Abstract
Critically ill pediatric patients frequently receive prolonged analgesia and sedation to provide pain relief and facilitate intensive care therapies. Iatrogenic withdrawal syndrome occurs when these drugs are stopped abruptly or weaned too rapidly. We investigated the validity and generalizability of the Withdrawal Assessment Tool-1 (WAT-1) in children during weaning of analgesics and sedatives. Of 308 children initially supported on mechanical ventilation for acute respiratory failure, 126 (41%) from 21 centers (median age 1.6 years; interquartile range: 0.6–7.7 years) were exposed to 5 or more days of opioids. Subjects were assessed for withdrawal symptoms using the WAT-1, an 11-item (12-point) scale, from the first day of weaning from analgesia/sedation until 72 hours after the last opioid dose. 836 daily WAT-1 assessments were completed, with a median WAT-1 score of 2 (0–4) over 6 (3–9) days per subject. There were no significant differences in WAT-1 scores as a function of age. Factor analyses confirmed that motor-related symptoms and behavioral state accounted for the most variance in WAT-1 scores. Supporting construct validity, cumulative opioid exposures were greater [40.2 (19.7–83.4) vs. 17.6 (14.6–39.7) mg/kg, P=0.004], length of opioid treatment before weaning was longer [7 (6–11) vs. 5 (5–8) days, P=0.004], and length of weaning from opioids was longer [10 (6–14) vs. 6 (3–9) days, P=0.008] in subjects with WAT-1 scores ≥ 3 compared to subjects with WAT-1 scores < 3. The WAT-1 shows good psychometric performance and generalizability when used to assess clinically important withdrawal symptoms in pediatric intensive care and general ward settings.
Keywords: drug withdrawal symptoms, opioid analgesia, benzodiazepine, sedation
1. Introduction
Over 90% of infants and children supported on mechanical ventilation in the Pediatric Intensive Care Unit (PICU) setting receive some form of sedative therapy, most commonly, various combinations of opioids and benzodiazepines. [21;25] Although analgesia is required for pain management and sedation is often indicated in pediatric patients who are unable to understand the imperative nature of critical care instrumentation and immobility, analgesic and sedative use is associated with morbidity and mortality. [5]
Iatrogenic withdrawal can occur with the abrupt discontinuation or too rapid weaning of opioids and/or benzodiazepines. An estimated 10–34% of all PICU patients are at risk for iatrogenic withdrawal [16;22] and for those exposed to greater than 5 to 10 days of opioids and benzodiazepines the risk is between 50–100%. [12;17] Classic signs of opioid withdrawal include neurological excitability, gastrointestinal dysfunction, autonomic instability, and poor organization of sleep states. [2] Benzodiazepine withdrawal symptoms are similar but also include agitation, visual hallucinations, facial grimacing, small amplitude choreic or choreoathetoid movements, and seizures. [8;24]
The accurate assessment of iatrogenic opioid and benzodiazepine withdrawal is necessary for effective prevention and treatment but this remains challenging. [15] The lack of adequate measures of iatrogenic withdrawal and the need for such tools to guide pain and sedation therapy has been repeatedly highlighted. [2;11;15;18] Several clinical assessment tools for critically ill children have been proposed. [9;10;14] The Withdrawal Assessment Tool-1 (WAT-1 [9], Figure 1) was a significant improvement over previous withdrawal symptom assessment scales in that it has fewer items, each of which can be objectively measured. It is performed only twice a day compared with the usual 6 to 12 times per day for other symptom assessment scales, which increases the likelihood that it will be used in clinical practice. The assessment of the WAT-1 parameters is easily integrated into the standard start-of-shift nursing assessment without additional time. Training can be accomplished through brief written instruction and bedside demonstration. In the initial psychometric testing, with 1040 assessments [median 11 per patient; interquartile range (IQR): 6–16] in 83 PICU patients at 2 hospitals, a WAT-1 score ≥ 3 had high sensitivity (0.87) and specificity (0.88) to predict nurses' numeric rating scale (0–10) scores > 4. [9] Predictive validity was suggested by correlations between the WAT-1 score and total opioid dose exposure prior to weaning, length of therapy, and the number of days of weaning. There was a strong correlation between WAT-1 scores and nurses' clinical judgment of withdrawal symptoms.
Figure 1.
Withdrawal Assessment Tool (WAT-1)
Further research to support the validity, reliability, and generalizability of the WAT-1 is needed and cut-off values for the diagnosis of withdrawal and decisions about treatment would be clinically useful. Therefore, the purpose of the present investigation was to further evaluate the psychometric properties and generalizability of the WAT-1 in children recovering from acute respiratory failure during weaning of analgesics and sedatives in 22 PICUs.
2. Materials and methods
2.1 Study design
A multicenter prospective repeated measures study was conducted to evaluate the psychometric properties of the WAT-1. Psychometric evaluation included examining response distributions overall and by age, factor structure, and construct validity by comparing scores across groups that were expected to differ (known groups validity) and analyzing the association of scores with other clinical variables (e.g., amount of drug exposure and length of weaning) hypothesized to be indicative of withdrawal severity (concurrent and predictive validity).
2.2 Patient enrollment
The study was conducted during the baseline, pre-randomization phase of the Randomized Evaluation of Sedation Titration fOr Respiratory FailurE (RESTORE) clinical trial which is designed to test a sedation management protocol in pediatric patients aged 2 weeks to 18 years intubated and supported on mechanical ventilation for acute respiratory failure. Patients were excluded from RESTORE if the primary reason for ventilation was cyanotic heart disease, immediate post-operative care, neuromuscular respiratory failure, or if care was considered futile by the child's family and medical team. During the baseline, pre-randomization phase of RESTORE (January to July 2009), all subjects received usual care in the 22 participating centers. Consent for data collection was obtained from all parents/guardians. Institutional Review Board approval was obtained for the use of baseline data for this study.
All subjects exposed to 5 or more days of continuous infusions, intermittent doses, or as needed bolus doses of opioids were to be assessed for withdrawal symptoms twice daily at 8 AM and 8 PM (and at other times if clinically indicated) from the day that opioid weaning started until 72 hours after the last opioid dose, regardless of benzodiazepine taper. The highest daily WAT-1 score was used in analyses. Subjects exited the study after hospital discharge or after 28 days.
2.3 Instrument description
The 11-item (12-point) WAT-1 consists of 1) a review of the patient's record for the past 12 hours, 2) direct observation of the patient for two minutes, 3) patient assessment using a progressive stimulus [4] routinely performed to assess level of consciousness, and 4) assessment of post-stimulus recovery as described elsewhere [9]. The WAT-1 was designed to be incorporated into normal shift assessments and care.
Train-the-trainer methods were used to instruct nurses in the 22 participating PICUs on the use of the WAT-1. Local nurse champions were trained first, and they then trained all bedside nurses in their respective PICUs. Training consisted of a didactic review of the data collection instrument and training videotapes, followed by completion of a post-test. Inter-rater reliability was established before and during the baseline phase of the RESTORE trial, with each site completing 3 rounds of testing during which at least 5 pairs of nurses performed simultaneous WAT-1 assessments. A total of 420 WAT-1 paired assessments were recorded to assess inter-rater reliability. The overall concordance rate for WAT-1 score < 3 versus WAT-1 score ≥ 3 was 97.4%. The Spearman rank correlation coefficient between simultaneous WAT-1 scores was 0.93 (P<0.001) and both nurses recorded identical scores for 83.1% of the pairs.
2.4 Additional data
Demographic and clinical data included age, race, ethnicity, mortality risk (Pediatric Risk of Mortality III–12 score) [19], Pediatric Cerebral Performance Category and Pediatric Overall Performance Category [7], cumulative and peak daily opioid dosage (morphine equivalents per kg of body weight), cumulative and peak daily benzodiazepine dosage (midazolam equivalents per kg of body weight), and administration of any other analgesia, sedation, or psychoactive medications. Definitive data in critically ill pediatric patients comparing analgesics and sedatives with regard to their potency or side effects are limited. However, based on several sources, the RESTORE Investigative Team compiled a relative potency scale for opioids and benzodiazepines [3;20;23;26]. All opioids were converted to morphine equivalents using the following conversions to equal 1 mg of morphine sulfate: 0.3 g remifentanil, 15 g fentanyl citrate, 0.15 mg hydromorphone hydrochloride, 0.3 mg methadone hydrochloride, 3 mg oxycodone, or 20 mg codeine phosphate. All benzodiazepines were converted to midazolam equivalents using the following conversions to equal 1 mg of midazolam: 0.2 mg clonazepam, 0.3 mg lorazepam, or 2 mg diazepam. Length of mechanical ventilation, length of PICU stay, and length of hospital stay were calculated at discharge or after 28 days.
2.5 Data analysis
Descriptive statistics were calculated, including means, standard deviations, medians, and interquartile ranges for continuous variables and frequency counts and percentages for categorical variables. Data were examined for skewness, outliers, and systematic missing data. The start of the opioid weaning period was defined as the first day of a decrease in daily dosage of ≥ 10% after 5 consecutive days of opioid treatment, the start of the benzodiazepine weaning period was defined as the day after the peak benzodiazepine dose, and the length of the preweaning and weaning phases of opioid and benzodiazepine therapy were computed to study discharge or to 28 days. Analyses comparing groups were conducted using the van Elteren test, an extension of the nonparametric Wilcoxon test that allows stratification by site, for continuous variables, Generalized Linear Mixed Models (GLMMs) including random effects for site for categorical variables, and proportional hazards regression using robust sandwich variance estimation to account for clustering within site for time to event variables. For analyses that included multiple observations on the same subject, we used GLMMs to account for intracluster correlation of data within site and within subject.
Confirmatory factor analyses were performed using principal components analysis with varimax rotation to examine the structural validity of the WAT-1. Based on a scree plot of initial eigenvalues, we examined 3-factor and 4-factor solutions for the total data set containing all assessments and for each age group separately. A factor loading of 0.4 was used as the threshold for inclusion of a symptom in a factor.
Construct validity of the WAT-1 was evaluated by comparing subjects who ever had a WAT-1 score ≥ 3 versus those with lower scores with respect to other indicators of the likelihood of withdrawal, including analgesia and sedative treatment during weaning, peak and cumulative opioid and benzodiazepine exposure, and the duration of the preweaning and weaning phases. As the data distributions were often skewed for these variables, we also calculated Spearman rank correlation coefficients between the peak WAT-1 scores per subject and these indicators of the likelihood of withdrawal.
Factor analyses were performed using SPSS (PASW Statistics 18, SPSS, Inc., Chicago, IL). All other analyses were performed using SAS (Version 9.2, SAS Institute, Inc., Cary, NC).
3. Results
3.1 Patient characteristics
Of 348 parents/guardians of eligible children approached for the study, 40 (11%) refused and 308 (89%) gave consent for their child to be enrolled. Of 308 enrolled children [median (IQR): 1.5 (0.4–7.6) years] supported on mechanical ventilation for acute respiratory failure in the 22 centers, 206 (67%) were exposed to 5 or more consecutive days of opioids. Children were excluded from the analysis because they died (n=3) or weaning was never commenced during the study period (n=16). Weaning was commenced in 187 (61%) children but a further 61 children were excluded because no WAT-1 assessments were completed after the start of weaning, resulting in a final sample of 126 children from 21 sites. Clinical and demographic characteristics of the study subjects are shown in Table 1. The majority of subjects were less than 2 years of age and ventilated for pneumonia or bronchiolitis for a median of 9.4 days (IQR: 5.9–13.3 days).
Table 1.
Demographic and clinical characteristics
| Results (N = 126) | |
|---|---|
| Age (median; IQRa) | 1.6 years; 0.6–7.7 years |
| Age group (N; %) | |
| 2 weeks-1.99 years | 66; 52% |
| 2.00–5.99 years | 23; 18% |
| 6.00+ years | 37; 29% |
| Gender (N; % male) | 64; 51% |
| Race (N; %) | |
| White | 88; 70% |
| Black/African American | 26; 21% |
| Other | 12; 10% |
| Ethnicity (N; %) | |
| Hispanic/Latino | 22; 18% |
| Not Hispanic/Latino | 101; 82% |
| Admission PCPCb > 1 (N; %) | 33; 26% |
| Admission POPCc > 1 (N; %) | 38; 30% |
| PRISM III-12d score (median; IQR) | 6.5 (3–12) |
| Risk of mortality (median; IQR) | 3% (1%–12%) |
| Primary reason for ventilation (N; %) | |
| Pneumonia | 47; 37% |
| Bronchiolitis | 32; 25% |
| Acute respiratory failure related to sepsis | 12; 10% |
| Asthma or reactive airway disease | 11; 9% |
| Pulmonary edema | 5; 4% |
| Thoracic trauma | 5; 4% |
| Aspiration | 4; 3% |
| Laryngotracheobronchitis (croup/trachetis) | 3; 2% |
| Other | 7; 7% |
| Length of mechanical ventilation (median; IQR) | 9.4 days; 5.9–13.3 days |
| Length of PICU stay (median; IQR) | 13.7 days; 9.6–21.4 days |
| Total length of stay (median; IQR) | 22 days; 15–28 days |
Interquartile range
Pediatric Cerebral Performance Category
Pediatric Overall Performance Category
Pediatric Risk of Mortality Version III using data collected within 12 hours of PICU admission
The 126 children had a total of 836 daily withdrawal symptom assessments completed after the start of weaning, with a median (IQR) WAT-1 score of 2 (0–4) over 6 (3–9) days per subject. The median (IQR) peak WAT-1 score per subject was 4 (3–6). In the PICU, the WAT-1 was recorded once daily for 23% of the days, twice daily for 29% of the days, and more than twice daily for 48% of the days. The WAT-1 scores were higher for assessments performed in the PICU [n=622; median (IQR): 2 (1–4)] compared with assessments performed after children were transferred to the general pediatric wards [n=214; 1 (0–2)].
3.2 Characteristics of analgesic/sedative administration and use of adjunctive agents
Characteristics of opioid and benzodiazepine exposure and weaning are shown in Table 2. All children received continuous infusions and/or scheduled intermittent doses of opioids for a median of 7 (IQR: 5–11) days and benzodiazepines for a median of 6 (IQR: 4–9) days prior to weaning. The median length of weaning was 9 days for both classes of drugs.
Table 2.
Opioid and benzodiazepine exposure and weaning
| Overall N = 126 | WAT-1 ever ≥ 3 N = 97 | WAT-1 always > 3 N = 29 | P-Value | |
|---|---|---|---|---|
| Median (IQRa) | ||||
| Preweaning opioid treatment cumulative dose (mg/kg)b | 22.8 (12.2–46.1) | 26.9 (13.4–47.2) | 15.0 (11.1–25.4) | 0.06 |
| Preweaning benzodiazepine treatment cumulative dose (mg/kg)c | 20.1 (7.2–44.8) | 24.7 (9.1–49.1) | 10.8 (5.0–22.4) | 0.03 |
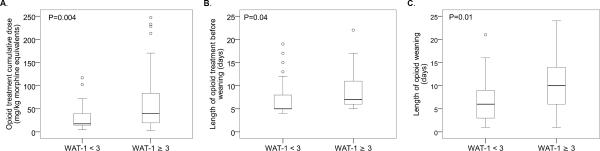
| Opioid treatment cumulative dose (mg/kg)b | 33.8 (17.4–74.3) | 40.2 (19.7–83.4) | 17.6 (14.6–39.7) | 0.004 |
| Benzodiazepine treatment cumulative dose (mg/kg)c | 27.4 (12.6–57.7) | 32.3 (15.9–65.7) | 18.0 (8.4–24.5) | 0.009 |
| Peak opioid dose (mg/kg)b | 5.1 (3.1–8.5) | 5.4 (3.1–8.8) | 4.1 (3.1–5.5) | 0.07 |
| Peak benzodiazepine dose (mg/kg)c | 4.3 (2.2–7.6) | 5.2 (2.6–9.3) | 3.0 (1.8–5.1) | 0.09 |
| Length of opioid treatment before weaning (days) | 7 (5–11) | 7 (6–11) | 5 (5–8) | 0.004 |
| Length of benzodiazepine treatment before weaning (days) | 6 (4–9) | 7 (5–9) | 5 (3–7) | 0.04 |
| Length of opioid weaning (days) | 9 (5–13) | 10 (6–14) | 6 (3–9) | 0.008 |
| Length of benzodiazepine weaning (days) | 9 (4–14) | 10 (5–14) | 6 (3–11) | 0.01 |
Interquartile range
Morphine equivalents
Midazolam equivalents
During their study course, 99 subjects (79%) received nonopioid/nonbenzodiazepine analgesic or sedative drugs that might influence total opioid exposure or response to weaning [5] as shown in Table 3. Prior to the start of weaning, 62 subjects (49%) received dexmedetomidine, ketamine, chloral hydrate, pentobarbital, propofol, and/or clonidine. The 62 children who received one or more of these six drugs prior to weaning did not have higher (≥ 3) WAT-1 scores or higher peak WAT-1 scores compared with the 64 children who were not given any of these drugs.
Table 3.
Nonopioid/nonbenzodiazepine analgesic or sedative drug use
| N subjects; % | N study days | |
|---|---|---|
| Dexmedetomidine | 46; 37% | 263 |
| Acetaminophen | 34; 27% | 107 |
| Ketamine | 32; 25% | 100 |
| Chloral hydrate | 26; 21% | 118 |
| Diphenhydramine | 23; 18% | 78 |
| Propofol | 21; 17% | 29 |
| Pentobarbital | 19; 15% | 82 |
| Clonidine | 17; 13% | 116 |
| Ibuprofen | 9; 7% | 26 |
| Ketorolac | 5; 4% | 10 |
| Phenobarbital | 4; 3% | 36 |
| Pentothal sodium | 4; 3% | 6 |
| Nalbuphine hydrochloride | 1; 1% | 6 |
| Gabapentin | 1; 1% | 4 |
| Naloxone hydrochloride | 1; 1% | 2 |
| Sertraline | 1; 1% | 1 |
We further examined the effects of clonidine during the weaning period because of its use as a second line agent for treatment of opioid withdrawal. [13] Eight children (6%) received clonidine for at least 50% of their weaning period, and they had higher peak WAT-1 scores [median (IQR): 7 (4.5–8.5) vs. 4 (3–6), P=0.003], higher cumulative opioid [50.5 (31.9–77.4) vs. 31.0 (16.5–74.3) mg/kg, P=0.02] and benzodiazepine [46.4 (23.8–85.7) vs. 25.6 (11.6–54.5) mg/kg, P=0.003] exposure, and longer lengths of weaning from opioids [14.5 (10.5–17.5) vs. 9 (4–13) days, P=0.04] and benzodiazepines [14 (12–17) vs. 9 (4–13) days, P=0.01] compared to the 118 children who were not given clonidine for at least 50% of their weaning period.
3.3 Factor structure
Motor-related symptoms and behavioral state accounted for the most variance in WAT-1 scores. A four-factor solution provided the best overall conceptual fit, explaining 56% of the variance in analysis of all WAT-1 assessments (Table 4). Motor-related symptoms (tremor, startle, uncoordinated/repetitive movements, and muscle tone) and yawning/sneezing comprised the factor that accounted for the most variance (25%). The second factor, accounting for 12% of the variance, was comprised of behavioral state (return to calm state and pre-stimulus state) and sweating, the third factor (10% of the variance) was comprised of gastrointestinal symptoms (vomiting and stooling), and the fourth factor (9% of the variance) was comprised of temperature and yawning/sneezing. Although yawning/sneezing had the lowest factor loadings, WAT-1 scores were significantly higher when yawning/sneezing occurred [median (IQR): 4 (3–6)] than when absent [1 (0–3), P<0.001], and therefore yawning/sneezing was retained in the factors.
Table 4.
Factor loadings for the main components of the WAT-1 (all age groups)
| Factor 1: Motor-related and yawning/sneezing | Factor 2: Behavioral state | Factor 3: Gastrointestinal | Factor 4: Temperature and yawning/sneezing | |
|---|---|---|---|---|
| Tremor | 0.71 | |||
| Startle to touch | 0.69 | |||
| Uncoordinated/repetitive movements | 0.63 | |||
| Muscle tone | 0.59 | |||
| Yawning or sneezing | 0.43 | 0.46 | ||
| Time to gain calm state: ≥ 2 minutes | 0.76 | |||
| SBS ≥ +1 or awake distressed | 0.69 | |||
| Any sweating | 0.53 | |||
| Any vomiting/wretching/gagging | 0.73 | |||
| Any loose/watery stools | 0.71 | |||
| Temperature > 37.8°C | 0.83 |
Although there were no significant differences in WAT-1 score as a function of age group, there were some differences in the frequency of individual symptoms as a function of age group (Table 5). The components of the factor solutions varied slightly by age group and explained a total of 55% (2 weeks-1.99 years), 62% (2.00–5.99 years), or 59% (6.00+ years) of the variance. Motor-related symptoms comprised the first factor for each age group, explaining 24%, 29%, and 25% of the variance, respectively.
Table 5.
Percent occurrence of withdrawal symptoms by age group
| Observation | Overall N = 836 | 2 wks-1.99 yrs N = 450 | 2.00–5.99 yrs N = 154 | 6.00+ yrs N = 232 | P-Valuea |
|---|---|---|---|---|---|
| From patient record, previous 12 hours | |||||
| Any loose/watery stools | 33 | 36 | 32 | 30 | 0.82 |
| Any vomiting/wretching/gagging | 14 | 17 | 5 | 14 | 0.08 |
| Temperature > 37.8°C | 15 | 12 | 6 | 27 | 0.001 |
| 2-minute pre-stimulus observation | |||||
| State behavior: SBSb ≥ +1 or awake distressed | 29 | 31 | 29 | 25 | 0.46 |
| Any sweating | 19 | 17 | 19 | 24 | 0.38 |
| Yawning or sneezing 2 or more times | 8 | 10 | 7 | 4 | 0.28 |
| Tremor: moderate/severe | 11 | 10 | 16 | 11 | 0.92 |
| Uncoordinated/repetitive movements: moderate/severe | 12 | 12 | 16 | 11 | 0.92 |
| 1-minute stimulus observation | |||||
| Startle to touch: moderate/severe | 16 | 21 | 15 | 7 | 0.002 |
| Muscle tone: increased | 19 | 24 | 20 | 10 | 0.01 |
| Post-stimulus recovery (time to gain calm state) | 0.25 | ||||
| 2–5 minutes | 19 | 24 | 18 | 12 | |
| > 5 minutes | 13 | 13 | 11 | 14 | |
| Peak WAT-1c, median (IQRd) | 2 (0–4) | 2 (1–4) | 1 (0–4) | 1 (0–3) | 0.11 |
Adjusted for clustering of observations within site and subject, N = 836 assessments
State Behavioral Scale
Withdrawal Assessment Tool-1
Interquartile range
3.4 Construct validity
3.4.1 High versus low WAT-1 scores
There were significant differences in the analgesia profiles of the 97 children (77%) who ever had a WAT-1 score ≥ 3 compared to those with lower scores, supporting construct validity. Subjects with higher WAT-1 scores (≥ 3) had greater cumulative opioid exposure, longer duration of opioid treatment before weaning, and longer duration of weaning from opioids compared to those whose symptoms were less severe (WAT-1 < 3; Table 2 and Figure 2). Similarly, subjects who ever had a WAT-1 score ≥ 3 had greater benzodiazepine exposure, longer duration of benzodiazepine treatment before weaning, and longer duration of weaning from benzodiazepines compared to subjects with WAT-1 scores < 3. Subjects with higher WAT-1 scores (≥ 3) also had a longer PICU length of stay [15.0 (10.4–22.3) vs. 10.9 (7.5–17.2) days, P=0.004] than those with WAT-1 scores < 3. The two groups were similar in terms of demographic characteristics.
Figure 2.
Boxplots of opioid exposure and weaning by Withdrawal Assessment Tool-1 (WAT-1) scores, comparing subjects who ever had a WAT-1 score ≥ 3 vs. those with lower scores
Peak WAT-1 scores for each subject correlated moderately with total cumulative opioid exposure (r=0.23, P=0.009), cumulative benzodiazepine preweaning (r=0.30, P<0.001) and total (r=0.33, P<0.001) exposure, benzodiazepine peak dose (r=0.31, P<0.001), length of opioid (r=0.20, P=0.02) and benzodiazepine (r=0.18, P=0.04) therapy before weaning, and length of opioid (r=0.33, P<0.001) and benzodiazepine (r=0.29, P=0.001) weaning.
3.4.2 Incidence of clinically significant iatrogenic withdrawal
Fifty-one episodes of clinically significant iatrogenic withdrawal were reported in 21 subjects. For the purposes of the RESTORE trial, these episodes were defined as “any patient receiving rescue therapy (defined as an opioid or benzodiazepine bolus or an increase in opioid or benzodiazepine infusion) to manage an increase in WAT-1 symptoms after the start of weaning (not for treatment of new pain or new sedation needs).” The median (IQR) WAT-1 score before rescue therapy was 6 (4–8) versus 2 (1–3) after rescue therapy (Wilcoxon signed rank test P<0.001).
4. Discussion
This study confirms and extends the preliminary psychometric evaluation of the WAT-1 [9] and provides strong evidence for its generalizability when used to assess opioid and benzodiazepine withdrawal symptoms in children. The WAT-1 demonstrated feasibility and utility as evidenced by its successful implementation and use by nurses in major pediatric centers across the USA.
We note that analgesia and sedation practices differed between the previous and current WAT-1 validation studies, with subjects in this study receiving approximately a 30% smaller cumulative and peak opioid dose and a 14% larger cumulative and 30% larger peak benzodiazepine dose. Despite the lower opioid exposure, more subjects had high WAT-1 scores (≥ 3) in the current study compared to the previous study (77% vs 64%). Length of treatment and weaning were within 10–20% of values between the two studies. The greatest difference in practice was in the use of other analgesic and sedative agents. In the previous WAT-1 study, 39% of patients received 1–3 nonopioid/nonbenzodiazepine drugs, principally ketamine and/or chloral hydrate. In the current study, 79% of patients received a wide range of other psychoactive drugs during their course, with 49% of patients receiving dexmedetomidine, ketamine, chloral hydrate, pentobarbital, propofol, and/or clonidine prior to opioid and benzodiazepine weaning. Use of clonidine has been suggested to reduce withdrawal intensity or duration of weaning in adults [13].It is plausible that patients would be more likely to receive clonidine if caregivers either observed them to have withdrawal signs or if the caregivers believed that the patients were at increased risk for withdrawal. However, further research is needed comparing the efficacy of clonidine with alternative treatments in reducing withdrawal symptoms in critically ill children.
Despite the differences in analgesia, sedation, and withdrawal symptom management practices, the factor structure and construct validity reported in the original WAT-1 validation were largely confirmed, with a few minor differences, which may be related to the performance of the WAT-1 in relation to age, frequency of symptom occurrence, or clinical practices. For example, although many group comparisons of cumulative exposure, length of preweaning exposure, and length of weaning showed statistically significant differences in withdrawal symptoms, the peak opioid dose showed only marginal differences. The small variations in symptom presentation due to age do not warrant separate weighting of items because that would diminish the clinical utility of the tool. Furthermore, the originally proposed cut-off score of WAT-1 ≥ 3 appears to be a reasonable designation of clinically significant symptoms as evidenced by the analyses presented.
The Neonatal Abstinence Score (NAS) was first shown to reduce the treatment time for neonates with prenatal drug exposure [6] and demonstrated the superiority of an assessment tool over subjective clinical assessment. The WAT-1 provides such an assessment for children experiencing iatrogenic withdrawal in either the intensive care or general ward setting. Therefore, withdrawal symptom assessment using WAT-1 should now be recommended for any child with risk factors for iatrogenic withdrawal. [1]
Important questions remain regarding the characterization of iatrogenic withdrawal risk factors, the differential symptom profile related to opioid versus benzodiazepine exposure, and the optimal speed of weaning and symptom treatment. Although these data were collected during the baseline, pre-randomization phase of a clinical trial, initial WAT-1 scores may have influenced the weaning process and thus impacted later scores. Clinical trials to reduce iatrogenic withdrawal and improve outcomes are needed. These trials should investigate the relative benefits and burdens of different analgesia and sedation regimens and should use WAT-1 assessment as a standard measure of symptom severity to determine effectiveness of symptom reduction so that results can be compared across studies. Using the WAT-1 with different analgesia and sedation regimens will also enable further testing of its validity, reliability, and generalizability. Individualized management of patient symptoms using WAT-1 may then be recommended, with the treatment titrated to the patient's change in responses over time.
The findings from the present study should be considered in light of the continued limited understanding of the mechanisms of iatrogenic opioid and benzodiazepine withdrawal and the continued lack of physiological biomarkers for withdrawal, pain, or hyperalgesia in children against which to compare the clinically observed symptoms. Research on this topic is further hampered by lack of pediatric data regarding opioid and benzodiazepine conversions. What little is known is extrapolated from adult data and based on single-dose ratios that are not corrected for tolerance. These are all important topics for future research. The concomitant administration of opioids and benzodiazepines also limits the interpretation of the findings, but reflects current clinical practice. Nevertheless, the present study was conducted within the context of a large scale trial in which the highest levels of design and methodological rigor were maintained.
In summary, iatrogenic withdrawal continues to be a common and clinically significant side effect of prolonged analgesia and sedation in critically ill pediatric patients. The WAT-1 shows good psychometric performance and generalizability when used to assess clinically important withdrawal symptoms in the pediatric intensive care unit setting. The WAT-1 has the advantage of rapid twice-daily assessment as opposed to more lengthy and frequent assessments required by other tools.
Acknowledgements
We thank the pediatric critical care nurses, physicians, patients and families for their support of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest declarations: None.
References
- [1].Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, Carcillo J, Newth CJ, Prodhan P, Dean JM, Nicholson C. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatr. 2010;125:e1208–e1225. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cho HH, O'Connell JP, Cooney MF, Inchiosa MA., Jr. Minimizing tolerance and withdrawal to prolonged pediatric sedation: case report and review of the literature. J Intensive Care Med. 2007;22:173–179. doi: 10.1177/0885066607299556. [DOI] [PubMed] [Google Scholar]
- [3].Curley MA, Hibberd PL, Fineman LD, Wypij D, Shih MC, Thompson JE, Grant MJ, Barr FE, Cvijanovich NZ, Sorce L, Luckett PM, Matthay MA, Arnold JH. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. Journal of the American Medical Association. 2005;294:229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Curley MA, Harris SK, Fraser KA, Johnson RA, Arnold JH. State Behavioral Scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. 2006;7:107–114. doi: 10.1097/01.PCC.0000200955.40962.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Devlin JW, Roberts RJ. Pharmacology of commonly used analgesics and sedatives in the ICU: benzodiazepines, propofol, and opioids. Crit Care Clin. 2009;25:431–49. vii. doi: 10.1016/j.ccc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [6].Finnegan LP, Kron RE, Connaughton JF, Emich JP. A scoring system for evaluation and treatment of the neonatal abstinence syndrome: A new clinical research tool. In: Morselli PI, Garatani S, Sereni F, editors. Basic and Therapeutic Aspects of Perinatal Pharmacology. Raven Press; New York: 1975. [Google Scholar]
- [7].Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- [8].Fonsmark L, Rasmussen YH, Peder C. Occurrence of withdrawal in critically ill sedated children. Crit Care Med. 1999;27:196–199. doi: 10.1097/00003246-199901000-00052. [DOI] [PubMed] [Google Scholar]
- [9].Franck LS, Harris SK, Soetenga DJ, Amling JK, Curley MA. The Withdrawal Assessment Tool-1 (WAT-1): an assessment instrument for monitoring opioid and benzodiazepine withdrawal symptoms in pediatric patients. Pediatr Crit Care Med. 2008;9:573–580. doi: 10.1097/PCC.0b013e31818c8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Franck LS, Naughton I, Winter I. Opioid and benzodiazepine withdrawal symptoms in paediatric intensive care patients. Intensive Crit Care Nurs. 2004;20:344–351. doi: 10.1016/j.iccn.2004.07.008. [DOI] [PubMed] [Google Scholar]
- [11].Franck LS, Vilardi J. Assessment and management of opioid withdrawal in ill neonates. Neonatal Network. 1995;14:39–48. [PubMed] [Google Scholar]
- [12].Franck LS, Vilardi J, Durand D, Powers R. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care. 1998;7:364–369. [PubMed] [Google Scholar]
- [13].Honey BL, Benefield RJ, Miller JL, Johnson PN. Alpha2-receptor agonists for treatment and prevention of iatrogenic opioid abstinence syndrome in critically ill patients. Ann Pharmacother. 2009;43:1506–1511. doi: 10.1345/aph.1M161. [DOI] [PubMed] [Google Scholar]
- [14].Ista E, van Dijk M, de Hoog M, Tibboel D. Duivenvoorden HJ. Construction of the Sophia Observation withdrawal Symptoms-scale (SOS) for critically ill children. Intensive Care Med. 2009;35:1075–1081. doi: 10.1007/s00134-009-1487-3. [DOI] [PubMed] [Google Scholar]
- [15].Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M. Withdrawal symptoms in children after long-term administration of sedatives and/or analgesics: a literature review. “Assessment remains troublesome”. Intensive Care Med. 2007;33:1396–1406. doi: 10.1007/s00134-007-0696-x. [DOI] [PubMed] [Google Scholar]
- [16].Jenkins IA, Playfor SD, Bevan C, Davies G, Wolf AR. Current United Kingdom sedation practice in pediatric intensive care. Paediatr Anaesth. 2007;17:675–683. doi: 10.1111/j.1460-9592.2006.02180.x. [DOI] [PubMed] [Google Scholar]
- [17].Katz R, Kelly HW, Hsi A. Prospective study on the occurrence of withdrawal in critically ill children who receive fentanyl by continuous infusion. Crit Care Med9. 22:763–767. doi: 10.1097/00003246-199405000-00009. [DOI] [PubMed] [Google Scholar]
- [18].Playfor S, Jenkins I, Boyles C, Choonara I, Davies G, Haywood T, et al. Consensus guidelines on sedation and analgesia in critically ill children. Intensive Care Med. 2006;32:1125–1136. doi: 10.1007/s00134-006-0190-x. [DOI] [PubMed] [Google Scholar]
- [19].Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- [20].Randolph AG, Wypij D, Venkataraman ST, Hanson JH, Gedeit RG, Meert KL, Luckett PM, Forbes P, Lilley M, Thompson J, Cheifetz IM, Hibberd P, Wetzel R, Cox PN, Arnold JH. Effect of mechanical ventilator weaning protocols on respiratory outcomes in infants and children: a randomized controlled trial. JAMA. 2002;288:2561–2568. doi: 10.1001/jama.288.20.2561. [DOI] [PubMed] [Google Scholar]
- [21].Rhoney DH, Murry KR. National survey on the use of sedatives and neuromuscular blocking agents in the pediatric intensive care unit. Pediatr Crit Care Med. 2002;3:129–133. doi: 10.1097/00130478-200204000-00007. [DOI] [PubMed] [Google Scholar]
- [22].Sfoggia A, Fontela PS, Moraes A, da Silva F, Sober RB, Noer RB, Bruno F, Einloft P, Garcia PC, Piva JP. Sedation and analgesia in children submitted to mechanical ventilation could be overestimated? J Pediatr (Rio J) 2003;79:343–348. [PubMed] [Google Scholar]
- [23].Taketomo CK, Hodding J, Krause DM. Pediatric dosage handbook. Lexi-Comp; Hudson, OH: 2011. [Google Scholar]
- [24].Tobias JD. Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Crit Care Med. 2000;28:2122–2132. doi: 10.1097/00003246-200006000-00079. [DOI] [PubMed] [Google Scholar]
- [25].Twite MD, Rashid A, Zuk J, Friesen RH. Sedation, analgesia, and neuromuscular blockade in the pediatric intensive care unit: survey of fellowship training programs. Pediatr Crit Care Med. 2004;5:521–532. doi: 10.1097/01.PCC.0000144710.13710.2E. [DOI] [PubMed] [Google Scholar]
- [26].Wilson WC, Smedira NG, Fink C, McDowell JA, Luce JM. Ordering and administration of sedatives and analgesics during the withholding and withdrawal of life support from critically ill patients. JAMA. 1992;19(267):949–953. [PubMed] [Google Scholar]