Background: FOXO3a is a forkhead transcription factor that mediates the effects of doxorubicin in cancer treatment.
Results: p38 regulates FOXO3a nuclear translocation and phosphorylates FOXO3a on Ser-7 upon doxorubicin treatment.
Conclusion: p38 phosphorylation of FOXO3a on Ser-7 contributes to its nuclear relocalization and activation in response to doxorubicin.
Significance: This study provides new information on FOXO3a regulation and the molecular mechanism of action of doxorubicin.
Keywords: Breast Cancer, Cancer Therapy, p38 MAPK, Signal Transduction, Transcription Factors, Nuclear Translocation
Abstract
FOXO3a is a forkhead transcription factor that regulates a multitude of important cellular processes, including proliferation, apoptosis, differentiation, and metabolism. Doxorubicin treatment of MCF-7 breast carcinoma cells results in FOXO3a nuclear relocation and the induction of the stress-activated kinase p38 MAPK. Here, we studied the potential regulation of FOXO3a by p38 in response to doxorubicin. Co-immunoprecipitation studies in MCF-7 cells demonstrated a direct interaction between p38 and FOXO3a. We also showed that p38 can bind and phosphorylate a recombinant FOXO3a directly in vitro. HPLC-coupled phosphopeptide mapping and mass spectrometric analyses identified serine 7 as a major site for p38 phosphorylation. Using a phosphorylated Ser-7 FOXO3a antibody, we demonstrated that FOXO3a is phosphorylated on Ser-7 in response to doxorubicin. Immunofluorescence staining studies showed that upon doxorubicin treatment, the wild-type FOXO3a relocalized to the nucleus, whereas the phosphorylation-defective FOXO3a (Ala-7) mutant remained largely in the cytoplasm. Treatment with SB202190 also inhibits the doxorubicin-induced FOXO3a Ser-7 phosphorylation and nuclear accumulation in MCF-7 cells. In addition, doxorubicin caused the nuclear translocation of FOXO3a in wild-type but not p38-depleted mouse fibroblasts. Together, our results suggest that p38 phosphorylation of FOXO3a on Ser-7 is essential for its nuclear relocalization in response to doxorubicin.
Introduction
Forkhead box O (FOXO) proteins are a subgroup of the forkhead transcription factors characterized by the conserved DNA-binding forkhead domain (1). FOXO3a is one of the four FOXO isoforms identified in mammals, and accumulated evidence shows that FOXO3a regulates a wide range of biological processes, including proliferation, apoptosis, protection against oxidative stress, and metabolism (2). It is now evident that the biological activity of FOXO3a is regulated predominantly by post-translational modifications, including phosphorylation, acetylation, and ubiquitination. Consistent with this notion, one of the first and potentially most important control mechanisms characterized for FOXO3a is its regulation by the PI3K/Akt (PKB) pathway (3), where the phosphorylation of FOXO3a by Akt results in the cytoplasmic accumulation and subsequent degradation of this transcription factor (4).
The MAPK pathways consist of a cascade of evolutionarily conserved kinases, which integrate a wide range of extracellular signals from the cell surface receptors with the transcription machinery in the nucleus. The p38 MAPK signaling cascade is one of the three canonical MAPK (i.e. ERK, p38, and JNK) pathways activated by stress signals (5, 6). Four p38 isoforms (p38α, p38β, p38δ, and p38γ) exhibiting differential tissue-specific expression patterns have been identified (7). Genetic ablation of specific p38 isoforms reveals the existence of functional redundancy between different p38 isoforms, and the serine/threonine kinase activities of the p38 members have been shown to regulate a broad spectrum of biological processes, including transcription and translation (8–10). The tumor-suppressive functions of p38 have also been demonstrated by studies using both cell lines and knock-out mouse models (11, 12). In addition, accumulated evidence has demonstrated that p38 is involved in cell cycle arrest (13), as well as the induction of apoptosis (14) and cellular senescence (15). Pharmacological inhibition of p38 has been shown to reduce the anti-tumor activities of a number of chemotherapeutic drugs, including doxorubicin, an anthracycline derivative (16–19).
Currently, anthracycline derivatives such as doxorubicin and epirubicin are the preferred treatment options for advanced or metastatic cancer. Anthracyclines are also used widely to treat malignancies, such as breast and ovarian cancers, when they are resistant to, or not suitable for, hormonal or molecular targeted therapy. Doxorubicin and epirubicin have been shown to function through inducing cell cycle arrest and cell death by apoptosis in different cancer cells (20–22). However, most anthracycline-based treatments will eventually fail and the patients will relapse because of acquired drug resistance (23, 24). The anticancer cytotoxicity of doxorubicin has been attributed to their ability to inhibit topoisomerase II and to promote the production of intracellular free radicals, but the exact mechanism of action still remains elusive. Converging evidence indicates that FOXO3a has a central role in mediating doxorubicin sensitivity and resistance in cancer (20, 25–29). Previously it has been demonstrated that JNK plays an essential role in mediating the cytotoxic function of paclitaxel in breast cancer cells by targeting FOXO3a. Accordingly, JNK can activate FOXO3a indirectly by repressing PI3K-AKT activity and directly through phosphorylating FOXO proteins, leading to their nuclear relocalization and transcriptional activation (30). Moreover, there is also evidence that activation of JNK can result in ERK and Akt inactivation, leading to FOXO3a nuclear translocation (31) and regulation of target genes, including p27Kip1 and Bim, important for cell cycle arrest and apoptosis (30, 32–35). Conversely, ERK has been reported to phosphorylate FOXO3a, resulting in its degradation through a MDM2-mediated ubiquitin-proteasome pathway and transcriptional inhibition (36). However, no information is yet available on the regulation of FOXO proteins by the p38 MAPK. In the present study, we explored the role of p38 in FOXO3a regulation in response to doxorubicin and characterized one of the major p38 phosphorylation sites involved.
EXPERIMENTAL PROCEDURES
Cell Culture
The human breast carcinoma cell line MCF-7, and HEK293 originated from the American Type Culture Collection were acquired from the Cell Culture Service, Cancer Research UK (London, UK), where they were tested and authenticated. Primary WT and p38α−/− mouse embryo fibroblasts (MEFs)2 were immortalized using the 3T3 protocol and maintained as described previously (37). Cell lines used in the present study were in culture for less than 6 months. All of the cells used were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mm glutamine, and 100 units/ml penicillin/streptomycin at 37 °C. SB202190 was obtained from Merck Biosciences, dissolved in Me2SO, and used at a final concentration of 1 μm. Doxorubicin was purchased from Sigma-Aldrich.
Plasmids and Transfections
The pCMV-FLAG-tagged human FOXO3a expression vector has previously been described. For transfections, the cells were seeded to a confluency of ∼50–70% and incubated with a mix of transfection reagents containing FuGENE 6 (Roche Applied Science) and the plasmid DNA. Site mutagenesis was performed using a Stratagene QuikChange site-directed mutagenesis kit with the sense oligonucleotides (5′-GACAAGGCAGAGGCACCAGCTGCGCCGGCCCCGCTCTCTCCG-3′) and the antisense oligonucleotides (5′-CGGAGAGAGCGGGGCCGGCGCAGCTGGTGCCTCTGCCTTGTC-3′).
Western Blotting and Antibodies
Western blotting was performed on whole cell extracts as described previously (55). Primary antibodies used were FOXO3a (antibody 06-951) (Upstate, Dundee, UK); P-FOXO3a-Thr-32 (antibody 9464), JNK (antibody 9252), P-JNK-Thr-183/Tyr-185 (antibody 9251), ERK1/2 (antibody 9102), P-ERK1/2-Thr-202/Tyr-204 (antibody 9101), p38 (antibody 9212), P-p38-Thr-180/Tyr-182 (antibody 9211), and P-c-Jun-Ser-73 (antibody 9164) (Cell Signaling Technologies, Hitchin, UK); and lamin B (antibody C-20) and β-tubulin (antibody H-235) (Santa Cruz Biotechnology, Wiltshire, UK). The sheep anti-P-FOXO3a-Ser-7 antibody was prepared by immunizing sheep with the peptide EAPApSPVPL, and the subsequent phosphorylation specific antibody was purified from serum by affinity chromatography. The primary antibodies were detected using horseradish peroxidase-linked anti-mouse, anti-rabbit, or anti-sheep conjugates as appropriate (DAKO, Ely, UK) and visualized using the ECL detection system (Amersham Biosciences).
Nuclear and Cytoplasmic Lysate Extraction
Nuclear and cytoplasmic extracts were prepared as previously described (56). The fractionation was done by using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Fisher, Horsham, UK) following the manufacturer's protocol.
Immunofluorescent Staining
Briefly, the cells were fixed with 4% paraformaldehyde (Sigma) and permeabilized with 0.1% Triton X-100 in PBS for 10 min. The samples were then blocked with 5% goat serum for 30 min and then incubated overnight with either the rabbit anti-FOXO3a (Cell Signaling), sheep anti-P-FOXO3a (Ser-7), or the mouse anti-FLAG antibody. Following washes with PBS, secondary goat anti-rabbit or rabbit anti-mouse or donkey anti-sheep IgG-FITC (1:500; Invitrogen) was added to the samples for an hour. The cells were then counterstained with DAPI (Sigma) before mounting. The images were captured using the Zeiss Axiovert 100 confocal laser scanning microscope and software Zeiss LSM 500 or ImageXpress (Molecular Devices) using the Nikon, Eclipse E400 fluorescent microscope (UK Labs-Direct, Ltd, Kilnhurst, UK).
Kinase Assays
GST-FOXO3a-HIS6 (2 μg) was incubated with 10 milliunits of active p38α in 50 mm Tris-HCl, pH 7.5, 0.1 mm Na3VO4, 0.1% (v/v) 2-mercaptoethanol, and 0.1 mm [γ-32P]ATP at 30 °C for the indicated time. The reaction was stopped by the addition of 4× LDS + 200 mm DTT, and the proteins were subjected to SDS-PAGE. The proteins were stained with Coomassie Blue, and incorporation of phosphate into GST-FOXO3a-HIS6 was determined by autoradiography followed by Cerenkov counting of excised protein bands in a Wallac 1409 liquid scintillation counter (Pegasus Scientific Inc., Rockville, MD).
Phosphopeptide Analysis
Tryptic phosphopeptides were separated on a Vydac (Hesperia, CA) 218TP54 C18 reverse phase column eluted with a linear gradient of increasing acetonitrile in 0.1% trifluoroacetic acid. Phosphorylation site determination was esssentially carried out as in Ref. 38, in which the identity of the peptide was determined by mass spectrometry (using an Applied Biosystems 4700 MALDI ToF ToF system), and the position of 32P labeling was identified by solid phase Edman sequencing (using an Applied Biosystems 494C ProCise sequencer (Carlsbad, CA)).
Statistical Analysis
Statistical analysis was performed using Student's t test and was considered significant at p ≤ 0.05 and very significant at p ≤ 0.01 All of the statistical analyses were performed with SPSS v.16 (SPSS Inc., Chicago, IL).
RESULTS
Doxorubicin Treatment of MCF-7 Cells Results in FOXO3a Nuclear Relocation and p38 Induction
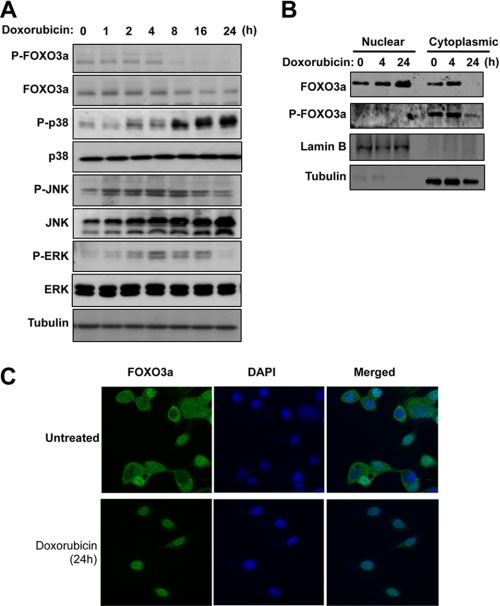
We have shown previously that FOXO3a plays an important role in mediating the cytotoxic effects of doxorubicin (21, 22). To investigate whether p38 has a role in the regulation of FOXO3a activity, we first investigated the expression patterns of FOXO3a and p38 in MCF-7 breast carcinoma cells following doxorubicin treatment. The results showed that doxorubicin caused a down-regulation of FOXO3a phosphorylation on Thr-32 (one of the sites phosphorylated by Akt), whereas there was an induction in activity of the three canonical MAPKs: p38, JNK, and ERK (Fig. 1A). Notably, JNK and ERK activity was induced earlier, peaking at 4 h and declining by 24 h, whereas p38 was induced later and persisted for at least 24 h. We next studied the subcellular distribution of FOXO3a by Western blotting the cytoplasmic and nuclear extracts from MCF-7 cells treated with doxorubicin for 0, 4, and 24 h (Fig. 1B). To control for cross-contamination of nuclear and cytoplasmic fractions, the extracts were also probed for β-tubulin and lamin B, which are cytoplasmic and nuclear markers, respectively. FOXO3a was detected in both the cytoplasmic and nuclear fractions at 0 and 4 h after doxorubicin treatment. Although comparable levels of FOXO3a were detected in both the cytoplasmic and nuclear fractions, it is likely that higher levels of FOXO3a resided in the cytoplasm, because the total amounts of protein yielded from cytoplasm were much higher compared with the nucleus. However, by 24 h, FOXO3a was only detected in the nuclear fractions, suggesting its nuclear relocalization in response to doxorubicin. In agreement, the phosphorylated FOXO3a (Thr-32; Akt site) was found predominantly in the cytoplasm, and its expression level decreased with doxorubicin treatment at 24 h. To confirm this further, we examined the subcellular distribution of the endogenous FOXO3a in response to doxorubicin by immunofluorescence staining. MCF-7 cells were treated with or without doxorubicin for 24 h, followed by staining for FOXO3a expression (Fig. 1C). Consistent with the cytoplasmic and nuclear fractionation results, FOXO3a was detected in both the cytoplasm and nucleus in the untreated MCF-7 cells but was detected predominantly in nucleus following doxorubicin treatment. Because p38 was activated with kinetics similar to those of the nuclear relocation of FOXO3a in response to doxorubicin, we investigated the possibility that p38 may directly regulate FOXO3a.
FIGURE 1.
Doxorubicn treatment induces p38 MAPK activity and FOXO3a nuclear accumulation in MCF-7 breast carcinoma cell line. A, MCF-7 cells were treated with 1 μmol/liter of doxorubicin for 0, 1, 2, 4, 8, 16, and 24 h. At the indicated time, the cells were collected and analyzed by Western blotting using the antibodies indicated. B, MCF-7 cells were treated with 1 μmol/liter of doxorubicin. At 0, 4, and 24 h, cells were collected for the preparation of nuclear and cytoplasmic extracts. 50 μg of nuclear and cytoplasmic lysates at different time points were then analyzed for FOXO3a, P-FOXO3a (Thr-32), lamin B, and β-tubulin expression by Western blotting. C, MCF-7 cells were seeded onto chamber slides and incubated with media in the absence or presence of doxorubicin for 24 h. Immunofluorescent staining was then performed using the pan-FOXO3a antibody and DAPI. All of the images shown are typical results obtained from at least 10 different fields.
Doxorubicin Promotes the Association between FOXO3a and p38α
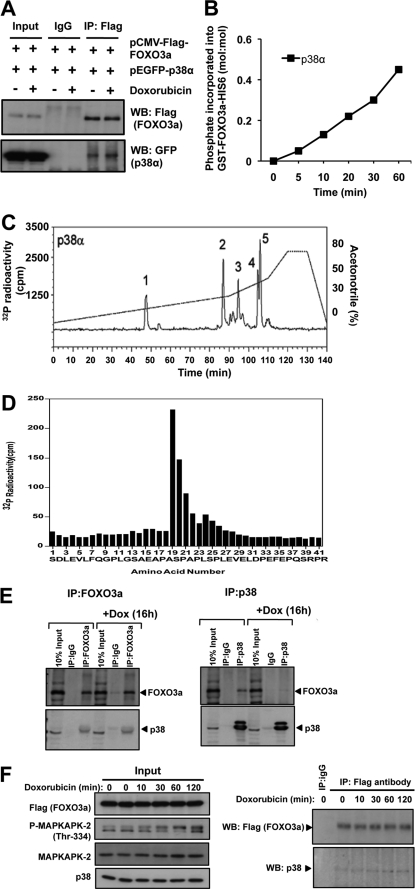
We next studied the potential interaction between p38α and FOXO3a in response to doxorubicin in a co-immunoprecipitation assay. HEK293 cells were co-transfected with the plasmids pCMV-FLAG-FOXO3a and pEGFP-p38α, with or without doxorubicin treatment. Immunoprecipitation was then performed using an anti-FLAG antibody or IgG as a negative control. The results showed that p38α complexes with FOXO3a in the absence as well as in the presence of doxorubicin treatment (Fig. 2A).
FIGURE 2.
p38α interacts with FOXO3a and phosphorylates the transcription factor at Ser-7. HEK293 cells were transfected with pCMV-FLAG-FOXO3a and pEGFP-p38α, followed by immunoprecipitation (IP) using anti-FLAG antibody. Whole cell lysates (Input), mock immunoprecipitation control (IgG), and immunoprecipitated samples were analyzed by Western blotting (WB) using the indicated antibodies. B, purified GST-FOXO3a-HIS6 (2 μg) protein was incubated with 10 milliunits of active recombinant p38α in the presence of [γ-32P]ATP at 30 °C for the times indicated. Incorporation of radiolabeled phosphate into GST-FOXO3a-HIS6 was determined following SDS-PAGE and autoradiography. The protein bands were stained, excised, and Cerenkov counted. C, the in vitro p38α-phosphorylated GST-FOXO3a-HIS6 was subjected to SDS-PAGE, excised from the gel, and digested with trypsin. The resultant peptides were then separated by HPLC on a Vydac C18 column developed with an acetonitrile gradient, as described. 32P radioactivity was detected using an online radioactivity detector. Peaks 1–5 are indicated. D, peak 5 fraction was analyzed by Edman degradation and mass spectrometry as described under “Experimental Procedures.” Amino acid residues 1–12 correspond to the N terminus of the GST tag. The analysis showed that Ser-7 is the residue phosphorylated by p38α. E, FOXO3a and p38 co-precipitation experiments were performed in MCF-7 after treatment with 1 μmol/liter doxorubicin for the times indicated. The precipitates were also blotted for P-MAPKAPK-2 and MAPKAPK-2 expression to gauge for the p38 kinase activity. F, co-precipitation experiments were also performed in MCF-7 with pCMV-FLAG-FOXO3a transfection using the anti-FLAG antibody after treatment with 1 μmol/liter doxorubicin for the times indicated.
p38α Mediates FOXO3a Phosphorylation at Ser-7 in vitro
We next questioned whether p38α can directly phosphorylate FOXO3a. To address this, in vitro, p38α kinase assays were performed using a bacterially expressed GST-FOXO3a fusion protein as substrate. The results showed that recombinant p38α was able to catalyze the increased incorporation of γ-32P into GST-FOXO3a in a time-dependent manner (Fig. 2B), thus demonstrating that p38α can phosphorylate FOXO3a in vitro. This finding also suggested that p38α interacts directly with FOXO3a. The abilities of other p38 isoforms (namely p38β, p38γ, and p38δ) in phosphorylating FOXO3a were also tested, and a similar trend was observed (data not shown). To determine the site(s) of phosphorylation in FOXO3a by p38α, the γ-32P-phosphorylated GST-FOXO3a protein was subjected to in-gel tryptic digestion, and the resultant tryptic peptides were separated by HPLC, revealing five major phosphopeptide-containing peaks (Fig. 2C). The same five phosphopeptide-containing peaks were also observed when GST-FOXO3a was γ-32P-phosphorylated in vitro by p38β, p38γ, and p38δ (supplemental Fig. S1). Subsequent mass spectrometric analysis together with solid phase Edman sequencing identified a major novel phosphorylation site in FOXO3a that mapped to the residue Ser-7 (Fig. 2, C and D, and supplemental Fig. S2). Although additional p38 phosphorylation sites (i.e. Ser-12, Ser-294, Ser-344, and Ser-425) were also identified (supplemental Figs. S2 and S3), these sites were also targeted by JNK (Ser-294 and Ser-425) (supplemental Fig. S4) and ERK (Ser-294, Ser-344, and Ser-425) (36). As a consequence, we focused on characterizing the biological consequence of FOXO3a-Ser-7 phosphorylation in response to doxorubicin treatment. We next examined whether the endogenous p38 interacts with FOXO3a in MCF-7 cells. Co-immunoprecipitation assays showed that p38 and FOXO3a exist in a complex before and after doxorubicin treatment, further supporting the idea that p38 binds to and phosphorylates FOXO3a in vivo (Fig. 2, E and F).
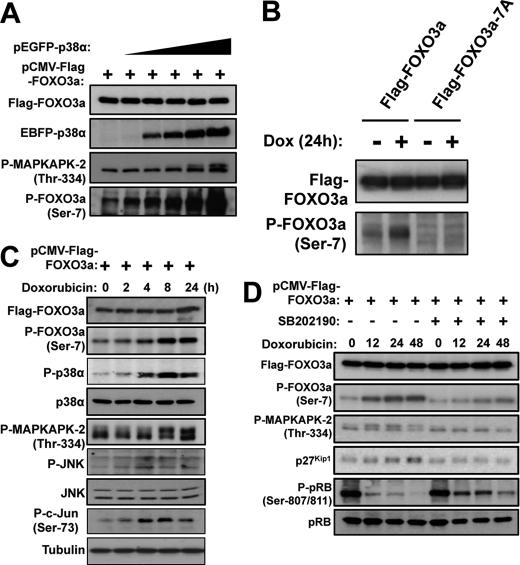
Phosphorylation of FOXO3a at Ser-7 in vivo
To study FOXO3a-Ser-7 phosphorylation and its functional significance in vivo, we generated and validated a phospho-specific antibody, anti-P-FOXO3a-(Ser-7) that recognizes FOXO3a when Ser-7 is phosphorylated (supplemental Fig. S5). On the basis of in vitro phosphorylation studies shown in Fig. 2B, we predicted that an elevated level of p38α would be accompanied by an increase in FOXO3a-Ser-7 phosphorylation. To confirm that p38 mediates FOXO3a phosphorylation on Ser-7 in vivo, MCF-7 cells were co-transfected with pCMV-FLAG-FOXO3a and increasing amounts of pEGFP-p38α. The transfected cells were then collected 24 h later, and lysates were immunoblotted with anti-P-FOXO3a-(Ser-7) (Fig. 3A). To confirm that the p38α transfection induces its kinase activity, we also monitored the level of MAPKAPK2 (Mitogen-Activated Protein Kinase-Activated Protein Kinase 2) phosphorylation by using a phospho-specific antibody that recognizes Thr-334 residue on MAPKAPK2, which is directly phosphorylated by p38α. As predicted, the level of MAPKAPK2-Thr-334 phosphorylation increased with the level of the transfected EBFP-p38α, indicating that the transfected p38α was biologically active. Importantly, the level of FOXO3a-Ser-7 phosphorylation was also up-regulated in a p38α-dependent manner, demonstrating that FOXO3a-Ser-7 phosphorylation is induced by p38α in cells. In addition, we also examined the ability of this P-FOXO3a(Ser-7) antibody to recognize a transfected wild-type FOXO3a or a FOXO3a mutant with Ser-7 converted to alanine in MCF-7 cells treated with doxorubicin. As predicted, this P-FOXO3a(Ser-7) antibody only recognized the wild-type but not the mutant FOXO3a in both Western blot analysis (Fig. 3B) and immunofluorescent staining (supplemental Fig. S6). Using the anti-P-FOXO3a(Ser-7) antibody, we next investigated the kinetics of FOXO3a-Ser-7 phosphorylation in response to doxorubicin treatment in the MCF-7 breast cancer cells. To this end, MCF-7 cells expressing FLAG-FOXO3a were treated with doxorubicin over a time course of 24 h, and Western blot analysis was then carried out on cell lysates collected at the various time points indicated (Fig. 3C). Doxorubicin treatment resulted in the activation of both p38 and JNK in MCF-7 cells, as indicated by the induction of MAPKAPK2-Thr-334 and c-Jun-Ser-73 phosphorylation, respectively. Importantly, an up-regulation of FOXO3a-Ser-7 phosphorylation was also observed at 8 h post-stimulation, demonstrating doxorubicin induces FOXO3a-Ser-7 phosphorylation in MCF-7 cells. Furthermore, the kinetics of FOXO3a-Ser-7 phosphorylation mirrored that of MAPKAPK2-Thr-334, and not c-Jun-Ser-73, suggesting that p38 is the predominant kinase responsible for phosphorylating FOXO3a on Ser-7 in vivo. To further examine the relationship between p38 and FOXO3a-Ser-7 phosphorylation, we monitored the effects of doxorubicin on FOXO3a-Ser-7 phosphorylation and some of the key cell cycle regulatory events in the absence or presence of the specific p38 inhibitor, SB202190. Consistent with the earlier results shown in Fig. 2B, doxorubicin treatment caused an increase in FOXO3a-Ser-7 phosphorylation; however, the induction of FOXO3a-Ser-7 phosphorylation in response to doxorubicin was markedly reduced in the presence of SB202190 (Fig. 3D). Moreover, the doxorubicin-induced FOXO3a activation, as indicated by the up-regulation of its target p27Kip1, was also impeded by p38 inhibition. Conversely, SB202190 partially restored pRb phosphorylation at Ser-807/Ser-811, suggesting the inverse relationship between FOXO3a-Ser-7 phosphorylation and cell cycle progression. Notably, the presence of basal FOXO3a-Ser-7 phosphorylation in the p38 inhibitor SB202190-treated lysates also suggests that this FOXO3a residue may also be targeted by kinases other than p38, and it is not known whether this represents a functional compensatory mechanism as a result of the loss of p38 activity. Taken together, these results suggest that doxorubicin induces p38 to mediate FOXO3a Ser-7 phosphorylation and activation, contributing to a cell proliferation arrest.
FIGURE 3.
Doxorubicin activates p38 MAPK to induce FOXO3a Ser-7 phosphorylation in MCF-7 cells. A, MCF-7 cells were transfected with pCMV-FLAG-FOXO3a and an increasing amount of pEGFP-p38α, followed by Western blot analyses using the indicated antibodies. B, MCF-7 cells transfected with pCMV-FLAG-FOXO3a or pCMV-FLAG-FOXO3a-A7 and treated with doxorubicin for 24 h were immunoprecipitated with the FLAG antibody followed by immunoblotting with the FOXO3a and P-FOXO3a (Ser-7) antibodies. C, MCF-7 cells were transfected with pCMV-FLAG-FOXO3a followed by doxorubicin treatment for the indicated times. The doxorubicin-treated cells were collected, and lysate samples were prepared and analyzed by Western blot analyses using the indicated antibodies. D, MCF-7 cells were transfected with pCMV-FLAG-FOXO3a, followed by doxorubicin treatment at the indicated times in the absence or presence of the p38 chemical inhibitor, SB202190. Cells lysate samples were then prepared and analyzed by Western blot analyses using the indicated antibodies. The results showed that p38 inhibition impedes doxorubicin-induced FOXO3a-Ser-7 phosphorylation and p27Kip1 accumulation and pRB hypophosphorylation in MCF-7 cells.
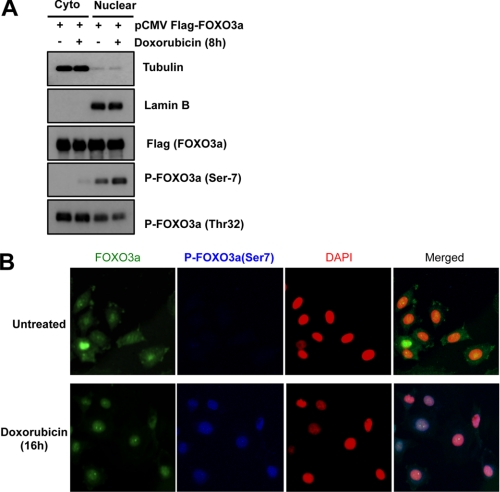
Ser-7 Phosphorylation Promotes FOXO3a Relocation to Nucleus
The findings that doxorubicin promotes FOXO3a-Ser-7 phosphorylation and its nuclear translocation led us to speculate that Ser-7 phosphorylation on FOXO3a by p38 has a role in relocating FOXO3a to the nucleus. To test this conjecture, we first examined the subcellular distribution of total and Ser-7 phosphorylated FOXO3a by Western blotting the cytoplasmic and nuclear extracts from FLAG-FOXO3a-expressing MCF-7 cells left untreated or treated with doxorubicin (Fig. 4A). β-Tubulin and lamin B were again used as markers to control for nuclear/cytoplasmic fraction cross-contamination. As expected, higher levels of Thr-32-phosphorylated FOXO3a were detected in the cytoplasmic fractions. By contrast, Ser-7-phosphorylated FOXO3a localized almost entirely in the nuclear fractions, and its level increased following doxorubicin treatment. Next, immunofluorescence microscopy was used to study the subcellular distribution of the endogenous total and Ser-7-phosphorylated FOXO3a in response to doxorubicin. MCF-7 cells were treated with or without doxorubicin for 24 h, followed by double staining for total and Ser-7-phosphorylated FOXO3a. As shown in Fig. 4B, a marked increase in nuclear FOXO3a was observed after doxorubicin treatment. Importantly, Ser-7-phosphorylated FOXO3a was only detected in the nuclei of the stimulated cells. Despite the presence of nuclear FOXO3a in the untreated cells, no Ser-7-phosphorylated FOXO3a was detected, suggesting that Ser-7 phosphorylation is synonymous with doxorubicin-activated and nuclear localized FOXO3a. Together, these subcellular localization studies indicate that Ser-7 phosphorylation is associated with doxorubicin-induced nuclear FOXO3a.
FIGURE 4.
Association of FOXO3a Ser-7 phosphorylation with nuclear localization in vivo. A, MCF-7 cells were transfected with pCMV-FLAG-FOXO3a, followed by doxorubicin treatment for 8 h. Whole cells were collected, and cytosolic (Cyto)/nuclear fractionation (Nuclear) procedures were performed. The resultant fractions were standardized according to protein content, followed by Western blot analyses using the indicated antibodies. B, MCF-7 cells were seeded into slide chamber and incubated with media in the absence or presence of doxorubicin for 16 h. Immunofluorescent staining was then performed using FOXO3a and P-FOXO3a (Ser-7) antibodies and DAPI. All of the images shown are typical results obtained from at least 10 different fields.
Phosphorylation-defective FOXO3a (Ala-7) Mutant Fails to Localize to Nucleus upon Doxorubicin Treatment
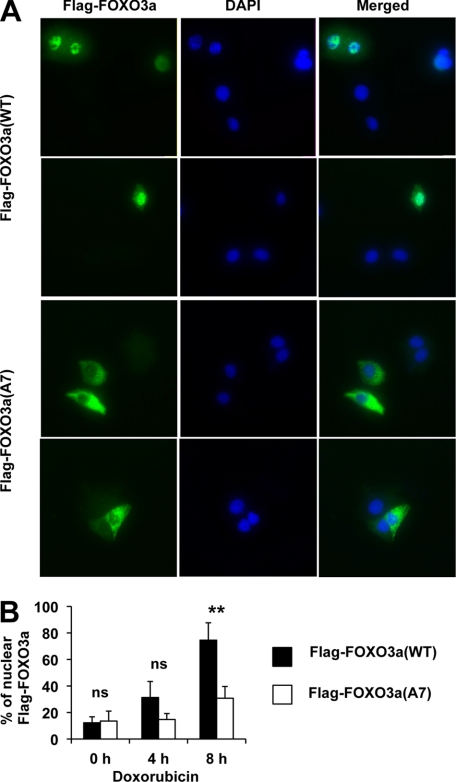
To directly evaluate the functional significance of FOXO3a-Ser-7 phosphorylation, we compared the nuclear translocation efficiency between wild-type FOXO3a and a Ser-7 phosphorylation-deficient FOXO3a mutant in response to doxorubicin. MCF-7 cells transfected with pCMV-FLAG-FOXO3a-WT or pCMV-FLAG-FOXO3a-A7 (with Ser-7 mutated to Ala-7) were left untreated or stimulated with doxorubicin. Immunofluorescent staining was then performed using the FLAG antibody, and the positively stained nuclei were scored against the total transfected population. The results showed that although a proportion of nucleus-localized wild-type FOXO3a and FOXO3a-A7 was comparable in untreated cells, there were significantly higher levels of wild-type FOXO3a compared with the mutant FOXO3a-A7 in the nucleus upon doxorubicin treatment at 8 h (Fig. 5). These results revealed the phosphorylation at Ser-7 plays a key role in relocating FOXO3a to the nucleus.
FIGURE 5.
Mutation of Ser-7 to Ala significantly affects the nuclear translocation of FOXO3a upon doxorubicin treatment. MCF-7 cells transfected with pCMV-FLAG-FOXO3a-WT or pCMV-FLAG-FOXO3a-A7 (Ala-7) were seeded into slide chambers, followed by doxorubicin stimulation for the times indicated. Immunofluorescent staining was then performed using FLAG antibody and DAPI. The subcellular localization of FLAG-FOXO3a was then examined by fluorescent microscopy. A, two representative fields of each type of transfection after 8 h of doxorubicin treatment from three independent experiments are shown. B, over 200 FLAG-positive transfected cells from three independent experiments were analyzed, and nuclear FLAG-FOXO3a staining was scored and expressed as a percentage against the total transfected populations. Representative data from three independent experiments are shown. Statistical analyses were done using Student's t test. *, p ≤ 0.05 significant; **, p ≤ 0.01 very significant. Significant differences in the nuclear localization of FLAG-FOXO3a-WT or FLAG-FOXO3a-A7 in the doxorubucin-treated MCF7 cells were detected at 8 h.
p38 Is Required for FOXO3a Nuclear Translocation upon Doxorubicin Treatment
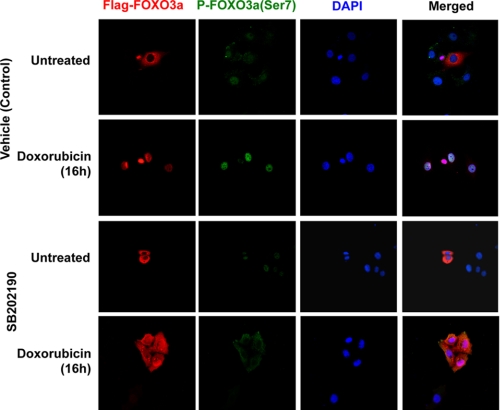
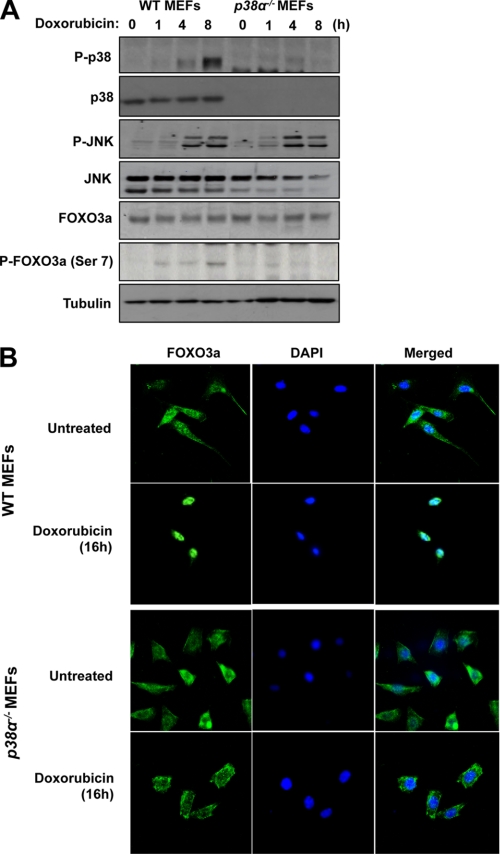
To examine whether p38 is required for the nuclear translocation of FOXO3a in response to doxorubicin treatment, MCF-7 cells transfected with FLAG-FOXO3a (WT) were cultured for 30 min in the absence or presence of the p38 inhibitor SB202190, before treatment with either the vehicle (control) or doxorubicin for 16 h. Immunofluorescent staining results showed that the transfected FOXO3a resided predominantly in the cytoplasm in both the untreated and the SB202190-pretreated MCF-7 cells (Fig. 6). Following doxorubicin treatment, FOXO3a accumulated almost exclusively in the nuclei of the MCF-7 cultured without SB202190, whereas substantial amounts of FOXO3a remained in the cytoplasm of the SB202190 and doxorubicin-treated cells. The result also revealed that doxorubicin caused an accumulation of P-FOXO3a (Ser-7) in the nucleus of control MCF-7 cells, whereas SB202190 treatment blocked the P-FOXO3a (Ser-7) accumulation. These results suggested that p38 has a role in the nuclear accumulation and Ser-7 phosphorylation of FOXO3a in response to doxorubicin. To demonstrate further that p38 has a role in the nuclear translocation of FOXO3a in response to doxorubincin treatment, we next compared the ability of doxorubicin to mediate FOXO3a nuclear relocation in WT and p38α null MEFs. To this end, we first studied the expression of p38 upon doxorubicin treatment in both the WT and p38α−/− MEFs (Fig. 7A). Western blot results showed that p38 and its active form P-p38 were detected as a single species in the WT but not in the p38α−/− MEFs. In contrast, the expression levels of the related JNK-MAPK and FOXO3a were at comparable levels in both the WT and p38α−/− MEFs. Moreover, the activated phosphorylated P-JNK was induced with similar kinetics in both cell types. These results suggest that p38α is the predominant p38 species in both the WT and p38α−/− MEFs and that p38 depletion does not have a discernible effect on JNK activity and FOXO3a expression in response to doxorubicin in these cells. Interestingly, whereas the levels of P-FOXO3a (Ser-7) accumulated in response to doxorubicin in the WT MEFs, the level of P-FOXO3a (Ser-7) was substantially depleted in the p38α−/− MEFs, further indicating that p38 mediates FOXO3a Ser-7 phosphorylation and that p38α is the predominant p38 species in MEFs. This is also consistent with the data from Fig. 6, which shows that inhibition of p38 using the pharmacological inhibitor SB202190 reduced the nuclear accumulation of P-FOXO3a (Ser-7) induced by doxorubicin. Confocal microscopy was next used to monitor the subcellular localization of FOXO3a in response to doxorubicin treatment. (Fig. 7B). In WT MEFs, endogenous FOXO3a was detectable in both the cytoplasmic and nuclear compartments of untreated cells but accumulated almost exclusively in the nuclei upon doxorubicin treatment. Similarly, FOXO3a also resided in the cytoplasm and nuclei of untreated p38-deficient MEFs but was not translocated predominantly to the nuclei upon doxorubicin treatment. Comparable results were also obtained from WT and p38α−/− MEFs transfected with ectopic FOXO3a (supplemental Fig. S7). Together, these data demonstrate that p38 is involved in the nuclear translocation of FOXO3a following doxorubicin treatment.
FIGURE 6.
Nuclear accumulation of FOXO3a upon doxorubicin treatment can be blocked by the p38 chemical inhibitor. MCF-7 cells transfected with pCMV-FLAG-FOXO3a were cultured on sterile coverslips and treated with 1 μm doxorubicin or remained untreated for 16 h in the absence or presence of the 10 μm p38 chemical inhibitor, SB202190. The cells were fixed in 4% paraformaldehyde after treatment, and the transfected FOXO3a was recognized by a mouse anti-FLAG antibody. FLAG-FOXO3a was visualized by the addition of Alexa 488 (red) labeled anti-mouse antisera. P-FOXO3a (Ser-7) was detected by donkey anti-sheep IgG-FITC (green) and DAPI (blue) was applied to visualize the nuclei.
FIGURE 7.
Doxorubicin induces the nuclear accumulation of FOXO3a in wild-type but not p38α null fibroblasts. Wild-type and p38α−/− MEFs were cultured with 1 μm doxorubicin for the times indicated and used for Western blot analysis and immunofluorescent staining. A, protein lysates were prepared at the times indicated, and protein expression levels were analyzed by Western blotting using antibodies against p38, P-p38, JNK, P-JNK, FOXO3a, P-FOXO3a (Ser-7), and β-tubulin. B, MEFs were cultured on sterile slide chambers and either left untreated or treated for 16 h with 1 μm doxorubicin, before being fixed in 4% paraformaldehyde. FOXO3a was visualized with a rabbit polyclonal antibody followed by the addition of ALEX488 (green) labeled anti-rabbit antisera. DAPI (blue) were also applied to visualize the nuclei.
DISCUSSION
FOXO3a has a central role in mediating the stress signals induced by numerous chemotherapeutic agents; however, the mechanistic information involved remains elusive. Treatment of breast cancer cells with doxorubicin induced cell cycle arrest and cell death, which is associated with induction of p38 activity and nuclear relocation of FOXO3a. In this study, we provide evidence that the doxorubicin-induced FOXO3a nuclear localization is mediated by the stress-activated protein kinase p38 through phosphorylation of the transcription factor on Ser-7. The substitution of Ser-7 with alanine markedly impairs the nuclear translocation of FOXO3a, thus highlighting the biological importance of this single phosphorylation event. We have made attempts but were unable to delineate the proliferative function of Ser-7 phosphorylation using phosphorylation mimicking and defective FOXO3a mutants (supplemental Fig. S8). This could be due to the fact that the overexpression approach could distort the subcellular localization of a proportion of the transfected FOXO3a, resulting in a substantial proportion of FOXO3a (A7) mutant mislocating to the nucleus, as shown in Fig. 5. Consistent with this idea, the FOXO3a (A7) mutant appeared to be marginally less effective in inducing the accumulation of p27Kip1 and cleaved PLAP, a marker for apoptosis. In addition, it is possible that other p38 phosphorylation sites also contribute toward FOXO3a nuclear relocalization. Indeed, our original phospho-mapping assay showed that p38 can potentially phoshorylate FOXO3a on four other sites, and some of these are also shared by JNK and ERK. Protein sequence alignment analysis shows that Ser-7 on FOXO3a is conserved between human and other mammals, indicative of an evolutionarily conserved function. The induction of Ser-7 phosphorylation by doxorubicin appears to be a universal event because it occurs all the cell lines examined (supplemental Fig. S9) Interestingly, other mammalian members of the FOXO family (FOXO1, FOXO4, and FOXO6) do not harbor this phosphorylation site, indicating that the mechanism identified is specific to FOXO3a. This could explain why FOXO3a, and not other FOXO proteins, is a key mediator of chemotherapeutic drug action, as well as certain environmental stress signals. The Ser-7 phosphorylation is specifically targeted by p38, because studies using kinase inhibitors showed that JNK, PI3K, and ERK inhibition has no effect on Ser-7 phosphorylation upon doxorubicin treatment (supplemental Fig. S10). In particular, the results suggested that doxorubicin does not modulate PI3K activity and inhibition of PI3K by LY294002 does not affect Ser-7 phosphorylation. As a result, Ser-7 phosphorylation FOXO3a relocation does not depend on previous phosphorylation by AKT. At present there is still little detailed knowledge available as to how FOXO3a cytosolic/nuclear translocation is regulated. Domains within FOXO3a that could function as nuclear localization signal (NLS) or nuclear export signal (NES) have been assigned but are supported by limited experimental evidence. However, it has been demonstrated that the nuclear export of FOXO1 is sensitive to leptomycin B treatment, indicating a Crm1-dependent export mechanism (4, 39). Moreover, phosphorylation of Ser-193 located within the atypical NLS (Gly-180 to Gly-221) of FOXO4 has been shown to impair the nuclear translocation of the transcription factor (40). This suggests that phosphorylation of residue(s) within and possibly in close proximity to the NLS/NES is likely to have a direct regulatory impact on the subcellular distribution of FOXO members. As for FOXO3a, Ser-7 is not located within the predicted NLS (Lys-242 to Lys-271) nor NES (Leu-369 to Leu-396). At present, only the DNA-binding domain of FOXO3a has been structurally resolved (41). However, when the crystal structure of full-length FOXO3a becomes available in the future, it will be interesting to examine the spatial arrangement of Ser-7 in relation to the NLS/NES domains, which should provide clues as to how Ser-7 phosphorylation affects FOXO3a structural conformation and subcellular distribution at the molecular level.
Our findings identify FOXO3a as a novel substrate of p38 MAPK in response to genotoxic and environmental stress; however, FOXO3a is not the only tumor suppressive transcription factor targeted by p38 in response to stress signals. For example, p38 has also been shown to phosphorylate and activate the tumor suppressors p53 and p73 in response to UV radiation and chemotherapeutic drugs (8, 19, 42). Although our results definitively show that p38-mediated FOXO3a-Ser-7 phosphorylation promotes the nuclear translocation of the transcription factor, we cannot exclude the possibility that this phosphorylation may also have other biological functions. For instance, p38-mediated phosphorylation of USF-1 at Thr-153 has been shown to facilitate acetylation, which in turn alters the transcriptional activity of USF-1 (43). Given that acetylation is one of the post-translational modifications that can influence FOXO3a transcriptional output (44, 45), it will be of interest to investigate the relationship between FOXO3a-Ser-7 phosphorylation and its acetylation in future studies.
Unchecked p38 activity has been linked to inflammatory disorders in humans. Consequently, p38 inhibitors have been one of the most intensively studied classes of therapeutics for inflammatory diseases, such as rheumatoid arthritis, psoriasis, and asthma (5). Conversely, in terms of cancer treatment, p38 inhibitors could promote drug insensitivity and have adverse effects. Consistent with this notion, the dependence of chemotherapeutic-induced cell death on p38 activation has been documented in previous studies. For instance, pharmacological inhibition of p38 is known to impede the anti-proliferative/pro-apoptotic effects of doxorubicin, cisplatin, paclitaxel, arsenite, and all-trans-retinoic acid in a wide range of cancer cell types (16, 46–48). In concordance, our results show that specific inhibition of p38 by SB202190 can alleviate doxorubicin-induced cell cycle inhibition. Accordingly, SB202190 also markedly down-regulates doxorubicin-induced FOXO3a-Ser-7 phosphorylation and p27Kip1 induction. Collectively, these data demonstrate Ser-7 on FOXO3a is a biologically relevant target of p38 in response to doxorubicin. In many cell types, the stress signals induced by reactive oxygen species have been shown to activate the MAP kinase pathways (57, 58). At the molecular level, the ability of reactive oxygen species in activating apoptosis signal-regulating kinase 1 (ASK1), an upstream activator of both p38 and JNK, has been documented in numerous studies (59–61). Given that doxorubicin is a potent inducer of reactive oxygen species in tumor cells (62–64), it is likely that the ASK1/p38 signaling axis may play a key role in relaying the doxorubicin signal to FOXO3a.
To date, the majority of the known FOXO3a phosphorylation sites have been shown to have inhibitory effects on its activity. Accordingly, phosphorylation mediated by Akt (PKB)/SGK (Thr-32, Ser-253, and Ser-315), IKKβ (Ser-644), ERK1/2 (Ser-294, Ser-344, and Ser-425), and DYRK1A (Ser-325), all promote the cytoplasmic accumulation of FOXO3a (49, 50). Although AMPK-mediated phosphorylation (Thr-179, Ser-399, Ser-413, Ser-555, Ser-588, and Ser-626) has been shown to activate FOXO3a, AMPK phosphorylation does not affect the cytoplasmic/nuclear distribution of the transcription factor (51, 52). Despite the fact that Akt phosphorylation is regarded as a marker for cytoplasmic localized FOXO (53), our data and that of others show that a significant level of Akt-phosphorylated FOXO3a resides in the nucleus (54). Furthermore, although Akt phosphorylation has been shown to promote FOXO protein cytoplasmic relocalization, its role in the nuclear relocation of FOXO proteins in response to stress signals remains unknown. To our knowledge, Ser-7 is the only phosphorylation site associated with the nuclear enrichment of FOXO3a, thus providing a useful molecular marker for nuclear FOXO3a.
In conclusion, our findings identify FOXO3a as a direct substrate of the stress-activated kinase p38 and Ser-7 as the novel p38 phosphorylation site for FOXO3a. This study also provides new information on the molecular mechanism of action of doxorubicin. In addition, we find that FOXO3a-Ser-7 phosphorylation is synonymous with the nuclear accumulation of the transcription factor and propose that this phosphorylation site can be exploited as a marker for nuclear FOXO3a and doxorubicin response in future investigations.
Supplementary Material
This work was supported by funds from Cancer Research UK (to K. K. H. and E. W.-F. L.), the Breast Cancer Campaign (to E. W.-F. L.), the Engineering and Physical Sciences Research Council (to D. J. K.), and Imperial College Healthcare NHS Trust-BRC Funding (to C.-Y. K.).

This article contains supplemental Figs. S1–S10.
- MEF
- mouse embryo fibroblast
- NLS
- nuclear localization signal
- NES
- nuclear export signal.
REFERENCES
- 1. Kaestner K. H., Knochel W., Martinez D. E. (2000) Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 14, 142–146 [PubMed] [Google Scholar]
- 2. van der Vos K. E., Coffer P. J. (2011) The extending network of FOXO transcriptional target genes. Antioxid. Redox Signal. 14, 579–592 [DOI] [PubMed] [Google Scholar]
- 3. Paradis S., Ruvkun G. (1998) Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 12, 2488–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biggs W. H., 3rd, Meisenhelder J., Hunter T., Cavenee W. K., Arden K. C. (1999) Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc. Natl. Acad. Sci. U.S.A. 96, 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coulthard L. R., White D. E., Jones D. L., McDermott M. F., Burchill S. A. (2009) p38MAPK: stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 15, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Nadal E., Posas F. (2010) Multilayered control of gene expression by stress-activated protein kinases. EMBO J. 29, 4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuadrado A., Nebreda A. R. (2010) Mechanisms and functions of p38 MAPK signalling. Biochem. J. 429, 403–417 [DOI] [PubMed] [Google Scholar]
- 8. Bulavin D. V., Saito S., Hollander M. C., Sakaguchi K., Anderson C. W., Appella E., Fornace A. J., Jr. (1999) Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18, 6845–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raingeaud J., Whitmarsh A. J., Barrett T., Dérijard B., Davis R. J. (1996) MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell Biol. 16, 1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X. Z., Ron D. (1996) Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science 272, 1347–1349 [DOI] [PubMed] [Google Scholar]
- 11. Bulavin D. V., Fornace A. J., Jr. (2004) p38 MAP kinase's emerging role as a tumor suppressor. Adv. Cancer Res. 92, 95–118 [DOI] [PubMed] [Google Scholar]
- 12. Han J., Sun P. (2007) The pathways to tumor suppression via route p38. Trends Biochem. Sci. 32, 364–371 [DOI] [PubMed] [Google Scholar]
- 13. Ambrosino C., Nebreda A. R. (2001) Cell cycle regulation by p38 MAP kinases. Biol. Cell 93, 47–51 [DOI] [PubMed] [Google Scholar]
- 14. She Q. B., Bode A. M., Ma W. Y., Chen N. Y., Dong Z. (2001) Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Res. 61, 1604–1610 [PubMed] [Google Scholar]
- 15. Haq R., Brenton J. D., Takahashi M., Finan D., Finkielsztein A., Damaraju S., Rottapel R., Zanke B. (2002) Constitutive p38HOG mitogen-activated protein kinase activation induces permanent cell cycle arrest and senescence. Cancer Res. 62, 5076–5082 [PubMed] [Google Scholar]
- 16. Deacon K., Mistry P., Chernoff J., Blank J. L., Patel R. (2003) p38 Mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol. Biol. Cell 14, 2071–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boronkai A., Bellyei S., Szigeti A., Pozsgai E., Bognar Z., Sumegi B., Gallyas F., Jr. (2009) Potentiation of paclitaxel-induced apoptosis by galectin-13 overexpression via activation of Ask-1-p38-MAP kinase and JNK/SAPK pathways and suppression of Akt and ERK1/2 activation in U-937 human macrophage cells. Eur. J. Cell Biol. 88, 753–763 [DOI] [PubMed] [Google Scholar]
- 18. St Germain C., Niknejad N., Ma L., Garbuio K., Hai T., Dimitroulakos J. (2010) Cisplatin induces cytotoxicity through the mitogen-activated protein kinase pathways and activating transcription factor 3. Neoplasia 12, 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanchez-Prieto R., Rojas J. M., Taya Y., Gutkind J. S. (2000) A role for the p38 mitogen-acitvated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 60, 2464–2472 [PubMed] [Google Scholar]
- 20. Millour J., de Olano N., Horimoto Y., Monteiro L. J., Langer J. K., Aligue R., Hajji N., Lam E. W. (2011) ATM and p53 regulate FOXM1 expression via E2F in breast cancer epirubicin treatment and resistance. Mol. Cancer Ther. 10, 1046–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hui R. C., Francis R. E., Guest S. K., Costa J. R., Gomes A. R., Myatt S. S., Brosens J. J., Lam E. W. (2008) Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol. Cancer Ther. 7, 670–678 [DOI] [PubMed] [Google Scholar]
- 22. Hui R. C., Gomes A. R., Constantinidou D., Costa J. R., Karadedou C. T., Fernandez de Mattos S., Wymann M. P., Brosens J. J., Schulze A., Lam E. W. (2008) The forkhead transcription factor FOXO3a increases phosphoinositide-3 kinase/Akt activity in drug-resistant leukemic cells through induction of PIK3CA expression. Mol. Cell Biol. 28, 5886–5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Savarese D. M., Hsieh C., Stewart F. M. (1997) Clinical impact of chemotherapy dose escalation in patients with hematologic malignancies and solid tumors. J. Clin. Oncol. 15, 2981–2995 [DOI] [PubMed] [Google Scholar]
- 24. Wong S. T., Goodin S. (2009) Overcoming drug resistance in patients with metastatic breast cancer. Pharmacotherapy 29, 954–965 [DOI] [PubMed] [Google Scholar]
- 25. Myatt S. S., Lam E. W. (2007) The emerging roles of forkhead box (Fox) proteins in cancer. Nat. Rev. Cancer 7, 847–859 [DOI] [PubMed] [Google Scholar]
- 26. Ho K. K., Myatt S. S., Lam E. W. (2008) Many forks in the path: cycling with FoxO. Oncogene 27, 2300–2311 [DOI] [PubMed] [Google Scholar]
- 27. Kwok J. M., Myatt S. S., Marson C. M., Coombes R. C., Constantinidou D., Lam E. W. (2008) Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression. Mol. Cancer Ther. 7, 2022–2032 [DOI] [PubMed] [Google Scholar]
- 28. Kwok J. M., Peck B., Monteiro L. J., Schwenen H. D., Millour J., Coombes R. C., Myatt S. S., Lam E. W. (2010) FOXM1 confers acquired cisplatin resistance in breast cancer cells. Mol. Cancer Res. 8, 24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McGovern U. B., Francis R. E., Peck B., Guest S. K., Wang J., Myatt S. S., Krol J., Kwok J. M., Polychronis A., Coombes R. C., Lam E. W. (2009) Gefitinib (Iressa) represses FOXM1 expression via FOXO3a in breast cancer. Mol. Cancer Ther. 8, 582–591 [DOI] [PubMed] [Google Scholar]
- 30. Sunters A., Madureira P. A., Pomeranz K. M., Aubert M., Brosens J. J., Cook S. J., Burgering B. M., Coombes R. C., Lam E. W. (2006) Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 66, 212–220 [DOI] [PubMed] [Google Scholar]
- 31. Wang X., Chen W. R., Xing D. (2011) A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J. Cell. Physiol. doi: 10.1002/jcp.22839 [DOI] [PubMed] [Google Scholar]
- 32. Sunters A., Fernández de Mattos S., Stahl M., Brosens J. J., Zoumpoulidou G., Saunders C. A., Coffer P. J., Medema R. H., Coombes R. C., Lam E. W. (2003) FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J. Biol. Chem. 278, 49795–49805 [DOI] [PubMed] [Google Scholar]
- 33. Kops G. J., Medema R. H., Glassford J., Essers M. A., Dijkers P. F., Coffer P. J., Lam E. W., Burgering B. M. (2002) Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol. Cell Biol. 22, 2025–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fernández de Mattos S., Essafi A., Soeiro I., Pietersen A. M., Birkenkamp K. U., Edwards C. S., Martino A., Nelson B. H., Francis J. M., Jones M. C., Brosens J. J., Coffer P. J., Lam E. W. (2004) FoxO3a and BCR-ABL regulate cyclin D2 transcription through a STAT5/BCL6-dependent mechanism. Mol. Cell Biol. 24, 10058–10071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dijkers P. F., Medema R. H., Pals C., Banerji L., Thomas N. S., Lam E. W., Burgering B. M., Raaijmakers J. A., Lammers J. W., Koenderman L., Coffer P. J. (2000) Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1). Mol. Cell Biol. 20, 9138–9148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang J. Y., Zong C. S., Xia W., Yamaguchi H., Ding Q., Xie X., Lang J. Y., Lai C. C., Chang C. J., Huang W. C., Huang H., Kuo H. P., Lee D. F., Li L. Y., Lien H. C., Cheng X., Chang K. J., Hsiao C. D., Tsai F. J., Tsai C. H., Sahin A. A., Muller W. J., Mills G. B., Yu D., Hortobagyi G. N., Hung M. C. (2008) ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat. Cell Biol. 10, 138–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dolado I., Swat A., Ajenjo N., De Vita G., Cuadrado A., Nebreda A. R. (2007) p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell 11, 191–205 [DOI] [PubMed] [Google Scholar]
- 38. Campbell D. G., Morrice N. A. (2002) Identification of protein phosphorylation sites by a combination of mass spectrometry and solid phase Edman sequencing. J. Biomol. Tech. 13, 119–130 [PMC free article] [PubMed] [Google Scholar]
- 39. Frescas D., Valenti L., Accili D. (2005) Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J. Biol. Chem. 280, 20589–20595 [DOI] [PubMed] [Google Scholar]
- 40. Brownawell A. M., Kops G. J., Macara I. G., Burgering B. M. (2001) Inhibition of nuclear import by protein kinase B (Akt) regulates the subcellular distribution and activity of the forkhead transcription factor AFX. Mol. Cell Biol. 21, 3534–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsai K. L., Lee A. C., Rivers P. A. (2001) Hospital re-admissions: an empirical analysis of quality management in Taiwan. Health Serv. Manage Res. 14, 92–103 [DOI] [PubMed] [Google Scholar]
- 42. Hildesheim J., Bulavin D. V., Anver M. R., Alvord W. G., Hollander M. C., Vardanian L., Fornace A. J., Jr. (2002) Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 62, 7305–7315 [PubMed] [Google Scholar]
- 43. Corre S., Primot A., Baron Y., Le Seyec J., Goding C., Galibert M. D. (2009) Target gene specificity of USF-1 is directed via p38-mediated phosphorylation-dependent acetylation. J. Biol. Chem. 284, 18851–18862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Corrado P., Mancini M., Brusa G., Petta S., Martinelli G., Barbieri E., Santucci M. A. (2009) Acetylation of FOXO3a transcription factor in response to imatinib of chronic myeloid leukemia. Leukemia 23, 405–406 [DOI] [PubMed] [Google Scholar]
- 45. Shiota M., Yokomizo A., Kashiwagi E., Tada Y., Inokuchi J., Tatsugami K., Kuroiwa K., Uchiumi T., Seki N., Naito S. (2010) Foxo3a expression and acetylation regulate cancer cell growth and sensitivity to cisplatin. Cancer Sci. 101, 1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cai B., Xia Z. (2008) p38 MAP kinase mediates arsenite-induced apoptosis through FOXO3a activation and induction of Bim transcription. Apoptosis 13, 803–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hallahan A. R., Pritchard J. I., Chandraratna R. A., Ellenbogen R. G., Geyer J. R., Overland R. P., Strand A. D., Tapscott S. J., Olson J. M. (2003) BMP-2 mediates retinoid-induced apoptosis in medulloblastoma cells through a paracrine effect. Nat. Med. 9, 1033–1038 [DOI] [PubMed] [Google Scholar]
- 48. Losa J. H., Parada Cobo C., Viniegra J. G., Sánchez-Arevalo Lobo V. J., Ramón y Cajal S., Sánchez-Prieto R. (2003) Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene 22, 3998–4006 [DOI] [PubMed] [Google Scholar]
- 49. Brunet A., Park J., Tran H., Hu L. S., Hemmings B. A., Greenberg M. E. (2001) Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell Biol. 21, 952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang J. Y., Hung M. C. (2009) A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 15, 752–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chiacchiera F., Simone C. (2010) The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle 9, 1091–1096 [DOI] [PubMed] [Google Scholar]
- 52. Greer E. L., Oskoui P. R., Banko M. R., Maniar J. M., Gygi M. P., Gygi S. P., Brunet A. (2007) The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem. 282, 30107–30119 [DOI] [PubMed] [Google Scholar]
- 53. Link W., Oyarzabal J., Serelde B. G., Albarran M. I., Rabal O., Cebriá A., Alfonso P., Fominaya J., Renner O., Peregrina S., Soilán D., Ceballos P. A., Hernández A. I., Lorenzo M., Pevarello P., Granda T. G., Kurz G., Carnero A., Bischoff J. R. (2009) Chemical interrogation of FOXO3a nuclear translocation identifies potent and selective inhibitors of phosphoinositide 3-kinases. J. Biol. Chem. 284, 28392–28400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen J., Gomes A. R., Monteiro L. J., Wong S. Y., Wu L. H., Ng T. T., Karadedou C. T., Millour J., Ip Y. C., Cheung Y. N., Sunters A., Chan K. Y., Lam E. W., Khoo U. S. (2010) Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS One 5, e12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Krol J., Francis R. E., Albergaria A., Sunters A., Polychronis A., Coombes R. C., Lam E. W. (2007) The transcription factor FOXO3a is a crucial cellular target of gefitinib (Iressa) in breast cancer cells. Mol. Cancer Ther. 6, 3169–3179 [DOI] [PubMed] [Google Scholar]
- 56. Essafi A., Gomes A. R., Pomeranz K. M., Zwolinska A. K., Varshochi R., McGovern U. B., Lam E. W. (2009) Studying the subcellular localization and DNA-binding activity of FoxO transcription factors, downstream effectors of PI3K/Akt. Methods Mol. Biol. 462, 201–211 [DOI] [PubMed] [Google Scholar]
- 57. Torres M., Forman H. J. (2003) Redox signaling and the MAP kinase pathways. Biofactors 17, 287–296 [DOI] [PubMed] [Google Scholar]
- 58. McCubrey J. A., Lahair M. M., Franklin R. A. (2006) Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 8, 1775–1789 [DOI] [PubMed] [Google Scholar]
- 59. Nagai H., Noguchi T., Takeda K., Ichijo H. (2007) Pathophysiological roles of ASK1-MAP kinase signaling pathways. J. Biochem. Mol. Biol. 40, 1–6 [DOI] [PubMed] [Google Scholar]
- 60. Hong S. W., Shin J. S., Lee Y. M., Kim D. G., Lee S. Y., Yoon D. H., Jung S. Y., Hwang J. J., Lee S. J., Cho D. H., Hong Y. S., Kim T. W., Jin D. H., Lee W. K. (2011) p34 (SEI-1) inhibits ROS-induced cell death through suppression of ASK1. Cancer Biol. Ther. 12, 421–426 [DOI] [PubMed] [Google Scholar]
- 61. Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2, 222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Myatt S. S., Brosens J. J., Lam E. W. (2011) Sense and sensitivity: FOXO and ROS in cancer development and treatment. Antioxid. Redox Signal. 14, 675–687 [DOI] [PubMed] [Google Scholar]
- 63. Ubezio P., Civoli F. (1994) Flow cytometric detection of hydrogen peroxide production induced by doxorubicin in cancer cells. Free Radic. Biol. Med. 16, 509–516 [DOI] [PubMed] [Google Scholar]
- 64. Sinha B. K., Mimnaugh E. G., Rajagopalan S., Myers C. E. (1989) Adriamycin activation and oxygen free radical formation in human breast tumor cells: protective role of glutathione peroxidase in adriamycin resistance. Cancer Res. 49, 3844–3848 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.