Abstract
Aggregatibacter actinomycetemcomitans is implicated in aggressive forms of periodontitis. Similarly to several other Gram-negative species, this organism produces and excretes a cytolethal distending toxin (CDT), a genotoxin associated with cell distention, G2 cell cycle arrest, and/or apoptosis in many mammalian cell types. In this study, we have identified A. actinomycetemcomitans outer membrane vesicles (OMVs) as a vehicle for simultaneous delivery of multiple proteins, including CDT, into human cells. The OMV proteins were internalized in both HeLa cells and human gingival fibroblasts (HGF) via a mechanism of OMV fusion with lipid rafts in the plasma membrane. The active toxin unit, CdtB, was localized inside the nucleus of the intoxicated cells, whereas OmpA and proteins detected using an antibody specific to whole A. actinomycetemcomitans serotype a cells had a perinuclear distribution. In accordance with a tight association of CdtB with OMVs, vesicles isolated from A. actinomycetemcomitans strain D7SS (serotype a), in contrast to OMVs from a D7SS cdtABC mutant, induced a cytolethal distending effect on HeLa and HGF cells, indicating that OMV-associated CDT was biologically active. Association of CDT with OMVs was also observed in A. actinomycetemcomitans isolates belonging to serotypes b and c, indicating that OMV-mediated release of CDT may be conserved in A. actinomycetemcomitans. Although the role of A. actinomycetemcomitans OMVs in periodontal disease has not yet been elucidated, our present data suggest that OMVs could deliver biologically active CDT and additional virulence factors into susceptible cells of the periodontium.
INTRODUCTION
Outer membrane vesicles (OMVs), naturally shed by most Gram-negative bacteria, can deliver toxins and other virulence factors to the host at relatively high concentrations without requiring close contact between the bacterial and target human cells, and they are believed to represent a key factor in effecting an inflammatory response in the host toward bacterial pathogens (4, 16, 36, 37, 67). Two main routes for delivery of OMV contents to various types of host cells have been recognized: receptor-mediated endocytosis of intact OMVs and fusion of OMVs with the host cell plasma membrane to liberate their cargo (3, 16). Release of OMVs, and their subsequent entry into the surrounding tissues and blood circulation may represent one mechanism of systemic stimulation that is of particular importance in chronic localized infections, such as periodontitis (33).
The Gram-negative bacterium Aggregatibacter (Actinobacillus) actinomycetemcomitans is implicated in aggressive forms of periodontitis (60, 62). The oral cavity is its natural habitat, but live bacteria also translocate from the oral cavity into the blood circulation and thereby to other body sites, as evidenced by the occurrence of severe nonoral A. actinomycetemcomitans infections (66). The mechanisms by which A. actinomycetemcomitans causes disease are not fully understood. OMVs released by this organism contain several proteins that may play a role in modulating the host response, although at present the contributions of individual OMV-associated factors are only beginning to be elucidated. For example, A. actinomycetemcomitans OMVs were demonstrated to be enriched with biologically active leukotoxin (LtxA) (15, 32), an RTX toxin (repeats in toxin) that lyses cells of the lymphocytic and monomyelocytic lineages (38, 59). Moreover, OmpA and the GroEL homologue of A. actinomycetemcomitans, which can activate several different types of mammalian cells, have been found in OMVs (22, 32, 55), and so has peptidoglycan-associated lipoprotein (PAL), which exhibits a proinflammatory activity on human whole blood (31).
Analogously to a variety of Gram-negative species (e.g., Escherichia coli, Haemophilus ducreyi, Shigella dysenteriae, Campylobacter spp., Helicobacter spp., and Salmonella enterica), A. actinomycetemcomitans produces and excretes a cytolethal distending toxin (CDT) (23, 61, 63). CDTs are genotoxins (i.e., they cause DNA damage in mammalian cells) and are the first bacterial protein toxins known to act in the nuclei of target cells (23, 40, 46). The toxicity of CDT is associated with G2 cell cycle arrest, progressive cellular distension, and/or apoptosis in many cell types (23, 61). CDT holotoxin is a tripartite complex comprised of CdtA, CdtB, and CdtC, which are all required for cytotoxicity (41). The CdtB protein is the active subunit and functions as a type I DNase (18, 40). To allow internalization of CdtB, CdtA and CdtC mediate the binding on the surface of target cells (2, 42, 43, 48). Membrane cholesterol has been reported to serve as an essential ligand for this binding (12). CdtB is then translocated to the nucleus via a yet-unknown mechanism (46, 49). It has been demonstrated that CdtB alone was sufficient to induce a cytotoxic effect both when expressed inside (e.g., after bacterial internalization) and when injected into target cells (17, 25).
A. actinomycetemcomitans is the only oral bacterial species known to produce CDT (70). A number of studies collectively support that CDT may be important in the pathogenesis of aggressive periodontitis (1, 19, 64), although the actual contribution of CDT to this disease is not yet understood. In vitro studies have revealed that CDT stimulated receptor activator of NF-κB ligand (RANKL) in human gingival fibroblasts (HGF) (9), which comprise the major cell population of the gingival connective tissue (5). It has been suggested that RANKL activation is the primary factor in acute alveolar bone absorption in aggressive periodontitis (9). Moreover, recent reports have revealed that purified A. actinomycetemcomitans CDT holotoxin caused structural damage to rat and human oral epithelia ex vivo (14). It also induced cell cycle arrest and damage in rat periodontal epithelial cells in vivo (50), which is consistent with CDT playing a part in early pathogenesis of periodontitis in the presence of A. actinomycetemcomitans.
A study using intestinal epithelial cells revealed that Campylobacter jejuni excretes biologically active CDT in association with OMVs, supporting that OMVs may represent a route to deliver CDT toxin to the surrounding environment, including infected host tissues (44). Release of OMV-associated CDT was also demonstrated by an extraintestinal E. coli isolate (11). These findings prompted us to investigate whether CDT may be secreted via OMVs also by A. actinomycetemcomitans.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A. actinomycetemcomitans serotype a smooth-colony strain D7SS (68), its cdtABC mutant derivative (47), and the rough-colony strains 2146 (serotype b) and 2292 (serotype c), which are part of the collection of oral clinical isolates of A. actinomycetemcomitans in our laboratory, were used in this study. A. actinomycetemcomitans strains were cultivated in air supplemented with 5% CO2, at 37°C as previously described (31), on blood agar plates (5% defibrinated horse blood, 5 mg hemin/liter, 10 mg vitamin K/liter, Columbia agar base), or in tryptic soy broth (TSB) supplemented with 0.6% yeast extract and 0.8% glucose (Becton, Dickinson and Company).
Cell lines, culturing, and treatment conditions.
HeLa cells (ATCC CCL-2) and HGF (7) were cultured in Advanced minimum essential medium (MEM) (Invitrogen) supplemented with 2 mM glutamine, 10% fetal calf serum, and 50 μg/ml gentamicin. The cells were cultivated at 37°C in a 5% CO2 atmosphere in air. Prior to experimental treatment, cells were washed with PBS and treated with trypsin (250 μg/ml in phosphate-buffered saline [PBS]) for 5 min at 37°C. Trypsin-treated cells were collected by centrifugation and then resuspended in MEM containing gentamicin (final concentration, 50 μg/ml).
Isolation of outer membrane vesicles.
OMVs were isolated by ultracentrifugation essentially as described earlier (31, 32). For this, A. actinomycetemcomitans strains were harvested from blood agar plates after 3 days of growth and suspended in phosphate-buffered saline (PBS), or, alternatively, strains were grown in broth culture to late exponential phase. Harvested plate-grown bacteria or broth cultures were then centrifuged at 8,000 × g (30 min, 4°C) in a JA-25.50 rotor (Beckman Instruments Inc.). Filtered (0.22 μm; Millipore) supernatants were centrifuged at 85,000 × g (2 h, 4°C) in a 70 Ti rotor (Beckman Instruments Inc.) to collect OMVs. Pellets were washed twice with PBS (85,000 × g; 2 h, 4°C) and then suspended in PBS and used as the OMV preparation. OMV preparations were checked for absence of bacterial contamination by cultivating small aliquots on blood agar plates in air supplemented with 5% CO2 at 37°C for 3 days. Fractionation of OMV preparations was performed by density gradient centrifugation essentially as described earlier (4, 29). In brief, OMV pellets were resuspended in 50 mM HEPES (pH 6.8) and then adjusted to 45% OptiPrep (Sigma-Aldrich) in a final volume of 150 μl. The sample was transferred to the bottom of a 4-ml ultracentrifuge tube, and then different OptiPrep-HEPES layers were sequentially added as follows: 900 μl of 35%, 900 μl of 30%, 660 μl of 25%, 660 μl of 20%, 400 μl of 15%, and 500 μl of 10%. Gradients were centrifuged at 180,000 × g (3 h, 4°C) in an SW 60 Ti rotor (Beckman Instruments Inc.), and fractions of equal volumes (200 μl) were removed sequentially from the top.
SDS-PAGE and Western immunoblotting.
The procedures used for SDS-PAGE and Western immunoblot analysis have been described previously (56). For detection of CdtA, CdtB, and CdtC, analogous to earlier studies on A. actinomycetemcomitans CDT (8, 10), we used rabbit polyclonal antisera specific for the H. ducreyi CDT holotoxin and its CdtA, CdtB, and CdtC protein units, respectively (69) (final dilution, 1:1,000). The CDT proteins of H. ducreyi and A. actinomycetemcomitans exhibit approximately 95% amino acid sequence homology (58). For immunoblots we also used rabbit polyclonal antisera specific for A. actinomycetemcomitans LtxA (final dilution, 1:10,000) (30), E. coli OmpA (1:10,000) (27), and whole cells of an A. actinomycetemcomitans serotype a isolate (1:1,000) (57) and a mouse monoclonal antibody specific for E. coli DnaK (Assay Designs) (1:10,000). Horseradish peroxidase (HRP)-conjugated anti-rabbit and anti-mouse secondary antibodies were used at final dilutions of 1:10,000 and 1:2,500, respectively. Immunoreactive bands were visualized using SuperSignal (Pierce) and the ChemiDoc XRS+ system (Bio-Rad).
AFM.
For atomic force microscopy (AFM), OMV preparations were diluted with ultrapure water (Millipore) and immediately placed on a freshly cleaved mica surface. The samples were incubated at room temperature for 5 min, gently washed with ultrapure water, and dried in a desiccator for at least 2 h. Imaging was performed using a Nanoscope V atomic force microscope (Veeco Instruments) with tapping mode. Final images were plane fitted in both the x and y axes and are presented in amplitude mode.
Dissociation assay.
A dissociation assay was carried out essentially as described previously (4). In brief, OMV preparations in PBS (approximately 20 μg/ml total protein) were treated with 1 M NaCl, 0.8 M urea, 1% SDS, or 1× PBS (60 min on ice). Samples were then centrifuged at 120,000 × g (2 h, 4°C) in an SW 60 Ti rotor (Beckman Instruments Inc.). The resulting supernatants were acetone precipitated and resolubilized in a volume of PBS equal to that for the pellets. Both pellets and supernatants were subsequently analyzed by Western immunoblotting.
Cytolethal distending and internalization assays.
To monitor CDT-dependent distension of HeLa cells and HGF exhibited by OMVs, cell aliquots of 1 ml (5 × 104 cells per ml) were incubated with OMV preparations such that the final OMV protein concentration was 10 μg/ml. Treated cells were then monitored to detect the earlier-reported CDT-dependent distension in HeLa and HGF cell sizes appearing after 1 to 4 days of incubation (7, 51, 63). To assess whether inhibition of retrograde transport affects internalization of A. actinomycetemcomitans OMV proteins, HeLa cell aliquots of 1 ml (5 × 104 cells per ml) were incubated with brefeldin A (final concentration, 10 μM) for 1 h prior to addition of OMVs. HeLa cells were then treated for 6 h with OMV preparations such that the final OMV protein concentration was 200 μg/ml. After incubations, cell samples were fixed (2% paraformaldehyde in PBS [pH 7.3] for 10 min). To monitor the localization of OMV proteins in HeLa cells and HGF, actin of the treated cell samples was stained using Alexa Fluor 488-phalloidin (Molecular Probes) containing 1% bovine serum albumin (BSA). Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) at 1:5,000. For antibody detection, fixed cells were incubated (60 min, 37°C) with rabbit polyclonal antibodies specific for whole cells of A. actinomycetemcomitans serotype a, E. coli OmpA, or H. ducreyi CdtB, each at a final dilution of 1:100. After three washes with PBS, the cells were incubated with tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-rabbit IgG (Sigma-Aldrich) (1:200) for 30 min at 37°C in the dark. Following three washes with PBS, the coverslips were subjected to confocal microscopy as described below.
Membrane fusion assay.
Membrane fusion assays with HeLa and HGF cells were performed essentially by following procedures described earlier (13). Briefly, A. actinomycetemcomitans OMVs were labeled with rhodamine isothiocyanate B-R18 (Molecular Probes) at a saturated concentration (1 mg/ml) for 1 h at room temperature. Fluorescence of this probe is quenched at high concentrations in bilayer membranes and is dequenched when the probe is diluted due to OMV fusion (13, 28). Unlabeled probe was removed by centrifugation at 100,000 × g (30 min, 4°C). After a washing step (PBS), B-R18-labeled OMVs were resuspended in 1 ml PBS (0.2 M NaCl). Subsequently, the host cell plasma membrane was labeled with fluorescein isothiocyanate (FITC)-conjugated cholera toxin B subunit (CtxB) (Sigma-Aldrich) at a concentration of 8 μg/ml for 1 h prior to the incubation with OMVs. CtxB binds to lipid raft-enriched GM1 ganglioside in the plasma membrane and is widely exploited as a marker to visualize lipid rafts (26). Alternatively, wheat germ agglutinin (WGA), which labels the plasma membrane, was used as a eukaryotic cell surface marker. For this, host cells were labeled with FITC-conjugated WGA (Sigma-Aldrich) at a concentration of 1 μg/ml for 1 h prior to the incubation with OMVs. B-R18-labeled OMVs were then applied to the apical sides of HeLa or HGF cells so that the OMVs were diluted 1:4 relative to the volume of MEM in the wells. Cells were incubated with OMVs for 30 min at 37°C. To inhibit OMV fusion with plasma membrane lipid rafts, the cholesterol-sequestering agent Filipin III (53) was added at a final concentration of 10 μg/ml at 30 min prior to the addition of OMVs. After the incubation with OMVs, cell samples were analyzed by confocal microscopy as described below.
Confocal microscopy.
For analysis by confocal microscopy, coverslips were mounted with Mowiol (Scharlau Chemie S.A., Sentmenat, Spain) containing antifade (p-phenylene diamine). Confocal microscopy was carried out using a Nikon D-Eclipse C1 confocal laser with a Nikon Eclipse 90i microscope. Images were captured with a Nikon color camera (24 bit), using aplan Apo Nikon 60× and 100× objectives. Fluorescence was recorded at 405 nm (blue; DAPI), 488 nm (green; FITC-CtxB, FITC-WGA, and Alexa Fluor 488-phalloidin), and 543 nm (red; TRITC and rhodamine isothiocyanate B-R18). Z-stack images were taken at 0.1-μm steps covering 2.8 to 5.8 μm using EZ-C1 3.80 imaging software. The images were adjusted and assembled in Adobe Photoshop 10.0. For calculation of cytoplasmic/nuclear fluorescence ratios, fluorescence levels were quantified using ImageJ on projected confocal stacks obtained from three independent experiments. For each calculation, single confocal slices from within the central region of the cells were analyzed, considering 20 cells. Data were analyzed using GraphPad Prism 4.03 (GraphPad, San Diego, CA). Statistical analysis was performed using the two-tailed Student t test. P values of less than 0.05 were regarded as statistically significant. Quantification of fluorophore colocalization in confocal stacks was done using NIS-Elements AR 3.2 software (Nikon). Colocalization coefficients were calculated from quantitative data obtained from five confocal stacks.
RESULTS
Identification of CDT protein components in association with A. actinomycetemcomitans OMVs obtained from plate-grown bacteria and broth cultures.
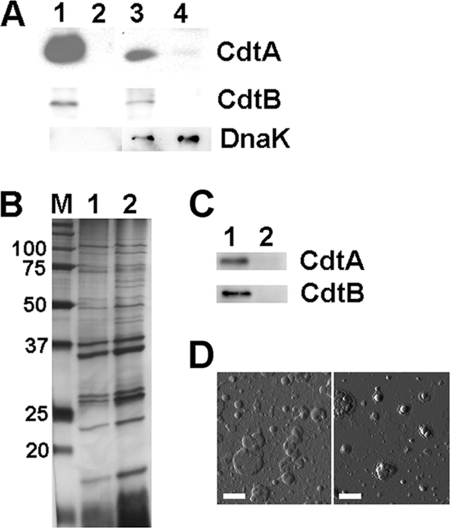
To investigate if CDT protein components are associated with A. actinomycetemcomitans OMVs, OMVs were isolated from the serotype a strain D7SS and its cdtABC triple mutant derivative cultivated on agar (see Materials and Methods). Western immunoblot analysis was then performed, using polyclonal antisera raised against the H. ducreyi CDT holotoxin and the CdtA, CdtB, and CdtC proteins. Two CDT protein components, CdtA and CdtB, were detected in both strain D7SS OMVs and whole-cell extracts, whereas they were absent from such samples of the cdtABC mutant (Fig. 1A). Moreover, we also could detect CdtA and CdtB in OMV preparations and whole-cell extracts from A. actinomycetemcomitans strains 2146 and 2292 (serotypes b and c, respectively) (data not shown), indicating that the OMV association was not restricted to strain D7SS or a characteristic of serotype a strains only. However, using the antiserum raised either against the CDT holotoxin or against CdtC, we could not detect CdtC protein with the present experimental setup, either in OMVs of strains D7SS, 2146, and 2291 or in whole-cell extracts (data not shown). This could be due to a lack of specificity of the antisera used, as they did not recognize the CdtC component of recombinant A. actinomycetemcomitans CDT holotoxin (data not shown). To rule out contamination from the cytoplasmic fraction of the bacterial cells in the OMV preparations, Western immunoblotting was performed using a monoclonal antibody specific for DnaK, which was used earlier as a cytoplasmic marker protein in studies with A. actinomycetemcomitans (55). According to our findings, DnaK was detected in whole-cell extracts of strain D7SS and of its corresponding cdtABC mutant but not in the OMV preparations (Fig. 1A).
Fig 1.
Detection of CdtA and CdtB in A. actinomycetemcomitans OMV preparations from plate-grown bacteria and broth cultures. (A) Immunoblot detection of CdtA, CdtB, and DnaK (lysis marker) in OMV preparations and in whole-cell preparation samples from A. actinomycetemcomitans strain D7SS (lane 1, OMVs; lane 3, whole cells) and from the strain D7SS cdtABC triple mutant (lane 2, OMVs; lane 4, whole cells), both cultivated on agar. (B) Silver-stained SDS-PAGE of OMV preparations obtained from strain D7SS cultivated on agar (lane 1), and in broth culture (lane 2). The sizes (kDa) of the proteins in the prestained molecular mass marker (M) are indicated along the left side. (C) Immunoblot detection of CdtA and CdtB in OMV preparations from strain D7SS (lane 1) and from the strain D7SS cdtABC triple mutant (lane 2), both grown in broth cultures. Polyclonal antisera specific for H. ducreyi CdtA, H. ducreyi CdtB, and E. coli DnaK were used for immunoblot detection. Protein samples equal to 10 μg (A and C) or 2.5 μg (B) were applied on the gels. (D) Atomic force micrographs of OMV preparations from strain D7SS cultivated on agar (left) and in broth culture (right). Bars = 200 nm.
As growth conditions are known to affect protein expression, OMVs were also isolated from A. actinomycetemcomitans broth cultures (see Materials and Methods). According to SDS-PAGE, the protein patterns of strain D7SS OMVs isolated from broth cultures and plate-grown bacteria were very similar, albeit not identical (Fig. 1B), suggesting that environmental conditions may affect the content of A. actinomycetemcomitans OMVs. Consistent with this, immunoblotting revealed a relative abundance of CdtA and CdtB in the OMVs extracted from broth cultures (Fig. 1C) that was different from that in OMVs from plate-grown bacteria (Fig. 1A). As judged by atomic force microscopy (Fig. 1D), vesicles obtained from broth cultures and plate-grown bacteria had very similar shapes, although the latter preparations also contained larger vesicles (with diameters of up to 200 nm). Since our results hitherto obtained indicated that CDT was associated with OMVs isolated from both plate-grown bacteria and broth cultures, we continued our analyses using mainly vesicles isolated from bacteria grown on agar.
Tight association of CdtB with A. actinomycetemcomitans OMVs.
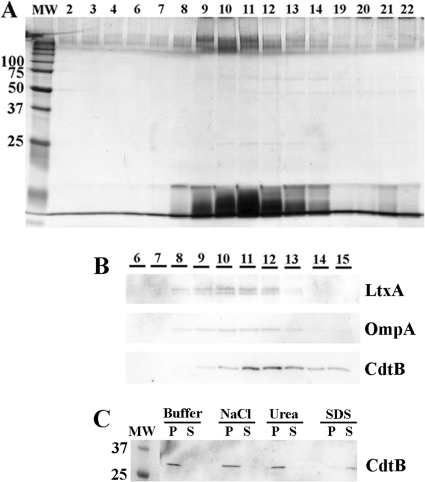
To rule out that the association of CDT protein components with A. actinomycetemcomitans OMVs was merely a consequence of coprecipitation of protein aggregates formed during OMV preparation, density gradient fractionation of OMV preparations was performed (see Materials and Methods). Equal volumes of the obtained gradient fractions were analyzed by SDS-PAGE for their protein and lipopolysaccharide (LPS) compositions. As indicated by the protein and LPS profiles, the vesicle preparations appeared to contain a major population of OMVs, peaking approximately in fractions 9 to 13 (Fig. 2A). Western immunoblotting (Fig. 2B) indicated that CdtB, the active toxin subunit, as well as LtxA and OmpA (the latter two used as OMV marker proteins based on previous data [32]) were mainly distributed in these fractions. However, the peak for CdtB was found in fractions 11 and 12, whereas both OMV marker proteins, OmpA and LtxA, were more centered in fractions 10 and 11. We assume that this difference may reflect different subpopulations of OMVs. To corroborate our observation that CdtB was intimately associated with the OMVs, a dissociation assay was performed (see Materials and Methods). As indicated in Fig. 2C, treatment of strain D7SS OMVs with a high salt concentration (1 M NaCl) did not extract CdtB from the vesicles to a greater extent than that observed with HEPES buffer alone; i.e., CdtB was recovered with OMVs in the pellet. Treatment with 0.8 M urea also did not affect the association of CdtB with OMVs (Fig. 2C). On the other hand, after solubilization of the OMVs with SDS (final concentration of 1%), CdtB could not be detected in the pellet but was instead released and remained in the supernatant after the subsequent centrifugation (Fig. 2C). Thus, we concluded that CdtB was tightly associated with A. actinomycetemcomitans OMVs.
Fig 2.
Tight association of CdtB with A. actinomycetemcomitans OMVs. (A) Silver-stained SDS-PAGE of proteins present in OptiPrep density gradient fractions of OMVs from A. actinomycetemcomitans strain D7SS. Density gradient fractions (15 μl applied on the gel) are numbered from left to right according to increasing density. The sizes (kDa) of the proteins in the prestained molecular mass marker (MW) are indicated along the left side. (B) Immunoblot detection of LtxA, OmpA, and CdtB in density gradient fractions of OMVs from strain D7SS. Polyclonal antisera specific for A. actinomycetemcomitans LtxA, E. coli OmpA, and H. ducreyi CdtB were used to detect LtxA, OmpA, and CdtB, respectively. Fifteen microliters of each of the gradient fractions 6 to 15, numbered according to panel A, was applied on the gel. (C) Dissociation assay using OMVs from strain D7SS. An OMV preparation in PBS was treated for 60 min on ice in the presence of PBS (buffer), NaCl (1 M), urea (0.8 M), or SDS (1%). The resulting pellets (P) and supernatants (S) after centrifugation were analyzed by immunoblotting, using a polyclonal antiserum specific for H. ducreyi CdtB. The sizes of proteins (kDa) in the prestained molecular mass marker (MW) are indicated.
A. actinomycetemcomitans OMVs exhibit CDT-dependent cytolethal distending activity.
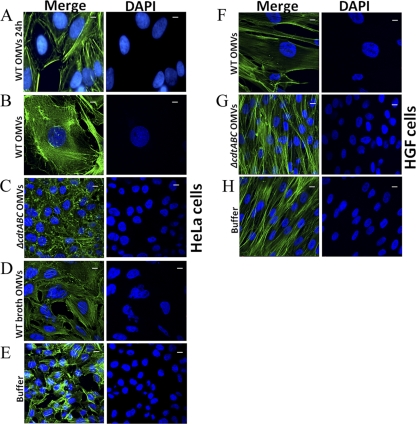
To investigate if A. actinomycetemcomitans OMVs carry biologically active CDT, we incubated HeLa cells and HGF with OMV preparations from A. actinomycetemcomitans strain D7SS and from the D7SS cdtABC triple mutant and then monitored the treated cells by confocal microscopy. According to our findings, strain D7SS OMVs isolated from both plate-grown bacteria (Fig. 3A, B, and F) and broth cultures (Fig. 3D and data not shown) induced a distinct enlargement of the intoxicated HeLa and HGF cells after 24 to 72 h of incubation. In contrast, this was not observed using OMVs from the cdtABC triple mutant (Fig. 3C and G) or when HeLa and HGF cells were treated with buffer (PBS) alone (Fig. 3E and H). Hence, we concluded that A. actinomycetemcomitans OMVs exhibited CDT-dependent cytolethal distending activity.
Fig 3.
Cytolethal distending effect exhibited by A. actinomycetemcomitans OMVs on HeLa cells (A to E) and HGF (F to H). Cells were treated with strain D7SS OMVs isolated from plate-grown bacteria (wild type [WT]) for 24 h (A) and for 72 h (B and F), and with D7SS OMVs isolated from broth cultures (WT broth) for 72 h (D). As controls, cells were treated for 72 h with OMVs isolated from strain D7SS cdtABC cultivated on agar (cdtABC) for 72 h (C and G) and with PBS (buffer) (E and H). After incubation, actin filaments and nuclei were stained with phalloidin (green) and DAPI (blue), respectively. The left panels show merged images from staining with both dyes, and the right panels show images from DAPI staining only. Magnification, ×1,000. Bars = 10 μm.
Internalization of A. actinomycetemcomitans OMV-associated proteins into HeLa cells and human gingival fibroblasts.
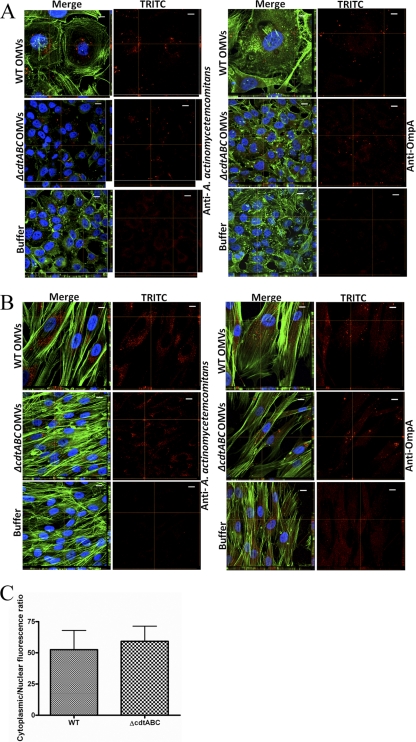
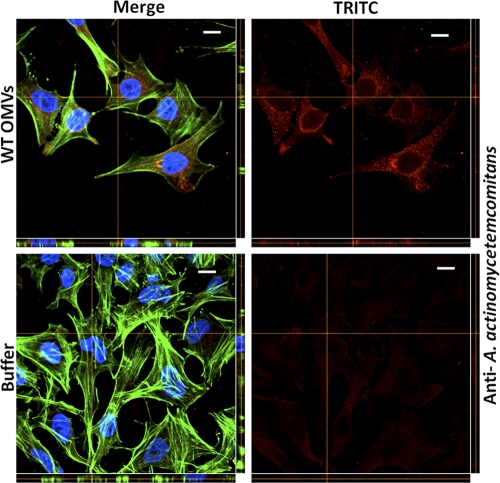
To investigate if A. actinomycetemcomitans OMVs may facilitate the delivery of proteins into cultured human cells, we carried out confocal microscopy analyses aimed at detecting internalized bacterial proteins. For this, we used an OmpA-specific antibody (major A. actinomycetemcomitans OMV protein [32]) and an antiserum specific for whole cells of A. actinomycetemcomitans serotype a, which reacts with OMV-associated proteins such as GroEL and PAL (54). We observed that a large majority of the OMV protein-associated fluorescence in HeLa cells and HGF treated with strain D7SS and D7SS cdtABC OMVs appeared to be distributed in the vicinity of, and surrounding, the nuclei of the intoxicated cells, whereas little or no signal was detected around the cell envelope (Fig. 4A and B). The essentially cytoplasmic distribution of OmpA was also demonstrated using fluorescence quantification software (Fig. 4C). On the other hand, the antibodies did not significantly stain the HeLa cells and HGF subjected to control treatment (buffer) (Fig. 4A and B). Similar observations were made using OMVs purified from strain D7SS by density gradient centrifugation and when we used OMVs isolated from broth cultures (data not shown). These findings are consistent with the suggestion that the OMV cargo (OmpA and additional A. actinomycetemcomitans proteins) was being internalized into the HeLa cells and HGF and that CDT per se was not required for this internalization.
Fig 4.
Perinuclear distribution of internalized A. actinomycetemcomitans OMV proteins in HeLa cells and HGF. A. actinomycetemcomitans strain D7SS (WT) and D7SS cdtABC (cdtABC) OMVs and PBS (buffer) were used to treat HeLa cells (A) and HGF (B) for 72 h. After treatment, cells were fixed and incubated with an FITC-conjugated antibody specific for E. coli OmpA or for whole cells of A. actinomycetemcomitans serotype a, as indicated. Actin filaments and nuclei were then stained with phalloidin (green) and DAPI (blue), respectively. Bound antibodies were detected with TRITC (red). The left panels show merged images from staining with the three dyes. The right panels show images from TRITC staining only. Confocal Z-stack projections are included in all images. The cross hairs indicate the positions of the xz and yz planes. Magnification, ×1,000. Bars = 10 μm. (C) Assessment of the cytoplasmic localization of OmpA. HeLa cells were treated with strain D7SS or D7SS cdtABC OMVs for 72 h. Shown are the means ± standard deviations (SD) for the cytoplasmic/nuclear ratio of red fluorescence from 20 cells from three experiments.
There is earlier reported evidence of internalized OMV proteins being trafficked into the cytosol of host cells through the retrograde pathway, i.e., via the Golgi apparatus and endoplasmic reticulum (ER), and/or accumulating in the ER (6, 35). To investigate this possibility, we used brefeldin A, which causes disassembly of the Golgi complex, inhibiting retrograde transport (20, 45). As evidenced by confocal microscopy, the presence of brefeldin A had no apparent effect on the internalization of A. actinomycetemcomitans OMV proteins. After 6 h of incubation with OMVs from strain D7SS, OMV proteins were clearly detectable in the cytosol of HeLa cells using the antiserum specific for whole cells of A. actinomycetemcomitans serotype a (Fig. 5), indicating that the internalization did not require an intact Golgi apparatus.
Fig 5.
Internalization of A. actinomycetemcomitans OMV proteins is independent of retrograde transport. A. actinomycetemcomitans strain D7SS OMVs (WT) and PBS (buffer) were used to treat HeLa cells for 6 h in the presence of brefeldin A (final concentration, 10 μM). After treatment, cells were fixed and incubated with a polyclonal antibody specific for whole cells of A. actinomycetemcomitans serotype a. Actin filaments and nuclei were then stained with phalloidin (green) and DAPI (blue), respectively. Bound antibodies were detected with TRITC (red). The left panels show merged images from staining with the three dyes. The right panels show images from TRITC staining only. Confocal Z-stack projections are included in all images. The cross hairs indicate the positions of the xz and yz planes. Magnification, ×1,000. Bars = 10 μm.
To assess if the active toxin component, CdtB, had entered into the nuclei of the intoxicated HeLa cells, we conducted confocal microscopy, using a CdtB-specific antiserum and a TRITC-conjugated secondary antibody, and then monitored the red fluorescence with quantification software. This revealed an apparent nuclear distribution of the fluorescence in HeLa cells treated with strain D7SS OMVs (Fig. 6). We also assessed the distribution of the weak background fluorescence observed when HeLa cells were incubated with OMVs from the strain D7SS cdtABC mutant or with PBS (buffer). According to our findings, the background fluorescence was equally distributed in the nucleus and cytoplasm (Fig. 6). Taken together, our observations are consistent with an essentially nuclear localization of CdtB.
Fig 6.
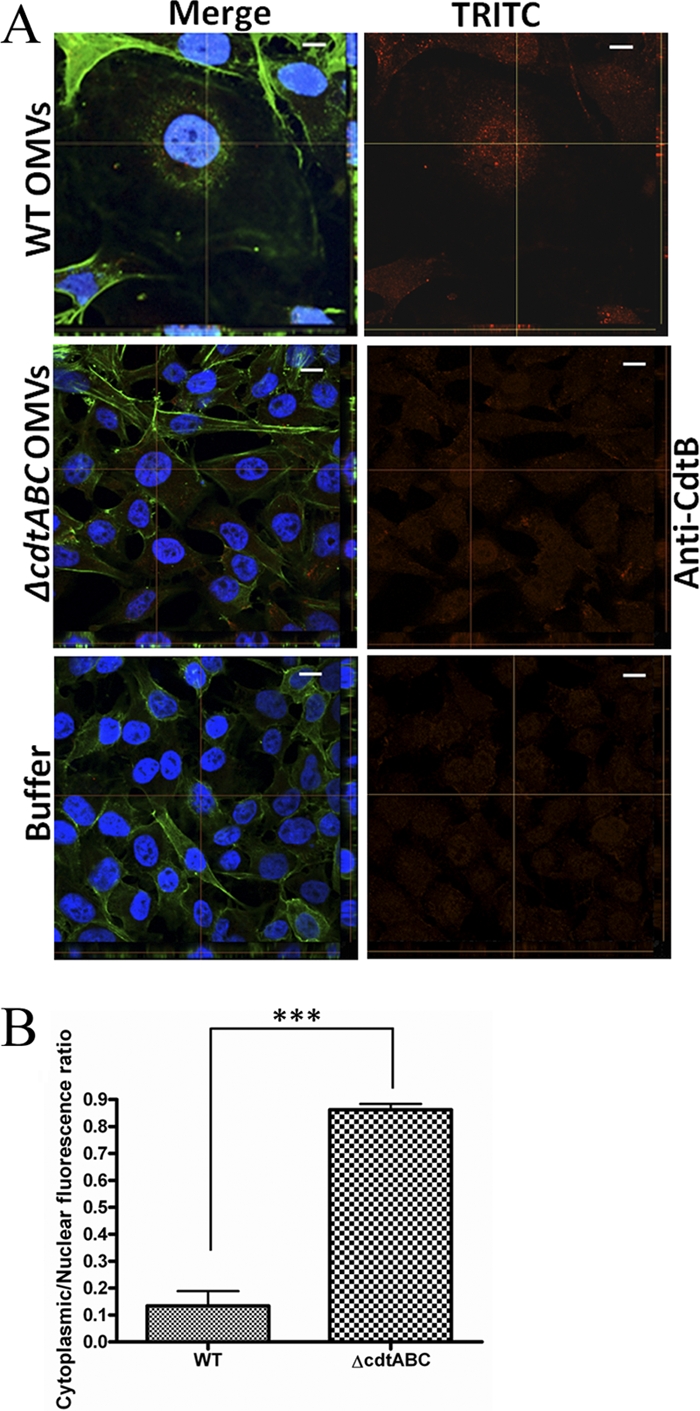
Nuclear distribution of internalized CdtB. (A) OMVs from A. actinomycetemcomitans strain D7SS (WT) or D7SS cdtABC (cdtABC) or PBS (buffer) were used to treat HeLa cells for 72 h. After treatment, cells were fixed and incubated with an antibody specific for H. ducreyi CdtB. Actin filaments and nuclei were then stained with phalloidin (green) and DAPI (blue), respectively. Bound antibodies were detected with TRITC (red). The left panels show merged images from staining with the three dyes. The right panels show images from TRITC staining only. Confocal Z-stack projections are included in all images. The cross hairs indicate the positions of the xz and yz planes. Magnification, ×1,000. Bars = 10 μm. (B) Assessment of the nuclear localization of CdtB. HeLa cells were treated with strain D7SS (WT) or D7SS cdtABC (cdtABC) OMVs for 72 h. Shown are the means ± SD for the cytoplasmic/nuclear ratio of red fluorescence from 20 cells from three experiments (P < 0.0001).
Thus, collectively our results support the idea that the release of A. actinomycetemcomitans OMVs could constitute a mechanism for simultaneous delivery of multiple proteins, including biologically active CDT, into susceptible human cells without requiring passage through the retrograde transport pathway.
Internalization of A. actinomycetemcomitans OMVs via membrane fusion is dependent on lipid raft microdomains in the host cell plasma membrane.
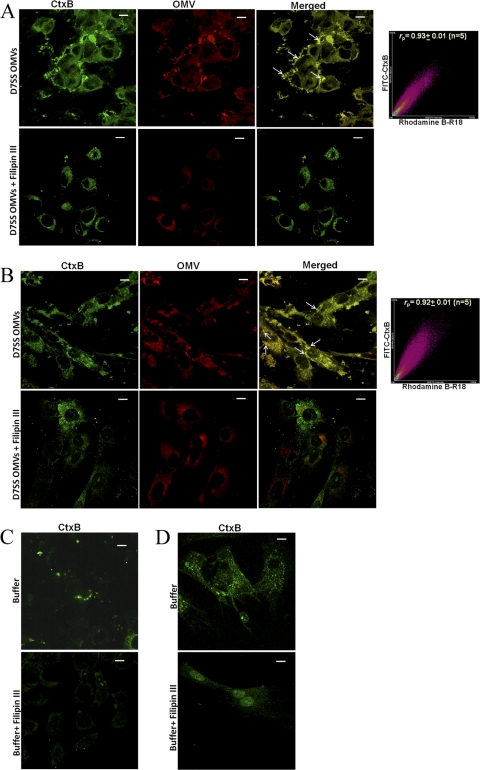
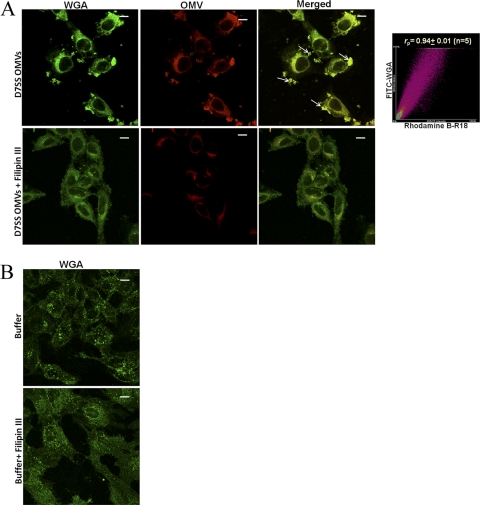
To investigate if A. actinomycetemcomitans OMVs may enter host cells via membrane fusion, we labeled strain D7SS OMVs with rhodamine isothiocyanate B-R18, which is dequenched upon fusion with the host cell plasma membrane, resulting in red fluorescence (13, 28). According to confocal microscopy analysis, FITC-conjugated CtxB subunit (a documented plasma membrane lipid raft marker [26]) colocalized with OMVs in both HeLa cells (Fig. 7A), and HGF (Fig. 7B) within 30 min of incubation. Quantitative analysis (Fig. 7A and B) revealed a high degree of colocalization of OMVs with CtxB. Essentially the same observations were made using FITC-conjugated WGA (a marker of host cell plasma membrane) in membrane fusion assays instead of CtxB (Fig. 8); i.e., confocal microscopy and quantitative analysis revealed a high degree of colocalization of OMVs with WGA in HeLa cells after 30 min of incubation. These findings together are consistent with the hypothesis that there was fusion of A. actinomycetemcomitans OMVs with the host cell plasma membrane. To investigate the possible requirement of lipid rafts for this fusion, we used the cholesterol-sequestering agent Filipin III to disrupt lipid raft domains, analogous to the procedure used in a similar study assessing Pseudomonas aeruginosa OMVs (13). Confocal microscopy revealed that this resulted in both reduced levels of fluorescence and no apparent colocalization of OMVs with either CtxB or WGA in HeLa and HGF cell samples incubated with strain D7SS OMVs for 30 min in the presence of Filipin III (Fig. 7 and 8). Moreover, Filipin III inhibited CtxB binding to lipid rafts as indicated by reduced green fluorescence levels in the treated HeLa and HGF cell samples (Fig. 7). We conclude that the OMV fusion with the plasma membrane was blocked upon disruption of lipid rafts. Hence, taken together our results are consistent with A. actinomycetemcomitans OMV-associated proteins being delivered to the host cell cytoplasm via membrane fusion, dependent on lipid raft microdomains in the plasma membrane.
Fig 7.
Fusion of OMVs from A. actinomycetemcomitans strain D7SS with HeLa cell and HGF lipid raft microdomains. (A and B) Colocalization of rhodamine B-R18-labeled OMVs with FITC-conjugated lipid raft marker CtxB in HeLa cells (A) and HGF (B) after 30 min of incubation in the presence or absence of Filipin III (final concentration, 10 μg/ml). Colocalization of OMVs (red) with CtxB (green) is indicated by small white arrows. Scattergrams of red and green pixels were plotted on graphs to obtain the colocalization coefficient (rp) between OMVs and CtxB. Shown is a representative plot. (C and D) HeLa cells (C) and HGF (D) subjected to control treatment (PBS [buffer] instead of OMVs). The merged images show the labeling with both dyes. Confocal Z-stack projections are included in all images. Magnification, ×1,000. Bars = 10 μm.
Fig 8.
Fusion of OMVs from A. actinomycetemcomitans strain D7SS with HeLa cell lipid raft microdomains. (A) Colocalization of rhodamine B-R18-labeled OMVs with FITC-conjugated plasma membrane marker WGA in HeLa cells after 30 min of incubation in the presence or absence of Filipin III (final concentration, 10 μg/ml). Colocalization of OMVs (red) with WGA (green) is indicated by small white arrows. Scattergrams of red and green pixels in confocal stacks were plotted on graphs to obtain the colocalization coefficient (rp) between OMVs and WGA. Shown is a representative plot. (B) HeLa cells subjected to control treatment (PBS [buffer] instead of OMVs). Confocal Z-stack projections are included in all images. The merged images show the labeling with both dyes. Magnification, ×1,000. Bars = 10 μm.
DISCUSSION
In this study, we have identified A. actinomycetemcomitans OMVs as a vehicle for the simultaneous delivery of multiple proteins, including biologically active CDT, into the cytosol of human cells. Our findings suggest that the different A. actinomycetemcomitans cultivation conditions used (growth on agar or in broth cultures) may affect the overall OMV protein composition, which is in accordance with earlier studies (52). Whether the observed difference in the relative abundance of CdtA and CdtB in OMV preparations from plate-grown bacteria and broth cultures may reflect the amount of CDT holotoxin in the vesicles is not known. Our observation by AFM that preparations from plate-grown bacteria also contained larger vesicles (with diameters of up to 200 nm) is consistent with earlier analyses using electron microscopy (31, 32). It cannot be excluded that the larger OMVs may be the result of vesicle fusion. However, despite these observed differences, essentially the same results were obtained regarding the cytolethal distending activity of OMVs and the perinuclear distribution of OMV proteins in intoxicated HeLa and HGF cells. Hence, for practical reasons we utilized mainly the OMVs isolated from bacteria grown on agar in further experiments.
The association of CdtA and CdtB with OMVs was confirmed in the smooth-colony serotype a strain D7SS and in two additional A. actinomycetemcomitans isolates, belonging to serotypes b and c, excluding that the OMV association was a characteristic of a single strain and/or serotype. Analogous to several previous studies on OMV-associated proteins (4, 29, 32), we used both density gradients and a dissociation assay to separate the OMVs from loosely associated proteins and to demonstrate the intimate association of the active toxin unit CdtB with A. actinomycetemcomitans OMVs. The lack of detection of CdtC in OMVs by immunoblotting appeared to be due to absence of cross-reactivity to the CdtC subunit by the antibodies used. Our preliminary liquid chromatography-tandem mass spectrometry (LC-MS/MS) data, indicating the presence of all three CDT subunits (CdtA, CdtB, and CdtC) in the A. actinomycetemcomitans secretome, which also contains OMVs (V. Zijnge, T. Kieselbach, and J. Oscarsson, unpublished data), suggest that CdtC, analogously to CdtA and CdtB, is also OMV associated. This is currently being further assessed by LC-MS/MS. OMV-associated secretion of CDT in A. actinomycetemcomitans is consistent with reports indicating the presence of CdtA, CdtB, and CdtC in the periplasmic compartment of A. actinomycetemcomitans cells and in both outer membrane and periplasmic fractions of E. coli used as a model organism (63, 65). We used both HGF and HeLa cells in our experiments demonstrating that OMV-associated CDT was biologically active. These cell models, which are used frequently to assess the cytotoxicities of CDTs from various species, including A. actinomycetemcomitans (7, 17, 43, 58, 63), allowed a straightforward comparison of our findings with previous studies. Most of the present experiments utilized A. actinomycetemcomitans strain D7SS, which was empirically selected as it releases significant amounts of OMVs (31) and CDT (10) and also is considered a minimally leukotoxic strain (34).
By confocal microscopy using two different polyclonal antibodies, specific for whole A. actinomycetemcomitans serotype a cells and OmpA, we obtained novel evidence that A. actinomycetemcomitans OMVs can deliver multiple proteins simultaneously into human cells. The OMV proteins were internalized into the cytosol, resulting in a preferentially perinuclear distribution in intoxicated HeLa cells and HGF. Notably, we obtained evidence that the OMV proteins were internalized in both cell types via a mechanism of OMV fusion with lipid rafts in the plasma membrane, which was detected after 30 min of incubation. Internalization of A. actinomycetemcomitans OMV proteins by membrane fusion was suggested in an earlier study, as the plasma membrane of HL60 cells was uniformly labeled with OMV-associated fluorescence within 2 min of treatment with OMVs (15). As very little OMV-associated fluorescence was detected within HL60 cells incubated with OMVs for up to 25 min, the authors suggested a model in which the OMVs were specifically localized to the cytoplasmic membrane and had possibly been incorporated into it by membrane fusion (15). Presumably, our present use of HeLa cells and HGF, which, in contrast to HL60 cells, lack the leukotoxin-receptor lymphocyte function-associated antigen 1 (LFA-1) (39), minimized cell lysis due to leukotoxin after intoxication with OMVs. The presence of leukotoxin would therefore not affect the assessment of internalized vesicle components inside the target cells after the initial association of OMVs with the plasma membrane. The results of this study are consistent with observations with Pseudomonas aeruginosa OMVs, which were demonstrated to fuse with lipid rafts in the plasma membrane to deliver multiple virulence factors directly to the cytosol of human airway epithelial cells (13). Moreover, analogous to the P. aeruginosa OMVs (13), internalization of A. actinomycetemcomitans OMV proteins was unaffected by inhibition of retrograde transport, suggesting that OMV proteins may be delivered directly to the host cell cytosol. This remains to be experimentally confirmed.
Dependence of plasma membrane lipid rafts for internalization of OMV proteins is also consistent with endocytic uptake of OMVs (21, 35). P. aeruginosa OMVs are so far the only OMVs demonstrated both to be internalized and to deliver cargo proteins via membrane fusion into different human airway epithelial cells (6, 13). Whether internalization via multiple pathways may be a characteristic of OMVs of additional organisms, including A. actinomycetemcomitans, is not known. OMV-associated toxins can act as adhesins in receptor-mediated endocytosis of OMVs (35). It was earlier excluded that OMV-associated leukotoxin was required for the interaction of A. actinomycetemcomitans OMVs with HL60 cells (15). Our present findings that proteins from the strain D7SS cdtABC mutant OMVs were also internalized indicate that CDT per se was neither required for the interaction of A. actinomycetemcomitans OMVs with the HeLa cells and HGF nor affected the relative distribution of OMV proteins within the intoxicated cells.
Our data support the notion that A. actinomycetemcomitans OMVs could constitute a mechanism for delivery of biologically active CDT into susceptible cells. According to our confocal microscopy analyses using a CdtB-specific polyclonal antibody, the active toxin unit had an essentially nuclear localization in HeLa cells treated with OMVs from A. actinomycetemcomitans strain D7SS. These observations are in accordance with previous findings using recombinant CdtB (24), suggesting that CdtB was directly translocated to the nucleus also under our experimental conditions. How this was achieved is not known, but it appeared not to involve OMVs per se entering into the nucleus, as judged by our confocal microscopy data indicating the absence of OMV-associated fluorescence other than CdtB from the nuclei of intoxicated HeLa cells. As OMV-mediated release of CDT has now been demonstrated in at least three bacterial species, A. actinomycetemcomitans, C. jejuni, and E. coli (references 11 and 44 and this work), this feature may be widespread among the organisms producing this toxin and could be advantageous for the bacteria during several different types of infections. Although the role of A. actinomycetemcomitans OMVs in periodontal disease has not yet been elucidated, our present data support that OMVs could deliver biologically active CDT and additional virulence factors into susceptible cells of the periodontium. Two recent studies have suggested that in the presence of A. actinomycetemcomitans, CDT toxicity may play a part in the early pathogenesis of periodontitis (14, 50). This would be consistent with OMVs promoting damage in the sulcular/junctional epithelium, which remains to be experimentally assessed.
Taken together, our results provide a molecular basis for the OMV-mediated delivery of multiple A. actinomycetemcomitans proteins, including biologically active CDT, into host cells. Further studies are needed to fully comprehend how this tentative virulence mechanism may contribute to human periodontal disease.
ACKNOWLEDGMENTS
We thank Elisabeth Granström for excellent technical assistance. A. actinomycetemcomitans CDT holotoxin and polyclonal antibodies raised against H. ducreyi CDT holotoxin, CdtA, CdtB, and CdtC were generously contributed by Teresa Lagergård. Gingival fibroblasts and the polyclonal anti-A. actinomycetemcomitans LtxA antibody were kindly provided by Anders Johansson.
This work was supported by grants from the Swedish Research Council (S.A. and S.N.W.) and by TUA grants from the County Council of Västerbotten, Sweden (J.O. and S.A.).
Footnotes
Published ahead of print 24 October 2011
REFERENCES
- 1. Ahmed HJ, et al. 2001. Prevalence of cdtABC genes encoding cytolethal distending toxin among Haemophilus ducreyi and Actinobacillus actinomycetemcomitans strains. J. Med. Microbiol. 50: 860–864 [DOI] [PubMed] [Google Scholar]
- 2. Akifusa S, Heywood W, Nair SP, Stenbeck G, Henderson B. 2005. Mechanism of internalization of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiology 151: 1395–1402 [DOI] [PubMed] [Google Scholar]
- 3. Amano A, Takeuchi H, Furuta N. 2010. Outer membrane vesicles function as offensive weapons in host-parasite interactions. Microbes Infect. 12: 791–798 [DOI] [PubMed] [Google Scholar]
- 4. Balsalobre C, et al. 2006. Release of the type I secreted α-haemolysin via outer membrane vesicles from Escherichia coli. Mol. Microbiol. 59: 99–112 [DOI] [PubMed] [Google Scholar]
- 5. Bartold PM, Walsh LJ, Narayanan AS. 2000. Molecular and cell biology of the gingiva. Periodontol. 2000 24: 28–55 [DOI] [PubMed] [Google Scholar]
- 6. Bauman SJ, Kuehn MJ. 2009. Pseudomonas aeruginosa vesicles associate with and are internalized by human lung epithelial cells. BMC Microbiol. 9: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belibasakis G, et al. 2002. Inhibited proliferation of human periodontal ligament cells and gingival fibroblasts by Actinobacillus actinomycetemcomitans: involvement of the cytolethal distending toxin. Eur. J. Oral Sci. 110: 366–373 [DOI] [PubMed] [Google Scholar]
- 8. Belibasakis GN, et al. 2005. The cytolethal distending toxin induces receptor activator of NF-κB ligand expression in human gingival fibroblasts and periodontal ligament cells. Infect. Immun. 73: 342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belibasakis GN, et al. 2005. Cytokine responses of human gingival fibroblasts to Actinobacillus actinomycetemcomitans cytolethal distending toxin. Cytokine 30: 56–63 [DOI] [PubMed] [Google Scholar]
- 10. Belibasakis GN, Mattsson A, Wang Y, Chen C, Johansson A. 2004. Cell cycle arrest of human gingival fibroblasts and periodontal ligament cells by Actinobacillus actinomycetemcomitans: involvement of the cytolethal distending toxin. APMIS 112: 674–685 [DOI] [PubMed] [Google Scholar]
- 11. Berlanda Scorza F, et al. 2008. Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli ΔtolR IHE3034 mutant. Mol. Cell Proteomics 7: 473–485 [DOI] [PubMed] [Google Scholar]
- 12. Boesze-Battaglia K, et al. 2009. Cytolethal distending toxin-induced cell cycle arrest of lymphocytes is dependent upon recognition and binding to cholesterol. J. Biol. Chem. 284: 10650–10658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bomberger JM, et al. 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 5: e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Damek-Poprawa M, Haris M, Volgina A, Korostoff J, Dirienzo JM. 2011. Cytolethal distending toxin damages the oral epithelium of gingival explants. J. Dent. Res. 90: 874–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Demuth DR, James D, Kowashi Y, Kato S. 2003. Interaction of Actinobacillus actinomycetemcomitans outer membrane vesicles with HL60 cells does not require leukotoxin. Cell. Microbiol. 5: 111–121 [DOI] [PubMed] [Google Scholar]
- 16. Ellis TN, Kuehn MJ. 2010. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 74: 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elwell C, Chao K, Patel K, Dreyfus L. 2001. Escherichia coli CdtB mediates cytolethal distending toxin cell cycle arrest. Infect. Immun. 69: 3418–3422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elwell CA, Dreyfus LA. 2000. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 37: 952–963 [DOI] [PubMed] [Google Scholar]
- 19. Fabris AS, DiRienzo JM, Wikstrom M, Mayer MP. 2002. Detection of cytolethal distending toxin activity and cdt genes in Actinobacillus actinomycetemcomitans isolates from geographically diverse populations. Oral Microbiol. Immunol. 17: 231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. 1988. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 263: 18545–18552 [PubMed] [Google Scholar]
- 21. Furuta N, et al. 2009. Porphyromonas gingivalis outer membrane vesicles enter human epithelial cells via an endocytic pathway and are sorted to lysosomal compartments. Infect. Immun. 77: 4187–4196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goulhen F, et al. 1998. Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect. Immun. 66: 5307–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guerra L, Cortes-Bratti X, Guidi R, Frisan T. 2011. The biology of the cytolethal distending toxins. Toxins 3: 172–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guerra L, et al. 2009. A novel mode of translocation for cytolethal distending toxin. Biochim. Biophys. Acta 1793: 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haghjoo E, Galan JE. 2004. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. U. S. A. 101: 4614–4619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harder T, Scheiffele P, Verkade P, Simons K. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141: 929–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Henning U, Schwarz H, Chen R. 1979. Radioimmunological screening method for specific membrane proteins. Anal. Biochem. 97: 153–157 [DOI] [PubMed] [Google Scholar]
- 28. Hoekstra D, de Boer T, Klappe K, Wilschut J. 1984. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry 23: 5675–5681 [DOI] [PubMed] [Google Scholar]
- 29. Horstman AL, Kuehn MJ. 2000. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem. 275: 12489–12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Johansson A, Sandström G, Claesson R, Hänström L, Kalfas S. 2000. Anaerobic neutrophil-dependent killing of Actinobacillus actinomycetemcomitans in relation to the bacterial leukotoxicity. Eur. J. Oral Sci. 108: 136–146 [DOI] [PubMed] [Google Scholar]
- 31. Karched M, et al. 2008. Vesicle-independent extracellular release of a proinflammatory outer membrane lipoprotein in free-soluble form. BMC Microbiol. 8: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kato S, Kowashi Y, Demuth DR. 2002. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 32: 1–13 [DOI] [PubMed] [Google Scholar]
- 33. Kebschull M, Demmer RT, Papapanou PN. 2010. “Gum bug, leave my heart alone!”—epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J. Dent. Res. 89: 879–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kelk P, Claesson R, Chen C, Sjöstedt A, Johansson A. 2008. IL-1β secretion induced by Aggregatibacter (Actinobacillus) actinomycetemcomitans is mainly caused by the leukotoxin. Int. J. Med. Microbiol. 298: 529–541 [DOI] [PubMed] [Google Scholar]
- 35. Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 23: 4538–4549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kouokam JC, et al. 2006. Active cytotoxic necrotizing factor 1 associated with outer membrane vesicles from uropathogenic Escherichia coli. Infect. Immun. 74: 2022–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64: 163–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lally ET, et al. 1989. Analysis of the Actinobacillus actinomycetemcomitans leukotoxin gene. Delineation of unique features and comparison to homologous toxins. J. Biol. Chem. 264: 15451–15456 [PubMed] [Google Scholar]
- 39. Lally ET, et al. 1997. RTX toxins recognize a β2 integrin on the surface of human target cells. J. Biol. Chem. 272: 30463–30469 [DOI] [PubMed] [Google Scholar]
- 40. Lara-Tejero M, Galan JE. 2000. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 290: 354–357 [DOI] [PubMed] [Google Scholar]
- 41. Lara-Tejero M, Galan JE. 2001. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 69: 4358–4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lara-Tejero M, Galan JE. 2002. Cytolethal distending toxin: limited damage as a strategy to modulate cellular functions. Trends Microbiol. 10: 147–152 [DOI] [PubMed] [Google Scholar]
- 43. Lee RB, Hassane DC, Cottle DL, Pickett CL. 2003. Interactions of Campylobacter jejuni cytolethal distending toxin subunits CdtA and CdtC with HeLa cells. Infect. Immun. 71: 4883–4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lindmark B, et al. 2009. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiol. 9: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lippincott-Schwartz J, et al. 1990. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell 60: 821–836 [DOI] [PubMed] [Google Scholar]
- 46. McSweeney LA, Dreyfus LA. 2004. Nuclear localization of the Escherichia coli cytolethal distending toxin CdtB subunit. Cell. Microbiol. 6: 447–458 [DOI] [PubMed] [Google Scholar]
- 47. Nalbant A, Chen C, Wang Y, Zadeh HH. 2003. Induction of T-cell apoptosis by Actinobacillus actinomycetemcomitans mutants with deletion of ltxA and cdtABC genes: possible activity of GroEL-like molecule. Oral Microbiol. Immunol. 18: 339–349 [DOI] [PubMed] [Google Scholar]
- 48. Nesic D, Hsu Y, Stebbins CE. 2004. Assembly and function of a bacterial genotoxin. Nature 429: 429–433 [DOI] [PubMed] [Google Scholar]
- 49. Nishikubo S, et al. 2003. An N-terminal segment of the active component of the bacterial genotoxin cytolethal distending toxin B (CDTB) directs CDTB into the nucleus. J. Biol. Chem. 278: 50671–50681 [DOI] [PubMed] [Google Scholar]
- 50. Ohara M, Miyauchi M, Tsuruda K, Takata T, Sugai M. 2011. Topical application of Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces cell cycle arrest in the rat gingival epithelium in vivo. J. Periodontal Res. 46: 389–395 [DOI] [PubMed] [Google Scholar]
- 51. Ohara M, Oswald E, Sugai M. 2004. Cytolethal distending toxin: a bacterial bullet targeted to nucleus. J. Biochem. 136: 409–413 [DOI] [PubMed] [Google Scholar]
- 52. Olofsson A, et al. 2010. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol. Microbiol. 77: 1539–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Orlandi PA, Fishman PH. 1998. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141: 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oscarsson J, Karched M, Thay B, Chen C, Asikainen S. 2008. Proinflammatory effect in whole blood by free-soluble bacterial components released from planktonic and biofilm cells. BMC Microbiol. 8: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paju S, et al. 2000. Localization of heat shock proteins in clinical Actinobacillus actinomycetemcomitans strains and their effects on epithelial cell proliferation. FEMS Microbiol. Lett. 182: 231–235 [DOI] [PubMed] [Google Scholar]
- 56. Paul-Satyaseela M, et al. 2006. Immunoproteomics of Actinobacillus actinomycetemcomitans outer-membrane proteins reveal a highly immunoreactive peptidoglycan-associated lipoprotein. J. Med. Microbiol. 55: 931–942 [DOI] [PubMed] [Google Scholar]
- 57. Saarela M, et al. 1992. Frequency and stability of mono- or poly-infection by Actinobacillus actinomycetemcomitans serotypes a, b, c, d or e. Oral Microbiol. Immunol. 7: 277–279 [DOI] [PubMed] [Google Scholar]
- 58. Shenker BJ, et al. 1999. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. 162: 4773–4780 [PubMed] [Google Scholar]
- 59. Simpson DL, Berthold P, Taichman NS. 1988. Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitans leukotoxin. Infect. Immun. 56: 1162–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Slots J, Genco RJ. 1984. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J. Dent. Res. 63: 412–421 [DOI] [PubMed] [Google Scholar]
- 61. Smith JL, Bayles DO. 2006. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit. Rev. Microbiol. 32: 227–248 [DOI] [PubMed] [Google Scholar]
- 62. Socransky SS, Haffajee AD. 1992. The bacterial etiology of destructive periodontal disease: current concepts. J. Periodontol. 63: 322–331 [DOI] [PubMed] [Google Scholar]
- 63. Sugai M, et al. 1998. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 66: 5008–5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tan KS, Song KP, Ong G. 2002. Cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Occurrence and association with periodontal disease. J. Periodontal Res. 37: 268–272 [DOI] [PubMed] [Google Scholar]
- 65. Ueno Y, et al. 2006. Biogenesis of the Actinobacillus actinomycetemcomitans cytolethal distending toxin holotoxin. Infect. Immun. 74: 3480–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van Winkelhoff AJ, Slots J. 1999. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontol 2000 20: 122–135 [DOI] [PubMed] [Google Scholar]
- 67. Wai SN, et al. 2003. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 115: 25–35 [DOI] [PubMed] [Google Scholar]
- 68. Wang Y, Goodman SD, Redfield RJ, Chen C. 2002. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J. Bacteriol. 184: 3442–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wising C, et al. 2002. Toxicity and immunogenicity of purified Haemophilus ducreyi cytolethal distending toxin in a rabbit model. Microb. Pathog. 33: 49–62 [DOI] [PubMed] [Google Scholar]
- 70. Yamano R, et al. 2003. Prevalence of cytolethal distending toxin production in periodontopathogenic bacteria. J. Clin. Microbiol. 41: 1391–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]