Background: CB2 cannabinoid receptors promote neural progenitor cell proliferation.
Results: CB2 receptors induce neural progenitor cell proliferation and neurogenesis via activation of mTORC1 signaling.
Conclusion: CB2 receptor/mTORC1-induced neural progenitor proliferation is relevant under physiological and pathological conditions such as cortical development and excitotoxicity-induced adult hippocampal neurogenesis.
Significance: Nonpsychotomimetic CB2 receptor-selective ligands are promising molecules to manipulate neurogenesis.
Keywords: Cannabinoid Receptors, Cell Proliferation, Development, Excitotoxicity, Neurogenesis, Neuroprogenitor Cell, CB2 Receptors, mTORC1
Abstract
The endocannabinoid system is known to regulate neural progenitor (NP) cell proliferation and neurogenesis. In particular, CB2 cannabinoid receptors have been shown to promote NP proliferation. As CB2 receptors are not expressed in differentiated neurons, CB2-selective agonists are promising candidates to manipulate NP proliferation and indirectly neurogenesis by overcoming the undesired psychoactive effects of neuronal CB1 cannabinoid receptor activation. Here, by using NP cells, brain organotypic cultures, and in vivo animal models, we investigated the signal transduction mechanism involved in CB2 receptor-induced NP cell proliferation and neurogenesis. Exposure of hippocampal HiB5 NP cells to the CB2 receptor-selective agonist HU-308 led to the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin complex 1 (mTORC1) pathway, which, by inhibiting its downstream target p27Kip1, induced NP proliferation. Experiments conducted with the CB2 receptor-selective antagonist SR144528, inhibitors of the PI3K/Akt/mTORC1 axis, and CB2 receptor transient-transfection vector further supported that CB2 receptors control NP cell proliferation via activation of mTORC1 signaling. Likewise, CB2 receptor engagement induced cell proliferation in an mTORC1-dependent manner both in embryonic cortical slices and in adult hippocampal NPs. Thus, HU-308 increased ribosomal protein S6 phosphorylation and 5-bromo-2′-deoxyuridine incorporation in wild-type but not CB2 receptor-deficient NPs of the mouse subgranular zone. Moreover, adult hippocampal NP proliferation induced by HU-308 and excitotoxicity was blocked by the mTORC1 inhibitor rapamycin. Altogether, these findings provide a mechanism of action and a rationale for the use of nonpsychotomimetic CB2 receptor-selective ligands as a novel strategy for the control of NP cell proliferation and neurogenesis.
Introduction
The endocannabinoids (eCBs)5 2-arachidonoylglycerol and anandamide are lipid signaling messengers involved in the homeostatic control of a large variety of functions of the nervous system (1). Thus, eCBs are produced on demand by activated postsynaptic cells and, by acting as retrograde messengers, control neurotransmitter release through presynaptic CB1 cannabinoid receptors (2). CB1 constitutes the most abundant neuronal G-protein-coupled receptor in some areas of the nervous system and is also involved in the control of neural cell proliferation/survival decision (3). CB1 receptors exert a neuroprotective action, at least in part by controlling excessive glutamate release and excitotoxicity (4). In addition, they contribute to long term neuronal plasticity by promoting NP proliferation and excitotoxicity-induced neurogenesis (5–7). The other type of cannabinoid G protein-coupled receptor, the CB2 cannabinoid receptor, is very abundant in some peripheral cells (e.g. lymphocytes and macrophages) and organs (e.g. spleen and thymus), and in the nervous system it is basically restricted to infiltrating immune cells and resident microglia/macrophages (8), oligodendrocyte progenitors (9), and neural progenitor/stem cells (NPs/NSC) (10). CB2 receptors control the pro-inflammatory status of immune cells by modulating their Th1/Th2 phenotype, and this activity has important implications for neuronal survival under neuroinflammatory conditions occurring in animal models of neurodegenerative diseases, such as multiple sclerosis, Alzheimer disease, and Huntington disease, and upon acute ischemic brain injury (11). Because of the lack of undesired psychoactive effects of CB2-selective ligands, therapeutic approaches aimed at targeting CB2 receptors rather than CB1 receptors are likely candidates to promote neuroprotection and neurorepair (12). CB2 receptors are present in embryonic stem cells (13) as well as in bone marrow-derived myeloid progenitors, in which they regulate cell proliferation and trafficking to the nervous system under neuroinflammatory conditions (14). In the nervous system, undifferentiated NSC/NPs also express functional CB2 receptors (10, 15), but the final fate of CB2-mediated newly born cell generation (10) is unknown; likewise, the signaling mechanism underlying CB2 receptor actions remains to be elucidated.
CB1 and CB2 receptors are coupled to heterotrimeric Gi proteins, inhibition of adenylyl cyclase, and activation of extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)/Akt (16). In addition, CB1 receptors have recently been shown to modulate mammalian target of rapamycin complex 1 (mTORC1) signaling, which is in turn responsible for the cognitive impairment induced by Δ9-tetrahydrocannabinol, the major active constituent of marijuana (17). mTORC1 is involved in the control of a plethora of cell functions by acting, for example, through the regulation of protein synthesis via phosphorylation of its downstream targets 70-kDa ribosomal protein S6 kinase (p70S6K) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) (18), which are essential elements in neuronal responses to synaptic activity and plasticity (19). In addition, mTORC1 is a major target of the PI3K/Akt pathway and thus also plays a central role in neural cell survival/death decision (18). For example, status epilepticus activates mTORC1, and this is required for the hippocampal alterations that contribute to the development of epilepsy, including mossy fiber sprouting, neuronal cell death, and neurogenesis (20). Considering this key position of mTORC1 in neural cell biology, as well as the involvement of the eCB system in finely tuning the balance between both excitatory and inhibitory neurotransmission (4, 21) and cell generation and death/survival (12, 22), here we investigated the signaling mechanism by which CB2 receptors control NP cell proliferation and, in particular, the potential role of mTORC1 in this process. We show that CB2 receptors present in NPs exert a proliferative effect that relies on the activation of the PI3K/Akt/mTORC1 axis and its downstream target p27Kip1. Furthermore, this CB2 receptor-induced NP proliferation via mTORC1 is relevant in pathophysiological conditions such as NP proliferation during cortical development and excitotoxicity-induced adult hippocampal neurogenesis.
EXPERIMENTAL PROCEDURES
Materials
Founders of the CB2 receptor knock-out mice colony were kindly donated by Nancy Buckley (National Institutes of Health, Bethesda) and were obtained by disrupting the CB2 receptor gene by using homologous recombination in the embryonic stem cell line 129 (23). The CB2 receptor-selective agonist HU-308 was kindly donated by Raphael Mechoulam (The Hebrew University, Jerusalem, Israel) and the HiB5 cells by Zaal Kokaia (Lund Stem Cell Center, Sweden). The antibodies employed in this study are detailed in supplemental Table 1.
Neural Progenitor Cultures
Multipotent self-renewing progenitors were obtained from embryonic E14.5 wild-type and nestin-GFP mice and grown as described previously (5) in chemically defined medium consisting of Dulbecco's modified Eagle's and F-12 media supplemented with N2 (Invitrogen), 0.6% glucose, nonessential amino acids, 50 mm Hepes, 2 μg/ml heparin-bound EGF, 20 ng/ml EGF, and 20 ng/ml FGF-2. Clonal neurospheres were derived from nonadherent dissociated cultures of NPs (1000 cells/ml), and experiments were carried out with early (up to 10) passage neurospheres. The HiB5 hippocampal progenitor cell line was grown as described (24) in Dulbecco's modified Eagle's medium supplemented with 2 mm glutamine, 1% penicillin/streptomycin, and 10% (v/v) fetal calf serum. HiB5 cell cultures were incubated in 5% CO2 at 33 °C, the proliferation-permissive temperature of the oncogenic tsA58 allele of the SV40 large T antigen. Incubation at 37 °C results in loss of proliferative capacity and neural differentiation. For Western blot and immunostaining analyses, HiB5 cells were pretreated with SR144528 (2 μm), LY-294,002 (5 μm), Akt inhibitor 1 (5 μm), rapamycin (50 nm), or PD98059 (10 μm) (10) for 30 min and subsequently treated with HU-308 for another 30 min. Stock solutions were prepared in dimethyl sulfoxide. No significant influence of dimethyl sulfoxide on any of the parameters determined was observed at the final concentration used (0.1% v/v). Control incubations included the corresponding vehicle content.
Proliferation and Cell Cycle Analyses
Neurosphere generation experiments were performed in 96-well dishes with 100 μl of medium. Neurospheres were pretreated with rapamycin (50 nm) for 30 min and then cultured in the continuous presence of HU-308 (50 nm) for 3 days. Subsequently the number of neurospheres per well was quantified. HiB5 cells were passaged, maintained, and analyzed at 33 °C. HiB5 cells were pretreated with SR144528 (2 μm) or rapamycin for 30 min, cultured in the continuous presence of HU-308 (50 nm) for 16 h, and with 5-bromo-2′-deoxyuridine (BrdU; 100 μg/ml) for 30 min followed by immunostaining. For cell cycle exit experiments, HiB5 cells were incubated with BrdU (100 μg/ml) for 30 min, treated with the indicated drugs for 48 h, and followed by immunostaining with rat monoclonal anti-BrdU and rabbit polyclonal anti-Ki-67 antibodies (supplemental Table 1). For flow cytometry analysis, HiB5 cells were trypsinized, permeabilized, and fixed in 1% (w/v) of bovine serum albumin and 30% ethanol/PBS and labeled with 5 μg/ml Hoechst 33342 (Molecular Probes, Leiden, The Netherlands). Fluorescence intensity was analyzed by using an LSR flow cytometer (BD Biosciences). Ten thousand cells per analysis were recorded.
Cell Transfection
HiB5 cells were transiently transfected 1 day after plating with 1 μg of pCMV6 mouse CB2 receptor-expressing vector or empty vector (Origene, Rockville, MD) by using Lipofectamine 2000 following the instructions of the manufacturer (Invitrogen).
Organotypic Brain Cultures
Cortical brain slices were obtained from E14.5 mice and cultured under semidry conditions in neurobasal medium, B27 (1%), N2 (1%), glutamine (1%), penicillin/streptomycin (1%), fungizone (1%), and ciprofloxacine (5 μg/ml) as described previously (25). Brain slices were treated with the indicated drugs for 1 or 16 h and subsequently incubated with BrdU for 1 h. At the end of the experiment, brain slices were processed in 10-μm sections, and slice sections from equivalent regions of the rostral to caudal axis were analyzed by immunofluorescence.
RT-PCR
RNA was obtained with the RNeasy Protect kit (Qiagen, Valencia, CA) using the RNase-free DNase kit and cDNA synthesis kit (Roche Applied Science). Amplification of cDNA was performed with the specific primers indicated in supplemental Table 2. CB2 receptor PCRs were performed using the following conditions: 1 min at 95 °C and 35 cycles (30 s at 95 °C, 30 s at 58 °C, and 1 min at 72 °C). Finally, after a final extension step at 72 °C for 5 min, PCR products were separated on 1.5% agarose gels. The rest of the transcripts were detected as described previously (6).
Western Blot
Cleared cell extracts were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membranes. After incubation with the correspondent primary antibodies (supplemental Table 1), blots were developed with the corresponding horseradish peroxidase-coupled secondary antibodies and enhanced chemiluminescence detection kit. Loading controls were performed with an anti-α-tubulin antibody. Densitometric quantification of the luminograms was performed using a GS-700 imaging densitometer (Bio-Rad) and MultiAnalyst software (Bio-Rad).
Animal Procedures
Animal procedures were performed according to the European Union guidelines (86/609/EU) for the use of laboratory animals. Mice were housed (five per cage) with food and water available ad libitum and maintained in a temperature-controlled environment on a 12-h light/dark cycle. Adult CB2 receptor knock-out mice (8 weeks old) and their respective wild-type littermates were obtained from heterozygote crosses (26). Mice were injected intraperitoneally with 100 mg/kg BrdU and vehicle (150 μl of PBS supplemented with 0.5 mg of defatted bovine serum albumin and 4% dimethyl sulfoxide) or 15 mg/kg HU-308, either alone or in combination with vehicle (150 μl PBS), or 6 mg/kg rapamycin (injected 30 min before vehicle/HU-308) daily for 5 days and perfused at either day 1 or 30 days later. For short term experiments, wild-type mice were administered a single intraperitoneal injection of 50 mg/kg BrdU and vehicle (150 μl PBS) or HU-308 (15 mg/kg) and perfused 3 h later. Kainate-induced excitotoxicity experiments were performed as described previously (6, 10). Animals were given a single injection of vehicle (150 μl PBS) or kainic acid (KA; 15 mg/kg) on the 1st day of treatment, alone or together with rapamycin, and sacrificed after 5 or 30 days.
Immunofluorescence and Confocal Microscopy
Fixed cell cultures, embryonic organotypic cortical sections (10 μm) attached to poly-l-lysine-coated slides, and adult coronal free-floating brain sections (30 μm) were processed as described (26). Briefly, after a 1-h blockade with PBS supplemented with 0.25% Triton X-100 and 10% goat serum, brain sections were incubated overnight at 4 °C with the indicated primary antibodies (supplemental Table 1), followed by incubation for 1 h at room temperature with secondary antibodies. The appropriate mouse, rat, and rabbit highly cross-adsorbed AlexaFluor 488, AlexaFluor 594, and AlexaFluor 647 secondary antibodies (Invitrogen) were used. Confocal fluorescence images were acquired by using Leica TCS-SP2 software (Wetzlar, Germany) and SP2 microscope with two passes by Kalman filter and a 1024X1024 collection box. In slices, the number of BrdU+, phospho-p27Kip1+, and phospho-S6 highly immunoreactive positive cells present in the VZ/SVZ of the developing cortex was quantified, after image conversion to grayscale, in ImageJ by using the threshold tool and normalized to the total area selected for quantification. In adult mice, BrdU- and phospho-S6-positive cells were quantified in the SGZ of the hippocampus in a minimum of five coronal sections per animal. A 1-in-10 series of hippocampal sections located between 1.3 and 2.5 mm posterior to bregma were analyzed, and positive cells were normalized to the SGZ area determined with ×10 objective. The absolute number of positive cells was calculated considering the total hippocampal volume as determined by the sum of the areas of the sampled sections multiplied by the distances between them.
Data Analysis
Data are presented as means ± S.E. Significant differences between the groups were evaluated using an analysis of variance test followed by a Bonferroni post hoc comparison in the case of parametric population and Mann-Whitney test in the case of nonparametric populations. p values < 0.05 were considered significant.
RESULTS
Selective CB2 Cannabinoid Receptor Activation Promotes Neural Progenitor Proliferation via PI3K/Akt/mTORC1 Signaling
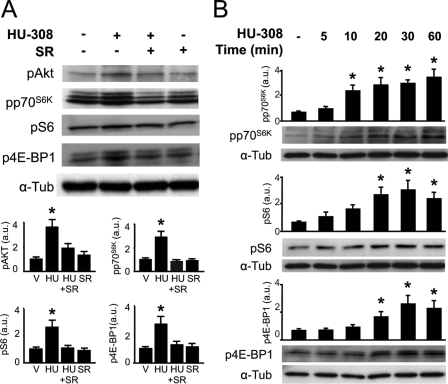
NP cells have been shown to express CB2 receptors in both neurosphere-derived progenitor cells and human/murine NSC lines (10, 15). To investigate the signal transduction mechanism of CB2 receptors in NP cell proliferation, we first employed the HiB5 rat NP line (24). HiB5 cells cultured in proliferating conditions (33 °C) express CB2 receptors, and after differentiation for 3 days at the restrictive temperature (37 °C), their expression was significantly reduced (supplemental Fig. 1). CB1 receptors were also present in HiB5 cells but with an opposite pattern of expression, as CB1 transcript levels increased with neural differentiation. In addition, transcripts of the putative eCB receptor GPR55 were also present in HiB5 cells, but their levels did not change with the differentiation status of the cells. CB2 receptors are known to be coupled to PI3K/Akt and ERK activation (9, 10). Thus, we analyzed their functional coupling to downstream signaling in proliferating HiB5 cells by treatment with the CB2-selective agonist HU-308 (27). HU-308 (50 nm) induced a rapid Akt activation as evidenced by Western blot analysis with anti-phospho-Ser-473-Akt antibody, and this effect was prevented by the CB2-selective antagonist SR144528 (2 μm) (Fig. 1A). Likewise, HU-308 induced a time-dependent increase in the phosphorylation of downstream targets of PI3K/Akt signaling, including p70S6K and its substrate the ribosomal protein S6, as well as 4E-BP1 (Fig. 1B). Phosphorylation of p70S6K at Thr-389 indicated the involvement of mTORC1 activity in HU-308 signaling, in agreement with the observed phosphorylation of S6 at Ser-235/236 and 4E-BP1 at Ser-37/46 (18), thus indicating the involvement of mTORC1 in CB2 receptor signaling. HU-308-induced phosphorylation of the mTORC1 downstream targets analyzed was prevented by co-incubation with SR144528 (Fig. 1A), therefore supporting the selectivity of HU-308 on CB2 receptors. As S6 phosphorylation constitutes a well established readout of mTORC1 activity, we performed immunofluorescence analysis of phospho-S6 in HiB5 cells (Fig. 2A) and NPs derived from transgenic nestin-GFP mice (supplemental Fig. 2A). HU-308 administration increased phospho-S6-positive cells (Fig. 2, A and B). To investigate the mechanism involved in mTORC1 activation by HU-308, pharmacological inhibition studies were performed with the PI3K inhibitor LY-294,002, the Akt inhibitor 1, and the mTORC1 inhibitor rapamycin in HiB5 cells. The three compounds prevented HU-308-induced p70S6K and S6 phosphorylation (Fig. 2, A–D), further supporting the involvement of PI3K/Akt/mTORC1 in CB2 receptor signaling. HU-308 alone or combined with the different inhibitors did not induce HiB5 cell death at the doses employed (supplemental Fig. 3). PI3K/Akt inhibitors and rapamycin reduced HU-308-induced p70S6K and S6 phosphorylation below basal levels in agreement with the involvement of this pro-survival signaling pathway in cortical development. The contribution of the ERK cascade to mTORC1 activation was analyzed with the MEK inhibitor PD98059. HU-308-induced ERK phosphorylation was prevented by PD98059, but this inhibitor failed to block HU-308-induced p70S6K phosphorylation and only exerted a marginal effect on HU-308-induced S6 phosphorylation (supplemental Fig. 4).
FIGURE 1.
CB2 cannabinoid receptors signal through the PI3K/Akt/mTORC1 axis on HiB5 neural progenitor cells. A, HiB5 cells were treated with HU-308 (50 nm) for 30 min alone or in the presence of SR144528 (SR, 2 μm). Western blot analysis was performed with anti-phospho-Akt, phospho-p70S6K, phospho-S6, and phospho-4E-BP-1 antibodies. Loading control was performed with anti-α-tubulin (a-Tub) antibody. Quantification of the relative phosphorylated protein and α-tubulin optical density is given in arbitrary units (a.u.). B, Western blot analysis was performed after HU-308 treatment for the indicated times with anti-phospho-p70S6K, phospho-S6, and phospho-4EBP-1 antibodies. Quantification of the relative phosphorylated protein was performed as above. Representative blots from four independent experiments are shown. *, p < 0.05 versus vehicle-treated cells.
FIGURE 2.
CB2 cannabinoid receptor activation of mTORC1 signaling depends on PI3K/Akt activation. A and B, HiB5 cells were treated with HU-308 (HU; 50 nm) for 30 min in the absence or presence of LY-294,002 (LY; 5 μm), Akt inhibitor 1 (Inh1; 5 μm), and rapamycin (Rapa; 50 nm). The number of phospho-S6+ HiB5 cells was quantified after incubation (as above) and immunofluorescence. Phospho-S6+ cells were normalized to total cell number as identified by Hoechst 33342 counterstaining. Representative images are shown for each condition. Veh, vehicle. Scale bar, 10 μm. C and D, Western blot analysis of phospho-p70S6K were quantified and referred to loading control performed with anti-α-tubulin (a-Tub) antibody. *, p < 0.05; **, p < 0.01 versus vehicle (V)-treated cells; #, p < 0.05; ##, p < 0.01 versus HU-308-treated cells.
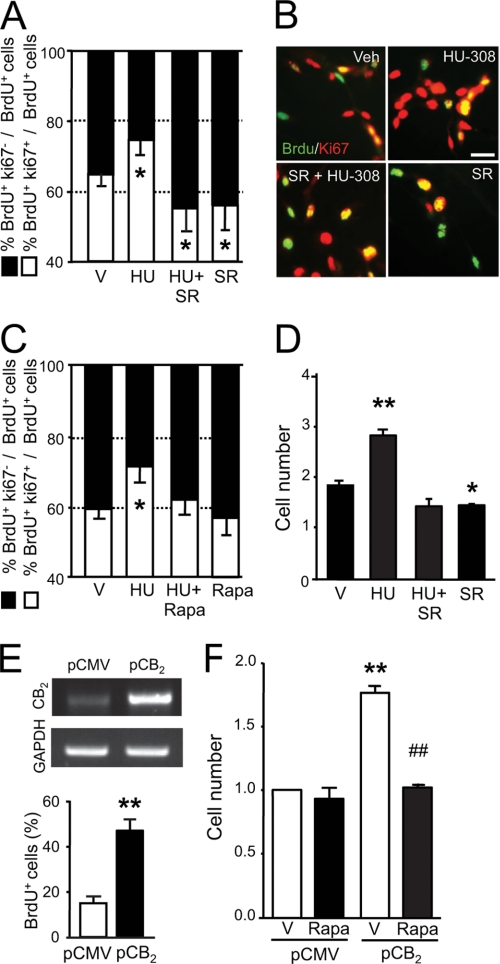
As CB2 receptors present in NPs control cell proliferation (10, 15), we investigated by flow cytometry analysis of DNA content their signaling mechanism in the regulation of cell cycle progression in HiB5 cells. Thus, HU-308 reduced the fraction of cells in the G0/G1 compartment while increasing the fraction of cells in the S phase. This G1-S phase progression was prevented by SR144528 (Fig. 3A) and rapamycin (Fig. 3, B and C), indicating the involvement of CB2 receptors and mTORC1 in HU-308-induced cell cycle regulation. Likewise, SR144528 and rapamycin prevented HU-308-induced cell proliferation as determined by BrdU-positive cell quantification (supplemental Fig. 2B). In addition, pharmacological inhibition of PI3K/Akt with LY-294,002 and Akt inhibitor 1 blocked HU-308-induced HiB5 cell proliferation (BrdU+ cells, vehicle, 25.94 ± 1.94; HU-308, 38.91 ± 3.24; LY-294,002+HU-308, 28.10 ± 2.16; I1+HU-308, 24.86 ± 1.08). The effect of HU-308 on cell proliferation was confirmed by using an NP-derived neurosphere formation assay. HU-308 administration increased neurosphere formation, and this pro-neurogenic action was prevented by mTORC1 inhibition (supplemental Fig. 2C). We further characterized CB2 receptor-mediated regulation of cell cycle maintenance by analyzing BrdU-labeled cells (in S phase) and quantification of double-labeled cells with BrdU and Ki67, an endogenous marker of cycling cells, after incubation with HU-308. HU-308 promoted HiB5 cell cycle maintenance as reflected by the increased BrdU+Ki67+ cell fraction and the reduction of the BrdU+Ki67− cell fraction (Fig. 4, A and B). In agreement with the flow cytometry studies, HU-308-induced cell cycle maintenance (BrdU+Ki67+ cells) was prevented by SR144528 and rapamycin (Fig. 4, A–C). HU-308 increased HiB5 cell number (Fig. 4D), and CB2 receptor overexpression increased BrdU+ cell number (Fig. 4E). Importantly, CB2 overexpression induced an increase in HiB5 cell number that was blunted by rapamycin (Fig. 4F). Overall, these results demonstrate that CB2 receptors in cultured NP cells evoke NP proliferation via PI3K/Akt/mTORC1 activation.
FIGURE 3.
CB2 cannabinoid receptor activation promotes G1/S phase progression of neural progenitors through activation of the PI3K/Akt/mTORC1 axis. A, HiB5 cells were treated with HU-308 (HU; 50 nm) alone or in the presence of SR144528 (SR, 2 μm), and cell cycle analysis was performed after DNA content quantification by flow cytometry analysis. The relative fraction of cells in G0/G1 and S phase is shown. B and C, cell cycle analysis was performed after HU-308 administration alone or in the presence of rapamycin (Rapa; 50 nm). The fraction of cells in G0/G1 and S phase and a representative DNA histogram of each condition are shown. *, p < 0.05; **, p < 0.01 versus vehicle (V)-treated cells; #, p < 0.05; ##, p < 0.01 versus HU-308-treated cells.
FIGURE 4.
CB2 cannabinoid receptor activation promotes neural progenitor cell cycle maintenance through mTORC1 activation. A and B, HiB5 cells were treated with HU-308 alone (HU; 50 nm) or in the presence of SR144528 (SR, 2 μm) for 48 h, and after immunofluorescence with anti-BrdU and anti-Ki67 antibodies (green and red, respectively), the percentage of BrdU+Ki67+ and BrdU+Ki67− cells was quantified. Total cell number was determined by Hoechst 33342 counterstaining. Representative images of each condition are shown. V, vehicle. Scale bar, 20 μm. C, cell cycle maintenance of HiB5 cells was determined after HU-308 administration alone or in the presence of rapamycin (Rapa; 50 nm) as above. D, neurosphere-derived NPs were treated with HU-308 with or without SR144528 (2 μm) as above, and the number of cells was quantified in each condition. E and F, HiB5 cells were transiently transfected with pCMV6-mCB2 or empty pCMV6 plasmids, and BrdU incorporation was quantified after 2 days (lower panel). Reverse transcription-PCR analysis of CB2 receptor and GAPDH as loading control (upper panel) is shown. HiB5 cell number was quantified in CB2 or control transfected cells after 2 days with and without rapamycin at 37 °C. Results correspond to four independent experiments. *, p < 0.05; **, p < 0.01 versus vehicle-treated cells; ##, p < 0.01 versus HU-308-treated cells or vehicle-treated CB2-transfected cells.
CB2 Cannabinoid Receptors Induce Cortical Progenitor Proliferation during Brain Development
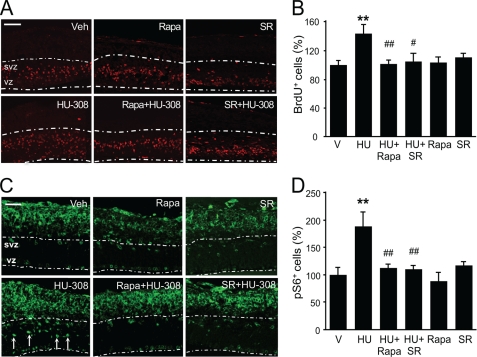
To investigate the relevance of CB2 receptor function in NSC proliferation in a more physiological environment, we employed organotypic mouse embryonic (E14.5) cortical slices. HU-308 induced NP cell proliferation as revealed by the increased BrdU-positive cell number in the VZ/SVZ, and this effect was prevented by SR144528 and rapamycin (Fig. 5, A and B). This increase in proliferation upon treatment with HU-308 was absent in CB2 receptor knock-out mice (data not shown). Moreover, short term HU-308 stimulation (1 h) induced Ser-235/236-S6 protein phosphorylation in undifferentiated VZ/SVZ cells (Fig. 5C, arrows) as well as in postmitotic neuroblasts that localize in the intermediate zone and developing cortical plate. The HU-308-induced increase in phospho-S6 immunoreactivity in the developing cortex was dependent on CB2 receptors and mTORC1 as it was blocked by SR144528 and rapamycin (Fig. 5, A–D). These results support that the proliferative action of CB2 receptor signaling in NPs is relevant during cortical development.
FIGURE 5.
CB2 cannabinoid receptor agonist HU-308 induces cortical progenitor proliferation in organotypic cultures via mTORC1 signaling. A and B, wild-type embryonic E14.5 cortical slices cultured in the presence of BrdU (10 μg/ml) were treated for 24 h with vehicle (Veh) or HU-308 alone (5 μm) or in the presence of SR144528 (SR; 25 μm) or rapamycin (Rapa; 250 nm). BrdU-positive cells in the ventricular and subventricular zone (white dashed line) were quantified and referred to the analyzed surface. C and D, phospho-S6-positive cells were quantified after immunofluorescence in slices treated for 1 h with vehicle or HU-308 alone or together with SR144528 or rapamycin. Results correspond to three independent experiments. Scale bars, A and C, 100 and 50 μm, respectively. **, p < 0.01 versus vehicle-treated slices; #, p < 0.05; ##, p < 0.01 versus HU-308-treated cells.
To investigate the mechanism by which CB2 receptors/mTORC1 control progenitor cell cycle progression, we focused on the cyclin-dependent kinase inhibitor p27Kip1. This protein inhibits the G1-S phase transition of NPs (28), regulates neuronal differentiation (29), and is a downstream target of mTORC1 (30). To test this possibility, E14.5 cortical slices were treated acutely with HU-308, and we quantified the number of phospho-Thr-157-p27Kip1-positive cells at the VZ/SVZ (Fig. 6, A and B). The HU-308-induced increase of phospho-p27 cell number was prevented by SR144528 and rapamycin. To investigate the mechanism of p27Kip1 regulation by CB2 receptors, HiB5 cells were treated with HU-308 in the presence or absence of PI3K/Akt/mTORC1 inhibitors. HU-308 induced a time-dependent increase in p27Kip1 phosphorylation (Fig. 6C) that was prevented by LY-294,002, Akt inhibitor 1, and rapamycin (Fig. 6D). As p27Kip1 activity may be inhibited by mTORC1 via the downstream serum- and glucocorticoid-inducible kinase 1 (SGK1), thus allowing G1-S progression (30), we analyzed the potential involvement of SGK1 as a molecular link between CB2 receptor-induced PI3K/Akt/mTORC1 activation and p27Kip1 inhibition. HU-308 induced SKG1 phosphorylation, which was prevented by rapamycin and the SGK1 inhibitor GSK-650394 (supplemental Fig. 5A). Furthermore, GSK-650394 prevented the HU-308-induced BrdU incorporation in HiB5 cells and the increase of neurosphere generation from NPs (supplemental Fig. 5, B and C). These results support that SGK1 may be responsible, at least in part, for the CB2 receptor/mTORC1-mediated modulation of p27Kip1 phosphorylation and progenitor cell proliferation.
FIGURE 6.
CB2 cannabinoid receptor-induced HiB5 cell proliferation is mediated by p27Kip1 inhibition. A and B, wild-type embryonic E14.5 cortical slices were treated for 1 h with vehicle (V) or HU-308 alone (HU; 5 μm) or in the presence of SR144528 (SR; 25 μm) or rapamycin (Rapa; 250 nm). Phospho-p27Kip1+ cells were quantified in the ventricular and subventricular zone (VZ/SVZ) after immunofluorescence and referred to the analyzed surface. Representative images are shown. C, HiB5 cells were treated with vehicle (Veh) or HU-308 (50 nm) for the indicated times, and p27Kip1 phosphorylation was determined by Western blot analysis. Loading control was performed with anti-α-tubulin antibody. D and E, HiB5 cells were treated for 30 min with vehicle or HU-308 (HU) alone or in the presence of LY294,002 (LY; 5 μm), Akt inhibitor 1 (Inh1; 5 μm), and rapamycin (Rapa; 50 nm). p27Kip1+ cells were quantified by immunofluorescence in each condition. Representative images are shown. Results correspond to three independent experiments. Scale bars, B and E, 100 and 10 μm, respectively. **, p < 0.01 versus vehicle-treated cells or slices; #, p < 0.05; ##, p < 0.01 versus HU-308-treated cells or slices.
CB2 Cannabinoid Receptors Promote Excitotoxicity-induced Hippocampal Progenitor Proliferation via mTORC1 Signaling
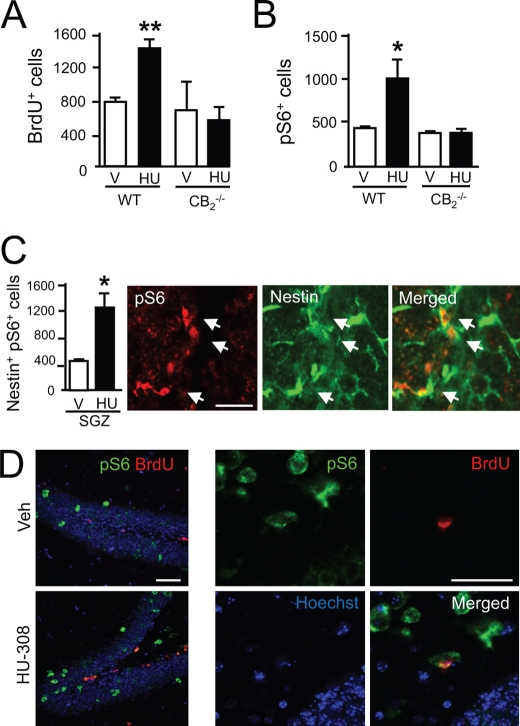
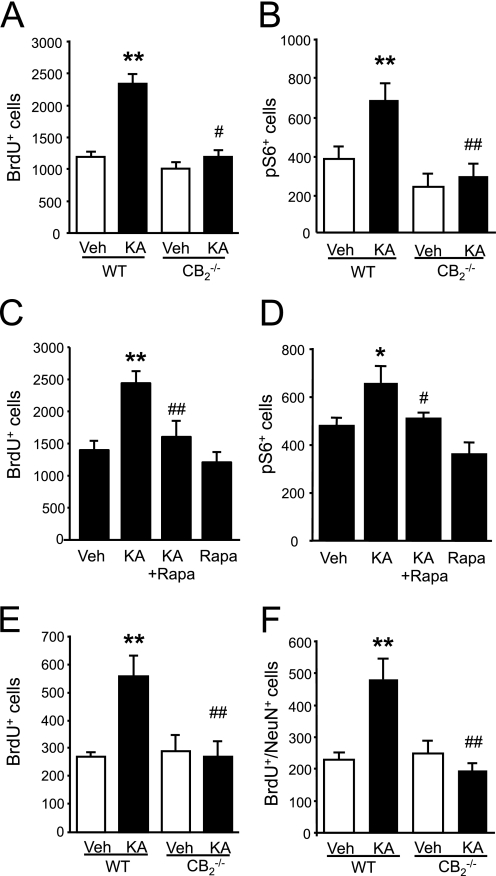
The aforementioned observations support that CB2 receptors expressed by NPs are candidate targets for pharmacological manipulation to expand NP populations by inducing cell proliferation and, importantly, that CB2 receptor action also occurs in the physiological NP niche of developing cortex slices. As both CB2 receptors (10) and mTORC1 (20) are involved in hippocampal neurogenesis and the plasticity responses to excitotoxicity, we analyzed whether CB2 receptors are coupled to mTORC1 signaling in the adult mouse hippocampus. HU-308 administration for 5 days increased NP proliferation as determined by quantification of BrdU+ cells (Fig. 7A) (10). NP mobilization by HU-308 was also associated with an increased number of phospho-S6+ cells, an effect that was absent in CB2 receptor-deficient mice (Fig. 7B). Detailed confocal immunofluorescence analyses showed that HU-308-increased proliferation occurred in concert with S6 phosphorylation as nestin-labeled cells co-localized with phospho-S6 immunoreactivity (Fig. 7C). After short term (3 h) administration, HU-308 selectively increased S6 phosphorylation in the subgranular zone (SGZ) (phospho-S6+ cells, vehicle, 438 ± 30; HU-308, 1230 ± 240, p < 0.01) where NPs reside but not in differentiated neurons located in the granular zone (phospho-S6+ cells, vehicle, 450 ± 54; HU-308, 522 ± 78, nonsignificantly different). Likewise, HU-308 stimulation (5 days) increased double-labeled pS6+BrdU+ newly born neurons (Fig. 7D). To determine the requirement of mTORC1 activation in HU-308-induced hippocampal NP proliferation, animals were co-administered with rapamycin, which blocked the BrdU+ and phospho-S6+ cell number increase induced by CB2 receptor activation (Fig. 8, A–D).
FIGURE 7.
HU-308-induced hippocampal progenitor proliferation is mediated by CB2 receptors. A and B, wild-type (WT) and CB2 receptor-deficient mice were treated with vehicle (V; white bars) or HU-308 (HU; 15 mg/kg, daily intraperitoneal administration, black bars) for 5 days, and NP proliferation was quantified as BrdU+ cell number. Immunoreactive phospho-S6+ cells in the SGZ were also quantified (n = 4 in each group). C, WT mice were treated with vehicle or HU-308 for 3 h (white and black bars, respectively), and after immunofluorescence with selective antibodies for phospho-S6 and nestin, the number of double phospho-S6+nestin+ cells in the SGZ was quantified (n = 4 in each group). Representative high magnification image of HU-308-increased phospho-S6 immunoreactivity in nestin+ cells is shown. D, representative images of wild-type mice treated for 5 days with vehicle or HU-308 indicating BrdU and phospho-S6 co-localization. Scale bar, 50 μm. *, p < 0.05; **, p < 0.01 versus vehicle-treated mice.
FIGURE 8.
HU-308-induced hippocampal progenitor proliferation is mediated by mTORC1 signaling. A and B, wild-type (WT) mice were treated with vehicle (V or Veh) or HU-308 (HU; 15 mg/kg, daily intraperitoneal administration) for 5 days alone or in the presence of rapamycin (Rapa; 6 mg/kg), and NP proliferation was quantified as BrdU+ cell number (n = 6 in each group). Representative immunofluorescence images are shown. C and D, immunoreactive phospho-S6 cells in the SGZ in the same treated animals were also quantified. Scale bars, 50 μm. *, p < 0.05; versus vehicle-treated mice; #, p < 0.05 versus HU-308-treated mice.
We next analyzed the involvement of CB2 receptors/mTORC1 signaling in excitotoxicity-induced hippocampal progenitor mobilization. CB2 receptor ablation impaired excitotoxicity-induced NP mobilization as quantified 5 days after KA injection (Fig. 9A) (10). Likewise, immunofluorescence quantification revealed that KA induced an increase of phospho-S6+ cells that did not occur in CB2 receptor-deficient mice (Fig. 9B). In agreement, excitotoxicity-induced NP proliferation (BrdU+ cells) and phospho-S6+ cell number increase were prevented by rapamycin co-administration (Fig. 9, C and D). Finally, we investigated the long lasting outcome of excitotoxicity-induced progenitor mobilization by quantifying 30 days after KA injection the number of newly born BrdU+ surviving cells together with the analysis of their expression of the mature neuronal marker NeuN (Fig. 9, E and F). Importantly, both the excitotoxicity-induced increase in BrdU+ surviving cells and neurogenesis (BrdU+NeuN+ cells) were blunted in CB2 receptor-deficient mice. These results reveal that CB2 receptors, through mTORC1 signaling, mediate NP proliferation after excitotoxicity, thus resulting in hippocampal neurogenesis.
FIGURE 9.
Excitotoxicity-induced hippocampal progenitor proliferation is mediated by CB2 cannabinoid receptor/mTORC1 signaling. A and B, wild-type (WT) and CB2 receptor-deficient mice were treated with vehicle (Veh; white bars) or kainic acid (KA, 15 mg/kg, black bars), and NP proliferation was determined by quantifying BrdU+ cells 5 days after injury. Immunoreactive phospho-S6 cells in the same animals were also quantified (n = 4 in each group). C and D, WT mice were treated with vehicle or KA (15 mg/kg) with or without rapamycin (Rapa; 6 mg/kg), and NP proliferation was determined by quantifying BrdU+ cells 5 days after injury. Immunoreactive phospho-S6 cells in the same animals were also quantified (n = 6 of each group). E and F, WT and CB2 receptor-deficient mice treated with vehicle or KA as described above and analyzed 30 days after injury. The number of BrdU+ cells and double BrdU+NeuN+ cells were quantified (n = 7 in each group). Scale bar, 50 μm. *, p < 0.05; **, p < 0.01 versus vehicle-treated mice; #, p < 0.05; ##, p < 0.01 versus HU-308-treated mice.
DISCUSSION
Here, we addressed the study of the signal transduction mechanism responsible for CB2 cannabinoid receptor-mediated regulation of NP proliferation by means of pharmacological and gene expression manipulation in several experimental models of varying cellular complexity and physiological relevance. Using the hippocampal HiB5 progenitor cell line, NP-derived neurosphere cultures, organotypic embryonic cortical cultures, and hippocampal adult neurogenesis experiments, we show that CB2 receptor activation promotes NP proliferation via PI3K/Akt/mTORC1 signaling both in vitro and in vivo. This proliferative effect of CB2 receptors on NPs appears to be mediated by mTORC1-induced p27Kip1 inhibition via SGK1, which in turn allows cell cycle progression. Our results also support that the proliferative effect of CB2 receptors in NPs is functional in neurogenic niches such as the developing cortex and the excitotoxicity-damaged adult hippocampal SGZ. CB2 receptors are present and functional in diverse stem/progenitor cell lineages, including embryonic stem cells (13) and myeloid progenitors (14). Within neural cells CB2 receptors are largely restricted to undifferentiated progenitor cells (10, 15) and, although still controversial, to discrete neuronal cell subpopulations (31). Biologically speaking, the notion that eCBs are signaling cues involved in the regulation of NSC/NP cell cycle progression and self-renewal is highlighted by the ability of cannabinoid receptors to contribute to the required proneurogenic niche that maintains NSC/NP cell populations during both brain development and adult neurogenesis (22, 32). On therapeutic grounds, the induction of progenitor cell proliferation and survival by CB2 receptors adds to the proliferative and neuroprotective role of CB1 receptors and opens new perspectives for the potential clinical utility of manipulating NP/stem cell mobilization with cannabinoid-based psychoactivity-devoid strategies (12).
CB2 Cannabinoid Receptors and mTORC1 in Brain Development
During brain development, cortical progenitor proliferation and self-renewal are tightly coordinated with the onset of neural differentiation, cell cycle exit, and cell migration (33). CB2 receptors present in undifferentiated neural cells promote mTORC1 signaling and downstream inhibition of the cell cycle inhibitor p27Kip1, which may play an important role in the balance of NP proliferation versus differentiation (28, 29). It is therefore conceivable that CB2 receptor down-regulation along neuronal differentiation allows NPs to progress beyond self-renewal (i.e. via loss of mTORC1-mediated p27Kip1 inhibition) and to commit to migrate and differentiate. In this regard, CB2 receptors have been recently shown to induce cell migration in explants of the postnatal SVZ enriched in progenitor cells, whereas the CB2-selective agonist JWH-133 exerts a positive action on SVZ-derived neuroblast migration toward the olfactory bulb (34). Likewise, in the developing cortex, radial migration of neuroblasts is coordinated by the orientation of the cell division plane with respect to the ventricular surface, which controls symmetric and asymmetric divisions. Thus, the apical polarity complex regulates the VZ/SVZ progenitor pool size by coordinating cell proliferation and differentiation through mTORC1 activity via the Pals1 protein (35). Maintenance of the balance between self-renewal and survival is associated with Pals1/mTORC1 activity and p27Kip1 phosphorylation. p27Kip1 exerts a dual role and, beyond cell cycle regulation, promotes neuronal differentiation and migration through distinct and separable mechanisms (29). CB2 receptor-mediated mTORC1 activation and downstream p27Kip1 inhibition may therefore constitute part of the molecular switch that coordinates cell proliferation and migration of VZ/SVZ cortical progenitors.
The signaling coupling of cannabinoid receptors to downstream effectors, and in particular to mTORC1, seems to be highly dependent on the pathophysiological cell context. On the one hand, CB1 receptors activate mTORC1 in hippocampal neurons, which, by regulating protein synthesis, is responsible for Δ9-tetrahydrocannabinol-induced cognitive impairment (17). On the other hand, CB1 receptors exert a proapoptotic action on transformed glial cells via Akt/mTORC1 inhibition (36). In addition, oligodendrocyte progenitor survival and differentiation is regulated by CB1 receptors via PI3K/Akt signaling (9) and cannabinoid agonists induce myelin basic protein expression and myelination during postnatal development (37) in a process that is blocked in vitro by rapamycin (38). This suggests a role of CB1 receptor-mediated PI3K/Akt/mTORC1 signaling in oligodendroglial cells, which is in agreement with the role of Rheb1-mediated activation of mTORC1 in myelination and oligodendrocyte differentiation (39). Importantly, the tuberous sclerosis complex proteins hamartin and tuberin function as upstream regulators of Rheb1 and therefore of mTORC1, and mutations of those proteins contribute to cortical dysplasia and intractable epilepsy (40). Hence, the role of CB2 receptor/mTORC1 signaling during cortical development may have important implications regarding the potential involvement of CB2 receptor misexpression in the development of tuberous sclerosis complex disorder (41).
CB2 Cannabinoid Receptors and mTORC1 in Epileptogenesis
The vast majority of the studies regarding the role of the endocannabinoid system in epileptogenesis has focused on the contribution of CB1 receptors (1) and, in particular, on the protective role of presynaptic CB1 receptor activation upon on-demand eCB synthesis as a consequence of excessive neuronal activity (4). Excitatory neuronal activity induced by KA administration induces seizures and a series of subsequent long term neuronal adaptive responses leading to epileptogenesis. Hippocampal progenitors respond to excitotoxicity with increased proliferation and neurogenesis, which may contribute to palliate neuronal cell loss or, on the contrary, participate in the generation of aberrant processes that contribute to the development of epilepsy (e.g. synaptic remodeling and axonal sprouting) (42). mTORC1 signaling is known to impact excitotoxicity-induced neuronal remodeling and follows a biphasic kinetic pattern with a rapid activation phase within hours and a subsequent sustained period that lasts several days (20, 43). Thus, mTORC1 inhibition prior to KA administration blocks acute and sustained seizure-induced mTORC1 activation, whereas late rapamycin administration fails to inhibit excitotoxicity-induced neurogenesis (20). This is in agreement with the dual contribution of mTORC1 signaling to cell proliferation (20, 44) and neuronal differentiation and migration (43, 45). The involvement of CB2 receptors in excitotoxicity-induced NP proliferation (10) via PI3K/Akt/mTORC1 activation (this study) suggests that CB2 receptor antagonists might be candidates to prevent seizure-induced neurogenesis, therefore attenuating the development of epileptogenesis.
CB2 receptors exert a prominent role in the regulation of microglial activation and neuroinflammation (8), and we found that in CB2−/− mice there is a complete absence of hippocampal NP proliferation induced by excitotoxicity (this study and see Ref. 10). This finding suggests that CB2 receptors, aside from actively promoting progenitor proliferation in a cell-autonomous manner, may be responsible for injury-induced microglial priming and for the release of neurogenesis-inducing factors (46). The role of the eCB system in epileptogenesis and long term neural plasticity is relevant not only in animal models but perhaps also in the human epileptic brain (1). Thus, CB1 receptors are regulated in a dynamic manner by synaptic activity and become down-regulated in the human epileptic hippocampus (47, 48). However, the possible involvement of CB2 receptors in these pathological events is still unknown. In opposition to the aforementioned potential benefit of blocking CB2 receptors expressed in the NP cell compartment to palliate epileptogenesis, the anti-inflammatory role of CB2 receptors in microglial and nervous system-infiltrating immune cells could contribute to attenuate neural cell loss (14, 26) and epileptogenesis (49). Altogether, these observations suggest that the presence of functional CB2 receptors in neurogenic niches in vivo might open new perspectives aimed at palliating the pathological consequences of aberrant neurogenesis, particularly in epileptogenesis.
Supplementary Material
Acknowledgments
We are indebted to M. Salazar, E. García-Taboada, and E. Resel for excellent experimental assistance; to G. Velasco for valuable manuscript comments, and to the rest of our laboratory members for providing an encouraging intellectual environment. We are also grateful to our collaborators that kindly contributed to this work by sharing reagents and molecular probes.
This work was supported by Ministerio de Ciencia e Innovación Grants PLE2009-0117 (to I. G.-R.) and SAF2009-08403 (to M. G.) and Comunidad de Madrid-Universidad Complutense de Madrid Grants S-SAL-2006/261 and 950344 (to M. G. and I. G.-R.).

This article contains supplemental Tables 1 and 2 and Figs.1–5.
- eCB
- endocannabinoid
- KA
- kainic acid
- mTORC1
- mammalian target of rapamycin complex 1
- NSC
- neural stem cell
- NP
- neural progenitor
- p70S6K
- 70-kDa ribosomal protein S6 kinase
- S6
- ribosomal protein S6
- SGZ
- subgranular zone
- VZ/SVZ
- ventricular/subventricular zone.
REFERENCES
- 1. Katona I., Freund T. F. (2008) Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 14, 923–930 [DOI] [PubMed] [Google Scholar]
- 2. Heifets B. D., Castillo P. E. (2009) Endocannabinoid signaling and long term synaptic plasticity. Annu. Rev. Physiol. 71, 283–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Galve-Roperh I., Aguado T., Palazuelos J., Guzmán M. (2008) Mechanisms of control of neuron survival by the endocannabinoid system. Curr. Pharm. Des. 14, 2279–2288 [DOI] [PubMed] [Google Scholar]
- 4. Marsicano G., Goodenough S., Monory K., Hermann H., Eder M., Cannich A., Azad S. C., Cascio M. G., Gutiérrez S. O., van der Stelt M., López-Rodriguez M. L., Casanova E., Schütz G., Zieglgänsberger W., Di Marzo V., Behl C., Lutz B. (2003) CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 [DOI] [PubMed] [Google Scholar]
- 5. Aguado T., Monory K., Palazuelos J., Stella N., Cravatt B., Lutz B., Marsicano G., Kokaia Z., Guzmán M., Galve-Roperh I. (2005) The endocannabinoid system drives neural progenitor proliferation. FASEB J. 19, 1704–1706 [DOI] [PubMed] [Google Scholar]
- 6. Aguado T., Romero E., Monory K., Palazuelos J., Sendtner M., Marsicano G., Lutz B., Guzmán M., Galve-Roperh I. (2007) The CB1 cannabinoid receptor mediates excitotoxicity-induced neural progenitor proliferation and neurogenesis. J. Biol. Chem. 282, 23892–23898 [DOI] [PubMed] [Google Scholar]
- 7. Trazzi S., Steger M., Mitrugno V. M., Bartesaghi R., Ciani E. (2010) CB1 cannabinoid receptors increase neuronal precursor proliferation through AKT/glycogen synthase kinase-3β/β-catenin signaling. J. Biol. Chem. 285, 10098–10109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stella N. (2009) Endocannabinoid signaling in microglial cells. Neuropharmacology 56, Suppl. 1, 244–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Molina-Holgado E., Vela J. M., Arévalo-Martín A., Almazán G., Molina-Holgado F., Borrell J., Guaza C. (2002) Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol 3-kinase/Akt signaling. J. Neurosci. 22, 9742–9753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palazuelos J., Aguado T., Egia A., Mechoulam R., Guzmán M., Galve-Roperh I. (2006) Nonpsychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 20, 2405–2407 [DOI] [PubMed] [Google Scholar]
- 11. Fernández-Ruiz J., Pazos M. R., García-Arencibia M., Sagredo O., Ramos J. A. (2008) Role of CB2 receptors in neuroprotective effects of cannabinoids. Mol. Cell. Endocrinol. 286, S91–S96 [DOI] [PubMed] [Google Scholar]
- 12. Mackie K. (2006) Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 46, 101–122 [DOI] [PubMed] [Google Scholar]
- 13. Jiang S., Fu Y., Williams J., Wood J., Pandarinathan L., Avraham S., Makriyannis A., Avraham S., Avraham H. K. (2007) Expression and function of cannabinoid receptors CB1 and CB2 and their cognate cannabinoid ligands in murine embryonic stem cells. PLoS ONE 2, e641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palazuelos J., Davoust N., Julien B., Hatterer E., Aguado T., Mechoulam R., Benito C., Romero J., Silva A., Guzmán M., Nataf S., Galve-Roperh I. (2008) The CB(2) cannabinoid receptor controls myeloid progenitor trafficking: involvement in the pathogenesis of an animal model of multiple sclerosis. J. Biol. Chem. 283, 13320–13329 [DOI] [PubMed] [Google Scholar]
- 15. Molina-Holgado F., Rubio-Araiz A., García-Ovejero D., Williams R. J., Moore J. D., Arévalo-Martín A., Gómez-Torres O., Molina-Holgado E. (2007) CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur. J. Neurosci. 25, 629–634 [DOI] [PubMed] [Google Scholar]
- 16. Pertwee R. G., Howlett A. C., Abood M. E., Alexander S. P., Di Marzo V., Elphick M. R., Greasley P. J., Hansen H. S., Kunos G., Mackie K., Mechoulam R., Ross R. A. (2010) International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB(1) and CB(2). Pharmacol. Rev. 62, 588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puighermanal E., Marsicano G., Busquets-Garcia A., Lutz B., Maldonado R., Ozaita A. (2009) Cannabinoid modulation of hippocampal long term memory is mediated by mTOR signaling. Nat. Neurosci. 12, 1152–1158 [DOI] [PubMed] [Google Scholar]
- 18. Foster K. G., Fingar D. C. (2010) Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J. Biol. Chem. 285, 14071–14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoeffer C. A., Klann E. (2010) mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 33, 67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zeng L. H., Rensing N. R., Wong M. (2009) The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J. Neurosci. 29, 6964–6972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monory K., Massa F., Egertová M., Eder M., Blaudzun H., Westenbroek R., Kelsch W., Jacob W., Marsch R., Ekker M., Long J., Rubenstein J. L., Goebbels S., Nave K. A., During M., Klugmann M., Wölfel B., Dodt H. U., Zieglgänsberger W., Wotjak C. T., Mackie K., Elphick M. R., Marsicano G., Lutz B. (2006) The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51, 455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galve-Roperh I., Aguado T., Palazuelos J., Guzmán M. (2007) The endocannabinoid system and neurogenesis in health and disease. Neuroscientist 13, 109–114 [DOI] [PubMed] [Google Scholar]
- 23. Buckley N. E., McCoy K. L., Mezey E., Bonner T., Zimmer A., Felder C. C., Glass M., Zimmer A. (2000) Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur. J. Pharmacol. 396, 141–149 [DOI] [PubMed] [Google Scholar]
- 24. Renfranz P. J., Cunningham M. G., McKay R. D. (1991) Region-specific differentiation of the hippocampal stem cell line HiB5 upon implantation into the developing mammalian brain. Cell 66, 713–729 [DOI] [PubMed] [Google Scholar]
- 25. Mulder J., Aguado T., Keimpema E., Barabás K., Ballester Rosado C. J., Nguyen L., Monory K., Marsicano G., Di Marzo V., Hurd Y. L., Guillemot F., Mackie K., Lutz B., Guzmán M., Lu H. C., Galve-Roperh I., Harkany T. (2008) Endocannabinoid signaling controls pyramidal cell specification and long range axon patterning. Proc. Natl. Acad. Sci. U.S.A. 105, 8760–8765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palazuelos J., Aguado T., Pazos M. R., Julien B., Carrasco C., Resel E., Sagredo O., Benito C., Romero J., Azcoitia I., Fernández-Ruiz J., Guzmán M., Galve-Roperh I. (2009) Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain 132, 3152–3164 [DOI] [PubMed] [Google Scholar]
- 27. Hanus L., Breuer A., Tchilibon S., Shiloah S., Goldenberg D., Horowitz M., Pertwee R. G., Ross R. A., Mechoulam R., Fride E. (1999) HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc. Natl. Acad. Sci. U.S.A. 96, 14228–14233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu J., Takagi Y., Harada J., Topalkara K., Wang Y., Sims J. R., Zheng G., Huang P., Ling Y., Scadden D. T., Moskowitz M. A., Cheng T. (2009) p27Kip1 constrains proliferation of neural progenitor cells in adult brain under homeostatic and ischemic conditions. Stem Cells 27, 920–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nguyen L., Besson A., Heng J. I., Schuurmans C., Teboul L., Parras C., Philpott A., Roberts J. M., Guillemot F. (2006) p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 20, 1511–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong F., Larrea M. D., Doughty C., Kwiatkowski D. J., Squillace R., Slingerland J. M. (2008) mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol. Cell 30, 701–711 [DOI] [PubMed] [Google Scholar]
- 31. Van Sickle M. D., Duncan M., Kingsley P. J., Mouihate A., Urbani P., Mackie K., Stella N., Makriyannis A., Piomelli D., Davison J. S., Marnett L. J., Di Marzo V., Pittman Q. J., Patel K. D., Sharkey K. A. (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332 [DOI] [PubMed] [Google Scholar]
- 32. Harkany T., Guzmán M., Galve-Roperh I., Berghuis P., Devi L. A., Mackie K. (2007) The emerging functions of endocannabinoid signaling during CNS development. Trends Pharmacol. Sci. 28, 83–92 [DOI] [PubMed] [Google Scholar]
- 33. Frank C. L., Tsai L. H. (2009) Alternative functions of core cell cycle regulators in neuronal migration, neuronal maturation, and synaptic plasticity. Neuron 62, 312–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oudin M. J., Gajendra S., Williams G., Hobbs C., Lalli G., Doherty P. (2011) Endocannabinoids regulate the migration of subventricular zone-derived neuroblasts in the postnatal brain. J. Neurosci. 31, 4000–4011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim S., Lehtinen M. K., Sessa A., Zappaterra M. W., Cho S. H., Gonzalez D., Boggan B., Austin C. A., Wijnholds J., Gambello M. J., Malicki J., LaMantia A. S., Broccoli V., Walsh C. A. (2010) The apical complex couples cell fate and cell survival to cerebral cortical development. Neuron 66, 69–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salazar M., Carracedo A., Salanueva I. J., Hernández-Tiedra S., Lorente M., Egia A., Vázquez P., Blázquez C., Torres S., García S., Nowak J., Fimia G. M., Piacentini M., Cecconi F., Pandolfi P. P., González-Feria L., Iovanna J. L., Guzmán M., Boya P., Velasco G. (2009) Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Invest. 119, 1359–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arévalo-Martín A., García-Ovejero D., Rubio-Araiz A., Gómez O., Molina-Holgado F., Molina-Holgado E. (2007) Cannabinoids modulate Olig2 and polysialylated neural cell adhesion molecule expression in the subventricular zone of postnatal rats through cannabinoid receptor 1 and cannabinoid receptor 2. Eur. J. Neurosci. 26, 1548–1559 [DOI] [PubMed] [Google Scholar]
- 38. Gomez O., Sanchez-Rodriguez A., Le M., Sanchez-Caro C., Molina-Holgado F., Molina-Holgado E. (2011) Cannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin (mTOR) pathways. Br. J. Pharmacol. 163, 1520–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zou J., Zhou L., Du X. X., Ji Y., Xu J., Tian J., Jiang W., Zou Y., Yu S., Gan L., Luo M., Yang Q., Cui Y., Yang W., Xia X., Chen M., Zhao X., Shen Y., Chen P. Y., Worley P. F., Xiao B. (2011) Rheb1 is required for mTORC1 and myelination in postnatal brain development. Dev. Cell 20, 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han J. M., Sahin M. (2011) TSC1/TSC2 signaling in the CNS. FEBS Lett. 585, 973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zurolo E., Iyer A. M., Spliet W. G., Van Rijen P. C., Troost D., Gorter J. A., Aronica E. (2010) CB1 and CB2 cannabinoid receptor expression during development and in epileptogenic developmental pathologies. Neuroscience 170, 28–41 [DOI] [PubMed] [Google Scholar]
- 42. Kokaia M. (2011) Seizure-induced neurogenesis in the adult brain. Eur. J. Neurosci. 33, 1133–1138 [DOI] [PubMed] [Google Scholar]
- 43. Buckmaster P. S., Ingram E. A., Wen X. (2009) Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J. Neurosci. 29, 8259–8269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goorden S. M., Hoogeveen-Westerveld M., Cheng C., van Woerden G. M., Mozaffari M., Post L., Duckers H. J., Nellist M., Elgersma Y. (2011) Rheb is essential for murine development. Mol. Cell. Biol. 31, 1672–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malagelada C., López-Toledano M. A., Willett R. T., Jin Z. H., Shelanski M. L., Greene L. A. (2011) RTP801/REDD1 regulates the timing of cortical neurogenesis and neuron migration. J. Neurosci. 31, 3186–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ehrhart J., Obregon D., Mori T., Hou H., Sun N., Bai Y., Klein T., Fernandez F., Tan J., Shytle R. D. (2005) Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. J. Neuroinflammation 2, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maglóczky Z., Tóth K., Karlócai R., Nagy S., Eross L., Czirják S., Vajda J., Rásonyi G., Kelemen A., Juhos V., Halász P., Mackie K., Freund T. F. (2010) Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia 51, Suppl. 3, 115–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ludányi A., Eross L., Czirják S., Vajda J., Halász P., Watanabe M., Palkovits M., Maglóczky Z., Freund T. F., Katona I. (2008) Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J. Neurosci. 28, 2976–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Auvin S., Mazarati A., Shin D., Sankar R. (2010) Inflammation enhances epileptogenesis in the developing rat brain. Neurobiol. Dis. 40, 303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.