Abstract
Background and Aims
The responsiveness of the central nervous system (CNS) is altered in patients with irritable bowel syndrome (IBS). However, due variations in experimental paradigms, analytic techniques, and reporting practices, little consensus exists on brain responses to visceral stimulation. We aimed to identify brain regions consistently activated by supraliminal rectal stimulation in IBS patients and healthy subjects (controls), by performing a quantitative meta-analysis of published studies.
Methods
Significant foci from with-in group statistical parametric maps were extracted from published neuroimaging studies that employed rectal distension. Voxel-based activation likelihood estimation was applied, pooling the results and comparing them across groups.
Results
Across studies, there was consistent activation in regions associated with visceral afferent processing (thalamus, insula, anterior mid-cingulate) among IBS patients and controls, but considerable differences in the extent and specific location of foci. IBS patients differed from controls in: 1) More consistent activations in regions associated with emotional arousal [pregenual anterior cingulate cortex (pACC), amygdala]; 2) Activation of a midbrain cluster, a region playing a role in endogenous pain modulation. Controls showed more consistent activation of the medial and lateral prefrontal cortex.
Conclusions
Patients with IBS have greater engagement of regions associated with emotional arousal and endogenous pain modulation, but similar activation of regions involved in processing of visceral afferent information. Controls have greater engagement of cognitive modulatory regions. These results support a role for CNS dysregulation in IBS. These findings provide specific targets for guiding development of future neuroimaging protocols to more clearly define altered brain-gut interactions in IBS.
Keywords: Irritable bowel syndrome, neuroimaging, visceral pain
Introduction
IBS is one of the most common persistent pain syndromes, characterized by chronic abdominal pain or discomfort and associated alterations in bowel habits. It is thought to result from a complex dysregulation of bidirectional brain-gut interactions.1, 2 Disease models were initially developed based on patient subjective reports of pain and discomfort, with observed modulation of symptoms and perceptual responses by stressful life events and experimental stressors respectively. Over the last decade, experimental paradigms utilizing various brain imaging techniques have supported as well as expanded these initial models, demonstrating that alterations in central sensory processing/modulation exist in IBS subjects 3–7 For example, we have previously reported evidence to suggest that activity in brain regions associated with pain modulation, attention, and emotional arousal appear to differ between IBS and healthy control subjects (controls) in conditions of controlled rectal distension.7–9 Most importantly, these differences could not be fully explained by the presence of visceral hypersensitivity (e.g. increased afferent input from the gut), as similar differences have been observed even in sham conditions or during the anticipation period before a visceral stimulus. Overall, neuroimaging findings suggest that IBS subjects appear to engage greater limbic regions in response to a real or potential visceral stressor, compared to controls.
Despite the growing body of literature on brain responses to visceral stimulation, reaching a consensus about published data has proved difficult due to the variety of experimental paradigms utilized (including subliminal, percept-related and stimulus related), analytic techniques, and reporting practices. Previous reviews of brain imaging studies using visceral stimulation have relied on low-powered conventional vote counting procedures to determine the brain regions activated consistently across studies.10–12 Derbyshire et al. reported on visceral stimulation studies in IBS and controls performed from 1997 to 2001 with PET or fMRI.11 This systemic review suggested that the most consistent activations seen across all subjects were in the insula, prefrontal, anterior cingulate, and primary sensory cortices and that differences between controls and IBS patients were localized to primary and secondary somatosensory cortices (S1, S2) and Brodmann area (BA) 39/40. Two more recent reviews also report activation of thalamus response to visceral stimulation in controls and IBS.10, 13
Newly emerging meta-analytic techniques for neuroimaging employ statistical analyses to empirically test and complement narrative literature reviews. 14–16 Activation Likelihood Estimation (ALE) has become the most prominent method of creating pooled analyses of neuroimaging data. Rather than simply counting the presence or absence of brain activity in an entire region of interest, these analyses incorporate the specific coordinates reported from individual studies to better determine convergent areas reliably activated across multiple studies. This allows for use of whole brain results, avoids potential disagreement in region of interest classification, and allows for determination of the statistical probability of cluster significance, rather than the descriptive reports of tabulation based studies.
The aim of this study was to apply a quantitative meta-analytic technique, ALE, to localize the brain regions most consistently activated during supraliminal lower gastrointestinal stimulation in IBS subjects and controls. We hypothesized that IBS subjects would display greater activity within regions involved in visceral afferent processing, emotional arousal and attention. We demonstrate that consistently activated brain regions can be reliably identified in control and IBS subjects undergoing lower gastrointestinal stimulation, despite the wide variety of study designs and subject inclusion criteria. In addition, we found significant differences between IBS and control subjects, most prominently in regions associated with emotional arousal and endogenous pain modulation, as well as in regions concerned with visceral afferent processing These findings are consistent with group differences in several neural networks we have previously hypothesized to be relevant in IBS pathophysiology.
Methods
Inclusionary/exclusionary criteria
Studies were included in the meta-analysis if the experimental design included supraliminal rectal balloon distension in male and/or female IBS subjects or controls. Supraliminal rectal distension in this setting includes both painful and non-painful stimuli. The analysis included both fMRI and PET studies. Foci were extracted from the results of with-in group analyses. Only activated foci were considered for analysis because deactivations were not consistently reported. Studies were excluded if the results were obtained after or during medical or psychological treatment (including placebo) or purposeful mood induction. Not all studies meeting inclusionary criteria reported within group analyses or specific information on significant foci. In these instances study authors were contacted regarding the data, and if available it was included. If data from the same sample was reported in more than one publication, only one publication was included in the analysis.
Statistical Analyses
A voxel-based ALE meta-analysis was applied to pool the results of multiple studies.14 Details of the mathematical algorithms can be seen in Turkeltaub 2002.16, 17 Briefly, given the uncertainty in reported spatial coordinates from a manuscript, each foci is treated as a probability distribution centered about a peak at the reported coordinate. The activation foci were modeled as the peaks of 3-D Gaussian distributions with a full-width half-maximum of 10 mm. The ALE statistic, representing the probability that at least one of the activation foci lies within a given voxel, was calculated at each voxel. A nonparametric permutation test was applied to test the null hypothesis that the foci are spread uniformly throughout the brain.18 A false discovery rate (FDR) of p=.05 was applied to threshold p values obtained from 5000 permutations for the ALE map. FDR controls the expected proportion of false positives among the suprathresholded voxels. The FDR threshold was determined from the observed p-value distribution, and hence is adaptive to the amount of signal in the data. ALE was used to pool and compare the results from IBS and controls. The minimum volume used to define a cluster was set to 100 mm3. Calculation of ALE statistics, permutation testing, thresholding, and cluster analysis were carried out with GingerALE Version 1.1 and 2.0 (www.brainmap.org).14 With-in group analyses were generated using random-effects approach in GingerALE version 2.0 which essentially weights the between subject variance for each study by sample size. Subtraction analyses were performed using the fixed-effects algorithm implemented in GingerALE version 1.1. We limit our interpretation of the subtraction analysis to regions identified in with-in group random effects analysis. Anatomic regions were labeled using the Talairach Daemon19 for all but the cingulate subregions, which were based on those described by Vogt et al. 20 Conjunction analysis for group results was performed by multiplying binarized versions of the thresholded ALE maps obtained for the within group analyses using FSLmaths. Because each of these maps was tested using a FDR of p=.05, this conjunction tests against the conjunction null at p <.05 (FDR). We overlayed the resultant conjunction map onto an anatomical template in Talairach and Tournoux (1988) space to visualize cluster overlays.21
Results
Studies meeting inclusionary criteria
18 studies 4, 7, 22–37 yielded tabulated coordinates for 13 inflation contrasts in IBS subjects and 12 inflation contrasts for control subjects (detailed in Table 1). The ALE analysis of IBS subjects incorporated 161 foci and the control subject analysis used 147 foci.
Table 1. Studies included in the meta-analysis.
| Study | Modality | Subjects | Supraliminal rectal inflation conditions |
|---|---|---|---|
| Berman et al. (2000)23 | PET | Study 1: 13 (7 F) IBS | 20, 45 and 60 mmHg |
| Study 2: 17 (6 F) IBS | |||
| Hobday et al (2001)27 | fMRI | 8 M Controls | average 11 p.s.i. |
| Lotze et al (2001)30 | fMRI | 8 Controls (4 F) | average 173.6ml |
| Naliboff et al (2001)13 | PET | 12 (2 F) IBS | 45 mmHg |
| 12 (2 F) Controls | |||
| Bonaz et al (2002)25 | fMRI | 11 (10 F) IBS | individually determined maximum tolerable volume |
| Verne et al (2003)36 | fMRI | 9 (6 F) IBS | 55 mmHg |
| 9 (6 F) Controls | |||
| Naliboff et al (2003)32 | PET | 42 (23 F) IBS | 45 mmHg |
| Andresen et al (2005)22 | fMRI | 8 (3 F) Controls | individually determined perception threshold +10 mmHg |
| 8 (5 F) IBS | |||
| Kwan et al (2005)29 | fMRI | 11 (7 F) Controls | individually determined moderate pain |
| 9 (6 F) IBS | |||
| Berman et al (2006)24 | fMRI | 13 (6 F) Controls | 25 and 45 mmHg |
| Naliboff et al (2006)33 | PET | 12 (8 F) IBS | 45 and 60 mmHg |
| Song et al (2006)35 | fMRI | 12 F IBS | pain detection +20% |
| 12 F Controls | |||
| Price et al (2007)34 | fMRI | 9 IBS | individually determined moderate pain (rating of 40 out of 100) |
| Berman et al (2008)7 | fMRI | 14 F IBS | 25 and 45 mmHg |
| 12 F Controls | |||
| Ringel et al (2008)4 | fMRI | 10 F Controls | 15 and 50 mmHg |
| 10 F IBS | |||
| Elsenbruch et al (2009)26 | fMRI | 15 F IBS | individually determined discomfort level |
| 12 F Controls | |||
| Kanazawa et al (2010)28 | PET | 32 M Controls | 20 and 40 mmHg |
| Moisset et al (2010)31 | fMRI | 11 F Controls | individually determined non-painful sensation and moderate pain |
| M: Male; F: Female |
Brain regions associated with lower gastrointestinal stimulation in healthy control subjects
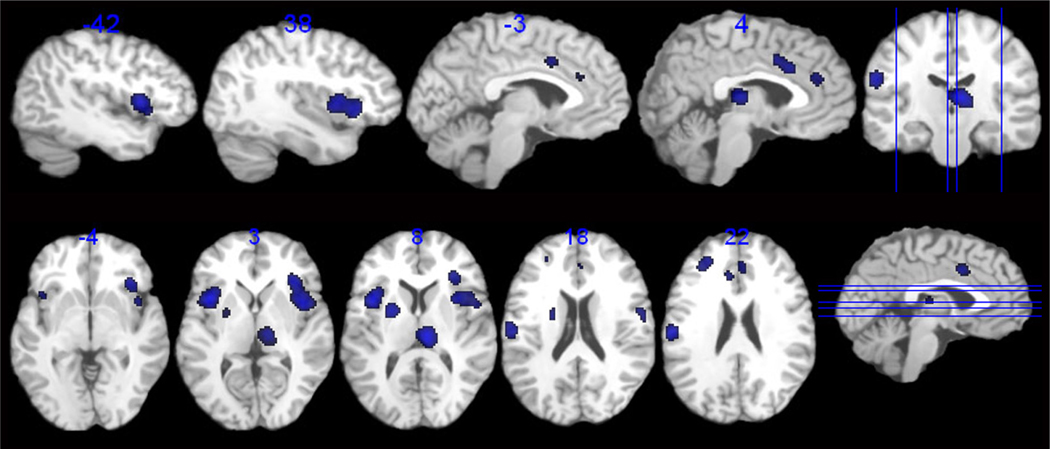
The within group analysis for control subjects is presented in Table 2 and Figure 1. Regions consistently activated in response to supraliminal lower gastrointestinal inflation across healthy control studies included those associated with visceral sensation [Bilateral (B) anterior insula (aINS), B anterior midcingulate cortex (aMCC), and right (R) thalamus], emotional arousal (R pACC, BA 32), and regions associated with attention and modulation of arousal [left (L) inferior parietal (BA 40), L lateral (BA 9/46) and R medial prefrontal cortex (BA 9/32)]. In addition consistent activation was seen in the putamen and the post central gyrus (BA 43).
Table 2. Controls.
Brain regions consistently activated and exceeding a false discovery rate threshold of p=.05 during supraliminal rectal balloon inflation in healthy controls.
| Label | Cluster # |
Volume (mm3) |
ALE Value |
Hemispher e |
x | y | z | Studies Contributin g |
|---|---|---|---|---|---|---|---|---|
| aINS | 1 | 5752 | 0.0025 | R | 42 | 6 | 2 | 7, 13, 24, 26–31, 35, 36 |
| aINS | 0.0024 | 36 | 22 | 0 | ||||
| Post central gyrus (BA 43) | 0.0013 | 58 | −8 | 16 | ||||
| aINS | 2 | 2968 | 0.0029 | L | −38 | 8 | 6 | 7, 22, 24, 29, 30, 36 |
| Thalamus | 3 | 2256 | 0.0027 | R | 8 | −24 | 8 | 13, 28, 29, 31, 36 |
| Inferior parietal lobule (BA 40) | 4 | 1744 | 0.0023 | L | −56 | −20 | 22 | 22, 27, 29, 30, 35 |
| Putamen | 5 | 1680 | 0.0025 | L | −22 | 0 | 12 | 28,31,13,,35 |
| aMCC (BA 24) | 6 | 1320 | 0.0018 | R | 6 | 16 | 30 | 22, 24, 27, 29 |
| 0.0017 | L | 0 | 6 | 36 | ||||
| Lateral prefrontal cortex (BA 9/46) | 7 | 1088 | 0.0019 | L | −26 | 40 | 24 | 13, 29, 35 |
| Inferior parietal lobule (BA 40) | 8 | 408 | 0.0018 | R | 52 | −30 | 30 | 28, 31 |
| pACC (BA 32) | 9 | 296 | 0.0013 | R | 4 | 36 | 22 | 22, 24 |
| aMCC/pACC (BA 32) | 10 | 272 | 0.0013 | L | −6 | 28 | 24 | 24, 36 |
| Medial prefrontal cortex (BA 9/32) | 11 | 168 | 0.0013 | R | 12 | 32 | 34 | 13, 31 |
Figure 1.
Regions showing consistent and reliable activation across all studies in healthy controls.
Brain regions associated with lower gastrointestinal stimulation in IBS subjects
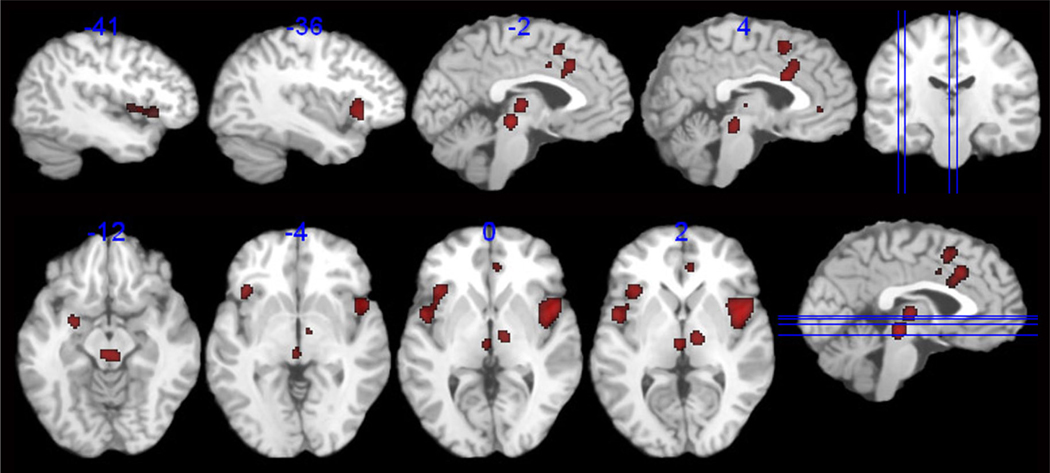
Significant consistent activations were observed in response to supraliminal lower gastrointestinal inflation for IBS subjects, in regions associated with visceral afferent processing (B a/mINS, B aMCC, B thalamus), emotional arousal (L amygdala, R inferior pACC) and attention (BA 6). A large midbrain region was also identified, and while it appears midline in the summary image, this region is the product of several more bilaterally positioned foci from 4 individual studies. While the spatial resolution in the brain stem is suboptimal across the included studies, foci comprising this region may include the nucleus cuneformis, red nucleus or periaqueductal gray (PAG). Consistent activations were also seen in the cerebellum and R Putamen. (see Table 3, Figure 2).
Table 3. IBS.
Brain regions consistently activated and exceeding a false discovery rate of p=.05 during supraliminal rectal balloon inflation in IBS patients.
| Region | Cluster # |
Hemispher e |
Volume (mm3) |
ALE Value |
x | y | z | Studies Contributing |
|---|---|---|---|---|---|---|---|---|
| a/mINS | 1 | R | 4304 | 0.0034 | 40 | 8 | 6 | 7, 13, 22, 23, 26, 29, 32–35 |
| Superior temporal gyrus (BA 22) | 2 | L | 1960 | 0.0020 | −48 | 2 | 2 | 7, 26, 29, 32, 35, 36 |
| aINS | 0.0021 | −34 | 20 | 4 | ||||
| aMCC (BA 32) | 3 | L | 1288 | 0.0021 | 0 | 18 | 34 | 7, 13, 33 |
| aMCC (BA 24) | R | 0.0021 | 2 | 12 | 28 | |||
| Midbrain | 4 | L | 1072 | 0.0025 | 0 | −28 | −8 | 7, 32, 33 |
| Cerebellum | R | 0.0020 | 6 | −30 | 14 | |||
| Thalamus | 5 | R | 960 | 0.0025 | 12 | −16 | 6 | 13, 22, 32, 36 |
| Superior frontal gyrus (BA 6) | 6 | R | 704 | 0.0019 | 2 | 10 | 48 | 22, 34, 35 |
| Thalamus | 7 | L | 496 | 0.0021 | −2 | −20 | 4 | 29, 33 |
| a/pMCC (BA 24) | 8 | L | 312 | 0.0014 | −10 | 4 | 36 | 7, 32 |
| Amygdala | 9 | L | 264 | 0.0018 | −24 | −4 | 12 | 23, 32 |
| pACC (BA 24) | 10 | R | 192 | 0.0014 | 6 | 36 | 2 | 7, 32 |
| Putamen | 11 | R | 112 | 0.0015 | 18 | 4 | 6 | 29, 36 |
Figure 2.
Regions showing consistent and reliable activation across all studies in IBS patients.
Group comparisons
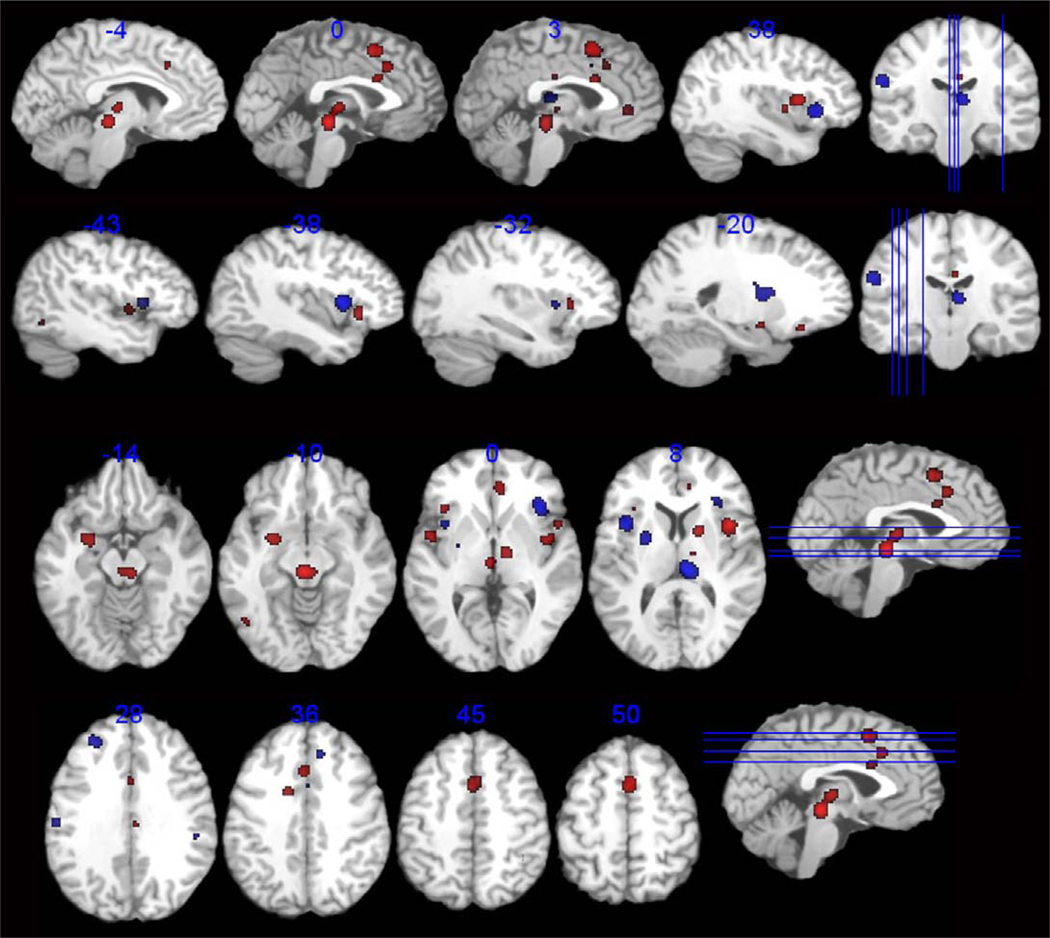
Group comparisons of brain regions consistently activated across studies can be seen in Table 4, and Figure 3. Conjunction analysis showed that regional overlap was evident in the right thalamus and bilaterally in the aMCC, pACC, and anterior insula. However, group differences in spatial extent and in subregions within a given region, were identified. For example, compared to controls, IBS subjects showed greater spatial extent of brain activity in regions of the B thalamus and B aMCC, regions associated with visceral afferent processing. Different insular regions were seen in IBS and control groups, with greater extension posteriorly to the mINS in IBS. IBS subjects showed thalamic activation in more medial regions, including the medial dorsal nucleus, while the control region was located caudally in the pulvinar nucleus. IBS showed greater consistent activity in the R pACC as well as L amygdala, regions associated with emotional arousal. IBS subjects had greater consistent activity of the R superior frontal cortex (BA 6) and of the midbrain, as noted in the within group analysis.
Table 4. GROUP DIFFERENCES.
Consistent differences in brain regions activated activations during supraliminal rectal balloon inflation in IBS patients compared to controls.
| IBS > HEALTHY CONTROL | |||||||
|---|---|---|---|---|---|---|---|
| Region | Hemisphere | Volume mm3 |
ALE Value | x | y | z | |
| 1 | Midbrain | R/L | 1704 | 0.0173 | 0 | −28 | −8 |
| Thalamus (medial dorsal nucleus) | L | 0.0131 | −2 | −20 | 2 | ||
| 2 | Thalamus | R | 1672 | 0.0150 | 10 | −14 | 0 |
| 3 | mINS | R | 1256 | 0.0161 | 40 | 8 | 8 |
| 4 | SMA (BA 6) | R | 920 | 0.0153 | 2 | 8 | 48 |
| 5 | Amygdala | L | 536 | 0.0123 | −24 | −4 | −12 |
| 6 | pACC (BA 24) | R | 472 | 0.0107 | 6 | 36 | 2 |
| 7 | Putamen | R | 400 | 0.0128 | 18 | 4 | 6 |
| 8 | aINS | L | 368 | 0.0104 | −36 | 20 | 0 |
| 9 | aMCC (BA 32) | R | 328 | 0.0112 | 0 | 18 | 36 |
| 10 | aINS | L | 312 | 0.0114 | −46 | 0 | 0 |
| 11 | aMCC (BA 24) | L | 288 | 0.0103 | −14 | 4 | 34 |
| 12 | aMCC (BA 24) | R | 240 | 0.0109 | 2 | 10 | 26 |
| HEALTHY CONTROL >IBS | |||||||
| Region | Hemisphere |
Volume mm3 |
ALE Value | x | y | z | |
| 1 | aINS | R | 1344 | −0.0107 | 36 | 22 | 0 |
| 2 | Thalamus (pulvinar) | R | 1312 | −0.0174 | 8 | −26 | 8 |
| 3 | Putamen | L | 1080 | −0.0134 | −22 | −2 | 12 |
| 4 | aINS | L | 968 | −0.0173 | −38 | 10 | 6 |
| 5 | Dorsolateral prefrontal cortex (BA 9/46) | L | 776 | −0.0126 | −26 | 42 | 26 |
| 6 | Inferior parietal lobule (BA 40) | L | 664 | −0.0153 | −56 | −20 | 24 |
| 7 | Pre/Post central gyrus (BA 43) | R | 344 | −0.0107 | 58 | −8 | 14 |
| 8 | Inferior parietal lobule (BA 40) | R | 168 | −0.0104 | 52 | −30 | 30 |
| 9 | Medial prefrontal cortex (BA 9/32) | R | 144 | −0.0095 | 12 | 32 | 34 |
Figure 3.
Selected axial and sagittal slices representing brain areas demonstrating difference greater activation in IBS (red) and greater activation in Controls (blue) across all studies.
Healthy controls showed greater consistent activity in some aspects of the B aINS, the pulvinar thalamus, the putamen, and post/precentral gyrus (BA 43, S2). BA 40, a region involved in stimulus driven somatosensory attentional processing, was seen in controls, but not IBS.38 In addition, the prefrontal cortex (L BA 9/46, R BA 9/32) was reliably activated in controls but not IBS subjects.
Discussion
The current ALE meta-analysis of supraliminal rectal inflation confirms the involvement of brain regions involved in visceral sensation, emotion arousal and attentional processes in both controls and IBS. Furthermore, the use of ALE techniques has allowed extension of these within group analyses to an IBS versus controls comparison showing group differences, particularly that IBS patients reliably engage known emotional arousal circuitry (amygdala, pACC) but healthy control subjects did not. The disparate results described in early studies of the brain’s response to visceral stimulation led some to raise a concern about the reliability and validity of the methodology in studying brain responses to lower intestinal distension. However, those inconsistent results appear to be primarily the result of different a priori choices, study designs and analytical methods, rather than an intrinsic limitation of the investigational approach. As hypothesized, IBS subjects undergoing lower intestinal stimulation showed a greater extent of brain activity than controls, specifically in regions associated with visceral afferent processing and emotional arousal. While the traditional identification of the individual brain regions involved in response to visceral stimulation has been important, in order to gain a fuller understanding of these regions in the pathophysiology of IBS, it is important to view them in the context of the brain networks in which they are involved. Advances in the past few years have informed our understanding of the networks involved in both visceral sensation (homeostatic afferent network) and the associated emotional response (emotional arousal network). The homeostatic afferent network is comprised of the thalamus, insula and aMCC. 39 This network encompasses the sensory input entering the thalamus from the brainstem, with projections to the primary interoceptive cortex (posterior INS), and to the aMCC, which mediates affective, motivational and motor aspects of the stimulus. 20, 40, 41 The emotional arousal network (including the locus coeruleus complex, amygdala, hypothalamus, parahippocampal gyrus, pACC, aINS, and orbitofrontal cortex) is engaged in the emotional processes modulating visceral responses such as anticipatory anxiety or fear 9, 42, 43. In the discussion of the results, we will focus on how the consistently involved brain regions described in the meta-analysis may operate within these functional networks. We will first discuss regions seen in both groups, and then discuss the observed group differences.
Brain regions engaged in both controls and IBS
Consistent with previous reviews, both control and IBS subjects showed activation of the thalamus, insula, and aMCC, though the specific regions seen in each group only partially overlap. These regions are key nodes within the homeostatic afferent network, as described by Mayer and colleagues. 39 Cortical regions associated with attentional processes were noted in both groups, though the specific location differed (BA 40 in control, BA 6 in IBS). Careful examination of attentional networks in IBS is lacking. The literature on attention suggests a role for BA 40 in the alerting response, as well as somatosensory attentional processing. 38, 44, 45 Brodmann area 6 is implicated in orienting to a stimulus and executive attention 44, 46 Just as limitations in study design preclude the differentiation between nociceptive and non-nociceptive aspects of ACC activation, control for attention was not a feature of most of the included paradigms, obviating a more specific interpretation.
IBS and control subjects also shared activation in the putamen, though the regions had no overlap in the conjunction analysis. While often noted as activated in visceral distension, the role of the putamen in these studies is largely unknown.
Brain regions showing greater engagement in IBS
Emotional arousal network
The most striking finding in comparison of IBS to controls was the greater engagement of specific nodes of the emotional arousal network (amygdala, pACC). Neither of these regions showed foci with greater consistent activity in controls. Additionally, activation of medial prefrontal cortex, a brain region known to negatively modulate emotional arousal was not seen in IBS. 47–49 The greater engagement of the emotional arousal network is consistent with a model of IBS characterized by increased anxiety, vigilance and altered autonomic responses.2 It has previously been suggested that greater engagement of emotional arousal circuitry may also play a role in central pain amplification.50, 51 A factor not accounted for in this type of analysis is the time course of network activation. Individual studies have suggested that in IBS subjects, regions of the emotional arousal network may be preferentially activated by the anticipation of visceral pain, and that greater engagement during anticipation of visceral pain may be seen primarily in female patients.9 It can be assumed that unless specific study paradigms were designed to differentiate brain responses to anticipation from the actual stimulus, brain responses during distension would be influenced by the anticipation response.
Homeostatic afferent network
IBS subjects also showed differential activation of regions in the homeostatic afferent network. Medial thalamic regions, including the medial dorsal nucleus, were seen with greater consistency in IBS. These thalamic nuclei have connectivity with the anterior cingulate and prefrontal cortices, consistent with stronger association of the afferent input to affective and motivation processing. 39, 52, 53 In comparison, the control subjects show posterior thalamic activity, in the pulvinar nucleus. Activation was seen across the groups in the insula, however the IBS groups shows great extension posteriorly into the mid-insula. A greater consistency of activation in the aMCC was seen in IBS compared to controls. This anterior region of the MCC is recognized to be activated by noxious visceral stimulation44, 45 though in a review examining its role in emotional processing, its most anterior portion has been associated with fearful emotion.45 Furthermore, more anterior activation of MCC extending into the superior portion of the pACC has been reported in studies of non-nociceptive pain, e.g. anticipated, imagined or empathy- related pain.43 Since the majority of the published studies were not designed to distinguish between the response to the visceral distension and the anticipation of such stimulation, a conclusion as to whether these subtle differences in the location and extent of regions may represent functional differences in network activity cannot be determined from the current analysis.
IBS subjects show consistent activation of a large region of the midbrain across studies, while no reliable midbrain regions are seen in the controls. None of the studies included in this analysis used a specific protocol for brainstem imaging, thus determination of which specific nuclei are included in this region is not feasible. Additionally, the ALE process leads to aggregation of multiple small adjacent regions, which may include the PAG, nucleus cuneformis, and the red nucleus. These regions, all of which are involved in emotional and pain modulatory functions, have been reported previously in studies imaging nociception.7, 54, 55,56 The nucleus cuneformis and PAG have both been implicated in descending pain facilitation as well as inhibition.57, 58 Greater descending pain facilitation or defects in descending inhibition may be mechanisms contributing to “central pain amplification”, e.g. the increased perceptual response to experimental and possibly physiological gut stimuli. Berman et al. (2008) described anticipation and distension related alterations in similar dorsal brain stem regions in IBS subjects, and specifically showed a lack of inhibition during anticipation, and greater activation during a painful stimulus.7 Clearly, nuclei in this region play important roles in perceptual responses and possibly in symptom generation. Imaging protocols optimized to study this brain region are required.
Brain regions and networks showing greater engagement in controls
Controls show greater reliable activation primarily in cortical regions involved in modulation of pain and emotion as well as attention, including lateral PFC, medial PFC and BA 40.48, 49, 59 The left lateral prefrontal cortex has been described in the cognitive control of emotion via reappraisal, and decreased activation of this region has been noted in women, in whom functional disorders are predominately seen. 60, 61 These findings are consistent with more effective down regulation of emotional arousal circuitry in controls, appropriate to the anticipation and sensation of an uncomfortable but tolerable visceral stimulus.
Limitations
This meta-analysis was limited by the availability of within group activation analyses from several previously published studies of lower gastrointestinal stimulation. However, it remains the only analysis of its kind in lower gastrointestinal distension and this quantitative meta-analytic approach significantly improves on the previously published systematic reviews. The individual studies analyzed included a variety of different stimulation paradigms and protocols, so by its nature this analysis displays mainly the most consistent and robustly activated regions involved in response to lower gastrointestinal stimulation. This approach may overlook regional activations or group differences specific to particular protocols. For example, previous studies have emphasized the importance of anticipation of visceral stimuli as an essential difference between IBS and control subjects and this analysis cannot differentiate between specific aspects of the experimental stimuli.7, 33, 35
Inhomogeneity in the patient populations of imaging studies of visceral sensation has been considered a barrier to interpretation 13 The analysis presented does not take into account bowel habit or sex, but despite this limitation, significant group differences are seen, suggesting at least some component of the central dysfunction in IBS are common across these sub-groups. This is consistent with the commonality of the cardinal symptoms (pain and discomfort) and frequently seen risk factors (psychological symptoms, early life trauma, co-morbid pain syndromes) seen in both gastrointestinal and non-gastrointestinal functional disorders. This meta-analysis cannot explicitly control for the presence of concomitant psychiatric or pain related disorders, or for the role of increased symptoms of anxiety or depression. Since the majority of studies did not control for such factors by rigorous patient selection (e.g. use of structured psychiatric interviews), it remains to be determined if the finding of greater engagement of emotional arousal circuitry is an essential component of IBS pathophysiology, or related to such comorbidity.
Previous reviews of the visceral stimulation imaging literature have noted within and between group differences in the somatosensory cortices, which we did not observe in the current analysis.10, 11 Part of this discrepancy can be accounted for by the fact that S1 and S2 are more robustly activated by upper compared to lower gastrointestinal stimulation and to create a more coherent analysis, we included only lower GI stimulation. Also, the inclusion of later studies, which were more likely to include specific coordinates, and the fact that some fMRI studies may have failed to include S1 and S2 due to a limited field of view may also have contributed to this difference.
CONCLUSIONS
The interpretation of combined neuroimaging and visceral stimulation studies has long been limited by relatively small sample sizes and diverse study designs. This analysis allows, for the first time, a quantitative meta-analysis of the rich existing dataset with an empirical approach. As expected, we show reliable activation of homeostatic afferent regions in both IBS and control subjects, but with greater extent in the IBS group. Patients with IBS have greater engagement of regions involved in emotional arousal network. These results support the role of central nervous system, IBS-control differences and most importantly, they will allow for more carefully designed experimental paradigms, to explore those networks which appear to best differentiate the central alterations in IBS responses to visceral stimulation.
Acknowledgments
Grant support: K23 DK073451(KT); K08 DK 071626 (JSL); R24 AT 00268; DK 48351, P50 DK 64530 (EAM.)
Abbreviations
- ALE
Activation Likelihood Estimate
- a
anterior
- B
bilateral
- BA
Brodmann area
- fMRI
functional magnetic resonance imaging
- control
healthy control
- INS
\ insula
- IBS
irritable bowel syndrome
- MCC
midcingulate cortex
- PAG
periaqueductal gray
- pACC
perigenual anterior cingulate gyrus
- PET
positron emission tomography
- SMA
supplementary motor area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Drossman DA, Dumitrascu DL. Rome III: New standard for functional gastrointestinal disorders. J Gastrointestin Liver Dis. 2006;15:237–241. [PubMed] [Google Scholar]
- 2.Mayer EA, Naliboff BD, Chang L, Coutinho SVV. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- 3.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 4.Ringel Y, Drossman DA, Leserman JL, Suyenobu BY, Wilber K, Lin W, Whitehead WE, Naliboff BD, Berman S, Mayer EA. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134:396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drossman DA, Ringel Y, Vogt BA, Leserman J, Lin W, Smith JK, Whitehead W. Alterations of brain activity associated with resolution of emotional distress and pain in a case of severe irritable bowel syndrome. Gastroenterology. 2003;124:754–761. doi: 10.1053/gast.2003.50103. [DOI] [PubMed] [Google Scholar]
- 7.Berman SM, Naliboff B, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller J, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. Journal of Neuroscience. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labus JS, Naliboff BD, Berman SM, Suyenobu B, Vianna EP, Tillisch K, Mayer EA. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage. 2009;47:952–960. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rapps N, van Oudenhove L, Enck P, Aziz Q. Brain imaging of visceral functions in healthy volunteers and IBS patients. J Psychosom Res. 2008;64:599–604. doi: 10.1016/j.jpsychores.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Derbyshire SW. Visceral afferent pathways and functional brain imaging. ScientificWorldJournal. 2003;3:1065–1080. doi: 10.1100/tsw.2003.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Light RJSP. Accumulating Evidence: Procedures for resolving contradictions among different research studies. Harvard Educational REview. 1971;41:429–471. [Google Scholar]
- 13.Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, Kuo B, Naliboff B, Tracey I. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil. 2009;21:579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wager TD, Lindquist M, Kaplan L. Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci. 2007;2:150–158. doi: 10.1093/scan/nsm015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- 18.Good P. Permutation Tests: A Practical Guide to Resampling Methods for Testing Hypothesis. Springer-Verlag; 1994. [Google Scholar]
- 19.Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Andresen V, Bach DR, Poellinger A, Tsrouya C, Stroh A, Foerschler A, Georgiewa P, Zimmer C, Monnikes H. Brain activation responses to subliminal or supraliminal rectal stimuli and to auditory stimuli in irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:827–837. doi: 10.1111/j.1365-2982.2005.00720.x. [DOI] [PubMed] [Google Scholar]
- 23.Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- 24.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Bueller JA, Ruby K, Mayer EA. Sex differences in regional brain response to aversive pelvic visceral stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R268–R276. doi: 10.1152/ajpregu.00065.2006. [DOI] [PubMed] [Google Scholar]
- 25.Bonaz B, Baciu M, Papillon E, Bost R, Gueddah N, Le Bas JF, Fournet J, Segebarth C. Central processing of rectal pain in patients with irritable bowel syndrome: an fMRI study. Am J Gastroenterol. 2002;97:654–661. doi: 10.1111/j.1572-0241.2002.05545.x. [DOI] [PubMed] [Google Scholar]
- 26.Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2009;59:489–495. doi: 10.1136/gut.2008.175000. [DOI] [PubMed] [Google Scholar]
- 27.Hobday D, Aziz Q, Thacker N, Hollander I, Jackson A, Thompson D. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain. 2001;124:361–368. doi: 10.1093/brain/124.2.361. [DOI] [PubMed] [Google Scholar]
- 28.Kanazawa M, Hamaguchi T, Watanabe S, Terui T, Mine H, Kano M, Fukudo S. Site-specific differences in central processing of visceral stimuli from the rectum and the descending colon in men. Neurogastroenterol Motil. 2009;22:173–180. e53. doi: 10.1111/j.1365-2982.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- 29.Kwan CL, Diamant NE, Pope G, Mikula K, Mikulis DJ, Davis KD. Abnormal forebrain activity in functional bowel disorder patients with chronic pain. Neurology. 2005;65:1268–1277. doi: 10.1212/01.wnl.0000180971.95473.cc. [DOI] [PubMed] [Google Scholar]
- 30.Lotze M, Wietek B, Birbaumer N, Ehrhardt J, Grodd W, Enck P. Cerebral activation during anal and rectal stimulation. Neuroimage. 2001;14:1027–1034. doi: 10.1006/nimg.2001.0901. [DOI] [PubMed] [Google Scholar]
- 31.Moisset X, Bouhassira D, Denis D, Dominique G, Benoit C, Sabate JM. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur J Pain. 2010;14:142–148. doi: 10.1016/j.ejpain.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 32.Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/s0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 33.Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Song GH, Venkatraman V, Ho KY, Chee MW, Yeoh KG, Wilder-Smith CH. Cortical effects of anticipation and endogenous modulation of visceral pain assessed by functional brain MRI in irritable bowel syndrome patients and healthy controls. Pain. 2006;126:79–90. doi: 10.1016/j.pain.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Verne GN, Himes NC, Robinson ME, Gopinath KS, Briggs RW, Crosson B, Price DD. Central representation of visceral and cutaneous hypersensitivity in the irritable bowel syndrome. Pain. 2003;103:99–110. doi: 10.1016/s0304-3959(02)00416-5. [DOI] [PubMed] [Google Scholar]
- 37.Naliboff BD, Derbyshire SW, Munakata J, Berman S, Mandelkern M, Chang L, Mayer EA. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. 2001;63:365–375. doi: 10.1097/00006842-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Coghill RC, Gilron I, Iadarola MJ. Hemispheric lateralization of somatosensory processing. J Neurophysiol. 2001;85:2602–2612. doi: 10.1152/jn.2001.85.6.2602. [DOI] [PubMed] [Google Scholar]
- 39.Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Craig AD. Interoception: The sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 41.Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain. 2008;138:362–374. doi: 10.1016/j.pain.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 43.Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. 2008;9:148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- 44.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res. 1977;136:393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez-Duque D, Posner MI. Brain imaging of attentional networks in normal and pathological states. J Clin Exp Neuropsychol. 2001;23:74–93. doi: 10.1076/jcen.23.1.74.1217. [DOI] [PubMed] [Google Scholar]
- 47.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 48.Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorenz JT, Irene . Brain Correlates of Psychological Amplification of Pain. In: Mayer EBMC, editor. Functional Pain Syndromes: presentation and pathophysiology. Seattle: IASP Press; 2009. pp. 385–404. [Google Scholar]
- 51.Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of visceral sensation. J Clin Neurophysiol. 2000;17:604–612. doi: 10.1097/00004691-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Taber KH, Wen C, Khan A, Hurley RA. The limbic thalamus. J Neuropsychiatry Clin Neurosci. 2004;16:127–132. doi: 10.1176/jnp.16.2.127. [DOI] [PubMed] [Google Scholar]
- 53.Hatanaka N, Tokuno H, Hamada I, Inase M, Ito Y, Imanishi M, Hasegawa N, Akazawa T, Nambu A, Takada M. Thalamocortical and intracortical connections of monkey cingulate motor areas. J Comp Neurol. 2003;462:121–138. doi: 10.1002/cne.10720. [DOI] [PubMed] [Google Scholar]
- 54.Dunckley P, Wise RG, Fairhurst M, Hobden P, Aziz Q, Chang L, Tracey I. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25:7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 56.Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci. 2006;26:4437–4443. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haws CM, Williamson AM, Fields HL. Putative nociceptive modulatory neurons in the dorsolateral pontomesencephalic reticular formation. Brain Res. 1989;483:272–282. doi: 10.1016/0006-8993(89)90171-6. [DOI] [PubMed] [Google Scholar]
- 58.Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. 2000;122:245–253. doi: 10.1016/s0079-6123(08)62143-3. [DOI] [PubMed] [Google Scholar]
- 59.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24:10364–10368. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Domes G, Schulze L, Bottger M, Grossmann A, Hauenstein K, Wirtz PH, Heinrichs M, Herpertz SC. The neural correlates of sex differences in emotional reactivity and emotion regulation. Hum Brain Mapp. 31:758–769. doi: 10.1002/hbm.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]