Abstract
γ-Secretase is a membrane embedded aspartyl protease complex with presenilin as the catalytic component. Along with β-secretase, this enzyme produces the amyloid β-protein (Aβ) of Alzheimer’s disease (AD) from the amyloid β-protein precursor (APP). Because of its key role in the pathogenesis of AD, γ-secretase has been a prime target for drug discovery, and many inhibitors of this protease have been developed. The therapeutic potential of these inhibitors is virtually negated by the fact that γ-secretase is an essential part of the Notch signaling pathway, rendering the compounds unacceptably toxic upon chronic exposure. However, these compounds have served as useful chemical tools for biological investigations. In contrast, γ-secretase modulators continue to be of keen interest as possible AD therapeutics. These modulators either shift Aβ production to shorter, less pathogenic peptides or inhibit the proteolysis of APP selectively compared to that of Notch. The various chemical types of inhibitors and modulators will be discussed, along with their use as probes for basic biology and their potential as AD therapeutics.
Keywords: protease, amyloid, active site, docking site, allosteric sites, chemical probes
γ-Secretase as a therapeutic target for Alzheimer’s disease
A considerable body of evidence supports the amyloid β-protein (Aβ), particularly its 42-residue variant (Aβ42), as a key initiator in the pathogenesis of Alzheimer’s disease (AD) (Tanzi & Bertram 2005, Goedert & Spillantini 2006). While ongoing research strives to discern which assembled form or forms of this aggregation-prone peptide are the critical deleterious species and what pathways mediate toxicity, it seems clear that safe and selective lowering of Aβ or Aβ42 levels in the brain should prevent or delay the onset of AD and may slow progression if effective agents are given early enough (i.e., before or soon after the first signs of cognitive impairment). For this reason, extraordinary efforts have been expended by many laboratories around the world over the course of two decades to identify agents that target Aβ or Aβ production. Such agents should be metabolically stable, brain accessible and safe enough for administration over many years, criteria that have been quite challenging to meet.
The proteases responsible for generating Aβ from its precursor protein APP, β- and γ-secretases, have been top targets for AD drug discovery (Ghosh et al. 2008, Wolfe 2008). The membrane-tethered β-secretase sheds the ectodomain of the type I membrane protein APP, leaving behind a 99-residue stub (C99) that is then cleaved by the membrane-embedded γ-secretase complex to release Aβ and the APP intracellular domain (AICD). β-Secretase has been an especially difficult target, as compounds that potently inhibit this pepsin-family aspartyl protease tend to have poor pharmaceutical and pharmacokinetic properties. In contrast, a variety of γ-secretase inhibitors (GSIs) have been reported, many of which are active in vivo, but this enzyme has presented its own challenges for drug discovery, as discussed below.
γ-Secretase is a complex of four integral membrane proteins, with presenilin-1 (PS1) or presenilin-2 (PS2) as the catalytic component of a noncanonical aspartyl protease (Wolfe 2006). Assembly of presenilin with the other components, Nicastrin, Aph-1 and Pen-2, leads to the cleavage of presenilin into two subunits, an N-terminal fragment (NTF) and C-terminal fragment (CTF), each of which contributes one transmembrane aspartate to the active site. The active protease complex hydrolyzes the transmembrane domain of APP to produce a heterogeneous set of secreted Aβ peptides, varying in their C-termini and ranging from 38–43 residues, and AICDs of 50 or 51 residues. Some 6–11 APP transmembrane residues are unaccounted for in considering the products Aβ and AICD; however, the enzyme apparently first cuts C99 at the so-called ε site to form Aβ48 or Aβ49 and AICD51 or AICD50 (Sato et al. 2003, Funamoto et al. 2004, Qi-Takahara et al. 2005). Release of AICD is followed by trimming of these long Aβ peptides every 3–4 residues until Aβ dissociates (Qi-Takahara et al. 2005). Indeed, the tri- and tetrapeptides have been identified by mass spectrometry, thereby accounting for the missing residues (Takami et al. 2009).
The γ-secretase complex cleaves a wide variety of other substrates besides APP, including APP-like proteins (APLP) 1 and 2, N- and E-cadherins, and Erb-B4 (Haapasalo & Kovacs 2011). Most problematic for AD drug discovery is the Notch family of receptors, which are involved in many different kinds of cell differentiation events. Ligand-activated proteolysis of these receptors releases the Notch intracellular domain (NICD), which translocates to the nucleus and interacts with transcription factors that regulate the expression of genes that control cell fate (Kopan & Ilagan 2009). Proteolysis of the Notch transmembrane domain by γ-secretase is an essential part of this signaling process, and blocking this process with GSIs can lead to specific toxic effects, including gastrointestinal bleeding and immunosuppression (Searfoss et al. 2003, Wong et al. 2004). As a consequence, recent efforts to identify AD drug candidates that target γ-secretase processing of APP have focused on strategies that have little or no effect on physiological Notch processing.
This review will describe γ-secretase inhibitors and modulators (GSIs and GSMs), both as chemical tools for biological investigation and as potential therapeutics for AD. Space limitations preclude comprehensive coverage of all reported compounds with these types of activities, and the reader is referred to excellent recent reviews for fuller discussion (Kreft et al. 2009, Pissarnitski 2007). Moreover, as results from clinical trials with GSIs and GSMs will be covered in another article in this special issue, this topic is not presented in depth here. The goal of this review is to highlight compounds that exemplify structural classes, possess desirable biological properties (e.g., potency, selectivity), serve as especially useful chemical probes, and represent promising candidate AD therapeutics both past and present.
Inhibitors
The first reported compounds shown to inhibit γ-secretase activity were peptide aldehyde-type calpain and proteasome inhibitors (Klafki et al. 1995, Higaki et al. 1995, Klafki et al. 1996). Despite their weak potency and lack of selectivity, these compounds were nevertheless the first chemical tools employed to address questions about γ-secretase. Because γ-secretase had yet to be isolated and identified, these compounds were tested in APP-transfected cells and found to increase levels of APP CTFs produced by α- and β-secretase (C83 and C99, respectively) and to inhibit the production of their γ-secretase cleavage products (p3 and Aβ, respectively). These compounds also revealed a pharmacological distinction between Aβ40 and Aβ42 production by γ-secretase (Klafki et al. 1996, Citron et al. 1996), a phenomenon since observed with many GSIs. Although this suggested distinct γ-secretases responsible for generating Aβ40 and Aβ42, subsequent work has demonstrated that this is not the case, as purification of tagged and overexpressed γ-secretase complexes of defined composition provides enzymes capable of generating both Aβ species (Fraering et al. 2004).
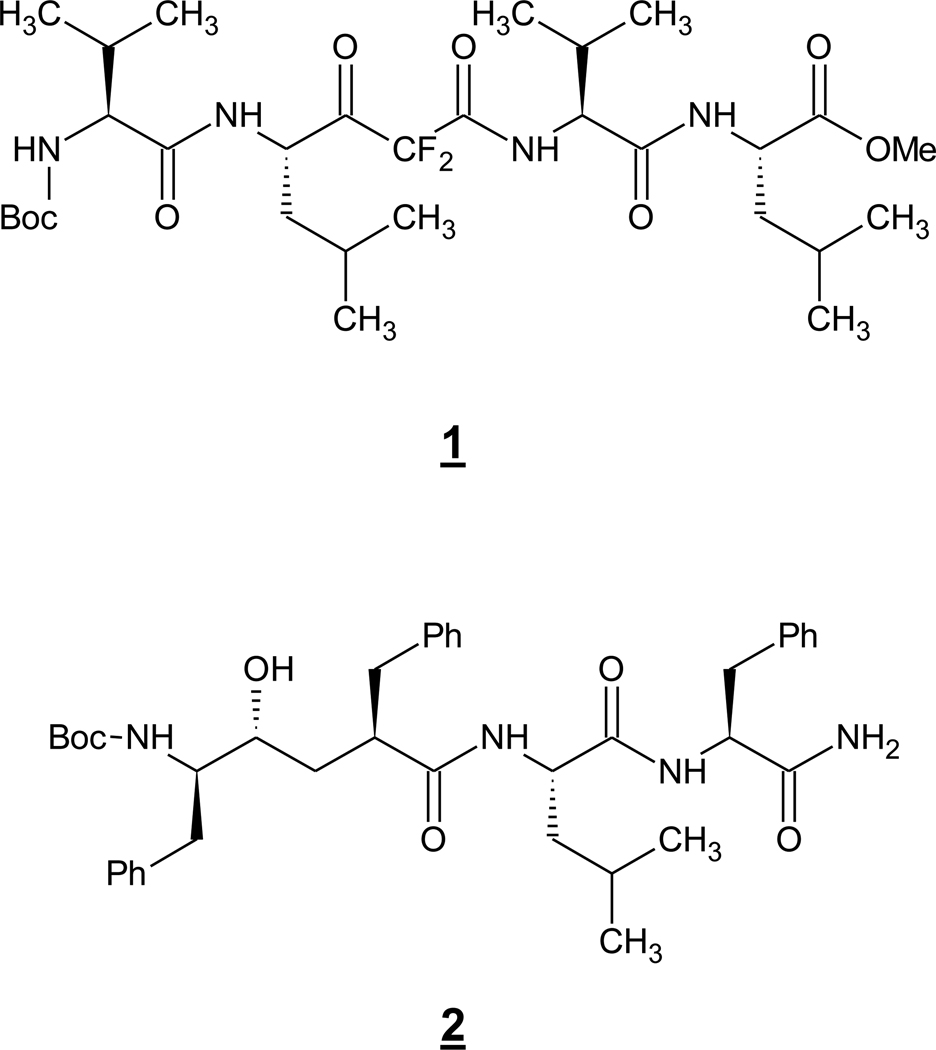
As peptide aldehydes typically inhibit serine and cysteine proteases, the fact that these compounds inhibited γ-secretase activity was initially interpreted as evidence that γ-secretases are in one or both of these protease classes. However, peptide aldehydes are readily hydrated to a form that resembles the transition state of aspartyl protease catalysis. Similarly, the first reported substrate-based inhibitor of γ-secretase activity, the difluoroketone peptidomimetic compound 1 (also called MW167 and DFK167, Fig. 1) (Wolfe et al. 1998) could in principle inhibit a serine or cysteine protease in its keto form or an aspartyl protease in its hydrated form. However, difluoroalcohol analogues of 1 also could inhibit γ-secretase activity (Wolfe et al. 1999). As this class of peptidomimetic only inhibits aspartyl protease, by virtue of mimicking the transition-state of aspartyl protease catalysis, γ-secretase was suggested to be such a protease. Conversion of one of these difluoroalcohol peptidomimetics into an affinity labeling reagent led to identification of PS1 NTF and CTF as the direct targets of this type of inhibitor (Esler et al. 2000). As difluoroalcohol peptidomimetics are transition-state analogues, this finding suggested that the active site of γ-secretase resides at the interface between these two presenilin subunits. Generation of a variety of such difluoroalcohols, varying in the identity of amino acid side chains, suggested that γ-secretase has relatively loose sequence specificity (Wolfe et al. 1999), a conclusion supported by later findings that the protease cleaves a wide variety of membrane proteins with no clear consensus sequence.
Figure 1.
Transition-state analogue inhibitors of γ-secretase.
Another class of transition-state analogue inhibitor of aspartyl proteases, hydroxyethylamines, were found to inhibit γ-secretase activity in cell culture as well (Shearman et al. 2000). The most potent compound 2 (or L-685,458)(Fig. 1), was used to validate the isolation of γ-secretase activity in the detergent-solubilized state and demonstrate that immunoprecipitation of presenilin brought down γ-secretase activity (Li et al. 2000a). As with the difluoroalcohols, conversion of this type of compound into affinity labeling reagents led to identification of PS1 NTF and CTF as the direct target of this active site-directed inhibitor, and the potency and specificity of these affinity reagents allowed determination that full-length (i.e., unprocessed) PS1 was not a target, consistent with the holoprotein being a zymogen (Li et al. 2000b). Use of a biotinylated version of 2 led to isolation of γ-secretase with copurification of nicastrin and provided evidence for separate substrate binding and active sites (Beher et al. 2003).
Structurally related to the hydroxyethylamines are hydroxyethylureas, which replace one of the chiral backbone carbon atoms of the hydroxyethylamines with an achiral nitrogen. This subtle difference greatly simplifies the synthesis of these transition-state analogues, allowing facile generation of a variety of analogues for analysis of structure-activity relationships (Esler et al. 2004). In this way, the pockets in the protease active site that interact with the inhibitor side chains can be readily probed. Moreover, covalent attachment of one such compound to resin provided an affinity chromatographic method for isolating (Esler et al. 2002b, Kimberly et al. 2003) and ultimately purifying γ-secretase (Fraering et al. 2004), demonstrating that the five components, PS1 NTF and CTF, Nicastrin, Aph-1 and Pen-2, are sufficient for protease activity and cleavage of APP and Notch substrates. The copurification of an endogenous APP substrate (C83) from the affinity matrix provided evidence for a substrate docking site on γ-secretase that is distinct from the active site, where the immobilized transition-state analogue binds (Esler et al. 2002b), findings similar to those seen with an immobilized hydroxyethylamine inhibitor (Beher et al. 2003).
Another type of substrate-based GSI is the helical peptide. Because γ-secretase cleaves APP within its transmembrane domain and single transmembrane domains typically fold into a helical conformation, short peptides of 6–10 amino acids were synthesized that contained the γ-secretase cleavage sites of APP but with selected residues replaced with the helix-inducing aminoisobutyric acid (Aib) (Das et al. 2003). Surprisingly, D-peptides as well as L-peptides could potently inhibit γ-secretase activity, but in either case structural modifications that disrupt the helical conformation resulted in dramatically reduced potency. Extension of the D-peptide series led to identification of a 13-residue helical peptide with an IC50 of 140 pM (Bihel et al. 2004).
This class of inhibitor was converted to affinity labeling reagents and, like the transition-state analogues, was found to directly bind to the PS1 NTF/CTF interface (Kornilova et al. 2005). However, competition experiments demonstrated that the helical peptide and transition-state analogue inhibitors bind to separate sites, consistent with previous evidence for an initial substrate docking site (Esler et al. 2002b, Beher et al. 2003). The finding that these two types of inhibitors bind to distinct sites at the NTF/CTF interface suggests the substrate passes between the two PS1 subunits when transitioning from docking site to active site. Moreover, in contrast to a 10-residue helical peptide inhibitor, a 13-residue helical peptide inhibitor could compete for binding to PS1 with a transition-state analogue as well with its shorter 10-residue counterpart, suggesting that the active site and docking site are in close proximity (i.e., the length of the three extra residues). Helical β-peptides (containing β-amino acids) can likewise inhibit γ-secretase and compete with Aib-containing helical α-peptide affinity probes for binding to PS1; that is, these β-peptides apparently also bind to the initial substrate docking site (Imamura et al. 2009).
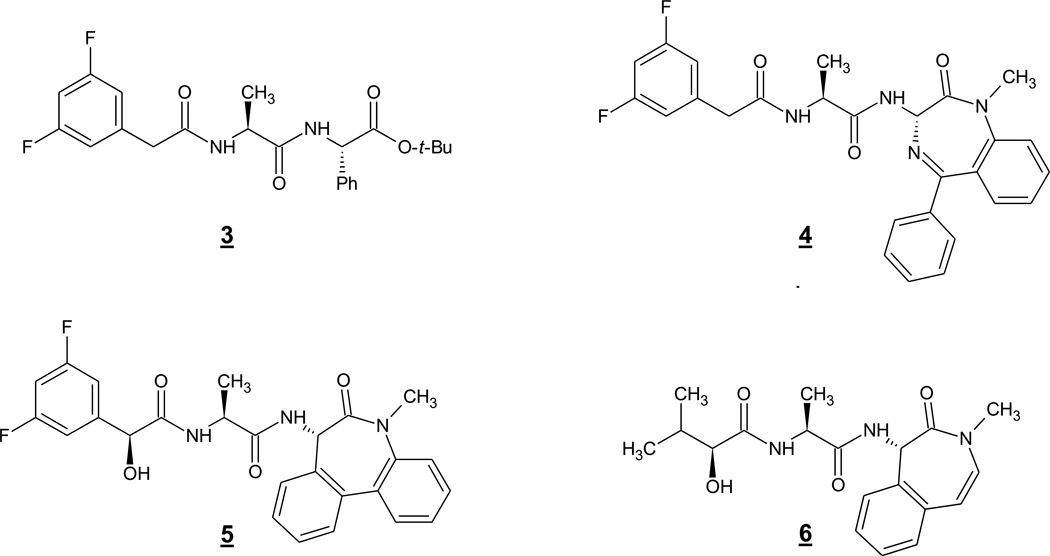
Another early prototype peptide-based inhibitor is compound 3 (or DAPT) (Fig. 2). This dipeptide analogue was the result of medicinal chemistry optimization of an initial hit from a high-throughput screening campaign (Dovey et al. 2001) and became an important research tool in the study of γ-secretase. Compound 3 showed good inhibitory potency (IC50 for Aβ lowering in cell-based assays of 20 nM) and was the first compound reported to be orally active in vivo, capable of lowering brain Aβ levels in an APP transgenic mouse model with an ED50 of 100 mg/kg. The conversion of this compound into a photoaffinity labeling reagent led to identification of the PS1 CTF as the direct target (Morohashi et al. 2006). This labeling could be blocked by transition-state analogue 2 or a helical peptide but only at elevated concentrations, suggesting that the binding site for 3 is distinct from the active site or the docking site, although it may overlap somewhat with these other sites. In this scenario, 3 may bind in the “transit path” between initial substrate docking site and active site. Related to 3 is the highly potent 4 (or compound E) (Fig. 2), in which the phenylglycine moiety is replaced by a benzodiazepine (Seiffert et al. 2000). This compound could inhibit Aβ production in cells with an IC50 of 300 pM. Surprisingly, a photoaffinity probe based on 4 labeled PS1 NTF but not the CTF, suggesting different binding pockets for the C-terminal phenylglycine of 3 and the C-terminal benzodiazepine of 4 (Fuwa et al. 2007). Nevertheless, because of their structural similarity and their ability to effectively block labeling by each other’s photoaffinity probes, the binding sites for these two compounds are likely to be otherwise closely similar.
Figure 2.
DAPT and related analogues.
Further modification of 4 led to the exquisitely potent and in vivo active compound 5 (or LY-411,575) (Fig. 2). With an IC50 for inhibition of cellular Aβ production of 30 pM and good drug-like properties, 5 was highly effective in reducing brain Aβ levels in APP transgenic mice upon oral dosing (ED50 < 1 mg/kg) (May et al. 2002). However, this compound also illustrated the toxicity issues that might be expected of a GSI with no selectivity for APP proteolysis vis-à-vis Notch. After treatment with 5 over the course of 15 days, gastrointestinal bleeding and immunosuppression due to peripheral inhibition of Notch signaling was observed (Wong et al. 2004). Despite this ominous result, nonselective GSIs of this type continued to be pursued on evidence from animal studies that careful dosing could identify a therapeutic window [e.g., (Hyde et al. 2006)].
Further modifications of 5 resulted in 6 (LY-450,139, semigacestat) (Fig. 2), a compound that advanced into phase III clinical trials, even though phase I and II trials demonstrated lowering of steady-state Aβ levels in the plasma but not in the cerebrospinal fluid (Siemers et al. 2005, Siemers et al. 2007, Fleisher et al. 2008). The phase III trial revealed severe gastrointestinal toxicity, immunomodulation and skin cancer, effects expected from inhibition of Notch proteolysis and signaling. Also of concern was the finding that cognition in the drug-treated group worsened compared to placebo-treated, raising the possibility that lowering brain Aβ levels may be the cause. However, as 6 is a nonselective GSI, the negative effect on cognitive function is more likely attributable to blocking the proteolysis of another substrate besides APP, stressing the need to identify selective inhibitors toward the development of AD therapeutics.
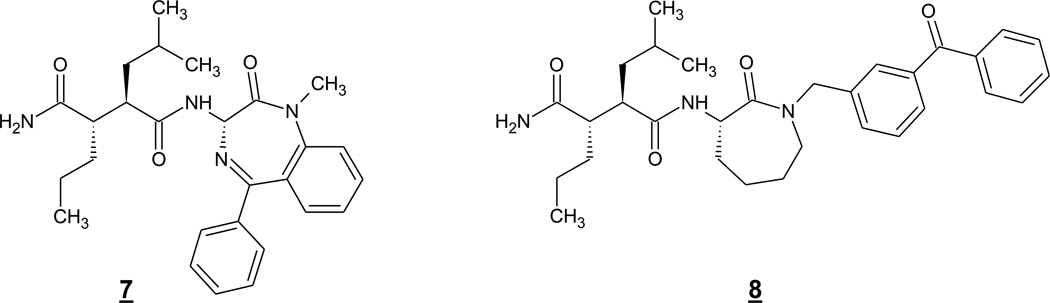
Two other nonselective inhibitors are of particular note, as they were employed as chemical probes for investigation of γ-secretase biology. One is the benzodiazepine 7 (or compound D) (Fig. 3) developed by what was then Dupont Pharmaceuticals (Seiffert et al. 2000) (since acquired by Bristol-Myers Squibb). Radiolabeling of this compound provided a tool to visualize the binding sites in rodent brain, which were found in the olfactory bulb, cerebral cortex, hippocampus and cerebellum (Yan et al. 2004). Brain regions labeled by 7 correlated with regions of PS1 gene expression. The other useful probe is the caprolactam succinamide 8 (or compound C) (Fig. 3) developed by Dupont Pharmaceuticals. This GSI was among the first to be converted into an affinity probe and shown to directly bind to presenilin (Seiffert et al. 2000).
Figure 3.
Malonamide inhibitors of γ-secretase.
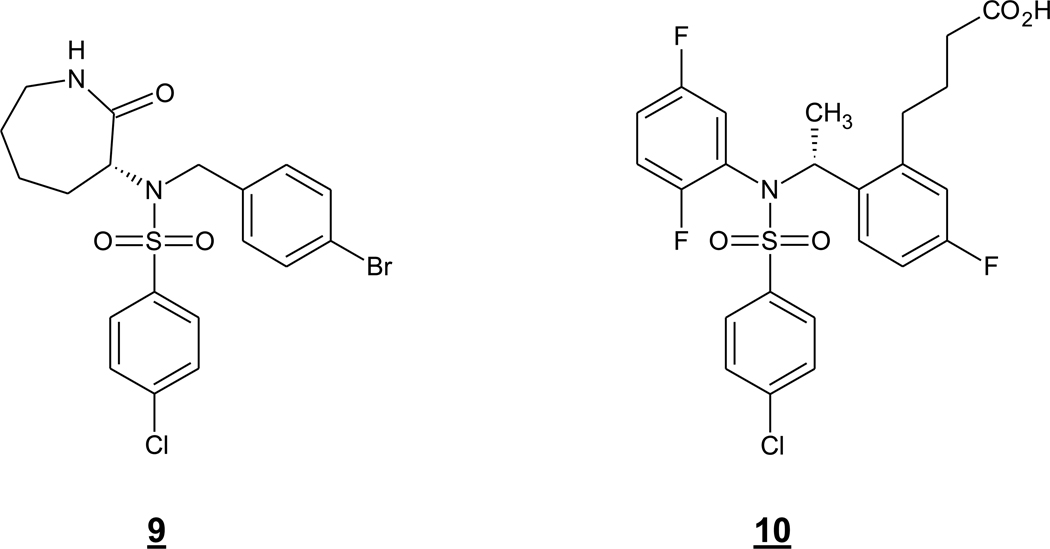
Some inhibitors have been reported to display selectivity for PS1-containing γ-secretase complexes over PS2-containing complexes, including the arylsulfonamides 9 (or ELN-318463) from Elan and 10 (or BMS-299,897) from Bristol-Myers Squibb (Fig. 4) (Zhao et al. 2008). Through the generation of PS1/PS2 chimeras and point mutations, specific residues (Leu172, Thr281 and Leu282) in PS1 were identified as necessary for the selective inhibition. Although PS1 appears to account for ~80% of total Aβ production (De Strooper et al. 1998), knockout of PS1 is perinatal lethal (Shen et al. 1997, Wong et al. 1997), and targeting PS1 selectively is not expected to prevent the toxic effects due to inhibition of Notch signaling. Mice deficient in PS2, however, are viable and fertile and develop only mild pulmonary fibrosis and hemorrhage with age (Herreman et al. 1999), and the 20% Aβ production remaining in PS1 deficient mice has been attributed to PS2 (De Strooper et al. 1998). A 20% reduction in brain Aβ may be sufficient for therapeutic purposes, and targeting PS2 selectively over PS1 could be worthwhile and may be possible. PS2 knockout mice, however, did not display any effect on APP processing (Herreman et al. 1999), although this may be due to compensation during development. Thus, at present there is conflicting evidence regarding whether selective inhibition of PS2-containing γ-secretase complexes would lower Aβ levels in the brain and do so effectively enough to prevent Aβ aggregation and neurotoxicity.
Figure 4.
PS1-selective γ-secretase inhibitors.
Modulators
Nonselective GSIs may have apparently insurmountable liabilities as AD therapeutic agents. Therefore, extensive efforts have been expended toward identifying γ-secretase modulators with more subtle effects on the activity of the protease. In general, modulators described to date fall into two main categories. The first are compounds that do not change the production of total Aβ but rather shift the spectrum of produced Aβ peptides toward shorter forms that are more soluble and less pathogenic. Specifically, these compounds lower Aβ42 levels and elevate Aβ37–39. Such compounds appear to have similar effects on the processing of the Notch receptor by γ-secretase; however, the release of the signaling molecule NICD is not inhibited, and toxic effects due to interference with Notch function are not observed. Compounds that alter Aβ production in this way are what are typically meant by the term γ-secretase modulator, or GSM, in the literature, even though other types of modulation are possible. The second type of modulator inhibits all cleavage of APP by γ-secretase, thereby blocking the production of all Aβ peptides, while allowing Notch proteolysis to continue, at least within a certain range of concentrations. Such compounds are typically referred to as Notch-sparing GSIs. In this review, both categories are considered modulators of γ-secretase activity, adjusting the activity as opposed to broadly inhibiting it. These two categories are denoted here as Aβ42-lowering GSMs and Notch-sparing GSMs.
Aβ42-lowering GSMs
The first type of Aβ42-lowering GSM to be reported was a subset of nonsteroid anti-inflammatory drug, or NSAIDs (Weggen et al. 2001). These included ibuprofen, sulindac sulfide and indomethacin but not naproxen or aspirin. The ability of these compounds to lower Aβ42 in cells lacking cyclooxygenase demonstrated that the mechanism of action does not involve this common NSAID target. In parallel with the reduction of Aβ42, the compounds also elevated Aβ38, suggesting a precursor-product relationship between these two Aβ peptides that has since been demonstrated as correct by the Ihara laboratory (Takami et al. 2009).
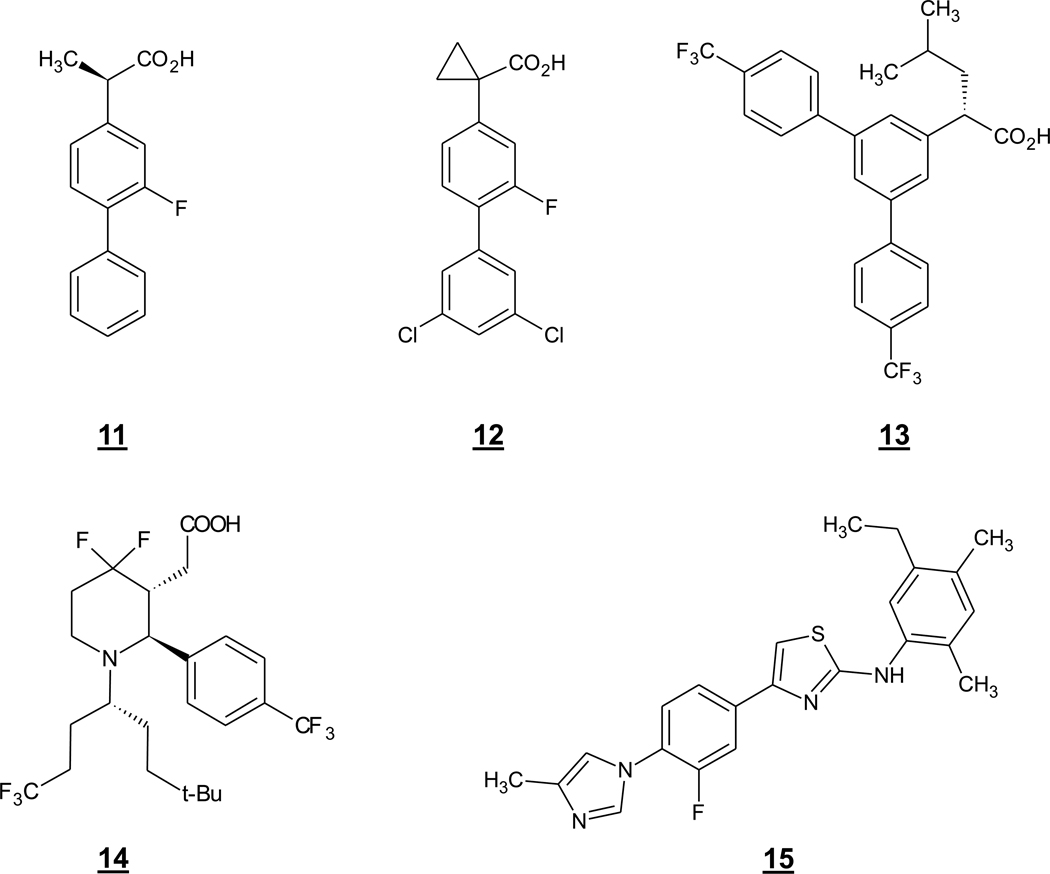
Subsequently, the R enantiomer of fluriprofen, compound 11 (Fig. 5), was found to be an Aβ42-lowering agent (Eriksen et al. 2003). As this enantiomer of a known drug is inactive toward cyclooxygenase and showed promising effects in APP transgenic mice (Kukar et al. 2007), 11 (also called Flurizan) entered into clinical trials for the treatment of AD, ultimately failing in phase III (Green et al. 2009) for reasons likely related to lack of potency and poor brain penetration. As for the molecular mechanism of this class of compounds, evidence suggests a direct effect on γ-secretase cleavage of APP (Weggen et al. 2003). The APP substrate C99 itself was identified as a target (Kukar et al. 2008), although other studies implicate the γ-secretase complex, particularly presenilin (Beher et al. 2004, Sato et al. 2006).
Figure 5.
Aβ42-lowering GSMs.
An arylacetic acid related to NSAIDs, compound 12 (or CHF5074) (Fig. 5) from Chiesi Farmaceutici, has been reported as an Aβ42-lowering agent that does not inhibit cyclooxygenase (Peretto et al. 2005). Although the potency of this compound is weak (IC50 of 41 µM for inhibition of cellular Aβ42 production), 12 lowered Aβ plaque burden, restored hippocampal neurogenesis, and reversed learning and memory deficits in APP transgenic mice (Imbimbo et al. 2007, Imbimbo et al. 2009, Imbimbo et al. 2010). Another arylacetic acid-type compound, 13 (or JNJ-40418677) (Fig. 5) from Johnson & Johnson and Jansen, was also found to safely reduce Aβ plaque burden upon chronic treatment in APP transgenic mice (Van Broeck et al. 2011). Piperidine acetic acids are another interesting class of Aβ-lowering GSM that are structurally related to but distinct from the arylacetic acids. Potencies approaching 200 nM for lowering Aβ42 in cell-based assays have been reported, and some of these compounds, exemplified by 14, can apparently get into the brain and reduce Aβ42 levels in rodents (Stanton et al. 2009).
A high throughput screening campaign followed by structure-activity optimization led to the discovery of a completely different structural class of Aβ42-lowering GSMs, 2-aminothiazoles that are exemplified by 15 (Fig. 5) (Kounnas et al. 2010). These compounds are not only structurally distinct from the Aβ42-lowering NSAIDs, but they also appear to work by a somewhat different mechanism and putatively through a different target within γ-secretase. Compounds such as 4 inhibit the production of both Aβ40 and Aβ42 without affecting total Aβ levels. In parallel, Aβ37 and Aβ38 are elevated. The potency of these agents are much higher than what is seen with any NSAID-like compounds, with IC50s as low as 5 nM for lowering Aβ42 in cell-based assays. The compounds could also inhibit Aβ40 and Aβ42 production in a cell-free assay, suggesting a direct effect on γ-secretase processing of APP.
Immobilization of one of these compounds to create an affinity matrix led to identification of PS1 NTF, PS1 CTF and Pen-2 from detergent-solubilized cellular extracts (Kounnas et al. 2010). Pen-2 was isolated quantitatively, suggesting that this small 10-kDa membrane protein component of γ-secretase is the direct target. However, as one of the detergents used for the affinity chromatography (Triton X-100) is known to completely dissociate the γ-secretase complex (Esler et al. 2002b) and no competition with free inhibitor was tested, the possibility of a nonspecific interaction cannot be ruled out. Regardless of the exact mechanism though, oral administration of 15 lowered brain Aβ42 levels in APP transgenic mice, and chronic daily dosing over 7 months substantially reduced Aβ deposition and was well tolerated, with no Notch-related toxicity observed.
Notch-sparing GSMs
Although inhibition of Notch signaling was identified as a potential problem for GSIs for AD in 1999, it was unclear if selective inhibition of APP processing over that of Notch was possible. Theoretically, the enzyme could possess a binding pocket for small molecules that allosterically regulates substrate selectivity, but whether such a site might actually exist was completely unknown. Isocoumarins were initially identified as selective inhibitors of Aβ production from C99 that did not affect processing of Notch (Petit et al. 2001). However, these compounds were not effect in cell-free assays (Esler et al. 2002a), and the direct target and affected cellular pathways remain unknown. Paul Greengard’s laboratory then found that the abl kinase inhibitor Gleevec (imatinib) could inhibit γ-secretase processing of C99 to Aβ with no effect on Notch processing (Netzer et al. 2003). This selective effect was also observed in Abl kinase knockout cells, indicating another target mediated the Aβ-lowering effect of Gleevec. Certain other compounds with kinase-inhibitor scaffolds could do the same.
A subsequent study showed that ATP and other nucleotides could increase the ability of purified γ-secretase to cleave APP substrate without affecting processing of a Notch substrate (Fraering et al. 2005). The nonhydrolyzable ATP-γS had the same effect, demonstrating that ATPase or kinase activities were not involved. Moreover, certain compounds from a library of commercially available kinase inhibitors could block the proteolysis of purified recombinant APP substrate and purified enzyme without inhibiting the cleavage of a purified recombinant Notch substrate, demonstrating that the compounds work by interacting directly with the enzyme, the substrate or both. An ATP photoaffinity probe labeled PS1 CTF, which could be blocked by ATP and the APP-selective inhibitors. Altogether, these results suggested that γ-secretase contains an allosteric site for small molecules that could selectively alter APP processing over that of Notch. The role of Gleevec per se, however, remained unclear, as pure Gleevec had no effect in the purified enzyme assay. The Greengard lab recently reported the identification of a γ-secretase-activating protein (GSAP) as the direct target of Gleevec via affinity labeling, and substantial evidence supported the ability of GSAP to regulate APP proteolysis by γ-secretase but not Notch, both in cells and in cell-free assays (He et al. 2010). Knockdown of GSAP in an APP transgenic mouse model reduced Aβ levels and plaque formation without apparent Notch-related toxic effects, suggesting that GSAP may be a worthwhile target for AD drug discovery.
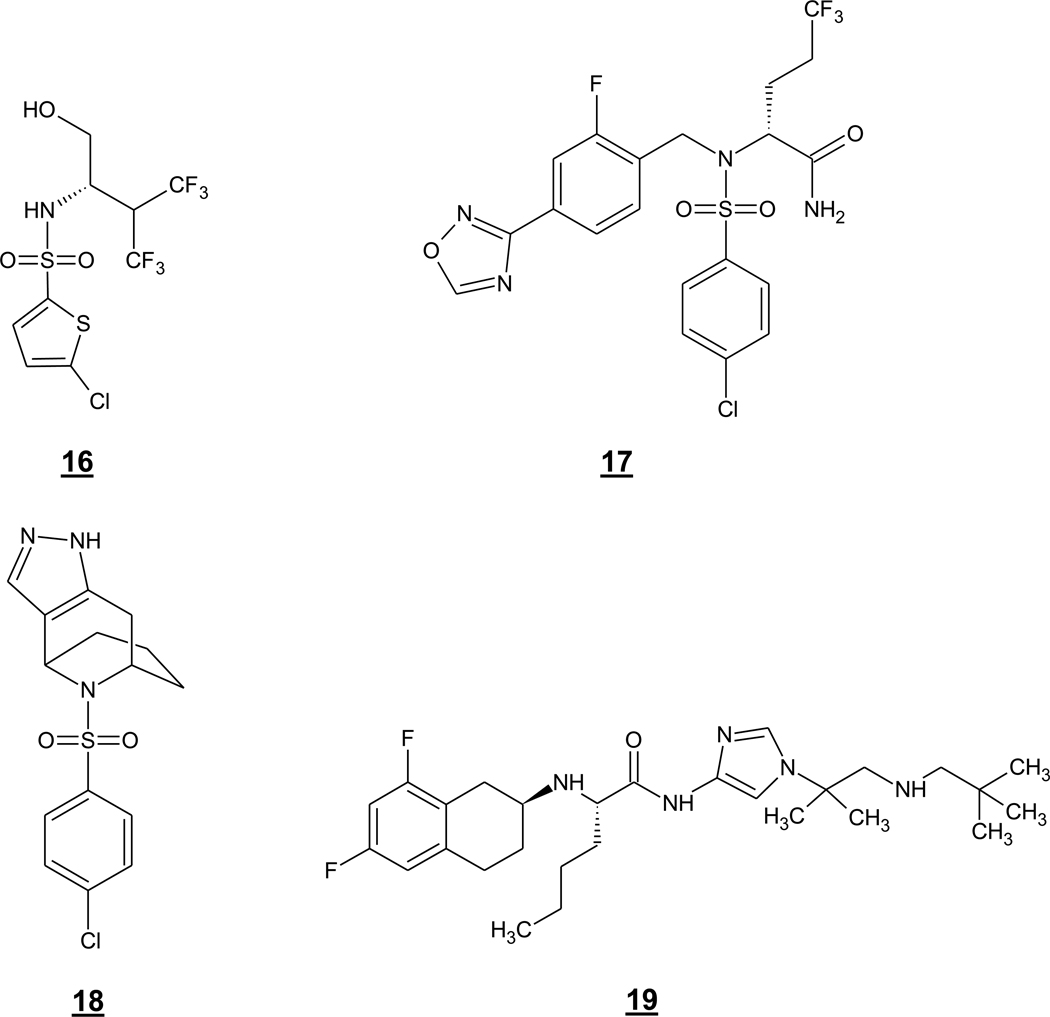
More recently, drug discovery efforts have identified Notch-sparing GSMs with better potencies and CNS drug-like characteristics. The thiophene-containing sulfonamide 16 (GSI-953, or begacestat) (Fig. 6), was reported by researchers at what was then Wyeth (now part of Pfizer) to potently inhibit cellular production of Aβ by γ-secretase with an IC50 of 15 nM, while inhibition of Notch signaling was 14-fold less effective (Mayer et al. 2008, Kreft et al. 2008). Note that these two assays are quite different, so the meaning of the 14-fold selectivity is unclear. This compound was also more metabolically stable than earlier prototypes developed at Wyeth and showed in vivo efficacy in an APP transgenic mouse model, reducing Aβ40 and Aβ42 in the brain by 37% and 25%, respectively, 4 hours after a 5 mg/kg oral dose (Martone et al. 2009). Compound 16 was also able to reverse contextual fear conditioning deficits in these mice. Lack of Notch-related toxic side effects encouraged moving forward with this compound in clinical trials, and single-dose administration in healthy volunteers produced a dose-dependent decrease in plasma Aβ levels (Martone et al. 2009). It remains unclear if the APP/Notch selectivity of this compound will be sufficient, as several previous GSIs could chronically lower brain Aβ levels in animal models without apparent Notch-related side effects. In general, a therapeutic window may be more readily identified in a genetically homogeneous animal population maintained in the same environment than in a heterogeneous population of AD patients living in a variety of environments.
Figure 6.
Notch-sparing GSMs.
Bristol-Myers Squibb has also reported the Notch-sparing GSM 17 (BMS-708163) (Fig. 6), which has advanced into clinical trials. Like the Wyeth compound, 17 is an arylsulfonamide, but this oxadiazole-substituted analogue is considerably more potent, with an IC50 of 0.30 nM for inhibiting cellular Aβ production, and more selective with respect to Notch, with an apparent selectivity of 193-fold (Gillman et al. 2010). As noted above for 16, the meaning of the selectivity index is unclear, as the APP and Notch processing assays were quite different, with the former measuring Aβ and the latter measuring a reporter signal. Compound 17 showed good pharmacokinetic properties in rats and dogs and also lowered brain and CSF Aβ40 in both species at 1–2 mg/kg oral doses. Notably, chronic dosing at 10 times the concentration required for lowering Aβ did not cause Notch-related toxic effects. The correlation between brain and CSF Aβ40 lowering activity in dogs suggested that CSF Aβ40 may serve as a surrogate biomarker for brain Aβ40 levels in humans. Compound 17 also lowered CSF Aβ40 and Aβ42 levels in healthy human volunteers with dosing up to 28 days.
Elan has also reported novel arylsulfonamides as Notch-sparing GSMs, exemplified by 9 (ELN318463) (Fig. 4) and 18 (ELN475516) (Fig. 6) (Basi et al. 2010). These compounds have been reported to display 120- and 82-fold selectivity, respectively, for inhibiting Aβ production in cells compared to inhibiting Notch signaling. Again, the differences between the assays (Aβ measurement vs. signaling reporter) may make the selectivity seem higher than what would be seen in more comparable assays. Indeed, the nonselective transition-state analogue inhibitor 2 showed 14-fold selectively in these cellular assays, and enzyme assays for APP and Notch substrates, in which the products were both measure by ELISA, revealed 51- and 14.5-fold selectivity for 9 and 18, respectively. In vivo lowering of brain Aβ in mice was observed after 7 days of dosing with 18 without overt signs of toxicity, although one week is likely not long enough to reveal Notch-related effects. Another Elan sulfonamide, ELND-006, which has advanced into clinical trials, has been reported to have a similar selectivity profile to 18. Whether this level of selectivity is sufficient for lower CSF Aβ in human without Notch-related toxicity upon chronic exposure remains to be seen.
Pfizer has also developed a Notch-sparing GSM 19, although this compound, PF-3084014 (Fig. 6), is not an arylsulfonamide but rather a novel tetralin imidazole (Lanz et al. 2010). This compound potently inhibited Aβ production in cells, with an IC50 of 1.2 nM, but inhibited Notch-dependent maturation of B- and T-lymphocytes in a fetal mouse thymus organ culture with IC50s of 1–3 µM. Again, comparing these two assays may not be appropriate, and so it is difficult to know what to make of the 1,000–3,000-fold APP/Notch selectivity of PF-3084014. As a benchmark, the relatively nonselective compound 5 showed an IC50 of 21 pM for lowering cellular Aβ production and a mean IC50 in the fetal thymus organ culture of 4 nM, a nearly 200-fold difference. Acute treatment in guinea pigs showed some selectivity for reducing brain Aβ40 over the more aggregation-prone Aβ42. Of further concern was the apparent elevation of Aβ43 levels. This longer Aβ variant has been recently reported to lead to cerebral plaque formation and neurotoxicity in APP/PS1 double transgenic mice (Saito et al. 2011) and may play an important role in AD pathogenesis. As 19 was administered subcutaneously or by osmotic pump to mice and guinea pigs, the oral bioavailability of this compound is unclear.
Perspective and Future Studies
γ-Secretase remains a target of keen interest for the potential prevention or treatment of AD. The focus, however, has clearly shifted toward modulators that minimize effects on Notch signaling function, with compounds that either shift the site of γ-secretase cleavage to produce shorter forms of Aβ or those that selectively inhibit APP processing by γ-secretase while allowing the enzyme to continue processing Notch. Inhibitors and modulators have also served as important research tools for the identification of the enzyme complex and probes for the topology of the active site, the substrate docking site and allosteric binding pockets. Present compounds under investigation may not have sufficient potency, brain penetration or selectivity to effectively lower brain Aβ while avoiding Notch-related toxicity.
Key questions remain: Is there a ceiling on the achievable APP/Notch selectivity of a GSM? If interference with Notch function can be avoided, will other toxic effects be revealed due to inhibition of intramembrane proteolysis of other γ-secretase substrates? Where are the allosteric binding sites on the γ-secretase complex with which GSMs interact? What are the topographies of these sites, and can this knowledge be leveraged for structure-based design? Does substrate contribute to the binding site of GSMs? Answering these questions should facilitate the development of optimal agents that would help provide the final test for the amyloid hypothesis: the prevention or treatment of AD by safely blocking Aβ production in the brain.
ACKNOWLEDGMENTS
The author would like to acknowledge the NIH for supporting work on γ-secretase inhibitors and modulators (NS41355 to MSW and AG15379 to Dennis Selkoe)
The abbreviations used are
- Aβ
amyloid β-peptide
- AICD
APP intracellular domain
- APLP
APP-like protein
- APP
amyloid β-protein precursor
- AD
Alzheimer disease
- BACE1
β-site APP-cleaving enzyme 1
- CTF
C-terminal fragment
- GSI
γ-secretase inhibitor
- GSM
γ-secretase modulator
- NICD
Notch intracellular domain
- NTF
N-terminal fragment
- PS1
presenilin-1
- PS2
presenilin-2
Footnotes
CONFLICT OF INTERESTS
The author declares no conflict of interests.
REFERENCES
- Basi GS, Hemphill S, Brigham EF, et al. Amyloid precursor protein selective gamma-secretase inhibitors for treatment of Alzheimer's disease. Alzheimers Res Ther. 2010;2:36. doi: 10.1186/alzrt60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher D, Clarke EE, Wrigley JD, Martin AC, Nadin A, Churcher I, Shearman MS. Selected non-steroidal anti-inflammatory drugs and their derivatives target gamma-secretase at a novel site. Evidence for an allosteric mechanism. J Biol Chem. 2004;279:43419–43426. doi: 10.1074/jbc.M404937200. [DOI] [PubMed] [Google Scholar]
- Beher D, Fricker M, Nadin A, et al. In vitro characterization of the presenilin-dependent gamma-secretase complex using a novel affinity ligand. Biochemistry. 2003;42:8133–8142. doi: 10.1021/bi034045z. [DOI] [PubMed] [Google Scholar]
- Bihel F, Das C, Bowman MJ, Wolfe MS. Discovery of a subnanomolar helical D-tridecapeptide inhibitor of γ-secretase. J Med Chem. 2004;47:3931–3933. doi: 10.1021/jm049788c. [DOI] [PubMed] [Google Scholar]
- Citron M, Diehl TS, Gordon G, Biere AL, Seubert P, Selkoe DJ. Evidence that the 42- and 40-amino acid forms of amyloid beta protein are generated from the beta-amyloid precursor protein by different protease activities. Proc Natl Acad Sci U S A. 1996;93:13170–13175. doi: 10.1073/pnas.93.23.13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron M, Westaway D, Xia W, et al. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- Das C, Berezovska O, Diehl TS, Genet C, Buldyrev I, Tsai JY, Hyman BT, Wolfe MS. Designed helical peptides inhibit an intramembrane protease. J Am Chem Soc. 2003;125:11794–11795. doi: 10.1021/ja037131v. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Dovey HF, John V, Anderson JP, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Sagi SA, Smith TE, et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esler WP, Das C, Campbell WA, et al. Amyloid-lowering isocoumarins are not direct inhibitors of γ-secretase. Nature Cell Biology. 2002a;4:E110–E111. doi: 10.1038/ncb0502-e110b. [DOI] [PubMed] [Google Scholar]
- Esler WP, Das C, Wolfe MS. Probing pockets S2–S4' of the gamma-secretase active site with (hydroxyethyl)urea peptidomimetics. Bioorg Med Chem Lett. 2004;14:1935–1938. doi: 10.1016/j.bmcl.2004.01.077. [DOI] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, et al. Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nature Cell Biology. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Ye W, Diehl TS, Selkoe DJ, Wolfe MS. Activity-dependent isolation of the presenilin/γ-secretase complex reveals nicastrin and a γ substrate. Proc Natl Acad Sci U.S.A. 2002b;99:2720–2725. doi: 10.1073/pnas.052436599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Raman R, Siemers ER, et al. Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch Neurol. 2008;65:1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraering PC, Ye W, Lavoie MJ, Ostaszewski BL, Selkoe DJ, Wolfe MS. gamma -Secretase substrate selectivity can be modulated directly via interaction with a nucleotide binding site. J Biol Chem. 2005;280:41987–41996. doi: 10.1074/jbc.M501368200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraering PC, Ye W, Strub JM, et al. Purification and Characterization of the Human gamma-Secretase Complex. Biochemistry. 2004;43:9774–9789. doi: 10.1021/bi0494976. [DOI] [PubMed] [Google Scholar]
- Funamoto S, Morishima-Kawashima M, Tanimura Y, Hirotani N, Saido TC, Ihara Y. Truncated carboxyl-terminal fragments of beta-amyloid precursor protein are processed to amyloid beta-proteins 40 and 42. Biochemistry. 2004;43:13532–13540. doi: 10.1021/bi049399k. [DOI] [PubMed] [Google Scholar]
- Fuwa H, Takahashi Y, Konno Y, et al. Divergent synthesis of multifunctional molecular probes to elucidate the enzyme specificity of dipeptidic gamma-secretase inhibitors. ACS Chem Biol. 2007;2:408–418. doi: 10.1021/cb700073y. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Gemma S, Tang J. beta-Secretase as a therapeutic target for Alzheimer's disease. Neurotherapeutics. 2008;5:399–408. doi: 10.1016/j.nurt.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman KW, Starrett JE, Parker MF, et al. Discovery and Evaluation of BMS-708163, a Potent, Selective and Orally Bioavailable Gamma-Secretase Inhibitor. ACS Medicinal Chemistry Letters. 2010;1:120–124. doi: 10.1021/ml1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Green RC, Schneider LS, Amato DA, Beelen AP, Wilcock G, Swabb EA, Zavitz KH. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: a randomized controlled trial. Jama. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo A, Kovacs DM. The Many Substrates of Presenilin/gamma-Secretase. J Alzheimers Dis. 2011 E-pub ahead of print. [Google Scholar]
- He G, Luo W, Li P, et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer's disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman A, Hartmann D, Annaert W, et al. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci U S A. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki J, Quon D, Zhong Z, Cordell B. Inhibition of beta-amyloid formation identifies proteolytic precursors and subcellular site of catabolism. Neuron. 1995;14:651–659. doi: 10.1016/0896-6273(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Hyde LA, McHugh NA, Chen J, et al. Studies to investigate the in vivo therapeutic window of the gamma-secretase inhibitor N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-oxo-6,7-di hydro-5H-dibenzo[b,d]azepin-7-yl]-L-alaninamide (LY411,575) in the CRND8 mouse. J Pharmacol Exp Ther. 2006;319:1133–1143. doi: 10.1124/jpet.106.111716. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Watanabe N, Umezawa N, Iwatsubo T, Kato N, Tomita T, Higuchi T. Inhibition of gamma-secretase activity by helical beta-peptide foldamers. J Am Chem Soc. 2009;131:7353–7359. doi: 10.1021/ja9001458. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP, Del Giudice E, Colavito D, et al. 1-(3',4'-Dichloro-2-fluoro[1,1'-biphenyl]-4-yl)-cyclopropanecarboxylic acid (CHF5074), a novel gamma-secretase modulator, reduces brain beta-amyloid pathology in a transgenic mouse model of Alzheimer's disease without causing peripheral toxicity. J Pharmacol Exp Ther. 2007;323:822–830. doi: 10.1124/jpet.107.129007. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP, Giardino L, Sivilia S, et al. CHF5074, a novel gamma-secretase modulator, restores hippocampal neurogenesis potential and reverses contextual memory deficit in a transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2010;20:159–173. doi: 10.3233/JAD-2010-1366. [DOI] [PubMed] [Google Scholar]
- Imbimbo BP, Hutter-Paier B, Villetti G, Facchinetti F, Cenacchi V, Volta R, Lanzillotta A, Pizzi M, Windisch M. CHF5074, a novel gamma-secretase modulator, attenuates brain beta-amyloid pathology and learning deficit in a mouse model of Alzheimer's disease. Br J Pharmacol. 2009;156:982–993. doi: 10.1111/j.1476-5381.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. γ-Secretase is a membrane protein complex comprised of presenilin, nicastrin, aph-1, and pen-2. Proc Natl Acad Sci U S A. 2003;100:6382–6387. doi: 10.1073/pnas.1037392100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klafki H, Abramowski D, Swoboda R, Paganetti PA, Staufenbiel M. The carboxyl termini of beta-amyloid peptides 1–40 and 1–42 are generated by distinct gamma-secretase activities. J Biol Chem. 1996;271:28655–28659. doi: 10.1074/jbc.271.45.28655. [DOI] [PubMed] [Google Scholar]
- Klafki HW, Paganetti PA, Sommer B, Staufenbiel M. Calpain inhibitor I decreases beta A4 secretion from human embryonal kidney cells expressing beta-amyloid precursor protein carrying the APP670/671 double mutation. Neurosci Lett. 1995;201:29–32. doi: 10.1016/0304-3940(95)12122-k. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilova AY, Bihel F, Das C, Wolfe MS. The initial substrate-binding site of gamma-secretase is located on presenilin near the active site. Proc Natl Acad Sci U S A. 2005;102:3230–3235. doi: 10.1073/pnas.0407640102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas MZ, Danks AM, Cheng S, et al. Modulation of gamma-secretase reduces beta-amyloid deposition in a transgenic mouse model of Alzheimer's disease. Neuron. 2010;67:769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft A, Harrison B, Aschmies S, et al. Discovery of a novel series of Notch-sparing gamma-secretase inhibitors. Bioorg Med Chem Lett. 2008;18:4232–4236. doi: 10.1016/j.bmcl.2008.05.064. [DOI] [PubMed] [Google Scholar]
- Kreft AF, Martone R, Porte A. Recent advances in the identification of gamma-secretase inhibitors to clinically test the Abeta oligomer hypothesis of Alzheimer's disease. J Med Chem. 2009;52:6169–6188. doi: 10.1021/jm900188z. [DOI] [PubMed] [Google Scholar]
- Kukar T, Prescott S, Eriksen JL, Holloway V, Murphy MP, Koo EH, Golde TE, Nicolle MM. Chronic administration of R-flurbiprofen attenuates learning impairments in transgenic amyloid precursor protein mice. BMC Neurosci. 2007;8:54. doi: 10.1186/1471-2202-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukar TL, Ladd TB, Bann MA, et al. Substrate-targeting gamma-secretase modulators. Nature. 2008;453:925–929. doi: 10.1038/nature07055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz TA, Wood KM, Richter KE, et al. Pharmacodynamics and pharmacokinetics of the {gamma}-secretase inhibitor, PF-3084014. J Pharmacol Exp Ther. 2010;334:269–277. doi: 10.1124/jpet.110.167379. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E, Wijsman EM, Nemens E, Anderson L, Goddard KA, Weber JL, Bird TD, Schellenberg GD. A familial Alzheimer's disease locus on chromosome 1. Science. 1995;269:970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- Li YM, Lai MT, Xu M, et al. Presenilin 1 is linked with gamma -secretase activity in the detergent solubilized state. Proc Natl Acad Sci U S A. 2000a;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Xu M, Lai MT, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000b;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- Martone RL, Zhou H, Atchison K, et al. Begacestat (GSI-953): a novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein gamma-secretase for the treatment of Alzheimer's disease. J Pharmacol Exp Ther. 2009;331:598–608. doi: 10.1124/jpet.109.152975. [DOI] [PubMed] [Google Scholar]
- May P, Altsteil L, Bender M, et al. Chronic treatment with a functional gamma-secretase inhibitor reduces Abeta burden and plaque pathology in PDAPP mice. Neurobiol. Aging. 2002;23:S133. [Google Scholar]
- Mayer SC, Kreft AF, Harrison B, et al. Discovery of begacestat, a Notch-1-sparing gamma-secretase inhibitor for the treatment of Alzheimer's disease. J Med Chem. 2008;51:7348–7351. doi: 10.1021/jm801252w. [DOI] [PubMed] [Google Scholar]
- Morohashi Y, Kan T, Tominari Y, et al. C-terminal fragment of presenilin is the molecular target of a dipeptidic gamma-secretase-specific inhibitor DAPT (N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester) J Biol Chem. 2006;281:14670–14676. doi: 10.1074/jbc.M513012200. [DOI] [PubMed] [Google Scholar]
- Netzer WJ, Dou F, Cai D, et al. Gleevec inhibits beta-amyloid production but not Notch cleavage. Proc Natl Acad Sci U S A. 2003;100:12444–12449. doi: 10.1073/pnas.1534745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretto I, Radaelli S, Parini C, et al. Synthesis and biological activity of flurbiprofen analogues as selective inhibitors of beta-amyloid(1)(-)(42) secretion. J Med Chem. 2005;48:5705–5720. doi: 10.1021/jm0502541. [DOI] [PubMed] [Google Scholar]
- Petit A, Bihel F, Alves da Costa C, Pourquie O, Checler F, Kraus JL. New protease inhibitors prevent gamma-secretase-mediated production of Abeta40/42 without affecting Notch cleavage. Nat Cell Biol. 2001;3:507–511. doi: 10.1038/35074581. [DOI] [PubMed] [Google Scholar]
- Pissarnitski D. Advances in gamma-secretase modulation. Curr Opin Drug Discov Devel. 2007;10:392–402. [PubMed] [Google Scholar]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, et al. Longer forms of amyloid beta protein: implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci. 2005;25:436–445. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Suemoto T, Brouwers N, et al. Potent amyloidogenicity and pathogenicity of Abeta43. Nat Neurosci. 2011;2011:2858. doi: 10.1038/nn.2858. [DOI] [PubMed] [Google Scholar]
- Sato T, Dohmae N, Qi Y, et al. Potential link between amyloid beta-protein 42 and C-terminal fragment gamma 49–99 of beta-amyloid precursor protein. J Biol Chem. 2003;278:24294–24301. doi: 10.1074/jbc.M211161200. Epub 22003 Apr 24221. [DOI] [PubMed] [Google Scholar]
- Sato T, Nyborg AC, Iwata N, Diehl TS, Saido TC, Golde TE, Wolfe MS. Signal peptide peptidase: biochemical properties and modulation by nonsteroidal antiinflammatory drugs. Biochemistry. 2006;45:8649–8656. doi: 10.1021/bi060597g. [DOI] [PubMed] [Google Scholar]
- Searfoss GH, Jordan WH, Calligaro DO, et al. Adipsin: a biomarker of gastrointestinal toxicity mediated by a functional gamma secretase inhibitor. J Biol Chem. 2003;278:46107–46116. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- Seiffert D, Bradley JD, Rominger CM, et al. Presenilin-1 and -2 are molecular targets for gamma -secretase inhibitors. J Biol Chem. 2000;275:34086–34091. doi: 10.1074/jbc.M005430200. [DOI] [PubMed] [Google Scholar]
- Shearman MS, Beher D, Clarke EE, et al. L-685,458, an Aspartyl Protease Transition State Mimic, Is a Potent Inhibitor of Amyloid beta-Protein Precursor gamma-Secretase Activity. Biochemistry. 2000;39:8698–8704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Siemers E, Skinner M, Dean RA, Gonzales C, Satterwhite J, Farlow M, Ness D, May PC. Safety, tolerability, and changes in amyloid beta concentrations after administration of a gamma-secretase inhibitor in volunteers. Clin Neuropharmacol. 2005;28:126–132. doi: 10.1097/01.wnf.0000167360.27670.29. [DOI] [PubMed] [Google Scholar]
- Siemers ER, Dean RA, Friedrich S, Ferguson-Sells L, Gonzales C, Farlow MR, May PC. Safety, tolerability, and effects on plasma and cerebrospinal fluid amyloid-beta after inhibition of gamma-secretase. Clin Neuropharmacol. 2007;30:317–325. doi: 10.1097/WNF.0b013e31805b7660. [DOI] [PubMed] [Google Scholar]
- Stanton MG, Hubbs J, Sloman D, et al. Fluorinated piperidine acetic acids as gamma-secretase modulators. Bioorg Med Chem Lett. 2009;20:755–758. doi: 10.1016/j.bmcl.2009.11.034. [DOI] [PubMed] [Google Scholar]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. gamma-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29:13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Van Broeck B, Chen JM, Treton G, Desmidt M, Hopf C, Ramsden N, Karran E, Mercken M, Rowley A. Chronic treatment with a novel gamma-secretase modulator, JNJ-40418677, inhibits amyloid plaque formation in a mouse model of Alzheimer's disease. Br J Pharmacol. 2011;163:375–389. doi: 10.1111/j.1476-5381.2011.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, Golde TE, Koo EH. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity. J Biol Chem. 2003;278:31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. The gamma-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry. 2006;45:7931–7939. doi: 10.1021/bi060799c. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Inhibition and modulation of gamma-secretase for Alzheimer's disease. Neurotherapeutics. 2008;5:391–398. doi: 10.1016/j.nurt.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS, Citron M, Diehl TS, Xia W, Donkor IO, Selkoe DJ. A substrate-based difluoro ketone selectively inhibits Alzheimer's γ-secretase activity. J Med Chem. 1998;41:6–9. doi: 10.1021/jm970621b. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Moore CL, Leatherwood DD, Ostaszewski B, Donkor IO, Selkoe DJ. Peptidomimetic probes and molecular modeling suggest Alzheimer's γ-secretases are intramembrane-cleaving aspartyl proteases. Biochemistry. 1999;38:4720–4727. doi: 10.1021/bi982562p. [DOI] [PubMed] [Google Scholar]
- Wong GT, Manfra D, Poulet FM, et al. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–12882. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- Wong PC, Zheng H, Chen H, et al. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- Yan XX, Li T, Rominger CM, Prakash SR, Wong PC, Olson RE, Zaczek R, Li YW. Binding sites of gamma-secretase inhibitors in rodent brain: distribution, postnatal development, and effect of deafferentation. J Neurosci. 2004;24:2942–2952. doi: 10.1523/JNEUROSCI.0092-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Yu M, Neitzel M, et al. Identification of gamma-secretase inhibitor potency determinants on presenilin. J Biol Chem. 2008;283:2927–2938. doi: 10.1074/jbc.M708870200. [DOI] [PubMed] [Google Scholar]