PEARLS
The majority of isolated cerebral zygomycosis is associated with intravenous drug abuse (IVDA)
Prompt diagnosis is challenging, but should be suspected in all otherwise healthy IV drug abusers who present focal neurologic deficits
Basal ganglia lesions on CT may be the most important diagnostic clue as it can facilitate early treatment
Clinicians should be aware of this disease as early medical treatment impacts its outcome.
CASE REPORT
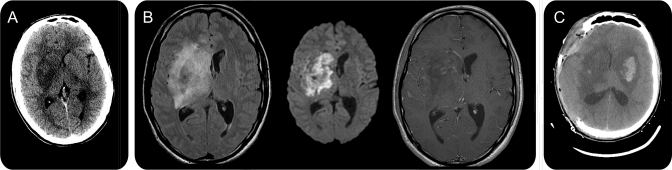
A 22-year-old man with a history of at least 1 year of IV heroin and crack cocaine abuse presented with a severe headache and difficulty walking. He reported symptom onset following heroin use the day before. He was found to have fever, nuchal rigidity, mild left facial droop, left hemiataxia, and mild encephalopathy. Serum studies revealed leukocytosis, a negative HIV test, no metabolic derangements, and negative bacterial cultures. Initial CT of his head revealed a large low-density lesion in the right basal ganglia with mild mass effect (figure, A), and thus lumbar puncture was not attempted. He was empirically given vancomycin, ceftriaxone, metronidazole, acyclovir, and trimethoprim/sulfamethoxazole. Over the next 24 hours, the patient developed left hemiplegia and severe encephalopathy. MRI revealed a predominantly T2 hyperintense and T1 hypointense lesion with diffusion restriction, minimal enhancement, and ill-defined margins in the right basal ganglia (figure, B). Gradient echo suggested a hemorrhagic component. These findings were consistent with an infectious or inflammatory cerebritis or focal ischemia, possibly from a vasculitis. The hemorrhagic component suggested a fungal infection such as Aspergillus spp or Mucor spp, so IV liposomal amphotericin B was added. He continued to deteriorate rapidly with development of decerebrate posture and seizures. The patient was intubated, placed on continuous EEG monitoring, and given anticonvulsants and intraventricular amphotericin B. Repeat CT demonstrated enlargement of the area of low attenuation in the right basal ganglia with mass effect on the lateral ventricle and 5.7 mm of midline shift. A new lesion in the left basal ganglia with petechial hemorrhage had also appeared (figure, C). He underwent emergent right hemicraniectomy to prevent herniation. Due to high risk of stereotactic biopsy of the deeper lesions, only a cortical biopsy was taken, and it revealed no pathologic changes. CSF demonstrated negative India ink stain, Gram stain, and cultures. Cryptococcus, Histoplasma, Aspergillus, and Blastomycosis immunoserologies were negative. After 15 days with no improvement, his pupils became fixed and dilated and CT revealed extension of the lesions to the cortex and brainstem. The patient died after care was withdrawn. Histopathology of his brain in autopsy revealed widespread areas consistent with abscess formation and bulbous thin-walled fungal hyphae with 90-degree branching and rare septations—all suggestive of zygomycosis.
Figure. Brain imaging.
(A) Initial head CT revealed a large low-density lesion in the right basal ganglia with mild mass effect. (B) MRI revealed a predominantly T2 hyperintense and T1 hypointense lesion with diffusion restriction, minimal enhancement, and ill-defined margins in the right basal ganglia. (C) Repeat head CT demonstrated enlargement of the original area of low attenuation in the right basal ganglia, mass effect on the lateral ventricle, and 5.7 mm of midline shift. A new lesion in the left basal ganglia with petechial hemorrhage had also appeared.
DISCUSSION
Zygomycosis is an uncommon disease caused by fungi of the phylum Zygomycota, which is identified by morphology in tissue sections. Historically, the infection was termed mucormycosis, but when tissue cultures are performed, species of the genera Rhizopus are identified more commonly than Mucor or Rhizomucor.1 The fungi are ubiquitous in the environment and the most common presentations are rhinocerebral, pulmonary, gastrointestinal, or cutaneous infection in patients with diabetes, malnutrition, or other immunocompromise. CNS involvement occurs typically through contiguous tissue spread and more rarely hematogenously. Isolated cerebral zygomycosis, as in our patient, is rare and has a high mortality.1
One review analyzed 31 reported cases of isolated CNS zygomycosis, of which 17 patients had a history of IVDA, 11 were immunocompromised, and the remaining 3 had no identified risk factors.2 Another review included 45 cases and found the association with IVDA to be even greater.1
Isolated CNS infection may lack systemic signs and symptoms, and initially manifest with a headache, encephalopathy, seizures, hemiparesis, or other focal neurologic deficit.2,3 The definitive diagnostic modality for zygomycosis is histopathology or culture from infected tissue. Adequate histologic preparations show necrotic tissue with neutrophilic infiltration and irregular branching, broad, nonseptate hyphae. Blood cultures are almost always negative and tissue cultures may be negative even when hyphae are seen.4 Molecular and serologic tests are in development, but not currently available for routine use.4,5
Imaging may be the most important clue to the early diagnosis of isolated CNS zygomycosis. The vast majority of IVDA-related cases have involvement of the basal ganglia.1,6 Contrast CT can show a wide range of enhancement patterns and there are only a few cases reported with MRI. As our case also demonstrated, an important feature is that unilateral basal ganglia mass lesions with low attenuation rapidly become bilateral and more extensive,6 highlighting the need for rapid diagnosis and treatment.
As soon as zygomycosis is suspected, treatment should be started immediately as it progresses rapidly and delay leads to poorer outcome. General treatment of zygomycoses includes reversal of the underlying condition, aggressive surgical debridement, and high-dose antifungal agents. In patients with an isolated CNS infection, most have an IVDA history with no underlying disease and surgical debridement of deep brain tissue is often problematic. The liposomal preparation of amphotericin B is recommended as the first-line antifungal treatment as it is less nephrotoxic than amphotericin B deoxycholate. In one study, posaconazole showed efficacy when amphotericin B is not tolerated or infection was refractory,7 but none of these patients had isolated CNS zygomycosis. The required duration of antifungals is unclear.
DISCLOSURE
Dr. Clark and Dr. Al Mohajer report no disclosures. Dr. Broderick has served on scientific advisory boards for Johnson & Johnson, Wyeth, and PhotoThera; holds patents re: Method for controlling the lysis of coagulated blood with apolipoprotein e4 phenotype; has served as a consultant for Novo Nordisk and Genentech, Inc. (all consulting fees and honoraria are placed in an education/research fund in Dr. Broderick's department of his institution); has received research support in the form of materials from Genentech, Inc., Novo Nordisk, Schering-Plough Corp., Concentric, EKOS Corporation, and Johnson & Johnson; and receives institutional research support from Boehringer Ingelheim, Genentech, Inc., Schering-Plough Corp., the NIH (NINDS U01 NS052220 [PI]; NINDS P50 NS44283 [PI]; NINDS R01 NS39512 [coinvestigator]; and NINDS R01 NS36695 [coinvestigator]).
REFERENCES
- 1. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634–653 [DOI] [PubMed] [Google Scholar]
- 2. Verma A, Brozman B, Petito CK. Isolated cerebral mucormycosis: report of a case and review of the literature. J Neurol Sci 2006;240:65–69 [DOI] [PubMed] [Google Scholar]
- 3. Sundaram C, Mahadevan A, Laxmi V, et al. Cerebral zygomycosis. Mycoses 2005;48:396–407 [DOI] [PubMed] [Google Scholar]
- 4. Chayakulkeeree M, Ghannoum MA, Perfect JR. Zygomycosis: the re-emerging fungal infection. Eur J Clin Microbiol Infect Dis 2006;25:215–229 [DOI] [PubMed] [Google Scholar]
- 5. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000;13:236–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hopkins RJ, Rothman M, Fiore A, Goldblum SE. Cerebral mucormycosis associated with intravenous drug use: three case reports and review. Clin Infect Dis 1994;19:1133–1137 [DOI] [PubMed] [Google Scholar]
- 7. Greenberg RN, Mullane K, van Burik JA, et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob Agents Chemother 2006;50:126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]