Abstract
The complexity of the human brain’s activity and connectivity varies over temporal scales and is altered in disease states such as schizophrenia. Using a multi-level analysis of spontaneous low-frequency fMRI data stretching from the activity of individual brain regions to the coordinated connectivity pattern of the whole brain, we investigate the role of brain signal complexity in schizophrenia. Specifically, we quantitatively characterize the univariate wavelet entropy of regional activity, the bivariate pairwise functional connectivity between regions, and the multivariate network organization of connectivity patterns. Our results indicate that univariate measures of complexity are less sensitive to disease state than higher level bivariate and multivariate measures. While wavelet entropy is unaffected by disease state, the magnitude of pairwise functional connectivity is significantly decreased in schizophrenia and the variance is increased. Furthermore, by considering the network structure as a function of correlation strength, we find that network organization specifically of weak connections is strongly correlated with attention, memory, and negative symptom scores and displays potential as a clinical biomarker, providing up to 75% classification accuracy and 85% sensitivity. We also develop a general statistical framework for the testing of group differences in network properties, which is broadly applicable to studies where changes in network organization are crucial to the understanding of brain function.
Keywords: schizophrenia, functional connectivity, network analysis, graph theory, resting state
Introduction
Recent evidence suggests that resting-state brain function measured by functional magnetic resonance imaging (fMRI) (Raichle and Snyder, 2007) is a sensitive marker of disease (Auer, 2008; Broyd et al., 2009) and a potentially important tool for the discovery of quantitative genetic phenotypes (Biswal et al., 2010). Resting-state functional connectivity, measured by correlations between blood oxygen level dependent (BOLD) time series, is profoundly disturbed in schizophrenia (Lynall et al., 2010; Skudlarski et al., 2010), supporting the dysconnectivity hypothesis (Friston, 1998) of the disease. Dysconnectivity refers to a change in the complex pattern of healthy functional interactions between brain regions, likely driven by alterations in underlying neurophysiological processes (Valli et al., 2011) such as synaptic plasticity or developmental wiring (Stephan et al., 2006).
Resting state brain function in both health and disease can be characterized at multiple levels, from the activity of single brain regions (univariate), to the functional interactions between a pair of regions (bivariate), to the coordinated pattern of connectivity between many brain regions (multivariate). Each provides a window into a unique dimension of brain function complexity that can be linked to cognitive function and its alteration in disease. At the lowest level, the predictability of a single brain signal can be examined using Lyapunov exponents, Hurst exponents, dimensional complexity, and multi-scale entropy (Subha et al., 2010), which vary with age and development (McIntosh et al., 2010; Meyer-Lindenberg and Bassett, 2008), task (Misic et al., 2010; Barnes et al., 2009; Meyer-Lindenberg and Bassett, 2008), and disease (Bosl et al., 2011; Breakspear, 2006). In schizophrenia, studies have reported both increases (Bob et al., 2009) and decreases (Meyer-Lindenberg and Bassett, 2008) in EEG signal complexity relative to healthy controls, and modulation by antipsychotics (Takahashi et al., 2010). At intermediate levels, pairwise functional connectivity can be measured by simple linear correlations or more complex synchronization or causal properties. Mounting evidence suggests that pairwise functional connectivity is particularly critical for the understanding of schizophrenia as it represents a neurogenetic risk mechanism for psychosis (Esslinger et al., 2009, 2011). At higher levels, complex network theory provides a framework in which to examine the multivariate pattern of functional interactions between brain signals, where the human brain is represented as a network whose nodes are brain regions and whose edges are their functional connections (Bassett and Bullmore, 2006, 2009; Bullmore and Sporns, 2009; Bullmore and Bassett, 2011). In schizophrenia, network organization extracted from multiple imaging modalities (EEG, fMRI, and MEG) appears to be both randomized (Rubinov and Sporns, 2009; Bassett et al., 2008; Lynall et al., 2010) and less cost-efficient (Bassett et al., 2009) compared to controls.
Here we examine the advantages of and relationships between uni-, bi-, and multivariate properties in identifying abnormalities in resting state function in schizophrenia. We hypothesize that regional activity directly constrains higher-order connectivity properties, and suggest that understanding this link is critical for the development of a neurophysiological interpretation of altered functional connectivity in schizophrenia. We further hypothesize that the added power of multivariate network measures will provide enhanced sensitivity to detecting disease effects on brain function, and that the patterns of both strong and weak connections will be differentially sensitive to disease state. In the context of our analysis, we introduce a statistical framework for the examination of group differences in functional connectivity based on functional data analysis (FDA) (Ramsay and Silverman, 2005) that has significant advantages over previously used methods of network comparison.
Methods
Data Acquisition and Preprocessing
Data from 29 participants with chronic schizophrenia (11 females; age 41.3 ± 9.3 (SD); 5 left-handed) and 29 healthy participants (11 females; age 41.1 ± 10.6 (SD); 2 left-handed) were included in this analysis (see Camchong et al. (2011) for detailed characteristics of participants and imaging data). Out of the 29 chronic schizophrenia patients: 16 were taking 1 atypical antipsychotic, 8 were taking 2 atypical antipsychotics, 1 was taking 1 typical antipsychotic, 1 was taking 1 atypical and 1 typical antipsychotic, 1 was taking 2 atypical and 1 atypical antipsychotics, and 2 were not taking any antipsychotic (Camchong et al., 2011).
A Siemens Trio 3 T scanner was used to collect imaging data, including a 6-min (TR=2 secs; 180 volumes) resting-state fMRI scan, in which participants were asked to remain awake with their eyes closed, a field map scan, and a T1 MPRAGE whole brain volumetric scan. The fMRI data was preprocessed using FEAT (FMRI Expert Analysis Tool) from FMRIB’s Software Library (FSL; Smith et al. (2004)) with the following pipeline: deletion of the first 3 volumes to account for magnetization stabilization; motion correction using MCFLIRT (Motion Correction using FMRIB’s Linear Image Registration Tool; Jenkinson et al. (2002)); B0 fieldmap unwarping to correct for geometric distortion using acquired field maps using PRELUDE (Phase Region Expanding Labeller for Unwrapping Discrete Estimates) and FUGUE52 (FMRIB’s Utility for Geometrically Unwarping EPIs); slice-timing correction using Fourier-space time-series phase-shifting; non-brain removal using BET (Brain Extraction Tool; Smith (2002)); regression against the 6 motion parameter time courses; registration of fMRI to standard space (Montreal Neurological Institute-152 brain); registration of fMRI to high resolution anatomical MRI; registration of high resolution anatomical MRI to standard space. Importantly, the two groups had similar mean root mean square (RMS) motion parameters: two-sample t-tests of mean RMS translational and angular movement were not significant (p = 0.14 and p = 0.12, respectively).
Each individual in the study completed a cognitive battery including tests that measured two domains of general cognitive abilities known to be altered in schizophrenia (Carroll, 1993; Heinrichs and Zakzanis, 1998; Aleman et al., 1999; Fuller et al., 2006; Potts et al., 2002): (1) the Weschler Adult Intelligence Scale-III (digit symbol, digit span, symbol search, letter-number sequence) and the Delis-Kaplan Executive Function System (trails numbers-letters test, tower test) to assess attention and concentration and (2) the California Verbal Learning Test II and the Weschler Memory Scales to assess memory. The symptomatology of participants in the schizophrenia cohort (diagnosed using the DSM-IV criteria (First et al., 1995)) was assessed using the scale for the assessment of negative symptoms (SANS) and the scale for the assessment of positive symptoms (SAPS) (Andreasen, 1982; Andreasen and Olsen, 1982).
Multi-Level Complexity in Brain Signals
Univariate Activity
We began by studying the complexity of resting state fMRI data using a univariate approach, that is, estimating the complexity of data extracted from individual brain regions. Average time series were extracted for each participant from 90 of the 116 anatomical regions of interest (ROIs) defined by the AAL atlas (Tzourio-Mazoyer et al., 2002) covering the whole brain and including cortical and subcortical regions but excluding the cerebellar regions and vermis. We focused our investigation on low frequency (0.06–0.125 Hz) oscillations in the BOLD signal which have previously been shown to be particularly sensitive to disease-related alterations in schizophrenia (Lynall et al., 2010). Consistent with Lynall et al. (2010), our data revealed that this frequency band was most sensitive to group differences in average connectivity as measured by correlation and mutual information. The frequency band of interest was isolated by applying the maximal overlap discrete wavelet transform to each time series (Percival and Walden, 2000) and selecting wavelet scale 2 (which corresponds approximately to the frequency range of interest and has center frequency 0.7143 Hz as determined by the MATLAB function centfrq for the Daubechies 4 wavelet used in this study).
While many measures exist to study the univariate complexity of these 90 regions, we chose to examine signal variation by employing the simple, temporally-independent measure of the Shannon wavelet entropy. We estimated the wavelet scale 2 entropy using methods developed in Coifman and Wickerhauser (1992) and Donoho and Johnstone (1994) and implemented in the MATLAB Wavelet Toolbox (function wentropy.m). The Shannon entropy of the wavelet coefficients is defined as:
| (1) |
where s is the signal of a single region in a given individual, si are the coefficients of s in the orthonormal wavelet basis, and where i indexes the wavelet coefficients of the scale 2 time series. As the wavelet entropy estimates were found to be non-normally distributed, we report median rather than mean values over subjects or ROIs, however, consistent results are found when using the mean.
Bivariate Pairwise Correlation
We next studied the complexity of the resting state fMRI data using a bivariate approach, by estimating the functional interactions between brain regions. We estimated the functional connectivity by computing the absolute value of the Pearson’s correlation between all possible pairs of time series, creating a 90×90 (N×N) connectivity matrix (Lynall et al., 2010). We then computed two simple measures of bivariate connectivity: strength and diversity (Lynall et al., 2010). The strength of node i is defined as the mean value of the ith column of the connectivity matrix and indicates the average connectivity for a single brain region to all others (Lynall et al., 2010; Bassett et al., 2010). The diversity of node i is defined as the variance of the ith column of the connectivity matrix and measures the variation in connectivity strength of a single brain region to all others. The average connectivity is defined as the average nodal strength over all brain regions. We note that while fine-grained analyses of voxel-wise connectivity have also been reported (Ferrarini et al., 2011; Hayasaka and Laurienti, 2010), here we focused our examination on average connectivity properties of resting state function derived from large anatomically-specified regions.
Multivariate Network Organization
While the mean and variance of the pairwise correlation matrix are useful measures of gross functional properties, neither characterizes the pattern of correlation values within the matrix. We quantitatively examine these patterns using complex network theory after converting the correlation matrix to a binary graph as described in the next section. Each graph can be characterized by a variety of diagnostic measures, although many of these measures are strongly correlated with one another (see for example Lynall et al. (2010)). Mathematically, many graph metric values are dependent on the number of nodes (brain regions) present in the graph. While the original connectivity matrix contains information from all brain regions, a threshold exists for all graphs such that nodes become disconnected from the graph, i.e., when no correlations for a node in the graph exceed the threshold applied during the graph construction process, so no edges connect that node to any other node in the graph. Therefore, a useful explanatory variable of interest is the size of the largest connected component (e.g., Achard et al. (2006)), defined as the largest number of nodes that form a connected group in the graph. In light of the dysconnectivity hypothesis of schizophrenia (Friston, 1998), this measure is particularly appropriate in the current context because it provides direct information regarding the global connectivity of the subject’s brain network. In addition, more complex graph metrics can be computed, including measures of global connectivity between all nodes (global efficiency (Latora and Marchiori, 2001) and betweenness centrality (Freeman, 1977)), local connectivity between subsets of nodes (clustering coefficient (Watts and Strogatz, 1998) and local efficiency (Latora and Marchiori, 2001)), and relationships between global and local connectivity (modularity (Leicht and Newman, 2008) and small-worldness (Watts and Strogatz, 1998)). See Bullmore and Bassett (2011) for detailed descriptions and applications, and Rubinov and Sporns (2009) for mathematical definitions of these 6 graph metrics.
Graph Construction
In order to perform the multivariate network analysis described above, it is necessary to convert the correlation matrices to binary graphs through the application of a threshold or set of thresholds (Bullmore and Bassett, 2011). The set of networks from a particular clinical group can be thresholded to create either equi-sparse graphs (different thresholds for each individual in order to ensure that all networks in the group have the same number of edges or ‘sparsity’; see for example Achard et al. (2006) and Bassett et al. (2006)) or equi-threshold graphs (the same threshold for each individual such that the networks usually have a different number of edges; see for example Hayasaka and Laurienti (2010), van den Heuvel et al. (2008), and van den Heuvel et al. (2009)). In both cases, network organization is most often examined over a wide range of sparsity values (Bassett and Bullmore, 2009; Bullmore and Sporns, 2009; Bullmore and Bassett, 2011). We chose to construct equi-sparse graphs in order to ensure the most direct mathematical comparability of graph metric values (Bullmore and Bassett, 2011).
Traditional Cumulative Thresholding
For each threshold over the full range of sparsity values from 0 (no node pairs connected) to 1 (all node pairs connected), we generate a binary matrix by setting all elements of the correlation matrix above that threshold to 1 and all elements below to 0. We choose threshold values that produce binary matrices with densities ranging from 0 to 1: for example, a density of 0.1 is obtained by using a threshold above which lie 10% of the elements of the correlation matrix. This “cumulative” thresholding procedure has been used extensively in previous functional brain network studies because it facilitates an investigation of a network composed of the x% statistically most significant connections (Bullmore and Bassett, 2011).
Alternative Windowed Thresholding
Graphs constructed by cumulative thresholding provide the most insight into the pattern of strong connections (for high thresholds). However, they provide less information regarding the pattern of weak connections. To address this weakness, we employed a windowed thresholding procedure in which graphs were constructed by retaining connections that fell in a threshold range rather than above a threshold (Schwarz and McGonigle, 2011). For example, a graph could be constructed from the 1% strongest connections, a second graph from the 1% next strongest connections, etc. In this way, 100 separate graphs can be constructed that facilitate the assessment of the pattern of different connection strengths: for example, we can investigate the topological organization of the 40 strongest connections separately from that of the 40 weakest connections. Windowed thresholding allows for an examination of independent sets of connections while the more traditional cumulative thresholding allows for an examination of additive, non-independent sets of connections.
Network Organization
Graph Curves
Both the windowed and cumulative thresholding procedures reduce full connectivity matrices (weighted graphs) into sets of binary matrices (single graphs or ‘graphlets’). As discussed in an earlier section, each graphlet may then be characterized using a variety of network measures (Costa et al., 2007; Rubinov and Sporns, 2009) to obtain unique graph metric values. The set of graph metric values derived from a given set of graphlets (and therefore a single full connectivity matrix or weighted graph) can be treated mathematically as a functional curve: the y-axis represents the graph metric value and the x-axis represents either connectivity density (in the case of cumulative thresholding) or window number (in the case of windowed thresholding). The complete network organization of each individual subject can then be characterized by one of the two curves (the cumulative curve or the windowed curve) depending on whether additive or independent network structure, respectively, are to be examined. Importantly, by transforming a correlation matrix into a complete set of binary graphs (also referred to as adjacency matrices), we are leveraging the inherent continuous structure of the correlation matrix. That is, in such a complex system, different neurophysiological drivers may be responsible for different portions of the network curves, and therefore a characterization of the entire curve is preferable to a characterization of a single point or portion of the curve.
Statistical Testing
To determine the existence of group differences in these curves, we utilized Functional Data Analysis (FDA), a statistical method that treats a curve as a function. FDA is a swiftly evolving branch of statistics used to characterize and compare curves (Ramsay and Silverman, 2005) and has been predominantly employed using MATLAB and R (Ramsay et al., 2009). Within FDA, there are several methods to compare sets of curves between groups. The simplest method is the non-parametric permutation test, which we employ here since the form of the graph-value-vs-density curves and their variance in the human population are currently unknown. Although beyond the scope of this paper, it is also possible to use parametric and semi-parametric tests based on complex statistical estimates of model parameters (for more information, see Ramsay and Silverman (2005)).
To perform the non-parametric test, we first treat each curve as a function (y = f(x)). For each graph metric, we compute the average curve for the healthy control group, ȳhc and the average curve for the schizophrenia group, ȳsz separately. We then compute the area, A, between the two curves ȳhc and ȳsz by summing the differences between y-values (graph metric values) of the two groups at each value of x (corresponding to windows for windowed curves and connection densities for cumulative curves): A = Σi |ȳhc(xi) − ȳsz(xi)|. The difference between the two groups was tested for significance using nonparametric permutation testing, whereby the group identity of each individual (a healthy control or a person with schizophrenia) was randomly reassigned without replacement. Average curves for the two pseudo groups were determined ( and ), and the area between the two curves was estimated, A′. This process was repeated for I iterations (here I = 10, 000) to create a set of I A′ values. The p-value of the true group difference, A, was defined as the number of A′ values that were greater than A divided by the number of iterations I.
Classification Using Support Vector Machines
We tested whether the size of the largest connected component in the graphs constructed by windowed thresholding could be used to predict diagnosis using a support vector machine (SVM) (Cristianini and Shawe-Taylor, 2000). The data was separated into a “training” data set and a “test” data set. We constructed 1000 training data sets by randomly sampling 14 healthy controls and 14 people with schizophrenia from their respective groups; for each training set, a test data set was chosen by randomly sampling 14 of the 15 subjects left in each group after removal of the training data (recall that the total size of each group was 29). After training a linear kernel SVM on the training data sets, we computed the average classification accuracy, sensitivity, and specificity of the SVM over the 1000 test data sets.
Software and Visualization
All computational and simple statistical operations (such as t-tests and correlations) were implemented using MATLAB (2009a, The MathWorks Inc., Natick, MA) software. Graph metrics were estimated using a combination of in-house software, the Brain Connectivity Toolbox (Rubinov and Sporns, 2009), and the MATLAB Boost Graph Library. Univariate, bivariate, and multivariate properties were visualized using surface projections in Caret (Van Essen Laboratory). The mapping of the image volume to the surface was performed using the Caret PALS-B12 (Population-Average, Landmark- and Surface-based) atlas, which is derived from 12 healthy young adult subjects. The image volume is mapped to each subject separately, and the average surface rendering is used for visualization; the inter-region boundaries are therefore blurred due to inter-subject differences, and the complete mapping therefore gives a realistic depiction of group effects.
Results
Cognitive Function and Symptom Scores
The memory and attention scores of the schizophrenia population were 6.91 (STD = 2.15) and 9.11 (STD = 1.77) respectively, while those for the healthy control population were 9.36 (STD = 1.94) and 12.43 (STD = 1.69); see Camchong et al. (2011) for further details and Table 1 for other demographic data. The schizophrenia cohort used in this study had an average SANS of 2.06 (STD = 0.79) and an average SAPS of 1.78 (STD = 0.64).
Table 1. Mean (and SD) Demographic and Diagnostic Characteristics of Participants.
| Schizophrenia | Controls | |
|---|---|---|
| Age | 41.3(9.28) | 41.1(10.6) |
| Education | 3.25(0.80) | 2.28(0.75) |
| SES | 3.62(0.98) | 5.46(1.40) |
| SANS | 10.31(3.66) | N/A |
| SAPS | 6.86(3.15) | N/A |
| Attention | 9.11(1.77) | 12.43(1.69) |
| Memory | 6.91(2.15) | 9.36(1.94) |
| Chronicity of Illness | 20.21(8.96) | N/A |
| MED | 35.41(41.00) | N/A |
Note: SES, Socioeconomic Status; SANS, Scales for Assessment of Negative Symptoms; SAPS, Scales for Assessment of Positive Symptoms; MED, chlorpromazine equivalents (available for 15 out of the 29 patients).
Examination of Univariate and Bivariate Complexity
Global Structure
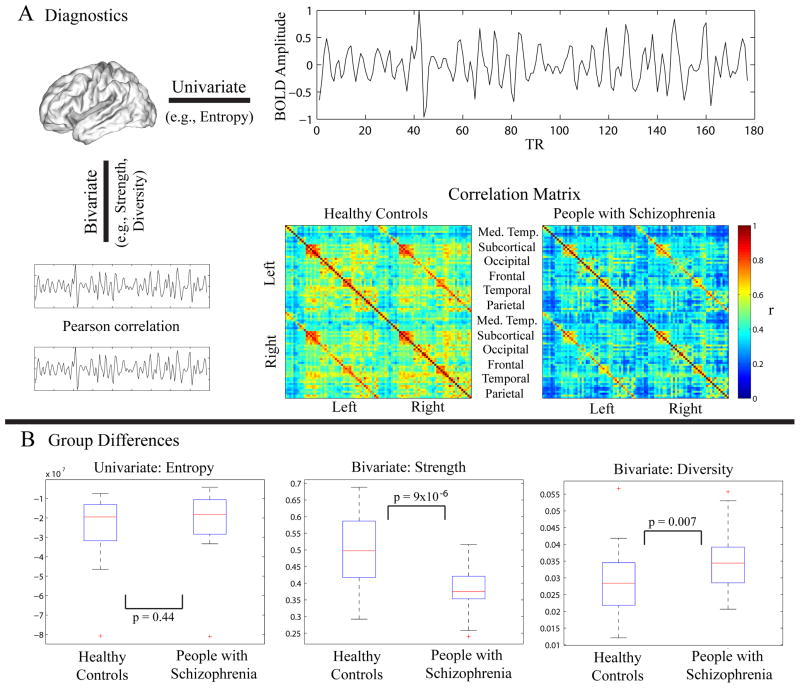
We first examined the complexity of resting state fMRI data using both univariate and bivariate measures (Fig 1A). The univariate complexity, measured by the average wavelet entropy of the regional mean time series extracted from all regions, was not significantly different between patients and controls (Fig 1B). Bivariate measures (strength and diversity), however, showed significant group differences. Strength was significantly decreased in patients, indicating that the magnitude of functional connectivity was lower in the schizophrenia cohort. Diversity, however, was significantly increased in patients, indicating an increased variance of that connectivity.
Figure 1. Examination of Functional Activity and Connectivity.
(A) Diagnostics. We characterized the fMRI data using both univariate and bivariate properties. We used the univariate property of wavelet entropy to characterize the average time series extracted from 90 cortical and subcortical regions defined by the AAL atlas. To estimate pairwise relationships, we computed the functional connectivity between any pair of regions using the Pearson correlation coefficient. Bivariate properties (strength and diversity) were estimated on the matrix of correlations between all possible pairs of ROIs. Strength is defined as the average of the column sum of the correlation matrix, while diversity is defined as the average of the column variance. Note that for ease of visualization, ROIs are ordered within the correlation matrices according to left and right hemisphere and then according to the respective functional module as defined by Salvador et al. (2005). (B) Group differences. While wavelet entropy was not significantly different between the two groups (2-sample t-test: t = −0.77, p = 0.44), strength was significantly higher in the controls (t = 4.86, p = 9.69 × 10−6) and diversity was significantly higher in the people with schizophrenia (t = 2.76, p = 0.007). Note: The edges of each box in these boxplots represent the 25th and 75th percentiles.
Regional Structure
In healthy controls, wavelet entropy was heterogeneously distributed throughout the brain with lowest wavelet entropy found in regions of the cuneus, precuneus, and temporal pole (Fig 2A). Strength showed a different spatial distribution, with highest values found in the temporal cortex, while diversity was highest in the precuneus and along the central sulcus (Fig 2B). The differences in the spatial distributions of these three measures may help to explain their differential sensitivity to disease state. Several regions in the posterior parietal, occipital, temporal, and central cortices showed group differences in wavelet entropy with p < .05, but none of these regions survived a multiple comparisons correction (Fig 2A). In contrast, both bivariate measures displayed broad sensitivities to disease state that survived Bonferroni correction (Fig 2B). Strength was significantly decreased in schizophrenia over the majority of the cortical and subcortical regions (62 out of 90 regions). Diversity was significantly increased in schizophrenia in bilateral superior frontal and left inferior temporal cortex.
Figure 2. Anatomy of Activity and Connectivity.
(Left) Spatial distribution of average wavelet entropy (A) as well as strength and diversity (B) in the healthy control group. (Right) Group differences in average wavelet entropy (A; regions for which p < .05 uncorrected), strength (B; p < .05, Bonferroni corrected) and diversity (B; p < .05, Bonferroni corrected).
Relationship Between Univariate and Bivariate Properties
It is intuitively plausible that the complexity of individual time series might influence their correlation with other time series, and indeed, Fig 3A shows that wavelet entropy has a positive correlation with strength: r = 0.39, p = 1 × 10−4 for the healthy controls and r = 0.51, p = 3 × 10−7 for the people with schizophrenia. A significant correlation was also observed between wavelet entropy and diversity in patients (r = 0.31, p = 0.002) but not in controls (r = −0.10, p = 0.34). Interestingly, the ability of wavelet entropy to predict functional connectivity is altered in disease: the magnitude of the correlation between wavelet entropy and strength is significantly larger in people with schizophrenia than in healthy controls (two-sample t-test, t = 2.34, p = 0.022), while the correlation between wavelet entropy and diversity is significantly lower (t = 3.40, p = 0.001); see Fig 3B.
Figure 3. Relationship Between Activity and Connectivity.
(A) Correlations between the complexity of activity (as measured by the time series wavelet entropy) and connectivity (as measured by the strength (Left) and diversity (Right) of the whole brain correlation matrices) for healthy controls (black) and people with schizophrenia (red). The correlation between group median wavelet entropy and mean strength was r = 0.39, p = 1 × 10−4 for the healthy controls and r = 0.51, p = 3 × 10−7 for the people with schizophrenia, while the correlation between wavelet entropy and diversity was r = −0.10, p = 0.34 for the controls and r = 0.31, p = 0.002 for the patients. Data points represent individual brain regions. (B) Boxplots showing significant group differences in Pearson’s r between strength and wavelet entropy (Left; two-sample t-test, t = 2.34, p = 0.022) and diversity and wavelet entropy (Right; t = 3.40, p = 0.001), computed for each individual. Note: the edges of each box are the 25th and 75th percentiles.
Examination of Multivariate Network Complexity
Network Organization
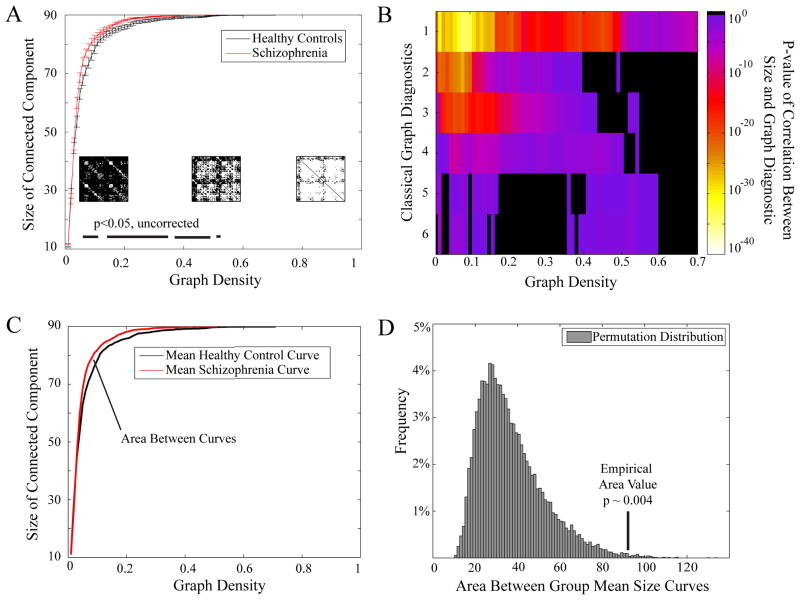
In addition to measuring strength and diversity, the correlation matrix can be examined in the context of complex network theory using a variety of graph theoretical measures (see Bullmore and Bassett (2011) and Methods). One of the simplest is the graph’s size, s, defined by the number of nodes in the largest connected component. We find that resting state fMRI networks, derived from both the healthy control and schizophrenic cohorts, become disconnected at density ~ 0.5 (Fig 4A). We note that the size of the graph, while a simple measure, is in fact a non-trivial predictor of a wide variety of other graph diagnostics used extensively in complex network analysis (Fig 4B) and therefore represents an important potential indicator of the underlying connectivity complexity that may be sensitive to disease state. Indeed, the size of the graph appears larger in the brain networks of people with schizophrenia, indicating that these networks have fewer disconnected nodes. This alteration is compatible with an increased network homogeneity in comparison to healthy controls. It has previously been unclear how to measure the statistical significance of these group differences given that multiple tests must be performed over an arbitrarily large number of graph densities. Instead, we therefore treat the entire size versus density curve as a function, and use functional data analysis (FDA) to determine that the significance of group differences in the shape of the curves was p = 0.004 by non-parametric permutation testing (see Methods and Fig 4C–D).
Figure 4. Group Differences in Patterns of Connectivity.
(A) The size of the largest connected component in brain graphs as a function of graph density for healthy controls (black) and people with schizophrenia (red). Error bars indicate standard deviation of the mean. Insets show binary matrices at three different graph densities; matrix elements shown in white indicate the existence of connections, while matrix elements shown in black indicate the absence of connections. Bars along the bottom indicate graph densities at which the p-value for a two-sample t-test showed significant group differences at p < .05 uncorrected. (B) P-values for the correlations between the size of the connected component and six more complex graph diagnostics (global efficiency, betweenness centrality, small-worldness, modularity, local efficiency, and clustering coefficient) as a function of density. For the majority of graph densities, the size of the connected component is a highly significant predictor of more complex graph measures: significant p-values are given by colors ranging from purple (p ~ .05) to white (p ~ 10−40) while non-significant p-values are show in black (p > 0.05). (C) & (D) Functional Data Analysis (FDA) can be used to test for group differences in graph metric curves like the size of the connected component as a function of graph density. (C) In order to determine the differences between the group curves shown in (A), we compute the area between the group mean curves. (D) We then permute group membership among the subjects to construct a permuted distribution (histogram shown in gray) and compare that to the ’empirical area value’ determined in (C). We find that the area between the two curves is significantly larger than expected in the null distribution: p = 0.004.
Effect of Correlation Strength on Network Organization
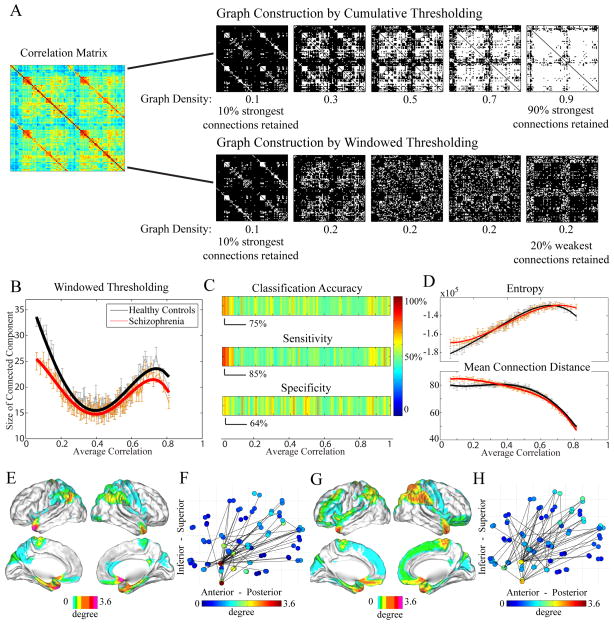
Given our finding that the pattern of resting state correlations (strength, diversity, and network size) is altered in schizophrenia, it is natural to ask whether this predictive power is affected by the strength of the correlations. To address this question, we use a windowed thresholding approach as described in the Methods section and depicted in Fig 5A to determine the network size as a function of threshold range and then compare the set of curves from the controls to the set from the patients (Fig 5B) using FDA. The shape of these curves is significantly different as determined by non-parametric permutation testing, p = 5 × 10−5. Interestingly, the most significant discrepancies between the two curves occur for graphs derived from weak correlations (Fig 5B), suggesting that the topological distribution of weak connections is particularly sensitive to disease state. To test this hypothesis, we used support vector machines to determine sensitivity to disease state, and found that the size of the graphs derived from weak correlations provide up to 75% classification accuracy, 85% sensitivity, and 64% specificity (Fig 5C). We note that the sensitivity of our findings (85%) is higher than that previously reported for other network classification efforts in Alzheimer’s disease (72%) (see Supekar et al. (2008)). Weak correlations were predominantly found between regions of low wavelet entropy and tended to link regions that were far apart in physical space (Fig 5D). The hubs of the weak correlation network included the olfactory cortex, temporal pole, angular gyrus, parahippocampus, amygdala, caudate, and pallidum (Fig 5E), which connected to a swath of posterior parietal cortex (Fig 5F). and their connectivity profiles in our patient cohort were significantly more diffuse (Fig 5G,H).
Figure 5. Examining Graph Structure.
(A) We can construct graphs using one of two methods: cumulative or windowed thresholding of the correlation matrix. In the commonly used cumulative thresholding procedure (top), matrix elements (graph links) with lower and lower correlation values are retained in the graph, such that the density (number of links) of the graph increases. In a windowed thresholding procedure (bottom), matrix elements (graph links) whose correlation values lie within a given correlation range (or window) are retained in the graph. The density of each graph is therefore determined by the correlation range. (B) Curves for the size of the largest connected component for both healthy controls (black) and people with schizophrenia (red) derived via the windowed thresholding procedure showed significant group differences (p < 5 × 10−5, via FDA permutation testing). (C) Using a support vector machine, the classification accuracy, sensitivity, and specificity of the size of the connected component as a function of window (data shown in (B)) were determined to be highest for windows that included weak correlations. Color bar indicates percent. (D) The median wavelet entropy (top) of nodes and the mean connection distance of the edges (bottom) present in the windows shown in (B). Windows constructed from weak correlations on average contain nodes of low wavelet entropy and edges linking distant regions. Node connectivity (E),(G) and average graph (F),(H) in a weak correlation window for healthy controls and people with schizophrenia, respectively. Color indicates average degree. The window chosen for this figure is the window that provided the highest classification accuracy and sensitivity shown in (C); however, results are consistent for a range of windows constructed from weak correlations. Note in comparing (A) and (B): While graph diagnostics are often plotted as a function of density when constructed by cumulative thresholding (networks of strong correlations lying on the left of the plot), when constructing graphs from windowed thresholding, diagnostics can be plotted as a function of average correlation (networks of strong correlations lying on the right of the plot).
Classification
Given the strong sensitivity of network properties of weak correlations to disease state, we asked whether this network structure could be related to cognitive variables and symptom scores. We found that the size of the graph was significantly correlated with composite attention (p = 4.5 × 10−5 uncorrected; Fig 6A), composite memory (p = 0.036 uncorrected; Fig 6B), and SANS scores (p = 0.001 uncorrected; Fig 6C), indicating a direct relationship between the pattern of weak correlations and external cognitive or behavioral variables. Individuals with low attention and memory scores and high SANS scores displayed smaller graphs whose nodes were spread over a broader swath of brain regions.
Figure 6. Cognitive Variables and Symptoms Scores.
Composite attention (A), composite memory (B), and SANS (C) scores as a function of the size of the connected component for healthy controls (black) and people with schizophrenia (red). For the two groups combined, the size of the connected component was significantly correlated with both attention (r = 0.50, p = 4.5 × 10−5 (A)) and memory (r = 0.27, p = 0.036 (B)); best fits are shown with a solid gray line. No significant correlations were found within the two groups separately; best fits are shown in the dotted black (red) line for the control (schizophrenia) population. The size of the connected component was also significantly correlated with the SANS scores in the schizophrenia population (r = −0.43, p = 0.018); best fit shown in the solid red line in (C). No significant correlations were found with the SAPS scores. Network size estimates are taken from the window in which the maximum classification accuracy and sensitivity were identified (see Fig 5C).
Discussion
Schizophrenia is a complex, well-studied but poorly-understood disease. Important factors slowing the progress of understanding the disease are many, and include the large variety in disease populations and the inherent difficulty in combining information from the range of neuroscientific inquiry, e.g., over data modalities, spatial scales, and analysis methods. These methods range from univariate analysis of the BOLD amplitude or EEG/MEG signal power (from which we identify focal processing abnormalities) to bivariate functional connectivity analysis (from which we identify altered relationships between voxels or regions of interest) to large-scale multivariate network analysis (from which we identify whole-brain disconnectivity patterns). While each of these analysis methods provides a piece of the disease picture, it is impossible to form a complete account of schizophrenia without building an understanding of the relationships between findings extracted from these diverse analytic streams.
To address this issue, our work draws together two important but previously separate approaches to the measurement of complexity in the brain system: 1) the classical approach of determining univariate estimates of the complexity of brain signals (Bullmore et al., 2009) and 2) the more recent multivariate approach based on the application of complex network theory (Bassett and Bullmore, 2006; Bullmore and Sporns, 2009; Bassett and Bullmore, 2009; Bullmore and Bassett, 2011). In particular, we show that a region with high wavelet entropy is more likely to have stronger functional connections to the rest of the brain than a region with low wavelet entropy, and the strength of this relationship is altered in schizophrenia. We further show that the organization of weak connections, which have historically been less well studied, have greater diagnostic power than that of strong connections. Finally, we introduce and employ a statistical framework for the comparison of functional connectivity structure between groups which we believe will prove generally useful in a broader context.
Altered Functional Connectivity in Schizophrenia
The intrinsic activity and connectivity of the human brain at rest is highly organized (Raichle and Snyder, 2007) while displaying significant variation between individuals and in disease states such as schizophrenia (see for example, Lynall et al. (2010) and Skudlarski et al. (2010)). Our results show that both strength and diversity are significantly different in the schizophrenia cohort, providing further supporting evidence for this conclusion. In a previous study, Lynall et al. (2010) reported group differences in strength and diversity that passed FDR correction (rather than the Bonferroni correction used in the present study) albeit within a smaller subset of the brain regions studied here (72 rather than 90). Our work confirms and extends Lynall’s findings, thereby joining a growing body of literature indicating abnormal resting state functional and structural dysconnectivity in schizophrenia (Zalesky et al., 2011; van den Heuvel et al., 2010; Skudlarski et al., 2010; Rubinov et al., 2009a).
Functional connectivity at rest is thought to support self-referential or introspective mental processes (Fair et al., 2008) that might be altered in people with schizophrenia, who display altered self-attribution and self-reference (Pauly et al., 2011), self-concept (Nelson et al., 2009), self-recognition (Waters and Badcock, 2010), and self-experience (Lysaker and Lysaker, 2010). Some have argued that failures of self-monitoring in schizophrenia can be explained mechanistically via altered neurophysiological phenomena, specifically aberrant N-methyl-D-aspartate receptor (NMDAR)-mediated synaptic plasticity (Stephan et al., 2009), and that large-scale functional connectivity measurements at rest might provide key insight into smaller-scale neurophysiological abnormalities in the disease. Recent work has directly linked resting state functional connectivity to patterns of the neurophysiological process of aerobic glycolysis (Vaishnavi et al., 2010). Abnormal connectivity in schizophrenia, therefore, may accompany abnormal glycolytic processes within the disease. Although previous work has provided strong genetic evidence for a link between regulatory enzymes in glycolysis and schizophrenia (Stone et al., 2004), a direct relationship between altered glycolysis and altered functional connectivity measurements in schizophrenia has yet to be validated.
Our results indicate that the univariate complexity measure of wavelet entropy was not as sensitive to disease state as higher order measures (strength and diversity). This finding highlights the importance of higher order statistics in the study of schizophrenia, consistent with previous studies. For example, in a study of scalp EEG data acquired from first episode schizophrenia patients, a dysregulation in the organization of interactions (multivariate) was identified rather than a decrease in the strength of those interactions (bivariate) (Breakspear et al., 2003). However, it is important to note that these findings do not dismiss the potential for some univariate measures to display disease sensitivity, particularly those which – like the bivariate interactions statistics – are based on the temporal evolution of the signal. Examples of such measures include the Renyi number, an entropy number based on time-frequency representations (Gonzalez et al., 2000), the multi-scale entropy (Subha et al., 2010), the Lyapunov exponent (Xie et al., 2008), the fractal dimension (Rubinov et al., 2009b), and the Hurst exponent (Bullmore et al., 2001).
Univariate Constraints on Functional Connectivity
The emergence of complex network theory as a means of quantitatively characterizing functional connectivity structure within a mathematical framework has led to a burgeoning body of neuroimaging literature (Bassett and Bullmore, 2009). However, it has not been clear how the network organization of functional connectivity is related to the more commonly studied activity of individual brain regions. In this work, we have taken the first step to address this issue. We have shown that the complexity of the time series from an individual brain region is strongly predictive of the functional connectivity to the rest of the brain. Our work therefore suggests that neurobiological drivers of brain complexity or ‘noise’ – such as those that have been linked to aging, development, and cognitive ability (McIntosh et al., 2010; Lippé et al., 2009; Garrett et al., 2010; Meyer-Lindenberg and Bassett, 2008) – might also constrain and predict functional connectivity. This relationship provides a critical and previously unexplored framework in which to discuss the neurophysiological interpretations of connectivity patterns: if neurotransmitter levels modulate BOLD activity (Valli et al., 2011), and activity is correlated with the organization of connectivity as reported here, then alterations in functional connectivity may be linked back to alterations in neurophysiological phenomena. Importantly, our preliminary results indicate that this relationship between activity and connectivity is highly sensitive to disease state, suggesting significant biological relevance. While the meaning of this finding for schizophrenia is as yet unclear, we can speculate that the altered relationship between entropy and correlation strength indicates that an underlying mechanism of brain oscillatory activity alters both the randomness of the signal and its temporal evolution. While our results are specific to the disease of schizophrenia, it would be of interest in future studies to determine whether similar alterations in univariate-bivariate relationships are evident in other diseases (e.g., Alzheimer’s). More generally, further work is necessary to more comprehensively examine the relationship between activity and connectivity in the context of task, disease, and development and the resulting impact on cognition.
Importance of Weak Connections
In the characterization of functional connectivity through complex network theory, previous works have generally focused on the examination of the most correlated regions – i.e., the strongest links in a network present at the sparsest network densities – to characterize the network’s organization and to test for significant effects of diagnostic group (Bassett and Bullmore, 2009; Bullmore and Bassett, 2011). The topological organization of weak correlations has remained largely unexplored. Here we take inspiration from the social sciences, where Granovetter’s work on the ‘strength of weak ties’ (Granovetter, 1983) points out the surprisingly strong influence of weak inter-relationships on the dynamics of a system. In this context, we note that the location of weak correlations (disconnections) may provide as much insight into brain function as the location of strong correlations (connections).
Using a windowed thresholding approach, we show that the organization of weak correlations is in fact more sensitive to the effect of diagnostic group than that of strong correlations (up to 75% classification accuracy, 85% sensitivity, and 64% specificity), providing a unique and previously uninvestigated method of network interrogation. The relative insensitivity of strong correlation networks to disease state is consistent with the idea that strong correlations may be held relatively constant in all human brains, whether healthy or diseased. We find that weak correlations typically link distant regions with low wavelet entropy and are specifically located between subcortical and temporal structures and the posterior parietal and midline cortices, all regions known to show structural and functional abnormalities in schizophrenia (Moberg et al., 1999; van den Heuvel et al., 2010; Arzy et al., 2006; Nenadic et al., 2010; Talamini et al., 2005; Koch et al., 2008; Salvador et al., 2010; Anticevic et al., 2011). The specificity of these correlations to long connections suggests the intriguing possibility that schizophrenia might target low frequency oscillatory neuronal activity thought to enable communication between distant areas (Kopell et al., 2000; Roopun et al., 2008).
Our finding that weak correlations are more diffusely localized in schizophrenia, leading to a smaller network size, is compatible with the idea that while disconnectivity between certain brain regions is expected in a healthy brain, the schizophrenic brain displays a broader disconnectivity that may underpin cognitive deficits evident in the disease. In support of this hypothesis, we show that the organization of weak correlations is not only sensitive to diagnostic group but also to cognitive scores – including attention and memory – and negative symptom scores, suggesting that the lack of connectivity in these regions is a highly relevant neurophysiological correlate.
Methodological Considerations
The construction of binary graphs from functional connectivity correlation matrices has historically required the choice of a threshold or range of thresholds over which to assess network structure (Bullmore and Bassett, 2011). Graph metrics are calculated at each threshold choice, and pointwise comparisons are made between the graph metric values of one group at a particular threshold and the values of a second group. However, the choice of these thresholds is rather arbitrary and unthresholded networks have in some cases proven more sensitive to disease state (Rubinov et al., 2009a). In this work we used a novel approach by employing FDA. FDA allows for a statistical comparison between groups of the shapes of the graph-metric-versus-network-density curves over the full range of possible densities. Not only is this method independent of arbitrary threshold choices, but it also facilitates a more comprehensive examination of the entire topological structure of a correlation matrix by incorporating the entire network density range. FDA can be used to compare any two sets of graph metric curves – clustering, path-length, efficiency, assortativity, etc. – and therefore represents a powerful tool for the statistically principled examination of group differences in network structure.
Several important potential limitations of the current work should be mentioned. First, the size of the sample (29 controls and 29 patients) is relatively small and does not allow us to assess the relative differences between subtypes of the disease. Secondly, all patients in the study were medicated, and therefore the effects of medication cannot be distinguished from the effects of the underlying disease state. However, we note that the chlorpromazine equivalents (available for 15 patients) were not significantly correlated with strength (r = −0.07, p = 0.78), diversity (r = −0.12, p = 0.63), entropy (r = 0.09, p = 0.71), or the size of the connected component over the correlation windows (r < 0.51, p > 0.05 FDR corrected). Thirdly, we note that while network size was significantly correlated with cognitive variables of attention and memory in the two groups combined (Figure 6A–B), no significant correlations were observed with these two variables for the two groups separately. Two potential explanations for this finding are that 1) no correlation exists between network size and attention/memory in the control or schizophrenic groups or 2) the size or cognitive range of our sample was not large enough to sensitively measure a correlation between these variables. From the current study, we cannot determine which of these two possibilities is the case. And finally, we have noted that the classification results we have obtained are higher than those previously reported in Alzheimer’s disease (Supekar et al., 2008). However, from the current study we cannot determine whether our increased sensitivity is due to our alternative methods employing weak correlations, a difference in schizophrenia versus Alzheimer’s disease, or our particular schizophrenia cohort.
Conceptually, it is also important to note that the approach presented in this study, consistent with other similar studies (e.g., Lynall et al. (2010) and Skudlarski et al. (2010)), considers a single static network structure as an average representation of the overall resting state functional connectivity over the experimental duration. However, evidence suggests that resting state functional connectivity architecture evolves in time (Park et al., 2011) and displays spatially distinct and temporally coherent subnetworks (Damoiseaux et al., 2006). One potential method for examining the dynamics of resting state function is the construction of dynamic network models, as have recently been extracted from task data during a simple motor learning paradigm (Bassett et al., 2011). Alternatively, multiple network models can be extracted from resting state time series using approaches based on independent components analysis (ICA), which when applied to schizophrenia (Calhoun et al., 2009) have identified several independent networks whose intra- and inter-network connectivity is altered in the disease (Jafri et al., 2008; Woodward et al., 2011).
Conclusion
Here we develop a novel multi-level framework for the analysis of univariate, bivariate, and multivariate estimates of complexity in brain signals. We identify a strong relationship between the univariate and bivariate measures, and find that this relationship is significantly altered in disease. Our results underscore the critical role of connectivity patterns in understanding brain function in schizophrenia and specifically highlight the as-yet-unexplored networks of weak correlations, which appear to be sensitive to diagnosis, cognition, and symtomatology. Furthermore, we develop and report a general framework for statistical testing of group differences in graph curves based on FDA, which is generalizable to the study of group differences in any network property.
Research Highlights.
Multi-level framework exposes relationship between brain activity and connectivity
Connectivity shows greater group differences than measures of resting state activity
Patterns of weak connections show potential as a clinical biomarker
Patterns of weak connections are correlated with cognitive performance and symptoms
Acknowledgments
Addendum
D.S.B. was supported by the David and Lucile Packard Foundation, PHS Grant NS44393 and the Institute for Collaborative Biotechnologies through contract no. W911NF-09-D-0001 from the U.S. Army Research Office. Additional support for this research was provided by the National Institute of Mental Health (R01MH060662), Training Grant T32DA007097 for J.C., and the Center for Magnetic Resonance Research (BTRR P41 RR008079 and NCC grant P30 NS057091).
Footnotes
Competing Interests: The authors declare that they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26 (1):63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: a meta-analysis. Am J Psychiatry. 1999;156 (9):1358–66. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia. definition and reliability. Arch Gen Psychiatry. 1982;39 (7):784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S. Negative v positive schizophrenia. definition and validation. Arch Gen Psychiatry. 1982;39 (7):789–94. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. 2011 doi: 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzy S, Seeck M, Ortigue S, Spinelli L, Blanke O. Induction of an illusory shadow person. Nature. 2006;443 (7109):287. doi: 10.1038/443287a. [DOI] [PubMed] [Google Scholar]
- Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the ‘resting’ brain. Magn Reson Imaging. 2008;26 (7):1055–64. doi: 10.1016/j.mri.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Barnes A, Bullmore ET, Suckling J. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One. 2009;4 (8):e6626. doi: 10.1371/journal.pone.0006626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Brown JA, Deshpande V, Carlson JM, Grafton ST. Conserved and variable architecture of human white matter connectivity. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Opin Neurol. 2009;22 (4):340–347. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28 (37):9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Meyer-Lindenberg A, Achard S, Duke T, Bullmore E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci USA. 2006;103:19518–19523. doi: 10.1073/pnas.0606005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Meyer-Lindenberg A, Weinberger DR, Coppola R, Bullmore E. Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci USA. 2009;106 (28):11747–11752. doi: 10.1073/pnas.0903641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Wymbs NF, Porter MA, Mucha PJ, Carlson JM, Grafton ST. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 2011;108 (18):7641–6. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107 (10):4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bob P, Susta M, Chladek J, Glaslova K, Palus M. Chaos in schizophrenia associations, reality or metaphor? Int J Psychophysiol. 2009;73 (3):179–85. doi: 10.1016/j.ijpsycho.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Bosl W, Tierney A, Tager-Flusberg H, Nelson C. EEG complexity as a biomarker for autism spectrum disorder risk. BMC Med. 2011;9:18. doi: 10.1186/1741-7015-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakspear M. The nonlinear theory of schizophrenia. Aust N Z J Psychiatry. 2006;40 (1):20–35. doi: 10.1080/j.1440-1614.2006.01737.x. [DOI] [PubMed] [Google Scholar]
- Breakspear M, Terry JR, Friston KJ, Harris AW, Williams LM, Brown K, Brennan J, Gordon E. A disturbance of nonlinear interdependence in scalp eeg of subjects with first episode schizophrenia. Neuroimage. 2003;20 (1):466–78. doi: 10.1016/s1053-8119(03)00332-x. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33 (3):279–96. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Barnes A, Bassett DS, Fornito A, Kitzbichler M, Meunier D, Suckling J. Generic aspects of complexity in brain imaging data and other biological systems. Neuroimage. 2009;47 (3):1125–34. doi: 10.1016/j.neuroimage.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Bassett DS. Brain graph models: Graphical models of the human brain connectome. Annu Rev Clin Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Long C, Suckling J, Fadili J, Calvert G, Zelaya F, Carpenter TA, Brammer M. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp. 2001;12 (2):61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10 (3):186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3(17) doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Macdonald AWr, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37 (3):640–50. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities, A survey of factor-analytic studies. Cambridge University Press; 1993. [Google Scholar]
- Coifman RR, Wickerhauser MV. Entropy-based algorithms for best basis selection. 1992;38 (2):713–718. [Google Scholar]
- Costa LDF, Rodrigues FA, Travieso G, Villas Boas PR. Characterization of complex networks: A survey of measurements. Advances in Physics. 2007;56 (1):167–242. [Google Scholar]
- Cristianini N, Shawe-Taylor J. An Introduction to Support Vector Machines and Other Kernel-based Learning Methods. Cambridge University Press; 2000. [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103 (37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho DL, Johnstone IM. Ideal de-noising in an orthonormal basis chosen from a library of bases. CRAS Paris, Ser I. 1994;319:1317–1322. [Google Scholar]
- Esslinger C, Kirsch P, Haddad L, Mier D, Sauer C, Erk S, Schnell K, Arnold C, Witt SH, Rietschel M, Cichon S, Walter H, Meyer-Lindenberg A. Cognitive state and connectivity effects of the genome-wide significant psychosis variant in znf804a. Neuroimage. 2011;54 (3):2514–23. doi: 10.1016/j.neuroimage.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, Witt SH, Rietschel M, Cichon S, Meyer-Lindenberg A. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324 (5927):605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105 (10):4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarini L, Veer IM, van Lew B, Oei NY, van Buchem MA, Reiber JH, Rombouts SA, Milles J. Non-parametric model selection for subject-specific topological organization of resting-state functional connectivity. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.02.028. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM-IV axis i disorderspatient edition (scid-i/p) 1995. [Google Scholar]
- Freeman LC. A set of measures of centrality based on betweenness. Sociometry. 1977;40:35–41. [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30 (2):115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. 2006;115 (2):266–75. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL. Blood oxygen level-dependent signal variability is more than just noise. J Neurosci. 2010;30 (14):4914–21. doi: 10.1523/JNEUROSCI.5166-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez ASL, Grave de Peralta Menendez R, Thut G, Spinelli L, Blanke O, Michel CM, Seeck M, Landis T. Measuring the complexity of time series: an application to neurophysiological signals. Hum Brain Mapp. 2000;11 (1):46–57. doi: 10.1002/1097-0193(200009)11:1<46::AID-HBM40>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granovetter M. The strength of weak ties: A network theory revisited. Sociological Theory. 1983;1:201–233. [Google Scholar]
- Hayasaka S, Laurienti PJ. Comparison of characteristics between region-and voxel-based network analyses in resting-state fMRI data. Neuroimage. 2010;50 (2):499–508. doi: 10.1016/j.neuroimage.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39 (4):1666–81. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. 2002;17 (2):825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Koch G, Ribolsi M, Mori F, Sacchetti L, Codecà C, Rubino IA, Siracusano A, Bernardi G, Centonze D. Connectivity between posterior parietal cortex and ipsilateral motor cortex is altered in schizophrenia. Biol Psychiatry. 2008;64 (9):815–819. doi: 10.1016/j.biopsych.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97:1867–72. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Leicht EA, Newman MEJ. Community structure in directed networks. Phys Rev Lett. 2008;100 (11):118703. doi: 10.1103/PhysRevLett.100.118703. [DOI] [PubMed] [Google Scholar]
- Lippé S, Kovacevic N, McIntosh AR. Differential maturation of brain signal complexity in the human auditory and visual system. Front Hum Neurosci. 2009;3:48. doi: 10.3389/neuro.09.048.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall ME, Bassett DS, Kerwin R, McKenna P, Muller U, Bullmore ET. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30 (28):9477–87. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysaker PH, Lysaker JT. Schizophrenia and alterations in self-experience: a comparison of 6 perspectives. Schizophr Bull. 2010;36 (2):331–40. doi: 10.1093/schbul/sbn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AR, Kovacevic N, Lippe S, Garrett D, Grady C, Jirsa V. The development of a noisy brain. Arch Ital Biol. 2010;148 (3):323–37. [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Bassett DS. Nonlinear and cooperative dynamics in the human brain: Evidence from multimodal neuroimaging. In: Fuchs A, Jirsa V, editors. Coordination: Neural, Behavioral and Social Dynamics. Vol. 17 of Understanding Complex Systems. Springer; Berlin/Heidelberg: 2008. pp. 161–181. [Google Scholar]
- Misic B, Mills T, Taylor MJ, McIntosh AR. Brain noise is task dependent and region specific. J Neurophysiol. 2010;104 (5):2667–76. doi: 10.1152/jn.00648.2010. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21 (3):325–40. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Nelson B, Fornito A, Harrison BJ, Yücel M, Sass LA, Yung AR, Thompson A, Wood SJ, Pantelis C, McGorry PD. A disturbed sense of self in the psychosis prodrome: linking phenomenology and neurobiology. Neurosci Biobehav Rev. 2009;33 (6):807–17. doi: 10.1016/j.neubiorev.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Nenadic I, Smesny S, Schlösser RG, Sauer H, Gaser C. Auditory hallucinations and brain structure in schizophrenia: voxel-based morphometric study. Br J Psychiatry. 2010;196 (5):412–413. doi: 10.1192/bjp.bp.109.070441. [DOI] [PubMed] [Google Scholar]
- Park B, Kim JI, Lee D, Jeong SO, Lee JD, Park HJ. Are brain networks stable during a 24-hour period? Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.07.049. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Pauly K, Kircher T, Weber J, Schneider F, Habel U. Self-concept, emotion and memory performance in schizophrenia. Psychiatry Res. 2011;186 (1):11–7. doi: 10.1016/j.psychres.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Percival DB, Walden AT. Wavelet Methods for Time Series Analysis. Cambridge University Press; 2000. [Google Scholar]
- Potts GF, O’Donnell BF, Hirayasu Y, McCarley RW. Disruption of neural systems of visual attention in schizophrenia. Arch Gen Psychiatry. 2002;59 (5):418–424. doi: 10.1001/archpsyc.59.5.418. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37 (4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Ramsay JO, Hooker G, Graves S. Functional Data Analysis with R and MATLAB (Use R) 2009. [Google Scholar]
- Ramsay JO, Silverman BW. Functional data analysis. Springer; 2005. [Google Scholar]
- Roopun AK, Kramer MA, Carracedo LM, Kaiser M, Davies CH, Traub RD, Kopell NJ, Whittington MA. Period concatenation underlies interactions between gamma and beta rhythms in neocortex. Front Cell Neurosci. 2008;2:1. doi: 10.3389/neuro.03.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AW, Williams LM, Breakspear M. Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009a;30 (2):403–16. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2009;52 (3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O, van Leeuwen C, Breakspear M. Symbiotic relationship between brain structure and dynamics. 2009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Sarró S, Gomar JJ, Ortiz-Gil J, Vila F, Capdevila A, Bullmore E, McKenna PJ, Pomarol-Clotet E. Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Hum Brain Mapp. 2010;31 (12):2003–2014. doi: 10.1002/hbm.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–1342. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, McGonigle J. Negative edges and soft thresholding in complex network analysis of resting state functional connectivity data. Neuroimage. 2011;55 (3):1132–46. doi: 10.1016/j.neuroimage.2010.12.047. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skud-larska BA, Pearlson G. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68 (1):61–9. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17 (3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy R, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (S1):208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiat. 2006;59 (10):929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35 (3):509–27. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WS, Faraone SV, Su J, Tarbox SI, Van Eerdewegh P, Tsuang MT. Evidence for linkage between regulatory enzymes in glycolysis and schizophrenia in a multiplex sample. Am J Med Genet B Neuropsychiatr Genet. 2004;127B (1):5–10. doi: 10.1002/ajmg.b.20132. [DOI] [PubMed] [Google Scholar]
- Subha DP, Joseph PK, Acharya UR, Lim CM. Eeg signal analysis: a survey. J Med Syst. 2010;34 (2):195–212. doi: 10.1007/s10916-008-9231-z. [DOI] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer’s disease. PLoS Comput Biol. 2008;4 (6):e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Cho RY, Mizuno T, Kikuchi M, Murata T, Takahashi K, Wada Y. Antipsychotics reverse abnormal eeg complexity in drug-naive schizophrenia: a multiscale entropy analysis. Neuroimage. 2010;51 (1):173–82. doi: 10.1016/j.neuroimage.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamini LM, Meeter M, Elvevåg B, Murre JM, Goldberg TE. Reduced parahippocampal connectivity produces schizophrenia-like memory deficits in simulated neural circuits with reduced parahippocampal connectivity. Arch Gen Psychiatry. 2005;62 (5):485–493. doi: 10.1001/archpsyc.62.5.485. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer B, Landeau D, Papathanassiou F, Crivello O, Etard N, Delcroix B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107 (41):17757–62. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli I, JS, Mechelli A, Bhattacharyya S, Raffin M, Allen P, Fusar-Poli P, Lythgoe D, O’Gorman R, Seal M, McGuire P. Altered medial temporal activation related to local glutamate levels in subjects with prodromal signs of psychosis. Biol Psychiatry. 2011;69 (1):97–9. doi: 10.1016/j.biopsych.2010.08.033. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30 (47):15915–26. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Boersma M, Hulshoff Pol HE. Small-world and scale-free organization of voxel-based resting-state functional connectivity in the human brain. Neuroimage. 2008;43 (3):528–39. doi: 10.1016/j.neuroimage.2008.08.010. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJ, Kahn RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29 (23):7619–24. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters FA, Badcock JC. First-rank symptoms in schizophrenia: reexamining mechanisms of self-recognition. Schizophr Bull. 2010;36 (3):510–7. doi: 10.1093/schbul/sbn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393 (6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Rogers B, Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr Res. 2011;130 (1–3):86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Cao Z, Weng X. Spatiotemporal nonlinearity in resting-state fmri of the human brain. Neuroimage. 2008;40 (4):1672–85. doi: 10.1016/j.neuroimage.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, Egan GF, Pantelis C. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69 (1):80–9. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]