Abstract
Background
A mathematical model describing mucociliary clearance in cystic fibrosis (CF) patients and its development with progressing course of the disease was developed. The approach should support the prediction of the disease state on the basis of measured bronchial clearance efficiencies.
Methods
The approach is based on the assumption of a steady-state steady-flow mucus transport through the tracheobronchial tree which enables the determination of airway generation-specific mucus velocities by using a measured tracheal mucus velocity and a realistic morphometric dataset of the human lung. Architecture of the tracheobronchial tree was approximated by a stochastic model, reflecting the intra-subject variability of geometric parameters within a given lung generation.
Results
As predicted by the appropriately validated mathematical approach, mucociliary clearance efficiency in CF patients is partly significantly decreased with respect to healthy controls. 24-h retention of patients with mild CF (FEV1 >70% of predicted) is reduced by 10% compared to healthy subjects, whilst 24-h retention of patients with moderate to severe CF (FEV1 <70% of predicted) differs by 25% from that of the healthy controls. These discrepancies are further enhanced with continuation of the clearance process.
Conclusions
The theoretical results lead to the conclusion that CF patients have a higher risk of inhaled particle accumulation and related particle overload in specific lung compartments than healthy subjects.
Keywords: Clearance model, stochastic lung structure, mucociliary clearance, 24-h retention
Introduction
Concerning its pathophysiology cystic fibrosis (CF) has to be regarded as a multi-organ disease that is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (1)-(3). Organs expressing CFTR among other include the lungs, pancreas, liver, reproductive tract, and sinuses, whereby CFTR mutations are commonly limited to the epithelial cells of these organic structures. In the lung airways, the basic genetic defect is responsible for chronic infections with bacterial pathogens that, due to their severity and chronicity, result in bronchiectasis and, subsequently, respiratory failure (1). In the past, several hypothesis linking mutations in the CFTR gene with the outbreak of bacterial infections have been subjected to an intense debate. Besides the inflammation-first hypothesis (4), the cell-receptor hypothesis (5),(6), and the salt-defensin or chemical-shield hypothesis (7)-(9) above all the isotonic fluid depletion/anoxic mucus hypothesis has excited enhanced medical interest. According to the last-named theory, a failure of the CFTR to secrete chloride is coupled with an abnormal absorption of sodium from the airway lumen. This intracellular increase in salt concentration has its consequence in a water uptake by the epithelial cells, resulting in a continuous volume depletion of the periciliary liquid (PCL) layer (10). Due to an increase in mucus viscosity induced by the water loss, higher amounts of inhaled bacteria (Pneumococcus aeruginosa, Staphylococcus aureus) are trapped in the mucus layer lining the airway epithelium. Within this unusual growth environment the bacteria encounter anaerobic conditions triggering their switch from non-mucoid to mucoid cell types (11).
Concerning the successful protection of lung airways from inhaled bacteria and other hazardous particles of natural or anthropogenic origin, mucociliary clearance has to be regarded as a primary innate defense system. According to several in vivo studies, airway walls loaded with organic or inorganic particulate mass are typically cleared within 6 h (12), whilst cleansing of the entire tracheobronchial tree is mainly completed within 24 h (13). In the healthy lung, mucociliary clearance takes place by the cilia beating-induced transport of the mucus layer towards the trachea, where it is either coughed out or swallowed (14). In order to produce a synchronous, wave-like movement of the cilia needed for an effective impelling of the mucus mass, the low-viscous and lubricant PCL is interposed between the mucus layer and the cell surface (Figure 1). Volume and composition of the PCL is chiefly controlled by two mechanisms, the first of which reflects the role of the mucus layer as a ‘liquid receptor’ and ‘liquid donator’. This specific property helps to preserve PCL height over a certain range of airway surface liquid (ASL) volumes (15). The second mechanism controlling PCL volume is represented by active transepithelial ion transport which has to be understood as a mix of sodium absorption and chloride secretion. Whilst uptake of sodium by epithelial cells via the Na+-K+-ATPase is coupled with cellular water absorption through the aquaporin water channels and a volume depletion of the PCL, release of chloride through the cAMP-mediated Cl−-channel results in a volume addition to the PCL (1).
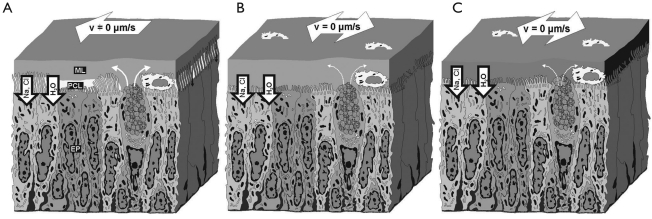
Figure 1. Pathogenic idea of CF lung disease (3),(4). In a healthy subject, the airway epithelium is lined by a mucus layer (ML) residing atop the so-called periciliary liquid layer (PCL); A. Due to the presence of the low-viscosity PCL, efficient mucociliary clearance is facilitated, whereby velocity of the mucus layer decreases from proximal to periphery lung generations. In CF airways, volume of both layers is successively depleted, leading to the complete removal of the PCL (B, C). The mucus layer becomes adherent to the epithelial surface and, as a consequence, mucociliary clearance slows down and stops.
In lungs affected by CF, cellular uptake of sodium is accompanied by defective cAMP/CFTR-mediated chloride secretion (see above), leading to a continuous depletion of the PCL. Concerning mucociliary particle clearance this reduction of the PCL causes a successive failure of the mucus transport insofar as dehydration is associated with a flattening of the mucus-driving cilia on the epithelial surface (Figure 1). This effect is further intensified by the circumstance that the mucus layer donates all the liquid feasible (about 50% of its normal volume) to the PCL, modifying it to a highly viscous mass with decreased elasticity. Due to a relatively little autofeedback between the removal of mucus from airway surfaces and the secretion of mucin by goblet cells, production of mucus components is continued. This implies the typical plaques and plugs that are frequently observed on CF airway surfaces (1). According to experimental findings outlined by Knowles and Boucher (16), thickness of the ASL of CF airway epithelia may be reduced by about two thirds (29 µm to 10 µm) within 24 h. As an important consequence of that, mucus rotational velocity in CF cell cultures may decrease from 45 µm s−1 at t=0 h to 6 µm s−1 at t=24 h, corresponding to a reduction of 88%.
Concerning the theoretical simulation of mucociliary clearance in the human respiratory tract several significant progresses have succeeded during the past 30 years, starting with respective mucus transport models in symmetric lung structures (17),(18) and finding its provisional end in the development of stochastic approaches of mucus transport (19)-(21). Theoretical description of mucociliary clearance in diseased lungs is so far limited to a low number of insufficiencies such as chronic obstructive pulmonary disease (COPD) with relatively limited number of modeling variables (22). Preliminary models on the clearance of deposited particulate mass from CF lungs have commonly assumed a viscosity-induced, linear decrease of mucus velocities with increasing grade of the disease, resulting in a final stand-still of this clearance mechanism (23).
In the study presented here, current theories of mucociliary clearance in CF patients are incorporated into a mathematical approach that has been previously outlined in several scientific contributions (19)-(21). Theoretical predictions carried out with the help of this actualized model address two main aims: First, they attempt to provide to a further improvement of inhalation therapy efficiencies by considering respective clearance effects on deposited medical aerosols; second, they contribute to the temporary optimization of mucolytics inhalation, being a fundamental problem regarding the life quality of CF patients.
Mathematical model of mucociliary clearance in CF lungs
General description of the mucociliary clearance model
The mathematical approach describing (fast) mucociliary clearance in the tracheobronchial tree of the human lung is founded upon the five-lobe lung structure provided by Yeh et al. (24) as well as ten fully asymmetric lung trees that were exclusively generated by stochastic calculation methods (25). For an appropriate computation of airway-specific mucus velocities, the modeling approach had to be simplified insofar as (i) mucus layer thickness was assumed to be constant within single airway bifurcations, (ii) travelling of the mucus mass was believed to take place with a constant net velocity, (iii) identical mucus production rates in the terminal bronchioles were considered, and (iv) thickness of the mucus layer lining the wall of a given airway tube was (negligibly) small with respect to the airway diameter (19),(20). Based upon the simplifications noted under point (i) to (iv), steady-state steady-flow mucus transport in a pre-selected airway bifurcation may be expressed by the equation
| [1] |
with Qp, Qd1, and Qd2 denoting the volume flow rate of the mucus mass in the parental section (p) and the two daughter sections (d1, d2) of that bifurcation. In general, the volume flow rate Qp in the parental section p of a given bifurcation is simply derived from the formula
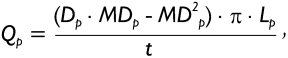 |
[2] |
where Dp, MDp, and Lp represent the airway diameter, the thickness of the (continuous) mucus blanket, and airway length, whereas t denotes a certain time unit (s or min). Since the term Lp/t corresponds to the velocity Vp of the mucus blanket in the parental tube, equation [1] can be re-written as follows:
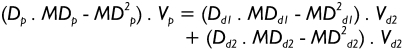 |
[3] |
In the formula noted above, all variables with the subscript d1 are attributed to daughter airway d1, whilst variables with the subscript d2 belong to daughter airway d2. According to assumption (iii) mentioned above mucus velocity has to be regarded as constant in the terminal bronchioles (Vt), so that mucus volume balance between the trachea, termed as airway generation 0, and the terminal bronchioles yields:
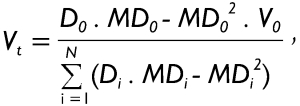 |
[4] |
In equation [4], all variables carrying the subscript 0 are attributed to the trachea. Variables Di and MDi denote the diameter and the thickness of the mucus layer in the i-th airway, and N indicates the total number of terminal bronchioles. Since tracheal mucus velocity V0 represents a measurable physiological parameter that commonly varies with both age and level of physical activity, mucus velocity in the terminal airways can be simply calculated from equation [4]. After computation of mucus velocities in the terminal bronchioles, mucus velocities of more proximal airway generations can be determined step by step by application of equation [3]. It has to be noted in this context that computations conducted on the basis of the procedure described here are remarkably simplified in the case of a symmetric lung structure, since diameters and mucus velocities are equal in all airways of a given generation.
An essential parameter indicating the efficiency of mucociliary clearance in a pre-selected airway is the clearance rate (CR) which is generally expressed by the formula
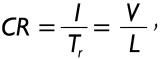 |
[5] |
where Tr is the residence time (i.e., the time for the mucus mass to be transported through an airway under conditions of steady-state and steady-flow), V the velocity of the mucus layer and, as a consequence, of the particles loaded on it, and L the length of the airway tube. Assuming that deposition of particles on the airway surface is rather inhomogeneous due to the combined influence of various deposition forces, residence time in that airway initially targeted by a particle may range from 0 to the time value derived from equation [5]. A solution for this phenomenon of deposition-dependent residence times in the initial airways of the clearance path is the calculation of an average Tr that is derived from individual residence times after particle deposition by Browian motion, gravitational settling, and inertial impaction, respectively.
By defining each airway as an independent compartment that is characterized by an initial mass of deposited particles (mi), by a particulate mass obtained from the daughter generation (md1, md2), and by a number of particles delivered to the more proximal airway generation (mout), the following mass balance equation can be formulated:
| [6] |
As outlined in detail by Asgharian and colleagues (19), the term dmi/dt is commonly defined by the product of deposition fraction, minute ventilation, and particle concentration. The term dmout/dt, on the other hand, is simply expressed by the formula:
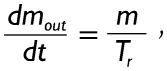 |
[7] |
thereby suggesting a constant outflow of mucus mass and removal of captured particulate matter with time. Equation [7] has also to be applied for the description of dmd1/dt and dmd2/dt, whereby individual measures for m and Tr being valid in the daughter tubes have to find their use.
Solution of equation [6] is founded upon an approach, in which clearance in each airway of the stochastic lung structure is computed at consecutive small time increments. Thereby, computation ranges from the start of exposure to a certain time point of the post-exposure period (e.g. 48 h). Due to the mathematical method of small time increments equation [6] can be solved independently for each airway, obtaining the following resultant equation:
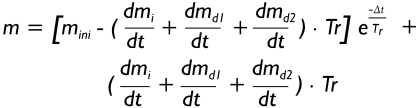 |
[8] |
In equation [8] mini denotes the initial mass that can be found in the airway at the start of time increment Δt. As a main simplification the term (dmi/dt + dmd1/dt + dmd2/dt) is constant during each time increment, but may be subject to a change between time increments. Regarding the terminal bronchioles, which according to equation [4] represent the starting points of clearance computations, dmd1/dt = dmd2/dt = 0, so that equation [8] is significantly simplified to
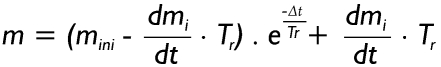 |
[9] |
The mucus mass cleared from the terminal bronchioles (mout) is determined by combining equations [7] and [9] and integrating the right side of the newly generated formula in the interval [0, Δt]:
| [10] |
In order to prevent a high number of integrations within the modeling procedure, equation [10] may be considerably simplified by re-formulation of equation [6] and combination of expressions [6] and [10]:
| [11] |
After mass balance computations for the terminal airways have been completed, the same procedure has to be carried out for the parental tubes of these outermost bronchioles, whereby equation [8] has to be used instead of equation [9], and mini is set to 0 at the start of exposure. Calculations are continued for all airways of the tracheobronchial tree, until the trachea (generation 0) is reached.
Model modifications for the prediction of muco-ciliary clearance in CF patients
For an appropriate prediction of mucociliary clearance in CF patients the theoretical approach described in the preceding section has to be refined in three main aspects. First, thickness of the unsupplied mucus layer is decreased by about 65% within 24 h, reflecting its continuous dehydration due to elevated ion concentrations in the underlying epithelial cell. Second, viscosity of the mucus mass is increased and function of the mucus-repelling cilia is subject to a successive impairment. Third, airway calibers are reduced due to epithelial edema and the non-feedback secretion of mucus by goblet cells and subepithelial glands. The effect of mucus thickness modification due to dehydration is considered in the advanced model insofar as the parameter MD is computed for each time point according to the equation
| [12] |
where k denotes the rate of mucus reduction that is on the order of 0.044 h−1. Values for MD computed with the help of equation [12] are subsequently inserted into the formulae [2]-[4]. Since mucus thickness is not homogeneously changed in the airway tubes of a CF patient, MD is further subjected to a stochastic variation within the interval [MD – a, MD + a], where the parameter a represents a certain percentage (e.g. 10%) of the MD value derived from equation [12].
As indicated by equations [2]-[4], a (stochastically variable) change of mucus thickness throughout the whole tracheobronchial tree has only minor effects on mucus velocities in the airways. Considering a lower increase of MD in the parental tube with respect to its daughters, mucus velocity in the parental airway is further increased in order to preserve the steady-state steady-flow frame conditions noted above. The effects of increasing mucus viscosity and progressive malfunction of the epithelial cilia are realized by definition of a time-dependent change (decrease) of the velocity V according to the expression:
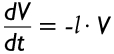 |
[13] |
Similar to the mucus reduction rate k in formula [12], l describes the reduction of mucus velocity per time. According to the experimental findings of Knowles and Boucher (16) this essential parameter commonly takes values of 0.084 h−1.
An interesting aspect arises regarding a reliable reduction of airway calibers in CF patients due to illness-caused modifications of the bronchial epithelium. This essential problem was solved insofar as airway diameters were commonly reduced by using the airway resistance that was determined for all CF patients. With the help of this parameter a mean airway scaling factor could be computed (27), which was subsequently subjected to a random variation. Therefore, in patients with mild CF reduction of the airway calibers varied between 0 and 30%, whereas in patients with moderate to severe CF respective reduction ranged from 20 to 50%. The trachea was not included into the scaling procedure.
Model validation and parameter setting for advanced predictions
In order to check the validity of the mucociliary clearance model outlined above, the clearance experiments of Lindström et al. (26) were subjected to a theoretical simulation. For their experimental study the authors selected eleven patients (four males/seven females) with an age of 18.7+/-2.5 years, suffering from mild to moderate CF. Pulmonary function of the volunteers was measured by forced expirograms, and airway resistance (Raw) was analyzed with the help of a panting technique within a whole-body plethysmograph. Based on the obtained data for lung function, patients were categorized, thereby distinguishing between a group with mild lung disease (FEV1 >70% of predicted) and a group with moderate to severe lung disease (FEV1 <70 % of predicted). Inhalation experiments were conducted with eleven CF patients as well as twelve healthy control subjects. All participants inhaled a monodisperse aerosol containing 111In-labelled teflon particles with a geometric diameter of 6 µm (mean aerodynamic diameter: 6.2 µm). Inhalative flow uniformly amounted to 0.05 L s−1, which should enable a deposition of the relatively large particles in the small ciliated airways. Radioactivity emerging from the inhaled particulate mass was measured immediately after inhalation and after further 24 h, 7 d, 14 d, and 21 d.
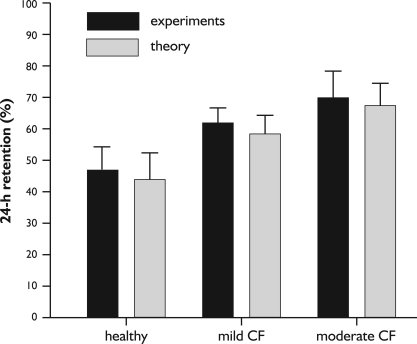
For an appropriate simulation of the experiments noted above, particle properties and inhalation parameters determining particle deposition prior to mucociliary clearance exactly corresponded with the experimental setup. Any impairment of the lung architecture due to the disease (e.g., decrease of the airway calibers, emphysematous increase of the alveoli) was approached with the help of the airway resistance measured for all patients, thereby determining a mean airway scaling factor (see above). Whilst in healthy subjects mucus velocity in single airways was derived from a mean tracheal mucus velocity of 5.5 mm min−1, in patients with mild CF a mucociliary clearance index (MCI) of 60-80% (reduction of mucus velocities by 20-40%) and in patients with moderate to severe CF a mucociliary clearance index of 40-60% (reduction of mucus velocities by 40-60%) was assumed. Comparisons between experimental and theoretical results are summarized in Figure 2, thereby using the 24-h retention or A-value, which is commonly regarded to express (mucociliary) clearance efficiency within the first 24 h following particle exposure. Regarding the healthy subjects, 24-h retention of the slowly inhaled particulate mass amounts to +/- 8.5% and corresponding theoretical value is 45.2+10.6%. In patients suffering from mild CF, a 24-h retention of 62+/- 5% (experiment) and 59.3+/- 6.7% (theory) could be determined, whereas in patients with moderate to severe CF respective parameters take values of 71+/- 9% (experiment) and 68.4+/- 8.5% (theory). It has to be noted that these values also consider those particle fractions that are either removed by slow bronchial clearance or subjected to the alveolar innate defense system. Based on theoretical deposition calculations using the setup parameters noted above, the overall fraction of slowly cleared particles of the diameter class used by the experimentators amounted to ca. 40%.
Figure 2. Comparison between 24-h retention values of healthy subjects, patients with mild CF, and patients with moderate to severe CF derived from clearance experiments [26] and respective results obtained from theoretical computations (mean +/-95%-ci).
For advanced predictions of mucociliary clearance in CF patients, diameters of inhaled uniform-density particles was varied between 100 nm and 10 µm, whilst inhalative flow was uniformly increased to 250 mL s−1 to create a more realistic breathing scenario. In order to simulate a possible degradation of the disease with time, mucus thicknesses, mucus velocities, and airway calibers were continuously modified according to the mathematical functions stated above.
Results
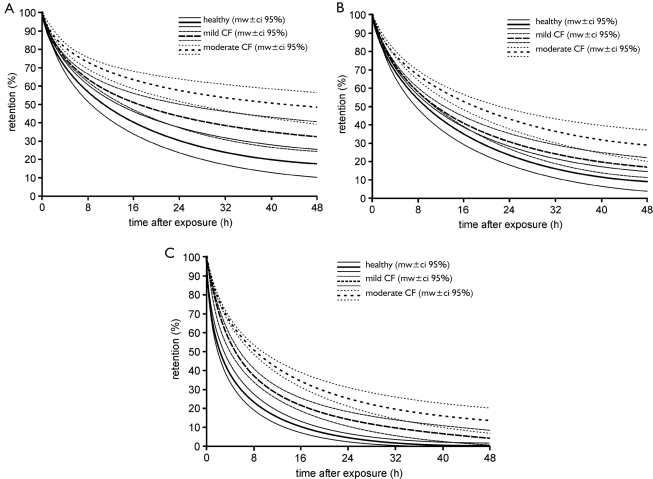
As already suggested by clearance experiments, which were carried out under conditions of extremely slow inhalation (26), mucociliary clearance is subject to a continuous decrease in efficiency with progressive course of the CF disease. Respective theoretical predictions conducted under the assumption of a physiologically realistic breathing scenario and coupled particle deposition clearly demonstrate that differences in fast particle clearance between healthy subjects and CF patients may become significant (P<0.05), after patients have reached a specific stage of the disease. In the graphs of Figure 3 retention curves (mean values +/- standard deviations) are compared between healthy subjects, patients suffering from mild CF, and patients being affected by moderate to severe CF. Particle retention was computed within the time interval reaching from 0 h after aerosol exposure to 48 h after aerosol exposure, whereby mucociliary transport times exceeding 2 d commonly indicate an enhanced particle deposition in small ciliated airways being located at the outermost periphery of the tracheobronchial tree. Concerning the fast clearance of particles with a diameter of 0.1 µm (Figure 3A, 4), in healthy subjects 68.2+/- 7.5% of the deposited mass are removed from the airways after 24 h, and 80.1+9.2% are cleared after 48 h. In patients with mild CF particulate mass cleared after 24 h and 48 h amounts to 54.2+/- 7.9% and 66.3+/- 8.1%, respectively, corresponding to a 17%-decrease in clearance efficiency with respect to healthy people. In patients with moderate to severe CF the trend already predicted for mild CF is further continued: after 24 h only 41.8+/- 6.7% of all particles deposited in the tracheobronchial tree are removed with the help of the mucociliary escalator, and after 48 h this value increases to 49.2+/- 9.5%. Therefore, clearance efficiency compared to the healthy subjects has decreased by about 42%. Mucociliary clearance of particles with a diameter of 1 µm differs from that of the 0.1-µm particles insofar as higher amounts of deposited mass are removed after 24 h and 48 h (Figure 3B, 4). In healthy subjects percentages of cleared particulate matter amount to 76.2+/- 6.6% (24 h) and 89.6+/- 5.8% (48 h). In patients suffering from mild CF these values are decreased to +/- for the percent values following below 68.9+/- 5.4% (24 h) and 81.1+/- 6.1% (48 h) which corresponds to a 9%-decline in clearance efficiency with respect to the healthy subjects. In people with moderate to severe CF the fraction of particles removed by the mucociliary escalator is further decreased to 57.8+/- 5.3% after 24 h and to 69.5+/- 7.1% after 48 h. Here, fast clearance efficiency is subject to a 30%-reduction with respect to healthy people. Regarding the mucociliary clearance of particles with a diameter of 10 µm, efficiency of particulate matter removal has to be generally regarded as rather high (Figure 3C, 4). In healthy subjects fractions of cleared particles after 24 h and 48 h amount to 95.8+/- 4.3% and 100+/- 2.1%, indicating an almost complete evacuation of particulate mass deposited in the tracheobronchial tree after 1 d. In patients with mild CF respective clearance fractions are decreased to 85.3+/-4.2% (24 h) and 94.9+/- 5.3% (48 h) which is equal to a relative reduction in clearance efficiency of 10%. In the lungs of patients with moderate to severe CF mucociliary clearance of 10-µm particles has been completed by 77.2+/- 5.5% after 24 h and by 86.4+/- 7.3% after 48 h. This corresponds to a 17%-decrease in clearance efficiency with respect to healthy people.
Figure 3. Retention curves (mean +/- 95%-ci) of healthy subjects, patients with mild CF, and patients with moderate to severe CF calculated with the help of the advanced mucociliary clearance model introduced in this contribution. A: mucociliary clearance of particles with a diameter of 0.1 µm; B: mucociliary clearance of 1-µm particles; C. mucociliary clearance of 10-µm particles.
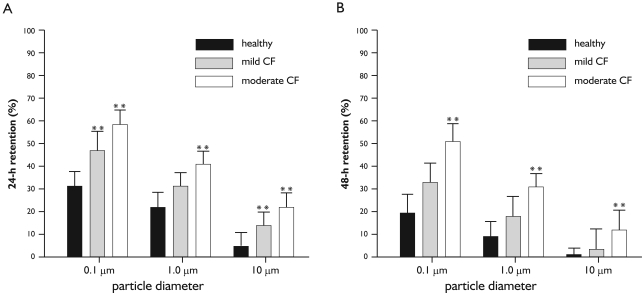
Figure 4. Intercomparison of retention values between healthy subjects, patients with mild CF, and patients with moderate to severe CF for particles adopting diameters of 0.1 µm, 1 µm, and 10 µm (mean +/- 95%-ci). A: behaviour of 24-h retention among the three health categories; B: behaviour of 48-h retention among the three health categories. Computed significances (*: P<0.05; **: P<0.001) are related to the predicted retention values of healthy subjects (black columns), respectively.
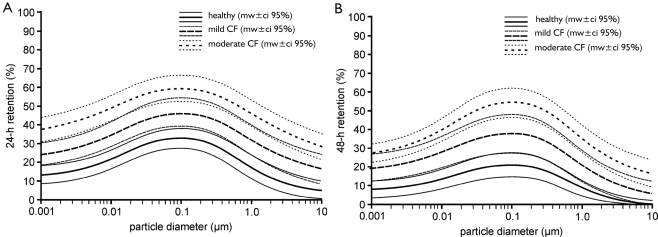
In order to obtain a more detailed insight into the relationship between mucociliary clearance efficiency and particle size, 24-h retention as well as 48-h retention were plotted against particle diameter, thereby considering a particle size range from 0.001 µm to 10 µm (Figure 5). In general, the retention functions generated with the computer model are characterized by two sections, i.e., a nearly increasing retention for particle sizes ranging from 0.001 µm to 0.1 µm, and a decrease in retention for particle sizes ranging from 0.2 µm to 10 µm. Since velocity of the particle transport on the mucus layer is regarded as constant among the size classes, differences in retention exclusively result from discrepancies and deposition, i.e., 0.1-µm particles penetrate to deeper lung regions and thus need more time to be evacuated towards the trachea. Concerning the comparison of healthy subjects with CF patients suffering from mild or moderate to severe disease grade, the results provided in Figure 3 find their general confirmation. As reflected in Figure 5, fractions of particles retained in the tracheobronchial tree after 24 h and 48 h are subject to a partly significant (P<0.05) elevation with progressing grade of disease. Whilst 24-h retention of patients with mild CF is increased by about 10% with respect to the healthy controls, respective retention values of patients with moderate to severe CF exhibit an increase of about 20% compared to the healthy controls. A similar phenomenon is observable for the 48-h retention, where differences between healthy subjects and mild CF patients amount to 12% and differences between healthy controls and moderate/severe CF patients yield 30%.
Figure 5. Dependence of 24-h retention (A) and 48-h retention (B; mean +/- 95%-ci) on particle size for particle diameters ranging from 0.001 µm to 10 µm.
Discussion
As largely underlined by experimental studies conducted during the past decade (26),(28),(29), lung diseases may have a remarkable effect on the fast clearance of particulate matter deposited on the epithelial walls of the tracheobronchial tree. Regarding CF, which is caused by a defect on the CFTR gene, this influence on mucociliary clearance is given by (i) a continuous dehydration and increase in viscosity of the mucus mass and (ii) a successive impairment of mucus-repelling cilia due to these modifications of mucus consistency (1)-(3). The CF-induced phenomena located on the surface of the bronchial epithelium result in a permanent decrease of the mucus transport velocity and, as a consequence of that, lead to a decline of the fast clearance efficiency, which is necessary to protect the lungs from a particle overload after abnormal exposure to ambient aerosols (30),(31). In the worst case, mucociliary clearance may come to a complete stand-still, representing the failure of a significant innate defense system. Theoretical models like that presented here may help to understand the mechanisms being concealed behind the gradual failure of bronchial clearance in CF patients and to manage the administration of mucolytics for a (partial) regeneration of the bronchial cleansing system (32).
Theoretical simulation of mucociliary clearance in the human lung dates back to the 1970s and early 1980s (18),(19), whereby early models used a symmetric (deterministic) lung structure, within which clearance paths of particles deposited in the same airway generation are characterized by identical lengths and transport times. The mathematical approach presented here differs significantly from those early models insofar as the idealistic lung structure was replaced by a more realistic one. Within a stochastic lung clearance paths of particles deposited in a specific airway generation have individual, partly highly variable lengths, thereby reflecting the so-called ‘intra-subject variability’ of mucociliary clearance (20),(21). Validation of a model limited to the fast clearance phase in the tracheobronchial tree is quite difficult, since experimental studies are not able to distinguish easily between single types of bronchial clearance (fast and slow; (21)-(23)) and alveolar clearance that may need months to years, until particulate mass is completely removed (33). In the case given here, mucociliary clearance was limited to the mean particle fraction which had been removed from the lungs of healthy controls 1 d after aerosol exposure (ca. 40%; Figure 2). The remaining fraction was assumed to be either subjected to slow bronchial clearance or affected by alveolar clearance. Earlier experimental investigations yielded evidence that the slow bronchial clearance fraction decreases linearly with increasing geometric diameter of the deposited particulate matter (21),(22). Assuming a normal breathing scenario (22), slowly cleared fraction of 6-µm particles like those used for the clearance experiments described above would amount to several percent. Due to the aerosol administration by extremely slow inhalation, inertial impaction of the particles is declined to a minimum and gravitational settling, preferentially taking place in the peripheral airways, reaches its maximum. This implies significant enhancements of the slow bronchial clearance fraction and, due to increased alveolar deposition, also of the particulate fraction cleared from the alveoli (34).
As exhibited by the theoretical predictions of the validated model, mucociliary clearance in CF patients differs from that of healthy controls insofar as respective clearance efficiency, e.g. expressed by the mucociliary clearance index (MCI), becomes continuously decreased with rising grade of the disease. Whilst mucociliary clearance of patients with mild CF is already characterized by partly significant retardations with respect to the healthy subjects, mucociliary clearance of patients with moderate to severe CF is commonly reduced in its effectiveness to <70%, corresponding to a considerable impairment of the pulmonary innate defense system and, as a consequence, of life quality. As underlined by several results obtained from clearance experiments with CF patients (26),(28),(29), mucociliary clearance in diseased lungs is successively slowed down compared to healthy controls, but a complete stand-still seems to be excludable for patients suffering from a mild to moderate form of the insufficiency. Slow-inhalation experiments could further yield evidence that whole clearance paths reaching from the terminal bronchioles to the trachea are either not or only insignificantly affected by CF-induced modifications on the airway surfaces (see above). This phenomenon may lead to the assumption that CF does not affect the cells of the tracheobronchial epithelium in a homogeneous fashion, but rather arises at single ‘hot spots’, from which the further spreading of the disease is initiated. This patchy occurrence of cells or cell clusters with defect CFTR gene at early stages of CF may be also confirmed by a missing coordination of cells within the epithelial layer, leading e.g. to a continued secretion of mucin granules despite of their failing transport (1).
From the theoretical results presented in this contribution the following general conclusions may be drawn: (i) Due to a continuous retardation of (fast) mucociliary clearance with progressing CF disease, CF patients exposed to ambient aerosols (above all to certain kinds of bioaerosols) are increasingly endangered by long-term particle accumulation and cellular particle overload compared to healthy controls, strongly suggesting their stay in habitats with clean atmosphere; (ii) Administration of mucolytics seems to reverse the CF-induced process of decreasing mucociliary clearance efficiency only in parts, because epithelial cilia, being subjected to a permanent mechanical stress, become disintegrated and paralyzed within a certain time span. Hence, CF, being understood according to the cell-biological theory of Knowles and Boucher (16), may be compared to a certain degree to immotile cilia syndrome (ICS); (iii) Although the validated model seems to provide reliable predictions of mucociliary clearance in CF-affected lungs, it has to be subjected to further improvements in near future. These among other include a precise simulation of particle clearance routes apart from their transport on the mucus layer.
Footnotes
No potential conflict of interest.
References
- 1.Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1359–71. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 2.Cutting GR. Genotype defect: Its effect on cellular function and phenotypic expression. Sem Respir Crit Care Med. 1994;15:356–63. [Google Scholar]
- 3.Welsh MJ, Tsui TF, Boat LC, Beaudet AL. Cystic Fibrosis. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Stanbury JB, Wyngaarden JB, Fredrickson DS, editors. Cystic Fibrosis. Vol. 7. New York: McGraw-Hill; 1995. pp. 3799–876. [Google Scholar]
- 4.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–82. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 5.Poschet JF, Boucher JC, Tatterson L, Skidmore J, Van Dyke RW, Deretic V. Molecular basis for defective glycosylation and Pseudomonas pathogenesis in cystic fibrosis lung. Proc Natl Acad Sci U S A. 2001;98:13972–7. doi: 10.1073/pnas.241182598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imundo L, Barasch J, Prince A, Al-Awqati Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc Natl Acad Sci U S A. 1995;92:3019–23. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–36. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 8.Widdicombe JH. Altered NaCl concentration of airway surface liquid in cystic fibrosis. Pflugers Arch. 2001;443:S8–10. doi: 10.1007/s004240100636. Suppl. 1. [DOI] [PubMed] [Google Scholar]
- 9.Bals R, Weiner DJ, Meegalla RL, Wilson JM. Transfer of a cathelicidin peptide antibiotic gene restores bacterial killing in a cystic fibrosis xenograft model. J Clin Invest. 1999;103:1113–7. doi: 10.1172/JCI6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–15. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 11.Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109:317–25. doi: 10.1172/JCI13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wanner A, Salathé M, O'Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 13.Lippmann M, Yeates DB, Albert RE. Deposition, retention, and clearance of inhaled particles. Br J Ind Med. 1980;37:337–62. doi: 10.1136/oem.37.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert RE, Lippmann M, Briscoe W. The characteristics of bronchial clearance in humans and the effects of cigarette smoking. Arch Environ Health. 1969;18:738–55. doi: 10.1080/00039896.1969.10665482. [DOI] [PubMed] [Google Scholar]
- 15.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol. 2001;118:223–36. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–7. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee PS, Gerrity TR, Hass FJ, Lourenco RV. A model for tracheobronchial clearance of inhaled particles in man and a comparison with data. IEEE Trans Biomed Eng. 1979;26:624–30. doi: 10.1109/tbme.1979.326544. [DOI] [PubMed] [Google Scholar]
- 18.Cuddihy RG, Yeh HC. Respiratory tract clearance of particles and substances dissociated from particles. In: Mohr U, editor. Inhalation toxicology: The design and interpretation of inhalation studies and their use in risk assessment. Berlin: Springer; 1988. pp. 169–93. [Google Scholar]
- 19.Asgharian B, Hofmann W, Miller FJ. Mucociliary clearance of insoluble particles from the tracheobronchial airways of the human lung. J Aerosol Sci. 2001;32:817–32. [Google Scholar]
- 20.Sturm R, Hofmann W. Stochastic modeling predictions for the clearance of insoluble particles from the tracheobronchial tree of the human lung. Bull Math Biol. 2007;69:395–415. doi: 10.1007/s11538-006-9143-3. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann W, Sturm R. Stochastic model of particle clearance in human bronchial airways. J Aerosol Med. 2004;17:73–89. doi: 10.1089/089426804322994488. [DOI] [PubMed] [Google Scholar]
- 22.International Commission on Radiological Protection (ICRP) Publication 66. Oxford: Pergamon Press; 1994. Human respiratory tract model for radiological protection; p. 478. [Google Scholar]
- 23.Sturm R, Hofmann W. Mechanistic interpretation of the slow bronchial clearance phase. Radiat Prot Dosimetry. 2003;105:101–4. doi: 10.1093/oxfordjournals.rpd.a006201. [DOI] [PubMed] [Google Scholar]
- 24.Yeh HC, Schum GM, Duggan MT. Anatomic models of the tracheobronchial and pulmonary regions of the rat. Anat Rec. 1979;195:483–92. doi: 10.1002/ar.1091950308. [DOI] [PubMed] [Google Scholar]
- 25.Koblinger L, Hofmann W. Monte Carlo modeling of aerosol deposition in human lungs. Part I: Simulation of particle transport in a stochastic lung structure. J Aerosol Sci. 1990;21:661–74. [Google Scholar]
- 26.Lindström M, Camner P, Falk R, Hjelte L, Philipson K, Svartengren M. Long-term clearance from small airways in patients with cystic fibrosis. Eur Respir J. 2005;25:317–23. doi: 10.1183/09031936.05.00120103. [DOI] [PubMed] [Google Scholar]
- 27.King M, Chang HK, Weber ME. Resistance of mucus-lined tubes to steady and oscillatory airflow. J Appl Physiol. 1982;52:1172–6. doi: 10.1152/jappl.1982.52.5.1172. [DOI] [PubMed] [Google Scholar]
- 28.Brown JS, Zeman KL, Bennett WD. Ultrafine particle deposition and clearance in the healthy and obstructed lung. Am J Respir Crit Care Med. 2002;166:1240–7. doi: 10.1164/rccm.200205-399OC. [DOI] [PubMed] [Google Scholar]
- 29.Smaldone GC, Foster WM, O'Riordan TG, Messina MS, Perry RJ, Langenback EG. Regional impairment of mucociliary clearance in chronic obstructive pulmonary disease. Chest. 1993;103:1390–6. doi: 10.1378/chest.103.5.1390. [DOI] [PubMed] [Google Scholar]
- 30.Oberdörster G. Lung particle overload: implications for occupational exposures to particles . Regul Toxicol Pharmacol. 1995;21:123–35. doi: 10.1006/rtph.1995.1017. [DOI] [PubMed] [Google Scholar]
- 31.Levy LS. Review: The ‘particle overload’ phenomenon and human risk assessment. Indoor Built Env. 1995;4:254–62. [Google Scholar]
- 32.Henke MO, Ratjen F. Mucolytics in cystic fibrosis. Ped Resp Rev. 2007;8:24–9. doi: 10.1016/j.prrv.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Morrow PE. Alveolar clearance of aerosols. Arch Int Med. 1973;131:101–8. [PubMed] [Google Scholar]
- 34.Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thoracic Soc. 2004;1:315–20. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]