Abstract
Melanoma is notoriously resistant to chemotherapy, but variable responses to biotherapies, including the IFNs and IL-2, provide intriguing avenues for further study. Systemic IL-2 treatment has provided significant clinical benefit in a minority of metastatic melanoma patients, leading to long term survival in a few cases. We hypothesize that one previously unidentified mechanism of effective IL-2 therapy is through direct upregulation of the tumor suppressor IL-24 in melanoma tumor cells resulting in growth suppression. In this study five melanoma cell lines were treated with high dose recombinant human IL-2. Three (A375, WM1341, WM793) showed statistically significant increases in IL-24 protein; two (WM35, MeWo) remained negative for IL-24 message and protein. This increase was abolished by preincubating with anti-IL-2 antibody or blocking with antibodies against the IL-2 receptor chains. These IL-2 responsive melanoma cell lines expressed IL-2Rβ and γ mRNA. The IL-2Rβγ complex was functional, as measured by IL-2-induced STAT activation as well as IL-15 signaling through its shared receptor complex. IL-24 upregulation was observed in response to either IL-2 or IL-15. Cell growth was significantly decreased by treatment of IL-24 positive cells with IL-2 or IL-15, while no effect was seen in negative cells. Incubating the IL-24 inducible-cells with anti-IL-24 antibody as well as transfecting with IL-24 siRNA effectively reversed the growth suppression seen with IL-2. Thus, we have shown that one mechanism of clinically effective IL-2 therapy may be the direct action of IL-2 on a biologically distinct subset of melanoma cells leading to upregulation of the tumor suppressor IL-24.
Keywords: IL-24, melanoma, growth suppression, IL-2
Introduction
IL-24 is a tumor-suppressor/cytokine that is expressed in cells of the immune system including monocytes and activated T-cells, and in skin cells including keratinocytes and melanocytes [1–5]. The tumor-suppressor function of IL-24 was first described by Fisher (reviewed in 1) in terminally differentiated melanoma cells. And therefore the cytokine was initially named “melanoma differentiation-association gene 7 (MDA-7)” (1,2). IL-24 receptors are expressed primarily in the skin on keratinocytes [6, 7], but also found on endothelial cells, lung, testis, ovarian cells, and some cancer cells including melanoma [8] They were shown to consist of the class II heterodimeric receptors of IL-22R1/IL-20R2 and IL-20R1/IL-20R2 [9]. IL-24 protein which is expressed in some primary melanoma tumors is subsequently lost during melanoma tumor invasion and progression but remains detectable in a small number of advanced metastatic tumors [10–13]. IL-24 causes growth suppression and apoptosis in tumor cells both in vitro and in vivo, including lung, pancreatic, and breast cancer cells, while demonstrating no apparent toxicity to normal cells [14–16]. Data from our laboratory further demonstrated the cytotoxicity of IL-24 to human melanoma cell lines. These data are consistent with the tumor suppressor functions attributed to IL-24.
IL-24 is currently being studied in clinical trials for its potential therapeutic usefulness. In addition to its direct tumor suppressive effects, several studies confirm a bystander effect of the secreted protein from Ad.mda-7 gene therapy [8] such that IL-24 secreted from Ad.mda-7 transfected cells induced cell death in non-transfected tumor cells.
Preliminary data from our laboratory which has been expanded in this report revealed that co-culture of A375, a human melanoma cell line, with high doses of IL-2 resulted in endogenous production of IL-24 in a dose dependent manner. However, another melanoma cell line, MeWo, did not show upregulation of IL-24 following IL-2 treatment. We have reported previously that stimulation of normal peripheral blood mononuclear cells (PBMC) with IL-2 resulted in upregulation of IL-24 protein probably due to stabilization of IL-24 mRNA [17]. These observations suggest a possible mechanism by which IL-2 acts directly on the melanoma cells causing upregulation of the tumor suppressor IL-24.
Recombinant human IL-2 treatment has been used to treat metastatic melanoma patients since 1985, resulting in significant clinical benefits and long term survival in a minority of melanoma patients [18–20]. Objective responses have been observed in 15% to 20% of melanoma patients, with 6% to 8% of patients achieving complete durable responses [21, 22]. IL-2 antitumor activity is thought to be mediated by activation of natural killer cells (NK) to lymphokine activated killing activity (LAK) [23, 24], in essence enhancing the patient’s immune activity against melanoma. The toxic side effects associated with high dose IL-2 therapy severely limit its use to patients with healthier organ function and performance status, and thus it remains an obstacle to widespread treatment with IL-2. Presently it is impossible to predict responders prior to use of this costly and toxic therapy. Therefore, it is beneficial to establish a reliable marker to identify melanoma patients who are most likely to respond to IL-2 therapy, as well as propose an additional mechanism of growth control that may be operating in vivo.
We now propose that one mechanism by which systemic IL-2 is able to generate a durable response in melanoma patients is through direct activation of IL-24 mediated tumor growth suppression via the IL-2R on some melanoma cells. IL-2R chains are reported to be present on the surfaces of cutaneous melanoma cells [25–29], and IL-2Rβ has been identified in a number of cancer cell lines including small cell lung cancer, squamous cell carcinoma of the head and neck [30, 31], and melanoma. We show here, through in vitro experiments, that IL-2 leads to upregulation of IL-24 in a subset of melanoma cells resulting in IL-24 induced growth suppression. We propose that melanoma tumor cell IL-24 expression may be a potential predictive marker for IL-2 therapy.
Materials and Methods
Cell lines and cell culture
Two human metastatic melanoma cell lines, A375 and MeWo, were obtained from the American Type Culture Collection (Rockville, MD). The WM1341, WM793, and WM35 human primary melanoma cell lines were developed by Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA) [32]and were obtained from Dr. Robert Kerbel (University of Toronto, Toronto, Ontario, Canada) [33, 34]. All cell lines were validated in 2009 by STR DNA fingerprinting using the AmpF STR Identifier kit according to manufacturer instructions (Applied Biosystems, Foster City CA). The STR profiles were compared to known ATCC fingerprints (ATCC.org), to the Cell Line Integrated Molecular Authentication database (CLIMA) version 0.1.200808 (http://bioinformatics.istge.it/clima/) [35]and to the MD Anderson fingerprint database. The STR profiles matched known DNA fingerprints or were unique. Cell lines used were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 100 U/mL of penicillin, 100 mg/mL of streptomycin, 2 mM L-glutamine, and HEPES buffer with 5% fetal bovine serum (Life Technologies, Inc., Grand Island, NY). Experiments were run in supplemented RPMI with 2% charcoal stripped FBS (CSFBS). Serum was charcoal stripped to remove endogenous hormones and steroids by incubating overnight at 4°C with a mixture of charcoal, dextran, sucrose, magnesium chloride, and HEPES buffer from Sigma-Aldrich (St. Louis, MO) following the manufacture’s protocol.
Reagents
Human recombinant IL-2 was obtained from Chiron (Emeryville, CA). The goal of this project was to mimic the conditions present in high dose IL-2 patients during therapy. Patients receive 720,000 IU/kg/dose every 8 hours intravenously over 15–20 minutes with a maximum of 28 doses per course [36]. A study examining different cytokine levels in patients’ serum showed that peak IL-2 levels averaged around 4000 U/ml and remained well above 1000 U/ml after the 4th dose [37]. Based on these data, we chose 1000U/ml as a reasonable conservative dose at which to test the effects of high dose IL-2 on melanoma cells. IL-2 was therefore used at 1000 U/ml in all experiments. IL-15 was purchased from eBioscience (San Diego, CA). 7G11, a mouse anti-human IL-24 monoclonal antibody (Introgen Therapeutics, Houston, TX), was used for Western blotting and for blocking IL-24 during growth assays. Mouse anti-human IL-2 monoclonal antibodies were purchased from BD BioSciences (San Jose, CA). Anti-IL-2 receptor (IL-2Rα, IL-2Rβ, and IL-2Rγ) antibodies were purchased from R&D Systems (Minneapolis, MN). Preimmune normal mouse IgG (Sigma-Aldrich, St. Louis, MO) was used as a negative control for receptor blocking studies. Anti-actin antibody from Santa Cruz Biotechnology (Santa Cruz, CA) was used as a standard loading control for all Western blot staining. Lipopolysaccharide (LPS) was purchased from Sigma-Aldrich (St. Louis, MO).
Western blotting analysis
Cells were treated with brefeldin A (Sigma-Aldrich, St. Louis, MO) at 10μg/ml four hours prior to harvesting, washed with cold PBS, and subsequently harvested into PBS. Western blot band protein quantification was performed using densitometry analysis with Scion Image software (Scion Corporation, Fredrick, MD). Differences in protein levels between treatment conditions were determined by normalizing test proteins to β-actin controls within each sample.
Phospho protein analysis by flow cytometry
Following the procedure described by Schulz et al. [38] cells were analyzed for expression of phosphorylated STATs. Briefly cells were treated with 1000 U/ml IL-2 for 12–18 hr prior to fixation with 1.6% paraformaldehyde followed by permeabilization at 4°C with methanol. Cells were stained with PE mouse anti STAT5 (pY694), PE mouse anti STAT3 (pY705), or PE anti STAT1 (pY701). These antibodies were purchased from BD Biosciences. Immunofluorescence was analyzed on a FACSCalibur with Cell Quest software (BD Biosciences).
Detection of secreted IL-24 protein
Cells were plated at 1×106 cells/mL and cultured in supplemented RPMI-1640 medium (Invitrogen, Carlsbad, CA) + 2% CSFBS with 1000U/ml IL-2. Supernatants were harvested at 24 hours and secreted IL-24 levels were determined by ELISA (R&D Systems, Inc., Minneapolis, MN).
RT-PCR
RNA extraction was accomplished using the Qiagen RNAeasy (Valencia, CA) following the manufacturers protocol using the primers described in Table 1. RT-PCR assays were performed in the Quantitative Genomics Core Laboratory (QGCL) at The University of Texas Health Sciences Center in Houston, Texas. All PCR assays were designed and validated by QGCL staff to ensure they pass the minimum requirements for efficiency, sensitivity and selectivity.
Table 1.
IL-24 and IL-2R chain PCR primers and efficiency
| hIL-24 (IL-24) accession #: NM_006850 |
1484(+) AAGCAGATCCTCAATAAACATTTC 1556(−) ACCAAGGGAAAGGGATGATG PCR amplicon length =77 bases Lowest limit of detection = 190 copies PCR efficiency = 98% |
| hIL-2Ra (IL2RA) accession #: NM_000417 |
1061(+) CTAAATGGTCGCCCAGGAG 1128(−) TGTGATGTGACTTCAGAGCTT PCR amplicon length = 90 bases Lowest limit of detection = 160 copies (RT-PCR) PCR efficiency = 99% |
| hIL-2Rb (IL2RB) accession #: NM_000878 |
2037(+) CTCCCTCGTTAATCACAGGAT 2124(−) AGGACTGATATTGGTGAATAGCT PCR amplicon length = 90 bases Lowest limit of detection = 160 copies (RT-PCR) PCR efficiency = 99% |
| hIL-2Rg (IL2RG) accession #: NM_000206 |
380(+) CCACCTCTACCAAACATTTGTT 450(−) GCATCTGTGTGGCCTGTC PCR amplicon length = 80 bases Lowest limit of detection = 180 copies (RT-PCR) PCR efficiency = 99% |
Growth suppression assays
Cells were plated at 5x105 cells per well in 24 well plates, serum starved overnight, and then treated with RPMI + 2% CSFBS with or without IL-2 at 1000U/ml and other indicated conditions at day 0. Each condition was performed in triplicate. Cells were then harvested and viable cells counted using trypan blue dye exclusion at days 2 through 6 to assess cell growth. Mean values were determined for each condition and used for analysis.
siRNA Assay
siGENOME SMARTpool for human IL-24 and siGENOME Non-Targeting siRNA Pool were purchased from Dharmacon, Inc. (Chicago, IL). Oligofectamine was obtained from Invitrogen. Cells were serum starved overnight and then transfected with 2 nM IL-24 siRNA or control siRNA in OptiMEM media. Fresh RPMI + 4% FBS was added 4 hours after the transfection. Cells were transfected a second time 24 hours after the initial transfection and harvested 24 hours later. RNA was extracted and analyzed for IL-24 mRNA levels by RT-PCR. For growth suppression assays, supplemented RPMI + 2% CSFBS media and IL-2 were added 24 hours after the second transfection. This time point was designated day 0, and viable cells were counted at day 6 using the trypan blue dye exclusion assay.
Statistical Analysis
Means and standard errors for each of the variables were determined and the Student’s t test was utilized to evaluate the statistical significance of the experimental results. All experiments were performed at least three times unless otherwise indicated. Statistical significance was set at P < 0.05.
Results
Melanoma Cell Lines Express IL-24 mRNA
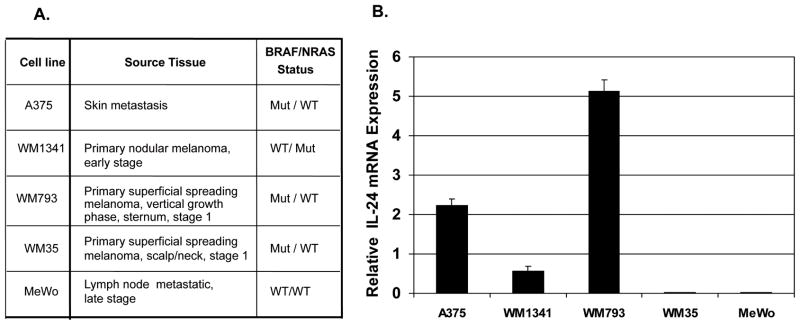
Five melanoma cell lines representing various stages of disease and mutation status (Fig. 1A) were examined by RT-PCR for the presence of IL-24 message. A375, WM1341, and WM793 were shown to contain IL-24 message while WM35 and MeWo had very low or undetectable levels of IL-24 mRNA (Fig. 1B).
Figure 1. Melanoma cells have message for IL-24.
Five melanoma cell lines, from both primary and metastatic tumors of different mutational status (A) were analyzed for IL-24 mRNA (B). Total RNA was extracted and analyzed for IL-24 mRNA by RT-PCR. Each sample was assayed in triplicate and results presented as the relative expression of IL-24 mRNA determined by densitometry and normalized against βactin.
IL-2 upregulates IL-24 protein production and secretion in IL-24 positive cell lines
Melanoma cells were treated with IL-2 at 1000 U/ml in RPMI + 2% CSFBS for 24 hours. Western blots are shown here for the three responsive cell lines (Fig. 2A). IL-24-expressing cell lines, A375, WM793 and WM1341 showed statistically significant increases in levels of IL-24 protein when treated with high dose IL-2 compared to cells cultured in media alone (Fig. 2A). Treatment with LPS served as a positive control causing increased expression of IL-24 in all three cell lines. IL-24 protein was visualized as monomeric species with molecular weights ranging from 18 to 32 kDa [39]. IL-24 contains three distinct N-glycosylation sites which accounts for the immunoreactivity of multiple bands that our IL-24 antibody detects during Western blot analysis.
Figure 2. High dose IL-2 upregulates IL-24 expression and secretion in IL-24 positive melanoma cells.
IL-24 expression in melanoma cells treated with IL-2 (1000 U/ml), for 24 hours was determined by immunoblotting with anti IL-24 monoclonal (7G11)(A).Treatment with LPS (100 ng/ml) was included as a positive control. The graph shows relative IL-24 expression levels determined by densitometry and normalized against β-actin expression using Scion Image Software. *P < 0.05 compared to cells without IL-2 stimulation. These experiments (A) were carried out five times. Shown are mean +/− SE of these results. Levels of secreted IL-24 were determined by ELISA in cells treated with IL-2 (1000 U/ml) (B). Pre-treatment with anti-hIL-2 antibody (1μg/ml) blocked this response in WM1341 (C) as shown by Western analysis with expression levels determined by densitometry and normalized againstβ-actin expression (C).
A375 exhibited an average increase in IL-24 levels of 43.0% (p=0.01) when treated with IL-2, while WM1341 showed an increase of 31.6% (p<0.01) and WM793 had an increase of 27.4% (p<0.01). In contrast, WM35 and MeWo cells did not produce detectable levels of IL-24 regardless of treatment with IL-2 or LPS (data not shown); this is consistent with our mRNA data showing little or no IL-24 message in these two cell lines.
In support of these observations, ELISA analysis showed that IL-2 treatment also caused an increase in IL-24 secretion in A375 by 36% (p=0.02), in WM1341 by 44% (p<0.01), and in WM793 by 28% (p=0.01) (Fig. 2B). WM35 and MeWo cells did not secrete any detectable levels of IL-24.
To determine whether the increases in IL-24 levels were specifically due to the actions of IL-2, we neutralized IL-2 by preincubating with a mouse-anti-IL-2 antibody for 1 hour at room temperature prior to stimulating the cells. Neutralization of IL-2 resulted in an inhibition of its effects on IL-24 bringing levels down to below baseline. A representative Western blot from the IL-24 positive cell line WM1341 is shown (Fig. 2C). These results indicate that the elevations of IL-24 in these cells are a direct result of treatment with IL-2.
Blocking IL-2R in Melanoma Cells Suppresses IL-2 Effects
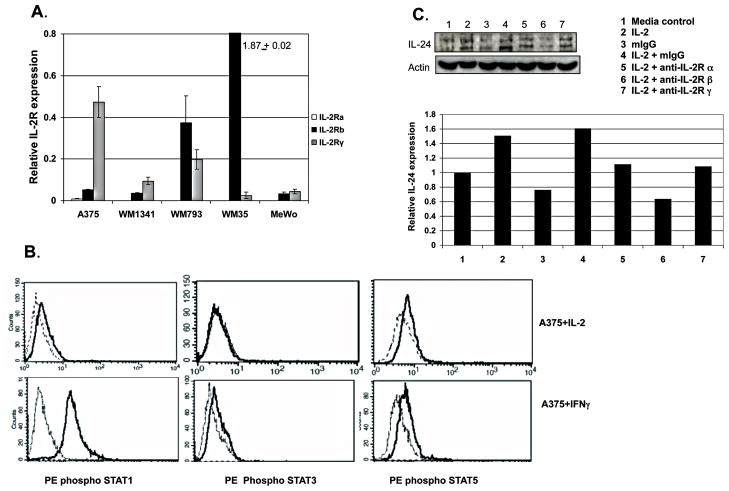
Since IL-2 has a direct effect on some melanoma cells, we examined IL-2 receptor expression on these cells. IL-2 mediates its effects on T cells by binding and signaling through the IL-2 receptor [40]. The receptor complex is composed of three chains -α, β, and γ which in different combinations form intermediate and high affinity receptors for IL-2 [41, 42]. Each of the five cell lines was found to have measurable IL-2Rβ and IL-2Rγ mRNA when analyzed by RT-PCR (Fig. 3A). IL-2Rα message was below the limit of detection of our assay in all the cells analyzed.
Figure 3. Functional IL-2Rβ and IL-2Rγ are expressed in melanoma cell lines and blocking IL-2R suppresses IL-2 effects.
IL-2 Rβ and IL-2Rγ are expressed on melanoma cells as determined by RT-PCR (A). These receptors are functional as shown by activation of STAT1 and 5 after stimulation with IL-2. Shown (B) are phospho STAT1 (left histograms, upper row) and phosphp STAT5 (right histogram, upper row) expression in A375 stimulated overnight with media (dotted line) or IL-2 (solid line) and measured by phospho flow cytometry. STAT3 is not activated by IL-2 treatment (center histograms, upper row, solid line). Positive controls for these experiments are A375 treated with IFNγ (solid line histogram) where phosphoSTAT1 (left histograms, lower row) as well as phospho STAT3 (center histogram, lower row) and phospho STAT5 (right histogram, lower row) cells are detected. Pre treatment of WM1341 with anti IL-2Rβ or anti-IL-2Rγ inhibited the expression of IL-24 in cells treated with IL-2 as measured by immunoblotting (C). The graph shows relative IL-24 expression levels determined by densitometry and normalized against β-actin.
We demonstrated that these receptors on melanoma cells were functional by stimulating with IL-2 and examining cells for downstream signaling events. Ligand binding to IL-2Rβ and IL-2Rγ leads to activation of STAT3 and STAT5 and in some cells STAT1 [43, 44]. Activation of STAT1, 3, or 5 was determined by phospho STAT analysis by flow cytometry. Shown in Figure 3B are the results of stimulating A375 with IL-2 and intracellular staining for phospho STAT1, 3 and 5. Activation of STAT1 and STAT5 was demonstrated by an increase in anti phospho STAT1 and phospho STAT5 binding to the IL-2 stimulated populations (solid line histogram), compared to cells treated in media alone (dotted line histogram). IL-2 did not cause activation of STAT3 in A375. The control condition for these experiments was A375 stimulated with IFNγ which resulted in activation of STAT1 and to a lesser extent STAT3 and STAT5.
To confirm that IL-2 signals specifically through its receptor to upregulate IL-24 in these cells, the IL-2R was blocked by preincubating WM1341 (Fig. 3C) and A375 cells (data not shown) with antibodies specific for each of the three IL-2 receptor chains. Blocking the IL-2R chains prior to adding IL-2 resulted in a decrease of 10% to 50% in IL-24 levels as compared to unblocked cells. This inhibition was most dramatic when IL-2Rβ was blocked, suggesting that the IL-2Rβ chain is essential for IL-2 upregulation of IL-24 expression.
IL-15 upregulates IL-24 expression in melanoma cell lines
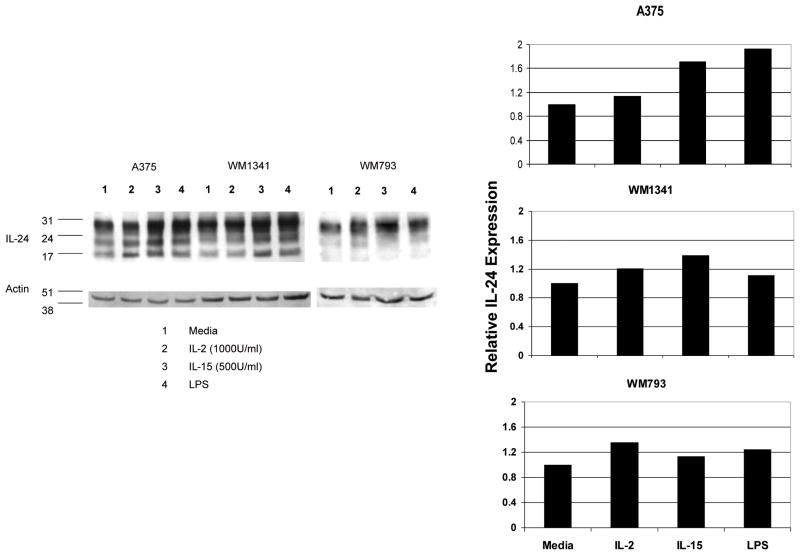
Having demonstrated the importance of IL-2Rβ on these melanoma cells, we examined whether IL-15, which shares both the IL-2Rβ chain and IL-2Rγ chain with IL-2 [45, 46], would affect the expression of IL-24 in melanoma cells Cells were stimulated with IL-15 (500 U/ml) for 24 hours and cell lysates were analyzed by Western blot analysis for IL-24 protein. All three IL-24 positive cell lines showed upregulation of IL-24 when treated with IL-15 (Fig. 4). Significantly, in A375 and WM1341, this increase was higher than that seen with high dose IL-2 stimulation. These data, together with the IL-2 receptor blocking experiment, confirm the significance of IL-2Rβ for IL-24 upregulation.
Figure 4. IL-15 upregulates IL-24 expression in some melanoma cells.
Melanoma cells incubated with media alone, IL-2 (1000U/ml), IL-15 (500U/ml), or LPS (100ng/ml) for 24 hours show increased expression of IL-24 as measured by immunoblotting with anti-IL-24 monoclonal antibody. The graph shows relative IL-24 expression levels determined by densitometry and normalized against β-actin.
IL-2 and IL-15 cause growth suppression in IL-24 expressing melanoma cells
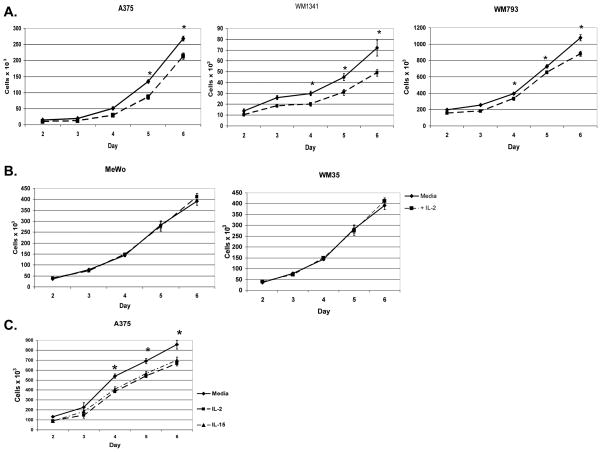
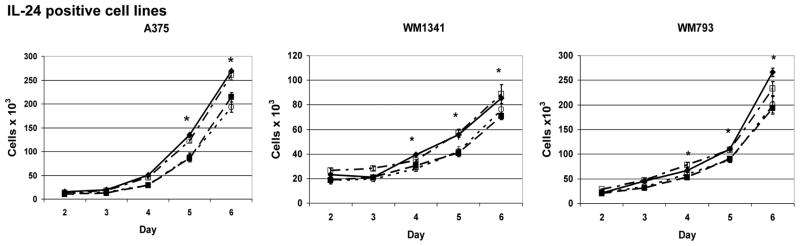
RT-PCR showed that IL-2Rβ and IL-2Rγ message are present in melanoma cell lines (Fig. 3A). It has been reported that several other cancers including squamous cell carcinoma, renal cell carcinoma, and gastric carcinomas are growth inhibited by IL-2 signaling specifically through the IL-2Rβ chain [31, 47]. We hypothesized that the growth of melanoma cell lines expressing IL-2R may be affected by IL-2. Therefore, cells were grown with or without IL-2 added at day 0 and cell viability assessed on days 2 through 6. WM1341 and WM793 cells treated with IL-2 had significantly fewer viable cells by day 4 (p ≤ 0.01) while A375 showed statistically significant growth suppression with IL-2 by day 5 (p = 0.04) (Fig. 5A). By day 6, IL-2 treated cells exhibited 18–32% less growth than untreated cells. The growth of the IL-24 negative cell lines WM35 and MeWo was unaffected by treatment with IL-2 (Fig. 5B). Although WM35 cells had the highest level of IL-2Rβ (Fig. 3A), IL-2 had no effect on its growth, suggesting that IL-2-mediated growth suppression is dependent on the IL-24 expression status of the cells.
Figure 5. IL-2 and IL-15 cause growth suppression in IL-24 expressing melanoma cells.
Melanoma cells, those that express IL-24 protein (A) or those that are negative for IL-24 (B) were grown with IL-2 at 1000 U/ml (■) or media alone (◆) for 6 days. A375 was grown with IL-15 at 500 U/ml (▲), IL-2 at 1000 U/ml (■), or in media alone (◆)(C). Cell growth was measured daily for 6 days by trypan blue exclusion. Results are reported as total cell number per culture. All experiments were set up in triplicate cultures and repeated twice. Shown are representative results for each cell line. *P < 0.05 comparing the total cell number of cells grown in media alone compared to those grown with IL-2.
We also tested the effect of IL-15 treatment on growth suppression in A375 cells (Fig. 5C). Results showed that cells treated with IL-15 demonstrated significant growth suppression of approximately 19–25% over days 4 through 6 (p=0.02 by day 4). These results are comparable to those cells treated with IL-2.
IL-2 mediated growth suppression is dependent on IL-24
Based on our data which showed that treatment with IL-2 caused increased IL-24 protein production and secretion in some melanoma cell lines, we predicted that these amplified levels of IL-24 would subsequently cause growth suppression in those cell lines. Therefore, we attempted to block the effects of the secreted IL-24 with an anti-IL-24 monoclonal antibody and by silencing IL-24 mRNA with IL-24 siRNA.
Melanoma cell lines were treated with anti-IL-24 monoclonal antibody to block the effects of secreted IL-24. In these cells anti-IL-24 antibody effectively reversed the growth suppression induced by IL-2 treatment (Fig. 6). These data indicate that IL-2 mediated growth suppression in melanoma cell lines is due to IL-24.
Figure 6. IL-2 melanoma cell growth suppression is dependent on IL-24.
Melanoma cells, those that express IL-24 protein, were grown with IL-2 at 1000 U/ml (■)or media alone (◆) for 6 days. Cells were pre incubated with anti IL-24 monoclonal antibody and grown with IL-2 (□); or pre incubated with mouse IgG and grown with IL-2 (○) for 6 days. Cell growth was measured daily for 6 days by trypan blue exclusion. Results are reported as total cell number per culture. All experiments were set up in triplicate cultures and repeated twice. Shown are representative results for each cell line. *P < 0.05 comparing the total cell number of cells grown in media alone compared to those grown with IL-2.
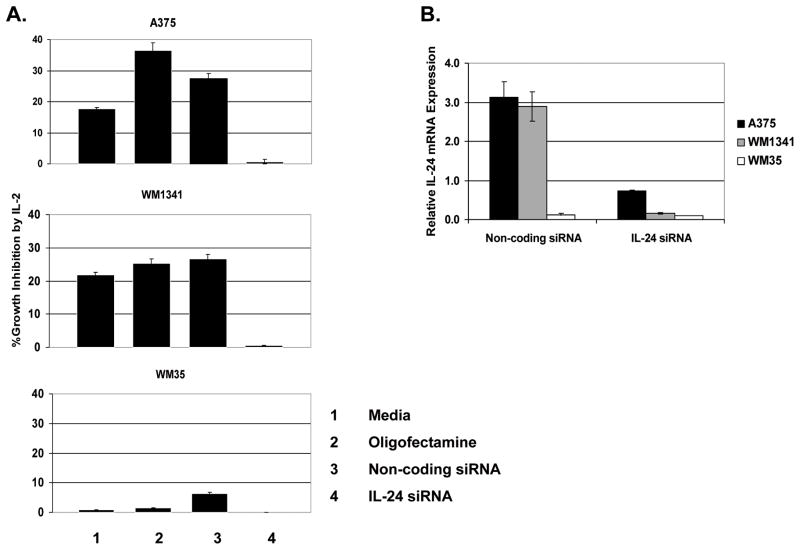
To directly block IL-24 production A375, WM1341, and WM35 were treated with IL-24 siRNA or a non-coding siRNA negative control for 48 hours prior to the addition of high dose IL-2 on day 0. IL-24 knock-down was verified by RT-PCR for IL-24 message in cells transfected with either non-coding control siRNA or IL-24 siRNA (Fig. 7B). Cell viability was assessed at day 6 (Fig. 7A). Results of growth suppression assays showed that in the two IL-24 positive cell lines, A375 and WM1341, silencing IL-24 at the mRNA level neutralized the IL-2 mediated growth suppressive effects previously observed; in these cell lines, there was no significant difference seen between the IL-24 silenced cells that were cultured with IL-2 and those without IL-2. In A375 and WM1341, IL-2 maintained its growth suppressive effects within all of the control groups (p≤0.05). In WM35, an IL-24 negative cell line, treating with IL-24 siRNA had no effect on growth between IL-2 treated and non-treated cells. These data further confirm that IL-2 mediated growth suppression is directly dependent on IL-24.
Figure 7. Silencing IL-24 reverses IL-2 induced growth suppression.
IL-24 siRNA inhibits IL-2-induced growth suppression in melanoma cells (A). Growth was assessed at day 6 in siRNA transfected melanoma cells treated with IL-2 at day 0. Data are presented as % Growth Inhibition, which is equal to [1− (total cell number of cells grown with IL-2/total cell number of cells grown in media alone)] × 100%. IL-24 siRNA specifically knocked down the expression of IL-24 in these cells compared to cells treated with non-coding siRNA as shown by RT-PCR analysis (B). Results are expressed relative to β-actin.
Discussion
We have established a novel mechanism of IL-2 action in melanoma by demonstrating that high dose IL-2 had a direct effect on a subset of melanoma cells. These cells expressed functional IL-2 receptors and upregulated their expression of IL-24 mRNA, protein expression and secretion, leading to growth suppression. Furthermore, we showed that IL-2 mediates this activity by signaling through the IL-2 receptor, in particular the IL-2Rβ chain in these cells. Finally, we demonstrated that IL-15, which shares the IL-2Rβ chain with IL-2, also upregulated IL-24 protein expression and resulted in growth suppression in IL-24 positive cells.
IL-2 mediates its actions by binding to its receptor resulting in activation of Ras/MAPK, JAK/Stat, and PI3-kinase/Akt signaling pathways leading to downstream gene transcriptional regulation [43, 44, 48]. As an immunotherapeutic agent, IL-2 directs its anti-tumor functions through cell-mediated immunity. Although reports exist of IL-2R and direct IL-2 effects on cancer cells, the mechanism of action is still unclear. One group has reported that IL-2 at low doses in the absence of serum appeared to stimulate proliferation of some melanoma cell lines but not others during the first 24 hours, while cell growth was unaffected, or somewhat suppressed, at higher doses around 500U/ml over the same time period [25].
IL-2 has been shown to directly inhibit the growth of human squamous cell carcinoma of the head and neck (SCCHN) both in vitro and in nude mice bearing SCCHN xenografts [31, 47, 49, 50]. Growth inhibition in SCCHN cells was completely reversed by blocking with an anti-IL-2Rβ antibody and partially reversed by blocking with an anti-IL-2Rα antibody; this indicated the importance of the intermediate affinity IL-2R for the growth suppressive effect of IL-2 in cancer cells. These reports support our data that IL-2 signals through the IL-2Rβ chain and has a direct growth suppressive effect on melanoma cells.
While a valuable and established cancer immunotherapy, IL-2 is successful in a subset of patients and can result in severe toxic side effects. The availability of positive predictive biomarkers of IL-2 response would significantly improve targeted therapy for melanoma. Accordingly, our study suggests that IL-24 may be such a marker. It is possible that the patients with durable responses to high dose IL-2 therapy have melanoma cells that increase their expression of IL-24 in response to IL-2. Therefore, establishing the IL-24 status of melanoma tumors could help identify patients who would benefit most from IL-2 treatment despite its negative side effects.
We have shown that upregulation of IL-24 through the actions of IL-2 results in IL-24-mediated melanoma growth suppression. In addition the increased secretion of IL-24 could also have a paracrine effect by impacting neighboring cells within the tumor microenvironment. This is highly possible since it has been reported that the IL-24 receptor chains are present on melanoma cell lines [8]. Even if only a fraction of melanoma cells within a tumor are capable of responding to IL-2, the remaining unresponsive cells which may possess the IL-24 receptor could be indirectly affected by the secreted IL-24 from responding cells resulting in their growth arrest. Of the individual cell lines used in this paper, FACs analysis confirms that 6–12% of cells within each cell line expressed IL20R1 and 17–23% expressed IL22R1 (data not shown).
Based on the data contained in this manuscript and the available literature reviewed, we have presented a novel model for the direct effects of IL-2 on melanoma cells. We propose that treatment with IL-2 can upregulate IL-24 protein expression in some tumor cells leading to melanoma tumor growth suppression. Currently, no reliable marker exists to identify and predict the melanoma patients who are most likely to respond positively to IL-2 therapy. The results from this study offer insights into several potential predictive biomarkers for targeted IL-2 therapy. Potential markers include the presence of IL-24 or coexpression of IL-24 and the IL-2Rβ chain in melanoma cells. Other potential markers previously presented in the literature include VEGF as high serum levels of VEGF were correlated with lack of clinic response to IL-2 [51]. IL-24 has been shown to inhibit VEGF production at a translational level in human prostate cancer cells, consistent with our model [52].
This study also offers evidence to support the exploration of IL-15 either alone or in combination with other treatments as a potential therapy for melanoma. We have shown IL-15 to be equally capable of causing IL-24 dependent melanoma cell growth suppression in comparison with IL-2, and the existing literature observes that IL-15 has fewer side effects and toxicity than high dose IL-2 therapy. It has been published that NSAIDs can also induce IL-24 protein expression and subsequent apoptosis and growth arrest both in vitro and in vivo [53]. It is possible that IL-2 or IL-15 could be used in conjunction with therapies such as NSAIDs which might synergistically intensify the IL-24 dependent growth suppressive effects in cancer cells.
Cancers are incredibly complex diseases, and increasing our understanding of the molecular mechanisms of both the diseases and the treatments is essential. Selection of an appropriate therapy should be tailored to the individual patient based on his specific tumor and immunologic profile. It is our hope that this work suggests IL-24 as a as potential predictive marker for IL-2 responsiveness, and provides the basis of an identifiable targeted therapy for melanoma in a subset of patients. Our laboratory is currently testing tumors from patients treated with high dose IL-2 for expression of IL-2R as well as IL-24 mRNA and protein.
Acknowledgments
The authors thank the Quantitative Genomics Core Laboratory (QGCL) at The University of Texas Health Sciences Center in Houston, Texas. STR DNA fingerprinting was done by the Cancer Center Support grant funded Characterized Cell Line core, NCI # CA16672.
Grant Support: This work was supported by National Institutes of Health Grant P50 CA093459 (to EAG), the Ruth L. Kirschstein National Research Service Award (National Institutes of Health, Center for Clinical and Translational Science (CCTS) T32 Training Award) 1-UL1- RR024148-01 (to EJ), by a Multidisciplinary Research Program award provided by M. D. Anderson Cancer Center and the Cancer Center Support Grant CA016672 (to E.A.G.), and in part by the generosity of the Dr. Miriam and Mr. Sheldon Adelson Medical Research Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors have no conflicts to disclosure.
References
- 1.Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, et al. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2(4 Suppl 1):S23–S37. [PubMed] [Google Scholar]
- 2.Huang EY. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20:7051–7053. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 3.Caudell EG, Mumm JB, Poindexter N, Ekmekcioglu S, Mhashilkar AM, Yang XH, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168(12):6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 4.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J Immunol. 2002;168(11):5397–5402. doi: 10.4049/jimmunol.168.11.5397. [DOI] [PubMed] [Google Scholar]
- 5.Poindexter NJ, Williams RR, Powis G, Jen E, Caudle AS, Chada S, et al. IL-24 is expressed during wound repair and inhibits TGFalpha-induced migration and proliferation of keratinocytes. Exp Dermatol. 2010;19(8):714–722. doi: 10.1111/j.1600-0625.2010.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Liang P. Interleukin-24 and its receptors. Immunology. 2005;114(2):166–170. doi: 10.1111/j.1365-2567.2005.02094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolk K, Haugen HS, Xu W, Witte E, Waggie K, Anderson M, et al. IL-22 and IL-20 are key mediators of the epidermal alterations in psoriasis while IL-17 and IFN-gamma are not. J Mol Med. 2009;87(5):523–536. doi: 10.1007/s00109-009-0457-0. [DOI] [PubMed] [Google Scholar]
- 8.Chada S, Mhashilkar AM, Ramesh R, Mumm JB, Sutton RB, Bocangel D, et al. Bystander activity of Ad-mda7: human MDA-7 protein kills melanoma cells via an IL-20 receptor-dependent but STAT3-independent mechanism. Mol Ther. 2004;10(6):1085–1095. doi: 10.1016/j.ymthe.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Tan Z, Zhang R, Kotenko SV, Liang P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J Biol Chem. 2002;277(9):7341–7347. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- 10.Ekmekcioglu S, Ellerhorst J, Mhashilkar AM, Sahin AA, Read CM, Prieto VG, et al. Down-regulated melanoma differentiation associated gene (mda-7) expression in human melanomas. Int J Cancer. 2001;94(1):54–59. doi: 10.1002/ijc.1437. [DOI] [PubMed] [Google Scholar]
- 11.Ellerhorst JA, Prieto VG, Ekmekcioglu S, Broemeling L, Yekell S, Chada S, et al. Loss of MDA-7 expression with progression of melanoma. J Clin Oncol. 2002;20(4):1069–1074. doi: 10.1200/JCO.2002.20.4.1069. [DOI] [PubMed] [Google Scholar]
- 12.Ekmekcioglu S, Ellerhorst JA, Mumm JB, Zheng M, Broemeling L, Prieto VG, et al. Negative association of melanoma differentiation-associated gene (mda-7) and inducible nitric oxide synthase (iNOS) in human melanoma: MDA-7 regulates iNOS expression in melanoma cells. Mol Cancer Ther. 2003;2(1):9–17. [PubMed] [Google Scholar]
- 13.Chada S, Sutton RB, Ekmekcioglu S, Ellerhorst J, Mumm JB, Leitner WW, et al. MDA-7/IL-24 is a unique cytokine-tumor suppressor in the IL-10 Family. Int Immunopharmacol. 2004;4(5):649–667. doi: 10.1016/j.intimp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Chada S, Bocangel D, Ramesh R, Grimm EA, Mumm JB, Mhashilkar AM, et al. mda-7/IL24 kills pancreatic cancer cells by inhibition of the Wnt/PI3K signaling pathways: identification of IL-20 receptor-mediated bystander activity against pancreatic cancer. Mol Ther. 2005;11(5):724–733. doi: 10.1016/j.ymthe.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 15.Saeki T, Mhashilkar A, Swanson X, Zou-Yang XH, Sieger K, Kawabe S, et al. Inhibition of human lung cancer growth following adenovirus-mediated mda-7 gene expression in vivo. Oncogene. 2002;21(29):4558–4566. doi: 10.1038/sj.onc.1205553. [DOI] [PubMed] [Google Scholar]
- 16.Saeki T, Mhashilkar A, Chada S, Branch C, Roth JA, Ramesh R. Tumor-suppressive effects by adenovirus-mediated mda-7 gene transfer in non-small cell lung cancer cell in vitro. Gene Ther. 2000;7(23):2051–2057. doi: 10.1038/sj.gt.3301330. [DOI] [PubMed] [Google Scholar]
- 17.Poindexter NJ, Walch ET, Chada S, Grimm EA. Cytokine induction of interleukin-24 in human peripheral blood mononuclear cells. J Leukoc Biol. 2005;78(3):745–752. doi: 10.1189/jlb.0205116. [DOI] [PubMed] [Google Scholar]
- 18.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 19.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6 (Suppl 1):S11–S14. [PubMed] [Google Scholar]
- 20.Tarhini AA, Kirkwood JM, Gooding WE, Cai C, Agarwala SS. Durable complete responses with high-dose bolus interleukin-2 in patients with metastatic melanoma who have experienced progression after biochemotherapy. J Clin Oncol. 2007;25(25):3802–3807. doi: 10.1200/JCO.2006.10.2822. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 22.Parmiani G, Rivoltini L, Andreola G, Carrabba M. Cytokines in cancer therapy. Immunol Lett. 2000;74(1):41–44. doi: 10.1016/s0165-2478(00)00247-9. [DOI] [PubMed] [Google Scholar]
- 23.Grimm EA, Robb RJ, Roth JA, Neckers LM, Lachman LB, Wilson DJ, et al. Lymphokine-activated killer cell phenomenon. III. Evidence that IL-2 is sufficient for direct activation of peripheral blood lymphocytes into lymphokine-activated killer cells. J Exp Med. 1983;158(4):1356–1361. doi: 10.1084/jem.158.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer- resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Vazquez MD, Boyano MD, Canavate ML, Gardeazabal J, de Galdeano AG, Lopez-Michelena T, et al. Interleukin-2 enhances the growth of human melanoma cells derived form primary but not from metastatic tumours. Eur Cytokine Netw. 2000;11(4):654–661. [PubMed] [Google Scholar]
- 26.Plaisance S, Rubinstein E, Alileche A, Han DS, Sahraoui Y, Mingari MC, et al. Human melanoma cells express a functional interleukin-2 receptor. Int J Cancer. 1993;55(1):164–170. doi: 10.1002/ijc.2910550129. [DOI] [PubMed] [Google Scholar]
- 27.He YG, Mayhew E, Mellon J, Niederkorn JY. Expression and possible function of IL-2 and IL-15 receptors on human uveal melanoma cells. Invest Ophthalmol Vis Sci. 2004;45 (12):4240–4246. doi: 10.1167/iovs.04-0599. [DOI] [PubMed] [Google Scholar]
- 28.McMillan DN, Kernohan NM, Flett ME, Heys SD, Deehan DJ, Sewell HF, et al. Interleukin 2 receptor expression and interleukin 2 localisation in human solid tumor cells in situ and in vitro: evidence for a direct role in the regulation of tumour cell proliferation. Int J Cancer. 1995;60(6):766–772. doi: 10.1002/ijc.2910600606. [DOI] [PubMed] [Google Scholar]
- 29.Alileche A, Plaisance S, Han DS, Rubinstein E, Mingari C, Bellomo R, et al. Human melanoma cell line M14 secretes a functional interleukin 2. Oncogene. 1993;8(7):1791–1796. [PubMed] [Google Scholar]
- 30.Meazza R, Marciano S, Sforzini S, Orengo AM, Coppolecchia M, Musiani P, et al. Analysis of IL-2 receptor expression and of the biological effects of IL-2 gene transfection in small-cell lung cancer. Br J Cancer. 1996;74(5):788–795. doi: 10.1038/bjc.1996.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weidmann E, Sacchi M, Plaisance S, Heo DS, Yasumura S, Lin WC, et al. Receptors for interleukin 2 on human squamous cell carcinoma cell lines and tumor in situ. Cancer Res. 1992;52(21):5963–5970. [PubMed] [Google Scholar]
- 32.Herlyn M, Kath R, Williams N, Valyi-Nagy I, Rodeck U. Growth-regulatory factors for normal, premalignant, and malignant human cells in vitro. Adv Cancer Res. 1990;54:213–234. doi: 10.1016/s0065-230x(08)60812-x. [DOI] [PubMed] [Google Scholar]
- 33.Cornil I, Theodorescu D, Man S, Herlyn M, Jambrosic J, Kerbel RS. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proc Natl Acad Sci U S A. 1991;88(14):6028–6032. doi: 10.1073/pnas.88.14.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu C, Rak JW, Kobayashi H, Kerbel RS. Increased resistance to oncostatin M-induced growth inhibition of human melanoma cell lines derived from advanced-stage lesions. Cancer Res. 1993;53(12):2708–2711. [PubMed] [Google Scholar]
- 35.Romano P, Manniello A, Aresu O, Armento M, Cesaro M, Parodi B. Cell Line Data Base: structure and recent improvements towards molecular authentication of human cell lines. Nucleic Acids Res. 2009;37(Database issue):D925–D932. doi: 10.1093/nar/gkn730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margolin KA, Rayner AA, Hawkins MJ, Atkins MB, Dutcher JP, Fisher RI, et al. Interleukin-2 and lymphokine-activated killer cell therapy of solid tumors: analysis of toxicity and management guidelines. J Clin Oncol. 1989;7(4):486–498. doi: 10.1200/JCO.1989.7.4.486. [DOI] [PubMed] [Google Scholar]
- 37.Konrad MW, Hemstreet G, Hersh EM, Mansell PW, Mertelsmann R, Kolitz JE, et al. Pharmacokinetics of recombinant interleukin 2 in humans. Cancer Res. 1990;50(7):2009–2017. [PubMed] [Google Scholar]
- 38.Schulz KR, Danna EA, Krutzik PO, Nolan GP. Single-cell phospho-protein analysis by flow cytometry. Curr Protoc Immunol. 2007;78:8.17.1–8.17.20. doi: 10.1002/0471142735.im0817s78. [DOI] [PubMed] [Google Scholar]
- 39.Mhashilkar AM, Schrock RD, Hindi M, Liao J, Sieger K, Kourouma F, et al. Melanoma differentiation associated gene-7 (mda-7): a novel anti-tumor gene for cancer gene therapy. Mol Med. 2001;7(4):271–282. [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi T, Minami Y. The IL-2/IL-2 receptor system: a current overview. Cell. 1993;73 (1):5–8. doi: 10.1016/0092-8674(93)90152-g. [DOI] [PubMed] [Google Scholar]
- 42.Waldmann TA. The multi-subunit interleukin-2 receptor. Annu Rev Biochem. 1989;58:875–911. doi: 10.1146/annurev.bi.58.070189.004303. [DOI] [PubMed] [Google Scholar]
- 43.Johnston JA, Bacon CM, Finbloom DS, Rees RC, Kaplan D, Shibuya K, et al. Tyrosine phosphorylation and activation of STAT5, STAT3, and Janus kinases by interleukins 2 and 15. Proc Natl Acad Sci U S A. 1995;92(19):8705–8709. doi: 10.1073/pnas.92.19.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou J, Schindler U, Henzel WJ, Wong SC, McKnight SL. Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995;2(4):321–329. doi: 10.1016/1074-7613(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 45.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13(12):2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bamford RN, Grant AJ, Burton JD, Peters C, Kurys G, Goldman CK, et al. The interleukin (IL) 2 receptor beta chain is shared by IL-2 and a cytokine, provisionally designated IL-T, that stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91(11):4940–4944. doi: 10.1073/pnas.91.11.4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasumura S, Lin WC, Weidmann E, Hebda P, Whiteside TL. Expression of interleukin 2 receptors on human carcinoma cell lines and tumor growth inhibition by interleukin 2. Int J Cancer. 1994;59(2):225–234. doi: 10.1002/ijc.2910590215. [DOI] [PubMed] [Google Scholar]
- 48.Frank DA, Robertson MJ, Bonni A, Ritz J, Greenberg ME. Interleukin 2 signaling involves the phosphorylation of Stat proteins. Proc Natl Acad Sci U S A. 1995;92(17):7779–7783. doi: 10.1073/pnas.92.17.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sacchi M, Snyderman CH, Heo DS, Johnson JT, d’Amico F, Herberman RB, et al. Local adoptive immunotherapy of human head and neck cancer xenografts in nude mice with lymphokine-activated killer cells and interleukin 2. Cancer Res. 1990;50 (10):3113–3118. [PubMed] [Google Scholar]
- 50.Sacchi M, Vitolo D, Sedlmayr P, Rabinowich H, Johnson JT, Herberman RB, et al. Induction of tumor regression in experimental model of human head and neck cancer by human A-LAK cells and IL-2. Int J Cancer. 1991;47(5):784–791. doi: 10.1002/ijc.2910470527. [DOI] [PubMed] [Google Scholar]
- 51.Sabatino M, Kim-Schulze S, Panelli MC, Stroncek D, Wang E, Taback B, Kaufman HL, et al. Serum vascular endothelial growth factor and fibronectin predict clinic response to high dose interleukin-2 therapy. J Clin Oncol. 2009;27(16):2645–52. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue S, Branch CD, Gallick GE, Chada S, Ramesh R. Inhibition of Src kinase activity by Ad-mda7 suppresses vascular endothelial growth factor expression in prostate carcinoma cells. Mol Ther. 2005;12(4):707–15. doi: 10.1016/j.ymthe.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Zerbini LF, Czibere A, Wang Y, Correa RG, Otu H, Fisher PB, Libermann TA, et al. A novel pathway involving melanoma differentiation associated gene-7/interleukin-24 mediates nonsteroidal anti-inflammatory drug-induced apoptosis and growth arrest of cancer cells. Cancer Res. 2006;66(24):11922–31. doi: 10.1158/0008-5472.CAN-06-2068. [DOI] [PubMed] [Google Scholar]