Abstract
Studies of both vertebrates and invertebrates have suggested that specialists, as compared to generalists, are likely to suffer more serious declines in response to environmental change. Less is known about the effects of environmental conditions on specialist vs. generalist parasites. Here, we study the evolutionary strategies of malaria parasites (Plasmodium spp.) among different bird host communities. We determined the parasite diversity and prevalence of avian malaria in three bird communities in the lowland forests in Cameroon, highland forests in East Africa, and fynbos in South Africa. We calculated the host specificity index of parasites to examine the range of hosts parasitized as a function of the habitat, and investigated the phylogenetic relationships of parasites. First, using phylogenetic and ancestral reconstruction analyses we found an evolutionary tendency for generalist malaria parasites to become specialists. The transition rate at which generalists become specialists was nearly four times as great as the rate at which specialists become generalists. We also found more specialist parasites and greater parasite diversity in African lowland rainforests as compared to the more climatically variable habitats of the fynbos and the highland forests. Thus, with environmental changes, we anticipate a change in the distribution of both specialist and generalist parasites with potential impacts on bird communities.
Keywords: Plasmodium, avian malaria, habitat specificity, specialist, generalist
Introduction
The existence of both generalist and specialist species raises several questions: why have these strategies evolved and for a given set of environmental conditions, is there an optimal strategy? Much attention has been paid to how species distributions and populations might be affected by environmental change (Pounds et al. 1999; Both & te Marvelde 2007; Gordo 2007; Hitch & Leberg 2007). In addition, the majority of research on specialization has been performed on both vertebrates and invertebrates, with most studies suggesting that niche specialists have suffered extensive declines as compared to generalists (insects: Hugues et al. 2000; Kotze & O’Hara 2003; reptiles: Foufopoulos & Ives 1999; fish: Munday 2004; birds: Owens & Bennett 2000, Julliard et al. 2004; mammals: Harcourt et al. 2002, Fisher et al. 2003), and that specialists are more likely to go extinct when faced with global environmental changes (McKinney 1997; Devictor et al. 2008; Colles et al. 2009; Ekroos et al. 2010; Clavel et al. 2010).
However, it remains unclear how habitat and environmental changes will impact parasites (Mostowy & Engelstaedter 2011). In contrast to their hosts, the environment of endoparasites is composed of at least two ecologically different dimensions: the host, and the environment of the host (Thomas et al. 2002). In addition, in the case of non free-living forms, such as vector-borne parasites, the modification of vector environment is a pivotal component. Therefore, environmental changes, such as habitat fragmentation and global climate change, can influence the evolutionary trajectories of parasites by affecting interactions between the pathogen and the arthropod vector, the host, or a combination of both (Zell 2004; Brooks & Hoberg 2008; Lafferty 2009; Sehgal 2010; Tabachnick 2010; Massad et al. 2011).
Malaria parasites are among the most intensively investigated vector-borne parasites, and a major cause of human mortality (WHO 2009). In addition, they have been studied extensively in birds due to their diversity of hosts and nearly worldwide distribution (Valkiūnas 2005). Effects of habitat characteristics and climate on avian Plasmodium prevalence have been investigated at various geographical scales (Merilä et al. 1995; Bensch & Åkesson 2003; Wood et al. 2007; Svensson & Ricklefs 2009; Bonneaud et al. 2009; Chasar et al. 2009; Ishtiaq et al. 2010; Sehgal et al. 2010; Knowles et al. 2010; Garamszegi 2011). Also to our knowledge, studies have focused on the effects of environmental variables on the host specificity of parasites (Poulin et al. 2011) but it is the first time regarding avian malaria parasites. To understand whether generalist or specialist vector-borne parasites are likely to have a competitive advantage under global environmental change, studies must take into account not only the environmental conditions under which these parasites exist, but also the geographical distribution of their hosts, parameters themselves linked to the habitat.
The distinction between specialist and generalist categories can be perplexing; Hellgren et al. (2009) demonstrated that parasites with the ability to successfully infect a wide variety of host species can also be the most prevalent in a single host species. Specialist and generalist strategies might be best thought of as extremes along a continuum. As an example, evolutionary relationships of avian blood parasites have revealed that several lineages of Plasmodium exhibit extreme generalist host–parasitism strategies, whereas other lineages appear to have been constrained to certain host families, or individual species (Ricklefs & Fallon 2002; Ricklefs et al. 2004; Beadell et al. 2009). It has been suggested that specialization represents an evolutionary "dead-end" which limits further evolution (Kelley & Farrell 1998; Snyder & Loker 2000; Nosil 2002). However, several studies have indicated that generalists can be repeatedly evolved from specialist lineages (Poulin et al. 2006; Johnson et al. 2009; Gomez et al. 2010).
Due to infections with a high diversity of both specialist and generalist parasites, African passerine birds provide an excellent model system to test hypotheses about the evolutionary strategies of generalist versus specialist parasites (Beadell et al. 2009). To date, however, most of the studies on avian malaria in Africa have been carried out in West and Central Africa (Kirkpatrick & Smith 1988; Waldenström et al. 2002; Sehgal et al. 2005; Hellgren et al. 2007; Beadell et al. 2009; Bonneaud et al. 2009; Chasar et al. 2009; Loiseau et al. 2010). Less is known about the diversity and prevalence of these parasites in other regions of Africa. Threatened habitats in East Africa, such as highland forests (Hall et al. 2009; Giam et al. 2010) and the fynbos (a natural Mediterranean shrubland vegetation occurring in a small belt along the west and south coasts of South Africa; Midgley et al. 2002; Fairbanks et al. 2004), are particularly interesting because they exhibit more variable environmental conditions in terms of temperature and rainfall than lowland rainforests (Bodker et al. 2003). We set out to compare these habitats (lowland, highland and fynbos) because of their distinct environmental characteristics that contribute to different communities of birds and vectors with varying degrees of specialization within their habitats (DeKlerk et al. 2002). In the near future, the communities of birds in these habitats will likely suffer declines due to rapid environmental changes (Berg et al. 2010).
We predict that habitats subject to highly temporally variable environmental conditions, as exemplified by montane highlands or the fynbos, will have a greater impact on the environment of the vectors since they are temperature sensitive (Tonnang et al. 2010; Paaijmans et al. 2009) and the community of birds, and should harbor more generalist parasites (i.e. more adaptable) than the lowlands, where the climate is more constant throughout the year (i.e. more prone to specialization). Therefore, for each of the three habitats, the objectives of this study were to: i) determine the parasite diversity and prevalence of avian malaria, ii) determine the host and habitat specificity, iii) examine the parasites phylogenetic relationships, particularly with regard to the transition rates between generalist and specialist states. Understanding how current habitat variability affects the host specificity of pathogens, and how strategies have changed during the evolution of this parasite group, will aid in predicting optimal adaptive strategies under rapid global environmental change.
Methods
Sample sites
Blood samples were collected in three distinct habitats across the African continent (36 sites; coordinates given in Electronic Supplemental Information Table S1 and distances between sites in Tables S6–S8; see also map in Fig. S1). The characteristics of each site were determined using a set of environmental variables that included bioclimatic metrics from the WorldClim data set (Hijmans et al. 2005), http://www.worldclim.org), Normalized Difference Vegetation Index (NDVI; Huete et al. 2006), the percentage of tree cover (Hansen et al. 2002) and elevation data from the Shuttle Radar Topography Mission (SRTM) (see Electronic Supplemental Information part A, for description of the bioclimatic and habitat variables; Sehgal et al. 2011). Bioclimatic metrics from the WorldClim data set showed that lowland forest sites experience high annual mean temperature, low temperature seasonality, high annual precipitation and moderate precipitation seasonality. Highland sites are characterized by moderate annual mean temperatures, temperature seasonality, and moderate annual precipitation but with high precipitation seasonality. Fynbos sites experience relatively low annual mean temperatures, high temperature seasonality, low annual precipitation, and moderate precipitation seasonality.
We captured 8 bird species from 5 families among the 9 West African lowland forest sites (N = 575; Table S2), 11 bird species from 5 families at 17 sites in East African highland forests (N = 406; Table S3) and 6 bird species from four families at 10 sites in South Africa in the fynbos (N = 383; Table S4). We found Plasmodium spp. infections in 8, 5 and 12 sites respectively (Table 1). Two families of birds are represented in all three habitats, Nectariniidae (sunbirds) and Pycnonotidae (bulbuls) and two further families are present in both West and East Africa, Turdidae (thrushes) and Muscicapidae (Old World flycatchers). Each location was sampled using mist-nets. Blood samples were collected from the brachial vein and stored in lysis buffer (10 mM Tris-HCL pH 8.0, 100 mM EDTA, 2% SDS) or in dimethyl sulfoxide.
Table 1.
Numbers of screened and infected individuals as well as the number of Plasmodium lineages are given per site in each habitat.
| Habitat | Site | N Individuals | N Infected | N Lineages | Prevalence (%) |
|---|---|---|---|---|---|
| Lowland | Ngoila | 93 | 30 | 8 | 32.3 |
| Zoebefame | 93 | 32 | 11 | 34.4 | |
| Bobo Camp | 96 | 40 | 9 | 41.7 | |
| Douni | 39 | 5 | 5 | 12.8 | |
| Mvono | 25 | 5 | 2 | 20.0 | |
| Beh | 48 | 13 | 5 | 27.1 | |
| Mvia | 45 | 18 | 6 | 40.0 | |
| Mokoko | 63 | 19 | 9 | 30.2 | |
| Fynbos | Beaufort West | 4 | 1 | 1 | 25.0 |
| Jonkershoek | 256 | 25 | 5 | 9.8 | |
| Koeberg | 4 | 1 | 1 | 25.0 | |
| Cape peninsula | 18 | 1 | 1 | 5.6 | |
| Bontebok NP | 6 | 5 | 1 | 83.3 | |
| Highland | Kilimanjaro | 2 | 1 | 1 | 50.0 |
| WestUsambara | 43 | 1 | 1 | 2.3 | |
| EastUsambara | 19 | 4 | 2 | 21.1 | |
| Nguru | 56 | 13 | 5 | 23.2 | |
| Rubeho | 48 | 9 | 3 | 18.8 | |
| Uluguru | 29 | 3 | 2 | 10.3 | |
| Udzungwa | 41 | 10 | 3 | 24.4 | |
| Livingstone | 18 | 1 | 1 | 5.6 | |
| Mahenge | 7 | 1 | 1 | 14.3 | |
| Misuku | 11 | 1 | 1 | 9.1 | |
| Nyika | 18 | 1 | 1 | 5.6 | |
| Namuli | 15 | 3 | 2 | 20.0 |
Parasite screening
DNA was extracted from whole blood following a DNeasy kit protocol (Qiagen, Valencia, California). Successful DNA extraction was verified with primers that amplify the brain-derived neurotrophic factor (BDNF) (Sehgal & Lovette 2003). For Plasmodium detection, we used a nested PCR to amplify a fragment of cytochrome b (524 bp) with the primers HAEMF/HAEMR2 – HAEMNF/HAEMNR2 following Waldenström et al. (2004). We included positive controls using samples with known infections as evident from microscopy results (Valkiūnas et al. 2008a), as well as negative controls using purified water in place of DNA template. The PCR products were run out on a 2% agarose gel using 1×TBE, and visualized by ethidium bromide staining under ultraviolet light to check for positive infections. PCR products were purified using ExoSap (following the manufacturer’s instructions, USB Corporation, Cleveland, Ohio). We identified lineages by sequencing the fragments (BigDye [R] version 3.1 sequencing kit, Applied Biosystems) on an ABI PRISM 3100 (TM) automated sequencer (Applied Biosystems). In some cases co-infection are not detected by PCR; however Valkiūnas et al. (2008b) demonstrated that both of the PCR and microscopy work showed similar prevalence.
Habitat classification
To ensure that the spatial pattern of the selected sample sites was not responsible for the results obtained for the difference in prevalence, we tested for spatial autocorrelations (Electronic Supplemental Information, part B). In addition, in order to determine whether differences in environmental variables could accurately classify each habitat type, we constructed classification trees using a random forest model (RandomForest; Liaw & Wiener 2002) in R (R Development Core 2004), with climate variables (extracted for each site) as predictors, and habitat type as a response variable.
Under a random forest classification model, climate variables correctly classified 97% of sites (35/36, classification error = 0.0588) as belonging to one of the three habitat types (lowland forest, fynbos, highland forest). The variables most important in determining these classifications were temperature seasonality (BIO4) and minimum temperature of the coldest month (BIO6). These results indicate that each of the three habitats can be distinguished from one another solely by environmental characteristics, particularly ground-based bioclimatic measurements.
Parasite prevalence and distribution
Potential differences in parasite prevalence between habitats were tested using generalized linear mixed-effects models (hereafter, GLMM) with binomial distribution of errors and logit link function. The dependent variable was the proportion of infected birds in each site (weighted by the number of individuals sampled) and explanatory variables were the habitat type (factor, fixed effect variable), parasite lineage and site (factors, random effect variables; assuming that site was nested within habitat). The host-parasite assembly rule was investigated using ad hoc randomization tests. Based on the prevalence of parasites in each habitat (regardless of host species), the abundance of birds of all species (estimated by sample sizes), and assuming that host-parasite associations are random, a theoretical distribution of the number of species infected by each lineage in each habitat was constructed (null model). Actual numbers of infected species were then compared to these expectations (details presented in Electronic Supplemental Information, part C).
Host specificity index
Host specificity of the parasite lineages was measured as the number of host species in which parasites were found. To get a measure that included both the diversity of host species and the taxonomic distance between these species, we used the modified version of the host specificity index STD (Poulin & Mouillot 2003; Hellgren et al. 2009). Therefore, a host specificity index has been calculated for each parasite lineage, with high values representing generalist parasites and low values specialist parasites. We calculated i) the host specificity index with only our data, i.e. local specificity index and ii) the host specificity index with the data available in Genbank and MalAvi database (Bensch et al. 2009), i.e. global specificity index. Indeed, we found lineages previously discovered at different locations in different host species, we combined all available data to estimate a global host specificity index. Host specificity indices are given in Table S5.
For each site in each habitat, STD index for a parasite lineage was weighted by the percentage of individuals infected by this parasite lineage to take into account the number of times that a parasite has been found. Potential differences in STD between habitats were tested using both linear (hereafter, LM) and linear mixed-effects models (LMM). In both types of models, dependent variables were the local and global STD in each site (log transformed). In LM, the habitat type was the only explanatory variable. In LMM, explanatory variables were the habitat type (factor, fixed effect variable), parasite lineage and site (factors, random effects variables; assuming that site was nested in habitat). STD values were weighted by sample sizes in each site to correct for heterogeneity in sampling variance. LM was used to test for global differences in parasites’ host specificity between habitats (pairwise differences between habitats were subsequently tested using post hoc Tukey test). LMM was used to assess whether particular parasite lineages show differences in STD in the different habitats. GLM, LMM and GLMM models were fitted in the R 2.10.1 framework (R Development Core Team 2009), specifically using the lme4 package (Crawley 2007).
In addition, we performed a mixed model to test whether the global host specificity indices of parasites were associated with the geographical range of their host. (as a repeated variable). The geographical range was determined using data available from BirdLife International (http://www.birdlife.org/index.html) and was used as a fixed factor in the model, and the host species was used as a random factor (SAS 1999).
Phylogenetic analyses and evolutionary relationships
Sequences obtained were aligned using Sequencher 4.8 (GeneCodes, Ann Arbor, MI). A phylogenetic tree was constructed using 34 mitochondrial cytochrome b sequences of avian Plasmodium spp. We compared these sequences to all sequences from Plasmodium lineages previously deposited in Genbank and the MalAvi database (http://mbioserv4.mbioekol.lu.se/avianmalaria/index.html; Bensch et al. 2009). Our sequencing analyses also identified birds infected with Haemoproteus lineages; although not used in these analyses, prevalence and lineage data of these parasites in these populations are available upon request. All unique sequences were verified via duplicate sequencing. GenBank accession numbers are shown in Fig. 1. All individual sequences were grouped into a consensus that was 500 bp long, with P. gallinaceum (GenBank accession number NC008288) as the outgroup. We first determined the model of sequence evolution that best fit the data using MrModeltest (Nylander 2004). Bayesian analysis of the sequence data was then conducted with MrBayes version 3.1.2 (Huelsenbeck & Ronquist 2001) using the model of sequence evolution obtained from MrModeltest (GTR+G+I). Two Markov chains were run simultaneously for 10 million generations and sampled every 200 generations, for a total of 50,000 trees each, sampled from the posterior distribution. For the “burn-in”, 25% of the trees were discarded from the posterior distribution. The remaining trees were used to calculate the posterior probabilities. Phylogenetic analyses were also implemented using maximum likelihood (ML) in PAUP* 4.0 (Swofford 2003). The support for the individual branches was estimated using ML bootstrap analyses with 100 replicates. The topology of both the Bayesian and the ML trees were almost identical, with only small differences in some branches that lacked significant support from either method.
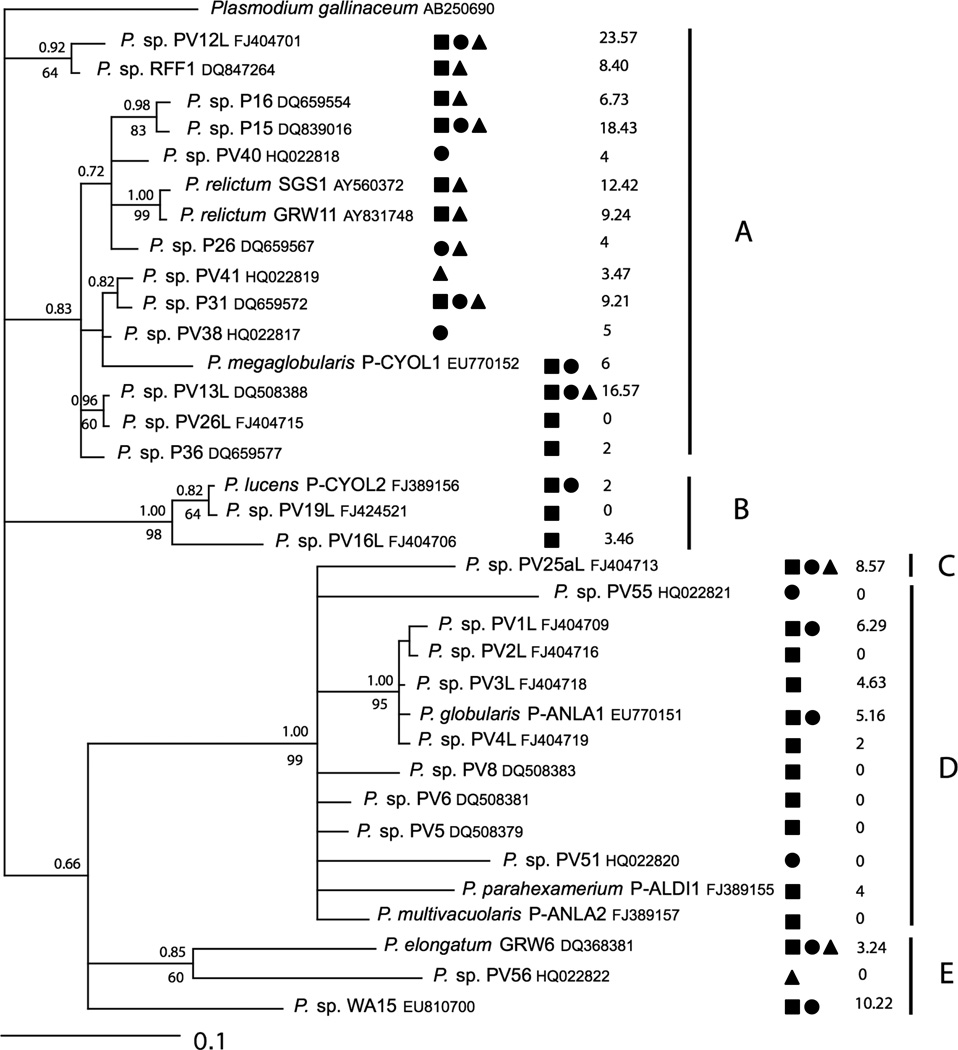
Figure 1.
Phylogenetic relationships in a consensus tree of the 34 Plasmodium lineages found in the three communities of birds based on cytochrome b sequences. Plasmodium gallinaceum was used as the outgroup. GenBank accession numbers of all sequences are indicated. Numbers located on the top of the branches indicate Bayesian probability values and below, ML bootstrap support (100 replications, only values above 50% are shown). Symbols depict the different habitats (square: lowland rainforest; circle: highland forest; triangle: fynbos). The STD index is given for each lineage. The bars indicate three groups of parasites (A: host generalist; B: host specialist; C: host specialist).
In addition, we performed ancestral state reconstruction analysis under a maximum likelihood framework to estimate the states of strategies (generalist vs. specialist) on phylogenies using BayesTraits (Pagel 1999; www.evolution.rdg.ac.uk). Using the consensus tree obtained from phylogenetic analyses and the STD values of sampled branches, we performed ancestral character state reconstructions at each node of the consensus tree. Likelihood scores were acquired from BayesTraits and tested (via likelihood ratio tests) for a model that did not control rates of change between states (generalist to specialist and vice versa), as compared to a model that forced these rates to be equal (Electronic Supplemental Information, part D).
Results
Parasite prevalence, diversity and distribution
In total, from 25 bird species (1,364 individuals), we found 34 Plasmodium lineages. In the lowland rainforest habitat, we screened 575 individuals, of which 177 were infected with a total of 18 Plasmodium lineages (Table S2). In the highland forest, 406 individuals were screened, of which 51 were infected with a total of 13 Plasmodium lineages (Table S3). In the fynbos habitat, we screened 383 individuals, of which 51 were infected with a total of 9 Plasmodium parasite lineages (Table S4).
Several lines of evidence suggested that parasites were not randomly distributed across habitats and host species. First, using a GLMM (with site and parasite lineage as random factors), we showed that that the probability of Plasmodium infection for a given individual varied between the lowland and the two other habitat types (fynbos: t = −2.7; P = 0.005; highland: t = -3.0; P = 0.002). Second, a randomization model indicated that seven Plasmodium lineages were preferentially associated with some host species in the lowland rainforest habitat (i.e., the number of host species infected by these parasite lineages was significantly lower than expected with random association between parasites and individual birds, see details in Electronic Supplemental Information, part B). No such association was found for the highland forest and fynbos habitats.
Host specificity index
A first comparison between habitats revealed that local STD indices were variable across habitats (ANOVA, F2,84 = 27.4, P < 10−4). Post hoc tests indicated that this global effect was due to differences between the lowland rainforest and the two other habitat types (P < 10−4 for both pair comparisons). Similar analysis with global STD showed that there was also a global difference between habitats (ANOVA, F2,84 = 7.7, P = 0.0008), but only the lowland and highland habitats differed from each other (P = 0.005). In order to check whether these differences were due only to the observed differences in parasite prevalence between habitats (see above), we ran a LMM model with site and parasite lineage as random factors. The analysis indicated that the lowland still differed from the two other habitat types with respect to local (fynbos: t = 1.9; P = 0.05; highland: t = 4.2; P <10−4) and global (fynbos: t = 2.7; P = 0.006; highland: t = 4.8; P <10−4) STD, even when controlling for lineage.
We also found that the global host specificity index was negatively affected by the geographical range of the hosts (F1,34 = 9.22; P = 0.0045). Parasites with a high host specificity index (i.e. generalist parasites) were found in hosts with a limited geographical range. In contrast, hosts with large geographical ranges tended to be infected by parasites with a low host specificity index (i.e. specialist parasites).
Evolutionary relationships
The phylogenetic relationship from the consensus tree showing the 34 Plasmodium lineages revealed groups of lineages with different host and habitat specificities that we designated as clusters A, B and C (Fig. 1). Cluster A represents habitat generalist Plasmodium lineages showing a high host specificity index. Cluster B represents specialist lineages found only in four species of sunbirds (Nectariniidae: Cyanomitra olivacea, C. cyanolaema, C. oritis and Hedydipna collaris). The cluster C represents Plasmodium lineages primarily from lowland forests that have a low host specificity index. STD values from cluster A were significantly different from cluster B (Kruskal-Wallis test, χ2 = 4.82; P = 0.028) as well as from the cluster C (Kruskal-Wallis test, χ2 = 8.23; P = 0.0041), but the values from cluster B and C did not significantly differ from one another.
Ancestral reconstruction analysis and likelihood ratio tests revealed a significant difference between the transition rates between generalist and specialist states (Equal rates L = −21.810, Unequal rates L = −16.358), with the rate at which generalists become specialists nearly four times (70.41) that of which specialists become generalists (17.63).
Discussion
It has been established that the degree of specialization of parasites lies along a continuum; on one end extreme specialists may be restricted to a single host species, and on the other generalists are capable of parasitizing a diverse set of avian hosts. However, there is little information about factors that can contribute to the evolution of host specificity. With ever increasing evidence for rapid global change, it is especially pertinent to establish how variation in environmental conditions can be linked to parasite strategies. Here, we take advantage of an unprecedented compilation of blood samples and field locations to compare diversity of malaria parasites across wide regions in Africa. In addition, to our knowledge, this is the first attempt to investigate avian malaria parasites and quantify a specificity index of host-parasite communities in relation to habitat characteristics. Using these host specificity data and our reconstructed phylogeny of these parasites, this study supports the hypotheses that i) the diversity of parasites and prevalence differ among host communities and habitats in Africa, ii) specialist parasites evolve from generalists more often than generalists evolve from specialists, and iii) the degree of host specialization is associated to habitat type and host geographical range.
We found that the prevalence and diversity of parasites varied considerably among three environmentally distinct habitats, and that host specificity indices of parasites differed significantly according to habitat. Results indicate that habitat type limits the geographical range of the host species, which in turn influences parasite specialization. The data revealed that generalist parasite lineages tend to infect bird communities with restricted geographical ranges as compared to those communities with larger ranges. Conversely, bird species with more extensive geographical ranges tend to be infected mostly with specialist parasites. Indeed, a parasite can specialize on a single host when the host occurs across an extensive environmental range, but would be at risk of rapid extinction in specializing on host species with limited geographical ranges. Only generalist parasites, with the ability to infect multiple species, would likely infect these birds and persist. It is worth noting that the host specificity index is based not only on our data but also data available from large databases that involve a significant number of screened hosts, in order to obtain the most reliable index possible. Although we cannot extend our conclusions to all lowland, highland, or fynbos habitats in Africa, we hypothesize that the trends observed represent an advance in our understanding of mechanisms involved in parasite strategy evolution, and they further provide testable predictions for areas outside our study sites.
Random forest analysis used to classify the sites indicates that the three habitats clearly differ in terms of climatic conditions. Temperature seasonality (BIO04) as well as the minimum temperature of the coldest month (BIO06) distinguish each of the three habitat types. Temperature variables can impact both hosts and vectors and consequently the abundance and diversity of parasites (Paaijmans et al. 2010). This is in accordance with several studies of human associated malaria parasites and their vectors in the highlands of East Africa and elsewhere. Both the survival of vector populations, and the development time of the parasite vary with diurnal temperature, and in field studies, temperature variation has been related to the abundance of vector populations (Koenraadt et al. 2006; Minakawa et al. 2006; Afrane et al. 2008; Paaijmans et al. 2009; Chaves et al. 2010; La Pointe et al. 2010; Paaijmans et al. 2010). This type of climate variability (short-term fluctuations around the mean climate state) would therefore play an important role in host specialization of malaria parasites. For instance, altitude is inversely correlated to temperature. In the Usambara Mountains (Tanzania), the difference between the lowest village on the warm plains at 300 m and the coldest village at 1,700 m is 9°C (Bodker et al. 2003). Fewer vector species are expected in colder habitats, and these habitats may be likely to harbor more generalist parasites. In this study, however, one limitation is that we lack information on the vector ecology. The parasite-vector interactions and specificity are difficult to predict since the mosquito vectors could be impacted in different ways by environmental conditions. To date, two studies have been carried out in the lowland forests of Cameroon, revealing that species of four genera of mosquitoes, Aedes, Coquillettidia, Culex and Mansonia, are potential vectors of avian malaria (Njabo et al. 2009; Njabo et al. 2010). However, very little is known about the vectors transmitting avian malaria in Tanzania or South Africa. Collecting vectors and identifying the host specificity of their malaria parasites in the highland forest and the fynbos would be an important step in assessing the strategies of generalist parasites in these habitats.
In our phylogenetic analyses of Plasmodium lineages, we found clusters with different host and habitat specificities. Implementing ancestral reconstruction methodologies, we found that specialized lineages tend to be phylogenetically derived. Similar results have been seen in other parasitic systems, such as schistosomes (Snyder & Loker 2000). Our results support the evolutionary directionality of malaria parasites from generalist towards specialist, as the transition from generalist to specialist occurs at a significantly faster rate than the transition from specialist to generalist. In addition to this evolutionary transition, ecological factors drive host specialization. Indeed, it is becoming increasingly clear that the disturbance of habitat leads to homogenization of biodiversity (Laliberte & Tylianakis 2010; Hewitt et al. 2010; Clavel et al. 2010; Freedman et al. 2010) and will also lead to a loss of diversity of birds with restricted ranges. For instance, the mountains in Tanzania exhibit a high diversity of endemic birds, which are extremely sensitive to disturbance (Hall et al. 2009; Giam et al. 2010). In addition, we expect that some generalist vertebrate hosts and vectors will extend their habitat ranges because of habitat disturbance (Ogden et al. 2008; Marini et al. 2009; Gonzalez et al. 2010), and carry with them novel lineages of parasites. This increased range sizes of parasites could be associated with increased parasite virulence, and have serious impacts on host health (Garamszegi 2006). Our findings have conservation implications, and are important in understanding the consequences of environmental change for malaria parasites. Ultimately, to fully test the hypotheses presented here, it will be important to assess the actual fitness effects of generalist versus specialist parasites on their hosts through experimental infections. The resulting information would be invaluable in assessing effects of global changes on the virulence of malaria parasites.
Supplementary Material
Acknowledgments
We thank the Governments of the Republic of Cameroon, Tanzania (The Tanzanian Commission for Science and Technology and Tanzanian Wildlife Research Institute), Malawi (Museums of Malawi), Mozambique (National Museum Maputo) and South Africa (CapeNature, Northern Cape Conservation) for permission to conduct field research. We thank Anthony Chasar, Kevin Njabo, Dennis Anye Ndeh, Jerome Fuchs, Graeme Oately, Mhairi McFarlane, Martim Melo, Claire Spottiswoode, Penn Lloyd, Hanneline Smit, Ângela Ribeiro, Peter Ryan, Potiphar Kaliba, Jacob Kuire, Jan Bolding Kristensen, Louis Hansen, Jay McEntee and Jon Fjeldså, for their help in the field and Raul Sedano and Karine Princé for their help with statistical analyses. We are also grateful to François Balloux and the anonymous reviewers for helpful comments on the manuscript. This research was supported by grants from the National Geographic Society, National Environmental Research Council, and the National Science Foundation DEB-9726425 and IRCEB9977072 and joint NSF-NIH (USA) Ecology of Infectious Diseases Program award EF-0430146 to TBS and RNMS. The work was also partially supported by the award NIH Grant 5SC2AI089120 to RNMS, as well as the University of California at Berkeley’s committee on research, and the Department of Science and Technology and National Research Foundation of South Africa.
Footnotes
Data Accessibility:
New DNA sequences: Genbank accessions HQ022817- HQ022822
Climate data for each site deposited in the DRYAD repository entry : doi:10.5061/dryad.h12kh08n
REFERENCES
- Afrane YA, Little TJ, Lawson BW, Githeko AK, Yan G. Deforestation and vectorial capacity of Anopheles gambiae Giles mosquitoes in malaria transmission, Kenya. Emerging Infectious Diseases. 2008;14:1533–1538. doi: 10.3201/eid1410.070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadell JS, Covas R, Gebhard C, Ishtiaq F, Melo M, Schmidt BK, Perkins S, Graves GR, Fleischer R. Host associations and evolutionary relationships of avian blood parasites from West Africa. International Journal of Parasitology. 2009;39:257–266. doi: 10.1016/j.ijpara.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch S, Åkesson A. Temporal and spatial variation of hematozoans in Scandinavian willow warblers. Journal of Parasitology. 2003;89:388–391. doi: 10.1645/0022-3395(2003)089[0388:TASVOH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bensch S, Hellgren O, Perez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Molecular Ecology Resources. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Berg M, Kiers ET, Driessen G, van der Heijden M, Kooi BW, Kuenen F, Liefting M, Verhoef H, Ellers J. Adapt or disperse: understanding species persistence in a changing world. Global Change Biology. 2010;16:587–598. [Google Scholar]
- Bodker R, Akida J, Shayo D, Kisinza W, Msangeni HA, Pedersen EM, Lindsay SW. Relationship between altitude and intensity of malaria transmission in the Usambara Mountains, Tanzania. Journal Medical Entomology. 2003;40:706–717. doi: 10.1603/0022-2585-40.5.706. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Sepil I, Milá B, Buermann W, Pollinger J, Sehgal RNM, Valkiūnas G, Iezhova TA, Saatchi S, Smith TB. The prevalence of avian Plasmodium is higher in undisturbed tropical forests of Cameroon. Journal of Tropical Ecology. 2009;25:439–447. [Google Scholar]
- Both C, te Marvelde L. Climate change and timing of avian breeding and migration throughout Europe. Climate Research. 2007;35:93–105. [Google Scholar]
- Brooks DR, Hoberg EP. How will global climate change affect parasite-host assemblages? Trends of Parasitology. 2008;23:571–574. doi: 10.1016/j.pt.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Chasar A, Loiseau C, Valkiūnas G, Iezhova T, Smith TB, Sehgal RNM. Prevalence and diversity patterns of avian blood parasites in degraded African rainforest habitats. Molecular Ecology. 2009;18:4121–4133. doi: 10.1111/j.1365-294X.2009.04346.x. [DOI] [PubMed] [Google Scholar]
- Chaves LF, Koenraadt CJ. Climate change and highland malaria: fresh air for a hot debate. Quarter Review of Biology. 2010;85:27–55. doi: 10.1086/650284. [DOI] [PubMed] [Google Scholar]
- Clavel J, Julliard R, Devictor V. Worldwide decline of specialist species: towards a global functional homogenization? Frontiers in Ecology and the Environment. 2010 [Google Scholar]
- Colles A, Liow LH, Prinzing A. Are specialists at risk under environmental change? Neoecological, paleoecological and phylogenetic approaches. Ecology Letters. 2009;12:849–863. doi: 10.1111/j.1461-0248.2009.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley MJ. The R Book. Chichester: John Wiley & Sons Ltd; 2007. [Google Scholar]
- De Klerk HM, Crowe TM, Fjeldsa J, Burgess ND. Biogeographical patterns of endemic terrestrial Afrotropical birds. Diversity and Distributions. 2002;8:147–162. [Google Scholar]
- Devictor V, Julliard R, Clavel J, Jiguet F, Lee A, Couvet D. Functional biotic homogenization of bird communities in disturbed landscapes. Global Ecology and Biogeography. 2008;17:252–261. [Google Scholar]
- Ekroos J, Heliola J, Kuussaari M. Homogenization of lepidopteran communities in intensively cultivated agricultural landscapes. Journal Applied of Ecology. 2010;47:459–467. [Google Scholar]
- Fairbanks DHK, Hughes CJ, Turpie JK. Potential impact of viticulture expansion on habitat types in the Cape Floristic Region, South Africa. Biodiversity and Conservation. 2004;13:1075–1100. [Google Scholar]
- Fisher DO, Blomberg SP, Owens IPF. Extrinsic versus intrinsic factors in the decline and extinction of Australian marsupials. Proceeding of the Royal Society of London B. 2003;270:1801–1808. doi: 10.1098/rspb.2003.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foufopoulos J, Ives AR. Reptile extinctions on land-bridge islands: life-history attributes and vulnerability to extinction. American Naturalist. 1999;153:1–25. doi: 10.1086/303149. [DOI] [PubMed] [Google Scholar]
- Freedman AH, Buermann W, Mitchard ETA, DeFries RS, Smith TB. Human impacts flatten rainforest-savanna gradient and reduce adaptive diversity in a rainforest bird. PloS ONE. 2010;5 doi: 10.1371/journal.pone.0013088. e13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamszegi LZ. The evolution of virulence and host specialization in malaria parasites of primates. Ecology Letters. 2006;9:933–940. doi: 10.1111/j.1461-0248.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- Garamszegi LZ. Climate change increases the risk of malaria in birds. Global Change Biology. 2011;17:1751–1759. [Google Scholar]
- Giam X, Bradshaw CJA, Tan HTW, Sodhi NS. Future habitat loss and the conservation of plant biodiversity. Biological Conservation. 2010;143:1594–1602. [Google Scholar]
- Gomez JM, Verdu M, Perfectti F. Ecological interactions are evolutionarily conserved across the entire tree of life. Nature. 2010;465:918–921. doi: 10.1038/nature09113. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Wang O, Strutz SE, Gonzalez-Salazar C, Sanchez-Cordero V, Sarkar S. Climate change and risk of Leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. Plos Neglected Tropical Disease. 2010;4 doi: 10.1371/journal.pntd.0000585. e585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordo O. Why are bird migration dates shifting? A review of weather and climate effects on avian migratory phenology. Climate Research. 2007;35:37–58. [Google Scholar]
- Hall J, Burgess ND, Lovett J, Mbilinyi B, Gereau RE. Conservation implications of deforestation across an elevational gradient in the Eastern Arc Mountains, Tanzania. Biological Conservation. 2009;142:2510–2521. [Google Scholar]
- Hansen MC, DeFries RS, Townshend JRG, Sohlberg R, Dimiceli C, Carroll M. Towards an operational MODIS continuous field of percent tree cover algorithm: examples using AVHRR and MODIS data. Remote Sensing of Environment. 2002;83:303–319. [Google Scholar]
- Harcourt AH, Copeton SA, Parks SA. Rarity, specialization and extinction risk in primates. Journal of Biogeography. 2002;29:445–456. [Google Scholar]
- Hellgren O, Waldenström J, Perez-Tris J, Szollosi E, Hasselquist D, Krizanauskiene A, Ottosson U, Bensch S. Detecting shifts of transmission areas in avian blood parasites - a phylogenetic approach. Molecular Ecology. 2007;16:1281–1290. doi: 10.1111/j.1365-294X.2007.03227.x. [DOI] [PubMed] [Google Scholar]
- Hellgren O, Perez-Tris J, Bensch S. A jack-of-all-trades and still a master of some: prevalence and host range in avian malaria and related blood parasites. Ecology. 2009;90:2840–2849. doi: 10.1890/08-1059.1. [DOI] [PubMed] [Google Scholar]
- Hewitt J, Thrush S, Lohrer A, Townsend M. A latent threat to biodiversity: consequences of small-scale heterogeneity loss. Biodiversity and Conservation. 2010;19:1315–1323. [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. [Google Scholar]
- Hitch AT, Leberg PL. Breeding distributions of north American bird species moving north as a result of climate change. Conservation Biology. 2007;21:534–539. doi: 10.1111/j.1523-1739.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Huete AR, Didan K, Shimabukuro YE, Ratana P, Saleska SR, Hutyra LR, Yang W, Nemani RR, Myneni R. Amazon rainforests green-up with sunlight in dry season. Geophysical Research Letters. 2006;33 L06405. [Google Scholar]
- Hughes JB, Daily GC, Ehrlich PR. Conservation of insect diversity: a habitat approach. Conservation Biology. 2000;14:1788–1797. doi: 10.1111/j.1523-1739.2000.99187.x. [DOI] [PubMed] [Google Scholar]
- Ishtiaq F, Clegg SM, Phillimore AB, Black R, Owens IPF, Sheldon BC. Biogeographical patterns of blood parasite lineage diversity in avian hosts from southern Melanesian islands. Journal of Biogeography. 2010;37:120–132. [Google Scholar]
- Johnson KP, Malenke JR, Clayton DH. Competition promotes the evolution of host generalists in obligate parasites. Proceeding of the Royal Society of London B. 2009;276:3921–3926. doi: 10.1098/rspb.2009.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julliard R, Jiguet F, Couvet D. Common birds facing global changes: what makes a species at risk? Global Change Biology. 2004;10:148–154. [Google Scholar]
- Kelley ST, Farrell BD. Is specialization a dead end? The phylogeny of host use in Dendroctonus bark beetles (Scolytidae) Evolution. 1998;52:1731–1743. doi: 10.1111/j.1558-5646.1998.tb02253.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick CE, Smith TB. Blood parasites of birds in Cameroon. Journal of Parasitology. 1988;74:1009–1013. [PubMed] [Google Scholar]
- Knowles SCL, Wood MJ, Alves R, Wilkin TA, Bensch S, Sheldon BC. Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Molecular Ecology. 2010 doi: 10.1111/j.1365-294X.2010.04909.x. [DOI] [PubMed] [Google Scholar]
- Koenraadt CJM, Paaijmans KP, Schneider P, Githeko AK, Takken W. Low larval vector survival explains unstable malaria in the western Kenya highlands. Tropical Medecine and International Health. 2006;11:1195–1205. doi: 10.1111/j.1365-3156.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- Kotze DJ, O’Hara RB. Species decline – but why? Explanations of carabid beetle (Coleoptera, Carabidae) declines in Europe. Oecologia. 2003;135:138–148. doi: 10.1007/s00442-002-1174-3. [DOI] [PubMed] [Google Scholar]
- Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- Laliberte E, Tylianakis JM. Deforestation homogenizes tropical parasitoid-host networks. Ecology. 2010;91:1740–1747. doi: 10.1890/09-1328.1. [DOI] [PubMed] [Google Scholar]
- LaPointe DA, Goff ML, Atkinson CT. Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai'i. Journal of Parasitology. 2010 doi: 10.1645/GE-2290.1. [DOI] [PubMed] [Google Scholar]
- Levin II, Outlaw DC, Vargas FH, Parker PG. Plasmodium blood parasite found in endangered Galapagos penguins (Spheniscus mendiculus) Biological Conservation. 2009;142:3191–3195. [Google Scholar]
- Liaw A, Wiener M. Classification and regression by randomForest. R News. 2002;2:18–22. [Google Scholar]
- Loiseau C, Iezhova T, Valkiunas G, Chasar A, Hutchinson A, Buermann W, Smith T, Sehgal RNM. Spatial variation of haemosporidian parasite infection in African rainforest bird species. Journal of Parasitology. 2010;96:21–29. doi: 10.1645/GE-2123.1. [DOI] [PubMed] [Google Scholar]
- Marini MA, Barbet-Massin M, Lopes LE, Jiguet F. Predicted climate-driven bird distribution changes and forecasted conservation conflicts in a neotropical savanna. Conservation Biology. 2009;23:1558–1567. doi: 10.1111/j.1523-1739.2009.01258.x. [DOI] [PubMed] [Google Scholar]
- Massad E, Coutinho FAB, Lopez LF, da Silva DR. Modeling the impact of global warming on vector-borne infections. Physics of Life Reviews. 2011;8:169–199. doi: 10.1016/j.plrev.2011.01.001. [DOI] [PubMed] [Google Scholar]
- McKinney ML. Extinction vulnerability and selectivity: combining ecological and paleontological views. Annual Review of Ecology, Evolution and Systematics. 1997;28:495–516. [Google Scholar]
- Merilä J, Bjorklund M, Bennett GF. Geographic and individual variation in haematozoan infections in the greenfinch, Carduelis chloris. Canadian Journal of Zoology. 1995;73:798–1804. [Google Scholar]
- Midgley GF, Hannah L, Millar D, Rutherford MC, Powrie LW. Assessing the vulnerability of species richness to anthropogenic climate change in a biodiversity hotspot. Global Ecology and Biogeography. 2002;11:445–451. [Google Scholar]
- Minakawa N, Omukunda E, Zhou GF, Githeko A, Yan GY. Malaria vector productivity in relation to the highland environment in Kenya. American Journal of Tropical Medecine and Hygiene. 2006;75:448–453. [PubMed] [Google Scholar]
- Mostowy R, Engelstaedter J. The impact of environmental change on host-parasite coevolution dynamics. Proceeding Royal Society London B. 2011;278:2283–2292. doi: 10.1098/rspb.2010.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday PL. Habitat loss, resource specialization, and extinction on coral reefs. Global Change Biology. 2004;10:1642–1647. [Google Scholar]
- Njabo K, Cornel AJ, Sehgal RNM, Loiseau C, Buermann W, Harrigan R, Pollinger J, Valkiunas G, Smith T. Coquillettidia (Culicidae, Diptera) mosquitoes are natural vectors of avian malaria in Africa. Malaria Journal. 2009;8:193. doi: 10.1186/1475-2875-8-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njabo K, Cornel AJ, Bonneaud C, Toffelmier E, Sehgal RNM, Valkiūnas G, Russell AF, Smith TB. Nonspecific patterns of vector, host and avian malaria parasite associations in a central African rainforest. Molecular Ecology. 2010;20:1049–1061. doi: 10.1111/j.1365-294X.2010.04904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil P. Transition rates between specialization and generalization in phytophagous insects. Evolution. 2002;56:1701–1706. doi: 10.1111/j.0014-3820.2002.tb01482.x. [DOI] [PubMed] [Google Scholar]
- Nylander JAA. Evolutionary Biology Centre. Uppsala University; 2004. MrModeltest v2. Program distributed by the author. [Google Scholar]
- Ogden NH, St-Onge L, Barker IK, Brazeau S, Bigras-Poulin M, Charron D, Francis CM, Heagy A, Lindsay LR, Maarouf A, Michel P, Milord F, O'Callaghan C, Trudel L, Thompson RA. Risk maps for range expansion of the Lyme disease vector, Ixodes scapularis, in Canada now and with climate change. International Journal of Health Geographics. 2008;7:24. doi: 10.1186/1476-072X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens IPF, Bennett PM. Ecological basis of extinction in birds: habitat loss versus human persecution and introduced predators. Proceedings of the National Academy of Science. 2000;97:12144–12148. doi: 10.1073/pnas.200223397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Read AF, Thomas MB. Understanding the link between malaria risk and climate. Proceedings of the National Academy of Science. 2009;106:13844–13849. doi: 10.1073/pnas.0903423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Blanford S, Bell AS, Blanford J, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proceedings of the National Academy of Science. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M. The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Systematic Biology. 1999;48:612–622. [Google Scholar]
- Poulin R, Mouillot D. Parasite specialization from a phylogenetic perspective: a new index of host specificity. Parasitology. 2003;126:473–480. doi: 10.1017/s0031182003002993. [DOI] [PubMed] [Google Scholar]
- Poulin R, Krasnov BR, Shenbrot GI, Mouillot D, Khoklova S. Evolution of host specificity in fleas: is it directional and irreversible? International Journal of Parasitology. 2006;36:185–191. doi: 10.1016/j.ijpara.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Poulin R, Krasnov BR, Mouillot D. Host specificity in phylogenetic and geographic space. Trends in Parasitology. 2011;27:355–361. doi: 10.1016/j.pt.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Pounds JA, Fogden MP, Campbell JH. Biological response to climate change on a tropical mountain. Nature. 1999;398:611–615. [Google Scholar]
- R Development Core Team: R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2004. 2004. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing.V. 2.7.2. 2009 [ http://www.R-project.org].
- Ricklefs RE, Fallon SM. Diversification and host switching in avian malaria parasites. Proceeding Royal Society London B. 2002;269:885–892. doi: 10.1098/rspb.2001.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE, Fallon SM, Bermingham E. Evolutionary relationships, cospeciation, and host switching in avian malaria parasites. Systematic Biology. 2004;53:111–119. doi: 10.1080/10635150490264987. [DOI] [PubMed] [Google Scholar]
- SAS. SAS user's guide. Cary, NC, USA: SAS Institute Inc; 1999. [Google Scholar]
- Sehgal RNM, Lovette IJ. Molecular evolution of three avian neurotrophin genes: Implications for proregion functional constraints. Journal of Molecular Evolution. 2003;57:335–342. doi: 10.1007/s00239-003-2484-8. [DOI] [PubMed] [Google Scholar]
- Sehgal RNM, Jones HI, Smith TB. Blood parasites of some West African rainforest birds. The Journal of Veterinary Medical Science. 2005;67:295–301. doi: 10.1292/jvms.67.295. [DOI] [PubMed] [Google Scholar]
- Sehgal RNM. Deforestation and avian infectious diseases. J. Exp. Biol. 2010;213:955–960. doi: 10.1242/jeb.037663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal RNM, Buermann W, Harrigan RJ, Bonneaud C, Loiseau C, Chasar A, Sepil I, Valkiūnas G, Iezhova T, Saatchi S, Smith TB. Spatially explicit predictions of blood parasites in a widely distributed African rainforest bird. Proceeding of the Royal Society London B. 2011;278:1025–1033. doi: 10.1098/rspb.2010.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SD, Loker ES. Evolutionary relationships among the Schistosomatidae (Platyhelminthes : Digenea) and an Asian origin for Schistosoma. Journal of Parasitology. 2000;86:283–288. doi: 10.1645/0022-3395(2000)086[0283:ERATSP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Svensson LME, Ricklefs RE. Low diversity and high intra-island variation in prevalence of avian Haemoproteus parasites on Barbados, Lesser Antilles. Parasitology. 2009;136:1121–1131. doi: 10.1017/S0031182009990497. [DOI] [PubMed] [Google Scholar]
- Swofford DL. Phylogenetic Analysis Using Parsimony (*and Other Methods) Sunderland, Massachusetts: Sinauer Associates; 2003. PAUP*. Version 4. [Google Scholar]
- Tabachnick WJ. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. Journal of Experimental Biology. 2010;213:946–954. doi: 10.1242/jeb.037564. [DOI] [PubMed] [Google Scholar]
- Thomas F, Brown SP, Sukhdeo M, Renaud F. Understanding parasite strategies: a state-dependent approach? Trends of Parasitology. 2002;18:387–390. doi: 10.1016/s1471-4922(02)02339-5. [DOI] [PubMed] [Google Scholar]
- Tonnang HEZ, Kangalawe RYM, Yanda PZ. Predicting and mapping malaria under climate change scenarios: the potential redistribution of malaria vectors in Africa. Malaria Journal. 2010;9:111. doi: 10.1186/1475-2875-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkiūnas G. Avian malaria parasites and other haemosporidia. Boca Raton, Florida: CRC Press; 2005. p. 946. [Google Scholar]
- Valkiūnas G, Iezhova TA, Loiseau C, Chasar A, Smith TB, Sehgal RN. New species of haemosporidian parasites (Haemosporida) from African rainforest birds, with remarks on their classification. Parasitology Research. 2008a;103:1213–1228. doi: 10.1007/s00436-008-1118-x. [DOI] [PubMed] [Google Scholar]
- Valkiūnas G, Iezhova TA, Krizanauskiene A, Palinauskas V, Sehgal RN, Bensch S. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. Journal of Parasitology. 2008b;94:1395–1401. doi: 10.1645/GE-1570.1. [DOI] [PubMed] [Google Scholar]
- van Riper C, van Riper SG, Goff ML, Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecoloical Monograph. 1986;56:327–344. [Google Scholar]
- Waldenström J, Bensch S, Kiboi S, Hasselquist D, Ottoson U. Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Molecular Ecology. 2002;11:1545–1554. doi: 10.1046/j.1365-294x.2002.01523.x. [DOI] [PubMed] [Google Scholar]
- Waldenström J, Bensch S, Hasselquist D, Ostman O. A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. Journal of Parasitology. 2004;90:191–194. doi: 10.1645/GE-3221RN. [DOI] [PubMed] [Google Scholar]
- Warner RE. The role of introduced diseases in the extinction of the endemic Hawaiian avifauna. Condor. 1968;70:101–120. [Google Scholar]
- Whiteman NK, Goodman SJ, Sinclair BJ, Walsh T, Cunningham AA, Kramer LD, Parker PG. Establishment of the avian disease vector Culex quinquefasciatus Say, 1823 (Diptera : Culicidae) on the Galapagos Islands, Ecuador. Ibis. 2005;147:844–847. [Google Scholar]
- World Health Organization. Malaria. WHO Fact Sheet No. 94. 2009
- Wood MJ, Cosgrove CL, Wilkin TA, Knowles SCL, Day KP, Sheldon BC. Within-population variation in prevalence and lineage distribution of avian malaria in blue tits, Cyanistes caeruleus. Molecular Ecology. 2007;16:3263–3273. doi: 10.1111/j.1365-294X.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- Zell R. Global climate change and the emergence/re-emergence of infectious diseases. International Journal of Medical Microbiology. 2004;293:16–26. doi: 10.1016/s1433-1128(04)80005-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.