Abstract
Escherichia coli K-12 suffers acetic acid stress during prolonged incubation in glucose minimal medium containing a limiting concentration of inorganic phosphate (0.1 mM Pi), which decreases the number of viable cells from 6 × 108 to ≤10 CFU/ml between days 6 and 14 of incubation. Here we show that following two serial transfers into Pi-limiting medium, evolved mutants survived prolonged incubation (≈107 CFU/ml on day 14 of incubation). The evolved strains that overtook the populations were generally PhnE+, whereas the ancestral K-12 strain carries an inactive phnE allele, which prevents the transport of phosphonates. The switching in phnE occurred with a high frequency as a result of the deletion of an 8-bp repeated sequence. In a mixed culture starved for Pi that contained the K-12 ancestral strain in majority, evolved strains grew through PhnE-dependent scavenging of probably organic phosphate esters (not phosphonates or Pi) released by E. coli K-12 between days 1 and 3, before acetic acid excreted by E. coli K-12 reached toxic levels. The growth yield of phnE+ strains in mixed culture was dramatically enhanced by mutations that affect glucose metabolism, such as an rpoS mutation inactivating the alternative sigma factor RpoS. The long-term viability of evolved populations was generally higher when the ancestral strain carried an inactive rather than an active phnE allele, which indicates that cross-feeding of phosphorylated products as a result of the phnE polymorphism may be essential for the spread of mutants which eventually help populations to survive under Pi starvation conditions.
INTRODUCTION
Bacteria such as Escherichia coli have evolved mechanisms to adapt to abrupt environmental changes that may result from the introduction of toxic products (e.g., HCl and H2O2) or from the deprivation of nutrients. In fact, cells starved for glucose (Glc), the preferred source of carbon (C), and for inorganic phosphate (HPO4−; Pi), the preferred source of phosphorus (P), use specific (the cyclic AMP [cAMP] receptor protein [CRP] and Pho regulons, respectively) and general (the RpoS regulon) adaptive responses. First, cells induce specific responses to Glc and Pi limitation with the aim of maintaining growth. Induced mechanisms allow cells to scavenge low levels of Glc and Pi through induction of the phosphotransferase system (PTS) and phosphate-specific transport (PST) system for Glc and Pi, respectively. These regulons may eventually help cells to grow on secondary sources of C (e.g., acetate previously excreted during growth on Glc or lactose) and of P (e.g., phosphite and phosphonates). The response to Glc starvation occurs primarily through the activation of the CRP-cAMP complex, when the transport of Glc via the PTS decreases and the response to Pi starvation occurs primarily through the activation of the two-component system PhoR-PhoB, when the concentration of Pi in the medium is <4 μM and the transport of Pi via the PST system decreases (13, 27, 36). Second, if cells cannot find C and P sources to maintain growth, they normally enter stationary phase. Starved cells accumulate a new sigma factor (σs, RpoS) of the RNA polymerase, which contributes to change the expression of >500 genes: the expression of σ70-dependent genes required for normal growth and for specific responses (including the CRP and Pho regulons) decreases, while σs-dependent genes are induced. The latter include genes for defense against extreme pH (e.g., gadB and gabD) and H2O2 (e.g., katE, dps, pdhR, and poxB) (20, 29, 34). Thus, the RpoS regulon is induced in advance, while stress is not occurring but could appear during prolonged stationary phase. In fact, there is evidence that RpoS-dependent processes help cells starved of Glc to resist oxidative stress and cells starved of Pi to resist oxidative and eventually acetic acid stresses (20–23).
Surprisingly enough, RpoS− mutants accumulate rapidly in populations incubated under C- and P-limiting conditions. During growth in Glc-limited chemostats, which normally induces the RpoS regulon because of the low growth rate, loss-of-function rpoS mutants may sweep the populations in a few generations. After 50 generations (17 days), the dominant rpoS mutant subpopulation may accumulate small-effect mutations that help evolved strains to grow on low levels of Glc. Finally, by 100 generations, rpoS+ cells may recover (8). Similarly, a population incubated for 37 days in a Pi-limited chemostat may evolve strains that contain several mutations. However, a single mutation in rpoS or in genes (e.g., spoT and hfq) that decrease RpoS levels is sufficient to provide a fitness benefit under conditions of Pi limitation (31). Thus, populations evolving in Glc- and Pi-limited chemostats appear to favor growth (through notably the enhanced expression of the σ70-dependent genes of the CRP and Pho regulons) at the expense of general stress response (through notably the reduced expression of σs-dependent genes). This solution may cause no significant problem, since cells are essentially in constant environments (that is, constant nutrient concentrations, osmolarity, partial O2 pressure [pO2], and pH).
In contrast, the spread of rpoS mutants might cause problems during prolonged incubation in batch cultures, in which environmental conditions may change dramatically as a result of the accumulation of secondary metabolites such as ammonia (during catabolism of amino acids) and acetic acid (during catabolism of Glc) (36). In fact, during prolonged incubation in batch cultures containing amino-acid-rich LB medium, evolved mutants that initially overtake the populations carry low-activity rather than null-activity rpoS alleles (38). This may satisfy a tradeoff between enhanced capacity to grow on scarce nutrients and sufficient RpoS-dependent defense against alkaline pH; at the entry into stationary phase, the pH of the LB medium increases from pH 7 up to pH 8.5 as a result of the degradation of amino acids and production of ammonia, which consumes protons (NH3 + H+ ⇆ NH4+) (6, 36).
We explored the phenotypic, metabolic, and genetic characteristics of strains that evolved during serial transfers in Pi-limiting batch cultures, in which cells are exposed to a decrease in internal pH as a result of the excretion, reentry, and dissociation inside the cell of acetic acid, which liberates protons (CH3COOH ⇆ CH3COO− + H+) (21). We show here that E. coli K-12 rapidly evolved heterogeneous and dynamic populations that survived prolonged incubation under aerobic, Pi starvation conditions. Many evolved strains that spread through the cultures exhibited an RpoS− phenotype. At first glance, such an evolution process was in good agreement with the fact that during prolonged incubation in batch monocultures starved for Pi, rpoS+ cells lose viability after 6 days of incubation as a result of the accumulation of acetic acid (30 mM at pH 4.8) (20, 21), whereas rpoS mutants eventually survive by growing between days 6 and 12 of incubation on low levels of acetate and on high levels of Pi released into the medium by rpoS mutants (11). However, we also show that single rpoS mutants could not grow in a mixed culture starved for Pi containing E. coli K-12 in majority. Two mutations isolated from an evolved strain were sufficient for growing in a mixed culture: a 1-bp deletion in rpoS that inactivated RpoS and an 8-bp deletion in phnE that removed a frameshift normally present in E. coli K-12 (17, 33). Activation of the Pho regulon was required primarily to induce the expression of the phnE+ allele. The role of the PhnE permease, which is primarily to transport phosphonates (C-P) (18, 35), was to scavenge probably organic phosphate esters (C-O-P) released by E. coli K-12 starved for Pi, thereby allowing growth recovery of evolved strains between days 1 and 3 of incubation before acetic acid excreted by E. coli K-12 reached toxic levels. Whereas most evolved strains were PhnE+, the long-term viability of evolved populations was generally higher when the ancestral strain carried an inactive rather than an active phnE allele, which indicates that cross-feeding of phosphorylated products, as a result of the phnE polymorphism, is essential for the spread of evolved mutants.
MATERIALS AND METHODS
Strains, media, and culture conditions.
ENZ strains derived from the E. coli K-12 strain ENZ535 (MG1655) (see Table S1 in the supplemental material). JW strains, which were derived from the E. coli K-12 strain BW25113, were used as donors to transduce single-gene in-frame deletions (Kmr) (2, 19). To construct Kms derivatives of deletion mutants, the kan cassette flanked by the FLP recombination target sites was removed by introducing the FLP recombinase-expressing plasmid pCP20 at 32°C and purifying clones at 42°C (2); Aps and Kms clones were tested for KatE− (ΔrpoS; ENZ1946), PhoA− (ΔphoB; ENZ2084), and MePn− (ΔphnE; ENZ2274) phenotypes (see below). In order to distinguish the different strains in mixed cultures, we transduced the lacY::Tn10 (Tcr) and ΔlacY::kan (Kmr) mutations into the ancestral and evolved strains. To backcross the rpoS1901 allele into the ancestral strain ENZ535, we transduced the cysC::Tn10 marker (7 kb apart from rpoS; frequency of cotransduction of 35%) (38) from ENZ2005 (cysC::Tn10 ΔrpoS::kan) into ENZ1901, selected a cysC::Tn10 rpoS1901 strain (Tcr Kms), retransduced the cysC::Tn10 region into the ancestral strain ENZ535, and determined that 35% (14/40) of the Tcr transductants exhibited an RpoS− phenotype. Finally, we transferred the cysC+ allele from ENZ535 into an rpoS1901 mutant strain, thereby giving rise to strain ENZ2041 (ENZ535 rpoS1901). The same strategy was used to transfer the rpoS819 allele from ZK819 (38) into ENZ535 (ENZ2039). The Pi-limiting MOPS (morpholineethanesulfonic acid) minimal medium contained 40 mM MOPS, 86 mM NaCl, 9.8 mM KCl, 9.5 mM NH4Cl, 40 mM glucose, and 0.1 mM K2HPO4 (pH 7.2) (21). Cultures (50 ml in 500-ml Erlenmeyer flasks) were agitated at 150 rpm in a covered water bath rotary shaker. For the experiments involving incubation in spent medium, the supernatants of 1-day-old 50-ml cultures in Pi-limiting medium were filter sterilized into 500-ml Erlenmeyer flasks and inoculated with 0.5 ml of cultures serially diluted into salts (86 mM NaCl, 10 mM KCl, and 9.5 mM NH4Cl). All incubations were performed at 37°C. The pHs of the media were determined at 25°C (21).
Measurement of cell viability.
To assess cell viability, serial dilutions were prepared in M9 buffer (19), and aliquots (20 μl) were spotted in triplicate onto LB medium plates, which were spread with 2,000 U catalase (21) and might contain kanamycin (30 μg/ml) or tetracycline (12 μg/ml). In the figures, the values of 10 CFU/ml in parentheses indicate that no CFU were detected when 5 20-μl portions of the cultures were directly plated.
Levels of glucose and acetic acid.
The culture supernatants were adjusted to pH 7, and the concentrations of glucose and acetate were determined by enzymatic tests (R-Biopharm). The values are the means from duplicate assays (standard deviations are ≤10% unless otherwise indicated).
RpoS and Glg phenotypes.
Isolated colonies were touched with a toothpick, cells were suspended in M9 buffer (200 μl), and aliquots (4 μl) were spotted onto LB medium plates, which were incubated for 24 h. Patches of cells, which were mostly in stationary phase, were exposed (i) to iodine vapor to determine the cellular levels of glycogen (Glg), estimated through the intensity of the brown color, and (ii) to H2O2 (2 μl of a 30% solution) to determine catalase activities (H2O2 → O2↑ + H2O), estimated through the time required for O2 bubbling. Because RpoS strictly controls the expression of katE, ΔrpoS cells (KatE− KatG+) bubble O2 after a delay of 5 s, whereas rpoS+ cells bubble O2 immediately (22). Various mutants (e.g., glgA, glgB, glgC, pgm, and galU mutants) in addition to ΔrpoS mutants produce low levels of glycogen (7). Thus, we used the terms RpoS− phenotype and Glg− phenotype for cells exhibiting Glg− KatE− (like ΔrpoS mutants) and Glg− KatE+ (like pgm mutants) phenotypes, respectively.
MePn phenotype.
Isolated colonies were touched with a toothpick, cells were suspended in salts (200 μl), and aliquots (4 μl) were spotted onto glucose-M9 minimal medium plates (19) and onto glucose-MOPS minimal medium plates (0.1 g/liter agarose) containing 0.05 mM methyl phosphonate (MePn) (Fluka) as the source of Pi. PhnE+ cells gave rise to confluent patches on MePn plates in 1 (rpoS+) or 2 (rpoS) days of incubation. PhnE− cells gave rise to isolated colonies after 3 to 4 days of incubation.
DNA sequencing.
PCR fragments were generated with PrimeSTAR HS DNA polymerase (Takara Bio Inc.). PCR products were purified using a QIAquick PCR purification kit (Qiagen), and sequencing was performed at GATC-Biotech.
RESULTS
Rapid and diverse evolution under Pi starvation conditions.
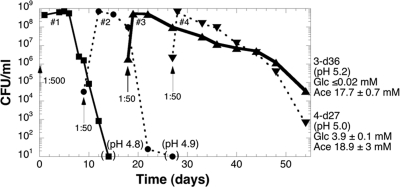
To determine whether the E. coli K-12 strain ENZ535 (MG1655) could evolve mutants which could survive prolonged incubation in Pi-limiting batch culture, we inoculated a culture in MOPS minimal medium containing excess glucose (40 mM) and a limiting concentration of Pi (0.1 mM) (21). Cells grew exponentially with a generation time of ≈1 h for 10 h and entered stationary phase when Pi was nearly exhausted (11). Thereafter, cells were diluted 1:50 into fresh Pi-limiting medium every 9 days of incubation and incubated further for up to 36 days. As shown in Fig. 1, the long-term viability of Pi-starved cells increased dramatically after 2 serial transfers: viability reached ≈107 CFU/ml after 14 days of incubation in the third and fourth subcultures, whereas viability was ≤10 CFU/ml after 14 days of incubation in the first culture. Similar results were obtained in eight other independent experiments (see Fig. S1 in the supplemental material, and data not shown).
Fig 1.
Adaptive evolution of E. coli K-12 under Pi starvation conditions. Strain ENZ535 (MG1655) was inoculated (0.1 ml) into 50 ml of Pi-limiting medium (time zero, indicated by the arrow labeled 1:500) and incubated further for 14 days (■). On day 9 of incubation, 1 ml of the culture (culture 1) was inoculated into 50 ml of fresh Pi-limiting medium (indicated by the arrow labeled 1:50), giving rise to subculture 2, which was incubated further for 18 days (●). On day 9 of incubation of culture 2, 1 ml was inoculated into 50 ml of fresh Pi-limiting medium (indicated by the arrow labeled 1:50), giving rise to subculture 3, which was incubated further for 36 days (▴). On day 9 of incubation of culture 3, 1 ml was inoculated into 50 ml of fresh Pi-limiting medium (indicated by the arrow labeled 1:50), giving rise to subculture 4, which was incubated further for 27 days (▾). The pH of the spent medium is indicated in parentheses. The final concentrations of glucose and of acetic acid (Ace) after 36 and 27 days of incubation in subcultures 3 and 4, respectively, are indicated.
The increase in the long-term viability of the evolved populations appeared to occur concomitantly with an increase in the consumption of glucose and a decrease in the production of acetic acid. For instance, an evolved culture with a high long-term viability contained 106 CFU/ml, ≤0.02 mM glucose, and ≤0.02 mM acetic acid at pH 6.9 after 60 days of incubation (data not shown), an evolved culture with a moderate long-term viability contained 3 × 104 CFU/ml, ≤0.02 mM glucose, and 18 mM acetic acid at pH 5.2 after 36 days of incubation (Fig. 1), and the first cultures contained ≤10 CFU/ml, 10 mM glucose, and 30 mM acetic acid at pH 4.8 after 14 days of incubation (11, 21).
Since final evolved populations survived prolonged incubation like rpoS mutants (11), we tested evolved cells for characteristic RpoS− phenotypes, namely, the Glg− (low production of glycogen) and KatE− (reduced catalase activity) phenotypes (see Materials and Methods). We found Glg− KatE− (like ΔrpoS mutants; referred to as RpoS−), Glg− KatE+ (like pgm mutants; referred to as Glg−), and Glg+ KatE+ (referred to as Glg+) cells; the three phenotypes, both in isolation and in association, occurred in proportions that fluctuated during prolonged incubation (see Fig. S1 in the supplemental material), which indicated that evolved populations were highly dynamic and that diverse subpopulations coexisted.
Evolved strains grow in mixed culture starved for Pi.
To determine how evolved strains behaved in a population starved for Pi, RpoS− (ENZ1901), Glg− (ENZ1902), and Glg+ (ENZ1903, ENZ1904, and ENZ1905) strains were isolated from two parallel final evolved populations (see Fig. S1B′ in the supplemental material); evolved and ancestral strains were marked with either Tc or Km resistance, grown in isolation for 1 day in Pi-limiting medium, added as a minority into 1-day-old saturated cultures of the ancestral strain ENZ535 grown in Pi-limiting medium, and incubated further for 8 days.
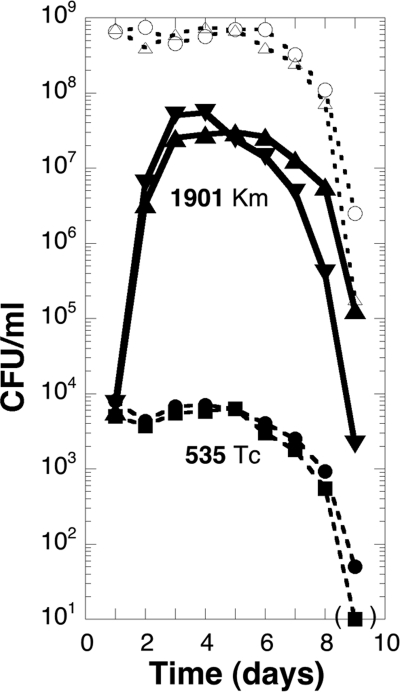
When the RpoS− evolved strain ENZ1901 (Kmr) and the ancestral strain ENZ535 (Tcr) were added together as a minority (each to ≈6 × 103 CFU/ml) into a saturated culture of ENZ535 (≈6 × 108 CFU/ml), the cell counts for the evolved strain ENZ1901 (Kmr) increased up to 3 × 107 to 6 × 107 CFU/ml between days 1 and 3 of incubation, whereas the cell counts for the ancestral strain ENZ535 (Tcr) did not change during this time period (Fig. 2). Similar results were found for mixed cultures containing a single strain in minority and when the Tcr and Kmr markers were switched between the evolved strain ENZ1901 and the ancestral strain ENZ535 (see Fig. S2 in the supplemental material). To determine more precisely the kinetics of growth of the evolved strain ENZ1901 (RpoS−) in a mixed culture, we added increasing concentrations of cells (from 102 up to 106 CFU/ml) into 1-day-old saturated cultures of the ancestral strain ENZ535 (≈6 × 108 CFU/ml); in all cases, the evolved strain ENZ1901 grew exponentially with a generation time of ≈3 h (versus ≈1 h in fresh medium) between days 1 and 2 to 3 of incubation up to a maximum yield of 3 × 107 to 6 × 107 CFU/ml (see Fig. S3 in the supplemental material).
Fig 2.
The evolved strain ENZ1901 grows in a culture of E. coli K-12 starved for Pi. Both evolved strain ENZ1901 (Kmr, ENZ2000) (▴ and ▾) and ancestral strain ENZ535 (Tcr, ENZ1797) (● and ■) were grown as monocultures in Pi-limiting medium for 1 day, diluted 103-fold, added (0.5 ml of each) into 50 ml of a 1-day-old culture of the ancestral strain ENZ535 in Pi-limiting medium, and incubated further for 8 days. CFU were determined on plates containing LB (○ and ▵), LB-Km (▴ and ▾), or LB-Tc (● and ■).
Expression of the growth-under-Pi-starvation (GPS) phenotype was not limited to RpoS− evolved strains; the evolved strains ENZ1902 (Glg−) and ENZ1903 (Glg+) also expressed a GPS phenotype, whereas the evolved strains ENZ1904 (Glg+) and ENZ1905 (Glg+) did not (see Fig. S4 in the supplemental material, and data not shown). The fact that evolved strains such as ENZ1901 (RpoS−) and ENZ1902 (Glg−) expressed a GPS phenotype may help explain the rapid spread of such mutants in evolved populations.
The evolved strain ENZ1901 (RpoS−) dies in pure culture starved for Pi.
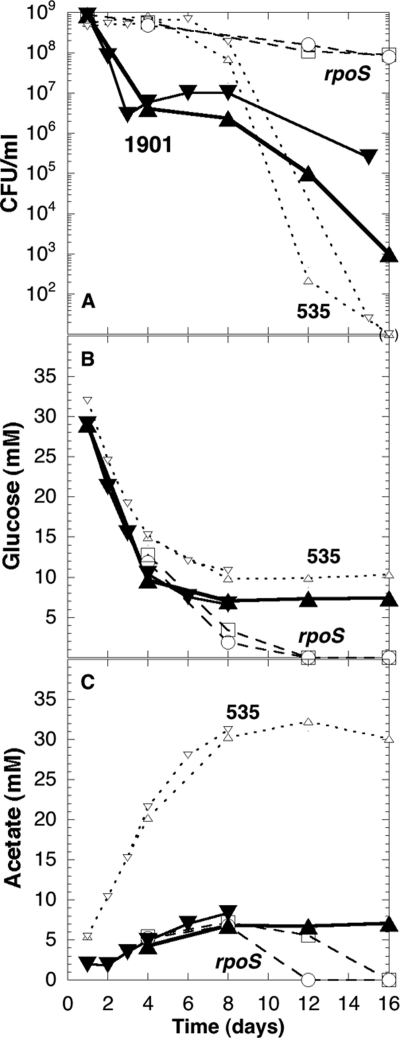
To determine whether changes in metabolism could help explain the behavior of evolved strains that grew in mixed cultures, we focused on studying the metabolic pattern of the evolved strain ENZ1901 (RpoS−). When incubated in isolation under Pi starvation conditions, ENZ1901 consumed higher levels of glucose and excreted lower levels of acetic acid than the ancestral strain ENZ535 (Fig. 3B and C). This pattern may help explain why evolved populations contained low levels of acetic acid. However, the viability of ENZ1901 declined precipitously in pure culture, whereas it increased in mixed culture during the first 3 days of incubation (Fig. 2 and 3A). Thus, intrinsic changes in metabolism and unique interactions with the ancestral strain could account for the GPS phenotype of the evolved strain ENZ1901.
Fig 3.
Viability and metabolic pattern of the evolved strain ENZ1901 in pure culture starved for Pi. Strains were inoculated (0.1 ml) into 50 ml of Pi-limiting medium (time zero) and incubated further for up to 16 days. The numbers of viable cells in the cultures (A) and the concentrations of glucose (B) and of acetate (C) in the spent medium were determined. Evolved strain ENZ1901 (Kmr, ENZ2000) (▴ and ▾), ancestral strain ENZ535 (Tcr, ENZ1797) (▵ and ▿), and ENZ535 carrying rpoS::Tn10 (ENZ985) (○) and ΔrpoS (ENZ2020) (□) were used.
The evolved strain ENZ1901 carries a null-activity rpoS allele.
Characteristics of the evolved strain ENZ1901, namely, low production of glycogen and reduced catalase activity, low excretion of acetic acid during glucose metabolism, and growth under starvation conditions, have been observed in strains carrying various mutations (e.g., mutations in rpoS, spoT, and hfq) that may somehow decrease the induction of the RpoS regulon (11, 29, 31, 38). To determine whether the evolved strain ENZ1901 carried a low- or null-activity rpoS allele, we transferred the rpoS gene from the evolved strain ENZ1901 into the ancestral strain ENZ535 using its cotransduction with the cysC::Tn10 marker (see Materials and Methods). The ENZ535 rpoS1901 reconstructed strain exhibited the same drastic RpoS− phenotype (Glg− KatE−) as ENZ535 ΔrpoS mutants (data not shown), which indicated that the evolved strain ENZ1901 carried a null-activity rpoS allele. Sequencing of rpoS (993 bp, 330 amino acids) in ENZ1901 revealed the deletion of 1 bp (G/C) in codon 214.
The low-activity rpoS819 allele confers a growth-advantage-in-stationary-phase (GASP) phenotype; rpoS819 single mutants grow and eventually take over a population of wild-type cells during prolonged incubation in rich LB medium (38). In contrast, the null-activity rpoS1901 and ΔrpoS alleles and the low-activity rpoS819 allele transduced into the ancestral strain ENZ535 could not confer a significant growth advantage in mixed cultures starved for Pi (see Fig. S5 in the supplemental material). Thus, another adaptive mutation(s) beyond the rpoS1901 mutation was required to account for the GPS phenotype.
PhoB-dependent mechanisms are required for expression of the GPS phenotype.
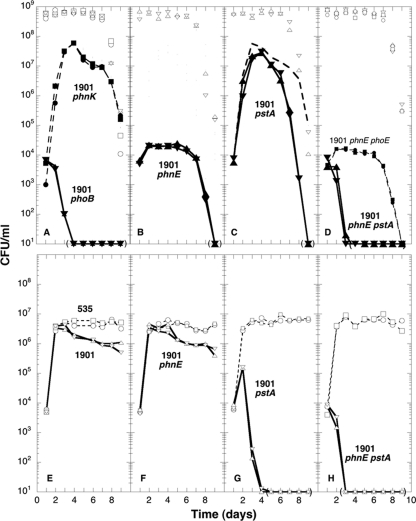
The Pho regulon is induced transiently in Pi-starved cells through a reversible phosphorylation of PhoB. Mutants express the Pho regulon constitutively as a result of a stable phosphorylation of PhoB (13, 32). Such mutations might occur in the evolved strain ENZ1901, which could provide an advantage over the ancestral strain to scavenge P-containing compounds during prolonged incubation. To test this possibility, we asked whether the inactivation of PhoB prevented the expression of the GPS phenotype. As shown in Fig. 4A, the evolved strain ENZ1901 carrying a ΔphoB (or ΔphoBR) mutation did not grow and lost viability in mixed culture. In addition, we found that ΔphoB (or ΔphoBR) mutants, which exhibit the same viability as the parental strain ENZ535 during prolonged incubation in monocultures starved for Pi (21), could not evolve surviving populations during serially propagated culture in Pi-limiting medium (data not shown). Thus, PhoB-dependent mechanisms might be required to evolve mutants and/or to allow evolved mutants to express a GPS phenotype.
Fig 4.
Growth of the evolved strain ENZ1901 in mixed culture and in spent medium. (A to D) Mixed cultures. Derivatives of the evolved strain ENZ1901 (Kmr) (solid symbols) were added into 1-day-old cultures of the ancestral strain ENZ535 (Tcr, ENZ1797) (open symbols) in Pi-limiting medium. (A) ENZ1901 ΔphoBR (ENZ2015) (▴), ENZ1901 ΔphoB (ENZ2079) (▾), and ENZ1901 ΔphnK (ENZ2268) (● and ■). (B) ENZ1901 ΔphnE (ENZ2271) (▴ and ▾). (C) ENZ1901 ΔpstA (ENZ2260) (▴ and ▾) and ENZ1901 (broken line) (the values are from Fig. S2A in the supplemental material). (D) ENZ1901 ΔphnE ΔpstA (ENZ2277) (▴ and ▾) and ENZ1901 ΔphnE ΔphoE (ENZ2278) (● and ■). (E to H) Spent media. The ancestral strain ENZ535 (Tcr, ENZ1797) (○ and □) and derivatives of the evolved strain ENZ1901 (Kmr) (▵ and ▿) were grown as monocultures in Pi-limiting medium for 1 day, diluted 103-fold, and added (0.5 ml of each) into 50 ml of filter-sterilized spent medium obtained from 1-day-old cultures of the ancestral strain ENZ535 in Pi-limiting medium. (E) ENZ1901 (ENZ2000). (F) ENZ1901 ΔphnE (ENZ2271). (G) ENZ1901 ΔpstA (ENZ2260). (H) ENZ1901 ΔphnE ΔpstA (ENZ2277).
The permease of the phosphonate utilization system is required for the GPS phenotype.
To determine whether the evolved strain ENZ1901 carried mutations in phoBR and/or in genes that belong to the Pho regulon, we transduced into ENZ1901 various Kmr markers that were inserted (i) in Pho genes (e.g., pstA, phoE, phoA, and ugpA) and (ii) close to Pho operons (i.e., phoBR and phnCDEFGHIJKLMNOP) (2) and tested Kmr transductants for the GPS phenotype. Only mutations identified by linkage near the beginning of the phnCDEFGHIJKLMNOP operon were required for the expression of the GPS phenotype (data not shown). Sequencing of the phnCDE genes in ENZ1901 revealed in phnE a deletion of 8 bp in tracts of 3 similar sequences of 8 bp called A and B (14), switching from the sequence 5′-ABB-3′ to 5′-AB-3′. Such an 8-bp deletion, which removes a frameshift that normally inactivates phnE in E. coli K-12, occurs at a high frequency, probably because of a strand slippage event during DNA replication (14, 17, 33).
The phnCDEFGHIJKLMNOP operon encodes 14 proteins essential for growth on phosphonates such as MePn: PhnE is the permease subunit in the PhnCDE ABC transporter, and PhnGHIJK form the complex essential for cleaving the C-P bond in phosphonates (15, 26, 35). We confirmed that the evolved strain ENZ1901 (phnE+) grew with the same efficiency on MePn or Pi as the sole source of P, whereas the ancestral strain ENZ535 (phnE::8 bp) did not grow on MePn but gave rise to variants at a high frequency of ≈2 MePn+ cells per 105 total cells (data not shown), compared with a typical frequency of ≈10−7 for a single-base substitution (10). Most interestingly, an ENZ1901 ΔphnK strain that expressed a GPS phenotype (Fig. 4A) could not grow on MePn as the sole source of P and did not give rise to any MePn+ variants (data not shown). These data indicated collectively that the ABC transporter PhnCDE, but not the C-P lyase PhnGHIJK, was required for the expression of the GPS phenotype.
PhnE scavenges organophosphates released by Pi-starved cells.
The PhnCDE system, which transports primarily phosphonates, can also transport organic phosphate esters and Pi (9, 18). Since growth of the evolved strain ENZ1901 in mixed culture did not require the C-P lyase activity, PhnE might be required to scavenge organophosphates, Pi, or both. We have shown previously that cells incubated in Pi-limiting medium (i) consume most of the available Pi during exponential growth, (ii) transiently accumulate organophosphates (e.g., NAD and uridine diphosphoglucose [UDPG]) at the entry into stationary phase, and (iii) release significant levels of Pi after 4 days of incubation (11, 22). Thus, ENZ1901 could scavenge through PhnE residual levels of Pi not consumed during exponential growth or Pi and/or organophosphates released during stationary phase.
To test these possibilities, we assessed the behavior of ENZ1901 ΔphnE mutants (i) in mixed culture and (ii) in 1-day-old spent medium (ENZ535 cells grown in Pi-limiting medium were removed on day 1 of incubation). ENZ1901 ΔphnE mutants grew normally in spent medium (Fig. 4F) but could barely grow in mixed culture (Fig. 4B), which indicated that the evolved strain ENZ1901 (PhnE+) grew in mixed culture primarily as a result of the PhnE-dependent scavenging of P-containing compounds released by the ancestral strain ENZ535 starved of Pi. However, ENZ1901 ΔphnE mutants (Fig. 4B) did not lose viability in mixed culture as rapidly as ENZ1901 ΔphoB mutants did (Fig. 4A), which indicated that PhoB should induce another essential gene(s) besides phnE. The phoE and pstA genes were likely candidates: the porin PhoE facilitates the diffusion of Pi and P compounds across the outer membrane, and the PstA permease is the primary transporter of Pi (13). Whereas ENZ1901 ΔphnE ΔphoE double mutants behaved like ENZ1901 ΔphnE single mutants (Fig. 4B and D), ΔphnE ΔpstA double mutants lost viability rapidly like ENZ1901 ΔphoB mutants (Fig. 4A and D), which indicated that the induction by PhoB of both phnE and pstA was required for the viability of the evolved strain ENZ1901 in mixed culture.
The synergistic effect of the ΔphnE and ΔpstA mutations was unexpected because ENZ1901 ΔpstA single mutants grew normally between days 1 and 3 of incubation in mixed culture (Fig. 4C). However, ENZ1901 ΔpstA mutants lost viability more rapidly than ENZ1901 after 4 days of incubation (Fig. 4C), which suggested that PstA might improve the viability of the evolved strain ENZ1901 through scavenging of Pi during prolonged incubation. Similarly, growth of the evolved and ancestral strains in spent medium (up to a total of ≈107 CFU/ml) might result primarily from the scavenging through PstA of residual levels of Pi left in the medium on day 1 of incubation (estimated as ≈1% of the initial concentration of Pi, that is, ≈1 μM) (Fig. 4E and G), and the limited growth (up to 2 × 105 CFU/ml) of ENZ1901 ΔpstA mutants in spent medium between days 1 and 2 (Fig. 4G and H) might result from the scavenging through PhnE of low levels of organophosphates already released by Pi-starved cells on day 1 of incubation.
These data indicated collectively that the viability of the evolved strain ENZ1901 in a mixed culture starved for Pi required primarily the Pho-dependent activity of the PhnE permease to scavenge probably organophosphates released by the ancestral strain ENZ535 until day 3 of incubation and secondarily the Pho-dependent activity of the PstA permease to scavenge (i) Pi left in the medium on day 1 of incubation and (ii) Pi released by the ancestral strain ENZ535 after day 4 of incubation. The PhnE activity was expressed uniquely by the evolved strain ENZ1901, whereas the PstA activity was expressed by both evolved and ancestral strains, which could compete for Pi in the medium especially at the entry into stationary phase, thereby limiting the growth of ENZ1901 ΔphnE mutants in mixed culture containing the ancestral strain in excess (Fig. 4B and F).
The rpoS mutant and phnE+ alleles are sufficient for expression of the GPS phenotype.
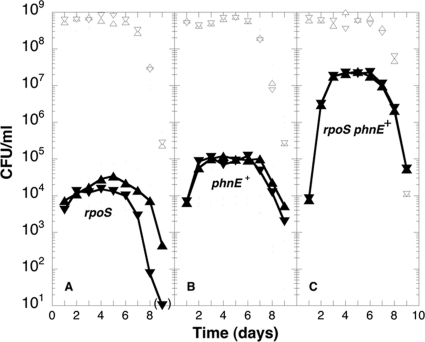
We transferred the phnE+ allele from the evolved strain ENZ1901 (by using cotransduction with ΔproP::Kan and assaying for the MePn+ phenotype) alone or in combination with the rpoS1901 allele into the ancestral strain ENZ535 and tested the ensuing reconstructed strains in mixed cultures. Whereas ENZ535 carrying the rpoS1901 (or ΔrpoS) allele alone grew barely from ≈5 × 103 up to ≈4 × 104 CFU/ml (Fig. 5A; see Fig. S5A and B in the supplemental material), ENZ535 carrying the phnE+ allele alone grew modestly up to ≈105 CFU/ml (Fig. 5B), and ENZ535 carrying both the rpoS1901 (or ΔrpoS) and phnE+ alleles grew up to ≈2 × 107 CFU/ml in 3 days (Fig. 5C), which indicated that the rpoS mutant and phnE+ alleles were sufficient to trigger a full GPS phenotype.
Fig 5.
The reconstructed strain ENZ535 rpoS1901 phnE+ exhibits a GPS phenotype. Derivatives of the ancestral strain ENZ535 (solid symbols) were tested in mixed cultures with ENZ535 (Tcr, ENZ1797) (open symbols) as a majority. (A) ENZ535 rpoS1901 (Kmr, ENZ2265) (▴ and ▾). (B) ENZ535 phnE+ (Kmr, ENZ2256) (▴ and ▾). (C) ENZ535 rpoS1901 phnE+ (Kmr, ENZ2263) (▴ and ▾); identical results were obtained with ENZ535 ΔrpoS phnE+ (Kmr, ENZ2259).
The phnE polymorphism is essential for the evolution of a population starved for Pi.
Although they exhibited different phenotypes, the five final evolved strains that we isolated, namely, ENZ1901 (RpoS− GPS+), ENZ1902 (Glg− GPS+), ENZ1903 (Glg+ GPS+), and ENZ1904 and -1905 (Glg+ GPS−) were MePn+ (data not shown). Thus, the phnE+ allele, associated with various adaptive mutations, might be essential for the spread of evolved mutants in a population starved for Pi. To determine whether the phnE polymorphism was required before the apparent fixation of the phnE+ allele in evolved populations, we conducted evolution experiments by using the E. coli K-12 strain ENZ535 and a phnE+ derivative. When the phnE+ strain was used as the starter strain, only one of 4 populations survived on day 30 of incubation after 2 serial transfers. In contrast, when E. coli K-12 (phnE::8 bp) was used as the starter strain, 3 of 4 populations survived after 30 days of incubation (see Fig. S6 in the supplemental material). The four latter evolved populations were diverse; almost all cells (94%) exhibited a PhnE+ phenotype on day 9 of incubation, but this proportion steadily decreased during prolonged incubation to reach 68% on day 30. The RpoS− phenotype was less abundant and decreased more rapidly, from 49% on day 9 to 14% between days 21 and 30. Thus, the phnE polymorphism might be essential for the spread of diverse subpopulations under Pi starvation conditions.
DISCUSSION
The E. coli K-12 strain ENZ535 (MG1655) loses viability after 6 days of incubation in a batch culture initially containing 40 mM glucose and a limiting concentration of Pi (0.1 mM), reaching ≤10 CFU/ml on day 14 of incubation, presumably as a result of the excretion of high levels of acetic acid (21). We show here that after generally 3 growth cycles of 9 days under Pi starvation conditions, ENZ535 evolved populations that survived during prolonged incubation (≈107 CFU/ml on day 14 of incubation). Evolved populations generally consumed all the glucose yet excreted low levels of acetic acid during prolonged incubation. This is reminiscent of ΔrpoS mutants in pure culture, which consume glucose but do not produce acetic acid and thus survive Pi starvation (≈108 CFU/ml on day 14 of incubation) (11). It appeared that ≈50% of the evolved populations contained, at least transiently, a majority of RpoS− cells.
The rapid spread of evolved strains may be accounted for by their unique capacity to grow in a mixed culture starved for Pi containing the ancestral strain ENZ535 in majority. However, the so-called growth-under-Pi-starvation (GPS) phenotype could not be triggered by single rpoS mutations. A clue about other mutations required for expression of the GPS phenotype was provided by the findings that both evolution of surviving populations and growth of evolved strains in mixed culture required the induction of the Pho regulon. The induction of two Pho-dependent genes was required for growth of an evolved strain in mixed culture, namely, phnE and pstA, which encode permeases for P compounds. PhnE and PstA might be primarily required to scavenge, respectively, organophosphates and Pi released until day 3 and after day 4 of incubation by the ancestral strain ENZ535 starved of Pi.
PhnE is normally cryptic in E. coli K-12 (14, 17, 18, 33). However, an evolved strain that exhibited a GPS phenotype carried a large deletion of 8 bp in phnE that restored an active phnE allele; such a deletion, which may result from a strand slippage event during replication of direct repeat motifs, occurred at a high frequency. Although most evolved strains were PhnE+, the polymorphism for phnE in evolving populations was essential since the parental E. coli K-12 strain evolved more surviving populations than a phnE+ derivative did during repeated cycles under Pi starvation conditions. Thus, cross-feeding of organophosphates between the ancestral E. coli K-12 strain (phnE::8 bp) in stationary phase and phnE+ variants might be essential for the spread of evolved mutants in populations starved for Pi. We have shown previously that Pi-starved cells transiently accumulate organophosphates at the entry into stationary phase (22). Organophosphates could be released and then transported inside phnE+ variants by the PhnCDE system, thereby providing an alternative source of P.
The two changes characterized in an RpoS− GPS+ evolved strain, that is, a 1-bp deletion in rpoS (conferring an RpoS− phenotype) and an 8-bp deletion in phnE (conferring a PhnE+ phenotype), were sufficient to express a full GPS phenotype. The lack of RpoS activity, which may result from various changes in rpoS sequence (8), may primarily maintain growth under Pi starvation conditions through a substantial activity of the tricarboxylic acid (TCA) cycle (11), and the essential role of PhnE may be to provide rapidly a source of P. We have shown previously that rpoS+ cells starved for Pi redirect the metabolism of pyruvate through PoxB, which causes the excretion of toxic levels of acetic acid by day 6 of incubation (20, 21). In contrast, rpoS mutants maintain the carbon flux through the TCA cycle, which prevents the accumulation of acetic acid and eventually allows the consumption of the low levels of acetic acid that are excreted. In fact, rpoS mutants resume growth on acetic acid when high levels of Pi are released by the bulk of rpoS mutants after 6 days of incubation (11). However, such a resumption of growth of rpoS mutants occurred in a pure culture but not in a mixed culture containing a minority of rpoS mutants. In the latter case, excess rpoS+ cells excreted high levels of acetic acid by day 6 of incubation, which prevented growth recovery of rpoS mutants and eventually killed both rpoS+ and rpoS cells. In contrast, an evolved strain carrying both the phnE+ and rpoS mutant alleles could resume growth in a mixed culture starved of Pi as soon as the ancestral strain in majority entered stationary phase and released organophosphates.
Could the PhnE- and RpoS-dependent processes of adaptation of the E. coli K-12 strain MG1655 under aerobic, Pi starvation conditions help explain the behavior of E. coli in the natural environment? We propose that the population polymorphisms in phnE and rpoS might help protect commensal E. coli strains during initial colonization of the human intestine. Several lines of evidence support this idea.
First, the E. coli K-12 laboratory strain MG1655, which is derived from the human isolate E. coli K-12 (3), is closely related to commensal gut strains of E. coli recently isolated from humans (28). Whereas E. coli K-12 contains a phn operon, the commensal E. coli HS strain contains no phn operon (25). Whereas E. coli K-12 contains a phnE::8 bp mutation, environmental E. coli strains isolated in water contain a phnE+ allele (14). This may result from the fact that environmental E. coli may generally use phosphonates as a source of Pi (35), which may provide a strong selection for the PhnE+ state. Thus, the polymorphism in phnE might occur in specific commensal E. coli strains such as E. coli K-12. In contrast, polymorphism in rpoS is common in the natural environment (8).
Second, bacteria might experience repeated Pi starvation periods in the distal part of the small intestine (ileum) and in cecum (21); the concentrations of nutrients, including amino acids, monosaccharides (e.g., glucose), and Pi, may vary considerably between feeds due to absorption in the proximal small intestine (duodenum), when C may be obtained through the metabolism of disaccharides (e.g., lactose) (12, 30). Interestingly, a Pi sensor that recognizes an increase in dietary phosphate concentrations, and consequently increases renal Pi excretion, may exist in the duodenum (4).
Third, the intestine after birth is sterile and contains relatively high oxygen levels (5, 24). Because E. coli can grow with and without oxygen (facultative anaerobe), it is generally the first to colonize the infant intestine in the first days after birth (24) and can reach high concentrations in ileum and cecum (5, 12). The spread of enterobacteria was also observed transiently after heavy antibiotic treatment in human (from 2 to 34%) and mouse (from 1 to 71%) gut microbiomes (1, 37). Aerobic respiration is essential for colonization of the mouse intestine by MG1655 (16).
Thus, during the first days of colonization of the ileum and cecum, it is tempting to speculate that E. coli K-12 might be periodically starved for Pi but could eventually convert lactose into pyruvate and oxygen into H2O (36). Such a metabolic activity might eventually decrease the viability of E. coli K-12 because aerobic pyruvate metabolism through the pyruvate dehydrogenase (PDH) complex, and eventually through RpoS-regulated pyruvate oxidase (PoxB), might cause the accumulation of toxic levels of acetic acid (21). However, the rapid sweep of the population by, notably, phnE+ rpoS evolved mutants, which could grow on organophosphates released by E. coli K-12 in stationary phase, might eventually allow survival of the whole evolved population during prolonged incubation under aerobic, Pi starvation conditions. Prolonged metabolism by evolved populations, which could consume acetic acid and oxygen in the small intestine, might eventually help in successful colonization of the colon by strictly anaerobic bacteria (16, 36).
Supplementary Material
ACKNOWLEDGMENT
We express our gratitude to Angélique Chanal (CNRS-LCB) for help in constructing strains and in sequencing evolved mutants.
Footnotes
Published ahead of print 4 November 2011
This article is dedicated to the memory of Benjamin Moreau.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Antonopolos DA, et al. 2009. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect. Immun. 77:2367–2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. doi:10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bachmann BJ. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p 2460–2488 In Neidhardt FC, et al. (ed), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC [Google Scholar]
- 4. Berndt T, et al. 2007. Evidence for a signaling axis by which intestinal phosphate rapidly modulates renal phosphate reabsorption. Proc. Natl. Acad. Sci. U. S. A. 104:11085–11090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Conway T, Krogfelt KA, Cohen PS. 29 December 2004, posting date Chapter 8.3.1.2, The life of commensal Escherichia coli in the mammalian intestine. In Bock A, et al. (ed), EcoSal—Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC: doi:10.1128/ecosal.8.3.1.2 [DOI] [PubMed] [Google Scholar]
- 6. Dri A-M, Moreau PL. 1994. Control of the LexA regulon by pH: evidence for a reversible inactivation of the LexA repressor during the growth cycle of Escherichia coli. Mol. Microbiol. 12:621–629 [DOI] [PubMed] [Google Scholar]
- 7. Eydallin G, et al. 2007. Genome-wide screening of genes affecting glycogen metabolism in Escherichia coli K-12. FEBS Lett. 581:2947–2953 [DOI] [PubMed] [Google Scholar]
- 8. Ferenci T. 2008. The spread of a beneficial mutation in experimental bacterial populations: the influence of the environment and genotype on the fixation of rpoS mutations. Heredity 100:446–452 [DOI] [PubMed] [Google Scholar]
- 9. Gebhard S, Cook GM. 2008. Differential regulation of high-affinity phosphate transport systems of Mycobacterium smegmatis: identification of PhnF, a repressor of the phnDCE operon. J. Bacteriol. 190:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gérard F, Dri A-M, Moreau PL. 1999. Role of Escherichia coli RpoS, LexA and H-NS global regulators in metabolism and survival under aerobic, phosphate-starvation conditions. Microbiology 145:1547–1562 [DOI] [PubMed] [Google Scholar]
- 11. Guillemet ML, Moreau PL. 2008. Fur-dependent detoxification of organic acids by rpoS mutants during prolonged incubation under aerobic, phosphate starvation conditions. J. Bacteriol. 190:5567–5575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hooper LV, Midtvedt T, Gordon JI. 2002. How host-microbial interaction shape the nutrient environment of the mammalian intestine. Annu. Rev. Nutr. 22:283–307 [DOI] [PubMed] [Google Scholar]
- 13. Hsieh H-J, Wanner BL. 2010. Global regulation by the seven-component Pi signaling system. Curr. Opin. Microbiol. 13:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iqbal S, Parker G, Davidson H, Moslehi-Rahmani E, Robson RL. 2004. Reversible phase variation in the phnE gene, which is required for phosphonates metabolism in Escherichia coli. J. Bacteriol. 186:6118–6123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jochimsen BJ, et al. 2011. Five phosphonate operon gene products as components of a multi-subunit complex of the carbon-phosphorus lyase pathway. Proc. Natl. Acad. Sci. U. S. A. 108:11393–11398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones SA, et al. 2007. Respiration of Escherichia coli in the mouse intestine. Infect. Immun. 75:4891–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Makino K, Kim SK, Shinagawa H, Amemura M, Nakata A. 1991. Molecular analysis of the cryptic and functional phn operons for phosphonate use in Escherichia coli. J. Bacteriol. 173:2665–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Metcalf WW, Wanner BL. 1991. Involvement of the Escherichia coli phn (psiD) gene cluster in assimilation of phosphorus in the form of phosphonates, phosphite, Pi esters, and Pi. J. Bacteriol. 173:587–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 20. Moreau PL. 2004. Diversion of the metabolic flux from pyruvate dehydrogenase to pyruvate oxidase decreases oxidative stress during glucose metabolism in nongrowing Escherichia coli cells incubated under aerobic, phosphate starvation conditions. J. Bacteriol. 186:7364–7368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreau PL. 2007. The lysine decarboxylase CadA protects Escherichia coli starved of phosphate against fermentation acids. J. Bacteriol. 189:2249–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moreau PL, Gérard F, Lutz NW, Cozzone P. 2001. Non-growing Escherichia coli cells starved for glucose or phosphate use different mechanisms to survive oxidative stress. Mol. Microbiol. 39:1048–1060 [DOI] [PubMed] [Google Scholar]
- 23. Nyström T. 2004. Stationary-phase physiology. Annu. Rev. Microbiol. 58:161–181 [DOI] [PubMed] [Google Scholar]
- 24. Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. 2007. Development of the human infant intestinal microbiota. PLoS Biol. 5:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rasko DA, et al. 2008. The pangenome structure of Escherichia coli: comparative genomic analysis of E. coli commensal and pathogenic isolates. J. Bacteriol. 190:6881–6893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rizk SS, Cuneo MJ, Hellinga HW. 2006. Identification of cognate ligands for the Escherichia coli phnD protein product and engineering of a reagentless fluorescent biosensor for phosphonates. Prot. Sci. 15:1745–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seeto S, Notley-McRobb L, Ferenci T. 2004. The multifactorial influences of RpoS, Mlc and cAMP on ptsG expression under glucose-limited and anaerobic conditions. Res. Microbiol. 155:211–215 [DOI] [PubMed] [Google Scholar]
- 28. Soupene E, et al. 2003. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J. Bacteriol. 185:5611–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spira B, Ferenci T. 2008. Alkaline phosphatase as a reporter of σs levels and rpoS polymorphisms in different Escherichia coli strains. Arch. Microbiol. 189:43–47 [DOI] [PubMed] [Google Scholar]
- 30. Vogel-Scheel J, Alpert C, Engst W, Loh G, Blaut M. 2010. Requirement of purine and pyrimidine synthesis for colonization of the mouse intestine by Escherichia coli. Appl. Environ. Microbiol. 76:5181–5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang L, et al. 2010. Divergence involving global regulatory gene mutations in an Escherichia coli population evolving under phosphate limitation. Genome Biol. Evol. 2:478–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wanner BL. 1996. Phosphorus assimilation and control of the phosphate regulon, p 1357–1381 In Neidhardt FC, et al. (ed), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC [Google Scholar]
- 33. Wanner BL, Boline JA. 1990. Mapping and molecular cloning of the phn (psiD) locus for phosphonate utilization in Escherichia coli. J. Bacteriol. 172:1186–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weber H, Polen T, Heuveling J, Wendisch VF, Hengge R. 2005. Genome-wide analysis of the general stress response network in Escherichia coli: σs-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 187:1591–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White AK, Metcalf WW. 2007. Microbial metabolism of reduced phosphorus compounds. Annu. Rev. Microbiol. 61:379–400 [DOI] [PubMed] [Google Scholar]
- 36. Wolfe AJ. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69:12–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Young VB, Schmidt TM. 2004. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J. Clin. Microbiol. 42:1203–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zambrano MM, Siegele DA, Almiron M, Tormo A, Kolter R. 1993. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science 259:1757–1760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.