Abstract
In urban areas with a predominance of early to mid-20th century housing stock, islands of children possessing blood lead levels (PbB) in excess of CDC guidelines (>10 μg/dL) exist. Many of these children are also exposed to environmental tobacco smoke (ETS). The current study examined the impact of Pb-exposure (PbB levels of 1-55 μg/dL) with/without concurrent ETS exposure on immune system function in 318 children aged 6-84 months from the urban area of Springfield-Greene County, Missouri. In this population, 36.5% of children possessed PbB levels >10 μg/dL, 62.9% of children came from smoking homes, 51.9% of children were under 2 years of age, and the population was WIC eligible and predominantly of white, non-Hispanic ethnicity. Multiple immune function markers including cell counts, IgE levels, sCD25 (sIL2R) and IL4 concentrations, and titers to common childhood immunizations were analyzed for correlation with Pb and/or ETS exposure. Increased IgE levels (p<0.01) were found in children with PbB levels within CDC Classes II-IV—this finding was primarily attributable to elevated IgE levels in the subpopulation of children with concurrent Pb and ETS exposure. A trend (0.05<p<0.01) of increases in % total T-cells (p=0.06) was also found in children with concurrent elevated PbB levels and ETS exposure. This trend was not found in the subset of children without ETS exposure nor was it present in the analysis of the entire population set. Conversely, alterations in median values for %Lymphocytes, % Granulocytes, and % Activated T-cells across Pb Classes were present in the subpopulation of children expose to Pb alone (without concurrent ETS exposure) though a clear trend was not evident. In the entire population set, a statistically significant correlation between ETS and PbB levels was found. This study indicates that prior reports of a correlation between elevated PbB levels and serum IgE levels may be strongly influenced by exposure to ETS. Findings from this study also indicate that Pb is an immune modulator and PbB levels may be influenced by ETS exposure.
Keywords: Pb-exposure, immune function, environmental tobacco smoke (ETS)
1. INTRODUCTION
Elevated PbB levels in children are linked to immunological, behavioral, and cognitive deficits (Min et al., 2009; Jones et al., 2009). Children between 12-24 months exhibit greater hand-to-mouth activity and thus are at increased risk of environmental Pb exposure from dust, soil, and water (Edwards et al., 2009; Gaitens et al., 2009). PbB levels have decreased among young children in the United States due to an effort to eliminate Pb sources in the environment. Jones et al (Jones et al., 2009) noted an 84% decrease across all ethnic groups in the number of U.S. children with PbB ≥ 10 between 1988-1991 and 1999-2004. However children living in housing built prior to 1950, common in poorer urban centers, are three times more likely to have PbB >5μg/dL (Dixon et al., 2009).
Pb-exposure suppresses specific lymphocyte cell populations (Karmaus et al., 2005; Lutz et al., 1994a), stimulates humoral immunity including B-cell proliferation (Massadeh et al., 2007; Lutz et al., 1999), and increases IgE levels (Lutz et al., 1994b; Lutz et al., 1999) in children. Significant increases in IgG, IgA, and IgM in children under age three with elevated PbB have been noted (Sarasua et al., 2000), but no relationship was found between elevated PbB levels and immunoglobulin levels for adults and children over 3 years of age. In previous studies of the Springfield population, Lutz et al. (1999) noted strong correlations between PbB and IgE (p = 0.0004) as well as significant differences in IgE levels across PbB risk classes (p = 0.05) among children aged 9 months to 6 years. Elevated PbB has been found to impair bronchial response in an IgE dependent manner (Min et al., 2008). Thus, the immunomodulatory effects of developmental Pb-exposure may predispose children to immune mediated respiratory diseases including asthma (Joseph et al., 2005).
Since cigarettes contain heavy metals such as cadmium and Pb, it has been recently suggested that exposure to environmental tobacco smoke (ETS) increases the level of Pb in the blood. An association (p = 0.047, OR = 2.87) between elevated PbB and parental smoking in the home has been observed (Friedman et al., 2005). These results were mirrored in a study of the correlation of hair Pb levels and number of smokers in the home (OR = 0. 229; 0.164–0.321 95% CI) (Ozden et al., 2007). In a study of urban children with a mean blood Pb level of 23.6 μg/dL, a lack of correlation between cotinine, a nicotine metabolite, and blood Pb was observed (Weaver et al., 1996). Our prior studies of the Springfield MO population (Lutz et al., 1994a) noted that children in higher PbB risk classes were more likely to come from a smoking home, and the average PbB level was higher in smoking versus non-smoking homes (12.05 μg/dL and 6.93 μg/dL, respectively).
The impact of ETS on the immunomodulatory effect of Pb-exposure in children has not been reported though ETS is frequently used as a covariate in data analysis. The current study examined the outcomes of a survey of the immune system of children from an urban population (Springfield MO) who were tested for environmental Pb exposure with or without concurrent ETS exposure. Three hundred eighteen children with blood Pb levels ranging from 1-55 μg/dL (62.9% with ETS exposure) were surveyed for markers of immune function including cell counts, IgE levels, and cytokine concentrations. A subset was also evaluated for possible effects on childhood immunization titers.
2. MATERIALS AND METHODS
2.1 Study population
The study population consisted of children aged 6 months to 6 years (<84 months) who were recruited through the Women, Infants, and Children (WIC) Nutritional Support Program and/or the Lead Poisoning Prevention Programs in Springfield-Greene County, MO. Subjects were recruited based on the result of a capillary blood sample testing for elevated PbB levels (≥10μg/dL). Due to possible false positive results from capillary sampling, a venous blood sample was drawn at the Public Health Dept within a few days to verify PbB levels, and children undergoing venous blood sampling were recruited for enrollment in the current study. Informed consent for participation was obtained from a parent or guardian according to NIH guidelines at the time of the venous sampling.
2.2 Sample collection
A blood sample of 8-10mL was taken via venipuncture with a 23 gauge needle and a 10ml uncoated syringe. The samples were immediately transferred to uncoated or heparin-coated Pb-free Vacutainer tubes (Becton-Dickinson) and stored at ambient air temperature. The samples were transported to St. John’s Regional Medical Center and the University of Missouri-Rolla on the same day for analysis (Lutz et al., 1999).
2.3 Blood Pb analysis
PbB levels were determined by graphite furnace atomic absorption spectrophotometry in the Environmental Laboratory at the Springfield-Greene Co. Department of Public Health. Each specimen was diluted tenfold in 5% Triton X solution and run in triplicate with samples of known Pb content (low, medium, and high; obtained from Wisconsin State Laboratory of Hygiene) (Lutz et al., 1999). Specimens were further diluted if absorbance values exceeded the calibrated range.
2.4 Cell Counts
A complete blood count was performed on heparinized blood at St. John’s Regional Medical Center to obtain percent lymphocytes, monocytes, and granulocytes.
2.5 Cytofluorimetry
Cytofluorimetry analysis determined percent T and B lymphocyte classes and various cell surface activation antigens (Lutz et al., 1999). The analysis was performed at the St. John’s Regional Medical Center’s CAP certified flow cytometry laboratory using a Becton-Dickinson FACS. Monoclonal antibody (mAb) reagents were used for immunophenotyping. The Simultest LeucoGATE reagent (Becton-Dickinson), which contained mAb to the CD45 human leukocyte antigen and CD14 monocyte antigen, was used to establish the optimal lymphocyte gate (Loken et al., 1990). Percent E-Rosette lymphocytes was determined using Coulter T11-RDI mAb (Bernard et al., 1984), percent T lymphocytes were analyzed with the B-D Leu 4-FITC mAb (Van Dongen et al., 1988), and percent B lymphocytes with the Coulter B4-FITC mAb (Reinherz et al., 1986). HLA-DR-BD’s phycoerythrin labeled anti-HLA-DR was used to determine the percent lymphocytes positive for the HLA-DR activation antigens (class II; on resting B and activated T cells) (Lampson and Levy, 1980); CD25-Coulter’s FITC labeled IL-2R1 mAb was used to determine percent CD25 (receptor for the cytokine interleukin 2, which is increased on activated T cells) (Fox et al., 1984). In addition, CD71 (transferrin receptor found on activated lymphocytes) was determined with CD71-Coulter’s FITC labeled T9 mAb (Sutherland et al., 1984), and percent CD28 (T lymphocyte cell adhesion molecule, which is a ligand for B7/BB-1) was determined with CD28-BD’s phycoerythrin labeled Leu 28 mAb (June et al., 1990). Non-specific binding was assessed with negative control mAbs of the specific subclasses.
2.6 Serum IgE
IgE levels were ascertained using microparticle enzyme immunoassays (MEIA) (IMX system, Abbott Laboratories) at St. John’s Regional Medical Center (Yman et al., 1981). Subclass-specific mouse monoclonal anti-human IgE was utilized to avoid cross-reactivity. The IMX system uses six standard calibrator solutions for human IgE, referenced against the WHO Second International Preparation. High (1200 IU/mL), medium (80 IU/mL), and low (1.5 IU/mL) controls were also included with each assay (Lutz et al., 1999).
2.7 Serum Rubella titers
A MEIA (IMX system, Abbott Laboratories) was used to assess the concentration of IgG antibodies directed against the specific immunogen. Externally referenced standard calibrator solutions and internal controls were also used with this assay.
2.8 Serum diphtheria and tetanus toxoid hemagglutination assay
Type “O” red blood cells were tanned in dilute tannic acid and conjugated with one of the specific toxoids or with phosphate buffered saline as a negative control. Diphtheria toxoid (1000 Lf/mL) and Tetanus toxoid (507 Lf/mL) were obtained from the Massachusetts State Health Department (Boston, MA) or Connaught Laboratories (Swiftwater, PA). Serum from children was serially diluted in 96-well round-bottomed microtiter plates from a 3-fold dilution to a 531,411 fold dilution (rows 1-12). Following addition of treated, tanned “O” blood cells samples were incubated for 1 hour at room temperature after which agglutination was determined. The maximum dilution at which agglutination occurred was recorded with expected values of >10,000-fold dilution. A known positive control (531,441-fold dilution) was obtained from Dr. Rebecca Buckley’s lab at Duke University Medical Center (Tiller and Buckley, 1978; Buckley and Dees, 1967).
2.9 Serum IL-4, soluble CD25, and soluble CD27
Cytokine interleukin 4 (IL4), soluble CD25 (receptor for IL-2), and CD27 levels were determined by ELISA (Human ELISA IL4 and CELLFREE Human ELISA sIL-2R kits, Endogen; soluble CD27 ELISA kits, Research Diagnostics, Inc) (Lutz et al., 1999). Standards, samples, and controls were added and another mAb to a second epitope on the protein was added (horseradish peroxidase conjugated). A colored product is formed in proportion to the amount of IL4, sCD25, or sCD27 present. Standards were run with each assay to prepare a standard curve used in interpolation, and controls were always included for quality control. The ELISAs were performed on serum samples frozen at −70°C.
2.10 Statistical analysis
Data were analyzed using SPSS 18.0 software. Spearman correlation coefficients were used to determine variation of the immune parameters with age. Assays showing p-values ≤ 0.05 were considered age-related and age was considered a covariate for analysis; all other parameters were grouped as non-age related without age as a covariate. Spearman correlation coefficients were then calculated to test for association with PbB (p-value ≤ 0.05 was considered significant) in children with/without ETS exposure. The equality of median values between PbB risk classes were tested using a Kruskal-Wallis test using residuals as data points for the entire population and subpopulations with/without concurrent environmental tobacco smoke exposure. The residuals were determined using a linear regression model with the immune system parameter as the dependent variable and PbB as the independent variable (age included for age-dependent variables). Due to the homogeneity of the population, inclusion/exclusion of covariates related to socioeconomic status (SES) and race did not alter the outcomes and thus data are reported as uncorrected. The Springfield population was predominantly white (89.3%) with all participants eligible for WIC and thus of low SES. The number of cigarettes smoked by the family with the child present (parental report of smoking behavior) was used as a covariate in the analysis of immune function in children with both ETS- and Pb-exposure.
3. RESULTS
The Springfield data were collected from 1992-1998. Participants were retrospectively assigned to PbB risk categories based on CDC guidelines from 1991 modified to include current levels of concern for cognitive impairment (Table 1). The cohort consisted of 318 children: 56.3% male and 43.7% female (Table 2A). More than half of the children were under 24 months of age (51.9%), with 38.4% in the 25-48 month age group, 8.2% in the 49-84 month age group, and 1.6% > 84 months. Of the 318 children, parental response to a survey question related to type/quantity of the child’s exposure to ETS was collected for 309 participants. Approximately two thirds (68.6%) of the children were from smoking homes and one third (31.4%) were from non-smoking homes. The population was ethnically homogeneous: 89.3% Caucasian, 6.0% African American, 2.2% Hispanic, 1.6% Asian, and 0.9% Native American.
Table 1. Risk classifications based on blood lead concentration (PbB)a.
| Class | Blood Pb (μg/dL) | Comment |
|---|---|---|
| IA | <5 | Not considered to be lead-poisoned |
| IB | 5-9 | Not considered to be lead-poisoned; potential cognitive impairment. |
| IIA | 10-14 | If many children in this range, community-wide childhood lead poisoning prevention activities triggered. Children in this range need frequent screening. |
| IIB | 15-19 | Nutritional and educational interventions and more frequent screening are necessary. If blood lead level persists in this range, an environmental investigation and intervention should be performed. |
| III | 20-44 | Environmental evaluation and remediation, and a medical evaluation are needed. Pharmacologic treatment of lead poisoning may be necessary. |
| IV | 45-69 | Medical and environmental interventions are needed, including chelation therapy. |
| V | >70 | This is considered to be a medical emergency. Medical and environmental management should begin immediately. |
Modified from the CDC statement on preventing lead poisoning in young children (1991)
Table 2A. Population demographics of children from Springfield, MO enrolled in the current study.
| Variable | Percent (%) a |
|---|---|
| Total n = 318 | |
| Gender | |
| Male | 56.3 |
| Female | 43.7 |
| Age (months) | |
| <25 | 51.9 |
| 25-48 | 38.4 |
| 49-84 | 8.2 |
| >84 | 1.6 |
| Race | |
| Caucasian | 89.3 |
| African American | 6.0 |
| Hispanic of Spanish descent | 2.2 |
| Asian or Pacific Islander | 1.6 |
| American Indian or Alaska Native | 0.9 |
Entire population WIC eligible, indicative of low SES
As shown in Table 2B, 15.8% of the participants were categorized as Class IA, 47.5% as Class IB, 24.9 % as Class IIA, 5.5% as Class IIB, and 5.8 % as Class III. When separated by parental report of smoking exposure, a shift towards higher PbB is evident for children from homes where parents smoke cigarettes. For children from smoking homes, 39.6% meet the classification of Pb-poisoned (Classes IIA, IIB, III, and IV) while 28.9% of children from non-smoking homes are classified as Pb-poisoned. A corresponding increase in mean PbB level was observed for children from smoking homes (p<0.03). A greater percentage of children from non-smoking homes (71.1%) were classified as non-Pb poisoned (CDC Class IA & IB) than children from smoking homes (59.9%). Together, these data support the general observation of higher PbB levels in children from smoking homes. A correlation of PbB and ETS exposure (reported as number of cigarettes smoked/day) was evident such that as ETS exposure increased, PbB increased in the study population (Table 2C; p=0.05).
Table 2B. PbB classification distribution, stratified by smoking status, of Springfield children included in the study.
| Pb Class | Total (n) |
Total (%) | ETS Exposed | |||
|---|---|---|---|---|---|---|
| 309 | 100 | No (n= 97) |
Percent (%)* |
Yes (n= 212) |
Percent (%)* |
|
| IA | 49 | 15.8 | 23 | 23.7 | 26 | 12.3 |
| IB | 147 | 47.5 | 46 | 47.4 | 101 | 47.6 |
| IIA | 77 | 24.9 | 19 | 19.6 | 58 | 27.3 |
| IIB | 17 | 5.5 | 2 | 2.1 | 15 | 7.1 |
| III | 18 | 5.8 | 7 | 7.2 | 11 | 5.2 |
| IV | 1 | <0.1 | 0 | - | 1 | <0.1 |
| Average PbB (μg/dL) (SEM)** |
9.23(0.33) | 8.62 (0.57) |
9.57 (0.42) |
|||
Percent of population non-ETS or ETS exposed calculated from total number of children in subgroup. Example: Of 97 non-ETS exposed children, 23 possessed PbB levels in Class IA for a percentage of 23.7% of non-ETS exposed children in Class IA
t-test for significance revealed significant difference between PbB values for non-smoking vs. smoking homes (p = 0.03)
Table 2C. A significant correlation of PbB level and ETS exposure exists for children from Springfield MO.
| Correlation coefficient (r) | p-value | |
|---|---|---|
| ETS and PbB | 0.113 | 0.05 |
A subset of data from 59 children without either Pb-exposure or ETS exposure (PbB <10 μg/dL; parental report of non-smoking home) was tested for dependence of variables on age since age of the child influences immune system maturity (Table 3). Children with either Pb-exposure and/or ETS exposure were excluded since both toxicants also modify immune system activity and maturation. The immune parameters identified as age-related were: % lymphocytes, IgE, sCD25 (sIL2R), and % granulocytes. The Rubella titer exhibited a trend toward age dependence. This may also be explained by time since immunization and was not included in the age-dependent parameters. All other immune measures were found to be non-age dependent.
Table 3. Spearman correlation between immune parameters and age for children without ETS exposure and PbB < 10μg/dL, a subset of the Springfield MO study population (n=59).
| Parameter | Age | |
|---|---|---|
| Correlation coefficient (ρ) |
p-value | |
| % Lymphocytes | −0.54 | <0.001** |
| IgE (IU/ml) | 0.61 | <0.001** |
| sCD25-sIL2R (IU/ml) |
−0.45 | <0.001** |
| % Granulocytes | 0.40 | 0.003** |
| Rubella titer | 0.22 | 0.07* |
| % Total T cells | −0.20 | 0.14 |
| % Activated Lymphocytes |
0.17 | 0.20 |
| % CD25+ | 0.17 | 0.21 |
| % E-Rosette (CD2+) |
−0.13 | 0.34 |
| % Activated T cells |
0.10 | 0.45 |
| sIL4 (pg/ml) | 0.13 | 0.47 |
| % Total B cells (CD19+) |
0.09 | 0.51 |
| % CD71+ | 0.09 | 0.52 |
| % CD28+ | −0.09 | 0.52 |
| % Monocytes | 0.07 | 0.60 |
| % CD27+ | −0.07 | 0.76 |
| Tetanus titer | 0.05 | 0.80 |
| Diptheria titer | −0.05 | 0.82 |
p < 0.05
0.05 < p < 0.10
The dataset was split into two subgroups for further analysis: children exposed to ETS or not exposed to ETS. A comparison of correlation of immune parameters and PbB in children from these subgroups is given in Table 4. Few correlations were evident in either group (age was included as a covariate for parameters identified from Table 3 as age-dependent). For the children without parental report of ETS exposure, the rubella titer response is positively correlated to PbB. The % Lymphocytes (p=0.08) exhibit a similar trend. These correlations and trends were not found in children with ETS exposure.
Table 4. Spearman correlation between immune parameters and blood lead levels (PbB): for children with and without ETS exposure.
| Parameter | Without ETS exposure | With ETS exposure | ||
|---|---|---|---|---|
| Correlation coefficient (ρ) |
p-value | Correlation coefficient (ρ) |
p-value | |
| Age Related | ||||
| % Lymphocytes |
−0.21 | 0.08* | −0.03 | 0.67 |
| IgE (IU/ml) | −0.08 | 0.50 | 0.12 | 0.24 |
| sCD25-sIL2R (IU/ml) |
−0.05 | 0.71 | 0.06 | 0.42 |
| % Granulocytes |
0.16 | 0.20 | 0.04 | 0.62 |
| % Total T cells | 0.09 | 0.43 | 0.06 | 0.42 |
| Non-Age Related | ||||
| % Activated Lymphocytes |
−0.08 | 0.46 | −0.002 | 0.98 |
| % CD25+ | −0.04 | 0.74 | 0.07 | 0.35 |
| % E-Rosette (CD2+) |
0.09 | 0.42 | 0.04 | 0.63 |
| % Activated T cells |
−0.08 | 0.50 | 0.04 | 0.65 |
| sIL4 (pg/ml) | 0.24 | 0.12 | 0.01 | 0.89 |
| % Total B cells (CD19+) |
−0.14 | 0.20 | 0.01 | 0.89 |
| % CD71+ | −0.13 | 0.26 | 0.06 | 0.45 |
| % CD28+ | 0.10 | 0.35 | −0.03 | 0.74 |
| % Monocytes | 0.004 | 0.98 | −0.07 | 0.34 |
| % CD27+ | 0.17 | 0.43 | −0.11 | 0.32 |
| Tetanus titer | 0.16 | 0.36 a | −0.09 | 0.42a |
| Diphtheria titer | 0.005 | 0.98a | −0.03 | 0.84a |
| Rubella titer | 0.29 | 0.04** a | −0.07 | 0.47a |
Time since immunization used as a covariate
p < 0.05
0.05 < p < 0.10
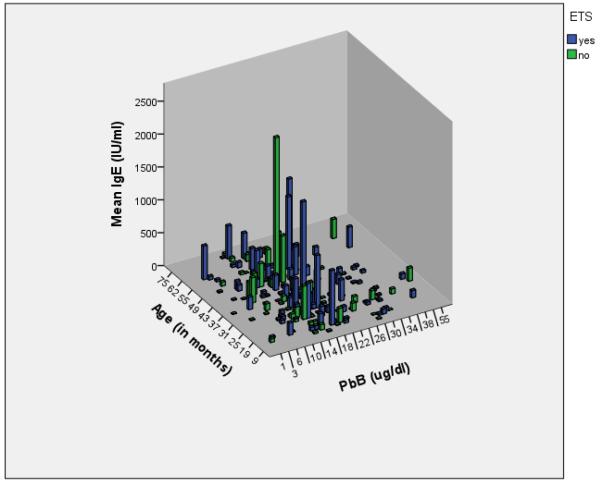
Estimations of the impact of Pb-exposure on median immune parameter values across Risk Categories were assessed for age-related immune parameters in the Springfield population (Table 5; Classes IIB-IV combined for analysis). Three separate population groups are shown (Entire, Table 5A; Pb+ETS, Table 5B; and Pb-only, Table 5C). In Table 5A, outcomes from the entire population of children enrolled in the current study including both ETS positive and ETS negative reported exposures are tabulated. A difference in medians across the PbB Classes for serum IgE levels was found with a generalized trend of increased median IgE levels in children considered Pb-poisoned as compared to children with Class IA or IB PbB levels. The association of individual IgE levels, Pb-level, and number of cigarettes reported by the parent is visible when the complete dataset is plotted according to age (Figure 1).
Table 5A. Effect of Pb on age related immune parameters; total population (ETS and non-ETS exposure).
| Parameter | Risk Class IA | Risk Class IB | Risk Class IIA | Risk Class IIB-IV | Kruskal p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | ||
| % Lymphocytes | 51.8 (22.8, 77.5) |
37 | 53.2 (17.2, 78.2) |
116 | 50.4 (10.5, 76.1) |
66 | 53.1 (29.8, 70.1) |
25 | 0.17 |
| IgE (IU/ml) | 13 (0.8, 892) |
51 | 12.6 (0, 2008) |
149 | 20.8 (0.4, 611.6) |
78 | 20.4 (3.6, 1756) |
35 | <0.01 |
| % Granulocytes | 41.3 (0, 69.8) |
37 | 39 (0, 73.9) |
115 | 41.4 (10.5, 80.9) |
66 | 40.7 (17.2, 66.8) |
25 | 0.78 |
Table 5B. Effect of Pb on age related immune parameters; subpopulation with ETS exposure.
| Parameter | Risk Class IA | Risk Class IB | Risk Class IIA | Risk Class IIB-IV | Kruskal p- value |
||||
|---|---|---|---|---|---|---|---|---|---|
| Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | ||
| % Lymphocytes | 50.6 (23.4, 77.5) |
21 | 51 (18.4, 78.2) |
76 | 51.5 (10.5, 72.1) |
52 | 52.3 (29.8, 70.1) |
20 | 0.95 |
| IgE (IU/ml) | 14.3 (0.8, 104) |
26 | 12 (0.0, 2008) |
98 | 22.1 (0.4, 611.6) |
58 | 21 (3.6, 1756) |
25 | 0.01 |
| % Granulocytes | 43.9 (0, 69.8) |
21 | 41.8 (0, 73.9) |
76 | 39.6 (10.5, 80.9) |
52 | 41.3 (17.2, 66.8) |
20 | 0.76 |
Table 5C. Effect of Pb on age related immune parameters; subpopulation without ETS exposure.
| Parameter | Risk Class IA | Risk Class IB | Risk Class IIA | Risk Class IIB-IV | Kruskal p- value |
||||
|---|---|---|---|---|---|---|---|---|---|
| Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | ||
| % Lymphocytes | 54.65 (22.8, 71.7) |
16 | 55.1 (17.2, 72.6) |
37 | 42.9 (17, 76.1) |
14 | 53.1 (30.9, 63.2) |
5 | 0.10 |
| IgE (IU/ml) | 12 (1.8, 892) |
23 | 14.3 (0.8, 1545) |
46 | 13.4 (1.6, 388) |
19 | 20.4 (3.8, 210) |
9 | 0.11 |
| % Granulocytes | 39.15 (21.5, 68.9) |
16 | 38.2 (11.1, 72.1) |
36 | 48.3 (17.5, 78.69) |
14 | 34.4 (31.5, 64.3) |
5 | 0.09 |
p < 0.05 Significant difference between means of risk groups.
0.05 < p < 0.10 Trend in differences between means of risk groups.
Figure 1.
Relationship between age-related serum IgE concentration, age in months, and PbB with ETS as a covariate.
In Table 5B, outcomes from age-related immune parameters for the children with reported ETS-exposure were tabulated with grouping according to PbB Risk Class. Median serum IgE levels were eIevated in children from Classes IIA-IV with concurrent ETS exposure as compared to children with low PbB levels (Class IA and IB) and ETS exposure (p=0.01). The correlation between PbB and IgE levels was not significant however (Table 4; p=0.24). The lack of correlation between the values reflects the high variability of this parameter across all PbB Classes due to the variable reactivity of the developing immune system.
In Table 5C, outcomes for the selected age-related immune parameters for the children without reported ETS-exposure are given. Median IgE levels across PbB Risk Classes IA, IB and IIA were not different. On first glance the median IgE level in Risk Classes IIB-IV (n=9) appears elevated but this finding was not significant due to the low number of children within the group and the high variability of the parameter. A trend in the median values for %Lymphocytes (p=0.10) and %Granulocytes (p=0.09) across the PbB Risk Classes was also observed for children with Pb-only exposure though this finding was not present in the total population nor in the subpopulation of children with Pb+ETS exposure. In comparison with Table 5A and 5B, it is apparent that the increased median IgE levels observed for Pb-poisoned children in the total population is driven by the ETS-exposed population subset. The limited number of children in Pb Class IIB-IV who are not ETS exposed likely reflects the observed association of ETS exposure and elevated PbB.
In Table 6, outcomes from the age-unrelated immune parameters are given. The outcomes for the total population are given in Table 6A. A lack of apparent impact of PbB on these immune parameters was noted. A trend towards an increase in median %Total T cells was found in Risk Classes IIA and IIB-IV for the Pb+ETS subpopulation (Table 6B) but not in the Pb-only subpopulation. Alterations in median %Activated T cells in children considered Pb-poisoned (Classes IIA-IV) (but without ETS exposure—Table 6C) were noted without a clear trend of impact but this outcome was not present in the total population nor the subpopulation with Pb+ETS exposure.
Table 6A. Effect of Pb on non-age related immune parameters; total population (ETS and non-ETS exposure).
| Parameter | Risk Class IA | Risk Class IB | Risk Class IIA | Risk Class IIB-IV | Kruskal p- value |
||||
|---|---|---|---|---|---|---|---|---|---|
| Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | ||
| % Total T cells | 69.2 (27.9, 84.4) |
43 | 70.2 (34.8, 90) |
124 | 73.55 (36, 85.2) |
68 | 68.8 (26.2, 90) |
26 | 0.20 |
| % Activated T cells |
3.3 (0, 40.4) |
43 | 2.85 (0.6, 27.3) |
124 | 3 (0.7, 17) |
68 | 3.5 (0.9, 65) |
26 | 0.16 |
Table 6B. Effect of Pb on non-age related immune parameters; subpopulation with ETS exposure.
| Parameter | Risk Class IA |
Risk Class IB |
Risk Class IIA |
Risk Class IIB-IV | Kruskal p- value a, b |
||||
|---|---|---|---|---|---|---|---|---|---|
| Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | ||
| % Total T cells | 69.2 (47, 84.4) |
23 | 69.4 (34.8, 86.8) |
81 | 73.95 (36, 85.2) |
52 | 71.3 (26.2, 89.1) |
18 | 0.06 |
| % Activated T cells |
3.2 (0, 16.8) |
23 | 2.8 (0.7, 27.3) |
81 | 3.35 (0.7, 17) |
52 | 3.2 (0.9, 65) |
18 | 0.42 |
Table 6C. Effect of Pb on non-age related immune parameters; subpopulation without ETS exposure.
| Parameter | Risk Class IA | Risk Class IB | Risk Class IIA | Risk Class IIB-IV | Kruskal p- value |
||||
|---|---|---|---|---|---|---|---|---|---|
| Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | ||
| % Total T cells | 69.3 (27.9, 79.8) |
19 | 72.2 (45.3, 90) |
39 | 72.2 (53.1, 83.1) |
15 | 65.5 (41, 84.3) |
7 | 0.34 |
| % Activated T cells |
3.5 (1.7, 40.4) |
19 | 3 (0.6, 26.9) |
39 | 2.6 (0.9, 16.8) |
15 | 5.2 (2.1, 23.1) |
7 | 0.06 |
p < 0.05 Significant difference between risk groups.
0.05 < p < 0.10 Trend in differences between means of risk groups.
The antibody production response triggered by childhood immunizations may also be affected by Pb- or ETS-exposure. As shown in Table 7, Rubella titers (corrected for time since immunization) for children from the study population were examined. For the complete population surveyed, median titer response between the PbB Classes did not vary. For the Pb+ETS-exposed subpopulation, significant decreases in median Rubella titers across Pb Classes were observed (p=0.03). For the Pb-only subpopulation, differences between Pb Class group medians for the Rubella titers (p=0.06), while suggestive, did not reach statistical significance and did not hold for Pb Class IIB-IV (n=9).
Table 7. Effect of Pb exposure on Rubella Titersa.
| Parameter | Risk Class IA | Risk Class IB | Risk Class IIA | Risk Class IIB-IV | Kruskal p- value |
||||
|---|---|---|---|---|---|---|---|---|---|
| Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | Median (Min, Max) |
N | ||
| Entire Population |
124.7 (0, 7500) |
51 | 79.3 (0, 500) |
149 | 58.75 (0, 7500) |
78 | 60.75 (0, 465.1) |
36 | 0.36 |
| Pb+ETS | 136.7 (5, 469) |
26 | 53.55 (0, 500) |
98 | 57.15 (0, 7500) |
58 | 35.8 (1, 425) |
26 | 0.03 |
| Pb only | 108.8 (0, 7500) |
23 | 99.1 (0, 500) |
46 | 87.3 (0, 500) |
19 | 173.6 (0, 465.1) |
9 | 0.06 |
Time since immunization as covariate.
p < 0.05 Significant difference between risk groups.
0.05 < p < 0.10 Trend in differences between means of risk groups.
Many immune response variables showed no statistically significant variations or trends within the total population or in the subpopulations of this study. Variations in median values across Pb Classes are given in Supplemental Table 1.
4. DISCUSSION
Pb-exposure induces neurocognitive impairment in children at low levels (< 5μg/dL) (Jedrychowski et al., 2009) as well as developmental delays (Jedrychowski et al., 2008), and has been shown to increase IgE levels leading to the development of asthma at low-to-moderate PbBs (Min et al., 2008; Blais, 2005). The current study focused on the modifications of immune function induced by Pb exposure with additional emphasis on the effect of concurrent ETS exposure. This study is a continuation of previous reports by Lutz et al. that describe outcomes prior to completion of recruitment/sample collection (first 45 months) in the Springfield MO cohort (Lutz et al., 1994a; Lutz et al., 1994b; Lutz et al., 1999).
Analyses performed with NHANES data indicate that ETS exposure increases PbB levels in both adults and children with the effect evident for lower PbB classifications (Mannino et al., 2003; Mannino et al., 2005). Willers et al. also reported a significant association between PbB in children and parental smoking, with a dose-response relationship between number of cigarettes smoked and PbB level (Willers et al., 1998). In the current study, as in the prior studies of the Springfield MO cohort, a significant correlation of PbB level and parental report of ETS exposure was found, though the magnitude of difference between the groups is less than in prior publications with the partial cohort. This is likely the result of two factors: 1) a gradual decline in the number of children with elevated PbB levels in this population due to active enforcement of Pb-containing paint abatement in rental housing by Springfield MO city government agencies; and 2) public health campaigns against smoking in the home or around children that were initiated during the course of this study. These factors resulted in lower overall PbB levels and less ETS exposure for children enrolled later in the study. The correlation of PbB and ETS exposure remained statistically significant throughout all reports from this cohort. Epidemiological studies, including those from the Springfield MO cohort, support an association of ETS exposure and low to moderate PbB levels in children.
It is not surprising that ETS exposure is associated with elevated PbB levels since cigarettes contain Pb, though the amount varies by brand. As an example, graphite furnace atomic absorption spectrometry revealed that sidestream smoke from a reference cigarette (1R4F) contained 43.8 ± 2.0 ng of Pb (Wagner et al., 2001). With smoke inhalation, absorption of Pb from particulate matter deposits in the bronchial tree is likely increased (Jedrychowski et al., 2006). Deposition of smoke contaminants on home surfaces, which are then ingested by young children with high hand-to-mouth activity, may also lead to Pb-exposure. As determined from parental reports of smoking behavior, approximately 42% of the children in the study were exposed to smoke from more than one-half pack of cigarettes per day. The number of cigarettes smoked around the child was included as a linear, rather than a categorical, variable in judging the impact of ETS on immune function. It is possible that underreporting of the number of cigarettes smoked around the child per day occurred in the study respondents as well as decreased parental attention to environmental conditions such as vigilance regarding Pb-contamination sources. Passive cigarette smoke exposure was assessed by parental response rather than by a biomarker such as cotinine since smoking patterns are fairly constant across time but biomarker response is dependent upon time since last exposure--a metric that was difficult to assess in the current population. It appears likely that PbB levels in children from the Springfield MO population are elevated due to a combination of sources of Pb contamination including paint, soil, water, and ETS exposure in many cases.
The immune system is developing in these children, resulting in a broad range of measures for each test. To examine whether increased PbB levels altered the distribution of immune parameter outcomes, the median values from risk categories were compared using the Kruskal-Wallis test with the covariates of age and number of cigarettes smoked around the child as appropriate. Though artificial compression of groups may mask statistically significant outcomes, we have chosen to combine the study cohort’s highest three exposure groups (Class IIB, III, and IV) for statistical analysis due to the small numbers of children in each of these groups. This resulted in a 4 group comparison of medians (Class IA, IB, IIA, Combined IIB-IV) descriptive of variations in immune function parameters associated with low to moderate Pb-exposure. The mean PbB level of the study population was centered near the division between non-Pb-poisoned and Pb-poisoned (Class IB and Class IIA).
The statistically significant correlation of IgE and PbB as well as the differences in IgE between PbB risk classes reported in the earlier study is confirmed in the larger Springfield cohort. This association is predominant in the children with concurrent Pb+ETS-exposure with a lesser trend also evident in the Pb-exposure only subgroup of this population. Lannerö et al. (2008) noted a dose-response relationship for ETS exposure and IgE sensitization (p = 0.019) and Krämer et al. (2004) noted a positive association between IgE and cotinine levels (nicotine metabolite; OR 1.39-1.62). Findings from the current study raise the question of whether the link between Pb-exposure and IgE levels is at least partially attributable to concurrent exposure to ETS. In previous studies, PbB has shown a positive correlation with IgE levels (Lutz et al., 1999; Sun et al., 2003) by acting upon IL4 synthesis (cytokine controlling immunoglobulin class switch to IgE production) and leading to an upregulation of IgE production by B cells (Heo et al., 2004).
Studies have also shown nicotine modulates the immune system through its effect on Th1, Th2, and B cells (Bendich et al., 1981; Jaremin, 1983; Kum-Nji et al., 2006; Shenker et al., 1997). Nicotine exhibits a suppressive effect on Th1 leading to a decrease in the production of the immunoglobulins IgA and IgG2 (Seymour et al., 2005; Zhang and Petro, 1996). Nicotine stimulates both Th2 and B cells; this results in a production of cytokines and interleukins (specifically IL4) by the Th2 cells and causes B cells to switch production of IgG1 to IgE (Fischer and Kö nig, 1994; Frazer-Abel et al., 2006; Holt, 1987). In conjunction, these effects manifest as an increased risk of allergies and asthma in ETS-exposed children. The stimulation of IgE production by ETS was also observed in our study. A stronger association of IgE and PbB levels for children exposed to ETS than for children without exposure to ETS was observed.
This study did not examine possible correlations of elevated PbB and ETS-exposure with allergy/asthma. Asthma in children is a heterogeneous syndrome with variable disease expression and response to environmental exposures. IgE triggers immediate-hypersensitivity reactions and late-phase responses with evidence that basal levels of IgE can augment humoral and cellular immune responses to allergens. Though elevation of total (and allergen specific) IgE levels are frequently associated with atopy and allergy, it has been difficult to demonstrate a precise role for IgE in asthma. IgE−/− mice do not exhibit increased inflammation of the bronchial mucosa or bronchial hyperresponsiveness following Aspergillus fumigatus challenge (Mehlhop et al., 1997). Allergic rhinitis, atopic dermatitis, and active anaphylaxis can also be triggered in IgE−/− mice (Oettgen et al., 1994; Miyajima et al., 1997). Activation and differentiation of CD4+ Th2 T cell dependent allergic responses are dependent on the enhanced production of the cytokines IL-4, IL-5, and IL-13 (Oettgen HC, and Geha RS., 1999) which leads to the production of IgE. Documentation of allergy/asthma was not attempted in the present study though measurement of serum IL-4 levels were measured in a subset of children in the latter stages of the study but without a finding of correlation with PbB-level or ETS-exposure.
The current study reports a magnification of effect by ETS exposure on Pb-exposure-associated elevation of IgE levels without a similar multiplicative effect on other immune parameters studied. Though an apparent correlation exists in this study, a clear cause and effect relationship has not been proven between PbB, ETS exposure, and IgE levels. The children in the current study were not undergoing chelation treatment and thus the impact of reduced Pb-levels (due to medical intervention) on IgE levels with/without ETS exposure could not be assessed. The relatively limited number of children enrolled in the Springfield cohort, coupled to the broad age ranges represented, was a barrier to analysis of associations of Pb and/or ETS with other markers of immune function.
Supplementary Material
Supplemental Table 1: Selected immune parameters not impacted by Pb Class or ETS exposure.
RESEARCH HIGHLIGHTS.
Pb levels associated with CSE.
Pb levels correlated with IgE
CSE influences Pb and IgE levels.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the contributions of the Springfield-Greene County Department of Public Health, the St. John’s Regional Medical Center Division of Immunology, and Lorena Canales for her contribution toward the statistical analyses. This work was supported by the following grants: R15 ES05369, R55 ESO6065 R01 ES06065, R21 DA027466, P30 ES014443, and P20 RR017702 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
University of Missouri-Rolla changed to Missouri University of Science and Technology in 2007.
6. REFERENCES
- Bernard A, Boumsel L, Dausset J, Milstein C, Schlossman SF, editors. Leucocyte typing. Springer; Berlin: 1984. [Google Scholar]
- Belisle EH, Strausser HR. Immune responses of rats chronically fed subclinical doses of lead. Clin Exp Immunol. 1981;43:189–194. [PMC free article] [PubMed] [Google Scholar]
- Blaiss MS. Epidemiology and pathophysiology of immunoglobulin E-mediated asthma. Allergy Asthma Proc. 2005;26:423–427. [PubMed] [Google Scholar]
- Buckley RH, Dees SC. Serum immunoglobulins. 3. Abnormalities associated with chronic urticaria in children. J Allergy. 1967;40:294–303. doi: 10.1016/0021-8707(67)90077-9. [DOI] [PubMed] [Google Scholar]
- Dixon SL, Gaitens JM, Jacobs DE, Strauss W, Nagaraja J, Pivetz T, Wilson JW, Ashley PJ. Exposure of U.S. children to residential dust lead, 1999-2004: II. The contribution of lead-contaminated dust to children’s blood lead levels. Environ Health Perspect. 2009;117:468–474. doi: 10.1289/ehp.11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M, Triantafyllidou S, Best D. Elevated blood lead in young children due to lead-contaminated drinking water: Washington, DC, 2001-2004. Environ Sci Technol. 2009;43:1618–1623. doi: 10.1021/es802789w. [DOI] [PubMed] [Google Scholar]
- Frazer-Abel AA, Baksh S, Fosmire SP, Willis D, Pierce AM, Meylemans H, Linthicum DS, Burakof SJ, Coons T, Bellgrau D, Modiano JF. Nicotine activates nuclear factor of activated T cells c2 (NFATc2) and prevents cell cycle entry in T cells. J Pharmacol Exp Ther. 2004;311:758–769. doi: 10.1124/jpet.104.070060. [DOI] [PubMed] [Google Scholar]
- Fischer A, König W. Modulation of in vitro immunoglobulin synthesis of human peripheral blood mononuclear cells by nicotine and cotinine. Clin Investig. 1994;72:225–232. doi: 10.1007/BF00189319. [DOI] [PubMed] [Google Scholar]
- Fox DA, Hussey RE, Fitzgerald KA, Actuo O, Poole CB, Palley L, Daley JF, Schlossman SF, Reinherz EL. Ta1, a novel 105 kd T cell activation antigen defined by monoclonal antibody. J Immunol. 1984;133:1250–1256. [PubMed] [Google Scholar]
- Friedman LS, Lukyanova OM, Kundiev YI, Shkiryak-Nizhnyk ZA, Chislovska NV, Mucha A, Zvinchuk AV, Oliynyk I, Hryhorczuk D. Predictors of elevated blood lead levels among 3-year-old Ukrainian children: a nested case-control study. Environ Res. 2005;99:235–242. doi: 10.1016/j.envres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Gaitens JM, Dixon SL, Jacobs DE, Nagaraja J, Strauss W, Wilson JW, Ashley PJ. Exposure of U.S. children to residential dust lead, 1999-2004: I. housing and demographic factors. Environ Health Perspect. 2009;117:461–467. doi: 10.1289/ehp.11917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo Y, Lee BK, Ahn KD, Lawrence DA. Serum IgE elevation correlates with blood lead levels in battery manufacturing workers. Hum ExperToxicol. 2004;23:209–213. doi: 10.1191/0960327104ht442oa. [DOI] [PubMed] [Google Scholar]
- Holt PG. Immune and inflammatory function in cigarette smokers. Thorax. 1987;42:241–249. doi: 10.1136/thx.42.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Flak E, Mróz E, Rauh V, Caldwell K, Jones R, Skolicki Z, Kaim I, Perera F. Exposure to environmental tobacco smoke in pregnancy and lead levels in maternal blood at delivery. Int J Occup Med Environ Health. 2006;19:205–210. doi: 10.2478/v10001-006-0034-5. [DOI] [PubMed] [Google Scholar]
- Jaremin B. Blast lymphocyte transformation (LTT), rosette (E-RFC) and leukocyte migration inhibition (MIF) tests in persons exposed to the action of lead during work. Report II. Bull Inst Marit Trop Med Gdynia. 1983;34:189–197. [PubMed] [Google Scholar]
- Jedrychowski W, Perera F, Jankowski J, Rauh V, Flak E, Caldwell KL, Jones RL, Pac A, Lisowska-Miszczyk I. Prenatal low-level lead exposure and developmental delay of infants at age 6 months (Krakow inner city study) Int J Hyg Environ Health. 2008;211:345–351. doi: 10.1016/j.ijheh.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Perera FP, Jankowski J, Mrozek-Budzyn D, Mroz E, Flak E, Edwards S, Skarupa A, Lisowska-Miszczyk I. Very low prenatal exposure to lead and mental development of children in infancy and early childhood: Krakow prospective cohort study. Neuroepidemiology. 2009;32:270–278. doi: 10.1159/000203075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, Brown MJ. Trends in blood lead levels and blood lead testing among US children aged 1 to 5 years, 1988-2004. Pediatrics. 2009;123:e376–385. doi: 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- Joseph CL, Havstad S, Ownby DR, Peterson EL, Maliarik M, McCabe MJ, Jr., Barone C, Johnson CC. Blood lead level and risk of asthma. Environ Health Perspect. 2005;113:900–904. doi: 10.1289/ehp.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Karmaus W, Brooks KR, Nebe T, Witten J, Obi-Osius N, Kruse H. Immune function biomarkers in children exposed to lead and organochlorine compounds: a cross-sectional study. Environ Health. 2005;4:5. doi: 10.1186/1476-069X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer U, Lemmen CH, Behrendt H, Link E, Schäfer T, Gostomzyk J, Scherer G, Ring J. The effect of environmental tobacco smoke on eczema and allergic sensitization in children. Br J Dermatol. 2004;150:111–118. doi: 10.1111/j.1365-2133.2004.05710.x. [DOI] [PubMed] [Google Scholar]
- Kum-Nji P, Meloy L, Herrod HG. Environmental tobacco smoke exposure: prevalence and mechanisms of causation of infections in children. Pediatrics. 2006;117:1745–1754. doi: 10.1542/peds.2005-1886. [DOI] [PubMed] [Google Scholar]
- Lampson L, Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980;125:293–299. [PubMed] [Google Scholar]
- Lannerö E, Wickman M, van Hage M, Bergström A, Pershagen G, Nordvall L. Exposure to environmental tobacco smoke and sensitisation in children. Thorax. 2008;63:172–176. doi: 10.1136/thx.2007.079053. [DOI] [PubMed] [Google Scholar]
- Loken M, Brosnan J, Bach B, Ault K. Establishing optimal lymphocyte gates for immunophenotypeing by flow cytometry. Cytometry. 1990;11:453–459. doi: 10.1002/cyto.990110402. [DOI] [PubMed] [Google Scholar]
- Lutz P, Jayachandran C, Gale N, Hewett J, Phillips P, Looney F, Bengsch H. Immunity in children with exposure to environmental lead: I. Effects on cell numbers and cell-mediated immunity. Environ. Geochem. Health. 1994a;16:167–77. doi: 10.1007/BF01747913. [DOI] [PubMed] [Google Scholar]
- Lutz P, Bauer S, Gale N, Hewett J, Phillips P, Looney F, Bengsch H. Immunity in children with exposure to environmental lead: II. Effects on humoral immunity. Environ. Geochem. Health. 1994b;16:179–189. doi: 10.1007/BF01747914. [DOI] [PubMed] [Google Scholar]
- Lutz PM, Wilson TJ, Ireland J, Jones AL, Gorman JS, Gale NL, Johnson JC, Hewett JE. Elevated immunoglobulin E (IgE) levels in children with exposure to environmental lead. Toxicology. 1999;134:63–78. doi: 10.1016/s0300-483x(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Albalak R, Grosse S, Repace J. Second-hand smoke exposure and blood lead levels in U.S. children. Epidemiology. 2003;14:719–27. doi: 10.1097/01.EDE.0000081998.02432.53. [DOI] [PubMed] [Google Scholar]
- Mannino DM, Homa DM, Matte T, Hernandez-Avila M. Active and passive smoking and blood lead levels in U.S. adults: data from the Third National Health and Nutrition Examination Survey. Nicotine Tob Res. 2005;7:557–564. doi: 10.1080/14622200500185264. [DOI] [PubMed] [Google Scholar]
- Massadeh AM, Al-Safi SA, Momani IF, Al-Mahmoud M, Alkofahi AS. Analysis of cadmium and lead in mice organs: effect of Nigella sativa L. (Black Cumin) on the distribution and immunosuppressive effect of cadmium-lead mixture in mice. Biol Trace Elem Res. 2007;115:157–167. doi: 10.1007/BF02686027. [DOI] [PubMed] [Google Scholar]
- Mehlhop PD, van de Rijn M, Goldberg AB, Brewer JP, Kurup VP, Martin TR, Oettgen HC. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci USA. 1997;94:1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JY, Min KB, Kim R, Cho SI, Paek D. Blood lead levels and increased bronchial responsiveness. Biol Trace Elem Res. 2008;123:41–46. doi: 10.1007/s12011-008-8099-6. [DOI] [PubMed] [Google Scholar]
- Min MO, Singer LT, Kirchner HL, Minnes S, Short E, Hussain Z, Nelson S. Cognitive development and low-level lead exposure in poly-drug exposed children. Neurotoxicol and Teratol. 2009;31:225–231. doi: 10.1016/j.ntt.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J Clin Invest. 1997;99:901–14. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- Oettgen HC, Geha RS. IgE in asthma and atopy: cellular and molecular connections. J Clin Invest. 1999;104:829–35. doi: 10.1172/JCI8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozden TA, Gökçay G, Ertem HV, Süoğlu OD, Kiliç A, Sökücü S, Saner G. Elevated hair levels of cadmium and lead in school children exposed to smoking and in highways near schools. Clin Biochem. 2007;40:52–56. doi: 10.1016/j.clinbiochem.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Reinherz EL, Haynes BF, Nadler LM, Berstein ID, editors. Leucocyte Typing II, vol 2. Springer; Berlin: 1986. [Google Scholar]
- Sarasua SM, Vogt RF, Henderson LO, Jones PA, Lybarger JA. Serum immunoglobulins and lymphocyte subset distributions in children and adults living in communities assessed for lead and cadmium exposure. J Toxicol Environ Health. 2000;60:1–15. doi: 10.1080/009841000156556. [DOI] [PubMed] [Google Scholar]
- Seymour BW, Peake JL, Pinkerton KE, Kurup VP, Gershwin LJ. Second-hand smoke increases nitric oxide and alters the IgE response in a murine model of allergic aspergillosis. Clin Dev Immunol. 2005;12:113–124. doi: 10.1080/17402520500116806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B, Matarazzo W, Hirsch R, Gray I. Trace metal modification of immunocompetence. I. Effect of trace metals in the cultures on in vitro transformation of B-lymphocytes. Cell Immunol. 1977;34:19–24. doi: 10.1016/0008-8749(77)90225-8. [DOI] [PubMed] [Google Scholar]
- Sun L, Hu J, Zhao Z, Li L, Cheng H. Influence of exposure to environmental lead on serum immunoglobulin in preschool children. Environ Res. 2003;92:124–128. doi: 10.1016/s0013-9351(02)00090-7. [DOI] [PubMed] [Google Scholar]
- Sutherland R, Delia D, Schneider C, Newman R, Kemshead J, Greaves M. Ubiquitous cell-surface glycoprotein on tumor cells is proliferation-associated receptor for transferrin. Proc Nat Acad Sci USA. 1981;78:4515–4519. doi: 10.1073/pnas.78.7.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiller TL, Jr, Buckley RH. Transient hypogammaglobulinemia of infancy: review of the literature, clinical and immunologic features of 11 new cases, and long-term follow-up. J Pediatr. 1978;92:347–353. doi: 10.1016/s0022-3476(78)80417-x. [DOI] [PubMed] [Google Scholar]
- Van Dongen JJ, Krissansen GW, Wolvers-Tettero IL, Comans-Bitter WM, Adriaansen HJ, Hooijkaas H, van Wering ER, Terhorst C. Cytoplasmic expression of the CD3 antigen as a diagnostic marker for immature T-cell malignancies. Blood. 1988;71:603–612. [PubMed] [Google Scholar]
- Wagner KA, McDaniel R, Self D. Collection and preparation of sidestream cigarette smoke for trace elemental determinations by graphite furnace atomic absorption spectrometry and inductively coupled plasma mass spectrometry. J AOAC Int. 2001;84:1934–1940. [PubMed] [Google Scholar]
- Weaver VM, Davoli CT, Murphy SE, Sunyer J, Heller PJ, Colosimo SG, Groopman JD. Environmental tobacco smoke exposure in inner-city children. Cancer Epidemiol Biomarkers Prev. 1996;5:135–137. [PubMed] [Google Scholar]
- Willers S, Schütz A, Attewell R, Skerfving S. Relation between lead and cadmium in blood and the involuntary smoking of children. Scand J Work Environ Health. 1988;14:385–389. doi: 10.5271/sjweh.1905. [DOI] [PubMed] [Google Scholar]
- Yman L, Roosdorp N, Schroder H, Andrae ML. Methods for the determination of IgE and allergen-specific antibodies. Excerpta Med; International Allergy Symposium; 1981.pp. 74–83. [Google Scholar]
- Zhang S, Petro TM. The effect of nicotine on murine CD4 T cell responses. Int J Immunopharmacol. 1996;18:467–478. doi: 10.1016/s0192-0561(96)00054-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Selected immune parameters not impacted by Pb Class or ETS exposure.