SUMMARY
G protein-coupled receptors form hetero-dimers and higher order hetero-oligomers, yet the significance of receptor heteromerization in cellular and behavioral responses is poorly understood. Atypical antipsychotic drugs, such as clozapine and risperidone all have in common a high affinity for the serotonin 5-HT2A receptor (2AR). However, closely related nonantipsychotic drugs, such as ritanserin and methysergide, while blocking 2AR function, lack comparable neuropsychological effects. Why some but not all drugs that inhibit 2AR-dependent signaling exhibit antipsychotic properties remains unresolved. We found that a heteromeric complex formed between the metabotropic glutamate 2 receptor (mGluR2) and the 2AR critically integrates the action of drugs affecting signaling and behavioral outcomes. Acting through the mGluR2/2AR heterocomplex, both glutamatergic and serotonergic drugs achieve a balance between Gi- and Gq-dependent signaling that predicts their psychoactive behavioral effects. These observations provide a novel mechanistic insight into antipsychotic action that may advance therapeutic strategies for schizophrenia.
INTRODUCTION
G protein-coupled receptors (GPCRs) are the most common cellular targets for drugs used in the clinic (Rosenbaum et al., 2009). The classical mechanism of receptor action states that binding of an agonist induces distinct conformational changes that enable GPCRs to couple to and activate heterotrimeric G proteins (e.g., G protein subtypes Gi/o, Gq/11, Gs or G12) (Oldham and Hamm, 2008). Although considerable biochemical and biophysical data are consistent with the ability of GPCRs to bind and activate G proteins in a monomeric form (Ernst et al., 2007; Whorton et al., 2007), several recent studies support the hypothesis that G protein coupling in cell membranes involves the formation of GPCR homomers (complexes formed by association of two or more components that belong to the same GPCR subtype) (Han et al., 2009; Lopez-Gimenez et al., 2007) and heteromers (in which two or more non-identical and independently functional GPCRs exist as a protein complex) (Carriba et al., 2008; Vilardaga et al., 2008). Oligomeric receptor complexes appear to exhibit distinct signaling properties when compared to monomeric receptors (Urizar et al., 2011; Milligan, 2009). The molecular mechanism(s) responsible for such changes in pharmacology are poorly understood, as is the physiological function of GPCR heteromeric complexes.
Atypical antipsychotics are drugs that have in common a high affinity for 2AR (Meltzer et al., 1989; Meltzer and Huang, 2008), and are widely used in the treatment of schizophrenia and other disorders involving psychosis (Ross et al., 2006). Interestingly, it has been recently recognized that most clinically effective antipsychotic drugs are, in fact, 2AR inverse agonists – ligands that preferentially bind and stabilize a GPCR in an inactive conformational state (Kenakin, 2002) – rather than simply neutral antagonists (Aloyo et al., 2009; Egan et al., 1998; Weiner et al., 2001) – ligands that compete for the same orthosteric binding site and prevent the cellular responses induced by agonists and inverse agonists. Yet, the mechanism connecting 2AR inverse agonism with antipsychotic effects has not been elucidated. Furthermore, a new class of potential antipsychotic drugs acting as agonists of mGluR2 recently received attention in preclinical (Woolley et al., 2008) and clinical studies (Patil et al., 2007, Kinon et al., 2011).
Previous work convincingly demonstrated that mGluR2 (a type C GPCR) and 2AR (a type A GPCR) form a specific heterocomplex in mammalian brain (González-Maeso et al., 2008) and in tissue culture preparations (González-Maeso et al., 2008; Rives et al., 2009). However, the signaling properties and the role of this receptor heterocomplex in the molecular mechanism of action of antipsychotic drugs remains unclear. Here, we proceeded to investigate how G protein signaling through the heteromeric mGluR2/2AR complex compares to homomeric signaling through either mGluR2 or 2AR expressed alone. Our results provide novel insights of integrated signaling through GPCR heteromers, and uncover a unifying mechanism of action of two families of antipsychotic drugs that target the mGluR2/2AR heteromeric complex. We developed a metric that predicts both the anti- or pro-psychotic effects of a wide range of serotonergic and glutamatergic ligands.
RESULTS
Heteromeric Assembly of mGluR2 and 2AR Enhances Glutamate-Elicited Gi Signaling and Reduces Gq Signaling
2AR is a Gq/11- (or simply Gq-) coupled GPCR that responds to the neurotransmitter serotonin (5-HT) (González-Maeso and Sealfon, 2009), whereas mGluR2 is a Gi/o- (or simply Gi-) coupled, pertussis toxin-sensitive GPCR that responds to the neurotransmitter glutamate (Glu) (Moreno et al., 2009).
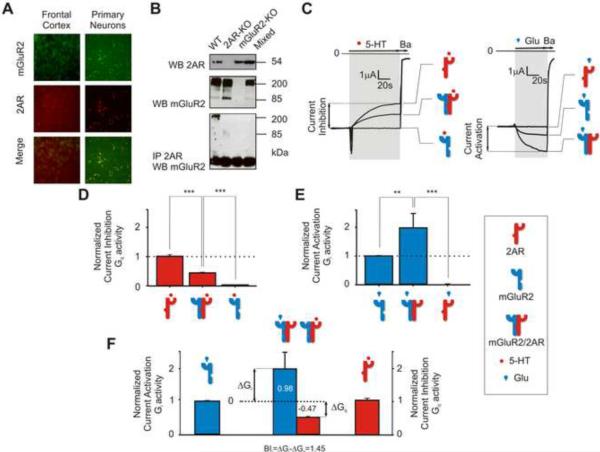
Similarly to what we showed previously by mRNA in situ hybridization (González-Maeso et al., 2008), 2AR and mGluR2 immunoreactivity co-localized in mouse cortical slices and neuronal primary cultures (Figure 1 A and Figure S1 F). In addition, the two receptors were co-immunoprecipitated in mouse frontal cortex membrane preparations (Figure 1 B). In order to investigate the signaling properties of the mGluR2/2AR heterocomplex, we utilized Xenopus oocytes, a heterologous system widely used for functional expression of recombinant ion channels (Barela et al., 2006). We expressed each of these GPCRs alone or together, but always with ion channels that served as reporters of GPCR-mediated signaling. We used inhibition of the IRK3 (Kir2.3) current to monitor Gq activity (Figure 1 C - left) (Du et al., 2004), and activation of the GIRK4* (or Kir3.4*) current to monitor Gi activity (Figure 1 C - right) (He et al., 1999; He et al., 2002) (see Experimental Procedures section).
Figure 1. Heteromeric Assembly of 2AR and mGluR2 enhances Glu-induced Gi Signaling and Reduces 5-HT-induced Gq Signaling.
2AR and mGlu2 co-localize and form a receptor complex in mouse frontal cortex. (A) Representative micrographs showing co-expression of endogenous 2AR (red) and mGluR2 (green) in mouse frontal cortex (left panels) and mouse cortical primary neurons (right panels). (Scale bar 25 μm). (See also Figure S1 E)
(B) Mouse frontal cortex membrane preparations were immunoprecipitated (IP) with anti-2AR antibody. Immunoprecipitates were analyzed by western blot (WB) with anti-mGluR2 antibody (lower blot). Mouse frontal cortex membrane preparations were also directly analyzed by WB with anti- 2AR antibody (upper blot) or anti-mGluR2 antibody (middle blot). 2AR-KO and mGluR2-KO mouse frontal cortex tissue samples were processed identically and used as negative controls. Frontal cortex tissue samples from 2AR-KO and mGluR2-KO mice were also homogenized together (mixed) and processed identically for immunoprecipitation and WB.
(C) (Left) Representative barium-sensitive traces of IRK3 currents obtained in response to 1 μM 5-HT in oocytes expressing 2AR alone, mGluR2 and 2AR together, or mGluR2 alone. (Right) Representative barium-sensitive traces of GIRK4* currents obtained in response to 1 μM Glu in oocytes expressing mGluR2 alone, mGluR2 and 2AR together, or 2AR alone. Barium (Ba) inhibited IRK3 and GIRK4* currents and allowed for subtraction of IRK3 and GIRK4*-independent currents. For illustrative purposes, traces with similar basal currents were chosen.
(D) Summary bar graphs of Gq activity measured as IRK3 current inhibition (mean ± SEM) following stimulation with 5-HT and (E), of Gi activity measured as GIRK4* current activation (mean ± SEM) following stimulation with Glu. IRK3 current inhibition was measured relative to basal currents and was normalized relative to that obtained by stimulating 2AR alone with 5-HT (100% or 1). GIRK4* current activation was measured relative to the basal currents and was normalized relative to that obtained by stimulating mGluR2 alone with Glu (100% or 1) (F) Calculation of the balance index (BI) as the difference of the increase in Gi-signaling in response to Glu from the mGluR2 homomeric level (ΔGi) and the decrease of Gq-signaling in response to 5-HT from the 2AR homomeric level (ΔGq). A reference BI (Bir=1.45) was calculated for the mGluR2/2AR complex in response to 1 μM Glu and 1 μM 5-HT using mean values (** p<0.01, *** p < 0.001). See also Figures S1, S2, and S3.
First, we pursued the question of how the signaling properties of the mGluR2/2AR heteromeric complex differ from the signaling properties of the homomeric receptors. We quantified the signaling elicited by Glu and 5-HT, the endogenous ligands of the mGluR2 and 2AR receptors, respectively, and compared the Gi and Gq activities in the absence or presence of the heteromeric receptor partner (Figure 1, C, D, and E). We found that co-expression of mGluR2 with 2AR reduced the Gq activity elicited by 5-HT to approximately 50% of the activity of the 2AR expressed alone (Figure 1, C-left panel and D). In contrast, co-expression of mGluR2 with 2AR increased the Gi activity elicited by Glu to approximately twice of that with the mGluR2 expressed alone (Figure 1, C-right panel and E). As anticipated, 5-HT and Glu did not affect mGluR2 or 2AR signaling, respectively, when the two receptors were expressed individually (Figure 1, C, D, and E).
The metabotropic glutamate receptor 3 (mGluR3), which shares a high degree of homology with mGluR2, does not form a receptor heterocomplex with the 2AR (González-Maeso et al., 2008). Exchanging the transmembrane domains required for mGluR2 heteromerization with 2AR either disrupts (mGluR2Δ) or rescues (mGluR3Δ) receptor complex formation and Gi cross-signaling upon 2AR receptor activation (González-Maeso et al., 2008). Control experiments showed that mGluR2Δ did not exhibit the effects of heteromerization with 2AR on Gi or Gq signaling, whereas activation of mGluR3Δ, which forms a receptor complex with the 2AR, induced an increase in Gi- and decrease in Gq- dependent signaling (Figure S1 A, B, C and D). Each of the mGluR chimeras, when expressed as homomers, showed intact Gi signaling (Figure S2 A, B and C) and cell surface localization (Figure S2 D).
We summarized the difference between Gi signaling and Gq signaling achieved by the formation of the complex using a new parameter: the balance index (BI). The BI combines the change in Gi activity (ΔGi) and the change in Gq activity (ΔGq) according to the formula (BI= ΔGi - ΔGq) (Figure 1F). Normalizing all the values to the homomeric Gi and Gq signaling, the formation of the complex alone yielded a BI of 1.45, which we shall refer to as the reference BI level (BIr).
We obtained the largest BIr when expressing mGluR2/2AR mRNAs in a 1:2 ratio (Figure S3 D). As shown in Figure S3 C, this ratio of mRNAs yielded a cell surface localization of receptor protein levels (compared to the total protein in the cell – see Figure S3 A, B) that suggested a higher order oligomeric complex between mGluR2 and 2AR.
Drugs that Bind 2AR Alter the Balance Between Gi and Gq Signaling
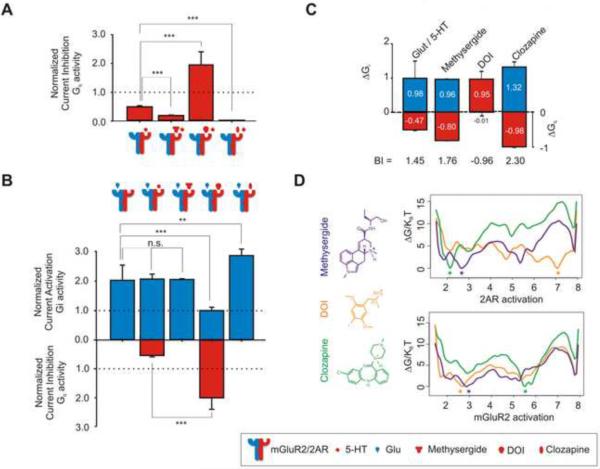
We next asked whether drugs bound to one receptor of the heteromer could affect the other receptor's signaling response to its endogenous neurotransmitter. We first investigated the effects of 2AR ligands (a neutral antagonist, a strong agonist, and an inverse agonist) on Glu-elicited Gi signaling by mGluR2. We define a “strong” agonist to be one that results in greater signaling than the endogenous agonist (e.g., DOI causing greater Gq signaling through 2AR than 5-HT – see Figure 2 B, red bars and Figure S4 A). In control experiments, the neutral antagonist (methysergide), the strong agonist (DOI) and the inverse agonist (clozapine) (Weiner et al., 2001) worked as expected to stimulate, or reduce 5-HT-induced Gq signaling, respectively (Figure 2 A).
Figure 2. Drugs that Target 2AR: Integrative Effects on Gi and Gq Signaling.
(A) Summary bar graphs of Gi activity (mean ± SEM) measured in oocytes expressing mGluR2/2AR following stimulation with 1μM 5-HT alone, or together with 10μM methysergide, 10μM DOI, or 10μM clozapine. Gq activity was normalized relative to that obtained by stimulation of 2AR alone with 5-HT (100% or 1, dotted line).
(B) Summary bar graphs of Gi activity (top) and Gq activity (bottom) (mean±SEM) measured in oocytes expressing mGluR2/2AR following stimulation with 1 μM Glu alone, or together with 10μM methysergide, 10μM DOI, or 10μM clozapine. Gi and Gq activity were normalized relative to the response to Glu and 5-HT respectively (100% or 1, dotted line).
(C) ΔGi referenced to the homomeric mGluR2 response to 1 μM Glu and ΔGq referenced to the homomeric 2AR response to 1 μM 5-HT together with 10 μM methysergide, 10 μM DOI, or 10 μM clozapine (** p<0.01, *** p<0.01, n.s. not significant)
(D) Metadynamics-based mechanistic interpretation of functional crosstalk between 2AR and mGluR2. (Top) Activation profile of 2AR in the presence of different ligands. Free-energy of the 2AR bound to the inverse agonist clozapine (green), the neutral antagonist methysergide (purple), and the dominant agonist DOI (orange), as a function of the position along the path connecting the inactive (s=1) to the active (s=8) states. (Bottom) Activation profile of mGluR2 in the presence of the different ligand-specific 2AR conformations. The three lines correspond to the activation free-energy profile of mGluR2 in dimeric complex through a TM4-TM4 interface with 2AR bound to the inverse agonist clozapine (green line), a TM4,5-TM4,5 interface with 2AR bound to the neutral antagonist methysergide (purple), and a TM4,5-TM4,5 interface with 2AR bound to the dominant agonist DOI (orange line). The most energetically stable states are indicated by a star, and the chemical structures of the three drugs are also shown.
Occupancy of the 2AR receptor by each of the three ligands influenced uniquely Glu-elicited signaling through mGluR2. Figure 2 B (blue bars) depicts the effects of methysergide, DOI, and clozapine on Glu-elicited Gi signaling through the complex. All values were normalized to the homomeric response of mGluR2 to Glu. As was shown in Figure 1, the formation of the complex doubled the extent of Gi signaling (200%). Although neither 5-HT nor methysergide affected Glu-elicited Gi signaling, DOI bound to 2AR decreased Gi signaling back to homomeric levels, and clozapine increased it by approximately 40% (240% greater than homomeric levels). In summary, while the neutral antagonist (methysergide) had no effect on Gi signaling, the strong agonist (DOI) decreased Gi levels back to homomeric levels, while the inverse agonist (clozapine) significantly increased Gi signaling.
Figure 2 B (red bars) summarizes the Gq activity associated with methysergide, DOI, and clozapine together with Glu in oocytes co-expressing mGluR2 and 2AR (note the difference with Figure 2 A where 5-HT is also present). Since these three drugs target 2AR, Gq signaling was only detected when the agonists 5-HT and DOI were applied.
Using the results obtained for both the Gi (Figure 2 B - blue bars) and Gq signaling (Figure 2 A and Figure 2 B - red bars), we calculated BI values for the three ligands in the co-presence of the endogenous ligands (summarized in Figure 2 C). All effects were disrupted when replacing mGluR2 by mGluR2Δ (see Supplemental Table S1), rescued by mGluR3Δ, and present when the Gq pathway was blocked by the regulator of G protein signaling subunit 2 (RGS2) (Figure S5 D and E). The 2AR ligand with the largest overall BI was the inverse agonist clozapine (BI=2.30; 140% increase in Gi and 100% decrease in Gq).
Could these ligands exert their effects by stabilizing different conformations of the receptor complex? We next investigated at the molecular level the conformational changes induced by the three 2AR ligands (i.e. the neutral agonist methysergide, the strong agonist DOI, and the inverse agonist clozapine) in atomistic representations of 2AR either alone or interacting with mGluR2 within an explicit lipid-water environment. To be able to observe large conformational changes in relatively short timescales, we used a combination of adiabatic biased molecular dynamics (ABMD) and metadynamics simulations (see “Experimental Procedures”, under “Computational Methods”). This approach has been recently validated on a prototypic GPCR (Provasi et al., 2011). First, we studied the effects of the three ligands (methysergide, DOI, and clozapine) on the activation free-energy profile of a protomeric 2AR (see Figure 2 D - top) and identified the most energetically favorable 2AR state for each ligand. In agreement with known efficacies of these ligands, the clozapine-bound 2AR conformation is inactive (i.e., 2RH1-like), the DOI-bound 2AR conformation is active (i.e., 3P0G-like), and the methysergide-bound conformation adopts an inactive state that is structurally different from the inactive state stabilized by clozapine.
To provide a structural context for the crosstalk between 2AR and mGluR2, we studied further the effects of the three 2AR ligands on mGluR2 conformations in the dimeric complex. The location of the dimeric interface was obtained for each ligand-specific 2AR conformation using an implicit membrane model Monte Carlo search (see Computational Methods). The reconstructed free-energy in the bottom part of Figure 2 D shows that when clozapine is bound to 2AR, the mGluR2 equilibrium is shifted toward a more activated conformation (i.e., 3DBQ-like), consistent with the functional up-modulation in Gi signaling. In contrast, methysergide and DOI bound to 2AR stabilized inactive (i.e., 1U19-like) states of mGluR2. Although no significant energetic and structural differences were noted between the TM regions of these inactive states of mGluR2, the functional down-modulation of Gi signaling induced by DOI, but not by methysergide, may be ascribed to possible different interactions between the receptor loop regions and the G-protein, which are not taken into account in our simulations. This computational approach that predicts the functional results (Figure 2 B) can be used to guide structure-based rational discovery of novel `biased' drugs that are capable of selectively activating specific signaling pathways.
Taken together, these results indicate that, in the heteromer, the Gi response elicited by Glu through mGluR2 can be modulated by ligands that bind the 2AR. While drugs such as the strong 2AR agonist DOI can greatly stimulate Gq signaling and decrease Gi signaling (henceforth referred to as dominant agonists), inverse agonists, such as clozapine, have the opposite effect, namely they abolish Gq signaling and increase Gi signaling.
Drugs that Bind mGluR2 Alter the Balance Between Gi and Gq Signaling
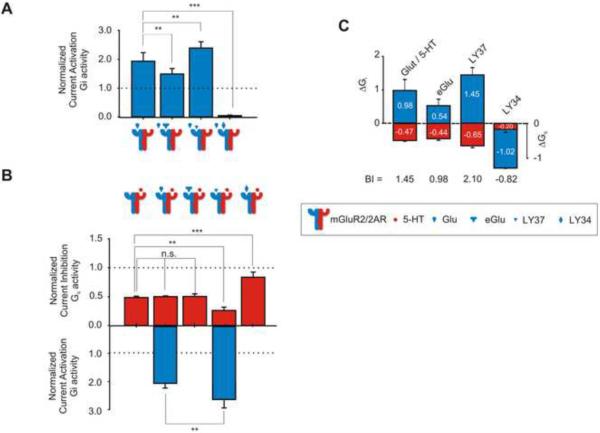
We proceeded to test how drugs bound to mGluR2 as part of the heteromeric complex affected the 5-HT-elicited Gq signaling of 2AR. The neutral antagonist ethylglutamic acid (eGlu), the strong agonist (LY37) (Figure S4 B) and the inverse agonist (LY34) [for full names, see “Experimental Procedures”, under “Drugs”] worked as expected to reduce, stimulate or abolish Glu-elicited Gi signaling through mGluR2, respectively (Figure 3 A). Consistent with its inverse-agonist properties, LY34 not only completely abolished Gi signaling, but also was able to reduce the basal Gi activity of the mGluR2 receptor even in the absence of Glu (Figure S4 C).
Figure 3. Drugs that Target mGluR2: Integrative Effects on Gi and Gq Signaling.
(A) Summary bar graphs of Gi activity (mean ± SEM) measured in oocytes expressing mGluR2/2AR following stimulation with 1μM Glu alone, or together with 10μM eGlu, 10μM LY37, or 10μM LY34. Gi activity was normalized relative to that obtained by stimulation mGluR2 alone with 5-HT (100% or 1, dotted line)
(B) Summary bar graphs of Gq activity (red) and Gi activity (blue) (mean± SEM) measured in oocytes expressing mGluR2/2AR following stimulation with 1μM 5-HT alone, or together with 10μM eGlu, 10μM LY37, or 10μM LY34. Gq and Gi activities were normalized relative to the response to 5-HT and Glu respectively (100% or 1, dotted line).
(C) ΔGi referenced to the homomeric mGluR2 response to 1 μM Glu and ΔGq referenced to the homomeric 2AR response to 1 μM 5-HT together with 10 μM methysergide, 10 μM DOI, or 10 μM clozapine (** p<0.01, *** p<0.01, n.s. not significant)
(see also Figures S4 and S5)
Occupancy of the mGluR2 receptor by each of the three ligands uniquely influenced the 5-HT-elicited signaling through 2AR. Figure 3 B (red bars) depicts the effects of eGlu, LY37, and LY34 on 5-HT-elicited Gq signaling through the complex. As before, the formation of the complex reduced to half the extent of Gq signaling (relative to the homomeric 2AR levels shown by the dotted line). Although neither Glu nor eGlu affected 5-HT-elicited Gq signaling, LY37 bound to mGluR2 decreased it even further (around 35%), while LY34 increased it almost back to homomeric levels (83%).
Figure 3 B (blue bars) summarizes the Gi activity associated with eGlu, LY37, and LY34 (in the absence of Glu) in response to 5-HT in oocytes co-expressing mGluR2 and 2AR. Since these three drugs target mGluR2, Gi signaling was only detected when the agonists Glu and LY37 were applied.
Using the data shown for Gi (Figure 3 A and Figure 3 B, blue bars) and Gq signaling (Figure 3B, red bars), we calculated the BI values for the three ligands (Figure 3 C). Similarly to 2AR drugs, the neutral antagonist eGlu only affected the mGluR2 side of signaling. The strong agonist LY37, however, affected both types of signaling through the complex and showed a dominant-agonist behavior as defined previously. Furthermore LY37, like DOI, was able to cross signal and elicit Gq signaling in the absence of Glu and 5-HT, respectively (Figure S4, F(5) and G(5)). The inverse agonist LY34 had the opposite two effects: it blocked Gi, but also potentiated Gq signaling, achieving almost 2AR homomeric levels (83%). All effects were disrupted when replacing mGluR2 by mGluR2Δ (see Supplemental Table S1), rescued by mGluR3Δ, and present when the Gi pathway was blocked by pertussis toxin (PTX) (Figure S5 A, B, and C). The largest overall signaling difference between Gi and Gq was obtained by the dominant agonist LY37 (BI=2.10).
In summary, our results thus far indicate that formation of the heteromeric complex favors Gi over Gq (i.e., increasing Gi and decreasing Gq) signaling by endogenous ligands. Dominant agonists of each receptor enhance signaling of the receptor they bind as part of the complex and inhibit signaling of their heteromeric receptor partner. On the other hand, inverse agonists of each receptor inhibit signaling of the receptor they bind as part of the complex, while enhancing signaling of their heteromeric receptor partner.
The Balance Index (BI) Predicts the Anti- or Pro-Psychotic Activity of Drugs Targeting mGluR2 or 2AR
Clozapine is superior to other antipsychotics not only with regard to extrapyramidal side effects, but also in several other clinical respects (Grunder et al., 2009). LY37 belongs to the class of the newly described Glu antipsychotics (Moreno et al., 2009; Patil et al., 2007). Our results thus far (Figures 2 and 3) indicate that although they act on different receptors, both drugs achieve through the mGluR2/2AR complex a similar effect on the relative Gi to Gq signaling activity, namely an increase in Gi activity concomitant with a decrease in Gq activity. We proceeded to test whether the extent of the difference between Gi-activity and Gq-activity elicited by different drugs was correlated with their psychoactive effects.
On the mGluR2 side, in addition to the dominant agonist LY37, we tested the neutral antagonist eGlu and the inverse agonist LY34, a drug that has been shown to increase locomotor activity and exploratory behavior in mice and might be considered pro-psychotic (Bespalov et al., 2007). On the 2AR side, besides the inverse agonist clozapine, we tested the following drugs: risperidone, another widely used atypical antipsychotic like clozapine; ritanserin, an antidepressant also used as an adjuvant therapeutic for schizophrenia; methysergide, a drug mainly used for migraines; and DOI, a propsychotic drug with lysergic acid diethylamide (LSD)-like effects.
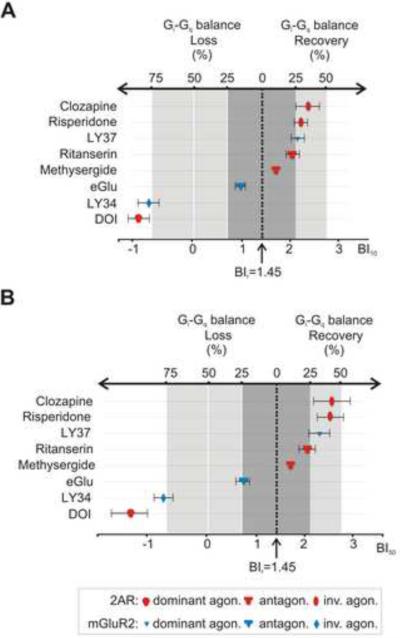
We calculated the BI values of these drugs at either a 10 μM (BI10, Figure 4 A) or at 50 μM concentrations (BI50, Figure 4B; Table S2 summarizes the BI values obtained for each of the drugs we tested). Since, in the presence of endogenous ligands and absence of drugs, the difference between Gi and Gq signaling is naturally kept in a balance that favors Gi over Gq (BIr), we converted the balance index scale in terms of the percentage recovery or loss of the Gi-Gq balance, taking a fractional occupancy of the receptor complex by the drug of 0.5 as a reference level for comparison (see Gi-Gq recovery/loss calculation in the Experimental Procedures section). Results from drugs that target mGluR2 (blue icons), versus those that target 2AR (red icons) were plotted in Figure 4.
Figure 4. Use of BI Index to Classify Anti-/Pro-psychotic Propensity of Drugs Targeting the mGluR2/2AR Complex.
Correlation maps between the balance index (BI) and percentage of Gi-Gq balance loss or recovery for different drugs assuming a fractional occupancy of the heteromer by the drug of 0.5 (see Experimental Procedures section). BIs were calculated for 10 μM (BI10) (A) and 50 μM (BI50) (B) concentrations of the drugs together with 1 μM Glu and 1 μM 5-HT and placed accordingly in the horizontal axis. BIr=1.45 corresponds to zero. Effects on the difference between Gi and Gq signaling is shown for drugs with known antipsychotic effects like clozapine, risperidone and LY37, for ritanserin, an antidepressant, for neutral antagonists methysergide and eGlu, for the psychedelic DOI, and for the propsychotic LY34 (see also Table S1).
As can be appreciated from Figure 4 (and Table S1), drugs with the most effective antipsychotic properties, regardless of the receptor they target (2AR: clozapine, risperidone; mGluR2: LY37), show the highest BI values. In contrast, drugs with the most effective propsychotic properties (2AR: DOI; mGluR2: LY34) show the lowest BI values. All of these drugs are either dominant agonists (antipsychotic for mGluR2 and propsychotic for 2AR) or inverse agonists (antipsychotic for 2AR and propsychotic for mGluR2). Neutral antagonists that target either receptors lie in between the dominant agonists and inverse agonists (between 25% recovery and 25% loss of the Gi-Gq signaling balance) and have not been shown to affect psychotic states.
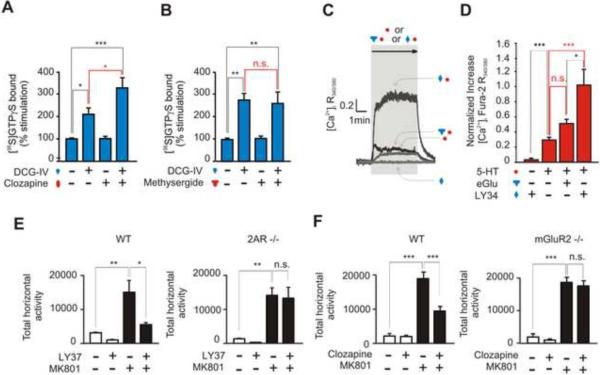
Inverse-Agonist Up-Modulation Occurs in Mouse Frontal Cortex
To study the relevance of the effects obtained in Xenopus oocytes, we examined the pattern of G protein coupling in mouse frontal cortex, a region that plays an important role in schizophrenia and antipsychotic action (González-Maeso and Sealfon, 2009). We first measured the mGluR2/2AR complex-dependent up-modulation of Gi signaling by a 2AR inverse agonist. Membrane preparations from mouse frontal cortex were incubated with the inverse agonist clozapine or the neutral antagonist methysergide (Figure S4 D and E), together with DCG-IV, a selective mGluR2/3 agonist. In oocytes, DCG-IV acted like the endogenous ligand Glu in stimulating Gi activity and in failing to down-regulate Gq signaling, unlike the dominant agonist LY37 (data not shown). Clozapine increased the DCG-IV-mediated Gi signaling (Figure 5A), while methysergide did not significantly affect Gi signaling (Figure 5B). Furthermore, clozapine failed to increase the DCG-IV-mediated Gi signaling in frontal cortex membrane preparations from 2AR knockout (KO) mice (Figure S6 C; see also Figure S6 A and B for LY37-dependent activation of Gq in wild-type but not in 2AR-KO mouse frontal cortex).
Figure 5. Up-Modulation of Gq Signaling by LY34 and Gi Signaling by Clozapine in Mouse Frontal Cortex.
DCG IV-stimulated [35S]GTPγS binding in mouse frontal cortex membranes followed by immunoprecipitation with anti-Gαi antibody in the presence or clozapine (A), methysergide (B), or vehicle. Activation of Gi was accomplished by DCG IV (10 μM). Data represent mean ± SEM (*p<0.05, **p<0.01, ***p<0.001, n.s. not significant) (see also Figure S6C).
(C) Representative traces of 5-HT-evoked elevation of intracellular calcium in mouse frontal cortex neurons as detected by ratiometric Fura-2 measurements. Measurements were obtained with 200 μM LY34 alone, 100 μM 5-HT (5-HT) alone, 100 μM 5-HT together with 200 μM eGlu (mGluR2 neutral antagonist), and 100 μM 5-HT together with 200 μM LY34 (mGluR2 inverse agonist).
(D) Bar graph summary of measured Fura-2 R340/380 change. Traces were normalized to the basal level, the steady-state fluorescence before perfusion of drugs. Data are mean ± SEM (* p<0.05, *** p<0.001, n.s. not significant).
(E) Summary bar graphs (mean± SEM) of the total MK801-induced locomotion as a summation of horizontal activity from t = 30 min to t =120 min. Injection time was at t = 0 min. Wild-type (WT, left) and 2AR-KO (right) mice were administered LY37 (5 mg/kg), or vehicle followed by MK801 (0.5 mg/kg) or vehicle (N = 5 − 6). (F) (Left) Wild-type mice were administered clozapine (1.5 mg/kg) or vehicle, followed by MK801 (0.5 mg/kg) or vehicle. (Right) mGluR2-KO mice were administered clozapine (1.5 mg/Kg) or vehicle (* p<0.05, ** p<0.01, *** p<0.001, n.s., not significant).
We next tested the mGluR2 inverse agonist up-modulation of Gq signaling in cortical primary cultures. Stimulation of Gq signaling in neurons is known to elicit a transient increase of intracellular calcium (via an IP3-mediated Ca2+ release from ER) that can be recorded using fluorescent calcium-sensitive dyes (Pichon et al., 2010). We used the calcium dye fura-2 to monitor changes in free intracellular calcium in response to 5-HT, and 5-HT with the mGluR2 inverse agonist LY34. As predicted, LY34 was able to boost the 5-HT response approximately five times (Figure 5, C and D), while by itself it showed no response. In contrast, co-application of the mGluR2 neutral antagonist eGlu with 5-HT, did not elicit a significant increase in intracellular calcium (Figure 5, C and D).
These data suggest that the effects of inverse agonists that bind 2AR or mGluR2 and boost their heteromeric partner receptor's signaling seen in vitro (see Figs. 1, 2, 3 above) also occur in cortical neurons in vivo (Figure 5, A – D).
2AR and mGluR2 Are Both Necessary for Antipsychotic-Like Behavior in Mice
We proceeded to explore the involvement of 2AR and mGluR2 in behavioral responses induced by the two antipsychotic drugs LY37 and clozapine and to compare their effects in wild-type and receptor KO mice. We determined the effects of the mGluR2/3 agonist LY37 on the locomotor behavior induced by MK801 in wild-type and 2AR-KO mice (Figure 5 E). Non-competitive NMDA receptor antagonists, such as phencyclidine (PCP) and ketamine are used as pharmacological models for schizophrenia in rodents because of their capacity in humans to evoke symptoms resembling those seen in this disease (Morris et al., 2005; Mouri et al., 2007; Patil et al., 2007). The potent and selective non-competitive NMDA receptor antagonist MK801 (dizocilpine) can also elicit ketamine-like symptoms in healthy volunteers (Reimherr et al., 1986). Activation of mGluR2, but not mGluR3, by LY37 has been shown to reduce the hyperlocomotion response induced by non-competitive NMDA antagonists in mouse models of schizophrenia (Woolley et al., 2008). We found that the locomotor activity elicited by MK801 in wild-type and 2AR-KO mice was indistinguishable. Remarkably, the MK801-stimulated activity was significantly attenuated by LY37 in wild-type mice, but it was not affected in 2AR-KO mice (Figure 5 E). As expected, the effect of LY37 on the MK801-locomotor response was absent in mGluR2-KO mice (Figure S7 B).
We next tested the role of mGluR2 in the antipsychotic-like effect induced by the atypical antipsychotic clozapine. Since clozapine binds with high affinity to 2ARs, and with lower affinity to dopamine D2 receptors (Meltzer et al., 1989), we first established the lowest dose of clozapine that induced an antipsychotic-like effect in mice. Clozapine significantly decreased the MK801-stimulated locomotor activity at doses ranging from 1.5 to 10 mg/kg (Figure S7 A). The locomotor activity induced by MK801 was similar in wild-type and mGluR2-KO mice. Notably, pre-treatment with 1.5 mg/kg clozapine significantly decreased the MK801-stimulated locomotion in wild-type mice, but not mGluR2-KO mice (Figure 5 F). The same dose of clozapine had no effect on 2AR-KO mice when compared to wild-type mice (Figure S7 C; see also Figure S7 A, B, and C for modulation of the MK801-induced locomotor activity by 10 mg/kg clozapine in wild-type, 2AR-KO and mGluR2-KO mice). The findings of Figure 5 F were also consistent with the absence of antipsychotic-like behavioral effects of methysergide (Figures S7, D and E). Our data do not exclude the possibility that the absence of antipsychotic-like effect of LY37 in 2AR-KO might also be affected by the lower expression of mGluR2 in 2AR-KO mice (González-Maeso et al., 2008; Moreno et al., 2011). Co-injection of LY37 and clozapine (1.5 mg/kg) did not affect the MK801-dependent locomotor response in either mGluR2-KO or 2AR-KO mice (data not shown).
Together, these findings demonstrate that the mGluR2-dependent antipsychotic-like behavioral response of LY37 requires the expression of the 2AR, and that the corresponding 2AR-dependent effect of clozapine requires the expression of the mGluR2.
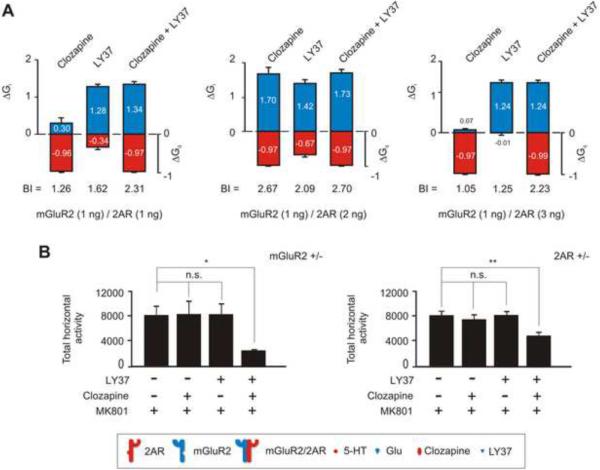
A Drug Combination Approach To Control Psychotic-like Behavior
We established earlier that an optimal mGluR2/2AR ratio of expression (1 ng of mGluR2 mRNA and 2 ng of 2AR mRNA injected) yielded the largest difference in Gi and Gq signaling (BIr=1.45) (Figure 1 F and Figure S3 D). However, injection of either a greater amount (3 ng) or a lesser amount (1ng) of 2AR mRNA than mGluR2 mRNA (1ng) decreased the BI (Figure S3 D). These findings may model the alterations in 2AR and mGluR2 expression that we have previously shown in postmortem human brain of untreated schizophrenic subjects (González-Maeso et al., 2008). Recent preclinical findings suggest that co-administration of suboptimal doses of atypical and Glu antipsychotics results in robust therapeutic-like behavioral effects and reduced unwanted side effects (Uslaner et al., 2009). We proceeded to test whether co-administration of clozapine (targeting 2AR) and LY37 (targeting mGluR2) could produce BI levels close to the BIr and compensate for alterations in the signaling crosstalk between mGluR2 and 2AR, caused by sub-optimal expression ratios. Figure 6 A shows the ΔGi and ΔGq values obtained in response to clozapine, LY37, and LY37 together with clozapine at concentrations of 50 μM. Co-administration of the two drugs increased significantly the BI in both suboptimal cases (left and right panels) compared to the optimal case (middle panel). These results reveal that co-administration of LY37 and clozapine could compensate for the loss in signaling capacity that is likely to result from decreased mGluR2/2AR heteromeric formation since cross signaling is decreased in suboptimal signaling receptor ratios.
Figure 6. Control of BI Through a Drug Combination Approach.
(A) BI calculations at 50 μM ligand concentrations for BI. ΔGi referenced to the homomeric mGluR2 (1 ng of mRNA) response to 1 μM Glu and ΔGq referenced to the homomeric 2AR (2 ng of RNA) response to 1 μM 5-HT and 1μM Glu. Responses to a concentration of 50uM clozapine, LY37, or LY37 together with clozapine were measured in oocytes injected with 1 ng mGluR2 mRNA and 1 ng (left), 2 ng (center) and 3 ng (right) or 2AR mRNA respectively.
(B) Summary bar graphs (mean± SEM) of the total MK801-induced locomotion as a summation of horizontal activity from t = 30 min to t =120 min. Injection time was at t = 0 min. mGluR2 heterozygotes (mGluR2 +/-) (left) and 2AR heterozygotes (right). Mice were administered vehicle, clozapine (1.5 mg/kg), LY37 (5 mg/kg), or both LY37 and clozapine, followed by MK801 (0.5 mg/kg) (N = 5 − 6). (* p<0.05, n.s. not significant).
Further behavioral experiments showed that administration of either clozapine or LY37 in mGluR2 or 2AR heterozygote mice did not affect the MK801-dependent locomotor response (Figure 6 B). However, co-administration of both antipsychotics in mGluR2 or 2AR heterozygotes significantly decreased the MK801-stimulated locomotor activity (Figure 6 B).
These results suggest that a combination of mGluR2 dominant agonists with 2AR inverse agonists are likely to synergize to achieve an optimal BI in cases where a suboptimal Gi – Gq signaling balance exists, as is potentially the case suggested by findings in postmortem human brain of schizophrenic subjects (González-Maeso et al., 2008).
DISCUSSION
Previous work demonstrated the physical association of the mGluR2 and 2AR into a functional heteromeric complex through which hallucinogenic drugs cross-signaled to the Gi side (González-Maeso et al., 2008). Despite this advance, it was not clear how the heteromeric complex signaled in response to ligands binding to either receptor and whether the differential pharmacology of this GPCR heteromer could be considered widely as a tractable therapeutic target for psychotic behavior. Our current studies indicate that the mGluR2/2AR heteromer functions to establish a Gi-Gq balance for the response to endogenous ligands (Glu, and 5-HT). We utilized a simple metric, the Balance Index (BI) that quantifies the change in Gi (increase) and Gq (decrease) signaling upon heteromerization relative to the homomeric signaling levels.
The BI can be modulated either by dominant agonists that take control over their counterpart receptors, or by inverse agonists that lift this control. This establishes a map between the ligand input to the heteromer (e.g., agonist/agonist, dominant agonist/agonist, neutral antagonist/agonist, inverse agonist/agonist) and its signaling output in terms of how specific ligands affect the BI. Our results are in agreement with results from a GPCR complex of D2 receptor homomers coupled to a single Gi protein subunit (Han et al., 2009). Our study further extends these previous findings and demonstrates a signaling crosstalk between 2AR and mGluR2, which are individually coupled to two different subtypes of G proteins (Gq and Gi). Our data indicate that signaling crosstalk through the mGluR2/2AR heterocomplex may be a causal mechanism for the induction of cellular and behavioral responses that differ from those of mGluR2 and 2AR homomers.
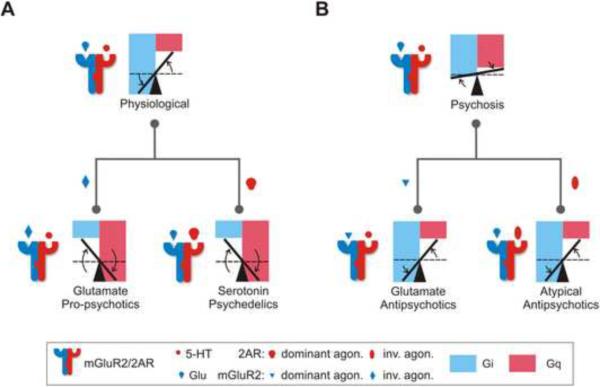
In our model (Figure 7), psychedelics invert the signaling balance through the complex (Gi signaling decreases, while Gq signaling increases, thus decreasing the BI), tipping the balance from being in favor of Gi (normal complex) to being in favor of Gq signaling (pro-psychotic) (Figure 7 A). Similarly, disease states involving psychosis, such as schizophrenia, would be expected to be associated with a variable disruption of the Gi-Gq balance (i.e., decrease in Gi, increase in Gq and a decrease in BI) (Figure 7 B), consistent with the mGluR2 down-regulation and 2AR up-regulation observed in untreated schizophrenic patients (González-Maeso et al., 2008). Such disruption would be reversed by antipsychotics that recover the Gi-Gq balance again in favor of Gi as in the normal complex (i.e., increasing Gi and decreasing Gq, thus increasing the BI) (Figure 7 B). The present study also suggests a unifying mechanism of action of atypical antipsychotics and the new Glutamate antipsychotics. Our findings suggest inverse agonism as a common feature of 2AR ligands with antipsychotic properties. We show that dysregulation of an optimal ratio of mGluR2 to 2AR expression via injection of different mRNA ratios greatly decreases BI values, and single application of 2AR inverse agonists or mGluR2 dominant agonists may not push the BI into the therapeutic range (see Figure 4). Yet co-administration of the most effective mGluR2 and 2AR drugs yields BI values in the therapeutic range. These findings in heterologous systems were paralleled in vivo using heterozygous mice for 2AR or mGluR2: co-injection of both clozapine and LY37 was needed to decrease the MK801-dependent locomotor activity. In some schizophrenic patients, atypical antipsychotics produce complete remission of psychotic symptoms. However, two-thirds of schizophrenic patients are considered treatment resistant, with persistent psychotic and other symptoms despite the optimal use of available antipsychotic medications (Lieberman et al., 2005). The absence of antipsychotic-like behavioral effect by injection of either LY37 or clozapine in 2AR or mGluR2 heterozygous mice, but not in the same mice co-injected with LY37 and clozapine, points towards potential beneficial use of combination therapy in treatment-resistant schizophrenia.
Figure 7. Gi-Gq Balance Model of the Mechanism of Action of Antipsychotic and Psychedelic Drugs through the mGluR2/2AR Complex.
Formation of the receptor complex establishes an optimal Gi-Gq balance in response to Glu and 5-HT (increase in Gi, decrease in Gq). A. Psychedelics (LY34 and DOI) invert the balance (strong Gi decrease, strong Gq increase). B. Disruption of the optimal balance in psychotic states (decrease in Gi, increase in Gq) can be compensated for by antipsychotics (LY37, clozapine, and risperidone) that recover the Gi-Gq balance (increasing Gi and decreasing Gq).
The metric (BI index) that we provide allows quantification and prediction of anti- / pro-psychotic effects of new drugs acting through the mGluR2/2AR receptor heterocomplex. Although long-term effects of drugs targeting mGluR2/2AR signaling are not taken into account in the way we have estimated the BI metric, the ability of this scale to predict appropriately the most effective anti- and pro-psychotic drugs acting through the receptor heterocomplex makes it a promising tool in predicting the efficacy of new drugs. This metric, as well as structural insights from ligand-specific heteromeric conformations, could be used extensively for screening new compounds with potential antipsychotic effects.
Our results pave the way toward a new understanding of the cellular signaling, function and pharmacology of other heteromeric G protein coupled receptors that have been implicated as therapeutic targets for the treatment of disease (Milligan, 2009). Provided that the receptor complex signaling output can classify accurately the behavior of drugs targeting the complex and used to treat disease, the case of the mGluR2/2AR complex can serve as a guiding example of development of therapeutic potency scales that can be used to classify existing drugs and predict the behavior of novel ones. Since the most effective antipsychotic drugs targeting the mGluR2/2AR complex all gave the highest BI values, it is likely that somehow signaling through this complex is uniquely coupled to specific targets. The mechanism of such signaling specificity as well as the detailed actions of the Gi versus Gq signaling through the mGluR2/2AR complex, aiming to achieve a homeostatic balance that ensures a normal non-psychotic state, are likely to become an active pursuit of future studies.
EXPERIMENTAL PROCEDURES
Drugs
(See Supplemental Experimental Procedures for details).
Molecular Constructs
(See Supplemental Experimental Procedures for details).
Expression of Recombinant Proteins in Xenopus oocytes
Oocytes were isolated and microinjected with equal volumes (50 nl), as previously described (Lopes et al., 2002). In all two-electrode voltage-clamp experiments (TEVC), oocytes were injected with 1 ng of mGluR2, 2ng of mGluR2ΔTM4,5, 2 ng of mGluR3, 2 ng of mGluR3ΔTM4,5, 2 ng of 2AR, 2 ng of GIRK4*, 2 ng of IRK3, 1 ng of PTX or 4 ng of RGS2, and were maintained at 18°C for 1–4 days before recording.
Two-Electrode Voltage-Clamp Recording and Analysis
Whole-cell currents were measured by conventional two-microelectrode voltage clamp (TEVC) with a GeneClamp 500 amplifier (Axon Instruments, Union City, CA), as previously reported. A high-potassium (HK) solution was used to superfuse oocytes (96 mM KCl, 1 mM NaCl, 1 mM MgCl2, 5 mM KOH/HEPES; pH 7.4) to obtain a reversal potential for potassium (EK) close to zero.
Inwardly rectifying potassium currents through GIRK4* and IRK3 were obtained by clamping the cells at −80 mV. In order to isolate Gi, GIRK4* was co-injected with RGS2 in order to eliminate the Gq component in the current. Basal IRK3 and GIRK4* currents were defined as the difference between inward currents obtained at −80 mV in the presence of 3 mM BaCl2 in HK solution and those in the absence of Ba2+ and measured for each trace. Current inhibition and current activation were measured respectively and normalized to basal current to compensate for size variability in oocytes.
Analysis of mGluR2 and 2AR protein levels and Surface expression assays (see Supplemental Experimental Procedures for details).
Computational Methods
Molecular modeling: Since there are no available crystal structures of the 2AR or mGluR2 available to date, we generated initial molecular models of these two receptors. Specifically, we built initial inactive conformations of 2AR or mGluR2 using a combination of homology modeling for the TM helices, and an ab initio loop prediction approach implemented in the Rosetta 2.2 code (Wang et al., 2007) for the loop regions of the receptors. According to specific structural and functional similarities, the β2 adrenergic (PDB 2RH1 (Cherezov et al., 2007) or rhodopsin (PDB 1U19 (Okada et al., 2004)) crystal structures were used as structural templates for the homology modeling of the TM regions of 2AR or mGluR2, respectively. We generated activation pathways for each receptor using the adiabatic biased MD (ABMD) algorithm (Paci et al., 1999), and a recently published simulation protocol (see [Provasi et. al., 2010] for details). Free-energy values were calculated using a Monte Carlo scheme.
(See Supplemental Experimental Procedures for additional details).
Experimental Animals
Experiments were performed on adult (8–12 weeks old) male 129S6/SvEv mice. 2AR-KO mice have been previously described (González-Maeso et al., 2007). mGluR2-KO mice were obtained from the RIKEN BioResource Center, Japan (see reference [Yokoi et al., 1996] for details), and backcrossed for at least ten generations onto a 129S6/SvEv background. All subjects were offspring of heterozygote breeding. For experiments involving genetically modified mice, 2AR wild type or mGluR2 wild type littermates were used as controls. Animals were housed at 12 h light/dark cycle at 23°C with food and water ad libitum. The Institutional Animal Use and Care Committee approved all experimental procedures at the Mount Sinai School of Medicine and Virginia Commonwealth University.
Measurement of Intracellular Ca2+
Measurement of intracellular free calcium was performed as described in the literature with minor modifications (Pichon et al., 2010) (see Supplemental Experimental Procedures for details).
Co-Immunoprecipitations and [3H]Ketanserin and [35S]GTPγS Binding Assays
Mouse frontal cortex membrane preparations and binding assays were performed as previously described with minor modifications (González-Maeso et al., 2008) (see Supplemental Experimental Procedures for details).
Cortical Primary Cultures and Immunocytochemistry
Mouse cortical primary neurons were cultured as previously reported (González-Maeso et al., 2008)
(see Supplemental Experimental Procedures for details).
Behavioral Studies
Locomotor and head-twitch behavioral studies were performed as previously described (González-Maeso et al., 2008). Motor function was assessed with a computerized three-dimensional activity monitoring system (AccuScanInstruments). The activity monitor has 32 infrared sensor pairs, with 16 along each side spaced 2.5cm apart. The system determines motor activity on the basis of the frequency of interruptions to infrared beams traversing the x, y and z planes. Total distance (cm) travelled and vertical activity were determined automatically from the interruptions of beams in the horizontal and vertical planes respectively.
Calculation of Gi-Gq Balance Recovery and Loss
The total Gi-Gq balance achieved by the mGluR2/2AR heteromer in the presence of a drug was calculated with the following equation:
where x is the fraction of heteromer that binds the drug, BId is the balance index of the drug at a fixed concentration, and BIr is the reference balance index of the complex (1.45). In the absence of drug (x=0) the total balance achieved will be BIr (1.45). If the drug has a BId=BIr, the total Gi-Gq balance is also BIr (1.45) for any fraction x of drug-bound heteromer. In a state where the mGluR2/2AR heteromer is signaling at a BI<BIr (disease state), a drug with a BId>BIr will be able to compensate for the total Gi-Gq balance loss and reestablish a total balance of 1.45 (BIr), if BId is sufficiently large.
The Gi-Gq balance recovery (R) was calculated with the following equation:
This value expressed as a percentage indicates the amount of Gi-Gq balance that could be recovered by a drug with a balance index BId. It is determined by the difference between the total balance achieved in the presence of the drug compared to BIr. A positive Gi-Gq balance (total balance>BIr) indicates that the drug is able to recover Gi-Gq balance (e.g. a drug with a BId of 2.3, with a fractional occupancy x=0.5 will have a total balance of 1.875, which will allow recovery up to ~30% loss from the reference level of 1.45). A negative Gi-Gq balance (total balance<BIr) indicates that the drug induces a loss in balance (e.g. a drug with a BId of 0.315 with a fractional occupancy x=0.5 will have a total balance of 0.315, which will result in ~80% loss from the reference level of 1.45).
In order to compare drugs, we established an arbitrary reference level of fractional occupancy of the heteromer by the drug (x=0.5). This allowed us to establish the differences in the ability of the drugs to recover or lose the Gi-Gq balance based on their BI at equal conditions. Changes in the fractional occupancy used for comparison (e.g. x=0.25) changed the magnitude but not the relative order in the classification of the drugs shown in Figure 4.
Statistical Methods
Statistical significance of behavioral experiments involving four groups and two treatments were assessed by two-factor ANOVA followed by Bonferroni's post hoc test. Statistical significance of behavioral experiments involving one treatment was assessed by Student's t-test.
Each electrophysiological experiment in Xenopus oocytes was performed in two batches. Every group in each experiment was tested in both batches. Data for both batches were compiled (n = 8–16) and one-way ANOVA tests applied followed by a multiple comparison procedure using Tukey's honestly significant difference test.
Intracellular calcium measurements were performed in three different isolations. Data for all isolations were compiled (n=7–11) and one-way ANOVA tests applied followed by a multiple comparison procedure using Tukey's honestly significant difference test.
[3H]-ketanserin binding experiments were performed 3–5 times in duplicate/triplicate. A one-site model versus a two-site model, as a better description of the data was determined by F test. [35S]GTPγS binding experiments were performed three times in triplicate. Two-way ANOVA tests were applied to the compiled data followed by a Bonferroni's post hoc test.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Sophia Gruszecki, Heikki Vaananen, Dr Jian Yang, and Dr. Basil Hanss for Xenopus oocyte isolation and are grateful to Drs. Kurt Houser for his support with the cortical primary neuronal cultures and J.A. Gingrich for his gift of 5HT2A-KO mice. The authors are also thankful to Drs. M. Scott Bowers, Louis J. De Felice, Frank Guarnieri (Virginia Commonwealth University), Lakshmi Devi (Mount Sinai School of Medicine), Jonathan Javitch (Columbia University), George Liapakis (University of Crete, Greece), and Herbert Meltzer (Vanderbilt University) for critical feedback on the manuscript, and to members of the Logothetis lab for useful feedback throughout this project. D.E.L. was partly supported for this work by NIH grant HL59949. J.G-M was supported by NIH grant 5R01MH084894, as well as NARSAD, Dainippon Sumitomo Pharma, and the Maltz Family Foundation Award. M. F. was supported by NIH grants MH084894, MH091360, and DA026434. The computations were supported in part by the National Science Foundation through TeraGrid advanced computing resources provided by the Texas Advanced Computing Center under grant TG-MCB080109N. J.L.M was the recipient of a postdoctoral fellowship from Ministerio de Ciencia e Innovación, Spain. R.M. was supported by NIH grant F30HL097582.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION Supplemental information includes seven figures (S1–S7), two tables (Table S1–S2) and Supplemental Experimental Procedures that are meant to be available online to support this article.
REFERENCES
- Aloyo VJ, Berg KA, Spampinato U, Clarke WP, Harvey JA. Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol. Ther. 2009;121:160–173. doi: 10.1016/j.pharmthera.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Barela AJ, Waddy SP, Lickfett JG, Hunter J, Anido A, Helmers SL, Goldin AL, Escayg A. An epilepsy mutation in the sodium channel SCN1A that decreases channel excitability. J. Neurosci. 2006;26:2714–2723. doi: 10.1523/JNEUROSCI.2977-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardenstein LM, Gurovich IY, Morozova MA, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Barducci A, Bussi G, Parrinello M. Well-tempered metadynamics: a smoothly converging and tunable free-energy method. Phys. Rev. Lett. 2008;100:020603. doi: 10.1103/PhysRevLett.100.020603. [DOI] [PubMed] [Google Scholar]
- Bespalov A, Jongen-Relo AL, van Gaalen M, Harich S, Schoemaker H, Gross G. Habituation deficits induced by metabotropic glutamate receptors 2/3 receptor blockade in mice: reversal by antipsychotic drugs. J. Pharmacol. Exp. Ther. 2007;320:944–950. doi: 10.1124/jpet.106.110684. [DOI] [PubMed] [Google Scholar]
- Bonomi D, Branduardi G, Bussi C. PLUMED: A portable plugin for free-energy calculations with molecular dynamics. Computer Physics Communications. 2009;180:1961–1972. [Google Scholar]
- Branduardi D, Gervasio FL, Parrinello M. From A to B in free energy space. J. Chem. Phys. 2007;126:054103. doi: 10.1063/1.2432340. [DOI] [PubMed] [Google Scholar]
- Carriba P, Navarro G, Ciruela F, Ferre S, Casado V, Agnati L, Cortes A, Mallol J, Fuxe K, Canela EI, Lluis C, Franco R. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat. Methods. 2008;5:727–733. doi: 10.1038/nmeth.1229. [DOI] [PubMed] [Google Scholar]
- Du X, Zhang H, Lopes C, Mirshahi T, Rohacs T, Logothetis DE. Characteristic interactions with phosphatidylinositol 4,5-bisphosphate determine regulation of Kir channels by diverse modulators. J. Biol. Chem. 2004;279:37271–37281. doi: 10.1074/jbc.M403413200. [DOI] [PubMed] [Google Scholar]
- Egan CT, Herrick-Davis K, Teitler M. Creation of a constitutively activated state of the 5-hydroxytryptamine2A receptor by site-directed mutagenesis: inverse agonist activity of antipsychotic drugs. J. Pharmacol. Exp. Ther. 1998;286:85–90. [PubMed] [Google Scholar]
- Ernst OP, Gramse V, Kolbe M, Hofmann KP, Heck M. Monomeric G protein-coupled receptor rhodopsin in solution activates its G protein transducin at the diffusion limit. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10859–10864. doi: 10.1073/pnas.0701967104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL. Expression of ion channels in Xenopus oocytes. In: Clare JJ, Trezise DJ, editors. expression and analysis of recombinant ion channels. WILEY-VCH Verlag GmbH & Co. KGaA; Weinheim: 2006. [Google Scholar]
- González-Maeso J, Rodriguez-Puertas R, Meana JJ. Quantitative stoichiometry of G-proteins activated by mu-opioid receptors in postmortem human brain. Eur. J. Pharmacol. 2002;452:21–33. doi: 10.1016/s0014-2999(02)02242-2. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- González-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225–232. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Grunder G, Hippius H, Carlsson A. The 'atypicality' of antipsychotics: a concept re-examined and re-defined. Nat. Rev. Drug Discov. 2009;8:197–202. doi: 10.1038/nrd2806. [DOI] [PubMed] [Google Scholar]
- Han Y, Moreira IS, Urizar E, Weinstein H, Javitch JA. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat. Chem. Biol. 2009;5:688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Yan X, Zhang H, Mirshahi T, Jin T, Huang A, Logothetis DE. Identification of critical residues controlling G protein-gated inwardly rectifying K+ channel activity through interactions with the beta gamma subunits of G proteins. J. Biol. Chem. 2002;277:6088–6096. doi: 10.1074/jbc.M104851200. [DOI] [PubMed] [Google Scholar]
- He C, Zhang H, Mirshahi T, Logothetis DE. Identification of a potassium channel site that interacts with G protein betagamma subunits to mediate agonist-induced signaling. J. Biol. Chem. 1999;274:12517–12524. doi: 10.1074/jbc.274.18.12517. [DOI] [PubMed] [Google Scholar]
- Hof PR, et al. Comparative cytoarchitectonic atlas of the C57BL/6 and 129/Sv mouse brains. Elsevier; Amsterdam: 2000. [Google Scholar]
- Kinon BJ, Zhang L, Millen BA, Osuntokun OO, Williams JE, Kollack-Walker S, Jackson K, Kryzhanovskaya L, Jarkova N, the HBBI Study Group A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J. Clin. Psychopharmacol. 2011;31 doi: 10.1097/JCP.0b013e318218dcd5. [DOI] [PubMed] [Google Scholar]
- Lazaridis T. Effective energy function for proteins in lipid membranes. Proteins: Structure, Function, and Bioinformatics. 2003:176–92. doi: 10.1002/prot.10410. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, Logothetis DE. Alterations in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–944. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Canals M, Pediani JD, Milligan G. The alpha1b-adrenoceptor exists as a higher-order oligomer: effective oligomerization is required for receptor maturation, surface delivery, and function. Mol. Pharmacol. 2007;71:1015–1029. doi: 10.1124/mol.106.033035. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Huang M. In vivo actions of atypical antipsychotic drug on serotonergic and dopaminergic systems. Prog. Brain Res. 2008;172:177–197. doi: 10.1016/S0079-6123(08)00909-6. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J. Pharmacol. Exp. Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br. J. Pharmacol. 2009 doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Holloway T, Albizu L, Sealfon SC, Gonzalez-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci. Lett. 2011;493:76–79. doi: 10.1016/j.neulet.2011.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JL, Sealfon SC, González-Maeso J. Group II metabotropic glutamate receptors and schizophrenia. Cell Mol. Life Sci. 2009;66:3777–3785. doi: 10.1007/s00018-009-0130-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr. Opin. Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem. Int. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat. Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Pichon X, Wattiez AS, Becamel C, Ehrlich I, Bockaert J, Eschalier A, Marin P, Courteix C. Disrupting 5-HT(2A) receptor/PDZ protein interactions reduces hyperalgesia and enhances SSRI efficacy in neuropathic pain. Mol. Ther. 2010;18:1462–1470. doi: 10.1038/mt.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provasi D, Camacho-Artacho M, Negri A, Mobarec JC, Filizola M. Ligand-Induced Modulation of the Free-Energy Landscape of G Protein-Coupled Receptors Explored by Adaptive Biasing Techniques. PLOS Computational Biology. 2011 doi: 10.1371/journal.pcbi.1002193. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimherr FW, Wood DR, Wender PH. The use of MK-801, a novel sympathomimetic, in adults with attention deficit disorder, residual type. Psychopharmacol. Bull. 1986;22:237–242. [PubMed] [Google Scholar]
- Rives ML, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin JP, Prezeau L. Crosstalk between GABA(B) and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 2009 doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Urizar E, Yano H, Kolster R, Galés C, Lambert N, Javitch JA. CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat Chem Biol. 2011;7:624–630. doi: 10.1038/nchembio.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Smith SM, Huszar SL, Pachmerhiwala R, Hinchliffe RM, Vardigan JD, Hutson PH. Combined administration of an mGlu2/3 receptor agonist and a 5-HT 2A receptor antagonist markedly attenuate the psychomotor-activating and neurochemical effects of psychostimulants. Psychopharmacology (Berl) 2009;206:641–651. doi: 10.1007/s00213-009-1644-y. [DOI] [PubMed] [Google Scholar]
- Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, Mackerell AD., Jr CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010;31:671–690. doi: 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nat. Chem. Biol. 2008;4:126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, et al. 5-hydroxytryptamine2A receptor inverse agonists as antipsychotics. J. Pharmacol. Exp. Ther. 2001;299:268–276. [PubMed] [Google Scholar]
- Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, et al. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.