Abstract
Activation of microglia, the resident macrophages of the brain, around the amyloid plaques is a key hallmark of Alzheimer’s disease (AD). Recent evidence in mouse models indicates that microglia are required for the neurodegenerative process of AD. Amyloid-β (Aβ) peptides, the core components of the amyloid plaques, can trigger microglial activation by interacting with several Toll-like receptors (TLRs), including TLR4. Here, we show that resveratrol, a natural polyphenol associated with anti-inflammatory effects and currently in clinical trials for AD, prevented the activation of murine RAW 264.7 macrophages and microglial BV-2 cells treated with the TLR4 ligand, lipopolysaccharide (LPS). Resveratrol preferentially inhibited NF-κB activation upon LPS stimulation by interfering with IKK and IκB phosphorylation, an effect that potently reduced the transcriptional stimulation of several NF-κB target genes, including TNF-α and IL-6. Consequently, downstream phosphorylation of STAT1 and STAT3 upon LPS stimulation was also inhibited by resveratrol. We found that resveratrol acted upstream in the activation cascade by interfering with TLR4 oligomerization upon receptor stimulation. Resveratrol treatment also prevented the pro-inflammatory effect of fibrillar Aβ on macrophages by potently inhibiting the effect of Aβ on IκB phosphorylation, activation of STAT1 and STAT3, and on TNF-α and IL-6 secretion. Importantly, orally administered resveratrol in a mouse model of cerebral amyloid deposition lowered microglial activation associated with cortical amyloid plaque formation. Together this work provides strong evidence that resveratrol has in vitro and in vivo anti-inflammatory effects against Aβ-triggered microglial activation. Further studies in cell culture systems showed that resveratrol acted via a mechanism involving the TLR4/NF-κB/STAT signaling cascade.
Keywords: Alzheimer’s disease, resveratrol, microglia, Aβ, lipopolysaccharide, TLR4, NF-κB, STAT
INTRODUCTION
Alzheimer’s disease is a neurodegenerative disorder characterized by selective and progressive loss of specific neuronal populations in the neocortex and hippocampus. The exact mechanism triggering neurodegeneration in AD remains unclear, but characteristic lesions implicating specific protein aggregates are invariably observed (Duyckaerts et al., 2009). These lesions include the amyloid plaques formed by the aggregation of amyloid-β (Aβ), a series of peptides derived from the sequential endoproteolysis of a longer precursor, the amyloid precursor protein (APP) (Marambaud and Robakis, 2005; Selkoe, 2001). APP is genetically linked to early-onset familial forms of AD and Aβ is thus considered to be a causative factor in AD. Cerebral Aβ deposition, however, is also observed in elderly non-demented individuals, suggesting that amyloid formation per se is not sufficient to trigger neurodegeneration. AD is also associated with the formation of lesions containing the tau protein called neurofibrillary tangles (NFTs) (Buee et al., 2000). Another key hallmark of AD is brain inflammation (Akiyama et al., 2000; Galimberti and Scarpini, 2011; Wyss-Coray and Mucke, 2002). Indeed, amyloid deposition is associated with activation of the surrounding microglia and the presence of a robust microglia-mediated inflammatory response (Landreth and Reed-Geaghan, 2009). Several inflammatory markers, such as cytokines and chemokines or proteins of the acute phase and complement are elevated in the AD brain. Extensive oxidative damage due to the production of reactive oxygen and nitrogen species is also observed within the AD brain. Furthermore, recent genome-wide association studies identified complement receptor type 1 (CR1), a protein implicated in the activated complement response, and CD33, a receptor expressed on cells of myeloid or lymphoid lineage and involved in the immune response, as significant susceptibility genes controlling the risk of developing AD (Harold et al., 2009; Lambert et al., 2009; Naj et al., 2011).
Microglia cells are derived from myeloid lineage progenitors and represent the resident mononuclear phagocytes of the central nervous system parenchyma. These cells are critically involved in cerebral inflammatory and immune responses (Ransohoff and Cardona, 2010). Like peripheral macrophages, microglial cells are activated by cytokines and other pro-inflammatory stimuli. This activation leads to specific intracellular signaling controlling the production by microglia of specific cell surface receptors, cytokines, and chemokines. The endotoxin lipopolysaccharide (LPS) for instance, a molecule found at the outer membrane of bacteria, can trigger stimulation of macrophages and microglial cells by activating an array of signal transduction pathways, which include the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), activator protein 1 (AP-1), and interferon regulatory factor 3 (IRF3). These transcriptional responses control the production of several cytokines, such as tumor necrosis factor-α (TNF-α) or interleukin-6 (IL-6). IL-6, in turn, promotes the activating phosphorylation of the STATs (signal transducer and activator of transcription), key transcription factors involved in the strengthening of the inflammatory response.
LPS specifically binds one type of receptor of the Toll-like receptor (TLR) family, TLR4. Following binding to LPS, TLR4 promotes signal transduction by activating intracellular pathways specific to two different adaptor proteins, myeloid differentiation primary response gene 88 (MyD88) and TIR domain-containing adapter molecule 1 (TRIF) (Kawai and Akira, 2007). The MyD88-dependent pathway activates the mitogen-activated protein kinase (MAPK) pathway and via IκB kinase (IKK) activation and IκBα inactivation leads to NF-κB transcriptional activation (Sanjo et al., 2003; Shim et al., 2005; Wang et al., 2001). On the other hand, the TRIF-dependent pathway activates the IKK-related kinases TRAF family member-associated NF-κB activator binding kinase-1 (TBK1) to activate IRF3 (Fitzgerald et al., 2003; Sharma et al., 2003).
Microglia are activated around the amyloid plaques in the AD brain (Bolmont et al., 2008; Bornemann et al., 2001). In cell cultures and animal models, fibrillar Aβ was found to interact with TLR4, TLR2, or the TLR co-receptor CD14 to activate the classical signaling pathways required for microglial activation, including NF-κB (Fassbender et al., 2004; Hanisch and Kettenmann, 2007; Udan et al., 2008; Walter et al., 2007). The role played by activated microglia in AD pathogenesis is unclear. On one hand, activation of microglia by Aβ may be beneficial by promoting CD14-, TLR4-, or TLR2-dependent phagocytosis and clearance of Aβ (Chen et al., 2006; Iribarren et al., 2005; Liu et al., 2005). On the other hand, microglial activation via TLR4- or CD14-dependent stimulation was proposed to lead to neuronal death by releasing potentially neurotoxic soluble factors or by contact-dependent neurotoxic mechanisms. Furthermore, monocytes activated by Aβ can induce neuronal death in vitro (London et al., 1996) and neurons cultured without microglia appear to be resistant to Aβ-induced neurotoxicity (Giulian et al., 1996). Recently, using two-photon in vivo imaging of neuronal loss in the intact brain, deletion of Cx3cr1, a microglial chemokine receptor critical for neuron-microglia communication, was found to prevent neuronal loss in a mouse model of amyloid deposition, demonstrating that microglia are involved in Aβ-mediated neuronal death in vivo (Fuhrmann et al., 2010).
Resveratrol (trans-3,4′,5-trihydroxystilbene) is a naturally occurring polyphenol found in abundance in red wine (Fremont, 2000). We and others have recently shown that this polyphenol is associated with anti-amyloidogenic and neuroprotective properties in vitro in cell lines and in vivo in mice (Karuppagounder et al., 2009; Marambaud et al., 2005; Vingtdeux et al., 2008; Vingtdeux et al., 2010). Resveratrol was also proposed to have anti-inflammatory effects in several systems, including in activated microglia (Zhang et al., 2010). However, the exact molecular mechanism by which resveratrol controls microglial activation remains uncertain. Furthermore, evidence is missing to support the notion that orally administered resveratrol can control microglial function in the brain. Here, we show that resveratrol is a potent inhibitor of the LPS-triggered inflammatory response in RAW 264.7 macrophages and microglial BV-2 cells. Resveratrol acted by preferentially antagonizing with the IKK/IκBα/NF-κB pathway upon LPS stimulation, an effect that inhibited NF-κB transcription and expression of several NF-κB target genes, including TNF-α and IL-6. Moreover, downstream activation of STAT1 and STAT3 upon stimulation with LPS was inhibited by resveratrol. Further studies showed that resveratrol also prevented the pro-inflammatory properties of fibrillar Aβ on macrophages by potently inhibiting the effect of Aβ on IκB phosphorylation, STAT1 and STAT3 activation, and on TNF-α and IL-6 secretion. Importantly, we found that resveratrol acted upstream in the activation cascade by interfering with TLR4 oligomerization upon TLR4 stimulation. Strikingly, in vivo studies in a mouse model of cerebral amyloid deposition showed that orally administered resveratrol lowered microglial activation associated with cortical amyloid plaque formation. Together, this work provides strong evidence that resveratrol has anti-inflammatory effects in vitro and in vivo against Aβ-triggered microglial activation. In vitro studies in cell culture systems also showed that resveratrol acted via a mechanism involving the TLR4/NF-κB/STAT signaling cascade. Because recent evidence in mouse models suggests that microglia are required for the neurodegenerative process of AD, this work provides further support for the current clinical trials aimed at assessing the beneficial effects of resveratrol intake against AD pathogenesis (see NIH registry of current clinical trials at http://clinicaltrials.gov/).
MATERIALS AND METHODS
Materials and Antibodies
Antibodies directed against total STAT3 and phospho-Tyr705 STAT3 (pSTAT3), total STAT1 and phospho-Tyr701 STAT1 (pSTAT1), total Akt and phospho-Ser473 Akt (pAkt), total IRF3 and phospho-Ser396 IRF3 (pIRF3), total IKKα and phospho-Ser176/180 IKKα/β (pIKKα/β), total IκBα and phospho-Ser32 IκBα (pIκBα), phospho-Ser536 NF-κB (pNF-κB), iNOS, Cox2, and TBK1 were obtained from Cell Signaling Technology (Danvers, MA). Antibody against phospho-Ser172 TBK1 (pTBK1) was from Epitomics (Burlingame, CA). Anti-actin antibody was from BD Transduction Laboratories (San Deigo, CA). Anti-Aβ-(1–17) 6E10 antibody was from Covance (Princeton, NJ). Anti-GFP antibody was from Molecular Probes, Invitrogen (Carlsbad, CA). Anti-FLAG M2 antibody, synthetic resveratrol (trans-resveratrol), and E. coli LPS were from Sigma-Aldrich (St. Louis, MO).
Cell culture and cell treatments
Murine macrophage cell line RAW 264.7 (American Type Culture Collection, Manassas, VA) and murine microglial cell line BV-2, kindly provided by Dr. Dennis J. Selkoe (Brigham and Women’s Hospital and Harvard Medical School, Boston, MA), were maintained in DMEM plus 10% FBS, penicillin, streptomycin, and 5 μg/ml puromycin at 37°C, 5% CO2. All cell lines were tested negative for mycoplasma contaminants (Zhao et al., 2008). RAW 264.7 and BV-2 cells were treated at a density of 2×106 cells per 35-mm well with the different drugs and for the indicated concentrations and incubation times. Murine bone marrow-derived pro-B cell line Ba/F3 stably expressing TLR4-GFP, TLR4-FLAG, MD-2, and CD14 were kindly provided by Dr. Kensuke Miyake (University of Tokyo, Tokyo, Japan) and were cultured in 10% FCS RPMI1640 supplemented with recombinant murine IL-3 (~70 U/ml). RAW 264.7 cells were stimulated with 10 ng/ml LPS, BV-2 cells with 100 ng/ml LPS, and Ba/F3 cells with 50 ng/ml LPS for the indicated periods of time. Before LPS stimulation, BV-2 and RAW 264.7 cells were pre-treated for 30 min with resveratrol (from a 100 mM stock solution in DMSO).
Immunoblotting
Protein extracts were analyzed by Western blotting (WB) using the antibodies listed above. Cell extracts obtained in Laemmli buffer were resolved on SDS-PAGE, followed by electrotransfer to nitrocellulose membranes. Following a blocking step in 5% milk in Tween-TBS, membranes were incubated with primary and secondary antibodies. Membranes were then developed and visualized with ECL (Pierce, Thermo Fisher Scientific Inc., Waltham, MA).
ELISA
Conditioned medium from cell cultures were collected and spun for 5 min at 14,000 × g at room temperature. Supernatants were analyzed for IL-6, TNF-α, and IL-1β levels by ELISA (R&D duo kits, R&D Systems, Minneapolis, MN), according to the manufacturer’s instructions.
TLR4 oligomerization assay
Ba/F3 cell lines stably expressing TLR4-GFP, TLR4-FLAG, MD-2, and CD14 (at a density of 40×106 cells/condition) were pre-treated or not with resveratrol for 2 hrs at the indicated concentrations. Cells were then incubated in the absence or presence of LPS for 20 min. Cells were rinsed once with ice-cold PBS, lysed with 1.5 ml lysis buffer [50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 4 mM EDTA, 1% Brij, 0.5% n-octyl-b-d-glucoside, 1× Complete protease inhibitor mixture (Roche Applied Sciences, Indianapolis, IN)] for 3 hrs at 4°C, and pre-cleared with protein-G beads for an additional 2 hrs. The samples were immunoprecipitated with anti-GFP antibody overnight at 4°C followed by addition of protein-G beads for 3 hrs at 4°C. The immunoprecipitates were washed twice in wash buffer [50 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.1% Brij, 0.1% n-octyl-b-d-glucoside, 1× Complete protease inhibitor mixture] and then subjected to SDS-PAGE and WB using anti-FLAG and anti-GFP antibodies.
NF-κB reporter assay
RAW 264.7 macrophages expressing a secreted embryonic alkaline phosphatase (SEAP) reporter construct inducible by NF-κB (RAW-Blue cells, Invivogen, San Diego, CA) were pre-incubated with resveratrol for 30 min and then treated with LPS for 8 hrs (1×105 cells/well). SEAP secretion was determined using the alkaline phosphatase substrate QUANTI-Blue (Invivogen), according to the manufacturer’s instructions. Absorbance was measured at 620 nm in a plate reader.
Fibrillar Aβ preparation and treatment
One milligram of Aβ1–42 (AnaSpec, Fremont, CA) was dissolved in 1000 μl HFIP (Hexa-Fluor-2-Propanol) in the manufacturer’s vial, vortexed briefly, incubated for 15 min in an ultra sonic water bath, and distributed into siliconized centrifuge tubes (100 – 200 μl per tube). Tubes were left at room temperature overnight to allow evaporation of the HFIP solution and stored at −20°C until use. 24 hrs before an experiment, one tube of Aβ preparation was resuspended in HFIP, followed by water [ratio H2O:HFIP (7:3)], sonicated in an ultra sonic water bath for 15 min. Tubes were stirred for 24 hrs and subjected to speed-vac for 10 min to allow evaporation of the HFIP solution. About 100 μM of fibrillar Aβ is recovered in the final solution. RAW 264.7 cells (at 0.8x106 cells/well) were treated for 24 hrs with 5 μM fibrillar Aβ.
Nuclear fractionation
Cells plated in 100-mm dishes were rinsed with ice-cold PBS and scrapped in 5 ml of ice-cold PBS. Cells were pelleted by centrifugation at 500 × g at 4°C for 5 min. Cell pellets were resuspended in 1 ml of sucrose buffer [20 mM Tris-HCl (pH 7.5), 0.25 M sucrose, 10 mM EGTA, 2 mM EDTA, and 1× Complete protease inhibitor mixture]. Cell suspension was homogenized with 15 strokes in a glass Dounce homogenizer with a Teflon pestle and centrifuged at 4°C at 750 x g for 10 min. Pellets (nuclear fractions) were washed 4 times with 1 ml of sucrose buffer containing 0.1 % Triton X-100, rinsed with 1ml of sucrose buffer, and lysed by sonication in 250 μl of 1% SDS PBS. Nuclear fractions were then analyzed by WB, as described above.
Mouse cytokine array
Conditioned medium from BV-2 and RAW 264.7 cell cultures pre-incubated with resveratrol and stimulated with LPS were analyzed for the secretion of selected mouse cytokines and chemokines on membrane arrays (Proteome Profiler Mouse Cytokine Array Panel A, R&D Systems), as per the manufacturer’s instructions.
Animal treatments and Immunohistochemistry
All animal experiments were performed according to procedures approved by the Feinstein Institute for Medical Research Institutional Animal Care and Use Committee. Fifteen-week-old male APP/PS1 transgenic mice (B6C3-Tg(APPswe, PSEN1dE9)85Dbo/J, The Jackson Laboratory, Bar Harbor, ME) were treated as described before (Vingtdeux et al., 2010). Briefly, mice were randomly assigned to resveratrol or control groups. The control groups received a standard AIN-93G diet (Testdiet), and the resveratrol groups received a standard AIN-93G diet supplemented with 0.35% resveratrol (Sigma-Aldrich). Supplemented diet was obtained as described before (Vingtdeux et al., 2010). Food intake and body weight were monitored weekly throughout the study. At 30 weeks of age, mice were sacrificed. Brains were excised and hemispheres were immediately fixed with 4% paraformaldehyde in 0.1 M PBS, pH 7.6. Paraformaldehyde-fixed brain hemispheres were then paraffin-embedded. Sections were incubated in 70% formic acid for 30 min before blocking in 5% milk in 0.25% Triton X-100 PBS for 1 h at room temperature. Sections were then incubated in the presence of primary antibody directed against Iba1 for 16 hrs at 4 °C. After washing, sections were incubated with 6E10 antibody for 2 hrs at room temperature. After washing, sections were then incubated with the appropriate secondary antibodies conjugated to Alexa fluorophores (Invitrogen). Finally, sections were stained with DAPI, mounted on glass slides using Vectashield (Vector laboratories, Burlingame, CA) and observed using a confocal microscope.
Statistical analyses
All data sets were analyzed using unpaired Student’s t-test. For the analysis of in vivo microglia activation, Wilcoxon test was used. Correlation between activated microglial cell number and plaque area was computed using Pearson correlation in the untreated and resveratrol-treated groups, separately. ANCOVA was used to test interaction between the groups.
RESULTS
Resveratrol inhibits cytokine secretion in LPS-stimulated BV-2 and RAW 264.7 cells
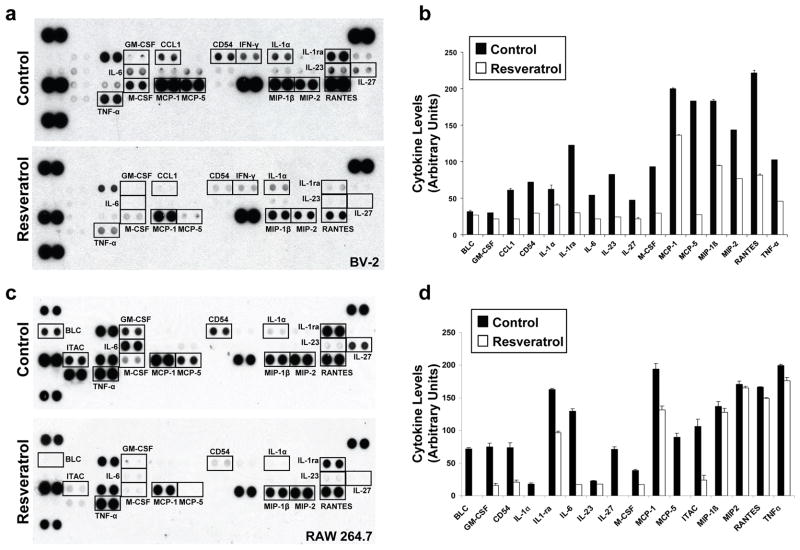
In order to assess the anti-inflammatory properties of resveratrol in microglial cells, we first assessed the effect of resveratrol treatment on cytokine production in LPS-stimulated BV-2 cells. Using cytokine arrays, we found that 50 μM resveratrol led to a robust decrease in the levels of multiple cytokines, including IL-6, M-CSF, MCP-1, MCP-5, CD54, IL-1ra, IL-27, and TNF-α (Figs. 1a and 1b). Resveratrol treatment had a very similar inhibitory effect on cytokine production in LPS-stimulated RAW 264.7 macrophages (Fig. 1c and 1d). IL-6, M-CSF, CD54, and IL-1ra levels, which were significantly reduced by resveratrol treatment in both BV-2 and RAW 264.7 cells, are transcriptionally controlled by NF-κB, suggesting that resveratrol may affect NF-κB activation upon LPS treatment.
Figure 1. Effect of resveratrol on cytokine secretion in LPS-stimulated BV-2 and RAW 264.7 cells.
Conditioned medium from BV-2 (a and b) and RAW 264.7 (c and d) cells pre-incubated in the absence (Control) or presence of resveratrol (50 μM, 30 min) and then stimulated for 8 hrs with LPS, was analyzed on cytokine arrays. Representative array analyses are shown in a and c. Histograms in b and d show the densitometric analysis and quantification of the immunoreactivity for the indicated cytokines from two independent experiments as in (a) and (c).
Resveratrol inhibits NF-κB signaling in LPS-stimulated BV-2 and RAW 264.7 cells
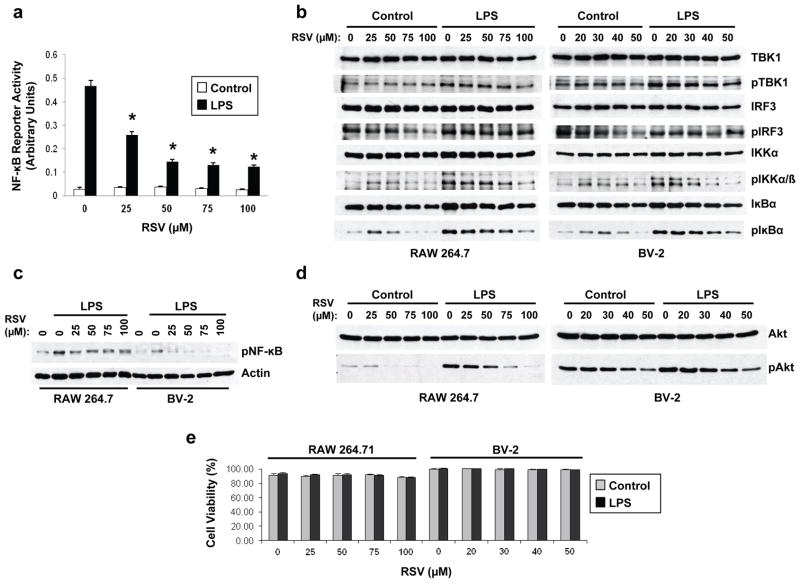
Using a NF-κB reporter assay and as previously reported (Youn et al., 2005), we found that resveratrol dose-dependently inhibited NF-κB activation in LPS-stimulated RAW 264.7 cells (Fig. 2a). TLR4 activation upon LPS binding leads to NF-κB activation via a MyD88-dependent pathway or a TRIF-dependent pathway. Previous work provided evidence indicating that resveratrol acts by specifically inhibiting the TLR4-mediated TRIF-dependent pathway, without affecting the MyD88-dependent pathway (Youn et al., 2005). In line with this previous study, we found a reduction in constitutively phosphorylated IRF3 in cells treated with resveratrol in the absence of LPS stimulation (Fig. 2b). We failed, however, to see any significant effect of resveratrol treatment on the levels of phosphorylated TBK1 or IRF3 upon LPS stimulation in both RAW 264.7 and BV-2 cells (Fig. 2b), suggesting that resveratrol did not inhibit NF-κB activation by interfering with the TRIF-dependent pathway. We confirmed the inhibitory effect of resveratrol on NF-κB activation in RAW 264.7 and BV-2 cells, by showing that resveratrol lowered the levels of phosphorylated IKKα, IκBα, and NF-κB in these cells upon stimulation with LPS (Figs. 2b and 2c). Furthermore, in LPS-stimulated BV-2 or RAW 264.7 cells, resveratrol potently inhibited the phosphorylation of Akt (Fig. 2d), a kinase controlled by MyD88 upon TLR4 activation (Monick et al., 2001; Salh et al., 1998). No cytotoxicity was observed in RAW 264.7 or BV-2 cells upon resveratrol treatment at concentrations required to inhibit NF-κB signaling (Fig. 2e).
Figure 2. Resveratrol inhibits NF-κB signaling in LPS-stimulated BV-2 and RAW 264.7 cells.
a, Conditioned medium from NF-κB reporter RAW 264.7 cells (RAW-Blue cells) pre-treated for 30 min with the indicated concentrations of resveratrol (RSV) and then stimulated or not for 8 hrs with LPS, was analyzed for SEAP reporter activity. Histogram shows the mean ± S.D. (*P < 0.001, n = 3 independent experiments). b, RAW 264.7 (left panels) and BV-2 (right panels) cells were pre-treated for 30 min with the indicated concentrations of RSV and then were stimulated (LPS) or not (Control) for 8 hrs with LPS. Total protein extracts were then analyzed by WB using antibodies directed against the indicated proteins. c, Nuclear protein extracts from RAW 264.7 (left panels) and BV-2 (right panels) cells treated as in (b) were analyzed by WB using antibodies directed against actin and phospho-NF-κB (pNF-κB). d, Total protein extracts from RAW 264.7 (left panels) and BV-2 (right panels) cells treated as in (b) were analyzed by WB using antibodies directed against total Akt and phospho-Akt (pAkt). e, Viability was evaluated by Trypan blue exclusion assays in RAW 264.7 and BV-2 cells treated as in (b). Histograms show the mean ± S.D. of two independent experiments using triplicate samples. Figs. 2b–2d show representative blots of three independent experiments.
Resveratrol inhibits STAT1/3 signaling in LPS-stimulated BV-2 and RAW 264.7 cells
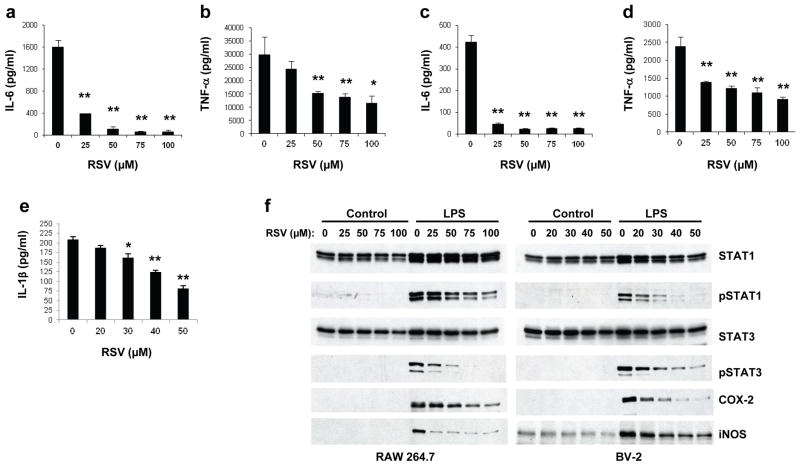
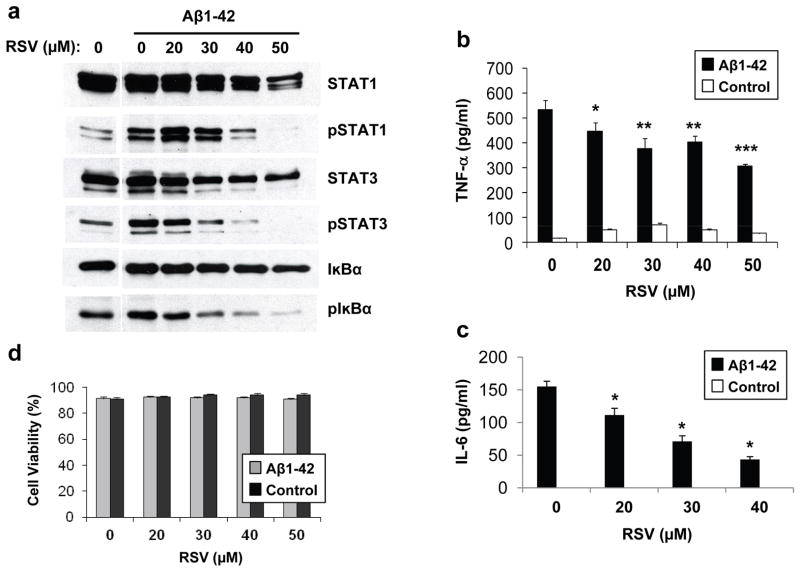
Cytokine arrays indicated that IL-6 and TNF-α secretion upon LPS stimulation were inhibited by resveratrol (Fig. 1). TNF-α and IL-6 are among the main pro-inflammatory cytokines released upon microglial activation and for which there is strong evidence of deregulation in AD (Sugama et al., 2009). Notably, TNFα and IL-6 are robustly elevated after Aβ treatment in cell culture systems or upon Aβ overexpression in APP transgenic organotypic brain slices (Patel et al., 2005; Udan et al., 2008). IL-6, upon binding to its receptor, is involved in the strengthening of the inflammatory response via the activation of the transcription factors STATs. Using ELISA, we confirmed the inhibitory effect of resveratrol treatment on LPS-stimulated IL-6 and TNF-α secretion in RAW 264.7 and BV-2 cells (Figs. 3a–3d), and on IL-1β secretion in RAW 264.7 cells (Fig. 3e). In line with these results, we observed a strong inhibition by resveratrol of STAT1 and STAT3 activation by LPS in these two cell lines (Fig. 3f). Furthermore, resveratrol dose-dependently lowered the expression of two downstream pro-inflammatory mediators, iNOS and COX-2, in both RAW 264.7 and BV-2 cells (Fig. 3f).
Figure 3. Resveratrol inhibits STAT1/3 signaling in LPS-stimulated BV-2 and RAW 264.7 cells.
a–d, Conditioned medium from RAW 264.7 (a and b) and BV-2 (c and d) cells pre-treated for 30 min with the indicated concentrations of resveratrol (RSV) and then stimulated for 8 hrs with LPS, was analyzed by ELISA for IL-6 (a and c) and TNF-α (b and d) secretion. e, Conditioned medium from RAW 264.7 cells pre-treated for 30 min with the indicated concentrations of RSV and then stimulated for 24 hrs with LPS, was analyzed by ELISA for IL-1β secretion. Histograms show the mean ± S.D. (*p<0.01, **p<0.001, n = 3 independent experiments). f, RAW 264.7 (left panels) and BV-2 (right panels) cells were pre-treated for 30 min with the indicated concentrations of RSV and then were stimulated (LPS) or not (Control) for 8 hrs with LPS. Protein extracts were analyzed by WB using antibodies directed against the indicated proteins. Fig. 3f shows representative blots of three independent experiments.
Resveratrol inhibits the LPS-mediated pro-inflammatory response by interfering with TLR4 oligomerization
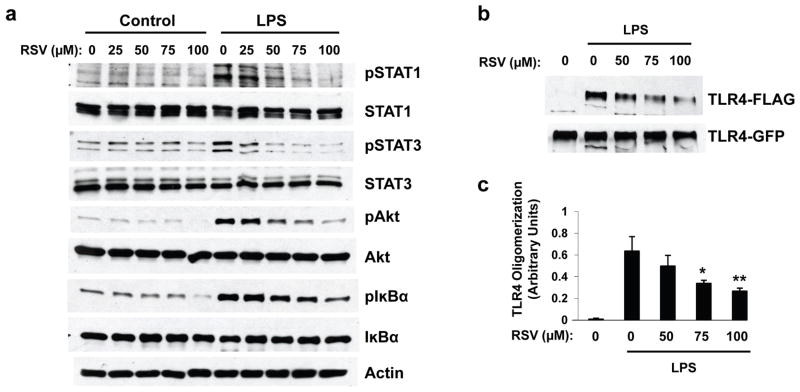
Because our data suggest that resveratrol acted upstream in the TLR4 activation cascade, we asked whether the natural polyphenol interferes with TLR4 oligomerization, a necessary step in the activation of the receptor upon ligand binding (Medzhitov et al., 1997; Rhee and Hwang, 2000; Zhang et al., 2002). To assess TLR4 oligomerization, we performed co-immunoprecipitation experiments in protein homogenates from pro-B Ba/F3 cells coexpressing two differently tagged versions of TLR4 (Saitoh et al., 2004). As expected, LPS treatment of Ba/F3 cells expressing TLR4 resulted in the phosphorylation of Akt, IκBα, STAT1, and STAT3 (Fig. 4a), confirming that these cells respond to LPS treatment by triggering NF-κB signaling activation. In line with our results in BV-2 and RAW 264.7 cells, resveratrol pre-incubation inhibited the phosphorylation of these proteins upon LPS stimulation (Fig. 4a). Under these conditions, we found that resveratrol treatment strongly reduced the levels of FLAG-tagged TLR4 co-immunoprecipitating with GFP-tagged TLR4, showing that resveratrol interfered with TLR4 oligomerization upon LPS stimulation, and this at concentrations consistent with its inhibitory effect on the Akt/NF-κB/STAT signaling cascade (Figs. 4b and 4c).
Figure 4. Resveratrol inhibits TLR4 oligomerization in LPS-stimulated Ba/F3 cells.
a, Ba/F3 cell lines stably expressing TLR4-GFP and TLR4-FLAG were pre-treated for 30 min with the indicated concentrations of resveratrol (RSV). Cells were then stimulated (LPS, 50 ng/ml) or not (Control) for 8 hrs with LPS. Protein extracts were analyzed by WB using antibodies directed against the indicated proteins. b, Transfected Ba/F3 cells were pre-treated for 2 hrs with the indicated concentrations of RSV and then were stimulated (LPS) or not (Control) for 20 min with LPS. Cell lysates were subjected to immunoprecipitation with anti-GFP antibody and immunoprecipitates were analyzed by WB using anti-GFP and anti-FLAG antibodies. c, Densitometric analysis and quantification of the ratio TLR4-FLAG/TLR4-GFP obtained from three independent experiments performed as in (b). Histogram shows the mean ± S.D (*p < 0.05, **p < 0.01). Figs. 4a and 4b show representative blots of three independent experiments.
Resveratrol inhibits Aβ-mediated microglial activation in vitro and in vivo
Microglial activation around amyloid plaques is a hallmark of AD. Strong evidence in cell cultures and mouse models indicates that aggregated Aβ is responsible for the activation of microglial cells. We found that treatment with fibrillar Aβ preparations resulted in a significant elevation of the phosphorylation of STAT1, STAT3, and IκBα (Fig. 5a), and of the secretion levels of TNF-α (Fig. 5b) and IL-6 (Fig. 5c). Importantly, pre-incubation with resveratrol dose-dependently inhibited the fibrillar Aβ-triggered increase of STAT1, STAT3, and IκBα phosphorylation (Fig. 5a), and of TNF-α and IL-6 secretion (Figs. 5b and 5c). No cytotoxicity was observed upon resveratrol and Aβ treatments at the tested concentrations (Fig. 5d). Thus, resveratrol can inhibit the microglial inflammatory responses triggered by both LPS and Aβ.
Figure 5. Resveratrol inhibits the Aβ-mediated pro-inflammatory response in RAW 264.7 cells.
a, RAW 264.7 cells pre-treated for 30 min with the indicated concentrations of resveratrol (RSV) were incubated for 24 hrs in the absence (Control) or presence of fibrillar Aβ1–42 preparations (5 μM, see Methods). Protein extracts were then analyzed by WB using antibodies directed against the indicated proteins. Representative blots of three independent experiments are shown. b and c, conditioned medium from RAW 264.7 cells treated as in (a) was analyzed by ELISA for TNF-α (b) and IL-6 (c) secretion. d, Viability was evaluated by Trypan blue exclusion assays in RAW 264.7 cells treated as in (b and c). Histograms show the mean ± S.D. (*p<0.05, **p<0.01, ***p<0.001, n=3 independent experiments).
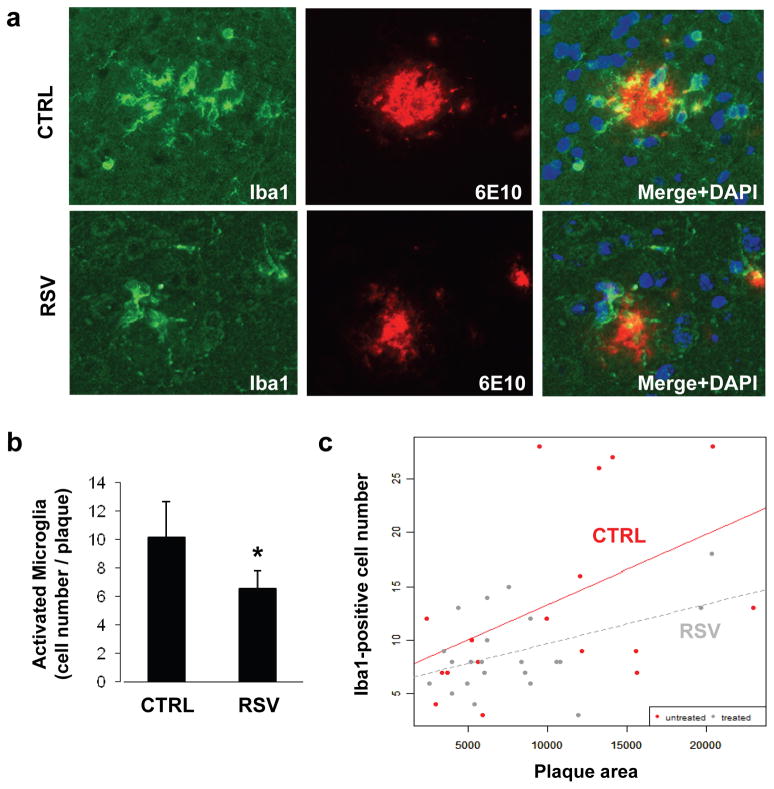
Based on these in vitro results in cell lines, we then tested the ability of resveratrol to prevent microglial activation in the brain of APP/PS1 mice, a model of cerebral amyloid deposition. These mice co-express two familial AD-linked mutant proteins, APP (carrying the Swedish mutation) and presenilin-1 (PSEN1ΔE9 mutation) (Jankowsky et al., 2004). By 30 weeks of age, these mice develop robust amyloid deposition in different brain regions, including the cerebral cortex (Jankowsky et al., 2004). These mice also develop strong microglial activation that accompanies cerebral amyloid plaque formation. Recently, we and others have reported that oral administration of resveratrol can significantly lower amyloid deposition in APP transgenic mice (Karuppagounder et al., 2009; Vingtdeux et al., 2010). We found that resveratrol, orally administered in mice for 15 weeks, can reach the brain to reduce Aβ levels and amyloid deposition in the cerebral cortex, showing that resveratrol is both bioavailable and bioactive in the brain after oral dosing (Vingtdeux et al., 2010). We followed the same protocol to evaluate the in vivo efficacy of resveratrol against Aβ-associated microglial activation in APP/PS1 mice. Two independent groups of 15-week-old male mice were fed an AIN-93G diet supplemented or not with 0.35% resveratrol for a period of 15 weeks. We determined that the mice ingested about 350 mg/kg of body weight daily of resveratrol from the supplemented diet during the 15-week period (Vingtdeux et al., 2010). The cortical region was analyzed by immunohistochemistry using an antibody directed against Iba1, a specific marker for activated macrophage/microglia (Imai and Kohsaka, 2002). We found a significant decrease in the total number of Iba1-positive cells surrounding the amyloid plaques (P < 0.05, Figs. 6a and 6b). In order to determine whether the reduction in the number of activated microglia is simply due to the anti-amyloidogenic effect of resveratrol in these mice (Vingtdeux et al., 2010), we also analyzed the correlation between Iba1-positive cell number and plaque area in the untreated and resveratrol-treated groups. As expected, cell numbers significantly correlated with plaque area in both groups (Fig. 6c). A clear trend towards a reduction in the number of activated microglia for the same plaque size was observed in resveratrol-treated mice, as compared to untreated controls (Fig. 6c), suggesting that resveratrol lowered inflammation, at least in part, independently of its effect on amyloid deposition. It is important to note that this effect was not statistically significant and thus will require careful validation in subsequent studies on additional groups. Together these data showed that resveratrol has in vivo efficacy against Aβ-associated microglial activation in mice.
Figure 6. Resveratrol inhibits Aβ-mediated microglial activation in APP/PS1 mice.
a, Shown are representative amyloid deposition and surrounding microglial activation staining in the cortex of control (CTRL, n = 5) and resveratrol-fed (RSV, n = 5) mice by immunohistochemistry using anti-Iba1 (green) and anti-Aβ (6E10, red) antibodies. Histogram in (b) shows the number of Iba-positive activated microglial cells per amyloid plaque. Histogram indicates the mean ± S.D. of counts taken in four independent sections per brain. *p < 0.05 (Wilcoxon test). c, Graph showing the interaction between the number of Iba1-positive cells and amyloid plaque area for the resveratrol-treated and untreated mouse groups. Correlation between cell number and plaque area was computed using Pearson coefficient in the untreated and treated groups, separately. ANCOVA was used to test interaction of treatment with area. Untreated group: Correlation = 0.47, p = 0.047; Treated group: Correlation = 0.45, p = 0.036; Interaction between the two groups: p = 0.42.
DISCUSSION
Several epidemiological data now suggest that moderate consumption of red wine is associated with a lower incidence of dementia and AD (Lindsay et al., 2002; Luchsinger et al., 2004; Orgogozo et al., 1997; Truelsen et al., 2002). Studies in AD mouse models have also shown that red wine intake may attenuate cerebral amyloid deposition and Aβ-associated cognitive deterioration (Ho et al., 2009; Wang et al., 2006). Resveratrol is found in relative abundance in red wine and has been associated with potential neuroprotective properties and thus could explain the beneficial effect of wine intake against AD (Marambaud et al., 2005; Ramassamy, 2006; Vingtdeux et al., 2008); however, the underlying mechanism responsible for this effect is uncertain. Resveratrol was found to delay Aβ-induced toxicity in different neuronal cell culture models (Han et al., 2004; Jang and Surh, 2003; Savaskan et al., 2003) and to exert anti-aggregation and anti-fibrillogenic effects on Aβ in vitro (Ono et al., 2003; Riviere et al., 2007). Furthermore, studies from our group and others have demonstrated the anti-amyloidogenic effect of resveratrol in cell cultures and mouse models for amyloid formation (Karuppagounder et al., 2009; Marambaud et al., 2005; Vingtdeux et al., 2010). In the current study, we provide strong evidence that resveratrol can significantly lower in vitro and in vivo the microglial inflammation triggered by Aβ. In vitro, we showed that this polyphenol prevented the activation of RAW 264.7 macrophages and microglial BV-2 cells by LPS or fibrillar Aβ treatments. At the molecular level, resveratrol appeared to act upstream in the activation cascade by interfering with TLR4 oligomerization upon stimulation of the receptor (Fig. 4). This inhibitory effect of resveratrol resulted in a significant attenuation of the signal transduction pathways downstream from MyD88, which include NF-κB, STAT1/3, and Akt (Figs. 2 and 3). In vivo, we found a robust decrease in the number of activated microglial cells surrounding amyloid plaques in APP/PS1 mice treated with resveratrol. This work not only confirmed that resveratrol is bioavailable and bioactive in the brain after oral dosing but also revealed the anti-inflammatory potential of this polyphenol in vivo against Aβ-mediated microglial activation. Together these data increase our understanding of the different neuroprotective properties of resveratrol against Aβ-associated toxicity.
Our previous work showed that, in neurons, resveratrol acts by promoting autophagic clearance of Aβ via a mechanism implicating the Ser/Thr protein kinase AMPK (Vingtdeux et al., 2010; Vingtdeux et al., 2011). The direct molecular target of resveratrol in vitro and in vivo and the exact mechanism by which AMPK is activated is not firmly established. Resveratrol was initially proposed to bind in vitro to the deacetylase SIRT1 and to activate its enzymatic activity (Borra et al., 2005; Howitz et al., 2003). SIRT1 may also represent a target for resveratrol metabolic functions in vivo in muscle tissue (Lagouge et al., 2006). Moreover, SIRT1 activation by resveratrol can lead to AMPK activation in several cell lines, including HepG2 hepatocytes and HEK293T cells (Hou et al., 2008; Lan et al., 2008). SIRT1 activation by resveratrol was also proposed to be required for the beneficial effect of this polyphenol against NF-κB activation in microglia stimulated by Aβ (Chen et al., 2005). It is important to note, however, that recent studies have shown that resveratrol can also activate AMPK independently of SIRT1 (Dasgupta and Milbrandt, 2007; Um et al., 2009) and that SIRT1 may not be a direct target of this polyphenol (Borra et al., 2005; Kaeberlein et al., 2005; Pacholec et al., 2010). In this context, it will be important to determine whether the inhibitory effect of resveratrol on TLR4 oligomerization and NF-κB/STAT signaling activation in microglial cells is due to direct interference via resveratrol binding to TLR4 or one of its co-receptors, or to indirect mechanisms implicating AMPK or SIRT1 activation.
Several naturally occurring polyphenols, including flavonoids, are characterized by their anti-inflammatory and immunomodulatory properties. The effects of theses polyphenols on inflammation has often been attributed to their actions on the NF-κB pathway (Gonzalez et al., 2011). The mechanism by which these different polyphenols interfere with NF-κB activation is, however, not fully elucidated. For instance, several flavonoids, such as EGCG (Youn et al., 2006), butein (Pandey et al., 2007), morin (Manna et al., 2007), fisetin (Sung et al., 2007), or gossypin (Kunnumakkara et al., 2007) have been shown to interfere with the NF-κB pathway by inhibiting IKK. Resveratrol has been proposed to act at another level by inhibiting the MyD88-independent pathway (Youn et al., 2005). The present study confirmed the inhibitory effect of resveratrol on the NF-κB pathway but failed to see any significant effect of resveratrol treatment on the levels of phosphorylated TBK1 or IRF3 upon LPS stimulation in both RAW 264.7 and BV-2 cells, suggesting that resveratrol acted without interfering with the TRIF-dependent pathway. Further studies focusing on the different cross talks between the MyD88- and TRIF-dependent pathways will be required to elucidate this apparent discrepancy in the mechanism of action of resveratrol on NF-κB activation.
We provided strong evidence that resveratrol acted upstream in the activation cascade by interfering with TLR4 oligomerization upon receptor stimulation. Activation of NF-κB in macrophages requires the phosphorylation of IKK by Akt and several flavonoids have been proposed to inhibit this pathway by interfering with the Akt activating kinase PI3K (Chen et al., 2007; Lee et al., 2006). Because our results also showed that resveratrol treatment interfered with Akt activation (Figs. 2d and 4a), it will be interesting to determine in future studies whether the effect of resveratrol on NF-κB activation could also be the result of a direct inhibitory effect on PI3K activity.
In summary, the present study provides strong evidence that resveratrol negatively controlled in vitro and in vivo microglial inflammation triggered by Aβ. Studies in cell cultures showed that resveratrol acted, at least in part, by interfering with TLR4 oligomerization upon activation of the receptor, a mechanism of action that resulted in the inhibition of the NF-κB/STAT/Akt signaling cascades. These findings provide further support for the current clinical trials aimed at assessing the beneficial effects of resveratrol administration against AD development.
Acknowledgments
We thank Dr. Dennis J. Selkoe (Brigham and Women’s Hospital and Harvard Medical School, Boston, MA) for kindly providing us with BV-2 cells and Dr. Kensuke Miyake (The Institute of Medical Science, University of Tokyo, Tokyo, Japan) for transfected Ba/F3 cells. This work was supported in part by the National Institutes of Health grant PO1 AT004511 (National Center for Complementary and Alternative Medicine Project 2 to P.M.). The authors declare no conflict of interest.
Abbreviations
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- Aβ
amyloid-β
- TLRs
Toll-like receptors
- LPS
lipopolysaccharide
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- AP-1
activator protein 1
- IRF3
interferon regulatory factor 3
- TNF-α
tumor necrosis factor-α
- IL
interleukin
- STAT
signal transducer and activator of transcription
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis CA, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornemann KD, Wiederhold KH, Pauli C, Ermini F, Stalder M, Schnell L, Sommer B, Jucker M, Staufenbiel M. Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice. Am J Pathol. 2001;158:63–73. doi: 10.1016/s0002-9440(10)63945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007;81:1602–1614. doi: 10.1016/j.lfs.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Chen K, Iribarren P, Hu J, Chen J, Gong W, Cho EH, Lockett S, Dunlop NM, Wang JM. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide. J Biol Chem. 2006;281:3651–3659. doi: 10.1074/jbc.M508125200. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyckaerts C, Delatour B, Potier MC. Classification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Walter S, Kuhl S, Landmann R, Ishii K, Bertsch T, Stalder AK, Muehlhauser F, Liu Y, Ulmer AJ, et al. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB J. 2004;18:203–205. doi: 10.1096/fj.03-0364fje. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer’s disease. Nat Neurosci. 2010;13:411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, Scarpini E. Inflammation and oxidative damage in Alzheimer’s disease: friend or foe? Front Biosci (Schol Ed) 2011;3:252–266. doi: 10.2741/s149. [DOI] [PubMed] [Google Scholar]
- Giulian D, Haverkamp LJ, Yu JH, Karshin W, Tom D, Li J, Kirkpatrick J, Kuo LM, Roher AE. Specific domains of beta-amyloid from Alzheimer plaque elicit neuron killing in human microglia. J Neurosci. 1996;16:6021–6037. doi: 10.1523/JNEUROSCI.16-19-06021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Ballester I, Lopez-Posadas R, Suarez MD, Zarzuelo A, Martinez-Augustin O, Sanchez de Medina F. Effects of flavonoids and other polyphenols on inflammation. Crit Rev Food Sci Nutr. 2011;51:331–362. doi: 10.1080/10408390903584094. [DOI] [PubMed] [Google Scholar]
- Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br J Pharmacol. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Chen LH, Wang J, Zhao W, Talcott ST, Ono K, Teplow D, Humala N, Cheng A, Percival SS, et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J Alzheimer’s Dis. 2009;16:59–72. doi: 10.3233/JAD-2009-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan K, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren T, Cohen R, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Imai Y, Kohsaka S. Intracellular signaling in M-CSF-induced microglia activation: role of Iba1. Glia. 2002;40:164–174. doi: 10.1002/glia.10149. [DOI] [PubMed] [Google Scholar]
- Iribarren P, Chen K, Hu J, Gong W, Cho EH, Lockett S, Uranchimeg B, Wang JM. CpG-containing oligodeoxynucleotide promotes microglial cell uptake of amyloid beta 1–42 peptide by up-regulating the expression of the G-protein- coupled receptor mFPR2. FASEB J. 2005;19:2032–2034. doi: 10.1096/fj.05-4578fje. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003;34:1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, Copeland NG, Lee MK, Younkin LH, Wagner SL, Younkin SG, Borchelt DR. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13:159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, Bedalov A, Kennedy BK. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Pinto JT, Xu H, Chen HL, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem Int. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Nair AS, Ahn KS, Pandey MK, Yi Z, Liu M, Aggarwal BB. Gossypin, a pentahydroxy glucosyl flavone, inhibits the transforming growth factor beta-activated kinase-1-mediated NF-kappaB activation pathway, leading to potentiation of apoptosis, suppression of invasion, and abrogation of osteoclastogenesis. Blood. 2007;109:5112–5121. doi: 10.1182/blood-2007-01-067256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landreth GE, Reed-Geaghan EG. Toll-like receptors in Alzheimer’s disease. Curr Top Microbiol Immunol. 2009;336:137–153. doi: 10.1007/978-3-642-00549-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Jung HS, Giang PM, Jin X, Lee S, Son PT, Lee D, Hong YS, Lee K, Lee JJ. Blockade of nuclear factor-kappaB signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. J Pharmacol Exp Ther. 2006;316:271–278. doi: 10.1124/jpet.105.092486. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hebert R, Helliwell B, Hill GB, McDowell I. Risk factors for Alzheimer’s disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Liu Y, Walter S, Stagi M, Cherny D, Letiembre M, Schulz-Schaeffer W, Heine H, Penke B, Neumann H, Fassbender K. LPS receptor (CD14): a receptor for phagocytosis of Alzheimer’s amyloid peptide. Brain. 2005;128:1778–1789. doi: 10.1093/brain/awh531. [DOI] [PubMed] [Google Scholar]
- London JA, Biegel D, Pachter JS. Neurocytopathic effects of beta-amyloid-stimulated monocytes: a potential mechanism for central nervous system damage in Alzheimer disease. Proc Natl Acad Sci U S A. 1996;93:4147–4152. doi: 10.1073/pnas.93.9.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- Manna SK, Aggarwal RS, Sethi G, Aggarwal BB, Ramesh GT. Morin (3,5,7,2′,4′-Pentahydroxyflavone) abolishes nuclear factor-kappaB activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-kappaB-regulated gene expression and up-regulation of apoptosis. Clin Cancer Res. 2007;13:2290–2297. doi: 10.1158/1078-0432.CCR-06-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marambaud P, Robakis NK. Genetic and molecular aspects of Alzheimer’s disease shed light on new mechanisms of transcriptional regulation. Genes Brain Behav. 2005;4:134–146. doi: 10.1111/j.1601-183X.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Monick MM, Carter AB, Robeff PK, Flaherty DM, Peterson MW, Hunninghake GW. Lipopolysaccharide activates Akt in human alveolar macrophages resulting in nuclear accumulation and transcriptional activity of beta-catenin. J Immunol. 2001;166:4713–4720. doi: 10.4049/jimmunol.166.7.4713. [DOI] [PubMed] [Google Scholar]
- Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, Gallins PJ, Buxbaum JD, Jarvik GP, Crane PK, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J Neurochem. 2003;87:172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, Renaud S, Breteler MB. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol. 1997;153:185–192. [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey MK, Sandur SK, Sung B, Sethi G, Kunnumakkara AB, Aggarwal BB. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-kappaB and NF-kappaB-regulated gene expression through direct inhibition of IkappaBalpha kinase beta on cysteine 179 residue. J Biol Chem. 2007;282:17340–17350. doi: 10.1074/jbc.M700890200. [DOI] [PubMed] [Google Scholar]
- Patel NS, Paris D, Mathura V, Quadros AN, Crawford FC, Mullan MJ. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer’s disease. J Neuroinflammation. 2005;2:9. doi: 10.1186/1742-2094-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000;275:34035–34040. doi: 10.1074/jbc.M007386200. [DOI] [PubMed] [Google Scholar]
- Riviere C, Richard T, Quentin L, Krisa S, Merillon JM, Monti JP. Inhibitory activity of stilbenes on Alzheimer’s beta-amyloid fibrils in vitro. Bioorg Med Chem. 2007;15:1160–1167. doi: 10.1016/j.bmc.2006.09.069. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Akashi S, Yamada T, Tanimura N, Kobayashi M, Konno K, Matsumoto F, Fukase K, Kusumoto S, Nagai Y, et al. Lipid A antagonist, lipid IVa, is distinct from lipid A in interaction with Toll-like receptor 4 (TLR4)-MD-2 and ligand-induced TLR4 oligomerization. Int Immunol. 2004;16:961–969. doi: 10.1093/intimm/dxh097. [DOI] [PubMed] [Google Scholar]
- Salh B, Wagey R, Marotta A, Tao JS, Pelech S. Activation of phosphatidylinositol 3-kinase, protein kinase B, and p70 S6 kinases in lipopolysaccharide-stimulated Raw 264.7 cells: differential effects of rapamycin, Ly294002, and wortmannin on nitric oxide production. J Immunol. 1998;161:6947–6954. [PubMed] [Google Scholar]
- Sanjo H, Takeda K, Tsujimura T, Ninomiya-Tsuji J, Matsumoto K, Akira S. TAB2 is essential for prevention of apoptosis in fetal liver but not for interleukin-1 signaling. Mol Cell Biol. 2003;23:1231–1238. doi: 10.1128/MCB.23.4.1231-1238.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaskan E, Olivieri G, Meier F, Seifritz E, Wirz-Justice A, Muller-Spahn F. Red wine ingredient resveratrol protects from beta-amyloid neurotoxicity. Gerontology. 2003;49:380–383. doi: 10.1159/000073766. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Cho BP, Joh TH, Hashimoto M, Kitani H. Possible roles of microglial cells for neurotoxicity in clinical neurodegenerative diseases and experimental animal models. Inflamm Allergy Drug Targets. 2009;8:277–284. doi: 10.2174/187152809789352249. [DOI] [PubMed] [Google Scholar]
- Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–1714. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- Truelsen T, Thudium D, Gronbaek M. Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology. 2002;59:1313–1319. doi: 10.1212/01.wnl.0000031421.50369.e7. [DOI] [PubMed] [Google Scholar]
- Udan ML, Ajit D, Crouse NR, Nichols MR. Toll-like receptors 2 and 4 mediate Abeta(1–42) activation of the innate immune response in a human monocytic cell line. J Neurochem. 2008;104:524–533. doi: 10.1111/j.1471-4159.2007.05001.x. [DOI] [PubMed] [Google Scholar]
- Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMPK-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2009 doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Chandakkar P, Zhao H, d’Abramo C, Davies P, Marambaud P. Novel synthetic small-molecule activators of AMPK as enhancers of autophagy and amyloid-beta peptide degradation. FASEB J. 2011;25:219–231. doi: 10.1096/fj.10-167361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Dreses-Werringloer U, Zhao H, Davies P, Marambaud P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2202-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Giliberto L, Zhao H, Chandakkar P, Wu Q, Simon JE, Janle EM, Lobo J, Ferruzzi MG, Davies P, Marambaud P. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao Z, Seror I, Humala N, Dickstein DL, Thiyagarajan M, Percival SS, Talcott ST, Pasinetti GM. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Youn HS, Lee JY, Fitzgerald KA, Young HA, Akira S, Hwang DH. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J Immunol. 2005;175:3339–3346. doi: 10.4049/jimmunol.175.5.3339. [DOI] [PubMed] [Google Scholar]
- Youn HS, Lee JY, Saitoh SI, Miyake K, Kang KW, Choi YJ, Hwang DH. Suppression of MyD88- and TRIF-dependent signaling pathways of Toll-like receptor by (−)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochem Pharmacol. 2006;72:850–859. doi: 10.1016/j.bcp.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Zhang F, Liu J, Shi JS. Anti-inflammatory activities of resveratrol in the brain: role of resveratrol in microglial activation. Eur J Pharmacol. 2010;636:1–7. doi: 10.1016/j.ejphar.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tay PN, Cao W, Li W, Lu J. Integrin-nucleated Toll-like receptor (TLR) dimerization reveals subcellular targeting of TLRs and distinct mechanisms of TLR4 activation and signaling. FEBS Lett. 2002;532:171–176. doi: 10.1016/s0014-5793(02)03669-4. [DOI] [PubMed] [Google Scholar]
- Zhao H, Dreses-Werringloer U, Davies P, Marambaud P. Amyloid-beta peptide degradation in cell cultures by mycoplasma contaminants. BMC Res Notes. 2008;1:38. doi: 10.1186/1756-0500-1-38. [DOI] [PMC free article] [PubMed] [Google Scholar]