Abstract
Background
Endomycocardial biopsies have demonstrated that subclinical myocarditis is a universal feature of acute Kawasaki disease (KD).
Methods
We investigated biochemical evidence of myocardial strain, oxidative stress, and cardiomyocyte injury in 55 acute KD subjects (30 with paired convalescent samples), 54 febrile control (FC), and 50 healthy control (HC) children by measuring concentrations of cardiovascular biomarkers.
Results
Levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and soluble ST2 (sST2) were elevated in acute vs. convalescent KD, FC, and HC (p≤0.0002), while γ-glutamyl transferase and alanine amino transferase as measures of oxidative stress were increased in acute vs. FC (p≤0.0008). Cardiac troponin I (cTnI) levels, using a highly sensitive assay, were elevated in 30% and 40% of paired acute and convalescent KD subjects, respectively, and normalized within two years of disease onset. NT-proBNP and sST2 negatively correlated with measures of diastolic function (MV E:A ratio and deceleration time), but only NT-proBNP positively correlated with the coronary artery Z score.
Conclusions
NT-proBNP and sST2 were elevated in acute KD subjects and correlated with impaired myocardial relaxation. These findings, combined with elevated levels of cTnI, suggest that both cardiomyocyte stress and cell death are associated with myocardial inflammation in acute KD.
Keywords: diastolic dysfunction, oxidative stress, myocarditis, coronary artery aneurysm
INTRODUCTION
Kawasaki disease (KD) is an acute inflammatory condition that involves both the arterial wall and the myocardium.[1] While patients uncommonly present with clinically significant systolic dysfunction in the acute phase, [2, 3] endomyocardial biopsies have documented a range of pathologic findings consistent with diffuse myocardial inflammation.[4-6] Most patients have normal myocardial systolic function after recovery from their acute illness, but diastolic dysfunction has been observed. [7, 8]
Protein biomarkers of cardiomyocyte strain, injury, and death are used to stratify risk and to monitor response to therapies in adults with congestive heart failure and ischemic heart disease.[9] Some biomarkers, such as troponin, are released by injured or dying cells, but do not directly participate in the pathologic process. Others, such as soluble ST2 (sST2), directly mediate injury and could be targets for therapeutic intervention.[10, 11] We tested a panel of cardiovascular biomarkers in acute and convalescent KD patients and compared the results to febrile and healthy controls as well as clinical and echocardiographic data to better understand the mechanisms of myocardial injury in acute KD.
METHODS
Patients
KD samples were from consecutive, unselected KD subjects for whom both plasma and serum samples were available. All KD subjects fulfilled American Heart Association diagnostic criteria for KD [12]. Acute KD samples were obtained prior to treatment with intravenous immunoglobulin (IVIG). N-terminal pro-B-type natriuretic peptide (NT-proBNP), sST2, serum cardiac troponin I (cTnI), γ-glutamyl transpeptisdase (GGT), and alanine amino transferase (ALT) concentrations were determined for the following subjects: 55 acute KD (30 of whom had paired convalescent samples; median 46 days, range 26-73 days after onset of KD), 54 age-similar febrile controls (FC), and 50 age-similar healthy controls (HC). cTnI levels were also determined for 17 KD subjects who had late convalescent serum obtained (median 431 days, range 347-757 days after onset of KD).
FC subjects were previously healthy children recruited from the Emergency Department at Rady Children’s Hospital San Diego and had ≥ 3 days of fever and at least one of the clinical signs of KD (rash, conjunctival injection, cervical lymphadenopathy, erythematous oral mucosa, and erythematous or edematous hands or feet). Among the 54 FC subjects, 8 had bacterial infection and 46 had viral infections (Table 1).
Table 1.
Diagnoses of febrile controls
| Diagnosis | n | |
|---|---|---|
| Bacterial Infection (n=8) |
Scarlet fever | 3 |
| Staphylococcal scalded skin syndrome | 2 | |
| Streptococcal pharyngitis | 3 | |
| Viral Infection (n=46) |
Measles | 1 |
| Culture-proven adenovirus | 11 | |
| Viral syndrome defined as self-limited, minor febrile illness with negative throat and rectal viral cultures |
34 |
HC subjects were children undergoing minor elective surgery for polydactyly. The Human Research Protection Program of the University of California, San Diego approved this research protocol and written informed consent was obtained from the parents of all subjects. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
We recorded age, sex, illness day at patient evaluation (first calendar day of fever= illness day 1), and clinical laboratory data. We normalized the hemoglobin concentration for age to allow valid comparisons across the age spectrum of our subjects. For KD subjects only, we recorded response to intravenous immunoglobulin (IVIG) and echocardiographic data. IVIG-resistance was defined as persistent or recrudescent fever (T ≥ 38°C) at least 24 hours after completion of the IVIG infusion (2 g/kg).
Echocardiography
Echocardiography was performed in acute KD subjects during their initial hospitalization and at 2 and 5 weeks post-IVIG. Dilatation of the right coronary artery (RCA) and left anterior descending coronary artery (LAD) was defined according to the American Heart Association criteria as a Z score of ≥ 2.5 (standard deviation units from the mean internal diameter normalized for body surface area) [12]. Aneurysms were defined as a focal region of the coronary artery 1.5 times the diameter of the adjacent segment. “Zworst” was defined as the larger of the Z scores for the RCA and LAD at any time point in the illness. The aortic root was measured by standard convention in the parasternal long axis view during mid-systole and the absolute dimension for the aortic sinus was normalized based on body surface area and considered dilated if the Z score was ≥ 2.0 [13]. Parameters of ventricular diastolic function included mitral inflow velocities during early diastolic filling (E wave velocity) and atrial contraction (A wave velocity), deceleration time (time, in milliseconds, from the peak of the E wave to the baseline), and Doppler measurement of tissue velocity (DTI) at the lateral mitral annulus (E’ velocity), septal mitral annulus, and lateral tricuspid annulus during early diastolic filling. DTI was only available for 19 subjects enrolled during the last year of the study. Data, calculated from the diastolic measurements, included the mitral E wave velocity/A wave velocity ratio and the E velocity/E’ velocity ratio. Values were compared to published normal values and categorized as either abnormal or normal [14, 15]. Fractional shortening (FS) was measured by standard methods (M mode) and normalized for age.
Biomarker assays
EDTA plasma NT-proBNP concentration was measured with a biotin-coupled anti-NT-proBNP antibody/streptavidin solid-phase chromatographic immunoassay (StatusFirst CHF NT-proBNP test devices, Nanogen, San Diego, CA; 99% for reference value for healthy adults=125 pg/mL), in combination with the DXpress Reader (Nanogen, San Diego, CA). Sodium citrate plasma sST2 levels were determined using the Presage sST2 assay kit (Critical Diagnostics, New York, NY; 99% for reference value for healthy adults= 50.2 ng/mL). Serum cTnI was measured using the Verisens human cTnI assay (Nanosphere, Northbrook, IL), a multi-step and automated assay using functionalized gold nanoparticles with signal enhancement by silver amplification (99% for reference value for healthy adults =0.0045 ng/mL). Plasma concentrations of GGT and ALT were measured using the VITROS GGT and ALT slides and the VITROS Chemistry Products Calibrator Kit 3 on VITROS Chemistry Systems.
Statistical analysis
Data were analyzed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) software, and presented as medians and interquartile range. Mann-Whitney U test was used for non-parametric data. Paired data for acute and convalescent KD were analyzed using a Wilcoxon signed rank test. Correlations between continuous variables were performed using Spearman’s test. Multivariable predictors of logNT-proBNP levels were identified by backward stepwise linear regression including clinical variables with significant univariable associations; they were confirmed with forward stepwise regression, which yielded the same results. Categorical data were analyzed with Fisher’s exact test. The p values were not adjusted for multiple testing and values <0.05 were considered significant.
RESULTS
Patient characteristics for the KD and FC groups are shown in Table 2. KD patients had a higher C-reactive protein (CRP) level, erythrocyte sedimentation rate (ESR), white blood cell count (WBC), and platelet count, and lower age-adjusted hemoglobin levels.
Table 2.
Clinical and laboratory characteristics of acute Kawasaki disease (KD) and febrile control (FC) subjects.
| Characteristics | Acute KD (n=55) |
FC (n=54) |
P |
|---|---|---|---|
| Median age, yrs. (range) | 2.83 (0.35–14.90) | 2.43 (0.15–13.49) | NS |
| Male, n (%) | 35 (64) | 31 (57) | NS |
| Median Illness Day (range) (first day of fever = Day 1) |
6 (3–10) | 4 (2–20) | 0.005 |
| Coronary artery status of subjects: n (%) |
Normal: 35 (64) Dilated: 11 (20) Aneurysm: 9 (16) |
NA | NA |
| IVIG resistant, n (%) | 17 (31) | NA | NA |
| CRP (mg/dL)* | 8.2 (5.2–18.5) | 2.2 (1.0–4.3) | <0.0001 |
| ESR (mm/h) | 62 (44–78) | 20 (15–38) | <0.0001 |
| WBC (×109/L) | 13.5 (10.7–18.5) | 8.7 (6.4–12.8) | <0.0001 |
| % Polymorphonuclear leukocytes |
56 (46–66) | 45 (31–63) | 0.03 |
| % Bands | 12 (8–21) | 8 (4–15) | NS |
| Absolute neutrophil count | 9520 (6519–13090) | 4636 (2695–6790) | <0.0001 |
| Age-adjusted Hgb, S.D. units | −1.25 (−2.33– −0.5) | −0.43 (−1.3–0.86) | 0.0004 |
| Platelet count (×109/L) | 405 (321–465) | 265 (213–349) | <0.0001 |
| ALT (IU/L) | 45 (24–102) | 24 (17–36) | 0.0008 |
| GGT (IU/L) | 45 (19–150) | 14 (12–17) | <0.0001 |
Laboratory data are presented as median (interquartile range).
IVIG = intravenous immunoglobulin, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, WBC = white blood cell count, Hgb = hemoglobin concentration, ALT = alanine amino transferase, GGT = γ-glutamyl transferase, NA = not available, NS = not significant,
N-terminal pro-B-type natriuretic peptide
Plasma NT-proBNP was significantly elevated in acute KD subjects compared to convalescent KD and control groups (p<0.0001) (Figure 1A, Table 3). NT-proBNP concentrations positively correlated with the internal diameter of the coronary arteries (RCA/LAD Zworst), sST2, ALT, and GGT levels (Table 4). Concentrations of NT-proBNP negatively correlated with age (Table 4), consistent with the observation that NT-proBNP concentrations are higher in infants and young children [16]. With respect to echocardiographic assessment of myocardial function, concentrations of NT-proBNP negatively correlated with measures of diastolic function (MV E:A ratio and deceleration time) and positively correlated with MV peak A wave suggesting that they were markers of impaired ventricular relaxation (Table 4).
Figure 1.
Plasma concentrations of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and soluble ST2 (sST2) in acute Kawasaki disease (KD), convalescent KD, febrile controls (FC), and healthy controls (HC). A) Comparisons of NT-proBNP concentrations (p<0.0001 for acute KD vs. conv. KD, FC, and HC). B) Comparisons of sST2 concentrations (p≤0.0002 for acute KD vs. conv. KD, FC, and HC). Box plot represents median (bar) with interquartile range (box), and T-bars show 5th-95th percentile. Data presented in a logarithmic scale. Outlying values are represented by black dots.
Table 3.
Comparison of cardiac biomarker levels in subjects with acute Kawasaki disease (KD), convalescent KD (conv. KD), febrile controls (FC), and healthy controls (HC)
| Biomarker Proteins |
Acute KD (n=55) |
Conv. KD (n= 30) |
FC (n=54) |
HC (n=50) |
|---|---|---|---|---|
| NT-proBNP (pg/mL) |
319.5 (117.3-651.6) |
41.1* (28.7-66.2) |
102.0* (46.1-197.3) |
65.2*† (45.0-112.3) |
| sST2 (ng/mL) | 46.1 (22.7-114.8) |
12.5* (9.9-15.3) |
28.4* (19.0-43.1) |
7.2*† (4.3-9.7) |
| cTnI (ng/mL) | 0.0014 (0.0003-0.0062) |
0.0030* (0.0014-0.0119) |
0.0013 (0.0004-0.0056) |
0.0008*† (0.0007-0.0012) |
Values are presented as median (interquartile range).
Significant at p<0.05 compared to acute KD by Mann Whitney U test and Wilcoxon signed rank test for paired acute and convalescent KD.
NT-proBNP, sST2, and cTnI levels in acute KD subjects were compared with n=20, n=30, and n=30 age-similar HC, respectively.
NT-proBNP = N-terminal pro-B-type natriuretic peptide; sST2 = soluble ST2; cTnI = cardiac troponin I.
Table 4.
Correlation of protein biomarker concentrations from acute KD subjects with demographic, echocardiographic, and laboratory data.
| Biomarker | sST2 (ng/mL) |
cTnI (ng/mL) |
Age at Onset (yrs) |
Illness Day |
CRP (mg/dL) |
ALT (IU/L) |
GGT (IU/L) |
MV A |
MV E:A |
DT (ms) |
DTI E:A |
RCA/LAD Zworst |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NT- proBNP |
−0.50† | 0.27 | −0.45* | −0.43* | 0.39* | 0.33 | 0.27 | 0.41 | −0.44 | −0.74† | NS | 0.41* |
| sST2 | 1.0† | NS | NS | −0.52† | NS | 0.54† | 0.58† | NS | NS | −0.53* | NS | NS |
Values represent Spearman rank correlation coefficient, r2. All values significant at P ≤ 0.05, unless otherwise noted: P < 0.005,
P < 0.0001. NS= not significant. MV A, MV E:A, and DT measured in only 28 patients. cTnI did not significantly correlate with measures of systolic or diastolic function, or clinical laboratory data. NT-proBNP = N-terminal pro-B-type natriuretic peptide; sST2 = soluble ST2; cTnI = cardiac troponin I; Illness day = first day of fever is Day 1; CRP = C-reactive protein; ALT= alanine amino transferase; GGT = γ-glutamyl transferase; MV A = mitral valve A-wave; MV E:A = ratio of mitral valve E-wave and A-wave; DT = deceleration time; DTI = Doppler tissue imaging; RCA/LAD Zworst= worst Z-score of either right coronary artery or left anterior descending coronary artery measured at 3 time points.
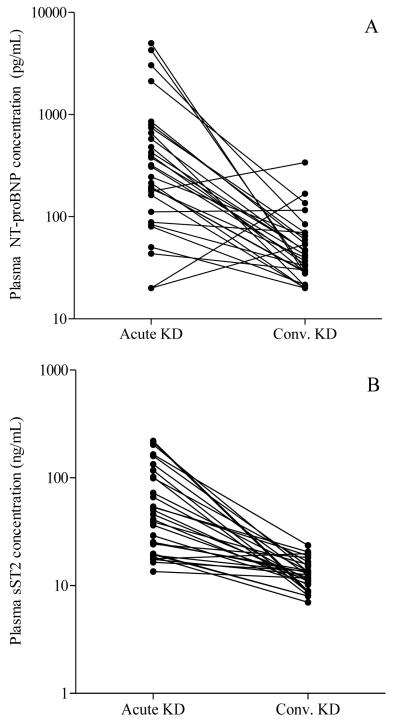
Multivariable predictors of logNT-proBNP levels included illness day (β= −0.33, p=0.017) and mitral valve E wave deceleration time (β= −0.64, p<0.001). For the majority of KD subjects, NT-proBNP concentrations declined by the convalescent phase (26 of 30) (Figure 2). Unexpectedly, a subset of the FC (n=3) discharged from the Emergency Department with self-limited febrile illnesses had NT-proBNP levels >1,000 pg/mL with sST2 levels 47-93 ng/mL and cTnI levels 0.0003-0.019 ng/mL, suggesting myocardial stress. The diagnoses in these 3 subjects were staphylococcal scalded skin syndrome (n=1) and viral syndrome (n=2).
Figure 2.
Concentrations of N-terminal pro-B-type natriuretic peptide (NT-proBNP) and soluble ST2 (sST2) in paired acute and convalescent (conv.) Kawasaki disease (KD) plasma samples plotted on a logarithmic scale. (A) NT-proBNP, n=30 (B) sST2, n=30
Six KD subjects had markedly elevated NT-proBNP levels (>1,000 pg/mL) associated with elevated levels of sST2 (5 subjects) and cTnI (4 subjects) (Table 5). Half were IVIG-resistant, and all had extremely elevated CRP levels. To compare echocardiographic parameters including FS and coronary artery internal dimensions, we converted these measurements to Z scores (standard deviation from the mean normalized for body surface area) to allow comparisons among patients of very different age and size. Measurement of left ventricular systolic function was abnormal in 3 of 6 subjects (FS Z score −2.65 to −3.5), and 4 of 6 had coronary artery dilatation (n=2) or aneurysms (n=2). The aortic root was dilated in only one of these 6 subjects. DTI was performed in only 2 of the 6 subjects and was normal in both. Mitral valve inflow E and A waves were fused and could not be evaluated in 5 subjects. The inflow velocity ratio was abnormal in the one subject who could be evaluated.
Table 5.
Acute Kawasaki disease subjects with plasma levels of NT-proBNP >1,000 pg/mL.
| Patient Number |
NT- pBNP (pg/mL) |
sST2 (ng/mL) |
cTnI (ng/mL) |
Age at Onset, (yrs.) |
Sex | Illness Day |
IVIG Resistant |
CRP (mg/dL) |
FS Z score |
RCA/LAD Zworst |
Aortic sinus Zworst |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1990 | 42 | 0.0012 | 2.8 | F | 6 | Y | 20.8 | −0.9 | 1.9 | 0.2 |
| 2 | 2117 | 102 | 0.0018 | 0.9 | M | 5 | Y | 37.6 | −1.3 | 5.6 | 0.6 |
| 3 | 3041 | 117 | 0.0081 | 4.3 | M | 7 | N | 26.9 | −3.5 | 1.7 | 0.4 |
| 4 | 4274 | 216 | 0.0134 | 2.4 | F | 7 | N | 16.4 | −2.6 | 3.0 | 0.4 |
| 5 | >5000 | >160 | 0.0444 | 4.0 | F | 5 | Y | 44.2 | −3.0 | 2.5 | 0.9 |
| 6 | >5000 | 107 | 0.0225 | 2.4 | M | 5 | N | 32.5 | −0.7 | 3.5 | 4.0 |
NT-proBNP = N-terminal pro-B-type natriuretic peptide; sST2 = soluble ST2; cTnI = cardiac troponin I; IVIG = intravenous immunoglobulin; CRP = C-reactive protein; FS = fractional shortening; Z score: standard deviation units from the mean normalized for body surface area; RCA/LAD Zworst= worst Z-score of either right coronary artery or left anterior descending coronary artery measured at 3 time points; Aortic sinus Zworst = worst Z-score of aortic sinus.
Soluble ST2
Plasma sST2 concentrations were significantly elevated in acute KD compared to convalescent KD and both control groups (p≤0.0002) (Table 3) and negatively correlated with deceleration time and illness day, suggesting that concentrations were highest in the earliest stages of the illness (Table 4). sST2 concentrations correlated strongly with ALT and GGT. For the majority of KD subjects, sST2 concentrations declined by the convalescent phase (29 of 30) (Figure 2).
Cardiac Troponin I
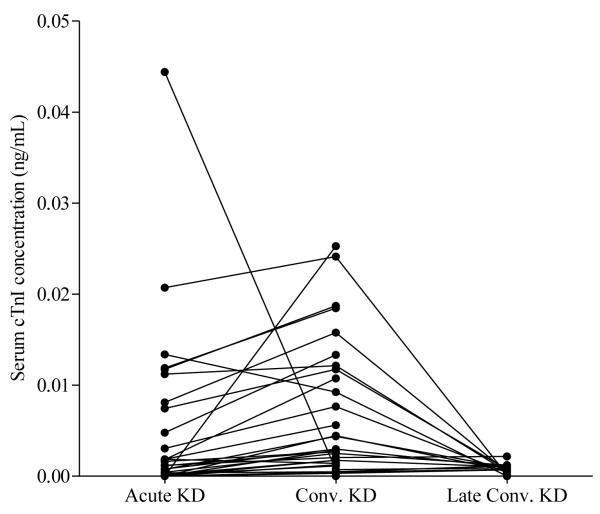
Elevated serum cTnI concentrations (>0.0045 ng/mL) were observed in 16 (29%) and 17 (31%) subjects in the acute KD and FC groups, respectively. Two of the 30 HC had values >99% for adults (0.007 and 0.006 ng/mL). The levels of cTnI were elevated in the convalescent as compared to the acute stage of KD (p=0.0003, n=30) and normalized in all 17 subjects with late convalescent samples (Table 3, Figure 3). Of the 9 subjects with aneurysms, 3 had elevated levels of cTnI (0.005, 0.008, and 0.021 ng/mL). For the KD subjects, there was a weak correlation of cTnI concentration with NT-proBNP (Table 4) and no significant correlation with the other biomarkers, measures of systolic or diastolic function, or measures of oxidative stress (data not shown).
Figure 3.
Cardiac troponin I (cTnI) concentrations in serial acute, convalescent (conv.), and late conv. (> 1yr.) serum samples from KD subjects.
DISCUSSION
Biomarkers associated with cardiomyocyte stress were elevated in the majority of acute KD subjects. Specifically, NT-proBNP correlated with markers of inflammation, oxidative stress, and echocardiographic measurements suggesting diastolic dysfunction. Biomarkers are emerging as valuable tools in assisting with disease prognosis and risk stratification for a variety of cardiovascular conditions. The natriuretic peptides are released in the setting of myocardial strain and are well-established for aiding in the diagnosis, prognostication, and monitoring of heart failure patients.[9] Previous studies have measured plasma BNP and NT-proBNP levels in acute and convalescent KD subjects and found results similar to those reported here.[17-22] Studies in Japanese KD patients found correlations between elevated plasma BNP levels and echocardiographic evidence of diastolic dysfunction and oxidative stress as assessed by urinary excretion of 8-isoprostane.[18, 20] An immunohistochemical study of KD autopsy tissues using an antibody to atrial natriuretic peptide revealed positive staining of cardiomyocytes adjacent to regions of fibrosis. Staining was most intense in patients who had clinical evidence of congestive heart failure prior to death.[23]
Impairment of diastolic function occurs in stages. Initially impaired relaxation, the consequence of inflammation and edema, is manifest by prolonged deceleration times and blunting of the mitral E wave, with atrial contraction (A wave) taking on a more central role in ventricular filling. With prolonged or severe inflammation, ventricular compliance can be affected, ultimately resulting in a compensatory increase in filling pressures, and a “pseudonormalization” of the inflow patterns. With further progression, abnormalities of ventricular compliance are seen resulting in an increase in filling pressures that result in a pronounced E wave and a shortened deceleration time. Our patients presented with a mixed picture, displaying increased A waves resulting in a reduced MV E:A ratio, and shortened deceleration times. Both NT-proBNP and sST2 negatively correlated with the mitral valve inflow E:A ratio and deceleration time, suggesting that as myocardial strain worsened, diastolic dysfunction became more pronounced. With respect to systolic function, 3 of the 6 subjects with NT-proBNP levels >1,000 pg/mL had FS more than 2 standard deviations below the mean for body surface area, and 4 of the 6 had elevated concentrations of cTnI.
sST2, a member of the IL-1 receptor family and a decoy receptor for IL-33, is released by cardiomyocytes and fibroblasts exposed to biomechanical stress.[10] sST2 levels, not previously measured in pediatric subjects, were significantly elevated in acute KD subjects compared to convalescent KD and HC subjects. Recent data suggest that sST2 may also be a mediator of myocardial injury. KD is likely to have an infectious etiology and IL-33 may play an important role in modulating the inflammatory response to pathogens.[24] Increased levels of IL-33 are associated with host protection against parasitic and viral infections and atherosclerosis, but can exacerbate Th2 T-cell and mast cell-mediated inflammatory diseases. Sequestration of IL-33 by sST2 could lead to increased inflammation in the setting of viral infection. Although elevated levels of sST2 are powerfully predictive of adverse events across a broad spectrum of cardiovascular conditions including heart failure and acute myocardial infarction, the mechanism by which sST2 mediates these effects is incompletely understood.[10, 25-28] sST2 may have a direct role in fibrosis or remodeling following myocardial injury.[29] The prognostic significance of sST2 levels in acute KD is unknown.
GGT catabolizes extracellular glutathione, the main thiol intracellular antioxidant in mammalian cells. Membrane-bound GGT is released into the serum from hepatocytes and the elevated levels in acute KD have been attributed to hepatobiliary inflammation, with the highest levels seen in association with hydrops of the gallbladder.[30] Results presented here, however, suggest that elevated GGT and ALT concentrations may be at least in part related to oxidative stress during the acute illness, with concentrations positively correlating with the biomarkers of cardiomyocyte strain.
cTnI, a measure of cardiomyocyte injury or death, was elevated in a third of both KD subjects and FC. A previous study that may have used a less sensitive assay did not detect elevated cTnI concentrations in children with acute KD.[31] Acute phase concentrations of cTnI did not correlate with markers of systemic inflammation, oxidative stress, or echocardiographic parameters, suggesting that other factors lead to myocardial necrosis or cardiomyocyte damage.[31] This was surprising as it would be logical to think that all of the effects on the myocardium were a result of systemic inflammation. However, this relationship did not hold for cTnI. The highly sensitive assay may detect variations in levels that are not physiologically significant with respect to myocardial function, though they seem to reflect disease severity to some degree since 4 of the 6 individuals with elevated NT-proBNP also had elevated cTnI. The elevation of cTnI in most KD subjects at the convalescent time point was also unexpected and may indicate that cardiomyocyte injury persists after systemic indicators of inflammation have returned to normal. The fact that levels of cTnI returned to normal in all subjects who had samples measured 1-2 years after disease onset suggests that the previous elevations were related to cardiomyocyte injury or death.
We recognize several limitations to our study. TDI was only performed on a subset of subjects as this imaging was not available until late into our study period. The timing of the first echocardiogram was not standardized with respect to IVIG infusion. To the extent that mitral valve inflow velocities are sensitive to preload, these measurements may have been influenced by the volume status of the patients in unpredictable ways. The volumes of plasma and serum samples were limited in this population of young infants and children, so not all measurements were performed on all patients. Limited data are available regarding the normal ranges of these biomarkers in the pediatric age group. Although this is the largest study of cardiovascular biomarkers in acute KD, the sample size was still small and thus the power to detect differences between groups was limited.
In summary, NT-proBNP and sST2 correlate with impaired myocardial relaxation as measured by echocardiography in acute KD subjects, and with increased oxidative stress as measured by elevated ALT and GGT levels. cTnI did not correlate with myocardial function, inflammation, or oxidative stress and was unique in having more elevated levels in many subjects in the convalescent but not late convalescent phase. Taken together, the results of this study suggest that myocardial stress and cardiomyocyte injury are common features of acute KD.
Acknowledgements
The authors wish to thank Joan Pancheri RN, BSN, for sample collection, Dee Anna Scherrer for sample preparation, and Albert Chiu for the analysis of Brahms biomarker proteins. We also thank the families who allowed their children to participate in this study.
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology.[32]
This work supported in part by grants from the Heart, Lung, Blood Institute, National Institutes of Health (HL69413 to JCB), from the American Heart Institute (09SDG2010231 to LBD), and from the Macklin Foundation (to JCB and LBD).
Footnotes
Work was conducted at University of California, San Diego, School of Medicine, 9500 Gilman Drive MC0641, La Jolla, CA 92093-0641.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Gordon JB, Kahn AM, Burns JC. When children with kawasaki disease grow up myocardial and vascular complications in adulthood. J Am Coll Cardiol. 2009;54:1911–20. doi: 10.1016/j.jacc.2009.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Crystal MA, Syan SK, Yeung RS, Dipchand AI, McCrindle BW. Echocardiographic and electrocardiographic trends in children with acute Kawasaki disease. The Canadian journal of cardiology. 2008;24:776–80. doi: 10.1016/s0828-282x(08)70683-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kanegaye JT, Wilder MS, Molkara D, Frazer JR, Pancheri J, Tremoulet AH, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123:e783–9. doi: 10.1542/peds.2008-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yutani C, Okano K, Kamiya T, Oguchi K, Kozuka T, Ota M, et al. Histopathological study on right endomyocardial biopsy of Kawasaki disease. Br Heart J. 1980;43:589–92. doi: 10.1136/hrt.43.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yonesaka S, Takahashi T, Matubara T, Nakada T, Furukawa H, Tomimoto K, et al. Histopathological study on Kawasaki disease with special reference to the relation between the myocardial sequelae and regional wall motion abnormalities of the left ventricle. Jpn Circ J. 1992;56:352–8. doi: 10.1253/jcj.56.352. [DOI] [PubMed] [Google Scholar]
- [6].Haneda N, Mori C. Histopathologic and coronary angiographic assessment of effectiveness of aspirin or aspirin-and-gammaglobulin in Kawasaki disease. Acta paediatrica Japonica; Overseas edition. 1993;35:294–7. doi: 10.1111/j.1442-200x.1993.tb03056.x. [DOI] [PubMed] [Google Scholar]
- [7].Arnold R, Goebel B, Ulmer HE, Gorenflo M, Poerner TC. An exercise tissue Doppler and strain rate imaging study of diastolic myocardial dysfunction after Kawasaki syndrome in childhood. Cardiol Young. 2007;17:478–86. doi: 10.1017/S1047951107000959. [DOI] [PubMed] [Google Scholar]
- [8].Tierney ES, Newburger JW, Graham D, Baker A, Fulton DR, Colan SD. Diastolic function in children with Kawasaki Disease. International journal of cardiology. 2009 doi: 10.1016/j.ijcard.2009.11.014. [DOI] [PubMed] [Google Scholar]
- [9].Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- [10].Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wiedermann FJ, Kaneider N, Egger P, Tiefenthaler W, Wiedermann CJ, Lindner KH, et al. Migration of human monocytes in response to procalcitonin. Critical care medicine. 2002;30:1112–7. doi: 10.1097/00003246-200205000-00025. [DOI] [PubMed] [Google Scholar]
- [12].Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- [13].Ravekes WJ, Colan SD, Gauvreau K, Baker AL, Sundel RP, van der Velde ME, et al. Aortic root dilation in Kawasaki disease. The American journal of cardiology. 2001;87:919–22. doi: 10.1016/s0002-9149(00)01541-1. [DOI] [PubMed] [Google Scholar]
- [14].Schmitz L, Xanthopoulos A, Koch H, Lange PE. Doppler flow parameters of left ventricular filling in infants: how long does it take for the maturation of the diastolic function in a normal left ventricle to occur? Pediatr Cardiol. 2004;25:482–91. doi: 10.1007/s00246-003-0605-1. [DOI] [PubMed] [Google Scholar]
- [15].Eidem BW, McMahon CJ, Cohen RR, Wu J, Finkelshteyn I, Kovalchin JP, et al. Impact of cardiac growth on Doppler tissue imaging velocities: a study in healthy children. J Am Soc Echocardiogr. 2004;17:212–21. doi: 10.1016/j.echo.2003.12.005. [DOI] [PubMed] [Google Scholar]
- [16].Nir A, Lindinger A, Rauh M, Bar-Oz B, Laer S, Schwachtgen L, et al. NT-pro-B-type natriuretic peptide in infants and children: reference values based on combined data from four studies. Pediatr Cardiol. 2009;30:3–8. doi: 10.1007/s00246-008-9258-4. [DOI] [PubMed] [Google Scholar]
- [17].Kawamura T, Wago M. Brain natriuretic peptide can be a useful biochemical marker for myocarditis in patients with Kawasaki disease. Cardiol Young. 2002;12:153–8. doi: 10.1017/s1047951102000318. [DOI] [PubMed] [Google Scholar]
- [18].Kurotobi S, Kawakami N, Shimizu K, Aoki H, Nasuno S, Takahashi K, et al. Brain Natriuretic Peptide as a Hormonal Marker of Ventricular Diastolic Dysfunction in Children with Kawasaki Disease. Pediatr Cardiol. 2005 doi: 10.1007/s00246-004-0812-4. [DOI] [PubMed] [Google Scholar]
- [19].Iwashima S, Ishikawa T, Ohzeki T. Brain natriuretic peptide levels in Kawasaki disease: a case report. Pediatr Int. 2009;51:415–8. doi: 10.1111/j.1442-200X.2009.02832.x. [DOI] [PubMed] [Google Scholar]
- [20].Takeuchi D, Saji T, Takatsuki S, Fujiwara M. Abnormal tissue doppler images are associated with elevated plasma brain natriuretic peptide and increased oxidative stress in acute Kawasaki disease. Circ J. 2007;71:357–62. doi: 10.1253/circj.71.357. [DOI] [PubMed] [Google Scholar]
- [21].Dahdah N, Siles A, Fournier A, Cousineau J, Delvin E, Saint-Cyr C, et al. Natriuretic Peptide as an Adjunctive Diagnostic Test in the Acute Phase of Kawasaki Disease. Pediatr Cardiol. 2009 doi: 10.1007/s00246-009-9441-2. [DOI] [PubMed] [Google Scholar]
- [22].de Benedictis FM, Colaneri M, Osimani P, Bettuzzi MG. Amino-Terminal Pro-B-Type Natriuretic Peptide in Refractory Kawasaki Disease. Pediatr Cardiol. 2009 doi: 10.1007/s00246-009-9416-3. [DOI] [PubMed] [Google Scholar]
- [23].Fujiwara T, Fujiwara H, Takemura G, Mukoyama M, Saito Y, Nakao K, et al. Expression and distribution of atrial natriuretic polypeptide in the ventricles of children with myocarditis and/or myocardial infarction secondary to Kawasaki disease: immunohistochemical study. American heart journal. 1990;120:612–8. doi: 10.1016/0002-8703(90)90019-t. [DOI] [PubMed] [Google Scholar]
- [24].Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 10:103–10. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- [25].Dieplinger B, Januzzi JL, Jr., Steinmair M, Gabriel C, Poelz W, Haltmayer M, et al. Analytical and clinical evaluation of a novel high-sensitivity assay for measurement of soluble ST2 in human plasma--the Presage ST2 assay. Clin Chim Acta. 2009;409:33–40. doi: 10.1016/j.cca.2009.08.010. [DOI] [PubMed] [Google Scholar]
- [26].Rehman SU, Mueller T, Januzzi JL., Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–65. doi: 10.1016/j.jacc.2008.07.042. [DOI] [PubMed] [Google Scholar]
- [27].Januzzi JL, Jr., Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, et al. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro-Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–13. doi: 10.1016/j.jacc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- [28].Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008;117:1936–44. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weir RA, Miller AM, Murphy GE, Clements S, Steedman T, Connell JM, et al. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol. 55:243–50. doi: 10.1016/j.jacc.2009.08.047. [DOI] [PubMed] [Google Scholar]
- [30].Ting EC, Capparelli EV, Billman GF, Lavine JE, Matsubara T, Burns JC. Elevated gamma-glutamyltransferase concentrations in patients with acute Kawasaki disease. Pediatr Infect Dis J. 1998;17:431–2. doi: 10.1097/00006454-199805000-00020. [DOI] [PubMed] [Google Scholar]
- [31].Checchia PA, Borensztajn J, Shulman ST. Circulating cardiac troponin I levels in Kawasaki disease. Pediatr Cardiol. 2001;22:102–6. doi: 10.1007/s002460010170. [DOI] [PubMed] [Google Scholar]
- [32].Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1–2. [Google Scholar]