SUMMARY
Valproic acid (VPA) is the most widely prescribed epilepsy treatment worldwide, but its mechanism of action remains unclear. Our previous work identified a previously unknown effect of VPA in reducing phosphoinositide production in the simple model Dictyostelium followed by the transfer of data to a mammalian synaptic release model. In our current study, we show that the reduction in phosphoinositide [PtdInsP (also known as PIP) and PtdInsP2 (also known as PIP2)] production caused by VPA is acute and dose dependent, and that this effect occurs independently of phosphatidylinositol 3-kinase (PI3K) activity, inositol recycling and inositol synthesis. In characterising the structural requirements for this effect, we also identify a family of medium-chain fatty acids that show increased efficacy compared with VPA. Within the group of active compounds is a little-studied group previously associated with seizure control, and analysis of two of these compounds (nonanoic acid and 4-methyloctanoic acid) shows around a threefold enhanced potency compared with VPA for protection in an in vitro acute rat seizure model. Together, our data show that VPA and a newly identified group of medium-chain fatty acids reduce phosphoinositide levels independently of inositol regulation, and suggest the reinvestigation of these compounds as treatments for epilepsy.
INTRODUCTION
Valproic acid (VPA; 2-propylpentanoic acid; Epilim) is a short branched-chain fatty acid that was serendipitously discovered in 1963 to prevent pentelenetetrazole (PTZ)-induced seizures (Meunier et al., 1963). Since then, its therapeutic role has expanded to include migraine and bipolar disorder prophylaxis, as well as a large list of potential new roles, including Alzheimer’s disease, cancer and HIV treatment (Lagace et al., 2005; Terbach and Williams, 2009). Despite considerable research investigating the molecular effect of VPA in mammalian systems, very few mechanisms of action have been identified that occur in the rapid time frame shown for seizure control (Lagace et al., 2005; Terbach and Williams, 2009). The introduction of new model systems for the analysis of VPA effects might thus aid in identifying and characterising the molecular actions of VPA, with an ultimate goal of developing new more potent epilepsy treatments.
The social amoeba Dictyostelium is a powerful, simple model system for cell biology and molecular pharmacology research because isogenic cell lines can be easily grown and analysed for biochemical changes following drug treatment (Williams et al., 2006; Williams, 2009; Williams, 2005a). We have employed Dictyostelium to understand the molecular mechanisms underlying VPA action in regards to both putative bipolar-disorder- and epilepsy-related signalling effects (Boeckeler et al., 2006; Eickholt et al., 2005; Williams et al., 1999; Williams et al., 2002; Xu et al., 2007; Ludtmann et al., 2011), and to study the mechanisms of uptake (Terbach et al., 2011). Our previous work has shown that, in Dictyostelium, both VPA and a structurally unrelated bipolar disorder treatment, lithium, chronically reduce the levels of inositol phosphate {e.g. inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3; also known as IP3 and InsP3]} (Eickholt et al., 2005; Shimshoni et al., 2007; Williams et al., 1999; Williams et al., 2002). We have also previously identified a VPA-induced acute reduction in phosphatidylinositol monophosphate [phosphatidylinositol phosphate (PtdInsP; also known as PIP)] and diphosphate [phosphatidylinositol bisphosphate (PtdInsP2; also known as PIP2)] synthesis in Dictyostelium, and shown that this effect is phenocopied by attenuation of phosphatidylinositol 3-kinase (PI3K)-dependent phosphoinositide signalling (Pawolleck and Williams, 2009; Xu et al., 2007). However, it remains unclear whether VPA-induced inositol phosphate and phosphoinositide effects are related, and whether signalling via type I PI3Ks is a direct target of VPA.
We now continue the investigation into the cellular effects of VPA using Dictyostelium as a model. Here, we use an in vivo assay to measure phosphoinositide production (Pawolleck and Williams, 2009; Xu et al., 2007) to show that VPA acutely reduces PIP and PIP2 levels, in a dose- and time-dependent manner. We then probe a range of potential mechanisms for this rapid effect (Fig. 1): first, we rule out PI3Ks as mediators of the effect of VPA (as we had previously suggested) (Xu et al., 2007), because cells lacking all type I PI3K activity in a single cell line still show VPA-sensitive phosphoinositide levels. Second, we dismiss inositol recycling, because ablation or overexpression of a range of enzymes involved in inositol recycling does not overcome the effect of VPA. Third, we can exclude de novo inositol biosynthesis, because VPA does not reduce inositol production in the timeframe examined here, but only after longer exposure (Shaltiel et al., 2004; Vaden et al., 2001). We then investigated a cohort of structurally simple fatty acids related to VPA for the ability to attenuate phosphoinositide levels. This identified a group of medium-length fatty acid compounds with greater potency than VPA in the reduction of phosphoinositide levels. Subsequent database analysis of these compounds identified related compounds that have been previously shown to be strongly effective seizure-control agents (Chapman et al., 1983; Keane et al., 1983; Liu and Pollack, 1994; Loscher and Nau, 1985). We then tested two of the newly identified compounds – nonanoic acid and 4-methyloctanoic acid (which have not been associated with seizure control) – in an in vitro seizure model, to show vastly improved seizure control compared with that of VPA. Thus, our results suggest that, contrary to expectation, VPA does not lower phosphoinositide levels via a reduction in inositol levels in Dictyostelium, and argues for a reappraisal of medium-chain fatty acids as potential new treatments for epilepsy and other VPA-treatable disorders.
Fig. 1.
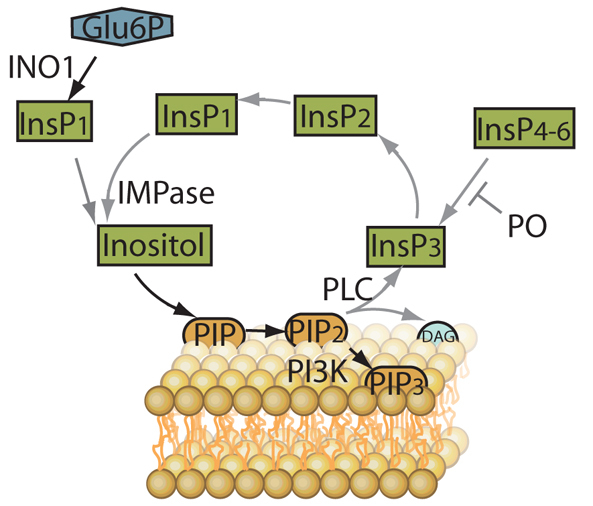
Schematic of phosphoinositide generation and recycling. Enzymes involved in recycling of the phosphoinositide head group (inositol) include phospholipase C (PLC), which catalyses the production of inositol trisphosphate (InsP3) and diacylglycerol (DAG) from phosphatidylinositol diphosphate (PIP2), which in turn is produced from phosphatidylinositol monophosphate (PIP). Prolyl oligopeptidase (PO) regulates the formation of InsP3 from higher order inositol phosphates containing 4–6 phosphates (InsP4–6). InsP3 is recycled via inositol bisphosphate (InsP2) and inositol monophosphate (InsP1) to enable inositol monophosphatase (IMPase) to catalyse the production of inositol. De novo inositol synthesis is catalysed from glucose 6-phosphate (Glu6P) by myo-inositol-3P synthase (INO1). Type 1 phosphatidylinositol 3-kinase (PI3K) is responsible for the phosphorylation of phosphinositides at the 3′ position of the inositol head group, with products including phosphatidylinositol-(3,4,5)-trisphosphate (PIP3).
RESULTS
VPA lowers phosphoinositide levels
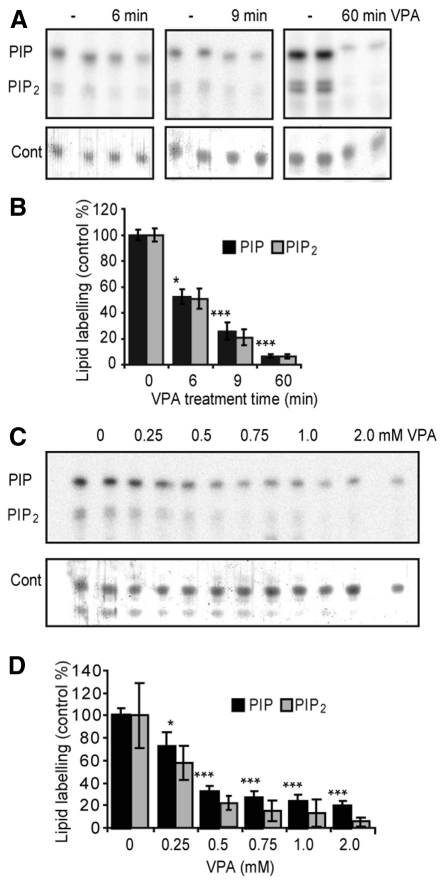
The effect of VPA on phosphoinositide production has not been characterised previously. Here we investigate this effect by permeabilising Dictyostelium cells with saponin and incubating cells with radiolabelled ATP (γ-32P-ATP) in the presence of phosphatase inhibitors (to block phospholipid dephosphorylation), followed by lipid extraction, thin layer chromatography (TLC) separation and phosphorimager quantification (as previously described) (Xu et al., 2007). Our method therefore eliminates potential effects due to VPA uptake and phosphoinositide degradation. Examining the time- and concentration-dependence of the action of VPA (Fig. 2A), we observed a rapid reduction in phosphoinositide phosphorylation after treatment with 0.5 mM VPA. The drug caused an approximate 48% reduction in both radiolabelled PIP and PIP2 production following a 6-minute treatment, a 74% and 79% reduction in PIP and PIP2 after 9 minutes, respectively, and around a 93% reduction in both phosphoinositides after 60 minutes of treatment (Fig. 2A,B). This reduction in phosphoinositide synthesis was also concentration dependent, with a 25% and 42% inhibition of PIP and PIP2 production, respectively, in the presence of 0.25 mM VPA following a 9-minute treatment, increasing to a 72% and 85% reduction with 1.0 mM VPA treatment, respectively, as compared with controls (Fig. 2C,D). Under these conditions, we measure the inhibitory effect (EC50) of VPA to be 154 μM. This acute and strong inhibition of phosphoinositide production caused by VPA makes this effect of potential therapeutic interest.
Fig. 2.
Time- and dose-dependent effect of VPA in reducing phosphoinositide production in Dictyostelium. Phosphoinositide synthesis was monitored by the incorporation of a radio-labelled phosphate into newly formed lipids, followed by extraction, TLC separation and quantification using a Typhoon phosphorimager (Pawolleck and Williams, 2009). (A) TLC separation of radio-labelled PIP and PIP2 in duplicate treatments of cells under control (–) conditions or treated with VPA (0.5 mM) for the times indicated. A loading control (Cont) shows total lipids. (B) Quantification of PIP and PIP2 production in cells treated with VPA (0.5 mM) for the times indicated. (C) TLC separation and (D) quantification of radio-labelled PIP and PIP2 production in duplicate for untreated (0) cells or following treatment with indicated VPA concentrations (for 9 minutes). Results are provided for triplicate experiments, each with duplicate samples ± s.d. *P<0.05; **P<0.01; ***P<0.001 for PIP levels.
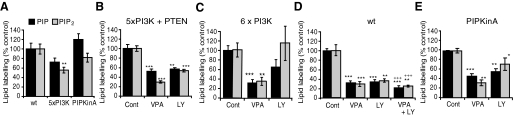
We next investigated potential mechanisms giving rise to this effect. Many routes can contribute to the regulation of the levels of PIP and PIP2 (for an overview, see Fig. 1). We started by analysing the role of PI3K activity as a VPA target, because our previous work had shown that both VPA treatment and pharmacological inhibition of PI3K activity (using LY294006) (Xu et al., 2007) mediated a reduction in Dictyostelium phosphoinositide levels and a reduction of glutamate release in isolated rat hippocampal cells. Studies such as this, analysing the role of phosphoinositide kinases using a pharmacological approach (with enzyme-class-specific inhibitors), can be hard to interpret owing to the large number of kinase enzymes and overlapping effects between inhibitors. We thus took advantage of Dictyostelium as a model system (whereby multiple genes can be ablated in a single cell line), and analysed the effect of VPA on PIP and PIP2 levels in a strain lacking all five type 1 PI3K genes and the single PTEN gene (Hoeller and Kay, 2007) (Fig. 3A,B). In the absence of VPA, these cells showed a 28% and 44% reduction in PIP and PIP2 levels, respectively, compared with wild-type cells. Hence, the ablation of these PI3K proteins seems to have a strong effect on PIP and PIP2 levels (Fig. 3A). We then tested the effect of VPA (0.5 mM) in these cells, identifying a reduction in PIP and PIP2 production by 48% and 70%, respectively, compared with untreated cells following a 9-minute treatment (Fig. 3B). Surprisingly, pharmacological inhibition of PI3K activity in these cells (using 50 μM LY294002) still caused a strong reduction in phosphoinositide levels, by 43% and 47% (for PIP and PIP2, respectively; Fig. 3B). We extended our investigation to a further cell line, lacking all five PI3K genes as well as deletion of an additional potential PI3K [pikH – a type-1-like PI3K protein lacking the Ras-binding domain that is characteristic of this family of enzymes (Hoeller and Kay, 2007)]. These cells still show a strong reduction in phospholipid production in the presence of VPA (68% and 65% for PIP and PIP2 respectively; Fig. 3C) but they did not show a significant reduction of phosphoinositide production in the presence of LY294002. We also found that the pharmacological inhibition of PI3K and the VPA-dependent reduction in phosphoinositide production are additive: LY294002 (50 μM) causes around a 65% inhibition of both PIP and PIP2, production (Fig. 3D), with an additional highly significant reduction in PIP and PIP2 production by 37% and 30%, respectively, following simultaneous addition of VPA (0.5 mM) (P<0.001). These data clearly indicate that VPA and LY294002 (and PI3K ablation) have different cellular effects. We also analysed a second, non-PI3K-related, phosphatidylinositol kinase, PIPKinA, which lacks nuclear phosphatidylinositol 4/5-kinase (PI4K and PI5K) activity (Guo et al., 2001). Ablation of PIPKinA showed no significant change in PIP and PIP2 production in the absence of VPA (Fig. 3A), and VPA treatment still caused a 54% and 68% reduction in PIP and PIP2 synthesis, respectively (Fig. 3E). These data indicate that type I PI3K and PIPKinA enzymes are not the target of VPA in attenuating phosphoinositide production in Dictyostelium.
Fig. 3.
Analysing phosphoinositide production and the effect of VPA treatment in phosphoinositide kinase null mutants. Phosphoinositide synthesis was measured in isogenic Dictyostelium cells in the absence of treatment (Cont), or following 9 minutes of treatment with VPA (0.5 mM) or 50 μM (LY294002), using wild-type cells, cells lacking five type 1 PI3K genes and the single PTEN gene (5×PI3K+PTEN); cells lacking five type 1 PI3K genes in addition to the pikH gene (6×PI3K); and cells lacking the single PIPKinA gene (PIPKinA). (A) Comparison of phosphoinositide production in untreated wild-type, 5xPI3K+PTEN null, and PIPKinA null cells. (B) VPA- and LY294002-sensitivity for 5×PI3K+PTEN null cells. (C) VPA- and LY294002-sensitivity for 6×PI3K null cells. (D) VPA- and LY294002-and combined-sensitivity for wild-type cells. (E) VPA- and LY294002-sensitivity for PIPKinA null cells. Results are provided for triplicate experiments with duplicate samples ± s.e.m. *P<0.05; **P<0.01; ***P<0.001 with respect to control and +++P<0.001 with respect to VPA treatment alone.
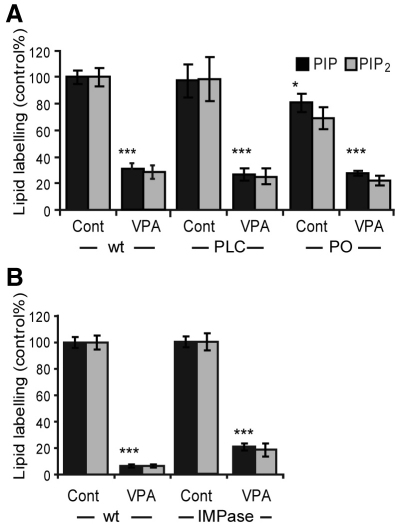
Second, we and others have shown that VPA attenuates inositol recycling through inositol phosphates in models ranging from Dictyostelium (Williams et al., 1999; Williams et al., 2002) to Caenorhabditis elegans (Tokuoka et al., 2008), rats and humans (Shaltiel et al., 2004; Shaltiel et al., 2007b). This effect could potentially play a role in the acute reduction of phosphoinositide levels outlined here (Fig. 1). To examine this, we analysed PIP and PIP2 levels in isogenic Dictyostelium mutants with the inositol recycling pathway blocked or activated. Cells lacking the single gene encoding phospholipase C (PLC) (Drayer et al., 1994), hence blocking the conversion of PIP2 to InsP3, showed no significant reduction in PIP and PIP2 levels compared with wild-type cells (Fig. 4A). Importantly VPA-dependent reduction in PIP and PIP2 production in these cells mirrors that for wild-type cells (around 74% for both PIP and PIP2). By contrast, cells with approximately threefold higher InsP3 levels caused by ablation of prolyl oligopeptidase (Williams et al., 1999; Williams et al., 2002; King et al., 2010) showed a slight decrease in PIP levels (and no significant change in PIP2 levels). Also, here VPA treatment reduced PIP and PIP2 signalling by around 67% (Fig. 4A) – similar to its effect in wild-type cells. We have also previously shown that inhibition of inositol monophosphatase (IMPase) activity by 10 mM lithium does not reduce phosphoinositide production following acute (9 minutes) treatment in Dictyostelium (King et al., 2009); however, extended lithium treatment (60 minutes) reduces PIP and PIP2 levels, and this effect is overcome by overexpression of IMPase. By comparison, overexpressing IMPase did not overcome extended VPA treatment (60 minutes; 0.5 mM) (Fig. 4B). These results show that elevating or reducing recycling of inositol through inositol phosphate species does not alter the effect of VPA on reducing PIP and PIP2 levels. Thus, the mechanism of action of VPA in reducing phosphoinositide levels seems to be independent of inositol recycling.
Fig. 4.
Analysing phosphoinositide production and the effect of VPA treatment following altered inositol recycling. (A) Comparison of phosphoinositide production in wild-type cells (wt), and cells lacking either phospholipase C (PLC) or prolyl oligopeptidase (PO) genes in the absence (Cont) or presence of VPA (0.5 mM; 9 minutes). (B) Comparison of phosphoinositide production in wild-type cells (wt), and cells over-expressing inositol monophosphatase (IMPase) following extended VPA treatment (60 minutes, 0.5 mM). Results are provided for triplicate experiments with duplicate samples ± s.d. *P<0.05; **P<0.01; ***P<0.001 for PIP levels.
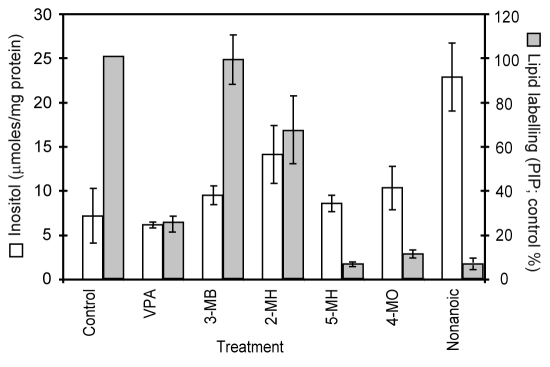
Third, VPA has been identified as an inhibitor of de novo inositol biosynthesis, indirectly blocking the production of inositol 1-phosphate from glucose-6-phosphate (Fig. 1) (Shaltiel et al., 2004; Vaden et al., 2001). We therefore tested a role for VPA in reducing inositol levels following acute treatment (9 minutes; 0.5 mM; Fig. 5), in a similar manner to phosphoinositide measurement experiments. Under these conditions, VPA does not reduce inositol levels, suggesting that a VPA-dependent effect on phosphoinositide production is independent of de novo inositol biosynthesis.
Fig. 5.
Comparison of inositol levels and phosphoinositide (PIP) synthesis following treatment with VPA and related fatty acids. Treatment of cells (9 minutes, 0.5 mM) with VPA, 3-methylbutanoic acid (3-MB), 2-methylhexanoic acid (2-MH), 5-methylhexanoic acid (5-MH), 4-methyloctanoic acid (4-MO) and nonanoic acid (NA) enabled the quantification for inositol levels (white) and phosphoinositol levels (grey) simultaneously. Results are provided for triplicate experiments with duplicate samples ± s.d. for PIP levels.
Identifying compounds that reduce phosphoinositide levels
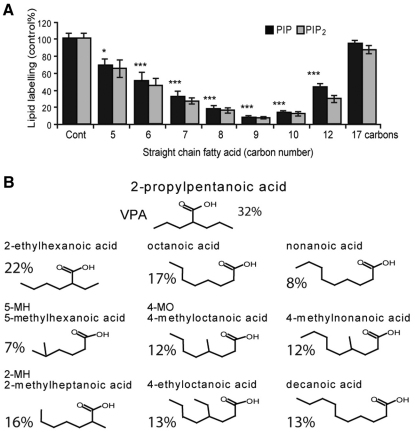
Next we utilised the acute effect of VPA on PIP and PIP2 production in Dictyostelium to investigate novel fatty acids and related structures for similar effects. We initially tested straight-chain fatty acids, ranging from 4 to 17 carbons (Fig. 6A; supplementary material Table S1), because the structure of VPA is based upon a five-carbon backbone. These compounds showed a wide breadth of inhibitory activity, with a peak effect occurring for octanoic (eight carbon; PIP and PIP2 reduction by around 84% for both), nonanoic (nine carbon; reduction by around 92% for both) and decanoic (ten carbon; reduction by 88% for both). Because VPA contains a three-carbon side chain branched from the second carbon, we tested a range of compounds within these medium-chain fatty acid structures with side chains of different length at various positions on the backbone (supplementary material Table S1; Fig. 6B). From these, a range of highly active compounds were identified with backbones of six to ten carbons, and short (ethyl or methyl) side chains branched on the second, fourth or fifth carbon. Active compounds showed a positive association with lipophilicity (supplementary material Fig. S1). Efficacy was also independent of acidity, because variable activity was shown with straight carbon acids of equivalent acidic function (pKa; supplementary material Table S2), although the acid head group is likely to be necessary because related alcohol and aldehyde compounds are non-active. These structural distinctions provide the first characterisation of VPA congeners for reducing phosphoinositide production and the identification of medium-chain fatty acids as highly active compounds for this effect. It is worth noting here that a small number of compounds function to elevate phosphoinositide levels, although we have not explored these compounds further in this paper.
Fig. 6.
Pharmacological inhibition of phosphoinositide signalling with VPA and straight-chain and branched medium chain fatty acids. A range of compounds related to VPA in structure were tested for attenuation of phosphoinositide turnover in Dictyostelium and show a stronger structure-activity relationship than VPA following 9 minutes of 0.5 mM treatment (for a complete list see supplementary material Table S1). (A) Straight chain fatty acids, from 5 carbon to 17 carbons, show the strongest inhibition of phosphoinositide production following treatment with 8–10 carbons. (B) A cohort of medium chain straight and branched fatty acids with short side-chains showed strong attenuation of phosphoinositide production (defined as ≤22% of PIP production compared with untreated cells, whereas VPA reduced production to 32% of untreated levels). These compounds have backbones of six to ten carbons long, with short (methyl or ethyl) side-chains branched from the second, fourth or fifth carbon. Results are provided for triplicate experiments with duplicate samples ± s.d. *P<0.05; **P<0.01; ***P<0.001 for PIP levels.
Because this study identified a range of compounds showing variable inhibitory effects on phosphoinositide production, we then assessed VPA and five related compounds for effects on inositol levels in acutely treated Dictyostelium cells (Fig. 5). Related compounds were selected to include a range of activities on phosphoinositide production (from no effect seen for 3-methylbutanoic acid to highly inhibitory for nonanoic acid). Unexpectedly, VPA and all five compounds increased inositol levels, providing an opposite effect to the acute inhibition of phosphoinositide production, and thus eliminating inositol depletion as a mechanism for this acute effect. A similar lack of correlation between inhibition of PIP and PIP2 production (shown here; supplementary material Table S1) and inositol phosphate level reduction is seen for various VPA congeners. For example, 2-ethyl-4-methyl-pentanoic acid and S-2-pentyl-4-pentynoic acid do not attenuate phosphoinositide production to the level shown by VPA but show a stronger reduction of inositol signalling (Eickholt et al., 2005), whereas 2M2P (Eickholt et al., 2005) and PIA (Shimshoni et al., 2007) show stronger activity than VPA for both effects. Interestingly, substituting the carboxylic acid moiety of compounds showing a strong reduction in phosphoinositide production (PIA, DIA, VPA) with a carboxamide group (yielding the corresponding amides PID, DID, VPD) reduces the inhibitory effect, indicating that amide derivatives are poorly effective for this effect.
Thus, our experiments show that acute VPA treatment lowers phosphoinositide levels in Dictyostelium. Mechanistically, we are able to rule out this effect occurring through PI3K-dependent activity, through inositol recycling and through de novo inositol production. We also found that fatty acids related to VPA can have even stronger effects, and these compounds further allowed us to decouple effects on inositol versus PIP and PIP2 production.
Novel compounds show enhanced efficacy in an in vitro epileptiform model
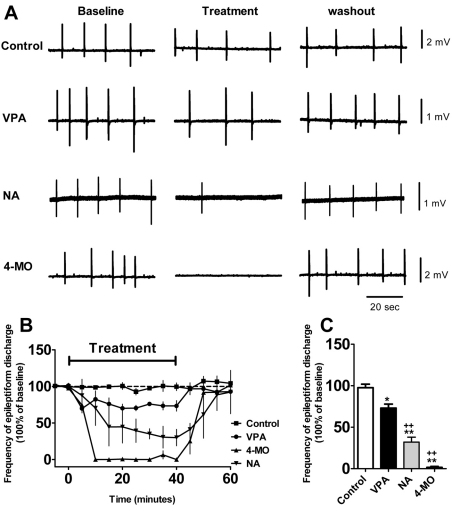
Early studies aimed at identifying more effective epilepsy treatments that are based upon VPA-like fatty acids focused on potential breakdown products of VPA, thus were generally short-chain fatty acids (with a five-carbon backbone), branched at the second carbon. Thus, few studies examined longer-chain fatty acids, and no study that we are aware of has focused on fatty acids branched at, for example, the fourth or fifth carbon. To analyse the potential efficacy of medium-chain fatty acids found in our study to control seizures, we tested two compounds in an in vitro low-Mg2+ seizure model (Armand et al., 1998), using rat hippocampal slices to measure frequency and amplitude of epileptiform discharges induced by low Mg2+ in the mammalian brain. The two compounds chosen were 4-methyloctanoic acid (hircinoic acid; a branched, eight-carbon backbone compound), because this fatty acid is endogenous to animal systems (Johnson et al., 1977), and nonanoic acid (a straight-chain nine-carbon backbone compound), and the effects were compared with those of VPA, with all compound concentrations (1 mM) based upon previous experimental protocols (Heinemann et al., 1994). VPA weakly reduced the frequency of recurrent short discharges in this model (Fig. 7). By contrast, application of an equimolar concentration of nonanoic acid markedly reduced the frequency of recurrent short discharges, and 4-methyloctanoic acid completely abolished the discharges 30–40 minutes after application. Both of these medium-chain fatty acids showed a highly significant efficacy compared with VPA (Fig. 7) and warrant future investigation.
Fig. 7.
Seizure control with VPA, nonanoic acid (NA) and 4-methyloctanoic acid (4-MO) using an in vitro acute low-Mg2+ model. (A) Trace samples of low Mg2+-induced burst discharges by application of VPA, NA and 4-MO (all at 1 mM) compared with control (DMSO only). (B) Summary of the frequency of low Mg2+-induced burst discharges following application of compound (n=5 for all) from time 0 to 40 minutes. Application of VPA resulted in a significant decrease in epileptiform discharge frequency, whereas application of NA showed a stronger effect, and 4-MO abolished discharges entirely. The epileptiform activities in all three treatments recovered during drug washout. (C) Comparison of the mean frequency of low Mg2+-induced burst discharges for the last 10 minutes during compound application with different treatments, demonstrating a significant effect of all compounds in attenuating seizure activity. *P<0.05, ** P<0.01 compared with control; +P<0.05, ++P<0.01, compared with VPA-treated group. Data are presented as means ± s.e.m.
DISCUSSION
VPA is used to treat a number of current medical conditions, including epilepsy, bipolar disorder and migraine, and its role is likely to expand widely to include cancer, HIV and Alzheimer’s treatment (for reviews, see Bialer and Yagen, 2007; Lagace et al., 2005; Terbach and Williams, 2009). Understanding how these conditions are controlled by VPA has proved highly complex because VPA triggers a variety of cellular changes with unknown primary targets, changes have not been related to specific clinical conditions and few structure-function studies have been carried out (Bialer and Yagen, 2007; Lagace et al., 2005; Nalivaeva et al., 2009; Terbach and Williams, 2009). In this study we used a non-sentient biomedical model system, the social amoeba Dictyostelium, to investigate potential mechanisms for the VPA-dependent reduction in phosphoinositide production (Figs 1–4). We then used this effect to identify compounds showing a stronger inhibitory effect, and showed two highly active compounds that are effective in reducing seizures in a mammalian epilepsy model.
In this study, we set about examining the potential mechanisms that lead to the acute and dose-dependent reduction in PIP and PIP2 production by VPA (Fig. 2). We started by first examining a potential role of PI3K as a primary target (Fig. 3), because our earlier work had implicated this activity (Xu et al., 2007). It is very difficult to identify the target(s) of a drug that regulates phosphoinositide production in vivo in a mammalian system, owing to the large number of kinases and phosphatases, and the promiscuous nature of the enzyme substrates. An advantageous approach to address this problem, facilitated in Dictyostelium, is to employ isogenic cell lines containing ablated genes. In this study, we analysed phosphoinositide production in two cell lines with ablated PI3Ks: cells lacking all five type I PI3Ks and PTEN activity (Hoeller and Kay, 2007) and cells lacking the five type I kinases and an additional unique related kinase lacking the Ras-binding domain common to this family of enzymes (Hoeller and Kay, 2007) (Fig. 3). Both cell lines still showed large reductions in phosphoinositide production following VPA treatment, albeit from a reduced basal level. Furthermore, pharmacological inhibition of PI3K activity (using LY294002) was enhanced by the addition of VPA in wild-type cells (Fig. 3D). These combined data suggest that VPA causes an effect on phosphoinositide production independent of type I PI3K activity. In addition to PI3K activity, we also assessed the effect of VPA on another phosphoinositide kinase mutant, lacking a nuclear phosphatidylinositol 4/5 kinase activity (Guo et al., 2001) (Fig. 3E). As shown for the PI3K-null cells, this mutant was still sensitive to the effect of VPA on phosphoinositide production, confirming that this gene product is not the VPA target.
In this paper, we assessed the VPA-dependent reduction of PIP and PIP2 production in isogenic cell lines lacking all type I PI3K homologues and a single phosphatidylinositol 4/5 kinase homologue; however, there are at least nine more potential phosphoinositide kinase proteins encoded within the genome with strong homology to the human kinases, and over 30 genes with related domains, and any one or several of these could be a target for VPA leading to the observed effect. However, all three phosphoinositide-kinase-ablated cell lines showed a reduced sensitivity to VPA than did wild-type cells [following 0.5 mM (9 minutes) VPA treatment, wild-type cells showed a 74% reduction in PIP production, whereas the 5×PI3K+PTEN mutant showed a 43% reduction, the 6×PI3K mutant a 68% reduction and PIPKinA a 54% reduction], suggesting that VPA might function through a more generalised effect on phosphoinositide production, through inhibition of either multiple phosphoinositide kinases or non-kinase activities related to phosphoinositide signalling (e.g. transferase proteins). This latter potential mechanism is supported by the increased levels of inositol observed following acute VPA treatment (Fig. 5). Further analysis of the lipid species involved, and the ablation of the remaining phosphoinositide kinase activities in the genome, might help to better understand this mechanism. The highly significant additive effect of VPA and LY294002 also suggests that off-target effects of LY294002, such as TOR signalling (Kortholt and van Haastert, 2008; Sasaki and Firtel, 2006), are also not the mechanisms of VPA-dependent reduction in phosphoinositide production.
We continued the investigation of potential mechanisms leading to the reduction in PIP and PIP2 production caused by VPA by examining mutants with altered inositol phosphate recycling (Fig. 1). VPA has been shown to reduce inositol phosphate levels (e.g. InsP3), leading to a dampening down of related cell signalling cascades, as described by the inositol depletion theory (Hallcher and Sherman, 1980; Williams et al., 2002; Williams, 2005b). We thus tested for a VPA-related effect on phosphoinositide production in cells containing no PLC activity (Drayer et al., 1994), in those with increased InsP3 levels (Williams et al., 1999; Williams et al., 2002) and in those overexpressing IMPase (King et al., 2010), to show that these mutants were still sensitive to the VPA-dependent effect (Fig. 4). Another potential mechanism for this effect is an indirect inhibition of the enzyme responsible for de novo inositol biosynthesis, myo-inositol-3P synthase (MIPS) (Shaltiel et al., 2004; Vaden et al., 2001). We also show no correlation between phosphinositide levels and inositol levels, because compounds that reduce phosphoinositide levels do not reduce free inositol in the same acute time period (Fig. 5). This data provides the first strong evidence for a potential mechanism of VPA (and related compounds) in targeting cellular mechanisms involving the acute elevation of inositol levels. Furthermore, a range of VPA-related compounds shown to inhibit MIPS activity (Shaltiel et al., 2007a; Shaltiel et al., 2007b) also do not cause a corresponding reduction of phosphoinositide production. Finally, a number of VPA congeners previously shown to reduce inositol phosphate signalling in Dictyostelium and mammalian dorsal root ganglia growth cones (Eickholt et al., 2005; Shimshoni et al., 2007) do not cause a corresponding inhibition of phosphoinositide production. These data together suggest that VPA reduces phosphoinositide production independently of inositol levels and inositol phosphate recycling. These results therefore provide a new mechanism of action of VPA in regulating phosphoinositide production.
Although the primary target for this new mechanism of VPA action is still to be identified, the characterisation of the effect enabled a structure-activity relationship study to be carried out. In studying VPA-related structures, the majority of research has centred around compounds with either a five-carbon backbone (with a branch point on the second carbon) – because these were thought to be reactive metabolites of VPA – or cyclic derivatives (Bialer and Yagen, 2007). Our analysis has, however, identified a previously unknown family of compounds with a six- to ten-carbon backbone (Fig. 6; supplementary material Table S1), showing inhibition of PIP and PIP2 production, opening up a new range of chemical structures that could be of potential therapeutic interest. Excitingly, early research in the analysis of VPA-related compounds in seizure control has identified three compounds related to this group that exhibit enhanced activity with respect to VPA: 2-propylheptanoic acid (Keane et al., 1983), 2-hexyloctanoic acid (Loscher and Nau, 1985) and 2-ethylhexanoic (Chapman et al., 1983). Although these early experiments support the potential relevance of the medium-chain fatty acids identified in this study for seizure control, our newly discovered compounds show a significantly broader range of structures (e.g. compounds with shorter side chains and with other branch points such as from the fourth and fifth carbon), and thus provide a new large family of compounds with potential clinical interest. These compounds might also prove to have reduced side effects, because straight chains and compounds with alternative branching (e.g. at the fourth or fifth carbon) would not be predicted to show teratogenic effects (Nau and Loscher, 1986), nor produce reactive metabolites associated with VPA and potentially giving rise to adverse effects. Further analysis of compounds causing elevated phosphoinositide levels, and an association with an in vivo effect, might also provide an important tool in the development of new VPA-related treatments.
To examine the potential seizure control efficacy of this broad category of compounds, we analysed two compounds in a low-Mg2+ model in vitro model of epileptiform activity (Armand et al., 1998), because this provides a standard in vitro model for drug-resistant seizure control (Heinemann et al., 1994). Both nonanoic acid and 4-methyloctanoic acid showed a much stronger (but still reversible) reduction in epileptiform activity compared with VPA (Fig. 7), suggesting that medium-chain fatty acids (identified in this study as causing a reduction in phosphoinositide synthesis) should be investigated as new treatments for seizure control. These further experiments would include in vivo experiments, because factors such as drug metabolism and brain penetration play an important role in this effect. Also, because VPA, nonanoic acid and 4-methyloctanoic acid do not cause an acute reduction in inositol levels, these data suggest that inositol depletion is not a seizure-protection mechanism. In support of this, pharmacological inhibitors of MIPS (through compounds structurally unrelated to VPA) do not control pilocarpine-induced seizures (Einat et al., 2008). Our data would instead support a role for a therapeutic action of VPA in reducing phosphoinositide synthesis in relation to seizure control.
A therapeutic role for the reduction in phosphoinositide production during epilepsy treatment has been supported by a range of other data. Increased phosphoinositide production has been shown during seizures (Van Rooijen et al., 1986), and ablation of PTEN [responsible for the dephosphorylation of phosphatidylinositol (3,4,5)-trisphosphate, (PIP3)] in a mouse model also gives rise to spontaneous seizures (Backman et al., 2001). These data have given rise to the suggestion that altered phospholipid signalling could provide a crucial role in the pathophysiology of epilepsy (Storey et al., 2002). The acute nature of phosphoinositide attenuation occurs with a similar speed to that of seizure control following intravenous VPA injection in a mouse seizure model (Honack and Loscher, 1992). The concentrations used in these experiments are also found in the therapeutic use of VPA (0.4–0.7 mM in plasma) (DSMV, IV, 2000), and an EC50 of 154 μM would provide a significant inhibition in mammalian systems, in which 90% of VPA is non-specifically bound to plasma proteins (Lagace et al., 2005). Clearly, further investigation into phosphoinositide production during seizures and the inhibition of this by VPA (and other fatty acids) in animal systems will be necessary to confirm this effect.
Our findings identify an effect of VPA in reducing phosphoinositide production in a simple biomedical model, Dictyostelium, and show that this effect occurs independently of PI3K activity and the recycling and de novo biosynthesis of inositol. We then identify a previously unknown family of medium-chain straight and branched compounds showing a stronger reduction in phosphoinositide synthesis than VPA. Translation of this simple model-based research to a preclinical model of seizure control (a low-Mg2+ hippocampal slice model of epileptiform activity) shows a large increase in efficacy for two of these compounds (nonanoic acid and 4-methyloctanoic acid) compared with VPA. These studies thus suggest that the re-investigation of medium-chain fatty acids could provide new, more effective and safer seizure-control treatments. Continued analysis of the biosynthetic pathways controlling phosphoinositide production in model and mammalian systems might also give rise to significant advances in understanding epilepsy and other VPA-treatable disorders such as bipolar disorder and migraine.
METHODS
All chemicals were provided by Sigma UK Ltd. Valproic acid congeners were provided by Sigma Aldrich UK, Alfa Aesar, ChemSampCo or The NCI/DTP Open Chemical Repository (http://dtp.cancer.gov).
Dictyostelium phospholipid labelling and inositol analysis
Dictyostelium mutant strains were kindly provided by Dictybase.org (PLC; DBS0236793) or by Rob Kay (University of Cambridge, UK; quintuple PI3K and PTEN; DBS0252654). A saponin-based cell permeabilisation protocol for Dictyostelium was adapted for these experiments (Pawolleck and Williams, 2009). Dictyostelium AX2 cells were developed for 5 hours as previously described (Boeckeler et al., 2006) (pulsed with cAMP to achieve final concentration of 25 nM), transferred to still dishes (2.5 cm), allowed to settle to give a confluent monolayer in KK2 (20 mM potassium phosphate buffer, pH 6.1) and pre-treated with compound (0.5 mM VPA or related compound, or 50 μM LY294002) for 3 minutes. At regular time intervals, buffer was replaced with labelling solution {139 mM sodium glutamate, 5 mM glucose, 5 mM EDTA, 20 mM PIPES, pH 6.6, 1 mM MgSO4.2H2O, 0.25% (w/v) saponin, 1× phosphatase inhibitor cocktails 1 and 2 (Roche) and 1 μCi/ml γ[32P]ATP (Perkin Elmer)} supplemented with compounds at defined concentrations. Following a 6-minute incubation, labelling solution was removed and cells were lysed in acidified methanol and phospholipids were separated as previously detailed (Pawolleck and Williams, 2009). Phospholipid labelling was quantified using a Typhoon phosphor-imager. Even loading was determined using total lipid stain with copper sulphate. Inositol levels were measured from 5-hour-developed cells (similar to phospholipid labelling), following lyophilisation, as previously described (Maslanski and Busa, 1990).
In vitro epilepsy model
The in vitro epilepsy model has been described previously (Armand et al., 1998): the rats (p21) were decapitated after killing by intraperitoneal injection with an overdose of pentobarbitone (500 mg/kg). The brain was removed and placed in ice-cold sucrose solution (87 mM NaCl, 2.5 mM KCl, 7 mM MgCl2, 0.5 mM CaCl2, 1.25 mM NaH2PO4, 75 mM sucrose, 25 mM glucose), equilibrated with 95% O2/5% CO2 (pH 7.4). Horizontal combined entorhinal cortex-hippocampus slices (350 μm) were prepared with a Leica vibratome (Leica VT1200S) and were then stored in an interface chamber that contained artificial cerebrospinal fluid solution (aCSF) containing 119 mM NaCl, 2.5 mM KCl, 4 mM MgSO4, 4 mM CaCl2, 26.2 mM NaHCO3, 1.0 mM NaH2PO4, 11 mM glucose, and gassed with 95% O2/5% CO2. They were stored for over 1 hour before being transferred to a submersion recording chamber continually perfused with carbogenated aCSF for recording. Field potential recordings were made by placing glass microelectrodes (∼1–2 MΩ) filled with aCSF solution in stratum radiatum of CA1. Bipolar stimulating electrodes were positioned in the Schaffer collateral/commissural fibre pathway in stratum radiatum to confirm slice viability. To generate rhythmic short recurrent discharges, Mg2+-free aCSF was then applied. Novel anticonvulsants were applied once the frequency and amplitude of the epileptiform discharges were stable over a period of 10 minutes. Anticonvulsant effects were evaluated by measuring the variation of frequency of the discharges every minute. The data acquired from 30 to 40 minutes after application of novel anticonvulsants were compared by ANOVA followed by post-hoc testing using the Tukey test, using SPSS statistical analysis. All experiments were conducted in accordance with the UK Home Office regulations under the Animal (Scientific Procedures) Act, 1986. Male Sprague-Dawley rats (P21-P30) were housed under a 12-hour light-dark cycle. The room temperature was maintained at 24–25°C and relative humidity at 50–60%. Standard rodent food and tap water were provided.
TRANSLATIONAL IMPACT.
Clinical issue
Valproic acid is one of the most highly prescribed treatments for seizure control worldwide. However, 46 years of research into the function of valproic acid has failed to identify its mechanism of action, so research has been slow in finding new improved compounds for seizure control. This research has mainly focused on mammalian systems, the standard and most widely accepted model for seizure control research. There is therefore a need to identify alternative model systems to examine the mechanisms underlying valproic acid’s action, and to ultimately develop improved treatments in which valproic acid is indicated, including epilepsy, bipolar disorder and migraine.
Results
The authors employed a simple model system, Dictyostelium, to examine the cellular effects of valproic acid. They identify a previously unidentified ability of valproic acid to decrease phosphoinositide synthesis in this organism. This effect does not occur through regulating inositol levels, which suggests a valproic acid-dependent effect on phosphoinositide phosphorylation. By testing structurally simple fatty acids related to valproic acid, they also identify a range of medium chain fatty acids that are more potent than valproic acid at reducing phosphoinositide levels. Finally, the authors show that two of these fatty acids are more active than valproic acid in an in vitro mammalian assay of seizure activity involving rat hippocampal slices.
Implications and future directions
This work suggests that valproic acid targets phosphoinositide signalling in a manner that is independent of cellular inositol signalling, and that a range of medium chain fatty acids might provide more effective seizure control treatments. Based on these results, investigation of these drugs in more complex mammalian models of seizures is warranted.
Supplementary Material
Acknowledgments
Thanks for strains and support by Dictybase.org.
Footnotes
FUNDING
This work was supported by the Wellcome Trust [project grant number 082640] to R.S.B.W.; the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) [grant number G0900775] to R.S.B.W.; the National Institutes of Health [grant number DK081367] to M.L.G.; and the Wayne State University Biology Department Graduate Enhancement grant to R.M.D.
COMPETING INTERESTS
The authors declare that they do not have any competing or financial interests.
SUPPLEMENTARY MATERIAL
Supplementary material for this article is available at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.008029/-/DC1
REFERENCES
- Armand V., Louvel J., Pumain R., Heinemann U. (1998). Effects of new valproate derivatives on epileptiform discharges induced by pentylenetetrazole or low Mg2+ in rat entorhinal cortex-hippocampus slices. Epilepsy Res. 32, 345–355 [DOI] [PubMed] [Google Scholar]
- Backman S. A., Stambolic V., Suzuki A., Haight J., Elia A., Pretorius J., Tsao M. S., Shannon P., Bolon B., Ivy G. O., et al. (2001). Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat. Genet. 29, 396–403 [DOI] [PubMed] [Google Scholar]
- Bialer M., Yagen B. (2007). Valproic acid: second generation. Neurotherapeutics 4, 130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckeler K., Adley K., Xu X., Jenkins A., Jin T., Williams R. S. (2006). The neuroprotective agent, valproic acid, regulates the mitogen-activated protein kinase pathway through modulation of protein kinase A signalling in Dictyostelium discoideum. Eur. J. Cell Biol. 85, 1047–1057 [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Meldrum B. S., Mendes E. (1983). Acute anticonvulsant activity of structural analogues of valproic acid and changes in brain GABA and aspartate content. Life Sci. 32, 2023–2031 [DOI] [PubMed] [Google Scholar]
- Drayer A. L., Van der Kaay J., Mayr G. W., Van Haastert P. J. (1994). Role of phospholipase C in Dictyostelium: formation of inositol 1,4,5-trisphosphate and normal development in cells lacking phospholipase C activity. EMBO J. 13, 1601–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSMV, IV (2000). American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Washington DC: American Psychiatric Association [Google Scholar]
- Eickholt B. J., Towers G., Ryves W. J., Eikel D., Adley K., Ylinen L., Chadborn N., Harwood A., Nau H., Williams R. S. (2005). Effects of valproic acid derivatives on inositol trisphosphate depletion, teratogenicity, GSK-3β inhibition and viral replication-A screening approach for new bipolar disorder drugs based on the valproic acid core structure. Mol. Pharmacol. 67, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H., Tian F., Belmaker R. H., Frost J. W. (2008). Myo-inositol-1-phosphate (MIP) synthase inhibition: in-vivo study in rats. J. Neural Transm. 115, 55–58 [DOI] [PubMed] [Google Scholar]
- Guo K., Nichol R., Skehel P., Dormann D., Weijer C. J., Williams J. G., Pears C. (2001). A Dictyostelium nuclear phosphatidylinositol phosphate kinase required for developmental gene expression. EMBO J. 20, 6017–6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. (1980). The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J. Biol. Chem. 255, 10896–10901 [PubMed] [Google Scholar]
- Heinemann U., Draguhn A., Ficker E., Stabel J., Zhang C. L. (1994). Strategies for the development of drugs for pharmacoresistant epilepsies. Epilepsia 35 Suppl. 5, S10–S21 [DOI] [PubMed] [Google Scholar]
- Hoeller O., Kay R. R. (2007). Chemotaxis in the absence of PIP3 gradients. Curr. Biol. 17, 813–817 [DOI] [PubMed] [Google Scholar]
- Honack D., Loscher W. (1992). Intravenous valproate: onset and duration of anticonvulsant activity against a series of electroconvulsions in comparison with diazepam and phenytoin. Epilepsy Res. 13, 215–221 [DOI] [PubMed] [Google Scholar]
- Johnson C. B., Wong E., Birch E. J. (1977). Analysis of 4-methyloctanoic acid and other medium chain-length fatty acid constituents of ovine tissue lipids. Lipids 12, 340–347 [DOI] [PubMed] [Google Scholar]
- Keane P. E., Simiand J., Mendes E., Santucci V., Morre M. (1983). The effects of analogues of valproic acid on seizures induced by pentylenetetrazol and GABA content in brain of mice. Neuropharmacology 22, 875–879 [DOI] [PubMed] [Google Scholar]
- King J., Keim M., Teo R., Weening K. E., Kapur M., McQuillan K., Ryves J., Rogers B., Dalton E., Williams R. S., et al. (2010). Genetic control of lithium sensitivity and regulation of inositol biosynthetic genes. PLoS. ONE 5, e11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. S., Teo R., Ryves J., Reddy J. V., Peters O., Orabi B., Hoeller O., Williams R. S., Harwood A. J. (2009). The mood stabiliser lithium suppresses PIP3 signalling in Dictyostelium and human cells. Dis. Model. Mech. 2, 306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortholt A., van Haastert P. J. (2008). Highlighting the role of Ras and Rap during Dictyostelium chemotaxis. Cell. Signal. 20, 1415–1422 [DOI] [PubMed] [Google Scholar]
- Lagace D. C., O’Brien W. T., Gurvich N., Nachtigal M. W., Klein P. S. (2005). Valproic acid: how it works. Or not. Clin. Neurosci. Res. 4, 215–225 [Google Scholar]
- Liu M. J., Pollack G. M. (1994). Pharmacokinetics and pharmacodynamics of valproate analogues in rats. IV. Anticonvulsant action and neurotoxicity of octanoic acid, cyclohexanecarboxylic acid, and 1-methyl-1-cyclohexanecarboxylic acid. Epilepsia 35, 234–243 [DOI] [PubMed] [Google Scholar]
- Loscher W., Nau H. (1985). Pharmacological evaluation of various metabolites and analogues of valproic acid. Anticonvulsant and toxic potencies in mice. Neuropharmacology 24, 427–435 [DOI] [PubMed] [Google Scholar]
- Ludtmann M. H., Boeckeler K., Williams R. S. (2011). Molecular pharmacology in a simple model system: implicating MAP kinase and phosphoinositide signalling in bipolar disorder. Semin. Cell Dev. Biol. 22, 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanski J. A., Busa W. B. (1990). A sensitive and specific mass assay for myo-inositol and inositol phosphates. In Methods in Inositide Research (ed. Irvin R. F.), pp. 113–126 New York: Raven Press Ltd [Google Scholar]
- Meunier H., Carraz G., Neunier Y., Eymard P., Aimard M. (1963). Pharmacodynamic properties of N-dipropylacetic acid. Therapie 18, 435–438 [PubMed] [Google Scholar]
- Nalivaeva N. N., Belyaev N. D., Turner A. J. (2009). Sodium valproate: an old drug with new roles. Trends Pharmacol. Sci. 30, 509–514 [DOI] [PubMed] [Google Scholar]
- Nau H., Loscher W. (1986). Pharmacologic evaluation of various metabolites and analogs of valproic acid: teratogenic potencies in mice. Fundam. Appl. Toxicol. 6, 669–676 [DOI] [PubMed] [Google Scholar]
- Pawolleck N., Williams R. S. (2009). Quantifying in vivo phosphoinositide turnover in chemotactically competent Dictyostelium cells. Methods Mol. Biol. 571, 283–290 [DOI] [PubMed] [Google Scholar]
- Sasaki A. T., Firtel R. A. (2006). Regulation of chemotaxis by the orchestrated activation of Ras, PI3K, and TOR. Eur. J. Cell Biol. 85, 873–895 [DOI] [PubMed] [Google Scholar]
- Shaltiel G., Shamir A., Shapiro J., Ding D., Dalton E., Bialer M., Harwood A. J., Belmaker R. H., Greenberg M. L., Agam G. (2004). Valproate decreases inositol biosynthesis. Biol. Psychiatry 56, 868–874 [DOI] [PubMed] [Google Scholar]
- Shaltiel G., Dalton E. C., Belmaker R. H., Harwood A. J., Agam G. (2007a). Specificity of mood stabilizer action on neuronal growth cones. Bipolar Disord. 9, 281–289 [DOI] [PubMed] [Google Scholar]
- Shaltiel G., Mark S., Kofman O., Belmaker R. H., Agam G. (2007b). Effect of valproate derivatives on human brain myo-inositol-1-phosphate (MIP) synthase activity and amphetamine-induced rearing. Pharmacol. Rep. 59, 402–407 [PubMed] [Google Scholar]
- Shimshoni J. A., Dalton E. C., Jenkins A., Eyal S., Kwan K., Williams R. S., Pessah N., Yagen B., Harwood A. J., Bialer M. (2007). The effects of CNS-active Valproic acid constitutional isomers, cyclopropyl analogues and amide derivatives on neuronal growth cone behaviour. Mol. Pharmacol. 71, 884–892 [DOI] [PubMed] [Google Scholar]
- Storey N. M., O’Bryan J. P., Armstrong D. L. (2002). Rac and Rho mediate opposing hormonal regulation of the ether-a-go-go-related potassium channel. Curr. Biol. 12, 27–33 [DOI] [PubMed] [Google Scholar]
- Terbach N., Williams R. S. (2009). Structure-function studies for the panacea, valproic acid. Biochem. Soc. Trans. 37, 1126–1132 [DOI] [PubMed] [Google Scholar]
- Terbach N., Shah R., Kelerman R., Klein P. S., Gordienko D. V., Brown N. A., Wilkinson C. J., Williams R. S. B. (2011). Identifying an uptake mechanism for the antiepileptic and bipolar disorder treatment valproic acid. J. Cell Sci. 124, 2267–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuoka S. M., Saiardi A., Nurrish S. J. (2008). The mood stabilizer valproate inhibits both inositol- and diacylglycerol-signaling pathways in Caenorhabditis elegans. Mol. Biol. Cell 19, 2241–2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaden D. L., Ding D., Peterson B., Greenberg M. L. (2001). Lithium and valproate decrease inositol mass and increase expression of the yeast INO1 and INO2 genes for inositol biosynthesis. J. Biol. Chem. 276, 15466–15471 [DOI] [PubMed] [Google Scholar]
- Van Rooijen L. A., Vadnal R., Dobard P., Bazan N. G. (1986). Enhanced inositide turnover in brain during bicuculline-induced status epilepticus. Biochem. Biophys. Res. Commun. 136, 827–834 [DOI] [PubMed] [Google Scholar]
- Williams R. S. (2009). Employing multiple models, methods and mechanisms in bipolar disorder research. Biochem. Soc. Trans. 37, 1077–1079 [DOI] [PubMed] [Google Scholar]
- Williams R. S., Boeckeler K., Graf R., Muller-Taubenberger A., Li Z., Isberg R. R., Wessels D., Soll D. R., Alexander H., Alexander S. (2006). Towards a molecular understanding of human diseases using Dictyostelium discoideum. Trends Mol. Med. 12, 415–424 [DOI] [PubMed] [Google Scholar]
- Williams R. S. B. (2005a). Pharmacogenetics in model systems: Defining a common mechanism of action for mood stabilisers. Prog. Neuropsychopharmacol. Biol. Psychiatry 29, 1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S. B. (2005b). Prolyl oligopeptidase and bipolar disorder. Clin. Neurosci. Res. 4, 233–242 [Google Scholar]
- Williams R. S. B., Eames M., Ryves W. J., Viggars J., Harwood A. J. (1999). Loss of a prolyl oligopeptidase confers resistance to lithium by elevation of inositol (1,4,5) trisphosphate. EMBO J. 18, 2734–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S. B., Cheng L., Mudge A. W., Harwood A. J. (2002). A common mechanism of action for three mood-stabilizing drugs. Nature 417, 292–295 [DOI] [PubMed] [Google Scholar]
- Xu X., Muller-Taubenberger A., Adley K. E., Pawolleck N., Lee V. W., Wiedemann C., Sihra T. S., Maniak M., Jin T., Williams R. S. (2007). Attenuation of phospholipid signaling provides a novel mechanism for the action of valproic acid. Eukaryot. Cell 6, 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.