Abstract
Background
Dystonia of the eyelids often spreads to affect other muscles in the craniocervical region. Certain blepharospasm-plus subphenotypes may be clinically unique.
Methods
Seven subjects with the subphenotype of late-onset blepharospasm with apraxia of eyelid opening and cervical dystonia with predominant anterocollis were identified from a database of over 1800 patients with primary dystonia.
Results
Blepharospasm was the first affected site in 6/7 subjects, followed by spread of the disease to the cervical muscles. Although four patients also had other forms of dystonia (laryngeal, lower face), none showed spread outside the craniocervical region. A family history of dystonia was present in 4/7. No mutations were identified in THAP1 or TOR1A. Overall, blepharospasm was difficult to treat, typically requiring both myectomy and substantial doses of botulinum toxin into the pretarsal orbicularis oculi muscles. In one subject, anterocollis markedly improved after deep brain stimulation.
Discussion
Delineation and characterization of craniocervical dystonia subphenotypes may serve to guide genetic and therapeutic studies, in addition to clinical interventions. The blepharospasm with apraxia of eyelid opening and anterocollis subphenotype can be therapeutically challenging.
Keywords: anterocollis, blepharospasm, craniocervical dystonia, THAP1, TOR1A
Introduction
Blepharospasm and cervical dystonia are two familiar manifestations of late-onset primary dystonia.1 Blepharospasm and cervical dystonia may occur in isolation or as part of more extensive anatomical involvement as segmental, multifocal, or generalized dystonia.2 Late-onset primary dystonia is concentrated in the craniocervical region of the body; the arms and legs are less frequently affected.1,3 Dystonia of the craniocervical region may involve contiguous or non-contiguous areas: (eyes and upper face) +/− (lower face) +/− (jaw and tongue) +/− (larynx) +/− (neck). Although various groupings of anatomical areas are possible in individual patients during the course of their disease, certain patterns do appear to be distinctive and have captured the attention of neurologists for over 100 years: Meige syndrome, Brueghel syndrome, and “blepharospasm-plus,” for example.2,4,5 Unfortunately, the non-possessive and possessive forms of Meige and Brueghel syndromes have been ambiguously ascribed by neurologists to anatomical variations of craniocervical dystonia that include the combination of blepharospasm and involuntary movements of lower facial and/or masticatory (jaw) muscles.2
Late-onset primary focal dystonia, particularly blepharospasm, may spread to adjoining or distant body parts.6–8 Most commonly, dystonia with onset in the eyelids (blepharospasm) extends to the lower face and masticatory muscles. In an important subset of patients, blepharospasm may also spread to the neck. In a retrospective study of 78 patients with blepharospasm in the United States, 31% developed manifest dystonia in the neck within 5 years of onset in the eyelids.6 Similar results were obtained in Italy, with 21% of 124 blepharospasm patients showing spread to the neck with follow-up periods of up to 15 years.8 Unfortunately, these reports did not detail the severity and positional characteristics of cervical dystonia in those subjects with concomitant blepharospasm.
In subjects with primary dystonia limited to the cervical region, isolated or predominant anterocollis is uncommon. In most neurology practices, the majority of subjects with anterocollis have a neurodegenerative disorder, or secondary cervical dystonia due to neuroleptic or antiemetic exposure.9–16 In two independent clinical series, 6.8% and 25% of subjects with cervical dystonia exhibited anterocollis, most commonly in combination with laterocollis and/or rotational torticollis.17,18 Conventionally, anterocollis can be difficult to treat with botulinum toxin.19 In fact, most clinical studies of chemodenervation for cervical dystonia have excluded subjects with isolated or predominant anterocollis.20
Herein, we describe the demographics and clinical features of seven patients with well-characterized primary late-onset segmental craniocervical dystonia with a distinctive combination of blepharospasm with apraxia of eyelid opening (AEO) and anterocollis. Given that the early-onset primary dystonias driven by Mendelian inheritance tend to exhibit characteristic clinical phenotypes, it is possible that subphenotypes of late-onset dystonia are also driven by relatively specific genetic variants and/or pathophysiological processes. Therefore, recognition and characterization of subphenotypes may prove useful for genetic, physiological, and therapeutic studies of dystonia.
Methods
Human studies were performed in accordance with institutional review board guidelines, and all subjects gave informed consent for genetic analyses, disclosure of medical and demographic information, and use of photographs. A total of 170 subjects with segmental dystonia were identified from our biorepository of over 1800 subjects with primary dystonia.21 Among the group of subjects with segmental dystonia, 114 had “blepharospasm-plus” subphenotypes of segmental craniocervical dystonia, of whom seven had a distinctive combination of anterocollis and blepharospasm. This subgroup was chosen for presentation because of their relatively unique combination of clinical features, including severe blepharospasm with AEO, limited or absent response to sensory tricks, and absence of head and appendicular tremors. Moreover, all patients were examined by the senior author (MSL) and had long disease durations. In addition, subjects #1–#6 were treated by the senior author.
AEO was defined as a non-paretic inability to open the eyes volitionally in the absence of overt visible contractions of the orbicularis oculi (OO) muscles.22 Subjects with dystonia that had spread outside the craniocervical region and variants of cervical dystonia (torticollis, laterocollis or retrocollis) in which the severity of rotational torticollis, laterocollis or lateral shift was equal to or exceeded the degree of anterocollis are not presented in this report. Sequence variants in exon 5 of TOR1A and THAP1 were excluded in all seven subjects.3,23 All three subscales of the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) were administered by the senior author before injections of botulinum toxin.24 Subjects were also examined by the senior author for the presence of non-physiological head and appendicular tremors (essential, dystonic, and other).
Results
Table 1 summarizes the demographic and clinical data of the seven subjects included in this study. In male subjects, dystonia first became manifest in their fourth or early fifth decade (44–52 years of age). In contrast, dystonia onset was 60 and 65 years, respectively, in the two female patients. At the time of this report, dystonia duration was between 7 and 15 years in all subjects. No subject showed clinical evidence of Parkinsonism, ataxia, spasticity, or posterior cervical (extensor) myopathy. Four patients had other sites of involvement, all within the craniocervical region. During the course of their disease, all subjects were given trials of oral agents for their dystonia and AEO. Treatments included benzodiazepines, tetrabenazine, anticholinergic agents, an analeptic (modafinil), a dopamine agonist (pramipexole), levodopa, and muscle relaxants. In aggregate, oral pharmacotherapy was only mildly beneficial in our cohort. Injections of onabotulinumtoxin A were employed to relieve the symptoms of blepharospasm (range: 47–110 units) and cervical dystonia (100–255 units). All patients received injections into the pretarsal portions of the OO muscles. Although complemented by the effects of botulinum toxin injections, all subjects required surgical intervention in the form of OO myectomy or blepharoplasty for adequate control of blepharospasm.
Table 1.
Demographic and Clinical Data
| Data | Subject #1 | Subject #2 | Subject #3 | Subject #4 | Subject #5 | Subject #6 | Subject #7 |
| Age at most recent evaluation (years) | 60 | 62 | 71 | 59 | 73 | 58 | 66 |
| Gender | Male | Male | Female | Male | Female | Male | Male |
| BSP onset (years) | 47 | 47 | 60 | 45 | 65 | 44 | 52 |
| CD onset (years) | 52 | 55 | 60 | 53 | 66 | 45 | 56 |
| AEO | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Other dystonia | Lower facial | None | Lower facial | None | None | Laryngeal | Lower facial |
| Time to spread (years) | 5 | 8 | 0 | 8 | 1 | 1 | 4 |
| Duration of BSP (years) | 13 | 15 | 11 | 14 | 7 | 14 | 14 |
| Pattern of spread | BSP→CD | BSP→CD | BSP = CD | BSP→CD | BSP→CD | BSP→CD | BSP→CD |
| TWSTRS: total score | 28.25 | 31.25 | 20 | 30.5 | 30.75 | 16 | 32 |
| TWSTRS: severity score | 17 | 15 | 15 | 13 | 20 | 13 | 14 |
| TWSTRS: disability score | 4 | 9 | 6 | 9 | 6 | 3 | 13 |
| TWSTRS: pain score | 7.25 | 7.25 | 1.5 | 8.5 | 4.75 | 0 | 5 |
| TWSTRS: anterocollis score | 3 | 3 | 1 | 2 | 2 | 1 | 2 |
| Cervical EMG | Tonic | Tonic | Tonic | Na | Tonic | Tonic | Na |
| Relief from sensory tricks | None | None | None | Limited | None | None | Limited |
| Non-physiological head and/or appendicular tremor | No | No | No | No | No | No | No |
| Family history | No | Sister - BSP | No | Sister -BSP | No | Father - segmental craniocervical dystonia | Several 1st & 2nd degree relatives with BSP+/-CD |
Abbreviations: BSP, blepharospasm; CD, cervical dystonia; EMG, electromyography; NA, not available or applicable; TWSTRS, Toronto Western Spasmodic Torticollis Rating Scale.
Subject #1 (Figure 1A–1C) is a Caucasian male with type II diabetes and hyperlipidemia. Blepharospasm onset was mildly asymmetrical, initially presenting in the left eye, followed by right eye involvement a few months later. Cervical dystonia, manifest as isolated anterocollis, appeared 5 years later. The severity of both blepharospasm and anterocollis has progressed slowly over time. Neither blepharospasm nor anterocollis has responded to trials of clonazepam (maximum dosage: 1 mg t.i.d.), tetrabenazine (maximum dosage: 25 mg t.i.d.), or trihexyphenidyl (maximum dosage: 5 mg t.i.d.). Baclofen (10 mg b.i.d.) has been associated with mild improvement in neck discomfort. This patient did not obtain satisfactory clinical improvement with injections of onabotulinumtoxin A and underwent limited OO myectomy at age 50 and, more extensive, integrated upper eyelid myectomy25 at age 56. Bilateral electromyographically (EMG)-guided injections of onabotulinumtoxin A (178–255 units) into the sternocleidomastoid, scalene, levator scapulae, longus colli, and longus capitis muscles were performed on three occasions, but provided only minimal benefit. In contrast, since his second myectomy procedure, this patient's blepharospasm and AEO have responded well to injections of onabotulinumtoxin A (8 units each upper eyelid pretarsal OO muscle, 47 units total).
Figure 1. Photographs of Three Subjects with Blepharospasm and Anterocollis.
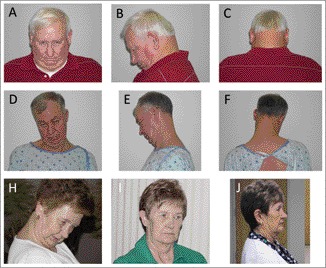
Frontal, lateral, and posterior views of subjects #1 (A–C) and #2 (D–F). Subject #5 before (H) and after ([I] 1 month; [J] 2 months) bilateral GPi DBS.
Subject #2 (Figure 1D–1F), a Caucasian male, has had dry eye symptoms that were treated with punctal plugs and, eventually, tear duct cauterization. Mild but non-sustained improvements in both blepharospasm and cervical dystonia were noted with benzodiazepines (clonazepam and alprazolam) and trihexyphenidyl. AEO showed no reliable response to amphetamines. A bedtime dose of cyclobenzaprine has helped to reduce nocturnal neck discomfort. Slight improvement in AEO and blepharospasm was noted after a limited bilateral OO myectomy and frontalis muscle sling procedure. Over the past 4 years, this patient's bilateral blepharospasm and AEO have responded satisfactorily to injections of onabotulinumtoxinA (20 units each upper eyelid pretarsal OO muscle, 94 units total). In contrast, cervical dystonia with severe anterocollis has shown only modest improvements with EMG-guided injections of onabotulinumtoxin A. Current medical problems include hyperlipidemia, hypertension, and type II diabetes mellitus. The patient's sister had a single episode of bilateral eyelid closure suggestive of blepharospasm that lasted approximately 3 months and resolved without specific treatment.
Subject #3, a Caucasian female with long-standing type I diabetes mellitus, noticed the simultaneous onset of segmental craniocervical dystonia (blepharospasm and cervical dystonia) at the age of 60, 2 weeks after the loss of her spouse. Blepharospasm and AEO did not improve with clonazepam or modafinil, but have responded well to injections of onabotulinumtoxin A (10 units each upper eyelid pretarsal OO muscle, 73 units total). Peri-oral dystonia has been well controlled with injections of onabotulinumtoxin A. Bilateral dermatochalasis was treated with blepharoplasty. In general, better clinical results were obtained with injections of onabotulinumtoxin A after blepharoplasty. Cervical dystonia manifest as anterocollis with minimal left rotational torticollis has responded well to EMG-guided injections of onabotulinumtoxinA (up to 220 units).
Subject #4, an African-American male, gets limited relief of both blepharospasm and cervical dystonia symptoms when he touches his left lateral brow. He was treated with limited OO myectomy at 47 years of age. His blepharospasm and AEO have been successfully managed with injections of onabotulinumtoxin A (16 units each upper eyelid pretarsal OO muscle, 100 units total) and low-dose trihexyphenidyl (2–4 mg daily). He has declined injections of botulinum toxin for treatment of his cervical dystonia, although it has become more severe over the past several years. A younger sister has isolated blepharospasm, reportedly responsive to injections of onabotulinumtoxin A.
Subject #5 (Figure 1G–1I), a Caucasian female, did not benefit from various combinations of medications including trihexyphenidyl, clonazepam, modafanil, pramipexole, levodopa, and cyclobenzaprine. Injections of onabotulinumtoxin A were moderately beneficial for blepharospasm, but produced minimal improvement in cervical dystonia. Additional reductions in blepharospasm severity and improved responses to injections of onabotulinumtoxin A were apparent after extensive integrated OO myectomy.25 However, owing to progression of anterocollis and persistent need for onabotulinumtoxin A injections to control blepharospasm, the subject underwent bilateral microelectrode-guided placement of bilateral globus pallidus pars interna (GPi) electrodes (Medtronic Kinetra). The benefits of surgery were apparent within weeks, and with her current settings (right GPi: case +, contact zero −, 2.5 V, 90 µs pulse width, 130 Hz; left GPi: case +, contact five −, 2.4 V, 60 µs pulse width, 130 Hz), there has been marked improvement of blepharospasm, AEO, and cervical dystonia. She no longer requires injections of onabotulinumtoxin A for blepharospasm/AEO, and her cervical dystonia is much improved with only slight anterocollis and minimal residual pain. Concomitant medical problems include restless legs syndrome, well controlled with pramipexole, and gastroesophageal reflux disease.
Subject #6 is a Caucasian male who underwent bilateral limited OO myectomy and frontalis muscle suspension owing to marginal benefits of onabotulinumtoxinA injections. Subsequently, an integrated upper eyelid myectomy25 was required to control blepharospasm and AEO. Since the more extensive myectomy, blepharospasm and AEO have responded well to injections (onabotulinumtoxin A: 10 units each upper eyelid pretarsal OO muscle, 62 units total). This patient has also obtained consistently good results with EMG-guided injections of onabotulinumtoxin A (up to 238 units) for treatment of cervical dystonia. At age 53, this subject began to exhibit episodic air hunger due to inspiratory laryngeal dystonia, a diagnosis confirmed by a neurolaryngologist via historical information in combination with videostroboscopy, direct laryngoscopy, and laryngeal EMG. Modest improvements in laryngeal dystonia were noted with EMG-guided injections of onabotulinumtoxin A into the thyroarytenoid muscles. Ultimately, however, a tracheostomy was required for symptom control. This patient's father, now deceased, was treated by the senior author for segmental craniocervical dystonia with severe jaw-opening, cervical dystonia with rotational torticollis and mild anterocollis, and very mild blepharospasm. Of note, subject #6's father did not show evidence of AEO or require injections for treatment of blepharospasm.
Subject #7, a Caucasian male, has blepharospasm and cervical dystonia manifest as isolated anterocollis. This patient has also exhibited mild lower facial dystonia and AEO. Sensory tricks for cervical dystonia (touching the side or back of the neck or chin) and blepharospasm (touching a lateral eyebrow) provide limited relief. He first underwent limited OO myectomy, which was followed by an integrated upper eyelid myectomy,25 and then by lower eyelid myectomy. More recently, he underwent OO myo-osseous fixation with titanium screws,26 which has been associated with marked subjective improvement in blepharospasm (∼90%). For cervical dystonia, he has obtained mild benefit from injections of onabotulinumtoxin A, performed without the use of EMG guidance. Family history of dystonia includes multiple individuals in four consecutive generations. The patient's mother and one of three brothers have blepharospasm. The patient's maternal grandmother (deceased) and one of two sisters (deceased) were reported to have had manifestations of blepharospasm and lower facial dystonia.
Discussion
Late-onset primary dystonia most commonly begins in the craniocervical region, and, although it may spread, tends to remain isolated to the craniocervical region. Isolated or disproportionate anterocollis is an uncommon form of primary cervical dystonia. Additionally, no explicit data on the combination of blepharospasm with subtypes of cervical dystonia have been published. Is the combination of blepharospasm and anterocollis a phenotype of segmental craniocervical dystonia that is physiologically, molecularly, and genetically unique and distinct, or simply two different focal manifestations of dystonia randomly affecting the same individual? Even though our data and study design do not answer those questions, the combination of blepharospasm and anterocollis does show evidence, albeit uncontrolled, of characteristic clinical features, including AEO, difficult-to-treat blepharospasm, absence of head and appendicular tremors, frequent family history of dystonia, and infrequent response to sensory tricks. From physiological and evolutionary perspectives, eyelid closure and anterocollis provide a coordinated motor plan to protect the eyes and face from oncoming threats. Ultimately, n-dimensional cluster analysis of high-resolution phenotypic data derived from a large multi-center cohort of subjects with segmental craniocervical dystonia will be required to determine whether creation of subphenotypes is scientifically valid. Although potentially limited by recall and referral bias, our descriptive analysis of a putative subphenotype does provide motivation for more exacting characterization of primary dystonia, which, at present, remains a clinical diagnosis, based solely on positive motor manifestations.
Our descriptive, largely retrospective report has several limitations. First, a rating scale for blepharospasm was not employed, and the TWSTRS was not completed at each visit. Second, the TWSTRS was not completed after washout of medications, including botulinum toxin. Third, the collection of subjects described herein may over-represent particularly complex cases owing to referral bias. Fourth, we did not compare our cohort with subjects with blepharospasm plus other forms of cervical dystonia. Lastly, our data do not allow us unambiguously to argue that that blepharospasm with AEO and anterocollis is a discrete entity rather than a probabilistic collection of numerous possible combinations of segmental craniocervical dystonia.
EMG showed tonic firing patterns in muscles known to generate head flexion. In addition, none of our patients had evidence of a focal or generalized myopathy with weakness of their posterior (extensor) cervical musculature. The marked improvement of cervical dystonia with deep brain stimulation (DBS) in subject #5, and positive response to injections of onabotulinumtoxin A injections in subjects #2, #3, and #6, also argue that the anterocollis noted in our cohort was actually driven by dystonia of the cervical musculature.
Although the treatment of anterocollis is notoriously difficult, recent advances in neuromodulation and botulinum toxin injection techniques are encouraging. The success of GPi DBS in subject #5 offers hope to patients similarly affected. Recent series support the efficacy of DBS in patients with blepharospasm, cervical dystonia, and more complex segmental craniocervical dystonia.27–29 However, the treatment of cervical dystonia predominantly manifest as anterocollis has not been explicitly reported. The use of EMG guidance for injections of botulinum toxin into the deep cervical musculature, particularly when coupled with imaging (e.g., fluoroscopy or computed tomography), may improve therapeutic outcomes.19,30
Concomitant AEO can be a major contributor to botulinum toxin refractoriness in a subset of patients with blepharospasm.31 Although the relative roles of the pretarsal OO and levator palpebrae (LP) muscles in AEO is rarely established in routine clinical practice, a significant percentage of patients do improve with pretarsal injections of botulinum toxin and/or upper eyelid myectomy.31,32 Although simultaneous OO and LP EMG was not performed in our cohort of subjects, the positive response to injections of botulinum toxin suggests that dystonic discharges of the OO muscle contributed to their AEO.32,33 Alternatively, reduced contraction of the pretarsal OO muscles and/or modulation of sensory pathways from orbital tissues might have helped to normalize central feedback loops responsible for activation of the LP muscle.33
Even after extensive upper eyelid myectomy, our patients still required botulinum toxin injections for control of their blepharospasm and AEO. A formal blepharospasm rating scale was not used to assess our subjects since their condition often demanded injections of botulinum toxin at intervals of less than 12 weeks, before the beneficial effects of toxin had entirely waned. Anecdotally, anterocollis contributed to disability by constricting normal access to upper portions of the visual fields.
Spread of dystonia to other sites after onset as blepharospasm has been well documented.6–8 For example, Svetel and colleagues7 observed 132 patients with primary blepharospasm and reported spread of dystonia in 33.3%, with the second site being affected in 1.2 years, on average, and the highest chance of spread occurring within the first 5 years after disease onset. In similar work, Abbruzzese et al.8 reported that only 3.4% of their patients demonstrated spread of dystonia outside the craniocervical region. Based on these data, the spread of blepharospasm to other craniocervical sites becomes increasingly uncommon with the passage of time and spreading outside the craniocervical region is distinctly unusual. Given the long duration of blepharospasm in our cohort of subjects (7–15 years), additional spread of dystonia is improbable.
Four of our subjects had a positive history of dystonia with variable expressivity and reduced penetrance. We offer the hypothesis that blepharospasm with AEO and anterocollis is a unique subphenotype of adult-onset primary craniocervical dystonia that might be due to distinct sequence variants in genes yet to be causally associated with dystonia. In this regard, phenotype–genotype correlations are well established in dystonia: early-onset leg involvement in DYT1 dystonia, writer's cramp and cervical dystonia in the myoclonus-dystonia (DYT11), and predominant craniocervical manifestations in DYT6 dystonia.3 Craniocervical subphenotype–genotype correlations should become possible as the genetic underpinnings of late-onset primary dystonia are exposed. Physiologically, we should consider the possibility that focal and segmental dystonias may be localized manifestations of generalized neural dysfunction.
Acknowledgments
This study was supported by the Neuroscience Institute at the University of Tennessee Health Science Center, Dystonia Medical Research Foundation, National Institutes of Health (NIH) grants R01NS048458 and R01NS069936, the NIH U54 Dystonia Coalition Pilot Projects Program, and the Parkinson's and Movement Disorder Foundation. The Dystonia Coalition is part of the NIH Rare Diseases Clinical Research Network. Funding and/or programmatic support for this project has been provided by NS067501 from the NIH Office of Rare Diseases Research and the National Institute of Neurological Disorders and Stroke. The views expressed in written materials or publications do not necessarily reflect the official policies of the Department of Health and Human Services, nor does mention of trade names, commercial practices, or organizations imply endorsement by the United States government.
Footnotes
Funding: This study was supported by the Neuroscience Institute at the University of Tennessee Health Science Center, Dystonia Medical Research Foundation, the National Institutes of Health, and the Parkinson's and Movement Disorder Foundation.
Competing Interests: OW has nothing to disclose. MSD has served on the scientific advisory board for the Dystonia Medical Research Foundation; serves on the editorial boards of Parkinsonism and Related Disorders and Tremor and Other Hyperkinetic Movements; serves on the speakers' bureaus for Lundbeck, Merz, and Teva Neuroscience; serves as an advisor for Merz; serves on the Xenazine Advisory Board for Lundbeck, Inc., and the Botulinum Toxin Type A Advisory Board for Allergan; receives research support from the National Institutes of Health (R01NS069936, R01NS058850, R01NS05772, R01NS06088, 5U01NS05259, 3U01AT000613, 1U54NS065701), Dystonia Medical Research Foundation, Bachmann-Strauss Dystonia & Parkinson Foundation, Merz, and HP Therapeutics Foundation; and receives royalty payments for Animal Models of Movement Disorders (Elsevier).
References
- 1.Defazio G, Abbruzzese G, Livrea P, et al. Epidemiology of primary dystonia. Lancet Neurol. 2004;3:673–678. doi: 10.1016/S1474-4422(04)00907-X. [DOI] [PubMed] [Google Scholar]
- 2.LeDoux MS. Meige syndrome: what's in a name. Parkinsonism Relat Disord. 2009;15:483–489. doi: 10.1016/j.parkreldis.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao J, Zhao Y, Bastian RW, et al. Novel THAP1 sequence variants in primary dystonia. Neurology. 2010;74:229–238. doi: 10.1212/WNL.0b013e3181ca00ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meige H. Les convulsions de la face. Ue forme clinique de convulsion faciale bilaterale et mediane. Rev Neurol (Paris) 1910;21:437–443. doi: 10.1136/jnnp.39.12.1204. [DOI] [Google Scholar]
- 5.Marsden CD. Blepharospasm-oromandibular dystonia syndrome (Brueghel's syndrome): a variant of adult onset torsion dystonia. J Neurol Neurosurg Psychiatry. 1976;39:1204–1209. doi: 10.1136/jnnp.39.12.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss EM, Hershey T, Karimi M, et al. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord. 2006;21:1175–1181. doi: 10.1002/mds.20919. [DOI] [PubMed] [Google Scholar]
- 7.Svetel M, Pekmezovic T, Jovic J, et al. Spread of primary dystonia in relation to initially affected region. J Neurol. 2007;254:879–883. doi: 10.1007/s00415-006-0457-8. [DOI] [PubMed] [Google Scholar]
- 8.Abbruzzese G, Berardelli A, Girlanda P, et al. Long-term assessment of the risk of spreads in primary late-onset dystonia. J Neurol Neurosurg Psychiatry. 2008;79:392–396. doi: 10.1136/jnnp.2007.124594. [DOI] [PubMed] [Google Scholar]
- 9.Uzawa A, Mori M, Kojima S, et al. Dopamine agonist-induced antecollis in Parkinson's disease. Mov Disord. 2009;24:2408–2411. doi: 10.1002/mds.22779. [DOI] [PubMed] [Google Scholar]
- 10.Kashihara K, Ohno M. Multiple system atrophy with antecollis that later changed to an extended posture: a case report. Mov Disord. 2009;24:939–940. doi: 10.1002/mds.22140. [DOI] [PubMed] [Google Scholar]
- 11.Czarnecki K, Kumar N, Josephs KA. Parkinsonism and tardive antecollis in frontotemporal dementia — increased sensitivity to newer antipsychotics. Eur J Neurol. 2008;15:199–201. doi: 10.1111/j.1468-1331.2007.02032.x. [DOI] [PubMed] [Google Scholar]
- 12.Van de Warrenburg BP, Cordivari C, Ryan AM, et al. The phenomenon of disproportionate antecollis in Parkinson's disease and multiple system atrophy. Mov Disord. 2007;22:2325–2331. doi: 10.1002/mds.21634. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M, Hirai T, Ito Y, et al. Pramipexole-induced antecollis in Parkinson's disease. J Neurol Sci. 2008;264:195–197. doi: 10.1016/j.jns.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Maeda K, Ohsaki T, Kuki K, et al. Severe antecollis during antipsychotics treatment: a report of three cases. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:749–759. doi: 10.1016/S0278-5846(98)00037-2. [DOI] [PubMed] [Google Scholar]
- 15.Jorens PG, Eycken MP, Parizel GA, et al. Antecollis in parkinsonism. Lancet. 1989;1:1320–1321. doi: 10.1016/S0140-6736(89)92706-2. [DOI] [PubMed] [Google Scholar]
- 16.Quinn N. Disproportionate antecollis in multiple system atrophy. Lancet. 1989;1:844. doi: 10.1016/S0140-6736(89)92300-3. [DOI] [PubMed] [Google Scholar]
- 17.Jankovic J, Leder S, Warner D, et al. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–1091. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 18.Papapetropoulos S, Tuchman A, Sengun C, et al. Antecollis: clinical features and treatment options. Med Sci Monit. 2008;14:427–430. [PubMed] [Google Scholar]
- 19.Glass GA, Ku S, Ostrem JL, et al. Fluoroscopic, EMG-guided injection of botulinum toxin into the longus colli for the treatment of anterocollis. Parkinsonism Relat Disord. 2009;15:610–613. doi: 10.1016/j.parkreldis.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Benecke R, Jost WH, Kanovsky P, et al. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology. 2005;64:1949–1951. doi: 10.1212/01.WNL.0000163767.99354.C3. [DOI] [PubMed] [Google Scholar]
- 21.Xiao J, Zhao Y, Bastian RW, et al. The c.-237_236GA>TT THAP1 sequence variant does not increase risk for primary dystonia. Mov Disord. 2011;26:549–553. doi: 10.1002/mds.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan DR, Anderson RL, Digre KB. Apraxia of lid opening in blepharospasm. Ophthalmic Surg. 1990;21:331–334. doi: 10.1186/1471-2350-10-24. [DOI] [PubMed] [Google Scholar]
- 23.Xiao J, Bastian RW, Perlmutter JS, et al. High-throughput mutational analysis of TOR1A in primary dystonia. BMC Med Genet. 2009;10:24. doi: 10.1186/1471-2350-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comella CL, Leurgans S, Wuu J, et al. Dystonia Study Group. Rating scales for dystonia: a multicenter assessment. Mov Disord. 2003;18:303–312. doi: 10.1002/mds.10377. [DOI] [PubMed] [Google Scholar]
- 25.Gillum WN, Anderson RL. Blepharospasm surgery: an anatomical approach. Arch Ophthalmol. 1981;99:1056–1062. doi: 10.1001/archopht.1981.03930011056015. [DOI] [PubMed] [Google Scholar]
- 26.Borodic GE. Orbicularis oculi myo-osseous fixation: a new treatment for benign essential blepharospasm and blepharospasm associated with diffuse facial dystonia (Meige syndrome) Ophthalmic Surg Lasers Imaging. 2010;41:360–369. doi: 10.3928/15428877-20100430-11. [DOI] [PubMed] [Google Scholar]
- 27.Ostrem JL, Marks WJ, Jr, Volz MM, et al. Pallidal deep brain stimulation in patients with cranial-cervical dystonia (Meige syndrome) Mov Disord. 2007;22:1885–1891. doi: 10.1002/mds.21580. [DOI] [PubMed] [Google Scholar]
- 28.Cacciola F, Farah JO, Eldridge PR, et al. Bilateral deep brain stimulation for cervical dystonia: long-term outcome in a series of 10 patients. Neurosurgery. 2010;67:957–963. doi: 10.1227/NEU.0b013e3181ec49c7. [DOI] [PubMed] [Google Scholar]
- 29.Sako W, Morigaki R, Mizobuchi Y, et al. Bilateral pallidal deep brain stimulation in primary Meige syndrome. Parkinsonism Relat Disord. 2011;17:123–125. doi: 10.1016/j.parkreldis.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Herting B, Wunderlich S, Glöckler T, et al. Computed tomographically-controlled injection of botulinum toxin into the longus colli muscle in severe anterocollis. Mov Disord. 2004;19:588–590. doi: 10.1002/mds.10704. [DOI] [PubMed] [Google Scholar]
- 31.Georgescu D, Vagefi MR, McMullan TF, et al. Upper eyelid myectomy in blepharospasm with associated apraxia of lid opening. Am J Ophthalmol. 2008;145:541–547. doi: 10.1016/j.ajo.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Krack P, Marion MH. “Apraxia of lid opening,” a focal eyelid dystonia: clinical study of 32 patients. Mov Disord. 1994;9:610–615. doi: 10.1002/mds.870090605. [DOI] [PubMed] [Google Scholar]
- 33.Aramideh M, Ongerboer de Visser BW, et al. Electromyographic features of levator palpebrae superioris and orbicularis oculi muscles in blepharospasm. Brain. 1994;117:27–38. doi: 10.1093/brain/117.1.27. [DOI] [PubMed] [Google Scholar]


