Abstract
Cavitation decreases the hydraulic conductance of the xylem and has, therefore, detrimental effects on plant water balance. However, cavitation is also hypothesized to relieve water stress temporarily by releasing water from embolizing conduits to the transpiration stream. Stomatal closure in response to decreasing water potentials in order to avoid excessive cavitation has been well documented in numerous previous studies. However, it has remained unclear whether the stomata sense cavitation events themselves or whether they act in response to a decrease in leaf water potential to a level at which cavitation is initiated. The effects of massive cavitation on leaf water potential, transpiration, and stomatal behaviour were studied by feeding a surfactant into the transpiration stream of Scots pine (Pinus sylvestris) seedlings. The stomatal response to cavitation in connection with the capacitive effect was also studied. A major transient increase in leaf water potential was found due to cavitation in the seedlings. As cavitation was induced by lowering the surface tension, the two mechanisms could be uncoupled, as the usual relation between xylem water potential and the onset of cavitation did not hold. Our results indicate that the seedlings responded more to leaf water potential and less to cavitation itself, as stomatal closure was insufficient to prevent the seedlings from being driven to ‘run-away’ cavitation in a manner of hours.
Keywords: Cavitation, leaf gas exchange, stomatal control, water potential, xylem transport
Introduction
Water columns in the xylem are usually under negative pressure and therefore vulnerable to cavitation (Tyree and Sperry, 1989). Excessive cavitation is unfavourable to plant function as it decreases the hydraulic conductance of the xylem and threatens the supply of water to the leaves. If a plant does not respond by stomatal closure to prevent excess cavitation, it would progressively spread to fill the entire xylem in ‘run-away’ cavitation and the plant could die of dehydration (Tyree and Sperry, 1988; McDowell et al., 2008). Various tree species have been shown to maintain stomatal conductance and xylem water potential above values that would lead to this excess cycle of xylem cavitation (Sperry and Pockman, 1993; Dang et al., 1997; Borghetti et al., 1998; Irvine et al., 1998; Salleo et al., 2000; Hubbard et al., 2001; Brodribb et al., 2003). Whether the stomata respond to leaf water potentials which correspond to the onset of significant amounts of cavitation or directly to a signal created by cavitation events themselves is not known (Whitehead et al., 1996; Nardini and Salleo, 2000; Salleo et al., 2000; Cochard et al., 2002).
Despite its unfavourable effect on xylem hydraulic conductance, cavitation is hypothesized to relieve water stress temporarily (Meinzer et al., 2001a). Following the entry of an air bubble into a conduit, water is quickly sucked out of the embolizing conduits into the surrounding tissue (Hölttä et al., 2007), and the water potential in the plant should be transiently increased (Lo Gullo and Salleo, 1992; Hölttä et al., 2009). Cavitation is therefore hypothesized to act as a water release mechanism. Its importance as a capacitive water release mechanism has been predicted to increase with increasing plant size (Hölttä et al., 2009). Water tension release due to cavitation has also been observed as temporary reversal of stem diameter shrinkage with increasing water loss in Norway spruce stems (Rosner et al., 2009, 2010).
This study was designed to quantify the capacitive effect of cavitation on plant water balance directly. Experimental results on the leaf water potential and transpiration rates during the capacitive phase of cavitation on Scots pine seedlings were compared with model predictions of an earlier theoretical study on the subject (Hölttä et al., 2009). The second goal was to answer the question of whether the stomata sense cavitation events directly or whether stomatal closure is merely ‘programmed’ to occur at water potentials which correspond to the inception of cavitation. In normal conditions, these two control mechanisms to avoid excess cavitation might be very difficult to differentiate as both typically occur simultaneously (Cochard et al., 2002). To uncouple these two mechanisms, a surfactant was used to lower the surface tension of water in the xylem transpiration stream so that cavitation would occur at substantially higher water potentials compared with natural conditions.
Materials and methods
Four-year-old grafted Scots pine seedlings (clone E618, Suomenniemi, Finland, 61°20′, 27°05′) were used as plant material. The seedlings had been grown in large pots outdoors at the Helsinki University campus in Viikki, Helsinki, Finland) until the start of the experiment and were well watered.
The experiments were conducted during June and July 2010 in a laboratory of the Department of Forest Sciences, University of Helsinki. Seedlings were cut at the base of the stem and then re-cut under water in a container. Two double saw-cuts, made radially approximately halfway to the stem, were performed at the base of the seedlings to reduce the total hydraulic conductance. This was done to promote the occurrence of lower water potentials to ensure the occurrence of cavitation.
The seedlings were illuminated from one side with a light source (HMS-100, Emil Niethammer GmbH, Germany) using a mercury lamp with a daylight spectrum (Metallogen HMI, 1200 W/GS, OSRAM GmbH, Berlin, Germany). Light intensity was approximately 1000 μmol m−2 s−1. The light intensity experienced by the seedling was ascertained with a PAR Sensor (Li-190 Quantum sensor, Li-Cor, Lincoln NE, USA). Aluminium foil was placed on the opposite side from the light source, behind the seedling, so that the whole seedling received illumination as uniform as possible. VPD in the laboratory was kept as constant as possible, being on average 2.05±0.40 kPa and 1.90±0.43 kPa, in treatment and control runs, respectively.
The surfactant used was Tween 80. The surfactant was added slightly above its micellar concentration (0.5% v/v), so that the surface tension of the water decreased to 36 N m−1 (Chu and So, 2001), that is, to half of its original value. Experiments conducted with both angiosperm (Crombie et al., 1985; Sperry and Tyree, 1988; Choat et al., 2004) and gymnosperm (Cochard et al., 2009) species have confirmed that cavitation water potential will increase in linear proportion to the decrease in surface tension. This linear relationship can be deduced from the Laplace equation (although it is not so obvious for tracheids with a torus-margo structure) and has also been experimentally confirmed for Scots pine (Cochard et al., 2009).
After the initial measurement phase when the seedling had reached steady-state, that is, transpiration rate and xylem water potential remained relatively constant, the surfactant was added to the water container where the cut seedling was placed. Water in the container was then mixed vigorously. The surfactant treatment was conducted with 11 replications (referred to hereafter as ‘treatment runs’).
To estimate potential errors and inaccuracies in our measurement protocol, six control runs were performed, where no surfactant was added to the water taken up by the seedlings. The control runs were otherwise performed in a similar way to the treatment runs and there was no difference in the time of the day, season or the size of the seedlings between the control and the treatment.
Gas exchange measurements
CO2 exchange and transpiration were followed by using the portable gas-exchange measuring system GFS-3000 (Heinz Walz GmbH, Effeltrich Germany). Four needle pairs were carefully placed in the measurement cuvette, through tiny holes in the insulation gaskets. This ensured that there were no leakages from the cuvette. The light level inside the cuvette was set at 1000 μmol m−2 s−1. Temperature and water vapour concentration inside the cuvette was set to track ambient values so that the needles inside the cuvette experienced the same conditions as the whole plant. Measurements were started approximately 1 h after the seedlings were detached from their roots and the saw-cuts made. After the measurement, the needles were photographed and the projected needle area was calculated by image analysis. The data were recorded as running averages over 10 min of measurements made every 10 s.
Other measurements
Needle water potential was measured with a custom-made pressure bomb. Needles for the measurements were excised randomly from the main stem. Running averages over five consecutive measurements of the leaf water potential values were calculated. This was done to ‘smooth’ the fluctuation in the leaf water potential measurement values that could have been caused by the slightly uneven positioning and water balance (especially during the capacitive phase) of the needles.
To quantify how much water had been lost from the xylem during the experiment, a sample of approximately 2 cm was taken from the stem base before and after (above the double saw-cuts) the experiment. The weight and volume of the stem sample was measured to determine fresh wood xylem density (mass of the fresh sample divided by its volume) after the bark, phloem, and cambium were peeled off. The relative water content of the needles was also calculated after the measurement using the measured fresh, saturated, and dry mass (all measured from the same needles).
Calculation of the apparent hydraulic conductance
Hydraulic conductance (k, mmol m2 Pa−1 s−1) of the whole xylem pathway is calculated by dividing the transpiration rate (E, mmol m−2 s−1) by the water potential difference over the pathway (P, Pa). The water potential difference (soil water potential minus leaf water potential) is now the same as the additive inverse of water potential in the leaves, because the pressure at the lower end of the stem is zero, as it is immersed in free water. Therefore,
| (1) |
The use of this equation implicitly assumes that xylem transport is in steady-state. The apparent hydraulic conductance is also calculated with equation (1), but now a steady-state need not be assumed. The apparent hydraulic conductance is always equal to the hydraulic conductance in steady-state, but will transiently differ from the ‘real’ hydraulic conductance if there is capacitance, that is, water movement to or from water stores along the pathway. For example, the apparent hydraulic conductance will increase while the hydraulic conductance remains constant when water from surrounding tissue, capillary spaces or cavitating conduits (Tyree and Zimmermann, 2002) is released into the transpiration stream.
Statistical analyses
A non-parametric Mann–Whitney test was used to detect significant differences in key variables between treatment and control groups. Non-parametric statistics were used due to the small and variable sample size of the data.
Differences in the regression models of stomatal conductance versus CO2 assimilation rate (of the type y=ax/(b+x)), or in the linear regression of leaf water potential versus stomatal conductance (of the type y=ax+b) between treatment and control runs were evaluated by calculating Student’s distribution t parameter for the difference in the slope parameter a between models, at and ac, where the subscripts refer to treatment or control points, respectively, as:
| (2) |
where set and sec are the standard errors associated with parameters at and ac, respectively. The P-value was computed from the t statistic and with df=nt+nc–4, where df is the degrees of freedom. Because the points in the treatment runs presented a larger distribution range compared with the points in the control runs, only those points from the treatment runs falling within the range encountered in the control runs were used in the analysis. Analyses were performed with SYSTAT 12 (Systat Software Inc, Chicago, IL, USA).
Model runs
Results from the experiment were compared with modelling predictions produced with a simplified version of an earlier published numerical model (Hölttä et al., 2009). The model calculates transient xylem water potential and the propagation of cavitation using xylem hydraulic conductance, a vulnerability curve to cavitation (Pammenter and Willingen, 1998), xylem sapwood cross-sectional area, plant bulk elastic modulus, plant height, and the transpiration rate as inputs. The model takes into account both the decrease in hydraulic conductance due to cavitation and water release to the transpiration stream by cavitation, i.e. the capacitive effect. The total xylem volume, xylem hydraulic conductance, and whole seedling transpiration rate (Table 1) were estimated from the experimental data and used as inputs to the model. The transpiration rate was estimated so that the model run transit time (total amount of water in xylem divided by transpiration rate) was equal to the time it took for the peak in apparent hydraulic conductance to be reached in the experiment after the addition of the surfactant. The transpiration value used was also confirmed to be of the right magnitude by a gravimetric measurement conducted in a few experiments where the weight loss rate from the seedling plus the pot was measured (not shown). The elastic modulus (Em), which specifies how much water potential changes for a given change in the amount of water in the whole plant (see equation S6 of the Supplementary data at JXB online) was estimated, assuming that approximately 1% of the transpiration need could be withdrawn from elastic storage within the plant.
Table 1.
Parameterization used in the model runs
| Parameter | Value | Reference |
| Tree height (h) | 0.5 m | Measured |
| Whole xylem volume (V) | 30×10–6m3 weight divided by 2.5) | Measured (seedling total fresh |
| Transpiration rate (E) | 7.5×10−9 m3s−1 | Estimated from the experimenta |
| Whole xylem hydraulic conductance (k) (without cavitation) | 3.5×10−15 m3Pa−1 s−1 | Calculated from measured transpiration and leaf water potential values |
| Proportion of resistance in the double ‘saw-cut’ | 50% | Measured |
| Parameter b in cavitation vulnerability curve | –3 MPa, modified in linear proportion to surface tension | Estimated from Cochard (1991) |
| Parameter a in cavitation vulnerability curve | 4.5×10−6 Pa−1, modified in inverse proportion to surface tension | Estimated from Cochard (1991) |
| Bulk elastic modulus of the seedling (Em) | 20 MPa | Estimated |
Estimated so that the model run transit time (amount of water in xylem divided by transpiration rate) was equal to the time it took for the peak in apparent hydraulic conductance to be reached in the experiment after the addition of the surfactant. The transpiration value used was also confirmed to be of the right magnitude by gravimetric measurements conducted in a few experiments (not shown).
The vulnerability curve, i.e. the function between xylem water potential and the proportional loss of hydraulic conductance due to cavitation was estimated from the literature. The value used for PLC 50 was –3.0 MPa (Cochard, 1992). This value may include a lot of uncertainty since xylem vulnerability could vary considerably in different parts of trees, and also between juvenile and mature wood in adult trees. However, the absolute value of PLC 50 is not critical for the interpretation of the results. The PLC 50 value could also be approximated from the dynamics of the apparent hydraulic conductance in the treatment runs. Leaf water potential is approximately –1.5 MPa at the time when the apparent hydraulic conductance is half in relation to the initial hydraulic conductance (see Results). This corresponds to a PLC 50 of –3.0 MPa when the lowered surface tension is taken into account.
The vulnerability curve to cavitation was corrected so that the total amount of cavitation at any given water potential increased in a linear proportion to the decrease in surface tension (Cochard et al., 2009). The amount of xylem volume filled by air was assumed to be linearly proportional to the loss of hydraulic conductance (Tyree et al., 1994). It is recognized, however, that more complicated relations could occur between the amount of water in the xylem and hydraulic conductance. Surface tension of the xylem sap was modelled to decrease in linear proportion to the penetration height of the surfactant into the seedling. The penetration height of surfactant was calculated to be the time elapsed from the addition of the surfactant multiplied by the sap flow velocity given by the model.
The model was run under a range of different scenarios and parameterizations. The purpose of the modelling was qualitatively to demonstrate the water relation dynamics during the experiments and also to demonstrate how the variability in some key parameters, such as plant size, stomatal control, and initial hydraulic conductance may cause the observed variability between the individual treatment runs. The model predictions were not, therefore, compared against the experimental results. The model is described and the model equations are given in the Supplementary data at JXB online.
Results
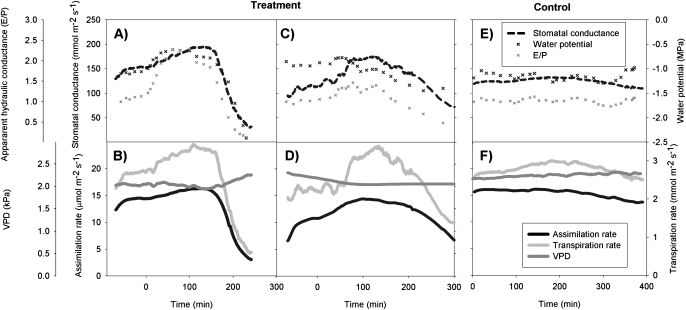
Figure 1 shows the dynamics of two typical treatment runs (A–D) and one control run (E, F). Leaf water potential and apparent hydraulic conductance (Fig. 1A, C) increased shortly after the addition of the surfactant. The same pattern was observed in all of the treatment runs. This so-called ‘capacitive phase’ reached its peak typically approximately 1 h after the addition of the surfactant, after which the leaf water potential and apparent hydraulic conductance started to decrease to a level much lower than that before the addition of the surfactant. Rapid stomatal closure (Fig. 1A, C) started when the capacitive phase of cavitation had passed and leaf water potential and apparent hydraulic conductance had decreased below their initial values. Stomatal conductance, leaf water potential, and other variables remained quite constant in the control runs (Fig. 1E, F), but small variations were detectable, mainly due to small variation in VPD.
Fig. 1.
Dynamics of leaf water potential, stomatal conductance, transpiration, and photosynthesis rates, VPD, and the apparent hydraulic conductance for two of the treatment runs (A–D) and one control run (E, F). Time zero corresponds to the addition of the surfactant in the treatment runs. These examples represent the typical behaviour observed during the treatment and control runs.
The apparent hydraulic conductance peaked, on average, at a value of 1.67±0.59 (mean ±standard deviation, normalized to its value at the time of the addition of the surfactant; see Table 2). The peak occurred, on average, 65±31 min after the addition of the surfactant. Stomatal conductance increased during the capacitive phase in seven of the 11 cases and the average peak stomatal conductance (normalized to the time of the addition of the surfactant) was 1.15±0.18 during the capacitive phase. The dynamics of the transpiration rate were very similar to the dynamics of stomatal conductance as VPD varied very little during each run. Leaf water potential increased during the capacitive phase in all except one case, and the average increase was 0.46±0.21 MPa relative to the time of the addition of the surfactant.
Table 2.
Characterization of key variables and evaluation of differences between treatment and control runs. With n(treatment)=11, n(control)=6
| Variable | Treatment (average and standard deviation) | Control (average and standard deviation) | Mann-Whitney (U) | P-value (Statistically significant at P<0.05 are in bold font) |
| Maximum apparent hydraulic conductance E/P (mmol m−2 s−1 Pa−1)* | 1.67±0.59 | 1.16±0.23 | 11 | 0.027 |
| E/P at the end of the experiment (mmol m−2 s−1 Pa−1) | 0.38±0.46 | 0.97±0.38 | 55 | 0.027 |
| Min E/P (mmol m−2 s−1 Pa−1)* | 0.38±0.46 | 0.80±0.18 | 53 | 0.044 |
| Time of maximum E/P(min)b | 65±31 | Not applicable | – | – |
| Maximum stomatal conductance (mmol m– s−1)a,d | 1.15±0.18 | 1.17±0.17 | 37 | 0.687 |
| Time of maximum stomatal conductance (min) | 62.7±48.1 | Not applicable | – | – |
| Stomatal conductance at the end of the experiment (mmol m−2 s−1)a | 0.426±0.344 | 1.03±0.28 | 60 | 0.007 |
| Minimum stomatal conductance (mmol m−2 s−1)* | 0.426±0.344 | 0.896±0.13 | 58 | 0.012 |
| Maximum leaf water potential (MPa) | –0.68±0.13 | –1.03±0.21 | 61 | 0.005 |
| Maximum leaf water potential (MPa)a | 0.76±0.22 | 0.93±0.11 | 53 | 0.044 |
| Minimum leaf water potential (MPa) | –2.0±0.91 | –1.39±0.27 | 20 | 0.190 |
| Water potential at stomatal closure (MPa) | –1.37±0.48 | Not applicable | – | – |
| Time of stomatal closure (min)b,c | 139.0±49 | Not applicable | – | – |
| E/Pat stomatal closure (mmol m−2 s−1 Pa−1)a | 0.95±0.29 | Not applicable | – | – |
| Initial stomatal conductance (mmol m−2 s−1) | 165.6±90.3 | 144.9±24.9 | 28 | 0.615 |
| Initial transpiration rate E(mmol m−2 s−1) | 3.17±1.50 | 2.62±0.53 | 28 | 0.615 |
| Initial CO2 assimilation rate (?mol m−2 s−1) | 16.2±6.0 | 16.6±3.4 | 36 | 0.736 |
| VPD (kPa) | 2.05±0.40 | 1.90±0.43 | 27 | 0.546 |
| Initial water potential (MPa) Fresh wood xylem densitye | –1.06±0.36 | –1.07±0.20 | 35.5 | 0.801 |
| (g cm−3) | 0.83±0.04 | 0.90±0.06 | 20 | 0.088 |
| Leaf relative water contentf (%) | 75±20 | 94±7 | 28 | 0.088 |
Expressed relative to value at time zero, i.e. at the time of the addition of the surfactant.
Time after the addition of the surfactant.
Stomatal closure was judged to occur when the decrease in the stomatal conductance (time averaged over 10 min) was more than 0.01 mmol m−2 s–1until the experiment ended or the stomatal conductance had decreased practically to zero. This corresponded well to the moment when stomatal conductance could be seen to decrease ‘sharply’ by a subjective inspection of the data.
Although the maximum stomatal conductance did not differ between the treatment and control, the time of the maximum stomatal conductance in the experiment was typically during the capacitive phase, while the maximum stomatal conductance in the control did not show any consistency.
nt=6, nc=4.
nt=7, nc=5.
Leaf water potential was, on average, –1.37±0.48 MPa when stomatal closure started. This happened, on average, 139±49 min after the addition of the surfactant. Apparent hydraulic conductance was, on average, 95±29% of its original value when stomatal closure started, that is, stomatal closure started when the capacitive phase was over. A significant negative correlation was found between initial water potential and maximum apparent hydraulic conductance (R= –0.62, n=11, P <0.05; not shown in Table 2), indicating that the capacitive effect was larger in magnitude when the seedling was initially closer to the cavitation threshold. The maximum apparent hydraulic conductance and leaf water potential, the minimum apparent hydraulic conductance, leaf water potential, and stomatal conductance were significantly different between treatment and control runs (Table 2). The initial conditions, for example, initial water potential, VPD, transpiration rate, and assimilation rate, did not significantly differ between treatment and control runs (Table 2).
Fresh wood xylem density above the double saw-cuts, determined after the measurement sequence, was, on average, 0.83±0.04 g cm−3 for the treated seedlings and 0.90±0.06 g cm−3 for the controls (U=20, P=0.088, nt=6, nc=4). Initial fresh wood xylem density (determined right after the cutting of the roots), at the bottom of the stem of the seedlings was 1.03±0.03 g cm−3. The leaf relative water content after the measurements tended to be smaller in the surfactant treated seedlings 75±20%, compared with the controls 94±7% (U=28, P=0.088, nt=7, nc=5).
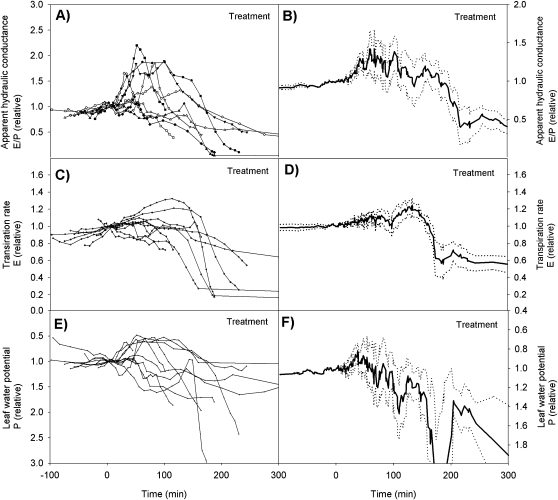
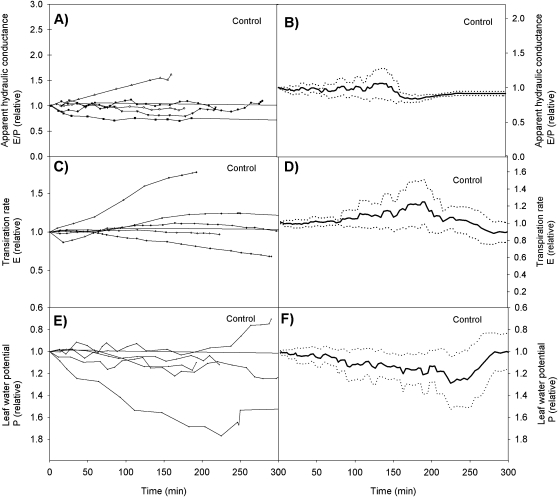
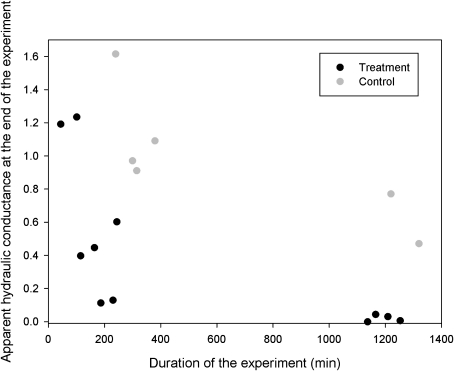
Apparent hydraulic conductance, transpiration rate and leaf water potential varied somewhat in magnitude and dynamics amongst the different treatment runs, but the general trend was similar showing an initial increase in apparent hydraulic conductance, transpiration rate, and leaf water potential, followed by a decrease (Fig. 2A–F). Variation in the control runs was small and did not show any consistency (Fig. 3A, F). In all cases where the treatment run was continued for several hours, the apparent hydraulic conductance decreased practically to zero (Fig. 4), signifying ‘run-away’ cavitation. The apparent hydraulic conductance decreased slightly also in the control runs when the experiment was continued for several hours.
Fig. 2.
Dynamics of the apparent hydraulic conductance for each treatment run (A), the averaged apparent hydraulic conductance with the 95% level confidence intervals (B), the transpiration rate for each treatment run (C), the averaged transpiration rate with the 95% level confidence intervals (D), the leaf water potential for each treatment run (E), and the averaged leaf water potential with the 95% level confidence intervals (F).
Fig. 3.
Dynamics of the apparent hydraulic conductance for each control run (A), the averaged apparent hydraulic conductance with the 95% level confidence intervals (B) the transpiration rate for each experiment (C), the averaged transpiration rate with the 95% level confidence intervals (D), the leaf water potential for each control run (E), and the averaged leaf water potential with the 95% level confidence intervals (F).
Fig. 4.
The apparent hydraulic conductance at the end of each run as a function of the duration of the run.
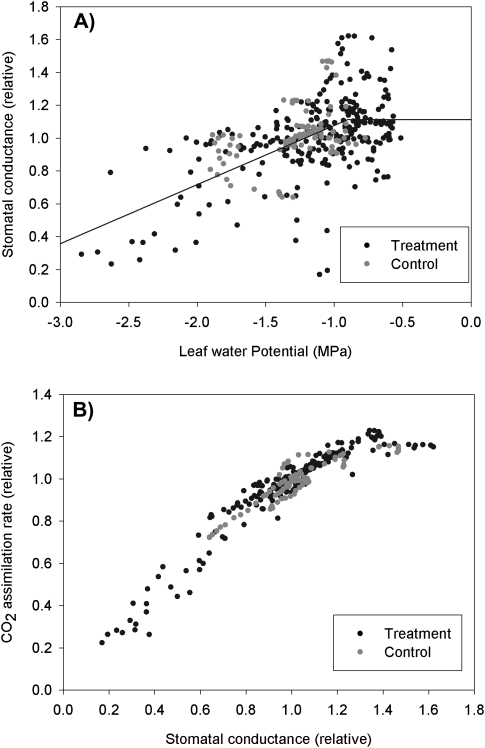
When data from all the runs was pooled together, no significant differences were found in the response of stomatal conductance to leaf water potential (t=0.467, df=284, P=0.641) (Fig. 5A), nor in the response of CO2 assimilation to stomatal conductance (t=1.193, df=318, P=0.234) (Fig. 5B) between treatment and control runs. Stomatal conductance was found to decrease with decreasing leaf water potential when leaf water potential was below a threshold value. When such a stepwise function with a linear decrease in stomatal conductance at low water potential was fitted to the data points, the best correlation coefficient (R2=0.34) was obtained when the threshold value for the decrease in stomatal conductance was set at –0.90 MPa.
Fig. 5.
Stomatal conductance as a function of leaf water potential (A) and CO2 assimilation rate as a function of stomatal conductance (B). All values are normalized in relation to time zero. The line in (A) is the fitted stepwise function with a linear relation between stomatal conductance and leaf water potential below a threshold value and a constant term above the threshold value. The threshold value that resulted in the highest correlation coefficient corresponded to a leaf water potential of –0.9 MPa. The equation for the fitted line is thus (G=1.11 for P > –0.90, and G=1.44+0.36P for P ≤ –0.9, where G is stomatal conductance).
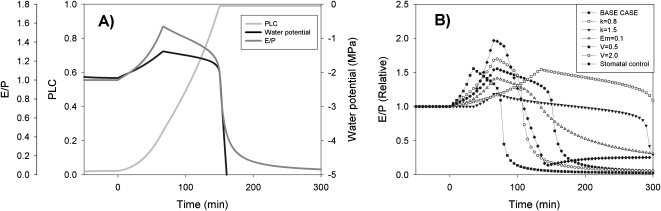
Results from the model runs demonstrated similar patterns in leaf water potential and apparent hydraulic conductance as in the treatment runs, increasing after the addition of the surfactant and decreasing after the initial phase (Fig. 6A). In the model runs the transpiration rate was kept constant, so that changes in the apparent hydraulic conductance were solely due to changes in xylem water potential (except in the case of ‘stomatal control’ in Fig. 6B). The exact dynamics of leaf water potential and apparent hydraulic conductance was dependent on parameterization of the model. Peak value in the apparent hydraulic conductance was always reached at the transit time, that is, when the surfactant arrived in the needles. Increasing xylem volume (V) therefore delayed the peak in apparent hydraulic conductance and increased the duration of the capacitive phase (Fig. 6B). Larger hydraulic conductance (k) caused cavitation to progress slower, so the peak in the apparent hydraulic conductance was lower and the capacitive phase lasted longer (Fig. 6B). Reducing the elastic modulus (Em) resulted in slower changes in the apparent hydraulic conductance as the increased elasticity buffered changes in leaf water potential (Fig. 6B). Increasing Em had only minor effect on the dynamics (not shown). The peak in the apparent hydraulic conductance became larger when transpiration rate increased (simulating stomatal opening) during the capacitive phase (see ‘stomatal control’ in Fig. 6B).
Fig. 6.
Modelled apparent hydraulic conductance, PLC, and leaf water potential in a model simulation using a simplified version of the model of Hölttä et al. (2009). The transpiration rate was kept constant in the model run (A). Modelled apparent hydraulic conductance when xylem conductance, xylem volume, bulk elastic modulus of the plant changes, or stomatal conductance is varied (B). k refers to the hydraulic conductance, V refers to xylem volume, and Em refers to the plant bulk elastic modulus. These values are expressed in relation to the ‘base case’ value, that is, the value expressed in Table 2. The transpiration rate was kept constant in the model runs except in the case ‘stomatal control’, where transpiration rate was raised linearly between 135–165 min to 1.3 times its original value, and then decreased from this value linearly between 180–240 min to 0.1 times its original value.
Discussion
Our results indicate that the stomata did not respond to cavitation directly. Stomatal closure was initiated only at water potentials typically observed in Scots pine (approximately –1.5 MPa; Irvine et al., 1998). However, the typical xylem water potential threshold value for the onset of cavitation did not hold in our experimental set-up as the surfactant had altered the vulnerability to cavitation, and thus rapid ‘run-away’ cavitation could not be prevented.
A temporary release of water stress due to cavitation was clearly observed in the experiment as the apparent hydraulic conductance increased shortly after the surfactant was added to the transpiration stream of Scots pine seedlings. The increase in apparent hydraulic conductance during the capacitive phase was mostly due to an increase in leaf water potential (Table 2). In most cases, stomatal conductance was also observed to increase concurrently with the increase in leaf water potential, and this was more pronounced when the initial leaf water potential was high, that is, when plant water status was good.
The duration of the capacitive phase was very short in the seedlings and, therefore, its physiological importance may only be marginal. However, the duration of the capacitive phase of cavitation would increase in a linear fashion in relation to the transit time of water in the xylem, which is equivalent to the total volume of water in the xylem divided by the whole plant transpiration rate (Hölttä et al., 2009). Transpiration rate has been theoretically predicted (West et al., 1999) and also experimentally confirmed (Meinzer et al., 2005) to scale roughly to the power of three-quarters of total plant volume. Several studies have also confirmed the reliance on water stored within a tree to increase with increasing tree size (Meinzer et al., 2001b; Phillips et al., 2003). The transit time of water was only slightly more than 1 h for the seedlings used in our experiment, judging both from the estimations of the total seedling transpiration rate and mass, and from the time it took for the apparent hydraulic conductance to reach its peak value in the experiments. A scaling exercise using the metabolic scaling theory (West et al., 1999) reveals that the transit time (τ) is proportional to plant height (L), that is, τ×α×L (see Supplementary data for the details at JXB online). Given a tree height of 50 cm and a transit time of 70 min in our experiment, the expected transit time in a 50 m tree would be expected to be approximately 15 d, if the average diurnal transpiration is assumed to be one-third of the peak transpiration rate (see Supplementary data for details of the calculation at JXB online). This estimation agrees well with, for example, Meinzer et al. (2006). The largest conifers have been shown to have transit times of well over one month (Meinzer et al., 2005). This indicates that the capacitive phase could last between 100 to 1000 times longer in the largest trees in relation to our experiment. The capacitive phase could be even much prolonged during a drought when the transpiration rate decreases due to stomatal control (Waring et al., 1979; Phillips et al., 2003; Hölttä et al., 2009).
The variation in the dynamics between the individual treatment runs could be explained by the differences in the hydraulic conductance, environmental conditions, size of the seedlings, or changes in transpiration, as the model demonstrated. Differences in the localization of cavitation in stem of the seedlings, which was not studied by the model, could also have caused variation between the treatment runs. For example, the model predicted the peak of the apparent hydraulic conductance to be larger during the capacitive phase when the plant was initially closer to the threshold of cavitation (i.e. low initial hydraulic conductance and/or low initial water potential). This trend was also observed in the experiments.
The pattern of embolism formation in different parts of the hydraulic pathway or the exact cavitation vulnerability characteristics of our seedlings were not quantified directly. However, this should not be critical for the interpretation of the results since the decrease in the conductance of the whole hydraulic pathway, that is, the ratio of transpiration rate to leaf water potential, clearly demonstrated that a substantial proportion of the total hydraulic conductance had been lost. Results from the fresh wood xylem density measurements also indicated that substantial, but not complete, water loss had occurred from the xylem conduits in the stem above the saw-cuts. Another potentially very important location of cavitation in the experiment is the xylem of needles. Leaves are typically are more vulnerable to cavitation than stems (Johnson et al., 2011), and xylem in leaves typically represent a major proportion of the resistance in the water transport system (Brodribb and Holrook, 2006). In addition, the xylem of conifer needles apparently embolizes and refills on a daily basis (Johnson et al., 2009). As practically all hydraulic conductance was lost when the experiment was continued for several hours, it seems that the peripheral parts of the seedling could have cavitated more than the stem, as would also be expected according to the hydraulic segmentation theory (Tyree and Zimmermann, 2002).
Lowering the surface tension would also be expected to result in the retrieval of stored water from the capillary spaces within the xylem and thus influence our results. However, previous studies (Tyree and Zimmermann, 2002) have demonstrated that water from capillary spaces, for example, in intercellular spaces, is already withdrawn at much higher water potentials compared with those experienced in our study. The control runs also showed some variation in the apparent hydraulic conductance within the runs. There could be many reasons for this. It could be that the runs were not strictly in steady-state so that water storage/release (Tyree and Zimmermann, 2002) occurred, or that some cavitation was occurring also in the control runs. The xylem hydraulic conductance could also have varied during the experiment due to changes in the xylem ionic concentration (Zwieniecki et al., 2001).
It is also possible that the surfactant itself induced some other effects on the seedlings. The surfactant used (Tween 80) may have increased the viscosity of the xylem sap slightly (by a few per cent) (Taylor et al., 2001; Lee et al., 2005) thus making the capacitive effect during cavitation even larger than it would appear from our results as a rise in viscosity would have contributed to a decrease in hydraulic conductance (k) after the surfactant was added. The similar behaviour in stomatal conductance and CO2 assimilation rate (Fig. 5) amongst surfactant-treated and control seedlings indicates that neither photosynthesis nor stomatal control was directly affected by the surfactant.
To our knowledge, this is the first time that the temporary water potential increase due to cavitation has been directly measured. In fact, it might not even be possible to have cavitation during increasing water potential when, simultaneously, the surface tension remains constant, which it did not in our case. This is because increasing water potential should prevent further cavitation from occurring (Hölttä et al., 2005). A rise in the apparent hydraulic conductance, however, can occur simultaneously with the progression of cavitation even in normal, constant surface tension conditions.
A change in the surface tension of xylem sap may also occur in natural conditions. Recently, surface tension was shown to be reduced by up to 27% below that of pure water in the xylem sap extracted from branches of P. tremuloides (Christensen-Dalsgaard et al., 2011). The development of embolism (Umebayashi et al.,2011), and the subsequent cause of mortality in Scots pine infected with the pine wilt disease is also the same as in our experiments: lowered surface tension in the xylem caused by volatile organic compounds emitted in defence against the pathogens, which ultimately resulted in ‘run-away’ cavitation (Kuroda, 1991). A similar fate has also been observed in various spruce species infected by blue-stain fungi (Kuroda, 2005). Importantly, our results indicate that, if xylem vulnerability to cavitation is altered, the stomata might be unable to respond to the loss of hydraulic conductance and prevent lethal ‘run away’ cavitation.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary data S1. Model description.
Supplementary data S2. Scaling of transit time to plant height according to the metabolic scaling theory.
Acknowledgments
T Hölttä and A Porcar-Castell received funding from the Academy of Finland projects No. 1132561 and No. 1138884, respectively. E Juurola was funded by the HENVI (project 470149021). Financial support by the Academy of Finland Centre of Excellence program (project No. 1118615) is gratefully acknowledged. We thank Herve Cochard for a discussion on the study and Minna Pulkkinen for her advice on the statistical analysis.
References
- Borghetti M, Cinnirella S, Magnani F, Saracino A. Impact of long-term drought on xylem embolism and growth in Pinus halepensis Mill. Trees. 1998;12:187–195. [Google Scholar]
- Brodribb TJ, Holrook NM. Declining hydraulic efficiency as transpiring leaves desiccate: two types of response. Plant, Cell and Environment. 2006;29:2205–2215. doi: 10.1111/j.1365-3040.2006.01594.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM, Edwards EJ, Gutiérrez MV. Relations between stomatal closure, leaf turgor and xylem vulnerability in eight tropical dry forest trees. Plant, Cell and Environment. 2003;26:443–450. [Google Scholar]
- Chu W, So WS. Modeling the two stages of surfactant-aided soil washing. Water Research. 2001;35:761–767. doi: 10.1016/s0043-1354(00)00292-x. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard KK, Tyree MT, Mussone PG. Surface tension phenomena in the xylem sap of three diffuse porous temperate tree species. Tree Physiology. 2011;31:361–368. doi: 10.1093/treephys/tpr018. [DOI] [PubMed] [Google Scholar]
- Choat B, Jansen S, Zwieniecki MA, Smets E, Holbrook M. Changes in pit membrane porosity due to deflection and stretching: the role of vestured pits. Journal of Experimental Botany. 2004;55:1569–1575. doi: 10.1093/jxb/erh173. [DOI] [PubMed] [Google Scholar]
- Cochard H. Vulnerability of several conifers to air embolism. Tree Physiology. 1992;11:73–83. doi: 10.1093/treephys/11.1.73. [DOI] [PubMed] [Google Scholar]
- Cochard H, Coll L, Le Roux X, Ameglio T. Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiology. 2002;128:282–290. [PMC free article] [PubMed] [Google Scholar]
- Cochard H, Hölttä T, Herbette S, Delzon S, Mencuccini M. New insights into the mechanisms of water-stress induced cavitation in conifers. Plant Physiology. 2009;151:949–954. doi: 10.1104/pp.109.138305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie DS, Milburn JA, Hipkins MF. Maximum sustainable xylem sap tensions in Rhododendron and other species. Planta. 1985;163:27–33. doi: 10.1007/BF00395893. [DOI] [PubMed] [Google Scholar]
- Dang QL, Margolis H, Coyea MR, Sy M, Collatz GJ. Regulation of branch-level gas exchange of boreal trees: roles of shoot water potential and vapor pressure difference. Tree Physiology. 1997;17:521–535. doi: 10.1093/treephys/17.8-9.521. [DOI] [PubMed] [Google Scholar]
- Hölttä T, Cochard H, Nikinmaa E, Mencuccini M. Capacitive effect of cavitation in xylem conduits. Plant, Cell and Environment. 2009;32:10–21. doi: 10.1111/j.1365-3040.2008.01894.x. [DOI] [PubMed] [Google Scholar]
- Hölttä T, Vesala T, Nikinmaa E, Perämäki M, Siivola E, Mencuccini M. Field measurements of ultrasonic acoustic emissions and stem diameter variations. New insight into the relationship between xylem tensions and embolism. Tree Physiology. 2005;25:237–243. doi: 10.1093/treephys/25.2.237. [DOI] [PubMed] [Google Scholar]
- Hölttä T, Vesala T, Nikinmaa E. A model of bubble growth leading to xylem conduit embolism: results from a dynamic model. Journal of Theoretical Biology. 2007;249:111–123. doi: 10.1016/j.jtbi.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Hubbard RM, Ryan MG, Stiller V, Sperry JS. Stomatal conductance and photosynthesis vary linearly with plant hydraulic conductance in ponderosa pine. Plant, Cell and Environment. 2001;24:113–121. [Google Scholar]
- Irvine J, Perks MP, Magnani F, Grace J. The response of Pinus sylvestris to drought: stomatal control of transpiration and hydraulic conductance. Tree Physiology. 1998;18:393–402. doi: 10.1093/treephys/18.6.393. [DOI] [PubMed] [Google Scholar]
- Johnson DM, McCulloh KA, Meinzer FC, Woodruff DR, Eissenstat DM, Philips N. Hydraulic patterns and safety margins, from stem to stomata, in three eastern US tree species. Tree Physiology. 2011;6:659–668. doi: 10.1093/treephys/tpr050. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Woodruff DR, McCulloh KA, Meinzer FC. Leaf hydraulic conductance, measured in situ, declines and recovers daily: leaf hydraulics, water potential and stomatal conductance in four temperate and three tropical tree species. Tree Physiology. 2009;29:879–887. doi: 10.1093/treephys/tpp031. [DOI] [PubMed] [Google Scholar]
- Kuroda K. Mechanism of cavitation development in the pine wilt disease. European Journal of Forest Pathology. 1991;21:82–89. [Google Scholar]
- Kuroda K. Xylem dysfunction in Yezo spruce (Picea jezoensis) after inoculation with the blue-stain fungus. Ceratocystis polonica. Forest Pathology. 2005;35:346–358. [Google Scholar]
- Lee J, Yang JS, Kim HJ, Baek K, Yang JW. Simultaneous removal of organic and inorganic contaminants by micellar enhanced ultrafiltration with mixed surfactant. Desalination. 2005;184:395–407. [Google Scholar]
- Lo Gullo MA, Salleo S. Water storage in the wood and xylem cavitation in 1-year-old twigs of Populus deltoides Bartr. Plant, Cell and Environment. 1992;15:431–438. [Google Scholar]
- McDowell N, Pockman WT, Allen CD, et al. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytologist. 2008;178:719–739. doi: 10.1111/j.1469-8137.2008.02436.x. [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Bond BJ, Warren JM, Woodruff DR. Does water transport scale universally with tree size? Functional Ecology. 2005;19:558–565. [Google Scholar]
- Meinzer FC, Brooks JR, Domec JC, Gartner BL, Warren JM, Woodruff DR, Bible K, Shaw DC. Dynamics of water transport and storage in conifers studied with deuterium and heat tracing techniques. Plant, Cell and Environment. 2006;29:105–114. doi: 10.1111/j.1365-3040.2005.01404.x. [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Clearwater MJ, Goldstein G. Water transport in trees: current perspectives, new insights and some controversies. Environmental and Experimental Botany. 2001a;45:239–262. doi: 10.1016/s0098-8472(01)00074-0. [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Goldstein G, Andrade JL. Regulation of water flux through tropical forest canopy trees: Do universal rules apply? Tree Physiology. 2001b;21:19–26. doi: 10.1093/treephys/21.1.19. [DOI] [PubMed] [Google Scholar]
- Nardini A, Salleo S. Limitation of stomatal conductance by hydraulic traits: sensing or preventing xylem cavitation? Trees. 2000;15:14–24. [Google Scholar]
- Pammenter NW, Willigen CV. A mathematical and statistical analysis of the curves illustrating vulnerability of xylem to cavitation. Tree Physiology. 1998;18:589–593. doi: 10.1093/treephys/18.8-9.589. [DOI] [PubMed] [Google Scholar]
- Phillips NG, Ryan MG, Bond BJ, McDowell NG, Hinckley TM, Cermak J. Reliance on stored water increases with tree size in three species in the Pacific Northwest. Tree Physiology. 2003;23:237–245. doi: 10.1093/treephys/23.4.237. [DOI] [PubMed] [Google Scholar]
- Rosner S, Karlsson B, Konnerth J, Hansmann C. Shrinkage processes in standard-size Norway spruce wood specimens with different vulnerability to cavitation. Tree Physiology. 2009;29:1419–1431. doi: 10.1093/treephys/tpp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner S, Konnerth J, Planck B, Salaberger D, Hansmann C. Radial shrinkage and ultrasound acoustic emissions of fresh versus pre-dried Norway spruce sapwood. Trees. 2010;24:931–940. doi: 10.1007/s00468-010-0464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleo S, Nardini A, Pitt F, Lo Gullo MA. Xylem cavitation and hydraulic control of stomatal conductance in laurel (Laurus nobilis L.) Plant, Cell and Environment. 2000;23:71–79. [Google Scholar]
- Sperry JS, Pockman WT. Limitation of transpiration by hydraulic conductance and xylem cavitation in. Betula occidentalis. Plant, Cell and Environment. 1993;16:279–287. [Google Scholar]
- Sperry JS, Tyree MT. Mechanisms of water stress-induced xylem embolism. Plant Physiology. 1988;88:581–587. doi: 10.1104/pp.88.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor T, Pennel K, Abriola LM, Dane JH. Surfactant enhanced recovery of tetrachloroethylene from a porous medium containing low permeability lenses. 1. Experimental studies. Journal of Contaminant Hydrology. 2001;48:325–350. doi: 10.1016/s0169-7722(00)00185-6. [DOI] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. Do woody plants operate near the point of catastrophic xylem dysfunction caused by dynamic water stress? Plant Physiology. 1988;88:574–580. doi: 10.1104/pp.88.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree MT, Sperry JS. Vulnerability of xylem to cavitation and embolism. Annual Reviews of Plant Physiology and Molecular Biology. 1989;40:19–38. [Google Scholar]
- Tyree MT, Davis S, Cochard H. Biophysical perspectives of xylem evolution: is there a tradeoff of hydraulic efficiency for vulnerability to dysfunction? IAWA Journal. 1994;15:335–360. [Google Scholar]
- Tyree MT, Zimmermann MH. Xylem structure and the ascent of sap. 2nd edn. Berlin, Germany: Springer; 2002. [Google Scholar]
- Umebayashi T, Fukuda K, Haishi T, Sotooka R, Zuhair S, Otsuki K. The developmental process of xylem embolisms in pine wilt disease monitored by multipoint imaging using compact magnetic resonance imaging. Plant Physiology. 2011;156:943–951. doi: 10.1104/pp.110.170282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring RH, Whitehead D, Jarvis PG. The contribution of stored water to transpiration in Scots pine. Plant, Cell and Environment. 1979;2:309–317. [Google Scholar]
- West GB, Brown JH, Enquist BJ. A general model for the structure and allometry of plant vascular systems. Nature. 1999;400:664–667. [Google Scholar]
- Whitehead D, Livingston NJ, Kelliher FM, Hogan KP, Pepin S, McSeveny TM, Byers JN. Response of transpiration and photosynthesis to a transient change in illuminated foliage area for a Pinus radiata D. Don tree. Plant, Cell and Environment. 1996;19:949–957. [Google Scholar]
- Zwieniecki MA, Melcher PJ, Holbrook NM. Hydrogel control of xylem hydraulic resistance in plants. Science. 2001;291:1059–1062. doi: 10.1126/science.1057175. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.