenodiagnosis on a domestic cat.
The study of strains SI5, SI8 and SIGR3 of T. cruzi, isolated from specimens of T. sordida and a domestic cat in the urban community in the district of Santo Inácio, Bahia, reinforces the need for entomological surveillance in Northeastern Brazil.
ACKNOWLEDGMENTS
We wish to thank Julio César Rente Ferreira Filho and Vagner José Mendonça, who collected Triatoma lenti, Eliane Góes Nascimento, from the Health Department of the State of Bahia/SESAB – Entomology Division, who sent us specimens of Triatoma melanocephala, and Roberto Monteiro de Lima, who provided this English version.
FINANCIAL SUPPORT
Financial support was provided by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasília, DF, Brazil), Programa de Apoio ao Desenvolvimento Cientifico da Faculdade de Ciências Farmacêuticas do Campus de Araraquara da Unesp and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo). The undertaken experiments comply with the current laws of the country in which they were performed.
REFERENCES
- Albuquerque S.. Considerações relativas ao comportamento biológico de amostras de uma cepa de Trypanosoma cruzi, obtidas por centrifugação diferencial. Tese Livre-Docente 2001 . Faculdade de Ciências Farmacêuticas de Ribeirão Preto. [Google Scholar]
- Andrade S. G.. Caracterização de cepas do Trypanosoma cruzi isoladas no Recôncavo Baiano. Revista de Patologia Tropical. 1974;3:65–121. [Google Scholar]
- Andrade S. G.. Morphological and behavioral characterization on Trypanosoma cruzi strains. Revista da Sociedade Brasileira de Medicina Tropical. 1985;18:39–46. [Google Scholar]
- Andrade S. G., Magalhães J. B.. Biodemes and Zymodemes of Trypanosoma cruzi strains: Correlations with clinical data and experimental pathology. Revista da Sociedade Brasileira de Medicina Tropical. 1997;30:27–35. doi: 10.1590/s0037-86821997000100006. [DOI] [PubMed] [Google Scholar]
- Anonymous. Recommendations from a Satellite Meeting. International Symposium to commemorate the 90th anniversary of the discovery of Chagas disease. Memórias do Instituto Oswaldo Cruz. 1999;94:429–432. doi: 10.1590/s0074-02761999000700085. [DOI] [PubMed] [Google Scholar]
- Araújo S. M., Chiari E.. Caracterização biológica de clones das cepas Y, CL e MR de Trypanosoma cruzi em camundongos C3H isogênicos. Memórias do Instituto Oswaldo Cruz. 1988;83:175–181. doi: 10.1590/s0074-02761988000200005. [DOI] [PubMed] [Google Scholar]
- Barata J. M. S., Rocha R. M., Rodrigues V. L. C. C., Ferraz A. N. F.. Primeiro caso autóctone de tripanossomíase Americana do Estado do Acre (Brasil) e sua relação com as cepas isoladas do caso humano e de triatomíneos silvestres da área. Revista de Saúde Pública. 1988;22:401–410. doi: 10.1590/s0034-89101988000500005. [DOI] [PubMed] [Google Scholar]
- Barreto M. P.. Tripanossomos semelhantes ao Trypanosoma cruzi em animais silvestres e sua identificação com o agente etiológico da doença de Chagas. Revista do Instituto de Medicina Tropical de São Paulo. 1965;7:305–315. [PubMed] [Google Scholar]
- Belda Neto F. M.. Estudos sobre a existência de correlação entre os dados biométricos e o grau de patogenicidade de amostras humanas do Trypanosoma cruzi Chagas, 1909. Tese Doutorado 1973 . Faculdade de Farmácia e Odontologia de Araraquara. [Google Scholar]
- Brener Z.. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Revista do Instituto de Medicina Tropical de São Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- Brener Z., Chiari E.. Variações morfológicas observadas em diferentes amostras de Trypanosoma cruzi. Revista do Instituto de Medicina Tropical de São Paulo. 1963;5:220–224. [PubMed] [Google Scholar]
- Brener Z., Chiari E.. Aspects of early growth of different Trypanosoma cruzi strains in culture medium. The Journal of Parasitology. 1965;51:922–926. [PubMed] [Google Scholar]
- Camargo E. P.. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosoma in liquid media. Revista do Instituto de Medicina Tropical de São Paulo. 1964;6:93–100. [PubMed] [Google Scholar]
- Cerqueira R. L., Kawarabayashi M., Guimarães A. C. S., Nakamura P. M., Ferraz S. N., Pinto P. L. S., Andrade H. F.. Santo Inácio Revisited: Protozoan diseases in an isolated village in northeastern Brazil after twenty years. American Journal of Tropical Medicine and Hgyiene. 1998;59:736–740. doi: 10.4269/ajtmh.1998.59.736. [DOI] [PubMed] [Google Scholar]
- Chagas C.. Nova tripanozomase humana. Estudos sobre a morfologia e o ciclo evolutivo do Schizotrypanum cruzi n. gen. n. gen. n. sp, agente etiológico de nova entidade mórbida do homem. Memórias do Instituto Oswaldo Cruz. 1909;1:159–218. [Google Scholar]
- Chagas C., Villela E.. Forma cardíaca da tripanosomíase americana. Memórias do Instituto Oswaldo Cruz. 1922;14:5–61. [Google Scholar]
- Chiari E.. Growth and differentiation of Trypanosoma cruziculture forms kept in laboratory for different periods of time. Revista do Instituto de Medicina Tropical de São Paulo. 1974;16:81–87. [PubMed] [Google Scholar]
- Coura J. R., Dias J. C. P.. Epidemiology, control and surveillance of chagas disease – 100 years after its discovery. Memórias do Instituto Oswaldo Cruz. 2009;104:31–40. doi: 10.1590/s0074-02762009000900006. [DOI] [PubMed] [Google Scholar]
- Dias E., Freitas Filho L.. Introdução ao estudo biométrico dos hemoflagelados do gênero Schizotrypanum. Memória do Instituto Oswaldo. 1943;38:427–436. [Google Scholar]
- Fernandes O., Souto R. P., Castro J.Á., Pereira J. B., Fernandes N. C., Junqueira A. C., Naiff R. D., Barrett T. V., Degrave W., Zingales B., Campbell D. A., Coura J. R.. Brazilian isolates of Trypanosoma cruzi from humans and triatomines classified into two lineages using mini-exon and ribosomal RNA sequences. American Journal of Tropical Medicine and Hyiene. 1998;58:807–811. doi: 10.4269/ajtmh.1998.58.807. [DOI] [PubMed] [Google Scholar]
- Koeberle F.. Chagas disease and Chagas syndromes: the pathology of American Trypanosomiasis. Advances in Parasitology. 1968;6:63–110. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- Lewis M. D., Ma, Jonathan, Yeo M., Carrasco J. H., Llewellyn S. M., Miles A. M.. Genotyping of Trypanosoma cruzi: systematic selection of assays allowing rapid and accurate discrimination of all known lineages. American Journal of Tropical Medicine and Hyiene. 2009;81:104–1049. doi: 10.4269/ajtmh.2009.09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhães J. B., Pontes A. L., Andrade S. G.. Comportamento das cepas Y e Peruana do Trypanosoma cruzi no camundongo, após passagem em diferentes meios. Memórias do Instituto Oswaldo Cruz. 1985;80:41–50. doi: 10.1590/s0074-02761985000100007. [DOI] [PubMed] [Google Scholar]
- Martins L. P. A., Castanho R. E. P., Rosa J. A., Silva L. C., Godoy C. A. P., Rosa R. M.. Caracterização biológica, histopatológica e análise de ácido nucléico de uma cepa de Trypanosoma cruzi da região de Marília, SP. Revista da Sociedade Brasileira de Medicina Tropical. 2003;36:35–39. doi: 10.1590/s0037-86822003000100006. [DOI] [PubMed] [Google Scholar]
- Martins L. P. A., Marcili A., Castanho R. E. P., Therezo A. L. S., Oliveira J. C. P., Suzuki R. B., Teixeira M. M. G., Rosa J. A., Sperança M. A.. Rural Triatoma rubrovaria from Southern Brazil Harbors Trypanosoma cruzi of Lineage IIc. American Journal of Tropical Medicine and Hyiene. 2008;79:427–434. [PubMed] [Google Scholar]
- Melo R. C., Brener Z.. Tissue tropism of different Trypanosoma cruzi strains. The Journal of Parasitology. 1978;64:475–482. [PubMed] [Google Scholar]
- Miles M. A., Lanhan S. M., De Souza A. A., Povoa D. G.. Further enzymic characters of Trypanosoma cruzi and their evaluation for strain identification. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1980;74:221–242. doi: 10.1016/0035-9203(80)90251-5. [DOI] [PubMed] [Google Scholar]
- Moncayo A., Silveira A. C.. Current epidemiological trends for Chagas disease in Latin América and future challengs in epidemiology, surveillance and health policy. Memórias do Instituto Oswaldo Cruz. 2009;104:17–30. doi: 10.1590/s0074-02762009000900005. [DOI] [PubMed] [Google Scholar]
- Nai G. A., Ferro L., Galle L. C., Quatrochi P. J., Giroto L. A.. Procedimentos de coloração dos preparados cito e histológicos – uma nova proposta. Laes & Haes. 2004;147:123–128. [Google Scholar]
- Pinto P. L. S.. Circulação e caracterização de Trypanosoma cruzi isolados de mamíferos silvestres capturados no Estado de São Paulo – Brasil. Tese Doutorado 2000 . Faculdade de Saúde Pública da Universidade de São Paulo. [Google Scholar]
- Rocha D. S., Juberg J., Rosa J. A., Schaefer C. W., Galvão C.. Description of eggs and instars of Triatoma baratai Carcavallo & Juberg, 2000 based on optical and scanning electron microscopy (Hemiptera: Reduviidae: Triatominae) Zootaxa. 2009;2064:1–20. [Google Scholar]
- Rossi L. R. L.. Estudo biométrico de formas epimastigotas e tripomastigotas de quatro cepas de Trypanosomes cruzi, CHAGAS, 1909 (KINETOPLASTIDAE, TRYPANOSOMATIDAE) Dissertação de Mestrado 2007 . Faculdade de Ciências Farmacêuticas de Araraquara. [Google Scholar]
- Silva R. A., Rodrigues V. L. C. C., Carvalho M. E., Pauliquévis C.. Programa de controle da doença de chagas no Estado de São Paulo: persistência de alta infestação por triatomíneos em localidades na década de 1990. Caderno de Saúde Pública. 2003;19:965–971. doi: 10.1590/s0102-311x2003000400019. [DOI] [PubMed] [Google Scholar]
- Souto R. P., Fernandes O., Macedo A. M., Campbell D. A., Zingales B.. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Molecular and Biochemical Parasitology. 1996;83:141–152. doi: 10.1016/s0166-6851(96)02755-7. [DOI] [PubMed] [Google Scholar]
- Sturm N. R., Vargas N. S., Westenberger S. J., Zingales B., Campbell D. A.. Evidence for multiple hybrid groups in Trypanosoma cruzi. International Journal for Parasitology. 2003;33:269–279. doi: 10.1016/s0020-7519(02)00264-3. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M., Ayala F. J.. The clonal theory of parasitic protozoa: 12 years on. Trends in Parasitology. 2002;18:405–410. doi: 10.1016/s1471-4922(02)02357-7. [DOI] [PubMed] [Google Scholar]
- Westenberger S. J., Barnabé C., Campbell D. A., Sturm N. R.. Two Hybridization Events Define the Population Structure of Trypanosoma cruzi. Genetics. 2005;171:527–543. doi: 10.1534/genetics.104.038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2010. http://www.who.int/mediacentre/factsheets/fs340/en/index.html http://www.who.int/mediacentre/factsheets/fs340/en/index.html . Acesso em: 10 ago. 2010.
- Zeledón R., Vieto P. L.. Comparative studies of Schizotrypanum cruzi Chagas, 1909 and S. verpertilionis Battaglia, 1904 from Costa Rica. Journal of Parasitology. 1958;44:499–502. [PubMed] [Google Scholar]
- Zingales B., Andrade S. G., Briones M. R. S., Campbell D. A., Chiari E., Fernandes O.. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Memórias do Instituto Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- Zingales B., Souto R. P., Mangia R. H., Lisboa C. V., Campbell D. A., Coura J. R., Jansen A., Fernandes O.. Molecular epidemiology of American trypanosomiasis in Brazil based on dimorphisms of rRNA and mini-exon gene sequences. International Journal for Parasitology. 1998;28:105–112. doi: 10.1016/s0020-7519(97)00178-1. [DOI] [PubMed] [Google Scholar]