Abstract
During the early stages of pregnancy, fertilized embryos must attach to the uterine epithelium, invade into the underlying uterine stroma, and the stroma must then differentiate in a process termed decidualization in order for a successful pregnancy to be initiated. The steroid hormone progesterone (P4) is an integral mediator of these early pregnancy events, exerting its effects via the progesterone receptor (PR). Insights gained from the use of mouse models and genomic profiling has identified many of the key molecules enlisted by PR to execute the paradigm of early pregnancy. This review describes several of the molecules through which the PR exerts its pleiotropic effects including ligands, receptors, chaperones, signaling proteins and transcription factors. Understanding these molecules and their concatenation is of vital importance to our ability to clinically treat reproductive health problems like infertility and endometriosis.
Keywords: Progesterone, Progesterone Receptor, Attachment, Implantation, Decidualization, Mouse Models
1. Introduction
According to the most recent data from the Centers for Disease Control and Prevention's National Survey of Family Growth, infertility is estimated to affect 2.1 million married women in the US. When the parameters describing unsuccessful pregnancy events are expanded to include reduced fecundity, defined as difficulties becoming pregnant or carrying a baby to term, it was estimated to affect 7.4 million married women, demonstrating the tremendous impact fertility problems have on female reproductive health. Although society and families have benefited from advancements in the field of assisted reproductive technology (ART), to which approximately 1% of US births can now be attributed to, the overall success rate of ART still remains low [Ola and Li 2006]. Many of the unsuccessful ART pregnancies can likely be attributed to the transfer of embryos into a nonreceptive uterus. In fact, recurrent implantation failure is considered to be an important limiting factor in the establishment of pregnancy by either ART or natural means [Norwitz et al., 2001].
In order for a successful pregnancy to be initiated, the maturation of a fertilized egg to an implantation-competent blastocyst must occur in precise synchrony with the succession of events that occur in the maternal uterus that render it receptive. This precise timing event has been termed ‘the window of receptivity’ and has been shown to be under the control of the steroid hormone estrogen (E2) [Ma et al., 2003]. During the window of receptivity, the trophectoderm layer of the blastocyst becomes juxtaposed with the uterine luminal epithelium in a process termed embryo attachment. Following attachment, the blastocyst invades through the luminal epithelium and into the underlying stroma and this process is called implantation. In most mammals, implantation triggers a rapid remodeling of the uterine stromal compartment resulting in a morphological and functional transformation including stromal cell proliferation and subsequent differentiation into large epithelioid cells in a process known as decidualization (reviewed in [Lee and DeMayo 2004]).
In humans and other menstruating primates, decidualization occurs independent of the presence of a conceptus during the midsecretory phase of the cycle following the postovulatory rise in progesterone [Finn 1987; Finn 1998]. The hallmarks of this differentiation and remodeling of the uterine endometrium in preparation of pregnancy are characterized by the transformation of the stromal cell to a rounded epithelioid cell secreting products such as prolactin (PRL) and insulin-like growth factor binding protein-1 (IGFBP-1), among many other factors [Daly et al., 1983; Gellersen et al., 2007; Irwin et al., 1989]. Decidualization is a progressive event, initiating around the spiral arteries of the superficial endometrium and eventually extending throughout the endometrium to the basal layer (reviewed in [Brosens et al., 2002]). The decidua plays a critical role in regulating trophoblast invasion, modulating the local immune response at the feto-maternal interface, and development of the placenta. Thus, an aberrant response of the endometrium to ovarian hormones results in a block in the initiation and progression of pregnancy and defective formation of the placenta, both of which are associated with recurrent miscarriages and infertility. Although E2 regulates the window of receptivity, progesterone (P4), produced by the corpus luteum following ovulation, is the predominant mediator of these events necessary for pregnancy (reviewed in [Franco et al., 2008; Lee and DeMayo 2004]).
The word progesterone was coined in the 1930s and is derived from the Latin pro and gestare to describe a substance which favors gestation. Indeed, P4 not only favors gestation, but it is absolutely required for it. Studies ablating P4 signaling either genetically or pharmaceutically demonstrate the paramount role of this steroid hormone and its signaling paradigm in pregnancy [Baulieu 1989; Baulieu 1991; Cheon et al., 2002; Lydon et al., 1995]. RU486, or mifepristone, was discovered in the early 1980's to be the first known P4 antagonist. Studies later determined that the administration of RU486 inhibited P4-mediated gene transcription, which, if administered postimplantation, ultimately lead to conceptus abortion [Baulieu 1989]. The abortifacient properties of RU486 later lead to it's use as an emergency contraceptive, demonstrating the critical role P4 signaling plays in pregnancy [WHO 1999].
P4 exerts its effects by binding its cognate receptor, the progesterone receptor (PR), which is a member of the nuclear receptor superfamily of transcription factors. There are two predominant isoforms of PR, PR-A and PR-B, each of which is transcribed from alternative start sites within the same gene (reviewed in [Li and O'Malley 2003]). Although PR-B is the larger of the two isoforms, containing an additional 164 N-terminal amino acids, PR-A is the predominant functional isoform in the uterus [Conneely and Lydon 2000; Conneely et al., 2001; Conneely et al., 2003; Mulac-Jericevic et al., 2000; Mulac-Jericevic et al., 2003]. The PR knockout (PRKO) mouse, lacking both PR-A and PR-B, displayed pleiotropic reproductive abnormalities and was shown to be infertile due to defects in multiple reproductive tissues including embryo implantation, uterine decidualization, and an abnormal response to E2 and P4 treatment [Lydon et al., 1995]. When individual PR-A and PR-B null mice (PRAKO and PRBKO, respectively) were generated, only the PRAKO female mice recapitulated the phenotype observed in PRKO mice; while in contrast, the PRBKO female mice were fertile [Conneely and Lydon 2000; Conneely et al., 2001; Conneely et al., 2003; Mulac-Jericevic et al., 2000; Mulac-Jericevic et al., 2003].
In humans, as in mice, both isoforms of PR are induced by estrogen and can be found in the epithelial and stromal compartments during the proliferative stage of the cycle [Koshiyama et al., 1995; Wang et al., 1994]. Transitioning into the secretory phase and during decidualization, PR-B is down-regulated in both compartments, while PR-A expression persists in the stromal compartment, indicating PR-A is the predominant mediator of decidualization in humans [Brosens et al., 1999; Wang et al., 1998]. Furthermore, it has been shown in vitro that PR-A is a stronger activator of decidual marker IGFBP-1 then PR-B [Gao et al., 2000]. However, the potential importance of PR-B should not be overlooked, because the ratio of PR-A to PR-B has been shown to play an important role in endometrial dysfunctions such as recurrent early pregnancy loss, endometriosis and endometrial cancer [Attia et al., 2000; Balleine et al., 2004; Carranza-Lira et al., 2000].
With advancements in gene targeting techniques and tools for genomic comparisons, many of the P4 mediators and regulators of pregnancy have been identified. With an emphasis on insights gained from mouse models, we herein concisely and comprehensively collate the prominent work thus far regarding P4 signaling, its modulators and effectors, and how this signaling axis regulates embryo implantation and subsequent decidualization (Table 1).
Table 1. Uterine Phenotypes of Genes that Mediate Progesterone Signaling During Early Pregnancy.
| Molecule | Protein | Expression | Fertility | Att | Impn | Dec | Ref |
|---|---|---|---|---|---|---|---|
| Esr1 | TF, SR | LE, GE, S, M | Infertile | No | No | Imp | [26] |
| Esr2 | TF, SR | LE, GE, S, M | Fertile | n/a | n/a | n/a | [67] |
| PR-A | TF, SR | LE, GE, S, M | Infertile | No | No | No | [21] |
| PR-B | TF, SR | LE, GE, S, M | Fertile | n/a | n/a | n/a | [21] |
| Fkbp4 | Chaperone | S | Infertile | No | No | No | [131, 148] |
| Fkbp5 | Chaperone | S | Fertile | n/a | n/a | n/a | [131] |
| SRC-1 | Co-reg | LE, GE→S | Fertile | n/a | n/a | Imp | [147] |
| SRC-1/2† | Co-reg | LE, GE→S | Infertile | n/a | n/a | No | [98] |
| SRC-2 | Co-reg | LE, GE→S | Sub/Infertile* | n/a | No | Imp | [58, 98] |
| SRC-3 | Co-reg | M | Subfertile | n/a | n/a | Yes | [58] |
| C/EBPβ | TF | S | Infertile | Yes | Yes | No | [5, 120] |
| COUP-TFII | TF | S | Sub/Infertile* | No | No | No | [69, 125] |
| Foxo1a | TF | S | n/a | n/a | n/a | n/a | n/a |
| Hand2 | TF | S | Infertile | No | No | No | [81] |
| Hoxa10 | TF | LE/GE→S | Subfertile | Imp | No | No | [85] |
| Klf9 | TF | S | Subfertile | n/a | Imp | n/a | [113] |
| Areg | Ligand | LE | Fertile | n/a | n/a | n/a | [31, 86] |
| Bmp2 | Ligand | S | Infertile | Yes | No | No | [78] |
| HB-EGF | Ligand | LE/GE→S | Subfertile | No | No | n/a | [30, 145] |
| Ihh | Ligand | LE, GE | Infertile | No | No | No | [75] |
| Wnt4 | Ligand | LE→S | Subfertile | Yes | No | Imp | [41] |
| Mig6 | Adaptor | LE, GE, S | Infertile | No | No | Yes | [64] |
| Cox1 | Enzyme | LE | Fertile | n/a | n/a | n/a | [136] |
| Cox2 | Enzyme | LE→S | Infertile | No | No | No | [83, 136] |
Abbreviations- Att: Attachment; Imn: Implantation; Dec: Decidualization; Ref: Reference TF: Transcription Factor; SR: Steroid Receptor; Co-reg: Coregulator; LE: Luminal Epithelium; GE: Glandular Epithelium; S: Stroma; M: Myometrium; Imp: Impaired; N/A: data not found
Straight knockout or heterozygote subfertile, conditional knockout infertile
→ Indicates transitioning expression between compartments
Double knockout
2. Mediators of PR Action
Execution of the P4/PR signaling paradigm of gestational regulation occurs via the manipulation of multiple levels of cellular and molecular biology. Molecules that affect the expression of the PR itself, those that regulate the PR's aptitude to respond to signals and the coregulators that enhance the PR's ability to activate transcription of its target genes all play important roles in the immediate initiation of the P4 response. In the following section, we focus on those molecules that facilitate PR signaling.
2.1 Steroid Hormone Regulation of PR Expression
One would be remiss to discuss the molecular mediators of pregnancy without first emphasizing the paramount role of the steroid hormones E2 and P4. The expression of both estrogen receptor (ER) and PR can be found in all compartments of the uterus, and embryo implantation has been shown to be critically dependent on their intricate balance and interaction [Rubel et al., 2010; Tan et al., 1999; Tibbetts et al., 1998]. On days 1 and 2 of pregnancy, preovulatory E2 stimulates uterine epithelial cell proliferation and growth. Then beginning on day 2.5 of pregnancy, luteal P4 attenuates this epithelial proliferation, promoting receptivity, and drives stromal proliferation. This PR-mediated transition from epithelial to stromal cell proliferation is requisite for successful implantation as it's been shown in several mouse models that a failure to do so results in a block in embryo attachment. While the role of PR was previously discussed herein, it should be noted that in the presence of P4, a negative-feedback loop manifests to down-regulate the expression of the PR, occurring most notably in the glandular epithelium. Thus, PR can regulate its own transcription [Tibbetts et al., 1998].
On the other hand, follicular E2 causes a transition in which the expression of epithelial PR is repressed while stromal PR expression is induced [Curtis Hewitt et al., 2002; Kastner et al., 1990; Kurita et al., 2000; Tibbetts et al., 1998]. Previous experiments utilizing neonatal tissue recombination models suggested that this E2 regulation of PR expression was dependent on stromal ERα [Kurita et al., 2000]. These findings were recently confirmed when the E2-dependent redistribution of PR expression remained intact in the Wnt7a-Cre ERαf/f mouse model containing epithelial-specific ablation of ERα [Winuthayanon et al., 2010]. Like PR, ER consists of two predominant isoforms, ERα (Esr1) and ERβ (Esr2). ERα appears to be the dominant mediator of uterine reproductive functions as knockout female mice for this isoform (ERaKO) were infertile while ERβ knockout (ERbKO) females did not exhibit impairments in receptivity [Curtis et al., 1999; Krege et al., 1998]. Interestingly, although ERbKO females were shown to be indistinguishable from wild type uteri on a molecular level, other studies indicate that ERβ regulates ERα expression and its loss can render phenotypic differences, causing the role of this ER isoform to remain unclear [Hewitt et al., 2003; Wada-Hiraike et al., 2006; Weihua et al., 2000]. Despite a potential modulatory role of ERβ, the complete infertility observed in ERaKO mice demonstrates that ERα is the prevailing mediator of E2 signaling in the uterus.
2.2 Regulation of PR Aptitude
P4 and PR regulate a plethora of different gene targets [Jeong et al., 2005]. Much of the specificity of PR target induction is dictated by the cellular context and interactions with other proteins. In the absence of P4, PR is found in the cytoplasm bound by a complex of heat shock proteins and immunophilins [Smith 2000]. The formation of such complexes functions to maintain receptor competence for hormone binding and transcriptional activation. Fkbp4 (Fkbp52) and Fkbp5 (Fkbp51), the expression of which overlaps with PR in the uterine stroma during implantation, are two immunophilins that have been shown to interact with PR. Although Fkbp5 knockout mice do not present with any overt reproductive phenotype, both male and female Fkbp4 knockout mice are infertile [Tranguch et al., 2005; Yang et al., 2006]. The fertility defect observed in these females was attributed to a complete failure in implantation and decidualization. Because Fkbp4 acts to maintain PR competency, the molecular basis of this infertility was shown to be due to dramatically reduced PR transcriptional activity, and thus reduced expression of many known target genes and mediators of fertility including Homeobox A-10 (Hoxa10), Indian Hedgehog (Ihh), and Amphiregulin (Areg). Additionally, it's well known that one of the functions of PR is to suppress epithelial E2 sensitivity and proliferation, a process which is necessary to permit embryo attachment to the luminal epithelium [Conneely et al., 2001; Lee et al., 2010]. Fkbp4 null females exhibit a failure of this process as indicated by elevation of E2 target genes such as lactoferrin (Ltf). Interestingly, P4 supplementation, although in a background dependant manner, was able to restore many of the defects observed in these mice. These results expose this mouse model as a good surrogate for studying P4 insensitivity, a characteristic observed in many human diseases including endometriosis, endometrial cancer, and in women who suffer from miscarriages.
2.3 Regulation of PR Activity
Upon binding of P4, progesterone receptors undergo a conformational change causing them to dissociate from heat shock proteins and chaperones and dimerize, thus gaining affinity for various coregulators and DNA response elements found within the genes they transcribe. One of the most well studied mediators of PR transcription is the p160/SRC (steroid receptor coactivator) family of coregulators, of which the three members are SRC-1, SRC-2 and SRC-3. These coregulators interact with and bind to the LBD of ligand-bound steroid hormone receptors and have been shown to modulate the activity of nuclear receptors including PR in vitro [Hofman et al., 2002]. SRC-1 and -2 are expressed in both the epithelial and stromal endometrium while SRC-3 expression is not detected in the preimplantation mouse uterus [Jeong et al., 2007].
Given the expression pattern of these molecules, one might predict that SRC-1 and -2 are the prime cofactors of PR transcription and indeed such is the case, with SRC-2 being the preeminent PR coactivator. SRC-1 knockout females are fertile but do exhibit a dampened response to exogenous steroid hormone treatment including a reduced increase in uterine size upon E2 treatment [Xu et al., 1998]. Additionally, SRC-1-/- females reveal a reduction in stromal differentiation when treated to undergo an artificially induced decidual response. Accordingly, although SRC-1 null females were fertile, SRC-1 plays an important role in mediating the response of the mouse uterus to steroid hormones.
Global ablation of SRC-2 resulted in a partial lethality phenotype accompanied by metabolic defects [Chopra et al., 2008; Gehin et al., 2002]. In SRC-2 null females that did survive to adulthood, there was an observed partial reduction in the artificial decidualization reaction as well as a hypofertility phenotype due to placental defects [Gehin et al., 2002; Jeong et al., 2007]. To circumvent the partial lethality and metabolic phenotypes that preclude effective adult uterine studies, a conditional ablation of SRC-2 was achieved by crossing a mouse model with floxed (flanked by loxP) SRC-2 alleles with the PR-Cre mouse model. [Mukherjee et al., 2006; Soyal et al., 2005]. This model excises alleles in tissues that express PR (pituitary gland, mammary gland, oviduct, corpus luteum of the ovary and all compartments of the murine uterus) as demonstrated when crossed with the Rosa26R reporter line which expresses the lacZ reporter gene in all tissues with Cre recombinase activity [Soriano 1999]. The mice generated from this cross (SRC-2d/d) were infertile and exhibited a reduction in the artificial decidualization reaction [Mukherjee et al., 2006]. Furthermore, microarray analysis has shown SRC-2 to be involved in in vivo P4-mediated gene transcription [Jeong et al., 2007]. These data demonstrate that SRC-2 is an integral mediator of pregnancy and P4 signaling.
Considering the similar phenotypes and overlapping expression profiles of SRC-1 and SRC-2, it was speculated that some form of functional compensation may occur between these coregulators. Hence, the SRC-2 conditional knockout mouse was crossed into the SRC-1-/- mouse model [Mukherjee et al., 2006]. Mice lacking both uterine SRC-1 and -2 were sterile with a complete ablation of decidualization, demonstrating that both of SRC-1 and -2 are necessary for decidualization. These mouse models are in contrast with the SRC-3 knockout mouse which is capable of a normal decidual response, although females are slightly subfertile [Jeong et al., 2007].
Distinct from the p160 family of coregulators, PR can also recruit secondary transcription factors such as Klf9 to act as cofactors. Krüppel-like factor (KLF)-9 is a transcription factor that has been shown to directly interact with and coactivate PR both in vitro and in vivo [Zhang et al., 2002; Zhang et al., 2003]. KLF9 expression is detected in the preimplantation stroma and its ablation results in uterine hypoplasia and female subfertility due to a reduction in the number of implantation sites [Simmen et al., 2004]. Although Klf9 is expressed in the stroma, its been shown that it can effect the response of adjacent luminal epithelial cells to P4 and that null females exhibit a partial P4 resistance [Simmen et al., 2004; Velarde et al., 2005]. Recently, it was demonstrated that KLF9 and bone morphogenic protein BMP2, a well known regulator of implantation, undergo cross-regulation in order to establish and maintain uterine receptivity [Lee et al., 2007; Pabona et al., 2010].
3. Effectors of PR Action
P4 and PR regulate implantation and decidualization via the activation of a cascade of various molecules. Periimplantation PR is known to propagate its signal by inducing the expression of many ligands and thus multiple signaling pathways. Some examples of these ligands that have been shown to play an important role in fertility include Ihh, Bmp2, wingless-related MMTV integration site 4 (Wnt4), and Erbb ligand heparin-binding epidermal growth factor (Hb-EGF) [Lee et al., 2006; Lee et al., 2007; Li et al., 2007; Takamoto et al., 2002; Wang et al., 1994; Xie et al., 2007]. Either by virtue of ligand induction or by other means, PR can also affect signaling by inducing the expression of signal modulators and/or transcription factors. Examples of the aforementioned molecules include adaptor proteins like mitogen inducible gene 6 (Mig6), cell cycle regulators like cyclin D3 and p53, and transcription factors like CCAAT/enhancer binding protein beta (C/EBPβ), Hand2, and chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII). In the following section we highlight several of the known effectors and propagators of PR signaling.
3.1.1 Signaling Ligands Induced by PR and the Pathways they Regulate
Multiple approaches aimed at indentifying P4 regulated genes using high density DNA microarrays have been conducted, including the employment of PRKO mice or PR antagonist RU486 [Cheon et al., 2002; Jeong et al., 2005] (as reviewed in [Lee et al., 2006]). Ihh, a member of the hedgehog family of morphogens, is one such P4-induced gene identified in this fashion [Matsumoto et al., 2002; Takamoto et al., 2002]. Interestingly, while Ihh expression is restricted to the endometrial epithelium and is observed with increasing intensity just prior to the time of implantation, its downstream effectors Patched-1 (Ptch1) and COUP-TFII are expressed in the endometrial stroma during this same period [Takamoto et al., 2002]. The expression pattern of these molecules alludes to a potential role of Ihh in the mediation of paracrine P4 signaling between the uterine epithelial and stromal compartments. Embryonic and perinatal lethality phenotypes observed in Ihh null mice prohibit the study of Ihh in the adult uterus [St-Jacques et al., 1999]. To bypass the observed lethality, conditional ablation of Ihh was achieved by crossing a floxed allele with the PR-Cre mouse model [Razzaque et al., 2005]. The resulting Ihhd/d females phenocopied the uterine defects observed in PRKO females, with an inability to support attachment, implantation, and decidualization [Lee et al., 2006]. Additionally, similar to the defects and elevated Ltf expression observed in Fkbp4-/- mice, the attachment defect observed in Ihhd/d females was shown to be due to a failure to attenuate E2 signaling in the preimplantation luminal epithelium as indicated by elevated expression of the secreted glycoprotein MUC-1 [Surveyor et al., 1995]. These results convey that Ihh is a major mediator of PR action in the uterus. Through findings of multiple studies, it's been shown that Ihh mediates decidualization and implantation via the activation of multiple signaling pathways including the Ihh-Ptch1-COUP-TFII-Bmp2-Wnt4 signaling axis and Erbb signaling [Kurihara et al., 2007; Lee et al., 2006; Lee et al., 2007].
Although it's not a ligand, COUP-TFII is one of the downstream mediators of Ihh signaling and its expression is lost in Ihhd/d females [Lee et al., 2006]. Although mice containing null alleles for COUP-TFII are embryonic lethal, a portion of female COUP-TFII+/- heterozygotes are viable and exhibit reduced fecundity attributed to both ovarian and uterine defects [Takamoto et al., 2005]. Given that PR is expressed in the ovarian granulosa cells while COUP-TFII is expressed in the theca cells, this differential expression permitted the utilization of the PR-Cre mouse model to further address the uterine specific defects observed in COUP-TFII+/- mice [Soyal et al., 2005; Takamoto et al., 2005]. The resulting COUP-TFIId/d mice resemble the conditional ablation of Ihh with failures in attachment and decidualization [Kurihara et al., 2007]. Also similar to Ihhd/d mice, conditional ablation of COUP-TFII results in enhanced E2 activity in the uterine epithelium as indicated by elevated levels of ERα, phosphorylated ERα (the active form) and ER target genes Ltf, Muc1, C3, and Clca3 [Braga and Gendler 1993; DeMayo et al., 2002; Driggers and Segars 2002; Jeong et al., 2006; Lagow et al., 1999; Sundstrom et al., 1989; Surveyor et al., 1995]. The suppression of ERα activity in the uterine epithelium was later shown to be one of the critical functions of COUP-TFII. The administration of the pure ERα antagonist ICI 182,780 was able to restore the defects observed in the conditionally ablated COUP-TFII females in attachment and decidualization, albeit to a lesser extent then what is normally observed in control mice [Lee et al., 2010]. These data indicate that one of the primary functions of COUP-TFII is the regulation of the window of receptivity by suppression of epithelial ERα activity.
3.1.2 Bmp2 Signaling
In addition to the role of COUP-TFII in E2 regulation, one of its functions is the induction of known decidual regulator Bmp2. Bmp2 is a member of the BMP family of morphogens. Its expression is observed in the uterine stroma near the site of attachment and persists through the early phases of decidualization [Paria et al., 2001; Ying and Zhao 2000]. BMP2 expression was shown to be lost in the stroma of COUP-TFIId/d uteri. Furthermore, Bmp2 was demonstrated to be downstream from the Ihh-COUP-TFII signaling axis functionally and genetically. When recombinant BMP2 was administered at the time of the application of the decidual stimulus, a marked rescue of the induction of uterine weight gain and differentiation, as indicated by alkaline phosphatase staining, could be observed in the stimulated uterine horns of COUP-TFII conditional mutants [Kurihara et al., 2007]. Bmp2 is yet another gene in which global ablation results in embryonic lethality and thus investigation of its functions in the adult uterus were executed using the PR-Cre mouse model [Lee et al., 2007; Zhang and Bradley 1996]. Female Bmp2d/d mice were infertile, however, unlike conditional Ihh or COUP-TFII mutants, blastocysts were able to attach to the luminal epithelium. The block in pregnancy progression was shown to occur due to a failure in implantation and subsequent decidualization when induced by either blastocysts or artificial stimulus [Lee et al., 2007]. Moreover, the expression of the Fkbp family of chaperones, including Fkbp-3, -4, and -5, was significantly reduced in Bmp2d/d females, indicating that Bmp2 may play an important role in maintaining P4 action during the postimplantion period by mediating a feed-forward loop of progesterone signaling. In addition to the critical role of Bmp2 in murine decidualization, it has been shown to play an important role in the in vitro decidualization of human endometrial stromal cells. The use of siRNA to attenuate Bmp2 expression prevents decidualization while the addition of recombinant BMP2 to the hormonal decidualization cocktail enhances it [Li et al., 2007].
3.1.3 Wnt Signaling
In investigating the mechanism by which Bmp2 regulates decidualization, microarray analysis revealed that the induction of Wnt ligands Wnt4 and Wnt6 was severely attenuated in the decidual-stimulated uterine horns of Bmp2d/d mice. WNT proteins are a highly conserved family of secreted glycoproteins that are critical for multiple processes including cell-cell communication, cell growth, and differentiation (reviewed in [Wodarz and Nusse 1998]). WNT signaling has been implicated in both uterine postnatal differentiation and the regulation of embryo implantation [Jeong et al., 2009; Li et al., 2005; Mohamed et al., 2005; Xie et al., 2008]. Ablation of Wnt7a or Wnt5a as well as conditional ablation of β-catenin results in altered postnatal uterine development while mice with an ablation of Wnt4 fail to develop the Mullerian duct, the precursor structure to the uterus [Jeong et al., 2009; Mericskay et al., 2004; Miller and Sassoon 1998]. Additionally, Wnt4-/- mice exhibit perinatal lethality due to kidney failure [Stark et al., 1994]. Wnt4 expression in the adult uterus is under the regulation of E2 and occurs at specific times throughout pregnancy. Although its expression can be weakly detected in the luminal epithelium at day 0.5 of pregnancy, its primary expression appears to be in the uterine stroma at day 4.5, persisting into the decidua [Daikoku et al., 2004; Hayashi et al., 2009; Hou et al., 2004; Katayama et al., 2006]. In addition to a potential role of Wnt4 in the regulation of murine pregnancy, it has been implicated in human decidualization as the use of siRNA to attenuate its expression was shown to prevent in vitro decidualization [Li et al., 2007].
Recently, Wnt4 was conditionally ablated using the PR-Cre mouse model and these Wnt4d/d females were severely subfertile and exhibited defects in postnatal uterine development [Boyer et al., 2010; Franco et al., 2010]. Upon conditional ablation of Wnt4, a reduction in the number of uterine glands as well as the focal accumulation of a layer of cells around the luminal epithelium expressing the basal cell marker p63 was observed [Koster and Roop 2007]. Furthermore, the observed focal stratification was exacerbated by prolonged exposure to E2, eventually developing to squamous cell metaplasia in a processes similar to what has been observed in humans who have been exposed in utero to the synthetic estrogen diethylstilbestrol (DES) [Goldberg and Falcone 1999]. The defects in fertility could possibly have been attributed to the lack of uterine glands in this model since their secretion of leukemia inhibitory factor (LIF) is critical for implantation and decidualization [Jeong et al., 2010; Stewart et al., 1992]. However, unlike other models in which LIF deficiency is a driver of infertility, the administration of recombinant LIF was unable to rescue the artificial decidualization response, indicating that uterine defects are the primary source of infertility in these mice [Chen et al., 2000; Jeong et al., 2010].
Although the uteri of Wnt4d/d female mice are able to support normal attachment, many blastocysts failed to invade through the luminal epithelium and induce a decidual response. Like Bmp2d/d conditional mutant females, Wnt4d/d females show a failure to induce uterine weight gain and differentiation following artificial decidualization; however, there is not a defect in decidual proliferation in these mice. In this case, the observed decidualization defects were attributed to mislocalization of FOXO1, resulting in elevated levels of apoptosis. When FOXO1 is found in the nucleus, it promotes the transcription of many proapoptotic genes [Huang and Tindall 2007]. Under the influence of P4, FOXO1 is normally shuttled to the cytoplasm, silencing expression of its target genes [Brosens and Gellersen 2006; Labied et al., 2006]. Wnt4 regulation of decidualization as well as FOXO1 localization is due in part to reduced expression of Fkbp4. Thus, Wnt4 is an important regulator of decidualization by mediating P4 actions to promote both stromal cell differentiation and survival.
3.1.4 P4 Regulation of Erbb Ligands and Subsequent Decidualization
It is well established that the uterus is a steroid hormone responsive organ and that crosstalk between growth factor and steroid hormone signaling occurs [Ignar-Trowbridge et al., 1992]. The Erbb family of growth factors and receptors is one such signaling family that has been increasingly implicated in female fertility [Lim et al., 1998]. Hb-EGF and Areg are two of the ligands that act on this pathway and both are regulated by P4 while Hb-EGF is also regulated by E2 [Das et al., 1995; Wang et al., 1994]. Uterine Hb-EGF expression is induced by blastocysts and can be found in the luminal epithelium just prior to attachment. Its expression can subsequently be observed in the proliferating stromal cells during the postimplantation period and persists through to the secondary decidual zone [Das et al., 1994]. When Hb-EGF was ablated either globally or conditionally with the PR-Cre, female mice were found to have reduced litter sizes with an inability to support implantation [Xie et al., 2007]. Mechanistically, Hb-EGF has been shown to regulate decidualization by upregulating the Cyclin D3-mediated polyploidization of uterine stromal cells [Das et al., 1999; Tan et al., 2002; Tan et al., 2004]. Interestingly, Areg expression was elevated in mice lacking Hb-EGF, suggesting that the partial uterine phenotype observed in these mice may be due to compensation by other Erbb ligands [Xie et al., 2007].
Areg expression is found transiently in the luminal epithelial cells at the time of implantation [Das et al., 1995]. However, Areg-/- female mice did not exhibit defects in fertility, further supporting the notion that there may be redundancy in the ligands [Luetteke et al., 1999]. Furthermore, mice null for Erbb ligands EGF or epiregulin also did not display fertility defects, indicating that while ablation of Hb-EGF renders female mice subfertile, ablation of no single gene is sufficient to cause complete infertility [Lee et al., 2004; Luetteke et al., 1999]. The potential for redundancy amongst the Erbb ligands suggest that perhaps the receptors may be the rate-limiting factor.
Erbb signaling requires ligand-dependent dimerization of the receptors (Erbb1-4) as either homo- or heterodimers, with different ligands exhibiting preferential binding of particular receptors [Ullrich and Schlessinger 1990]. It's known that HB-EGF exerts its effects by binding to ERBB1 (EGFR) and ERBB4. Egfr expression is observed primarily in the subepithelial stroma while Erbb4 expression is found primarily in the submyometrial stroma and myometrial connective tissues, suggesting that Egfr might be the predominant receptor of Hb-EGF mediated implantation and decidualization [Lim et al., 1998]. Further support for a potential role of Egfr in female fertility exists in that P4 has been shown to maintain EGFR expression during pregnancy and promote growth of the decidua [Dai and Ogle 1999; Ogle et al., 1999]. Notably, EGFR expression in the subepithelial stroma was reported to be reduced in Ihhd/d females [Lee et al., 2006]. While compelling data suggests Egfr likely plays an important role in female reproduction, bona fide in vivo evidence of a critical function is currently lacking due to the observed embryonic or perinatal lethality observed in Egfr-/- mice, depending on genetic background [Threadgill et al., 1995].
3.2 Progesterone Regulation of Signaling Modulators
Separate from the ability of P4 to induce ligands and their signaling pathways, multiple reports demonstrate that P4 can induce the expression of genes such as Mig6, Cyclooxygenase 2 (Cox2) and many cell cycle regulatory proteins which can all influence active signaling paradigms. ERBB receptor feedback inhibitor 1 (Errfi1, Mig-6) is a an adaptor protein containing multiple protein interaction domains including a Cdc42/Rac interactive binding (CRIB) domain, Src homology 3 (SH3) binding domain, and a 14-3-3 binding domain [Burbelo et al., 1995; Makkinje et al., 2000; Pirone et al., 2001]. It can be induced by various mitogens and can act as a negative feedback inhibitor of EGFR signaling [Anastasi et al., 2003; Fiorentino et al., 2000; Fiorini et al., 2002; Hackel et al., 2001]. Mig6 was identified as a P4 responsive gene using microarray analysis [Jeong et al., 2005]. Its expression was later shown to also be induced by E2 alone and synergistically by cotreatment of E2 and P4. In the preimplantation mouse uterus, Mig6 expression is detected in both the epithelium and stroma beginning at 2.5 days post coitum (dpc) [Kim et al., 2010]. Interestingly, Mig6 is involved in regulatory loop that feeds back to regulate P4 signaling. Mig6 null mice exhibit an inability of P4 to inhibit E2-induced uterine weight gain and target gene expression [Jeong et al., 2009]. Furthermore, ovariectomized Mig6-/- mice develop endometrial hyperplasia, which when challenged with exogenous E2, developed into invasive endometrioid-type endometrial adenocarcinoma. When Mig6 was conditionally ablated with the PR-Cre, E2 hypersensitivity similar to that which was observed in COUP-TFIId/d mice resulted in a block in implantation, rendering these mice infertile. The results from these studies demonstrate that Mig6 modulates the ability of P4 signaling to regulate ER activity.
3.3 Progesterone Mediation of Implantation via Prostaglandin Regulation
Following blastocyst attachment and throughout implantation there is an increase in endometrial stromal vascular permeability and this process is known to be mediated by prostaglandins (PG) [Chakraborty et al., 1996]. Prostaglandin-endoperoxide synthase 1 and -2 (Ptgs-1, Cox1; Ptgs-2, Cox2) are two enzymes that mediate the synthesis of PGs, and PG receptors have been shown to be induced by P4 [Lim et al., 1999]. While Cox1 is a constitutive enzyme and thought to play a basic housekeeping role, Cox2 is inducible and is found in the luminal epithelium and in underlying stromal cells at the site of blastocyst attachment [Lim et al., 1997]. Consistent with the expression pattern of these two enzymes, Cox1-/- females are fertile. Cox2-/- females are infertile with defects in ovulation, implantation and decidualization [Lim et al., 1997]. Although, in a background dependent fashion, Cox1 is able to compensate for Cox2 expression, improving fertility [Wang et al., 2004]. Furthermore, the use of selective inhibitors of either COX1 or COX2 separately had no significant effect on blastocyst implantation, but a combination of both inhibitors in lower doses resulted in a delay and impairment in implantation, ultimately leading to significant pregnancy loss. Administration of higher doses of inhibitors further reduced PG production and completely prevented implantation [Pakrasi and Jain 2007]. Lastly, it's been shown that uterine angiogenesis during decidualization is severely compromised in Cox2-/- mice, and that this is due to a loss of coordination and regulation of vascular endothelial growth factor (VEGF) signaling in the absence of COX2 derived PGs [Douglas et al., 2009; Matsumoto et al., 2002]. These results demonstrate the critical role of both angiogenesis and prostaglandin production during implantation.
3.4 Transcription Factors Regulated by P4
The final class of molecules highlighted in this review enlisted by P4 to execute the pregnancy paradigm is transcription factors. The ultimate biological aim of the uterus is the support and nourishment of the embryo, a process that requires the production of many different proteins. A few prominent examples of transcription factors that mediate this process that have not been elaborated upon here previously include Hoxa10, C/EBPβ and Hand2.
3.4.1 Hoxa10
Homebox (Hox) genes are a multigene family of developmentally regulated transcription factors that are well known to display distinct spatial and temporal patterns of expression [Krumlauf 1994]. Homebox A10 (Hoxa10), a member of the AbdB-like Hoxa gene family, is expressed in the uterine luminal and glandular epithelium from days 0.5 to 2.5, after which it shifts to the subepithelial stroma, persisting robustly in the stroma through decidualization to day 7.5 [Satokata et al., 1995]. This expression pattern along with the observation that Hoxa10 is induced by P4 implicated it might be involved in adult uterine function [Ma et al., 1998].
Indeed, Hoxa10-/- females were found to be subfertile with defects in attachment and decidualization. In investigating the cause of decreased fertility it was determined that most of the embryos found at the time of implantation were either degenerating or delayed in development. Approximately half of these embryos were unattached and those that did attach resulted in abnormal implantation sites due to defects in decidualization and stromal-cell proliferation [Benson et al., 1996; Lim et al., 1999; Satokata et al., 1995]. Furthermore, it was determined that a subset of P4 regulated genes, including Cox2 and prostaglandin receptors, were deregulated, indicating that Hoxa10 is required for successful implantation as a partial mediator of P4 signaling [Lim et al., 1999].
3.4.2 CCAAT/Enhancer Binding Protein β (C/EBPβ)
CCAAT/enhancer binding protein β is a member of the basic leucine zipper (bZIP) family of transcription factors that regulate diverse processes such as cell proliferation, differentiation and apoptosis [McKnight 2001; Ramji and Foka 2002; Wedel and Ziegler-Heitbrock 1995]. C/EBPβ is regulated by both ER and PR and mediates the actions of E2 and P4 during the periimplantation period. The expression of C/EBPβ is detected in the stroma on day 4.5 and remains robustly expressed in the decidua until day 9.0 [Bagchi et al., 2006; Mantena et al., 2006]. C/EBPβ-/- females are infertile with defects originating in both ovarian and uterine functions [Sterneck et al., 1997]. Uterine deficiencies were attributed to a failure in decidualization including impairments in proliferation and differentiation of the uterine stromal cells. Furthermore, C/EBPβ null mice displayed a reduction in E2-induced epithelial cell proliferation [Bagchi et al., 2006]. Recent work elucidating the mechanism by which C/EBPβ induces stromal cell proliferation indicates that the transcription factor plays a key role in the regulation of the G2 to M cell cycle checkpoint. It was shown that C/EBPβ binds directly to the promoter of cyclin B2 approximately 1kb upstream of the transcriptional start site and induces its expression in the presence of deciduogenic stimuli. Although C/EBPβ-/- uterine stromal cells are able to progress through the S phase and replicate their DNA, reduced expression of proliferative molecules cyclin B2 and cdc25C accompanied by elevated expression of p53 and cell cycle inhibitors p21 and p27 resulted in a block at the G2 to M transition [Wang et al., 2010]. Altogether, these results demonstrate that C/EBPβ is a key mediator of stromal cell proliferation and thus decidualization by regulating the expression of multiple cell cycle regulatory proteins.
3.4.3 P4 Inhibition of Epithelial Proliferation Mediated by Hand2
Several of the mouse models discussed here including those involving Ihh, COUP-TFII and Mig6 demonstrate that the attenuation of E2 activity in the uterine luminal epithelium by P4 is an absolute requirement for successful implantation. Although the physiological necessity of this event is well understood, the molecular mechanism by which it occurs has been unclear. Key findings of recent work illuminate a molecular pathway in which Hand2 conveys the antiproliferative effects of P4. Hand2 is a basic helix-loop-helix transcription factor known to play a critical role during cardiac morphogenesis (reviewed in [Srivastava 1999]). It was identified as a potential PR regulated gene by a microarray experiment in which RU-486 was administered to periimplantation mice to identify P4 pathways mediating implantation [Bagchi et al., 2005]. This notion was directly confirmed by the observation that treatment of mice with exogenous P4 stimulated robust induction of Hand2 in the uterine stroma. Conditional ablation of Hand2 using the PR-Cre resulted in infertility due to a failure in blastocyst attachment resulting from elevated epithelial E2 activity [Li et al., 2011]. Following gene expression profiling studies using stromal cells isolated from day 4 pregnant mice, it was determined that Hand2 functions to suppress stromal expression of multiple members of the fibroblast growth factor (FGF) family. It was then shown that stromal-derived FGFs act in a paracrine fashion, stimulating their receptors in the luminal epithelium and thus resulting in an ERK 1/2 mediated activation of ERα via phosphorylation [Li et al., 2011].
This work provides clear molecular details regarding the mediation of the antiproliferative effects of P4. However, it would be interesting to determine how other mouse models with similar phenotypes fit into this pathway. Given that the induction of COUP-TFII in the uterine stroma by P4 mediated expression of epithelial Ihh is also critical for the inhibition of epithelial E2 activity, one might speculate that these molecules are intertwined in some fashion with Hand2. Also, microarray data examining Ihhd/d uteri show reduced expression of Hand2 [Franco et al., 2010]. Furthermore, Mig6 is not only induced by P4 and expressed in both the epithelium and stromal compartments, but it has been shown to inhibit signaling pathways that activate ERK 1/2 [Fiorentino et al., 2000; Fiorini et al., 2002]. The potential for interactions and regulatory networks between these proteins warrants further investigation.
4. Conclusion and Future Challenges
Progesterone has long been considered the hormone of pregnancy [Allen 1970]. Advancements in technology and the use of mouse models have extensively enhanced our understanding of the molecular events regulating early pregnancy. The progesterone receptor exerts pleiotropic effects by regulating multiple types of proteins including ligands, receptors, chaperones, signaling proteins and transcription factors (Figure 1). Understanding these processes is not only vital to advancements in reproductive physiology but also in pathology, for these same signaling pathways go awry in diseases like endometriosis and endometrial cancer.
Figure 1.
Schematic highlighting the complex network of genes enlisted by PR during periimplantion uterine biology.
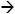 Indicates activation or induction. ┤indicates inhibition or repression. Dashed arrows represent potential induction lacking bona fide evidence.
Indicates activation or induction. ┤indicates inhibition or repression. Dashed arrows represent potential induction lacking bona fide evidence.
However, with an accelerating influx of data, an equal number of questions to be addressed arise. The eminent importance of the paracrine signaling and epithelial-stromal cross talk in the regulation of early pregnancy is made obvious by observations in many of the mouse models listed within this review. Although the use of the PR-Cre mouse model has been tremendously successful in the creation of conditional knockouts and advancements in our understanding, there is an imploring necessity for the generation of uterine compartment-specific mouse models expressing Cre-recombinase. Several models expressing Cre under the regulation of promoters specific to particular uterine compartments have recently been developed (Wnt7a and Sprr2f- epithelium; Amhr2- mesenchyme; Myh11- myometrium); however, depending on the functions of the gene of interest, the presence of Cre-recombinase activity in extrauterine tissues in these models has the potential to preclude effective uterine studies [Contreras et al., 2010; Jamin et al., 2002; Regan et al., 2000; Winuthayanon et al., 2010]. The advent of Cre models expressed solely in the uterus exhibiting compartment specificity would allow researchers to investigate important questions concerning proteins expressed in multiple compartments.
Additionally, it's well established that PR regulates a plethora of proteins, but it's likely there are many novel PR targets that regulate important functions that have yet to be identified. The use of chromatin immunoprecipitation coupled with DNA sequencing (ChIP-seq) will help identify PR target genes and the different hormonal conditions that drive PR recruitment to the promoters of each of these genes. The use of proteomics is needed to advance our understanding of what proteins PR is interacting with and thus driving its specificity and activity. A systems approach combining gene expression profiling by microarrays, transcription factor binding sites by ChIP-seq, and protein interactions using proteomics would be immensely valuable in the advancement of our understanding of PR target gene regulation. Lastly, the use of biomarkers to identify disease is prevalent in many other tissues. The identification of biomarkers indicative of infertility and malignant disease would be exceedingly beneficial in the diagnosis and treatment of these incapacitating conditions. The use of primary stromal cell culture has been the predominant method of interrogating gene function in human biology. While several techniques have begun to be utilized, including 3D culture of human endometrium and renal capsule xenograft experiments, the use of these systems has been sparing thus far. Hence, there remains a pressing demand for the development of tools that facilitate translation of findings from mice to humans.
Highlights.
In this review we discuss mediators and effectors of progesterone signaling
We collate data and insights gained from many mouse models
We discuss progesterone regulation of proliferation and differentiation
We examine how progesterone regulates implantation and uterine decidualization
Acknowledgments
The authors are supported by NIH grant RO1 HD042311 (to F.J.D) and the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54 HD07495, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michael J. Large, Email: large@bcm.edu.
Francesco J. DeMayo, Email: fdemayo@bcm.edu.
References
- 1.Allen WM. Progesterone: how did the name originate? South Med J. 1970;63:1151–5. doi: 10.1097/00007611-197010000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Anastasi S, Fiorentino L, Fiorini M, Fraioli R, Sala G, Castellani L, Alema S, Alimandi M, Segatto O. Feedback inhibition by RALT controls signal output by the ErbB network. Oncogene. 2003;22:4221–34. doi: 10.1038/sj.onc.1206516. [DOI] [PubMed] [Google Scholar]
- 3.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 4.Bagchi IC, Li Q, Cheon YP, Mantena SR, Kannan A, Bagchi MK. Use of the progesterone receptor antagonist RU 486 to identify novel progesterone receptor-regulated pathways in implantation. Semin Reprod Med. 2005;23:38–45. doi: 10.1055/s-2005-864032. [DOI] [PubMed] [Google Scholar]
- 5.Bagchi MK, Mantena SR, Kannan A, Bagchi IC. Control of uterine cell proliferation and differentiation by C/EBPbeta: functional implications for establishment of early pregnancy. Cell Cycle. 2006;5:922–5. doi: 10.4161/cc.5.9.2712. [DOI] [PubMed] [Google Scholar]
- 6.Balleine RL, Earls PJ, Webster LR, Mote PA, deFazio A, Harnett PR, Clarke CL. Expression of progesterone receptor A and B isoforms in low-grade endometrial stromal sarcoma. Int J Gynecol Pathol. 2004;23:138–44. doi: 10.1097/00004347-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Baulieu EE. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989;245:1351–7. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- 8.Baulieu EE. The steroid hormone antagonist RU486. Mechanism at the cellular level and clinical applications. Endocrinol Metab Clin North Am. 1991;20:873–91. [PubMed] [Google Scholar]
- 9.Benson GV, Lim H, Paria BC, Satokata I, Dey SK, Maas RL. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–96. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 10.Boyer A, Lapointe E, Zheng X, Cowan RG, Li H, Quirk SM, DeMayo FJ, Richards JS, Boerboom D. WNT4 is required for normal ovarian follicle development and female fertility. Faseb J. 2010;24:3010–25. doi: 10.1096/fj.09-145789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braga VM, Gendler SJ. Modulation of Muc-1 mucin expression in the mouse uterus during the estrus cycle, early pregnancy and placentation. J Cell Sci. 1993;105(Pt 2):397–405. doi: 10.1242/jcs.105.2.397. [DOI] [PubMed] [Google Scholar]
- 12.Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809–20. doi: 10.1210/endo.140.10.7070. [DOI] [PubMed] [Google Scholar]
- 13.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187:1416–23. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 14.Brosens JJ, Gellersen B. Death or survival--progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36:389–98. doi: 10.1677/jme.1.02060. [DOI] [PubMed] [Google Scholar]
- 15.Burbelo PD, Drechsel D, Hall A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J Biol Chem. 1995;270:29071–4. doi: 10.1074/jbc.270.49.29071. [DOI] [PubMed] [Google Scholar]
- 16.Carranza-Lira S, Blanquet J, Tserotas K, Calzada L. Endometrial progesterone and estradiol receptors in patients with recurrent early pregnancy loss of unknown etiology--preliminary report. Med Sci Monit. 2000;6:759–62. [PubMed] [Google Scholar]
- 17.Chakraborty I, Das SK, Wang J, Dey SK. Developmental expression of the cyclo-oxygenase-1 and cyclo-oxygenase-2 genes in the peri-implantation mouse uterus and their differential regulation by the blastocyst and ovarian steroids. J Mol Endocrinol. 1996;16:107–22. doi: 10.1677/jme.0.0160107. [DOI] [PubMed] [Google Scholar]
- 18.Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141:4365–72. doi: 10.1210/endo.141.12.7855. [DOI] [PubMed] [Google Scholar]
- 19.Cheon YP, Li Q, Xu X, DeMayo FJ, Bagchi IC, Bagchi MK. A genomic approach to identify novel progesterone receptor regulated pathways in the uterus during implantation. Mol Endocrinol. 2002;16:2853–71. doi: 10.1210/me.2002-0270. [DOI] [PubMed] [Google Scholar]
- 20.Chopra AR, Louet JF, Saha P, An J, Demayo F, Xu J, York B, Karpen S, Finegold M, Moore D, Chan L, Newgard CB, O'Malley BW. Absence of the SRC-2 coactivator results in a glycogenopathy resembling Von Gierke's disease. Science. 2008;322:1395–9. doi: 10.1126/science.1164847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conneely OM, Lydon JP. Progesterone receptors in reproduction: functional impact of the A and B isoforms. Steroids. 2000;65:571–7. doi: 10.1016/s0039-128x(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 22.Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179:97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 23.Conneely OM, Mulac-Jericevic B, Lydon JP. Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids. 2003;68:771–8. doi: 10.1016/s0039-128x(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 24.Contreras CM, Akbay EA, Gallardo TD, Haynie JM, Sharma S, Tagao O, Bardeesy N, Takahashi M, Settleman J, Wong KK, Castrillon DH. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis Model Mech. 2010;3:181–93. doi: 10.1242/dmm.004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis Hewitt S, Goulding EH, Eddy EM, Korach KS. Studies using the estrogen receptor alpha knockout uterus demonstrate that implantation but not decidualization-associated signaling is estrogen dependent. Biol Reprod. 2002;67:1268–77. doi: 10.1095/biolreprod67.4.1268. [DOI] [PubMed] [Google Scholar]
- 26.Curtis SW, Clark J, Myers P, Korach KS. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor alpha knockout mouse uterus. Proc Natl Acad Sci U S A. 1999;96:3646–51. doi: 10.1073/pnas.96.7.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai D, Ogle TF. Progesterone regulation of epidermal growth factor receptor in rat decidua basalis during pregnancy. Biol Reprod. 1999;61:326–32. doi: 10.1095/biolreprod61.1.326. [DOI] [PubMed] [Google Scholar]
- 28.Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, Dey SK. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol. 2004;18:1238–50. doi: 10.1210/me.2003-0403. [DOI] [PubMed] [Google Scholar]
- 29.Daly DC, Maslar IA, Riddick DH. Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol. 1983;145:672–8. doi: 10.1016/0002-9378(83)90572-0. [DOI] [PubMed] [Google Scholar]
- 30.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120:1071–83. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 31.Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705. doi: 10.1210/mend.9.6.8592515. [DOI] [PubMed] [Google Scholar]
- 32.Das SK, Lim H, Paria BC, Dey SK. Cyclin D3 in the mouse uterus is associated with the decidualization process during early pregnancy. J Mol Endocrinol. 1999;22:91–101. doi: 10.1677/jme.0.0220091. [DOI] [PubMed] [Google Scholar]
- 33.DeMayo FJ, Zhao B, Takamoto N, Tsai SY. Mechanisms of action of estrogen and progesterone. Ann N Y Acad Sci. 2002;955:48–59. doi: 10.1111/j.1749-6632.2002.tb02765.x. discussion 86-8, 396-406. [DOI] [PubMed] [Google Scholar]
- 34.Douglas NC, Tang H, Gomez R, Pytowski B, Hicklin DJ, Sauer CM, Kitajewski J, Sauer MV, Zimmermann RC. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology. 2009;150:3845–54. doi: 10.1210/en.2008-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Driggers PH, Segars JH. Estrogen action and cytoplasmic signaling pathways. Part II: the role of growth factors and phosphorylation in estrogen signaling. Trends Endocrinol Metab. 2002;13:422–7. doi: 10.1016/s1043-2760(02)00634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finn CA. Why do women and some other primates menstruate? Perspect Biol Med. 1987;30:566–74. doi: 10.1353/pbm.1987.0007. [DOI] [PubMed] [Google Scholar]
- 37.Finn CA. Menstruation: a nonadaptive consequence of uterine evolution. Q Rev Biol. 1998;73:163–73. doi: 10.1086/420183. [DOI] [PubMed] [Google Scholar]
- 38.Fiorentino L, Pertica C, Fiorini M, Talora C, Crescenzi M, Castellani L, Alema S, Benedetti P, Segatto O. Inhibition of ErbB-2 mitogenic and transforming activity by RALT, a mitogen-induced signal transducer which binds to the ErbB-2 kinase domain. Mol Cell Biol. 2000;20:7735–50. doi: 10.1128/mcb.20.20.7735-7750.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiorini M, Ballaro C, Sala G, Falcone G, Alema S, Segatto O. Expression of RALT, a feedback inhibitor of ErbB receptors, is subjected to an integrated transcriptional and post-translational control. Oncogene. 2002;21:6530–9. doi: 10.1038/sj.onc.1205823. [DOI] [PubMed] [Google Scholar]
- 40.Franco HL, Jeong JW, Tsai SY, Lydon JP, DeMayo FJ. In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin Cell Dev Biol. 2008;19:178–86. doi: 10.1016/j.semcdb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MK, Demayo FJ. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. Faseb J. 2010 doi: 10.1096/fj.10-175349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Franco HL, Lee KY, Broaddus RR, White LD, Lanske B, Lydon JP, Jeong JW, DeMayo FJ. Ablation of Indian hedgehog in the murine uterus results in decreased cell cycle progression, aberrant epidermal growth factor signaling, and increased estrogen signaling. Biol Reprod. 2010;82:783–90. doi: 10.1095/biolreprod.109.080259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J, Mazella J, Tang M, Tseng L. Ligand-activated progesterone receptor isoform hPR-A is a stronger transactivator than hPR-B for the expression of IGFBP-1 (insulin-like growth factor binding protein-1) in human endometrial stromal cells. Mol Endocrinol. 2000;14:1954–61. doi: 10.1210/mend.14.12.0564. [DOI] [PubMed] [Google Scholar]
- 44.Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC- 1 and p/CIP. Mol Cell Biol. 2002;22:5923–37. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–53. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg JM, Falcone T. Effect of diethylstilbestrol on reproductive function. Fertil Steril. 1999;72:1–7. doi: 10.1016/s0015-0282(99)00153-3. [DOI] [PubMed] [Google Scholar]
- 47.Hackel PO, Gishizky M, Ullrich A. Mig-6 is a negative regulator of the epidermal growth factor receptor signal. Biol Chem. 2001;382:1649–62. doi: 10.1515/BC.2001.200. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi K, Erikson DW, Tilford SA, Bany BM, Maclean JA, 2nd, Rucker EB, 3rd, Johnson GA, Spencer TE. Wnt genes in the mouse uterus: potential regulation of implantation. Biol Reprod. 2009;80:989–1000. doi: 10.1095/biolreprod.108.075416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17:2070–83. doi: 10.1210/me.2003-0146. [DOI] [PubMed] [Google Scholar]
- 50.Hofman K, Swinnen JV, Verhoeven G, Heyns W. Coactivation of an endogenous progesterone receptor by TIF2 in COS-7 cells. Biochem Biophys Res Commun. 2002;295:469–74. doi: 10.1016/s0006-291x(02)00698-8. [DOI] [PubMed] [Google Scholar]
- 51.Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18:3035–49. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 53.Ignar-Trowbridge DM, Nelson KG, Bidwell MC, Curtis SW, Washburn TF, McLachlan JA, Korach KS. Coupling of dual signaling pathways: epidermal growth factor action involves the estrogen receptor. Proc Natl Acad Sci U S A. 1992;89:4658–62. doi: 10.1073/pnas.89.10.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB. Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril. 1989;52:761–8. doi: 10.1016/s0015-0282(16)61028-2. [DOI] [PubMed] [Google Scholar]
- 55.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet. 2002;32:408–10. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 56.Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, DeMayo FJ. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005;146:3490–505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- 57.Jeong JW, Lee KY, Lydon JP, DeMayo FJ. Steroid hormone regulation of Clca3 expression in the murine uterus. J Endocrinol. 2006;189:473–84. doi: 10.1677/joe.1.06747. [DOI] [PubMed] [Google Scholar]
- 58.Jeong JW, Lee KY, Han SJ, Aronow BJ, Lydon JP, O'Malley BW, DeMayo FJ. The p160 steroid receptor coactivator 2, SRC-2, regulates murine endometrial function and regulates progesterone-independent and -dependent gene expression. Endocrinology. 2007;148:4238–50. doi: 10.1210/en.2007-0122. [DOI] [PubMed] [Google Scholar]
- 59.Jeong JW, Lee HS, Franco HL, Broaddus RR, Taketo MM, Tsai SY, Lydon JP, DeMayo FJ. beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene. 2009;28:31–40. doi: 10.1038/onc.2008.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW, Vande Woude GF, Giudice LC, Young SL, Lessey BA, Tsai SY, Lydon JP, DeMayo FJ. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci U S A. 2009;106:8677–82. doi: 10.1073/pnas.0903632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeong JW, Kwak I, Lee KY, Kim TH, Large MJ, Stewart CL, Kaestner KH, Lydon JP, DeMayo FJ. Foxa2 is essential for mouse endometrial gland development and fertility. Biol Reprod. 2010;83:396–403. doi: 10.1095/biolreprod.109.083154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. Embo J. 1990;9:1603–14. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Katayama S, Ashizawa K, Fukuhara T, Hiroyasu M, Tsuzuki Y, Tatemoto H, Nakada T, Nagai K. Differential expression patterns of Wnt and beta-catenin/TCF target genes in the uterus of immature female rats exposed to 17alpha-ethynyl estradiol. Toxicol Sci. 2006;91:419–30. doi: 10.1093/toxsci/kfj167. [DOI] [PubMed] [Google Scholar]
- 64.Kim TH, Lee DK, Franco HL, Lydon JP, Jeong JW. ERBB receptor feedback inhibitor 1 regulation of estrogen receptor activity is critical for uterine implantation in mice. Biol Reprod. 2010;82:706–13. doi: 10.1095/biolreprod.109.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koshiyama M, Konishi I, Nanbu K, Nanbu Y, Mandai M, Komatsu T, Yamamoto S, Mori T, Fujii S. Immunohistochemical localization of heat shock proteins HSP70 and HSP90 in the human endometrium: correlation with sex steroid receptors and Ki-67 antigen expression. J Clin Endocrinol Metab. 1995;80:1106–12. doi: 10.1210/jcem.80.4.7714077. [DOI] [PubMed] [Google Scholar]
- 66.Koster MI, Roop DR. Mechanisms regulating epithelial stratification. Annu Rev Cell Dev Biol. 2007;23:93–113. doi: 10.1146/annurev.cellbio.23.090506.123357. [DOI] [PubMed] [Google Scholar]
- 67.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–82. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krumlauf R. Hox genes in vertebrate development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 69.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurita T, Lee KJ, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–30. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- 71.Labied S, Kajihara T, Madureira PA, Fusi L, Jones MC, Higham JM, Varshochi R, Francis JM, Zoumpoulidou G, Essafi A, Fernandez de Mattos S, Lam EW, Brosens JJ. Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol Endocrinol. 2006;20:35–44. doi: 10.1210/me.2005-0275. [DOI] [PubMed] [Google Scholar]
- 72.Lagow E, DeSouza MM, Carson DD. Mammalian reproductive tract mucins. Hum Reprod Update. 1999;5:280–92. doi: 10.1093/humupd/5.4.280. [DOI] [PubMed] [Google Scholar]
- 73.Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW. Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol Cell Biol. 2004;24:8907–16. doi: 10.1128/MCB.24.20.8907-8916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee DK, Kurihara I, Jeong JW, Lydon JP, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of ERalpha activity by COUP-TFII is essential for successful implantation and decidualization. Mol Endocrinol. 2010;24:930–40. doi: 10.1210/me.2009-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, Toftgard R, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–9. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 76.Lee K, Jeong J, Tsai MJ, Tsai S, Lydon JP, DeMayo FJ. Molecular mechanisms involved in progesterone receptor regulation of uterine function. J Steroid Biochem Mol Biol. 2006;102:41–50. doi: 10.1016/j.jsbmb.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee KY, DeMayo FJ. Animal models of implantation. Reproduction. 2004;128:679–95. doi: 10.1530/rep.1.00340. [DOI] [PubMed] [Google Scholar]
- 78.Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–78. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J, Zhang JV, Cao YJ, Zhou JX, Liu WM, Fan XJ, Duan EK. Inhibition of the beta-catenin signaling pathway in blastocyst and uterus during the window of implantation in mice. Biol Reprod. 2005;72:700–6. doi: 10.1095/biolreprod.104.033837. [DOI] [PubMed] [Google Scholar]
- 80.Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282:31725–32. doi: 10.1074/jbc.M704723200. [DOI] [PubMed] [Google Scholar]
- 81.Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–6. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li X, O'Malley BW. Unfolding the action of progesterone receptors. J Biol Chem. 2003;278:39261–4. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- 83.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 84.Lim H, Das SK, Dey SK. erbB genes in the mouse uterus: cell-specific signaling by epidermal growth factor (EGF) family of growth factors during implantation. Dev Biol. 1998;204:97–110. doi: 10.1006/dbio.1998.9072. [DOI] [PubMed] [Google Scholar]
- 85.Lim H, Ma L, Ma WG, Maas RL, Dey SK. Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol. 1999;13:1005–17. doi: 10.1210/mend.13.6.0284. [DOI] [PubMed] [Google Scholar]
- 86.Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–50. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- 87.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O'Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–78. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 88.Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Dev Biol. 1998;197:141–54. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- 89.Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci U S A. 2003;100:2963–8. doi: 10.1073/pnas.0530162100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Makkinje A, Quinn DA, Chen A, Cadilla CL, Force T, Bonventre JV, Kyriakis JM. Gene 33/Mig-6, a transcriptionally inducible adapter protein that binds GTP-Cdc42 and activates SAPK/JNK. A potential marker transcript for chronic pathologic conditions, such as diabetic nephropathy. Possible role in the response to persistent stress. J Biol Chem. 2000;275:17838–47. doi: 10.1074/jbc.M909735199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mantena SR, Kannan A, Cheon YP, Li Q, Johnson PF, Bagchi IC, Bagchi MK. C/EBPbeta is a critical mediator of steroid hormone-regulated cell proliferation and differentiation in the uterine epithelium and stroma. Proc Natl Acad Sci U S A. 2006;103:1870–5. doi: 10.1073/pnas.0507261103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsumoto H, Ma WG, Daikoku T, Zhao X, Paria BC, Das SK, Trzaskos JM, Dey SK. Cyclooxygenase-2 differentially directs uterine angiogenesis during implantation in mice. J Biol Chem. 2002;277:29260–7. doi: 10.1074/jbc.M203996200. [DOI] [PubMed] [Google Scholar]
- 93.Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol. 2002;245:280–90. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- 94.McKnight SL. McBindall--a better name for CCAAT/enhancer binding proteins? Cell. 2001;107:259–61. doi: 10.1016/s0092-8674(01)00543-8. [DOI] [PubMed] [Google Scholar]
- 95.Mericskay M, Kitajewski J, Sassoon D. Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development. 2004;131:2061–72. doi: 10.1242/dev.01090. [DOI] [PubMed] [Google Scholar]
- 96.Miller C, Sassoon DA. Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development. 1998;125:3201–11. doi: 10.1242/dev.125.16.3201. [DOI] [PubMed] [Google Scholar]
- 97.Mohamed OA, Jonnaert M, Labelle-Dumais C, Kuroda K, Clarke HJ, Dufort D. Uterine Wnt/beta-catenin signaling is required for implantation. Proc Natl Acad Sci U S A. 2005;102:8579–84. doi: 10.1073/pnas.0500612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, Demayo FJ, Lydon JP, O'Malley BW. Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol. 2006;26:6571–83. doi: 10.1128/MCB.00654-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–4. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 100.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci U S A. 2003;100:9744–9. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 102.Ogle TF, Dai D, George P. Progesterone-regulated determinants of stromal cell survival and death in uterine decidua are linked to protein kinase C activity. Steroids. 1999;64:628–33. doi: 10.1016/s0039-128x(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 103.Ola B, Li TC. Implantation failure following in-vitro fertilization. Curr Opin Obstet Gynecol. 2006;18:440–5. doi: 10.1097/01.gco.0000233940.82296.49. [DOI] [PubMed] [Google Scholar]
- 104.Pabona JM, Zeng Z, Simmen FA, Simmen RC. Functional differentiation of uterine stromal cells involves cross-regulation between bone morphogenetic protein 2 and Kruppel-like factor (KLF) family members KLF9 and KLF13. Endocrinology. 2010;151:3396–406. doi: 10.1210/en.2009-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pakrasi PL, Jain AK. Effect of cyclooxygenase on “window of implantation” in mouse. Prostaglandins Leukot Essent Fatty Acids. 2007;77:147–53. doi: 10.1016/j.plefa.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 106.Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001;98:1047–52. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pirone DM, Carter DE, Burbelo PD. Evolutionary expansion of CRIB-containing Cdc42 effector proteins. Trends Genet. 2001;17:370–3. doi: 10.1016/s0168-9525(01)02311-3. [DOI] [PubMed] [Google Scholar]
- 108.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–75. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Razzaque MS, Soegiarto DW, Chang D, Long F, Lanske B. Conditional deletion of Indian hedgehog from collagen type 2alpha1-expressing cells results in abnormal endochondral bone formation. J Pathol. 2005;207:453–61. doi: 10.1002/path.1870. [DOI] [PubMed] [Google Scholar]
- 110.Regan CP, Manabe I, Owens GK. Development of a smooth muscle-targeted cre recombinase mouse reveals novel insights regarding smooth muscle myosin heavy chain promoter regulation. Circ Res. 2000;87:363–9. doi: 10.1161/01.res.87.5.363. [DOI] [PubMed] [Google Scholar]
- 111.Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ. Epithelial-stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med. 2010;28:27–35. doi: 10.1055/s-0029-1242990. [DOI] [PubMed] [Google Scholar]
- 112.Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–3. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- 113.Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L, Jr, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem. 2004;279:29286–94. doi: 10.1074/jbc.M403139200. [DOI] [PubMed] [Google Scholar]
- 114.Smith DF. Chaperones in progesterone receptor complexes. Semin Cell Dev Biol. 2000;11:45–52. doi: 10.1006/scdb.1999.0350. [DOI] [PubMed] [Google Scholar]
- 115.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 116.Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66. doi: 10.1002/gene.20098. [DOI] [PubMed] [Google Scholar]
- 117.Srivastava D. HAND proteins: molecular mediators of cardiac development and congenital heart disease. Trends Cardiovasc Med. 1999;9:11–8. doi: 10.1016/s1050-1738(98)00033-4. [DOI] [PubMed] [Google Scholar]
- 118.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–86. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–83. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 120.Sterneck E, Tessarollo L, Johnson PF. An essential role for C/EBPbeta in female reproduction. Genes Dev. 1997;11:2153–62. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–9. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 122.Sundstrom SA, Komm BS, Ponce-de-Leon H, Yi Z, Teuscher C, Lyttle CR. Estrogen regulation of tissue-specific expression of complement C3. J Biol Chem. 1989;264:16941–7. [PubMed] [Google Scholar]
- 123.Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology. 1995;136:3639–47. doi: 10.1210/endo.136.8.7628404. [DOI] [PubMed] [Google Scholar]
- 124.Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indianr hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16:2338–48. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- 125.Takamoto N, Kurihara I, Lee K, Demayo FJ, Tsai MJ, Tsai SY. Haploinsufficiency of chicken ovalbumin upstream promoter transcription factor II in female reproduction. Mol Endocrinol. 2005;19:2299–308. doi: 10.1210/me.2005-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–21. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111:99–113. doi: 10.1016/s0925-4773(01)00614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol. 2004;265:181–95. doi: 10.1016/j.ydbio.2003.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, et al. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–4. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 130.Tibbetts TA, Mendoza-Meneses M, O'Malley BW, Conneely OM. Mutual and intercompartmental regulation of estrogen receptor and progesterone receptor expression in the mouse uterus. Biol Reprod. 1998;59:1143–52. doi: 10.1095/biolreprod59.5.1143. [DOI] [PubMed] [Google Scholar]
- 131.Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102:14326–31. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–12. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 133.Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RC. Null mutation of Kruppel-like factor9/basic transcription element binding protein-1 alters peri-implantation uterine development in mice. Biol Reprod. 2005;73:472–81. doi: 10.1095/biolreprod.105.041855. [DOI] [PubMed] [Google Scholar]
- 134.Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta-/- mice. Proc Natl Acad Sci U S A. 2006;103:18350–5. doi: 10.1073/pnas.0608861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang H, Critchley HO, Kelly RW, Shen D, Baird DT. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod. 1998;4:407–12. doi: 10.1093/molehr/4.4.407. [DOI] [PubMed] [Google Scholar]
- 136.Wang H, Ma WG, Tejada L, Zhang H, Morrow JD, Das SK, Dey SK. Rescue of female infertility from the loss of cyclooxygenase-2 by compensatory up-regulation of cyclooxygenase-1 is a function of genetic makeup. J Biol Chem. 2004;279:10649–58. doi: 10.1074/jbc.M312203200. [DOI] [PubMed] [Google Scholar]
- 137.Wang JD, Zhu JB, Shi WL, Zhu PD. Immunocytochemical colocalization of progesterone receptor and prolactin in individual stromal cells of human decidua. J Clin Endocrinol Metab. 1994;79:293–7. doi: 10.1210/jcem.79.1.8027244. [DOI] [PubMed] [Google Scholar]
- 138.Wang W, Li Q, Bagchi IC, Bagchi MK. The CCAAT/enhancer binding protein beta is a critical regulator of steroid-induced mitotic expansion of uterine stromal cells during decidualization. Endocrinology. 2010;151:3929–40. doi: 10.1210/en.2009-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang XN, Das SK, Damm D, Klagsbrun M, Abraham JA, Dey SK. Differential regulation of heparin-binding epidermal growth factor-like growth factor in the adult ovariectomized mouse uterus by progesterone and estrogen. Endocrinology. 1994;135:1264–71. doi: 10.1210/endo.135.3.8070372. [DOI] [PubMed] [Google Scholar]
- 140.Wedel A, Ziegler-Heitbrock HW. The C/EBP family of transcription factors. Immunobiology. 1995;193:171–85. doi: 10.1016/s0171-2985(11)80541-3. [DOI] [PubMed] [Google Scholar]
- 141.Weihua Z, Saji S, Makinen S, Cheng G, Jensen EV, Warner M, Gustafsson JA. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A. 2000;97:5936–41. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.WHO. Comparison of three single doses of mifepristone as emergency contraception: a randomised trial. Task Force on Postovulatory Methods of Fertility Regulation. Lancet. 1999;353:697–702. [PubMed] [Google Scholar]
- 143.Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–7. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 145.Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, Demayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci U S A. 2007;104:18315–20. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xie H, Tranguch S, Jia X, Zhang H, Das SK, Dey SK, Kuo CJ, Wang H. Inactivation of nuclear Wnt-beta-catenin signaling limits blastocyst competency for implantation. Development. 2008;135:717–27. doi: 10.1242/dev.015339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–5. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 148.Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sanchez ER, Shou W. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol. 2006;20:2682–94. doi: 10.1210/me.2006-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ying Y, Zhao GQ. Detection of multiple bone morphogenetic protein messenger ribonucleic acids and their signal transducer, Smad1, during mouse decidualization. Biol Reprod. 2000;63:1781–6. doi: 10.1095/biolreprod63.6.1781. [DOI] [PubMed] [Google Scholar]
- 150.Zhang D, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RC. Direct interaction of the Kruppel-like family (KLF) member, BTEB1, and PR mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology. 2002;143:62–73. doi: 10.1210/endo.143.1.8590. [DOI] [PubMed] [Google Scholar]
- 151.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–86. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 152.Zhang XL, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RC. Selective interactions of Kruppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem. 2003;278:21474–82. doi: 10.1074/jbc.M212098200. [DOI] [PubMed] [Google Scholar]