Abstract
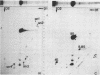
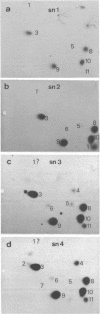
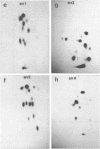
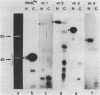
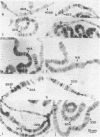
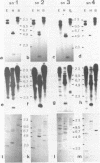
Four small nuclear RNAs (snRNAs) have been isolated from Drosophila melanogaster flies. They have been characterized by base analysis, fingerprinting, and injection into Axolotl oocytes. The size of the molecules and the modified base composition suggest that the following correlations can be made: snRNA1 approximately U2-snRNA; snRNA2 approximately U3-snRNA; snRNA3 approximately U4-snRNA; snRNA4 approximately U6-snRNA. The snRNAs injected into Axolotl oocytes move into the nuclei, where they are protected from degradation. The genes coding for these snRNAs have been localized by "in situ" hybridization of 125-I-snRNAs to salivary gland chromosomes. Most of the snRNAs hybridize to different regions of the genome: snRNA1 to the cytological regions 39B and 40AB; snRNA2 to 22A, 82E, and 95C; snRNA3 to 14B, 23D, 34A, 35EF, 39B, and 63A; snRNA4 to 96A. The estimated gene numbers (Southern-blot analysis) are: snRNA1:3; snRNA2:7; snRNA3:7; snRNA4:1-3. The gene numbers correspond to the number of sites labeled on the polytene salivary gland chromosomes.
Full text
PDF



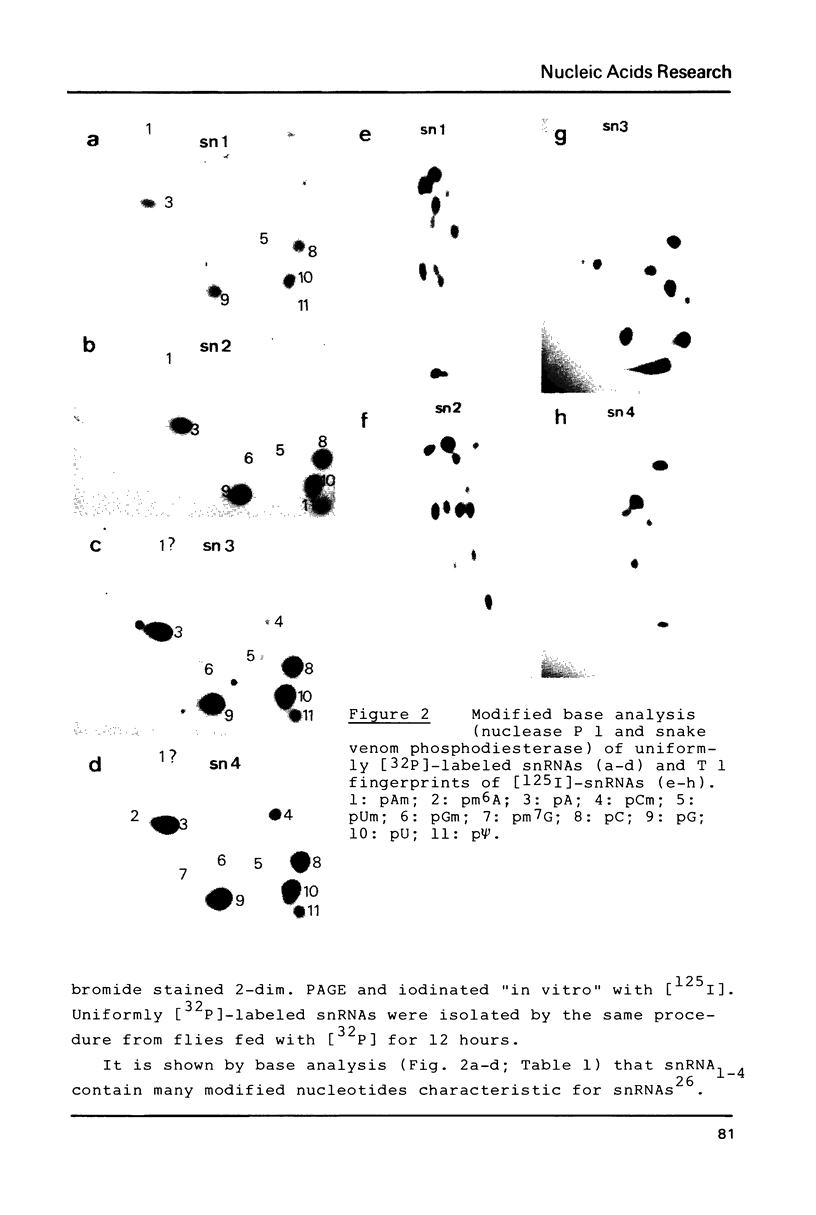







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bester A. J., Kennedy D. S., Heywood S. M. Two classes of translational control RNA: their role in the regulation of protein synthesis. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1523–1527. doi: 10.1073/pnas.72.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Gallinaro H., Jacob M., Sri-Widada J., Jeanteur P. Nucleotide sequences of nuclear U1A RNAs from chicken, rat and man. Nucleic Acids Res. 1980 Sep 25;8(18):4143–4154. doi: 10.1093/nar/8.18.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Lienhard S., Parisot R. F. Intracellular transport of microinjected 5S and small nuclear RNAs. Nature. 1982 Feb 18;295(5850):572–577. doi: 10.1038/295572a0. [DOI] [PubMed] [Google Scholar]
- Denison R. A., Van Arsdell S. W., Bernstein L. B., Weiner A. M. Abundant pseudogenes for small nuclear RNAs are dispersed in the human genome. Proc Natl Acad Sci U S A. 1981 Feb;78(2):810–814. doi: 10.1073/pnas.78.2.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudler R., Schmidt T., Bienz M., Kubli E. The genes coding for tRNA Tyr of Drosophila melanogaster: localization of determination of the gene numbers. Chromosoma. 1981;84(1):49–60. doi: 10.1007/BF00293362. [DOI] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Harada F., Ikawa Y. A new series of RNAs associated with the genome of spleen focus forming virus (SFFV) and poly(A)-containing RNA from SFFV-infected cells. Nucleic Acids Res. 1979 Oct 25;7(4):895–908. doi: 10.1093/nar/7.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Kato N. Nucleotide sequences of 4.5S RNAs associated with poly(A)-containing RNAs of mouse and hamster cells. Nucleic Acids Res. 1980 Mar 25;8(6):1273–1285. doi: 10.1093/nar/8.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K. Organization of sequences related to U6 RNA in the human genome. Nucleic Acids Res. 1981 Jul 24;9(14):3379–3388. doi: 10.1093/nar/9.14.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland L., Szyszko J., Krause M. Small nuclear RNAs from Drosophila KC-H cells; characterization and comparison with mammalian RNAs. Mol Biol Rep. 1982 Mar 31;8(2):97–101. doi: 10.1007/BF00778511. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of ribonucleic acids from mammalian tissues. Biochem J. 1956 Nov;64(3):405–408. doi: 10.1042/bj0640405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern H. Fractionation of nucleic acids with special regard to rapidly labeled RNA. Anal Biochem. 1975 Jul;67(1):147–156. doi: 10.1016/0003-2697(75)90282-1. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser T., Gesteland R. F. Human U1 loci: genes for human U1 RNA have dramatically similar genomic environments. Cell. 1982 May;29(1):257–264. doi: 10.1016/0092-8674(82)90110-6. [DOI] [PubMed] [Google Scholar]
- Monstein H. J., Westin G., Philipson L., Pettersson U. A candidate gene for human U1 RNA. EMBO J. 1982;1(1):133–137. doi: 10.1002/j.1460-2075.1982.tb01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Steitz J. A. Sequence of U1 RNA from Drosophila melanogaster: implications for U1 secondary structure and possible involvement in splicing. Nucleic Acids Res. 1981 Dec 11;9(23):6351–6368. doi: 10.1093/nar/9.23.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Huu M. C., Sippel A. A., Hynes N. E., Groner B., Schütz G. Preferential transcription of the ovalbumin gene in isolated hen oviduct nuclei by RNA polymerase B. Proc Natl Acad Sci U S A. 1978 Feb;75(2):686–690. doi: 10.1073/pnas.75.2.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Okada N., Tani T., Itoh Y., Itoh M. Nucleotide sequences of mouse genomic loci including a gene or pseudogene for U6 (4.8S) nuclear RNA. Nucleic Acids Res. 1981 Oct 10;9(19):5145–5158. doi: 10.1093/nar/9.19.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Lelay-Taha M. N., Sri-Widada J., Brunel C., Liautard J. P., Jeanteur P. Mouse DNA sequences complementary to small nuclear RNA U1. Nucleic Acids Res. 1982 Aug 11;10(15):4627–4640. doi: 10.1093/nar/10.15.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestayko A. W., Tonato M., Busch H. Low molecular weight RNA associated with 28 s nucleolar RNA. J Mol Biol. 1970 Feb 14;47(3):505–515. doi: 10.1016/0022-2836(70)90318-9. [DOI] [PubMed] [Google Scholar]
- Rein A., Penman S. Species specificity of the low molecular weight nuclear RNA's. Biochim Biophys Acta. 1969 Sep 17;190(1):1–9. doi: 10.1016/0005-2787(69)90149-x. [DOI] [PubMed] [Google Scholar]
- Ro-Choi T. S., Moriyama Y., Choi Y. C., Busch H. Isolation and purification of a nuclear 4.4 S ribonucleic acid of the Novikoff hepatoma. J Biol Chem. 1970 Apr 25;245(8):1970–1977. [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiha H., Glover D. M. Chracterisation of complete type II insertions in cloned segments of ribosomal DNA from Drosophila melanogaster. J Mol Biol. 1980 Jun 25;140(2):341–355. doi: 10.1016/0022-2836(80)90110-2. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Kristo P., Stumph W. E., Tsai M. J., O'Malley B. W. Structure and expression of a chicken gene coding for U1 RNA. Cell. 1981 Mar;23(3):671–680. doi: 10.1016/0092-8674(81)90430-x. [DOI] [PubMed] [Google Scholar]
- Schmidt T., Kubli E. The localization of tRNA5Asn, tRNAHis, and tRNAAla genes from drosophila melanogaster by in situ hybridization to polytene salivary gland chromosomes. Chromosoma. 1980;80(3):277–287. doi: 10.1007/BF00292685. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Van Arsdell S. W., Denison R. A., Bernstein L. B., Weiner A. M., Manser T., Gesteland R. F. Direct repeats flank three small nuclear RNA pseudogenes in the human genome. Cell. 1981 Oct;26(1 Pt 1):11–17. doi: 10.1016/0092-8674(81)90028-3. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Wise J. A., Weiner A. M. Dictyostelium small nuclear RNA D2 is homologous to rat nucleolar RNA U3 and is encoded by a dispersed multigene family. Cell. 1980 Nov;22(1 Pt 1):109–118. doi: 10.1016/0092-8674(80)90159-2. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Davidson N. The gross anatomy of a tRNA gene cluster at region 42A of the D. melanogaster chromosome. Cell. 1980 Nov;22(1 Pt 1):137–148. doi: 10.1016/0092-8674(80)90162-2. [DOI] [PubMed] [Google Scholar]
- Zapisek W. F., Saponara A. G., Enger M. D. Low molecular weight methylated ribonucleic acid species from Chinese hamster ovary cells. I. Isolation and characterization. Biochemistry. 1969 Mar;8(3):1170–1181. doi: 10.1021/bi00831a051. [DOI] [PubMed] [Google Scholar]