Abstract
Evidence exists that vitamin D has a potential antimicrobial activity and its deficiency has deleterious effects on general well-being and longevity. Vitamin D may reduce the risk of infection through multiple mechanisms. Vitamin D boosts innate immunity by modulating production of anti-microbial peptides (AMPs) and cytokine response. Vitamin D and its analogues via these mechanisms are playing an increasing role in the management of atopic dermatitis, psoriasis, vitiligo, acne and rosacea. Vitamin D may reduce susceptibility to infection in patients with atopic dermatitis and the ability to regulate local immune and inflammatory responses offers exciting potential for understanding and treating chronic inflammatory dermatitides. Moreover, B and T cell activation as well as boosting the activity of monocytes and macrophages also contribute to a potent systemic anti-microbial effect. The direct invasion by pathogenic organisms may be minimized at sites such as the respiratory tract by enhancing clearance of invading organisms. A vitamin D replete state appears to benefit most infections, with the possible noteworthy exception of Leishmaniasis. Antibiotics remain an expensive option and misuse of these agents results in significant antibiotic resistance and contributes to escalating health care costs. Vitamin D constitutes an inexpensive prophylactic option and possibly therapeutic product either by itself or as a synergistic agent to traditional antimicrobial agents. This review outlines the specific antimicrobial properties of vitamin D in combating a wide range of organisms. We discuss the possible mechanisms by which vitamin D may have a therapeutic role in managing a variety of infections.
Keywords: vitamin D, infections, antimicrobials
Introduction
Vitamin D deficiency is associated with several adverse health outcomes.1 A plethora of health benefits, including a boost in longevity with vitamin D replacement, is evident.2 Vitamin D has an emerging role in regulating inflammation and chemokine production3 as well as an important role in immunomodulation.4 These anti-inflammatory and anti-infective roles of Vitamin D are becoming increasingly important in a variety of skin diseases. A modulating effect of Vitamin D on the skin immune system is apparent from the neonatal period, with the altered regulatory T cell profile persisting to adulthood.5 Vitamin D and its analogues may have effects on, melanocytes, sebocytes and keratinocytes and offer an exciting option for treating many chronic inflammatory dermatitides.6 Biggs et al.7 demonstrated that vitamin D dependent induction of interleukin-10 by mast cells could contribute to the mast cell's ability to suppress cutaneous inflammation. In addition, vitamin D by modulating T cell profiles and production of antimicrobial peptides in the skin can potentially improve cutaneous inflammatory and vascular response as well as reduce secondary skin infection.8
Vitamin D may also improve survival in acute illness by boosting innate immunity.9 Vitamin D appears to have systemic antimicrobial effects10 that may be crucial in a variety of both acute and chronic illness. The current use of antimicrobials in the United States costs billions of dollars. Moreover, the overuse of antibiotics persists despite many efforts to address this problem and contributes to resistant organisms such as methicillin resistant staphylococcus aureus.11 Growing expenditures on prescription drugs represent a major challenge to many health care systems. Vitamin D use could potentially reduce inappropriate antibiotic prescription and boost therapeutic response when combined with appropriate antibiotic use. We review the possible mechanisms by which Vitamin D may modulate the antimicrobial response.
Vitamin D as an Immunomodulatory and Antimicrobial Agent
Vitamin (1,25-D3) acts as an immune system modulator.12 Nearly all cells display a specific vitamin D receptor (VDR), including B and T lymphocytes (both resting and activated), monocytes and dendritic cells.13 Vitamin D exerts its immunomodulatory activity on both mononuclear and polynuclear cell lines through its effects on the VDR.14 Vitamin D tends to favor a mononuclear phenotype, increasing VDR expression on monocytes and macrophages.13,15 Circulating vitamin D levels have a direct influence on macrophages, increase their “oxidative burst” potential (maturation and production of cytokines, acid phosphatase and hydrogen peroxide),16 and prevent excessive expression of inflammatory cytokines. Vitamin D also facilitates neutrophil motility and phagocytic function.17
Vitamin D may improve outcomes by reducing both local and systemic inflammatory responses as a result of modulating cytokine responses and reducing Toll-like receptor (TLR) activation.18 Studies in mice suggest that blocking TLR9 may have use in treating human sepsis.19 Vitamin D also modulates the immune system by direct effects on T-cell activation and on the phenotype and function of antigen-presenting cells, especially dendritic cells.20 Vitamin 1,25-D3 inhibits proliferation of T helper 1(Th1) cells [consequently impairing production of IL-2, tumor necrosis factor α and interferon (IFN)], as well as T helper 17 (Th17) cells, skewing cytokine production toward a T helper 2 (Th2) phenotype.21
A major component of the antimicrobial action of Vitamin D is through the production of peptides which have antimicrobial as well as anti-endotoxin activity. Vitamin D stimulates the expression of potent antimicrobial peptides, such as cathelicidin and β defensin 2,22 which exist in neutrophils, monocytes, natural killer (NK) cells and epithelial cells lining the respiratory tract.23 Macrophages, lymphocytes and monocytes have VDRs that, with 25(OH)D stimulation, increase the expression of these antimicrobial peptides.24,25 Jeng et al.18 noted a positive relationship between vitamin D levels and cathelicidin levels in acutely ill patients. Grant proposed this relationship on the basis of a synthesis of the epidemiological studies of septicemia in the United States.26
Cathelicidin is effective against gram-positive and gram-negative bacteria, fungi and mycobacteria at a variety of pathogen entry sites, including the skin and the mucosal linings of the respiratory and gastrointestinal systems.22 Patients with 25(OH) D levels less than 20 ng/mL may be unable to fully express cathelicidin,18 which could be associated with increased susceptibility to nosocomial infections such as pneumonia, sepsis and central line infections.21
Another antimicrobial peptide, human beta-defensin-2 (HBD)-2, may have special utility in multidrug resistant microbes from in vitro studies,27 although its overall role is less clearly defined. In lesions observed in psoriasis and atopic dermatitis, vitamin D promoted increased cathelicidin28 but decreased beta-defensin-2 production.29 This enhanced antimicrobial pep-tide production may improve skin lesions in psoriasis and atopic dermatitis.29 The ability of vitamin D analogs to alter antimicrobial peptide expression in psoriatic lesions extends our understanding of dermatologic therapies.30
Human beta-defensin-3 (HBD)-3 is an antimicrobial pep-tide that exhibits a broad spectrum antimicrobial activity against gram-positive/negative bacteria and fungi.31 It is possible that (HBD)-3 maybe more relevant than (HBD)-2 as shown in the severity of staphylococcal aureus skin infections.32 Augmentation of (HBD)-3 production in keratinocytes by antifungal agents such as Itraconazole may potentiate cutaneous defense against infection.33 A deficiency of antimicrobial peptide production may contribute to the susceptibility to staphylococcus aureus skin infections in patients with atopic dermatitis.8 Moreover, abnormal processing of cathelicidin peptides may contribute to cutaneous inflammation and vascular response seen in Rosacea.34
In addition to antimicrobial and immune benefits, antimicrobial peptides may contribute to host defense through wound repair35 and clearance of bacteria at various barrier sites.36 Vitamin D deficiency may also predispose patients to hypocalcemia, which impairs normal lymphocyte and neutrophil function.37
Antimicrobial Efficacy Against Different Infective Agents
Viruses.
Acute lower respiratory infections are one of the commonest reasons for hospital emergency department presentations, hospitalization and intensive care unit admissions among children.38 Evidence exists that vitamin D may have a protective role in influenza16,39 and other viral diseases, such as the risk of developing acquired immunodeficiency syndrome (AIDS) in human immunodeficiency virus (HIV),40 hepatitis,41 Avian flu,42 and other viral infections.
Studies dating back to the 1940s have associated a diet poor in vitamin D with susceptibility to experimental influenza viruses in mice.43 Influenza epidemics in North America and Europe generally reach peaks during December through March, the months during which ultraviolet-B (UVB) radiation exposure and serum levels of 25(OH)D are lowest in the population.44 Epidemics of influenza peak in the month after the winter solstice45 and have greater clinical severity with less sunlight.16 Low vitamin D levels may reduce AMP synthesis, which then is less likely to impede the influenza virus.16 A randomized controlled trial involving Japanese schoolchildren found a relative risk of influenza of 0.36 in those taking 1,200 IU/day compared with those taking 200 IU/day.39 This result was found to be related to influenza type A, with no effect for type B.
Sabetta et al. demonstrated that maintenance of a vitamin D serum concentration of 38 ng/mL or higher should significantly reduce the incidence of acute viral respiratory tract infections, including influenza, at least during the fall and winter in temperate zones.46 Similarly, variations in vitamin D production might explain the seasonality of childhood respiratory infections in Hawaii.47 During the 1918–1919 influenza pandemic, in the United States, an inverse correlation emerged between the case fatality rates and the mean vitamin D status of the population which was represented by solar UVB doses.48
In Indian children younger than 5 years, subclinical vitamin D deficiency was a significant risk factor for severe acute lower respiratory tract infections.49 Corroborative evidence has emerged in a study in which 94 children received vitamin D supplementation and were noted to have a lower incidence of respiratory tract infections from autumn through spring of the following year.50 The serum vitamin D3-binding protein (Gc), which B-cell membranes constitutively express in association with membrane immunoglobulin, could be involved in cell activation.51 It is the precursor for the principal macrophage-activating factor (MAF) and is reduced in all patients infected with the influenza virus. Sera from these patients contain α-N-acetylgalactosaminidase (Nagalase) that deglycosylates Gc protein, preventing it from converting to MAF, possibly contributing to immunosuppression.52
After the outbreaks of H1N1 influenza in 2009, Edlich et al.53 strongly recommended that all health care workers and patients be tested and treated for vitamin D deficiency to prevent exacerbation of respiratory infections. Vitamin D also reduces the production of proinflammatory cytokines, which may reduce the risk of cytokine storm in H1N1 infection.48
Other respiratory viruses such as respiratory syncytial virus (RSV) and parainfluenza 1 and 2 viruses present with a similar seasonal pattern,54 although the incidence of RSV seems to relate more to humidity and temperature than to UV radiation exposure.55 Vitamin D appears to decrease the inflammatory response to RSV infections in airway epithelium without jeopardizing viral clearance.56
These findings suggest that the immunomodulatory properties of vitamin D influence acute lower respiratory tract disease severity38 and may thus protect against asthma. A recent randomized controlled trial involving Japanese students found a reduced incidence of asthma in those taking vitamin D.39 Moreover, in children with asthma, decreased vitamin D status is associated with increased use of corticosteroids.57 Viral infections also constitute a major cause of recurrent otitis media. In a group of 16 young children undergoing tympanostomy tubes, suboptimal 25(OH)D levels were noted: 50% were deficient and another 31% were in the insufficient range.58
Individuals with 25(OH)D levels less than 10 ng/mL had 55% higher probability of a recent upper respiratory infection than individuals with levels greater than 30 ng/mL.23 In elderly patients, this concern is of greater magnitude; levels will need to be sufficient to compensate for an age-dependent decrease in immune function. Consuming 800 IU of oral vitamin D3 daily was insufficient for prevention of infection in the RECORD trial.59
One cannot extrapolate conclusions from studies with the influenza virus to other respiratory viruses. For example, AMPs do not inactivate rhinoviruses because these viruses lack a lipoprotein envelope, which appears to be prerequisite for the antimicrobial activity of most AMPs.60 Epstein-Barr virus transforms B lymphocytes into immortalized lymphoblasts. A VDR protein was found to be a binding partner of Epstein-Barr virus nuclear antigen 3 (EBNA-3), a member of the EBNA-3-protein family that can regulate transcription of cellular and viral genes. This latter protein blocks the activation of VDR-dependent genes and protects lymphoblast cell lines against vitamin D3-induced growth arrest and apoptosis.61
Despite a complex relationship that remains to be fully elucidated, vitamin D appears to contribute in defense against HIV. The role is based on both direct action (by promoting release of antiviral elements such as β-defensin 2 and cathelicidin),62 and indirect effects through VDR actions. Polymorphisms in the structure of the VDR gene directly affect host susceptibility to HIV infection, CD4 counts, immunological hyperactivity and rapidity of disease progression to full-blown AIDS.40,63 The G-A-T-G-L haplotype of VDR (which confers a diminished efficacy of the vitamin D pathway) protects against transmission of HIV type 1 (possibly secondary to diminished antiviral chemokine release and an unbalanced Th2 response),40 whereas in AIDS patients, the VDR BsmI BB and FokI heterozygosities are associated with a more accelerated CD4 drop and faster progression to AIDS.64,65
However, under certain circumstances, the vitamin D pathway may be implicated in promoting disease activity. Nevado et al. used U937 cells as a model for mononuclear cells (a target for HIV) and demonstrated that VDR and its ligand 1,25-D3 have a role in transactivation of the long terminal repeat sequence of HIV type 1, which is a crucial element in viral replication.66 The exact nature of this influence has yet to be fully elucidated. HIV entry into monocytes depends on chemokine receptor CCR5,67 and vitamin D has a dual role in potentially blocking this step in the infectious pathway. Promoting a predominantly Th2 response will induce release of cytokines (such as IL-13) known to downregulate CCR5 expression.68 Vitamin D also regulates production of RANTES,3 a natural ligand of CCR5 whose binding helps block viral entry.
Highly active antiretroviral therapy may be linked to significant changes in vitamin D status. Median 25(OH)D levels were significantly lower in white nonnucleoside reverse transcriptase inhibitor-treated patients than in white patients treated with pro-tease inhibitors.63 Moreover, nonnucleoside reverse transcriptase inhibitor and protease inhibitor treatment puts patients at risk of elevated parathyroid hormone levels,63 as shown with tenofovirlinked hyperparathyroidism, which is closely linked to vitamin D deficiency.69 However, vitamin D status did not affect CD4 cell recovery after initiation of highly active antiretroviral therapy.63
Host defense against human T-lymphotropic virus type 1 (HTLV-1) hinges on an effective Th1 response. Vitamin D seems to limit the magnitude of Th1 activation, providing a more effectively orchestrated response in a similar manner to tuberculosis (as discussed later). In patients infected with HTLV-1, a greater risk of developing tropical spastic paraparesis was associated with the ApaI polymorphism of VDR (although clinical course was unaltered).70
The potential benefit of a vitamin D-replete state against hepatitis is an interesting recent development.41 Chronicity of hepatitis B infection is also influenced by mutations in the VDR gene, with polymorphisms being associated with higher viral load, disease progression and severity.71 Of note, the t allele (resulting from a dimorphism at position 352) is associated with enhanced Th1 cellular immunity and promotes more efficient clearance of several viral infections, including hepatitis B and dengue virus.72,73 A potential benefit of vitamin D on the hepatitis C virus (HCV) is emerging; however, the data are preliminary. One study in patients with HCV demonstrated that vitamin D2 (but not D3) inhibits viral RNA replication, supposedly by inducing oxidative stress in a manner similar to the action of cyclosporine.74 Genotype 1 chronic HCV patients have low 25(OH)D serum levels, thus placing them at risk of severe fibrosis and low sustained viral response to IFN.75 Another study also reproduced these findings,76 where vitamin D supplementation improved the probability of achieving a sustained virological response after antiviral treatment with IFNα and ribavirin. Further, vitamin D-binding protein was among the three prominent candidate biomarkers of liver fibrosis, where vitamin D-binding protein levels were higher in the normal liver/mild fibrosis stage and lower in the advanced stage. Thus, vitamin D-binding protein level is potentially a way to predict the stage of liver fibrosis without biopsy.77 Vitamin D is linked not only to liver fibrosis but also to liver cirrhosis. A significant correlation exists between VDR genetic polymorphisms and the occurrence of hepatocellular carcinoma in patients with liver cirrhosis; this association is even more prominent in alcoholic patients.78
Deficient production of CCR5 has been linked to an increased susceptibility to HCV infection,79 supporting a potential deleterious role for vitamin D deficiency by favoring host infection through the aforementioned Th2 influence on CCR5.
Bacteria.
Recent discoveries have revealed the importance of the vitamin D-dependent generation of antimicrobial peptides in human host defense against Mycobacterium tuberculosis.80 Low vitamin D levels were associated with a fivefold-increased risk for progression to tuberculosis.81 The influence of vitamin D on tuberculosis can be traced back to the predrug era, when exposure to sunlight was considered an important adjuvant treatment modality. Yesudian et al. hypothesized that high-risk populations latently infected with the organism would develop full-fledged disease upon migrating from an area with high sun exposure to areas with diminished sunlight.82 Since the early 19th century, both environmental (i.e., sunlight) and dietary sources (cod liver oil) of vitamin D have been identified as treatments for tuberculosis.10
More than one study has associated polymorphisms in VDR with an increased susceptibility to tuberculosis,83,84 and vitamin D deficiency has been linked to a more severe form of the disease.85 The lower serum levels observed in African Americans may account for deficient production of cathelicidin and a greater susceptibility to infection.86 Cathelicidin is required for the 1,25-D3-triggered antimicrobial activity against intracellular M. tuberculosis.86 M. tuberculosis activates TLR2/1, enhancing 1,25-D3 production and VDR expression, with subsequent release of cathelicidin by monocytes.87
Vitamin D limits mycobacterial growth within macrophages and monocytes and, until recently, the precise mechanism governing this activity was subject to debate.88,89 Murine models suggest that 1,25(OH)2D-mediated induction of nitric oxide release by macrophage-like cell lines is a fundamental element in host defense, but these findings may not apply to human subjects.90 Vitamin 1,25(OH)2D may play an important role in limiting the pathological process in tuberculosis by downregulating the levels of matrix metalloproteinases (MMPs) and upregulating the levels of tissue inhibitor of MMP-1.91
A variety of other mechanisms have also been postulated. Autophagy also plays a crucial role in antimycobacterial resistance92 and contributes to immune surveillance of intracellular pathogens and vaccine efficacy. Vitamin D3 contributes to host immune responses against M. tuberculosis through cathelicidin.93 Yuk et al.94 have outlined how vitamin D induces autophagy and mediates co-localization of M. tuberculosis and AMPs within an autophagolysosome, leading to killing of the bacterium. Similarly, TLR2/1 activation leads to vitamin D3-dependent antimycobacterial activities. TLR2/1/CD14 stimulation by mycobacterial lipoprotein LpqH can activate antibacterial autophagy through activating VDR signaling and inducing cathelicidin.93
Analogously to HTLV-1, 1,25-D3 induces important cytokine downregulation activity and actually blocks mononuclear cell production of IFNγ and IL-12,95 modulating the immune response against M. tuberculosis, possibly reducing the risk of cytokine storm.
The effects of vitamin D on other mycobacterial diseases, such as leprosy, are also being studied. The presence of 1-α-hydroxylase in foamy macrophages (characteristic of granulomatous disease) may result in greater circulating levels of 1,25-D3 and subsequent hypercalcemia. Whether these metabolic changes alter disease course is unknown. More concrete evidence has emerged linking VDR polymorphisms with host susceptibility. The t allele (already highlighted as a marker of prominent Th1 immunity) has been linked with greater incidence of tuberculoid leprosy.96 In a highly endemic region of Brazil, the combination of the tt genotype and a negative Mitsuda reaction was associated with an incidence of leprosy 13 times higher than that in control subjects with a positive Mitsuda test.97
In vitro studies proved vitamin D3 has inhibitory activity on strains of Staphylococcus aureus, Streptococcus pyogenes, Klebsiella pneumoniae, Escherichia coli (E. coli) and other bacteria. In the presence of 50,000–90,000 IU/mL of vitamin D3, the organisms were killed or demonstrated marked growth inhibition.98 Gram-positive bacteria, invasive pneumococcal disease, meningococcal disease and group A streptococcal disease are more common when vitamin D levels are low, raising the possibility that pharmacological doses of vitamin D could be an effective adjuvant therapy.99
Excess wintertime mortality related to pneumococcal pneumonia has been noted for more than a century. The major predictor of invasive pneumococcal disease in Philadelphia was extended periods of low UV radiation, which may explain the observed wintertime seasonality. The virulence of the pathogen and the efficacy of host immune function boosted by 1,25-D3 may determine the outcomes of invasive disease.100 A double-blind individually randomized placebo-controlled trial involving young children in an inner-city hospital in Kabul showed that the risk of a repeat episode of pneumonia within 90 days of supplementation of oral 100,000 IU of vitamin D3 was lower in the intervention than in the placebo group.101
Bahr et al. examined group A streptococcal infections and found a striking association between vitamin D-binding protein (Gc2) and rheumatic fever in a homogeneous Arab population.51 Vitamin D plays an important role in mediating immune function of the skin through several pathways, including the enhanced release of AMPs.102 Vitamin D deficiency is associated with an increased risk of methicillin-resistant Staphylococcus aureus nasal carriage.103 Similar findings suggest that VDR polymorphisms may be associated with nasal carriage of S. aureus in individuals with type 1 diabetes mellitus.104 However, others found no association between VDR gene variation and S. aureus nasal carriage.105
Moreover, vitamin D deficiency has been linked to adverse and more costly outcomes in veterans with Clostridium difficile and methicillin-sensitive S. aureus infections.106 Animal studies also confirm a potential beneficial role for vitamin D treatment. In the turkey osteomyelitis complex, vitamin D treatment resulted in significantly lower isolation of bacteria from tissues and reduced mortality.107
The Tt genotype of VDR is associated with periodontal disease and may mediate this through its effects on the gram-positive bacteria that constitute the oral flora.108 Both dental caries and periodontal disease are caused largely by Streptococcus mutans.109 Although eating refined carbohydrates and poor dental hygiene contribute to dental caries, vitamin D can reduce the risk of caries by inducing production of cathelicidin and defensins, which have antibacterial properties.10 A cross-sectional study from the Third National Health and Nutrition Examination Survey (NHANES III) in the United States found a significant inverse correlation between periodontal disease (PD) and serum 25(OH)D concentrations.110
A study of British Bangladeshi adults, free of known diabetes or major illness, showed that a year of modest vitamin D supplementation decreased serum circulating MMP-9 levels by 69%.111 Two small randomized controlled trials of vitamin D and calcium supplementation and risk of PD revealed a higher rate of PD in the nontaker groups.112,113 Thus, vitamin D evidently reduces the risk of PD since it largely satisfied the criteria for causality in a biological system, as outlined by Grant and Boucher.114
Vitamin D-induced downregulation of the cytokine response may not always be associated with optimal host defense. The Th1 cytokine profile is vital for clearance of certain organisms and ancillary immune activity, and a limiting effect on this cytokine profile may result in reduced chances for overcoming infections. A study on cultured macrophages treated with 1,25-D3 demonstrated deficient activity against Listeria spp.115 This effect may be due to inhibition of IFNγ activation of macrophages, which would preclude reactive oxygen formation and thus effective bactericidal activity.115 However, Bruce et al.116 indicated that clearance of Listeria monocytogenes was delayed in the absence of the VDR.
Many examples document the benefits of having an optimal vitamin D level in gram-negative bacteria. Treatment with 25(OH)D protected 3A cells of the placenta against cell death after infection with E. coli.117 Through enhancing AMP expression, vitamin D may be a part of the defense against infections such as shigellosis, which reduce the endogenous expression of AMPs, resulting in serious infections.118 Vitamin D deficiency predisposes mice to colitis by means of dysregulated colonic antimicrobial activity and impaired homeostasis of enteric bacteria.119 In the same context, VDR activation reduces bacterial-induced intestinal NFκB activation and attenuates response to infection.120 Therefore, it is an important contributor to intestinal homeostasis and host protection from bacterial invasion and infection.120 Treatment with oral active vitamin D may be associated with a lower risk of peritonitis in peritoneal dialysis patients.121
Despite a decrease in mortality in the last decade, sepsis remains the tenth-leading cause of death in western countries122 and one of the commonest causes of death in intensive care units. Mortality in adult intensive care units may be partially linked to severe systemic inflammatory responses and sepsis.18 Vitamin D status may determine AMP levels in patients with sepsis in the intensive care unit.18 The epidemiology of septicemia in the United States and the variations of solar UVB, as well as the effects of vitamin D, support the hypothesis that both play important roles in reducing the risk of septicemia.26 The risk of diseases comorbid with septicemia are generally inversely correlated with serum 25(OH)D levels.26 Grant also demonstrated that vitamin D supplementation of mother and infant can reduce the risk of sepsis in infants123 and neonates.124 Activation of TLR4, the receptor for gram-negative bacteria's outer membrane lipopolysaccharide or endotoxin, may play a potential role in determining outcomes. Vitamin D, through its modulation, may have a role as adjunctive therapy in severe sepsis and septic shock.122 In veterans admitted to the intensive care unit, higher mortality and a longer stay were significantly linked to lower vitamin D status.125 A total of 17% of the intensive care patients in one study had undetectable levels of vitamin D,126 which may predispose to hypocalcemia. Zaloga et al.127 found that 20% of critically ill patients with bacterial sepsis had hypocalcemia and that their mortality rate was significantly higher (50%) than that of normocalcemic patients with sepsis (29%).
Protozoa.
Leishmania major is an obligatory intracellular organism residing within macrophages. Effective clearance will depend on appropriate macrophage activation (which occurs through IFNγ release by Th1 and NK cells) and production of nitric oxide.128 The presence of 1,25-D3 disrupts this pathway, as IFNγ secretion is blocked, impairing macrophage activation.129 Similarly, host defense against Toxoplasma gondii (T. gondii) relies on a Th1 cytokine profile response dominated by IFNγ and IL-2 production.130 The potential downregulation of this response by vitamin D can prove problematic in toxoplasmosis. Treatment with vitamin D dose-dependently inhibits both in vivo and in vitro growth of T. gondii, possibly by limiting tachyzoite proliferation within the parasitophorous vacuole because of activity at a cellular level.131 However, no difference occurred in the total number of infected cells regardless of the presence of vitamin D, perhaps refuting a role in cell invasion. Interestingly, vitamin D may be linked to increased mortality in mice infected with toxoplasmosis, presumably because of its downregulation of the Th1 cytokine response.132 This finding suggests a delicate balance in overall immune response against this organism, and further studies would need to be done to establish the role of vitamin D among other variables in determining outcomes.
Two other parasitic infections reportedly benefit from adequate circulating vitamin D levels. A murine model demonstrated that intraperitoneal injection with vitamin D conferred protection against inoculation with trypomastigotes of Trypanosoma cruzi, with histopathology revealing diminished tissue inflammation and parasitism.133 The intraerythrocytic forms of Plasmodium falciparum (P. falciparum) produce triacylglycerols through enhanced expression of diacylglycerol acyltransferase, an essential step in sustaining their proliferation within erythrocytes.134 Biosynthetic activity is particularly enhanced at the trophozoite and schizont stages, which are precisely the two forms at which higher serum levels of 25-D3 and 1,25-D3 have been demonstrated to hinder growth of P. falciparum.135 It has been proposed that the activity of vitamin D resides in altering phospholipid metabolism and precluding this crucial step. It is, however, not an easy hypothesis to sustain, given that erythrocytes have not been shown to have receptors for 25-D3135 therefore, a direct cellular effect is highly unlikely.
Fungi.
The role of vitamin D in combating fungal infections dates back to a case series published in 1954, in which three patients with severe refractory chromoblastomycosis showed marked improvement in their skin lesions after receiving repeated treatments with 600,000 IU of calciferol.136 Despite these early promising results—plus the ability of vitamin D to increase circulating NK cells, which may contribute to host defense against fungal organisms37—subsequent findings confirming similar results have been scarce. Thus, more studies are needed to support vitamin D as an adjuvant treatment modality for fungal infection.
For Candida albicans, patients with end-organ resistance to 1,25-D3 all had relative decrease in neutrophil fungicidal activity.137 In animal studies, Cantorna et al.138 demonstrated that 1,25-D3 may reduce the prevalence of opportunistic infections such as candidiasis.
Murray et al. described a patient with disseminated histoplasmosis and adrenal insufficiency treated with vitamin D and calcium supplementations.139 The patient was hypercalcemic (due to the granulomatous nature of the disease), and vitamin D supplements exacerbated this condition. Despite aggressive management, the patient later died. In light of this, avoiding vitamin D supplementation in invasive granulomatous fungal infections would be prudent, since it may induce or aggravate hypercalcemia and hypercalcemia may lead to impaired monocyte and neutrophil activity.140
Parasites.
A remarkable lack of data exists on the effect of vitamin D in parasitic infections. Chowdhury et al. showed that media enriched with vitamin D3 can exert a toxic effect on Hymenolepis microstoma, leading to degenerative changes in the worm.141 Similarly, a prior study reported that a diet lacking in vitamins A, D3 and E would predispose to an increase in the size of Hymenolepis diminuta due to host intestinal paralysis and worm migration to a position more favorable for growth.142
An 11-month trial in Kenyan children living in an endemic area for helminth infections yields some useful data. Subjects receiving supplements (consisting of 15 micronutrients, including the equivalent of 200 IU daily of vitamin D3) showed a decrease of borderline significance in the rate of reinfection with Schistosoma mansoni but no benefit with regards to rates of infection with Ascaris lumbricoides, Trichuris trichiura or hookworms.143 The relatively low dose of vitamin D3 supplementation given hinders drawing definitive conclusions.
Conclusions
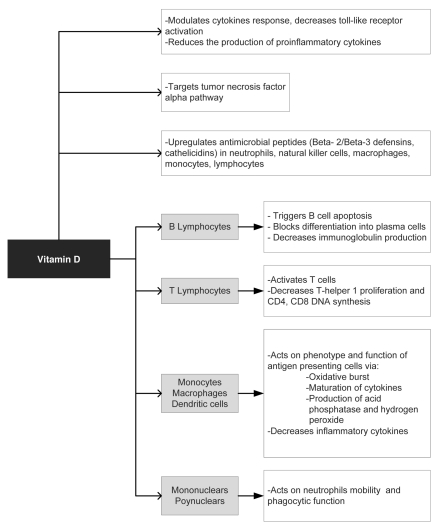
Abundant evidence is surfacing with regard to the role of vitamin D in modulating the immune and antimicrobial response. Figure 1 summarizes the potential antimicrobial mechanisms of vitamin D. Vitamin D also has diverse and potent local and systemic activities such as enhanced production of AMPs, which generally appears to favor the host in curtailing infection. Leishmaniasis may be the possible exception to this beneficence in that vitamin D may actually favor the invading organism.
Figure 1.
Potential antimicrobial mechanisms of vitamin D.
Much of the data supporting vitamin D has come from laboratory or epidemiologic settings. We urgently need clinical trials to confirm the efficacy of vitamin D as a potent antimicrobial and immunomodulating agent. Recent work suggest that in addition to low vitamin D levels, excessive 25(OH)D levels may also increase the risk of tuberculosis.144 A recently published randomized controlled trial demonstrated that the administration of four doses of 2.5 mg vitamin D(3) increased serum 25-hydroxyvitamin D concentrations in patients receiving intensive-phase treatment for pulmonary tuberculosis. Vitamin D significantly hastened sputum culture conversion in participants with the tt genotype of the TaqI vitamin D receptor polymorphism.145
Considering the plethora of general benefits that an adequate vitamin D state confers, as well as potential antimicrobial benefits, checking vitamin D status and maintaining adequate 25(OH)D levels seems prudent. It appears likely that VDR polymorphisms determine tissue responses to vitamin D.146 Some individuals may need much larger replacement doses than others, emphasizing the need to customize vitamin D replacement through monitoring. While optimal Vitamin D levels are not clearly defined, there is evidence that maintaining a vitamin D level 38 ng/ml or higher should significantly reduce the incidence of upper respiratory infection.46 In addition, Grant has proposed a significant reduction in mortality with a vitamin D level of 45 ng/ml.147 As such we believe it is reasonable to aim for 25(OH)D levels of about 40–45 ng/ml in patients.46
Vitamin D is emerging as an important and cost-effective option in the therapeutic armamentarium in reducing many infections either as a sole agent or as an adjunct to current antimicrobial agents.
Acknowledgements
We thank the Mountain Home VAMC library staff for their assistance.
This material is the result of works supported with resources and the use of facilities at the Mountain Home VAMC. The contents of this report do not reflect the position of the US Government and the Department of Veterans Affairs.
Disclosure Statement
W.B.G. receives or has received funding from the UV Foundation (McLean, VA), the Sunlight Research Forum (Veldhoven), Bio-Tech-Pharmacal (Fayetteville, AR), the Vitamin D Council (San Luis Obispo, CA), and the Danish Sunbed Federation (Middelfart).
References
- 1.Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DR. Vitamins in aging, health and longevity. Clin Interv Aging. 2006;1:81–91. doi: 10.2147/ciia.2006.1.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuoka M, Ogino Y, Sato H, Ohta T, Komoriya K, Nishioka K, et al. RANTES expression in psoriatic skin and regulation of RANTES and IL-8 production in cultured epidermal keratinocytes by active vitamin D3 (tacalcitol) Br J Dermatol. 1998;138:63–70. doi: 10.1046/j.1365-2133.1998.02027.x. [DOI] [PubMed] [Google Scholar]
- 4.Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. 2010;88:441–450. doi: 10.1007/s00109-010-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller HK, Malley RC, McGee HM, Scott DK, Wozniak T, Woods GM. Effect of UV radiation on the neonatal skin immune system—implications for melanoma. Photochem Photobiol. 2008;84:47–54. doi: 10.1111/j.1751-1097.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 6.Reichrath J. Vitamin D and the skin: an ancient friend, revisited. Exp Dermatol. 2007;16:618–625. doi: 10.1111/j.1600-0625.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 7.Biggs L, Yu C, Fedoric B, Lopez AF, Galli SJ, Grimbaldeston MA. Evidence that vitamin D(3) promotes mast cell-dependent reduction of chronic UVB-induced skin pathology in mice. J Exp Med. 2010;207:455–463. doi: 10.1084/jem.20091725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 9.Hewison M. Vitamin D and the intracrinology of innate immunity. Mol Cell Endocrinol. 2010;321:103–111. doi: 10.1016/j.mce.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4:1151–1165. doi: 10.2217/fmb.09.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranji SR, Steinman MA, Shojania KG, Gonzales R. Interventions to reduce unnecessary antibiotic prescribing: a systematic review and quantitative analysis. Med Care. 2008;46:847–862. doi: 10.1097/MLR.0b013e318178eabd. [DOI] [PubMed] [Google Scholar]
- 12.Toubi E, Shoenfeld Y. The role of vitamin D in regulating immune responses. Isr Med Assoc J. 2010;12:174–175. [PubMed] [Google Scholar]
- 13.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 14.Eleftheriadis T, Antoniadi G, Liakopoulos V, Galaktidou G. The effect of paricalcitol on osteoprotegerin production by human peripheral blood mono-nuclear cells. J Rheumatol. 2009;36:856–857. doi: 10.3899/jrheum.080987. [DOI] [PubMed] [Google Scholar]
- 15.Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, Kwiatkowski D. 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun. 1998;66:5314–5321. doi: 10.1128/iai.66.11.5314-5321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorente F, Fontan G, Jara P, Casas C, Garcia-Rodriguez MC, Ojeda JA. Defective neutrophil motility in hypovitaminosis D rickets. Acta Paediatr Scand. 1976;65:695–699. doi: 10.1111/j.1651-2227.1976.tb18005.x. [DOI] [PubMed] [Google Scholar]
- 18.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J Exp Med. 2008;205:1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canning MO, Grotenhuis K, de Wit H, Ruwhof C, Drexhage HA. 1-alpha,25-Dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol. 2001;145:351–357. doi: 10.1530/eje.0.1450351. [DOI] [PubMed] [Google Scholar]
- 21.Bikle DD. Vitamin D and the immune system: role in protection against bacterial infection. Curr Opin Nephrol Hypertens. 2008;17:348–352. doi: 10.1097/MNH.0b013e3282ff64a3. [DOI] [PubMed] [Google Scholar]
- 22.Bartley J. Vitamin D: emerging roles in infection and immunity. Expert Rev Anti Infect Ther. 2010;8:1359–1369. doi: 10.1586/eri.10.102. [DOI] [PubMed] [Google Scholar]
- 23.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwalfenberg GK. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55:96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- 25.Alitalo A. Human anti-infectious defence may be enhanced by vitamin D. Duodecim. 2010;126:1127–1134. [PubMed] [Google Scholar]
- 26.Grant WB. Solar ultraviolet-B irradiance and vitamin D may reduce the risk of septicemia. Dermatoendocrinol. 2009;1:37–42. doi: 10.4161/derm.1.1.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Routsias JG, Karagounis P, Parvulesku G, Legakis NJ, Tsakris A. In vitro bactericidal activity of human beta-defensin 2 against nosocomial strains. Peptides. 2010;31:1654–1660. doi: 10.1016/j.peptides.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Peric M, Lehmann B, Vashina G, Dombrowski Y, Koglin S, Meurer M, et al. UV-B-triggered induction of vitamin D3 metabolism differentially affects antimicrobial peptide expression in keratinocytes. J Allergy Clin Immunol. 2010;125:746–749. doi: 10.1016/j.jaci.2009.12.933. [DOI] [PubMed] [Google Scholar]
- 29.Vähävihu K, Ala-Houhala M, Peric M, Karisola P, Kautiainen H, Hasan T, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321–328. doi: 10.1111/j.1365-2133.2010.09767.x. [DOI] [PubMed] [Google Scholar]
- 30.Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Büchau A, et al. Vitamin D analogs differentially control antimicrobial peptide/“alarmin” expression in psoriasis. PLoS One. 2009;4:6340. doi: 10.1371/journal.pone.0006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y, Inokuchi S, Takazawa K, Umezawa K, Saito T, Kidokoro M, et al. Introduction of human β-defensin-3 into cultured human keratinocytes and fibroblasts by infection of a recombinant adenovirus vector. Burns. 2011;37:109–116. doi: 10.1016/j.burns.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Zanger P, Holzer J, Schleucher R, Scherbaum H, Schittek B, Gabrysch S. Severity of Staphylococcus aureus infection of the skin is associated with inducibility of human beta-defensin 3 but not human beta-defensin 2. Infect Immun. 2010;78:3112–3117. doi: 10.1128/IAI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanda N, Kano R, Ishikawa T, Watanabe S. The antimycotic drugs itraconazole and terbinafine hydrochloride induce the production of human β-defensin-3 in human keratinocytes. Immunobiology. 2011;216:497–504. doi: 10.1016/j.imbio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Peric M, Koglin S, Ruzicka T, Schauber J. Cathelicidins: multifunctional defense molecules of the skin. Dtsch Med Wochenschr. 2009;134:35–38. doi: 10.1055/s-0028-1105888. [DOI] [PubMed] [Google Scholar]
- 35.Hiemstra PS. The role of epithelial beta-defensins and cathelicidins in host defense of the lung. Exp Lung Res. 2007;33:537–542. doi: 10.1080/01902140701756687. [DOI] [PubMed] [Google Scholar]
- 36.White JH. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. J Steroid Biochem Mol Biol. 2010;121:234–238. doi: 10.1016/j.jsbmb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Matsuyama W, Takenaga S, Nakahara K, Kawabata M, Iwakiri Y, Arimura K, et al. Idiopathic hypoparathyroidism with fungal seminal vesiculitis. Intern Med. 1997;36:113–117. doi: 10.2169/internalmedicine.36.113. [DOI] [PubMed] [Google Scholar]
- 38.McNally JD, Leis K, Matheson LA, Karuananyake C, Sankaran K, Rosenberg AM. Vitamin D deficiency in young children with severe acute lower respiratory infection. Pediatr Pulmonol. 2009;44:981–988. doi: 10.1002/ppul.21089. [DOI] [PubMed] [Google Scholar]
- 39.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr. 2010;91:1255–1260. doi: 10.3945/ajcn.2009.29094. [DOI] [PubMed] [Google Scholar]
- 40.De la Torre MS, Torres C, Nieto G, Vergara S, Carrero AJ, Macías J, et al. Vitamin D receptor gene haplotypes and susceptibility to HIV-1 infection in injection drug users. J Infect Dis. 2008;197:405–410. doi: 10.1086/525043. [DOI] [PubMed] [Google Scholar]
- 41.Lange CM, Bojunga J, Ramos-Lopez E, Wagner MV, Hassler A, Vermehren J, et al. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J Hepatol. 2011;54:887–893. doi: 10.1016/j.jhep.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 42.Chan MC, Cheung CY, Chui WH, Tsao SW, Nicholls JM, Chan YO, et al. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young GA, Jr, Uunderdahl NR, Carpenter LE. Vitamin D intake and susceptibility of mice to experimental swine influenza virus infection. Proc Soc Exp Biol Med. 1949;72:695–697. doi: 10.3181/00379727-72-17545. [DOI] [PubMed] [Google Scholar]
- 44.Grant WB, Garland CF. The role of vitamin D3 in preventing infections. Age Ageing. 2008;37:121–122. doi: 10.1093/ageing/afm182. [DOI] [PubMed] [Google Scholar]
- 45.Hope-Simpson RE. The role of season in the epidemiology of influenza. J Hyg (Lond) 1981;86:35–47. doi: 10.1017/s0022172400068728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin D and the incidence of acute viral respiratory tract infections in healthy adults. PLoS One. 2010;5:11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grant WB. Variations in vitamin D production could possibly explain the seasonality of childhood respiratory infections in Hawaii. Pediatr Infect Dis J. 2008;27:853. doi: 10.1097/INF.0b013e3181817bc1. [DOI] [PubMed] [Google Scholar]
- 48.Grant WB, Giovannucci E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918–1919 influenza pandemic in the United States. Dermatoendocrinol. 2009;1:215–219. doi: 10.4161/derm.1.4.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wayse V, Yousafzai A, Mogale K, Filteau S. Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. Eur J Clin Nutr. 2004;58:563–567. doi: 10.1038/sj.ejcn.1601845. [DOI] [PubMed] [Google Scholar]
- 50.Linday LA, Shindledecker RD, Tapia-Mendoza J, Dolitsky JN. Effect of daily cod liver oil and a multi-vitamin-mineral supplement with selenium on upper respiratory tract pediatric visits by young, inner-city, Latino children: randomized pediatric sites. Ann Otol Rhinol Laryngol. 2004;113:891–901. doi: 10.1177/000348940411301108. [DOI] [PubMed] [Google Scholar]
- 51.Bahr GM, Eales LJ, Nye KE, Majeed HA, Yousof AM, Behbehani K, et al. An association between Gc (vitamin D-binding protein) alleles and susceptibility to rheumatic fever. Immunology. 1989;67:126–128. [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto N, Urade M. Pathogenic significance of alpha-N-acetylgalactosaminidase activity found in the hemagglutinin of influenza virus. Microbes Infect. 2005;7:674–681. doi: 10.1016/j.micinf.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Edlich RF, Mason SS, Dahlstrom JJ, Swainston E, Long WB, 3rd, Gubler K. Pandemic preparedness for swine flu influenza in the United States. J Environ Pathol Toxicol Oncol. 2009;28:261–264. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i4.10. [DOI] [PubMed] [Google Scholar]
- 54.Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States 1990–2004. Clin Infect Dis. 2006;43:1016–1022. doi: 10.1086/507638. [DOI] [PubMed] [Google Scholar]
- 55.Yusuf S, Piedimonte G, Auais A, Demmler G, Krishnan S, Van Caeseele P, et al. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol Infect. 2007;135:1077–1090. doi: 10.1017/S095026880600776X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NFkappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. 2010;184:965–974. doi: 10.4049/jimmunol.0902840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Searing DA, Zhang Y, Murphy JR, Hauk PJ, Goleva E, Leung DY. Decreased serum vitamin D levels in children with asthma are associated with increased corticosteroid use. J Allergy Clin Immunol. 2010;125:995–1000. doi: 10.1016/j.jaci.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Linday LA, Shindledecker RD, Dolitsky JN, Chen TC, Holick MF. Plasma 25-hydroxyvitamin D levels in young children undergoing placement of tympanostomy tubes. Ann Otol Rhinol Laryngol. 2008;117:740–744. doi: 10.1177/000348940811701006. [DOI] [PubMed] [Google Scholar]
- 59.Avenell A, Cook JA, Maclennan GS, Macpherson GC. Vitamin D supplementation to prevent infections: a sub-study of a randomised placebo-controlled trial in older people (RECORD trial, ISRCTN 51647438) Age Ageing. 2007;36:574–577. doi: 10.1093/ageing/afm091. [DOI] [PubMed] [Google Scholar]
- 60.Zhang G, Ross CR, Blecha F. Porcine antimicrobial peptides: new prospects for ancient molecules of host defense. Vet Res. 2000;31:277–296. doi: 10.1051/vetres:2000121. [DOI] [PubMed] [Google Scholar]
- 61.Yenamandra SP, Hellman U, Kempkes B, Darekar SD, Petermann S, Sculley T, et al. Epstein-Barr virus encoded EBNA-3 binds to vitamin D receptor and blocks activation of its target genes. Cell Mol Life Sci. 2010;67:4249–4256. doi: 10.1007/s00018-010-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 63.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, Sweep FC, Hermus AR, Bosch ME, et al. Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses. 2008;24:1375–1382. doi: 10.1089/aid.2008.0058. [DOI] [PubMed] [Google Scholar]
- 64.Barber Y, Rubio C, Fernandez E, Rubio M, Fibla J. Host genetic background at CCR5 chemokine receptor and vitamin D receptor loci and human immunodeficiency virus (HIV) type 1 disease progression among HIV-seropositive injection drug users. J Infect Dis. 2001;184:1279–1288. doi: 10.1086/324000. [DOI] [PubMed] [Google Scholar]
- 65.Nieto G, Barber Y, Rubio MC, Rubio M, Fibla J. Association between AIDS disease progression rates and the Fok-I polymorphism of the VDR gene in a cohort of HIV-1 seropositive patients. J Steroid Biochem Mol Biol. 2004;89:199–207. doi: 10.1016/j.jsbmb.2004.03.086. [DOI] [PubMed] [Google Scholar]
- 66.Nevado J, Tenbaum SP, Castillo AI, Sanchez-Pacheco A, Aranda A. Activation of the human immunodeficiency virus type I long terminal repeat by 1 alpha,25-dihydroxyvitamin D3. J Mol Endocrinol. 2007;38:587–601. doi: 10.1677/JME-06-0065. [DOI] [PubMed] [Google Scholar]
- 67.Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 68.Creery D, Weiss W, Graziani-Bowering G, Kumar R, Aziz Z, Angel JB, et al. Differential regulation of CXCR4 and CCR5 expression by interleukin (IL)-4 and IL-13 is associated with inhibition of chemotaxis and human immunodeficiency Virus (HIV) type 1 replication but not HIV entry into human monocytes. Viral Immunol. 2006;19:409–423. doi: 10.1089/vim.2006.19.409. [DOI] [PubMed] [Google Scholar]
- 69.Rosenvinge MM, Gedela K, Copas AJ, Wilkinson A, Sheehy CA, Bano G, et al. Tenofovir-linked hyperparathyroidism is independently associated with the presence of vitamin D deficiency. J Acquir Immune Defic Syndr. 2010;54:496–499. doi: 10.1097/qai.0b013e3181caebaa. [DOI] [PubMed] [Google Scholar]
- 70.Saito M, Eiraku N, Usuku K, Nobuhara Y, Matsumoto W, Kodama D, et al. ApaI polymorphism of vitamin D receptor gene is associated with susceptibility to HTLV-1-associated myelopathy/tropical spastic paraparesis in HTLV-1 infected individuals. J Neurol Sci. 2005;232:29–35. doi: 10.1016/j.jns.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 71.Li JH, Chen DM, Li Z, Liu Y, Gao JR, Zeng XJ, et al. Study on association between vitamin D receptor gene polymorphisms and the outcomes of HBV infection. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2006;23:402–405. [PubMed] [Google Scholar]
- 72.Bellamy R, Ruwende C, Corrah T, McAdam KP, Thursz M, Whittle HC, et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–724. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 73.Loke H, Bethell D, Phuong CX, Day N, White N, Farrar J, et al. Susceptibility to dengue hemorrhagic fever in vietnam: evidence of an association with variation in the vitamin D receptor and Fc gamma receptor IIa genes. Am J Trop Med Hyg. 2002;67:102–106. doi: 10.4269/ajtmh.2002.67.102. [DOI] [PubMed] [Google Scholar]
- 74.Yano M, Ikeda M, Abe K, Dansako H, Ohkoshi S, Aoyagi Y, et al. Comprehensive analysis of the effects of ordinary nutrients on hepatitis C virus RNA replication in cell culture. Antimicrob Agents Chemother. 2007;51:2016–2027. doi: 10.1128/AAC.01426-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petta S, Camma C, Scazzone C, Tripodo C, Di Marco V, Bono A, et al. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158–1167. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- 76.Bitetto D, Fabris C, Fornasiere E, Pipan C, Fumolo E, Cussigh A, et al. Vitamin D supplementation improves response to antiviral treatment for recurrent hepatitis C. Transpl Int. 2010 doi: 10.1111/j.1432-2277.2010.01141.x. [DOI] [PubMed] [Google Scholar]
- 77.Ho AS, Cheng CC, Lee SC, Liu ML, Lee JY, Wang WM, et al. Novel biomarkers predict liver fibrosis in hepatitis C patients: alpha 2 macroglobulin, vitamin D binding protein and apolipoprotein AI. J Biomed Sci. 2010;17:58. doi: 10.1186/1423-0127-17-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Falleti E, Bitetto D, Fabris C, Cussigh A, Fontanini E, Fornasiere E, et al. Vitamin D receptor gene polymorphisms and hepatocellular carcinoma in alcoholic cirrhosis. World J Gastroenterol. 2010;16:3016–3024. doi: 10.3748/wjg.v16.i24.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coenen M, Nattermann J. The role of CCR5 in HCV infection. Eur J Med Res. 2010;15:97–101. doi: 10.1186/2047-783X-15-3-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shapira Y, Agmon-Levin N, Shoenfeld Y. Mycobacterium tuberculosis, autoimmunity and vitamin D. Clin Rev Allergy Immunol. 2010;38:169–177. doi: 10.1007/s12016-009-8150-1. [DOI] [PubMed] [Google Scholar]
- 81.Talat N, Perry S, Parsonnet J, Dawood G, Hussain R. Vitamin D deficiency and tuberculosis progression. Emerg Infect Dis. 2010;16:853–855. doi: 10.3201/eid1605.091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yesudian PD, Berry JL, Wiles S, Hoyle S, Young DB, Haylett AK, et al. The effect of ultraviolet B-induced vitamin D levels on host resistance to Mycobacterium tuberculosis: a pilot study in immigrant Asian adults living in the United Kingdom. Photodermatol Photoimmunol Photomed. 2008;24:97–98. doi: 10.1111/j.1600-0781.2008.00339.x. [DOI] [PubMed] [Google Scholar]
- 83.Larcombe LA, Orr PH, Lodge AM, Brown JS, Dembinski IJ, Milligan LC, et al. Functional gene polymorphisms in canadian aboriginal populations with high rates of tuberculosis. J Infect Dis. 2008;198:1175–1179. doi: 10.1086/592049. [DOI] [PubMed] [Google Scholar]
- 84.Selvaraj P, Alagarasu K, Harishankar M, Vidyarani M, Narayanan PR. Regulatory region polymorphisms of vitamin D receptor gene in pulmonary tuberculosis patients and normal healthy subjects of south India. Int J Immunogenet. 2008;35:251–254. doi: 10.1111/j.1744-313X.2008.00764.x. [DOI] [PubMed] [Google Scholar]
- 85.Gibney KB, MacGregor L, Leder K, Torresi J, Marshall C, Ebeling PR, et al. Vitamin D deficiency is associated with tuberculosis and latent tuberculosis infection in immigrants from sub-Saharan Africa. Clin Infect Dis. 2008;46:443–446. doi: 10.1086/525268. [DOI] [PubMed] [Google Scholar]
- 86.Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 87.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crowle AJ, Ross EJ, May MH. Inhibition by 1,25(OH)2-vitamin D3 of the multiplication of virulent tubercle bacilli in cultured human macrophages. Infect Immun. 1987;55:2945–2950. doi: 10.1128/iai.55.12.2945-2950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rook GA, Steele J, Fraher L, Barker S, Karmali R, O'Riordan J, et al. Vitamin D3, gamma interferon and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 90.Denis M. Human monocytes/macrophages: NO or no NO? J Leukoc Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 91.Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clin Immunol. 2009;133:126–131. doi: 10.1016/j.clim.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Jo EK. Innate immunity to mycobacteria: vitamin D and autophagy. Cell Microbiol. 2010;12:1026–1035. doi: 10.1111/j.1462-5822.2010.01491.x. [DOI] [PubMed] [Google Scholar]
- 93.Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, et al. Mycobacterial Lipoprotein Activates Autophagy via TLR2/1/CD14 and a Functional Vitamin D Receptor Signaling. Cell Microbiol. 2010;12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Vidyarani M, Selvaraj P, Jawahar MS, Narayanan PR. 1,25-Dihydroxyvitamin D3 modulated cytokine response in pulmonary tuberculosis. Cytokine. 2007;40:128–134. doi: 10.1016/j.cyto.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Roy S, Frodsham A, Saha B, Hazra SK, Mascie-Taylor CG, Hill AV. Association of vitamin D receptor genotype with leprosy type. J Infect Dis. 1999;179:187–191. doi: 10.1086/314536. [DOI] [PubMed] [Google Scholar]
- 97.Goulart LR, Ferreira FR, Goulart IM. Interaction of TaqI polymorphism at exon 9 of the vitamin D receptor gene with the negative lepromin response may favor the occurrence of leprosy. FEMS Immunol Med Microbiol. 2006;48:91–98. doi: 10.1111/j.1574-695X.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 98.Feindt E, Stroder J. Studies on the antimicrobial effect of vitamin D. Klin Wochenschr. 1977;55:507–508. doi: 10.1007/BF01489010. [DOI] [PubMed] [Google Scholar]
- 99.Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13:6–20. [PubMed] [Google Scholar]
- 100.White AN, Ng V, Spain CV, Johnson CC, Kinlin LM, Fisman DN. Let the sun shine in: effects of ultraviolet radiation on invasive pneumococcal disease risk in Philadelphia, Pennsylvania. BMC Infect Dis. 2009;9:196. doi: 10.1186/1471-2334-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Manaseki-Holland S, Qader G, Isaq Masher M, Bruce J, Zulf Mughal M, Chandramohan D, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health. 2010;15:1148–1155. doi: 10.1111/j.1365-3156.2010.02578.x. [DOI] [PubMed] [Google Scholar]
- 102.Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zügel U, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 103.Matheson EM, Mainous AG, 3rd, Hueston WJ, Diaz VA, Everett CJ. Vitamin D and methicillin-resistant Staphylococcus aureus nasal carriage. Scand J Infect Dis. 2010;42:455–460. doi: 10.3109/00365541003602049. [DOI] [PubMed] [Google Scholar]
- 104.Panierakis C, Goulielmos G, Mamoulakis D, Maraki S, Papavasiliou E, Galanakis E. Staphylococcus aureus nasal carriage might be associated with vitamin D receptor polymorphisms in type 1 diabetes. Int J Infect Dis. 2009;13:437–443. doi: 10.1016/j.ijid.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 105.Claassen M, Nouwen J, Fang Y, Ott A, Verbrugh H, Hofman A, et al. Staphylococcus aureus nasal carriage is not associated with known polymorphism in the Vitamin D receptor gene. FEMS Immunol Med Microbiol. 2005;43:173–176. doi: 10.1016/j.femsim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 106.Youssef D, Bailey B, El Abbassi A, Copeland R, Adebonojo L, Manning T, et al. Healthcare costs of Staphylococcus aureus and Clostridium difficile infections in veterans: role of vitamin D deficiency. Epidemiol Infect. 2010;138:1322–1327. doi: 10.1017/S0950268809991543. [DOI] [PubMed] [Google Scholar]
- 107.Huff GR, Huff WE, Balog JM, Rath NC, Xie H, Horst RL. Effect of dietary supplementation with vitamin D metabolites in an experimental model of turkey osteomyelitis complex. Poult Sci. 2002;81:958–965. doi: 10.1093/ps/81.7.958. [DOI] [PubMed] [Google Scholar]
- 108.Borges MA, Figueiredo LC, Brito RB, Jr, Faveri M, Feres M. Microbiological composition associated with vitamin D receptor gene polymorphism in chronic periodontitis. Braz Oral Res. 2009;23:203–208. doi: 10.1590/s1806-83242009000200018. [DOI] [PubMed] [Google Scholar]
- 109.Islam B, Khan SN, Khan AU. Dental caries: from infection to prevention. Med Sci Monit. 2007;13:196–203. [PubMed] [Google Scholar]
- 110.Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80:108–113. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 111.Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, et al. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- 112.Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001;111:452–456. doi: 10.1016/s0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- 113.Garcia MN, Hildebolt CF, Miley DD, Dixon DA, Couture RA, Anderson Spearie CL, et al. One-year Effects of Vitamin D and Calcium Supplementation on Chronic Periodontitis. J Periodontol. 2011;82:25–32. doi: 10.1902/jop.2010.100207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grant WB, Boucher BJ. Are Hill's criteria for causality satisfied for vitamin D and periodontal disease? Dermato-endocrinol. 2010;2:30–36. doi: 10.4161/derm.2.1.12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Helming L, Bose J, Ehrchen J, Schiebe S, Frahm T, Geffers R, et al. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106:4351–4358. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 116.Bruce D, Whitcomb JP, August A, McDowell MA, Cantorna MT. Elevated non-specific immunity and normal Listeria clearance in young and old vitamin D receptor knockout mice. Int Immunol. 2009;21:113–122. doi: 10.1093/intimm/dxn129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, et al. Vitamin D induces innate antibacterial responses in human trophoblasts via an intracrine pathway. Biol Reprod. 2009;80:398–406. doi: 10.1095/biolreprod.108.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gudmundsson GH, Bergman P, Andersson J, Raqib R, Agerberth B. Battle and balance at mucosal surfaces—the story of Shigella and antimicrobial peptides. Biochem Biophys Res Commun. 2010;396:116–119. doi: 10.1016/j.bbrc.2010.03.081. [DOI] [PubMed] [Google Scholar]
- 119.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151:2423–2432. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu S, Liao AP, Xia Y, Li YC, Li JD, Sartor RB, et al. Vitamin D receptor negatively regulates bacterial-stimulated NFkappaB activity in intestine. Am J Pathol. 2010;177:686–697. doi: 10.2353/ajpath.2010.090998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rudnicki M, Kerschbaum J, Hausdorfer J, Mayer G, König P. Risk factors for peritoneal dialysis-associated peritonitis: the role of oral active vitamin d. Perit Dial Int. 2010;30:541–548. doi: 10.3747/pdi.2009.00108. [DOI] [PubMed] [Google Scholar]
- 122.Wittebole X, Castanares-Zapatero D, Laterre PF. Toll-like receptor 4 modulation as a strategy to treat sepsis. Mediators Inflamm. 2010;2010:568396. doi: 10.1155/2010/568396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grant WB. Vitamin D supplementation could reduce risk of sepsis in infants. World J Pediatr. 2010;6:185. doi: 10.1007/s12519-010-0034-1. [DOI] [PubMed] [Google Scholar]
- 124.Grant WB. Vitamin D supplementation of mother and infant could reduce risk of sepsis in premature infants. Early Hum Dev. 2010;86:133. doi: 10.1016/j.earlhumdev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 125.McKinney JD, Bailey BA, Garrett LH, Peiris P, Manning T, Peiris AN. Relationship between vitamin D status and ICU outcomes in veterans. J Am Med Dir Assoc. 2011;12:208–211. doi: 10.1016/j.jamda.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 126.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360:1912–1914. doi: 10.1056/NEJMc0809996. [DOI] [PubMed] [Google Scholar]
- 127.Zaloga GP, Chernow B, Cook D, Snyder R, Clapper M, O'Brian JT. Assessment of calcium homeostasis in the critically ill surgical patient. The diagnostic pitfalls of the McLean-Hastings nomogram. Ann Surg. 1985;202:587–594. doi: 10.1097/00000658-198511000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diefenbach A, Schindler H, Rollinghoff M, Yokoyama WM, Bogdan C. Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science. 1999;284:951–955. doi: 10.1126/science.284.5416.951. [DOI] [PubMed] [Google Scholar]
- 129.Ehrchen J, Helming L, Varga G, Pasche B, Loser K, Gunzer M, et al. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J. 2007;21:3208–3218. doi: 10.1096/fj.06-7261com. [DOI] [PubMed] [Google Scholar]
- 130.Jongert E, Verhelst D, Abady M, Petersen E, Gargano N. Protective Th1 immune responses against chronic toxoplasmosis induced by a protein-protein vaccine combination but not by its DNA-protein counterpart. Vaccine. 2008;26:5289–5295. doi: 10.1016/j.vaccine.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 131.Rajapakse R, Uring-Lambert B, Andarawewa KL, Rajapakse RP, Abou-Bacar A, Marcellin L, et al. 1,25(OH)2D3 inhibits in vitro and in vivo intracellular growth of apicomplexan parasite Toxoplasma gondii. J Steroid Biochem Mol Biol. 2007;103:811–814. doi: 10.1016/j.jsbmb.2006.12.058. [DOI] [PubMed] [Google Scholar]
- 132.Rajapakse R, Mousli M, Pfaff AW, Uring-Lambert B, Marcellin L, Bronner C, et al. 1,25-Dihydroxyvitamin D3 induces splenocyte apoptosis and enhances BALB/c mice sensitivity to toxoplasmosis. J Steroid Biochem Mol Biol. 2005;96:179–185. doi: 10.1016/j.jsbmb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 133.Silva ME, Silva ME, Silva ME, Nicoli JR, Bambirra EA, Vieira EC. Vitamin D overload and experimental Trypanosoma cruzi infection: parasitological and histopathological aspects. Comp Biochem Physiol Comp Physiol. 1993;104:175–181. doi: 10.1016/0300-9629(93)90026-z. [DOI] [PubMed] [Google Scholar]
- 134.Palacpac NM, Hiramine Y, Seto S, Hiramatsu R, Horii T, Mitamura T. Evidence that Plasmodium falciparum diacylglycerol acyltransferase is essential for intraerythrocytic proliferation. Biochem Biophys Res Commun. 2004;321:1062–1068. doi: 10.1016/j.bbrc.2004.07.070. [DOI] [PubMed] [Google Scholar]
- 135.Vial HJ, Thuet MJ, Philippot JR. Inhibition of the in vitro growth of Plasmodium falciparum by D vitamins and vitamin D-3 derivatives. Mol Biochem Parasitol. 1982;5:189–198. doi: 10.1016/0166-6851(82)90020-2. [DOI] [PubMed] [Google Scholar]
- 136.Bonilla E. Treatment of chromoblastomycosis with calciferol: report of three cases. AMA Arch Derm Syphilol. 1954;70:666–667. [PubMed] [Google Scholar]
- 137.Etzioni A, Hochberg Z, Pollak S, Meshulam T, Zakut V, Tzehoval E, et al. Defective leukocyte fungicidal activity in end-organ resistance to 1,25-dihydroxyvitamin D. Pediatr Res. 1989;25:276–279. doi: 10.1203/00006450-198903000-00012. [DOI] [PubMed] [Google Scholar]
- 138.Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29:369–375. doi: 10.1016/j.mam.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Murray JJ, Heim CR. Hypercalcemia in disseminated histoplasmosis. Aggravation by vitamin D. Am J Med. 1985;78:881–884. doi: 10.1016/0002-9343(85)90300-6. [DOI] [PubMed] [Google Scholar]
- 140.Drutz DJ, Cline MJ. Intermittent neutrophil-monocyte bactericidal defects in a patient with sarcoidosis. Am Rev Respir Dis. 1975;112:387–392. doi: 10.1164/arrd.1975.112.3.387. [DOI] [PubMed] [Google Scholar]
- 141.Chowdhury N. Effects of fat soluble vitamins (vitamin A, D3 and E) on axenically in vitro growth of Hymenolepis microstoma. Z Parasitenkd. 1978;56:29–38. doi: 10.1007/BF00925934. [DOI] [PubMed] [Google Scholar]
- 142.Addis CJ, Jr, Chandler AC. Further studies on the vitamin requirement of tapeworms. J Parasitol. 1946;32:581–584. [PubMed] [Google Scholar]
- 143.Olsen A, Thiong'o FW, Ouma JH, Mwaniki D, Magnussen P, Michaelsen KF, et al. Effects of multimicronutrient supplementation on helminth reinfection: a randomized, controlled trial in Kenyan schoolchildren. Trans R Soc Trop Med Hyg. 2003;97:109–114. doi: 10.1016/s0035-9203(03)90042-3. [DOI] [PubMed] [Google Scholar]
- 144.Nielsen NO, Skifte T, Andersson M, Wohlfahrt J, Søborg B, Koch A, et al. Both high and low serum vitamin D concentrations are associated with tuberculosis: a case-control study in Greenland. Br J Nutr. 2010:1–5. doi: 10.1017/S0007114510002333. [DOI] [PubMed] [Google Scholar]
- 145.Martineau AR, Timms PM, Bothamley GH, Hanifa Y, Islam K, Claxton AP, et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet. 2011;377:242–250. doi: 10.1016/S0140-6736(10)61889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Alvarez-Hernandez D, Naves-Diaz M, Gomez-Alonso C, Coto E, Cannata-Andia JB. Tissue-specific effect of VDR gene polymorphisms on the response to calcitriol. J Nephrol. 2008;21:843–849. [PubMed] [Google Scholar]
- 147.Grant WB. In defense of the sun: An estimate of changes in mortality rates in the United States if mean serum 25-hydroxyvitamin D levels were raised to 45 ng/mL by solar ultraviolet-B irradiance. Dermatoendocrinol. 2009;1:207–214. doi: 10.4161/derm.1.4.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]