Abstract
Xenobiotic particles can be considered in two genres: air pollution particulate matter and engineered nanoparticles. Particle exposures can occur in the greater environment, the workplace, and our homes. The majority of research in this field has, justifiably, focused on pulmonary reactions and outcomes. More recent investigations indicate that cardiovascular effects are capable of correlating with established mortality and morbidity epidemiological data following particle exposures. While the preliminary and general cardiovascular toxicology has been defined, the mechanisms behind these effects, specifically within the microcirculation, are largely unexplored. Therefore, the purpose of this review is several fold: first, a historical background on toxicological aspects of particle research is presented. Second, essential definitions, terminology, and techniques that may be unfamiliar to the microvascular scientist will be discussed. Third, the most current concepts and hypotheses driving cardiovascular research in this field will be reviewed. Lastly, potential future directions for the microvascular scientist will be suggested. Collectively speaking, microvascular research in the particle exposure field represents far more than a “niche”. The immediate demand for basic, translational, and clinical studies is high and diverse. Microvascular scientists at all career stages are strongly encouraged to expand their research interests to include investigations associated with particle exposures.
Keywords: particulate matter, microcirculation, nanoparticles, exposure, cardiovascular
INTRODUCTION
Xenobiotic indicates being foreign to a living organism. A particle is a piece or minute amount of something larger. Joined together in the context of humans, a “xenobiotic particle” is a piece of something foreign that has gained access to our body. Whether intentional or unintentional, airborne particle exposures have compromised human health since the first wood fire fouled a cave. Of course, other more immediate causes of human mortality and short life-spans during that period precluded the realization that pulmonary particle exposure directly affected human health.
In 1872, R.A. Smith published “Air and Rain. The Beginnings of a Chemical Climatology” and introduced the concept of airborne toxicants [133]. Convincing proof of principle would not exist until decades later in the densely populated regions of industrialized nations, where particle associated morbidity and mortality were realized on a massive scale. Commonly cited examples include the Meuse Valley, Belgium (1930) and Donora, Pennsylvania (1948) where dramatic increases in sudden death, illness, and hospitalizations during air pollution incidents were reported [48;93]. This observation has reliably repeated itself in every industrialized nation to date. Perhaps the most striking example of this relationship was in London (1952), where ~12,000 premature deaths were associated with smog exposure produced by combustion-based industrial processes [6].
Human particle exposures are inescapable in modern day environments. It is given that both the frequency and severity of these exposures will increase as population density swells and particle sources diversify. Furthermore and quite alarmingly, the potential for personal particle exposure is rising independently of population density [149] suggesting that the development of complex workplaces and domiciles have expanded the scope of human exposure risk from ambient air pollution, to now include occupational and domestic point sources.
Historically (and understandably), research on the health effects of particle exposure focused almost exclusively on pulmonary mechanisms. For decades, the prevailing dogma was that the target organ of inhaled particles was the lung, and associated morbidity or mortality resulted from compromised pulmonary function or overt failure. This paradigm began to shift between 1993 and 2004, when seminal epidemiological studies revealed associations between particle exposure and cardiovascular events [31;32;114;115;125]. The most commonly reported endpoints included arrhythmias, hypertension, ischemic events, and infarcts.
Today, cardiovascular investigations of particle exposure are just as prominent, if not more so than pulmonary studies in the literature. A strong and sustained research commitment by the National Institute for Environmental Health Sciences [52] and the Environmental Protection Agency [76] has populated the field with timely and critically relevant investigations by principal investigators, non-traditional collaborations, and even specialized research centers. This commitment has advanced the field considerably, and tremendously broadened our understanding of the cardiovascular outcomes associated with particle exposures. However, in most every endpoint, an Achilles’ heel exists specifically: the microcirculation has been largely ignored. Moreover, many of the macrovascular techniques currently employed in this field are incapable of illuminating and/or characterizing the sensitive mechanisms operating at the microvascular level. Comparatively speaking, an analysis of tracheal ring function would be considered a poor representation of what particle exposure does to bronchiolar or alveolar function; yet, aortic ring and conduit artery studies are generally considered representative of how particle exposure alters a host of microvascular functions. Examples of these technical disparities include peripheral resistance, ischemia-reperfusion, inflammation, leukocyte-endothelium interactions, and mechanotransduction. This has produced a plethora of excellent, but relatively blunt macrovascular studies that consistently indicate endothelial dysfunction, blood pressure disturbances and/or autonomic changes following xenobiotic particle exposure. However, these studies even taken as a whole, cannot make the critical link between exposure and mechanism in physiologically relevant terms. While it is respectfully acknowledged that all scientific endeavours begin at an introductory level, if they are to evolve, they must in their ultimate pursuit to provide meaningful conclusions, match the proper model with the specific problem.
Therefore, the purpose of this review is to enlighten, excite, and motivate fellow microvascular scientists of research opportunities that exist in the fertile field of particle exposure. Furthermore, it is a goal that interested microvascular scientists will expand their research interests to include particle exposures and perhaps even collaborate.
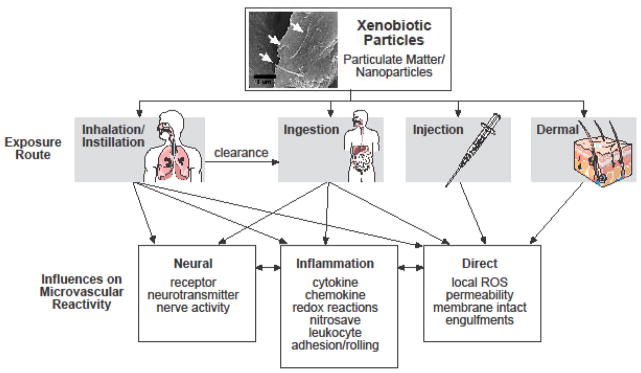
This will be achieved via three avenues. First, definitions, methodology and limitations will be discussed. As is the case with any niche, specific jargon carries implicit meaning, complex techniques are present, and the inherent limitations are not always obvious. Second, the most prominent hypotheses driving research in particle exposure and systemic health effects will be discussed. In Figure 1 a flow chart is presented to assist the readers navigation of these pathways and hypotheses, and to merge their collective influences on microvascular reactivity. It may surprise the reader how imbedded microvascular principles are in these hypotheses, yet formal investigations have yet to explore them. Third, future directions of the particle exposure research field will be discussed. As is the case with other research fields, and particularly so in terms of microvascular research, a host of factors such as co-morbidities, developmental state, sex and hormonal status may interact with particle exposures; yet it remains to be determined what the net outcome would be. If particle exposure research is to truly identify susceptible mechanisms and clinically relevant effects, for the greater goal of prevention and treatment strategies; then a significant portion of this research must be focused on identifying microvascular endpoints. Each subsequent section will highlight the indicated topic, and cite the most relevant research. Great effort will be made to incorporate potential microvascular endpoints in every regard, and cite appropriate references. However, it should be noted that a significant paucity of microvascular research studies exist in xenobiotic particle research.
Figure 1.
Flow diagram depicting the potential routes of xenobiotic particle exposures, the major hypotheses influencing microvascular reactivity, and the potential interplay between these routes and hypotheses.
DEFINITIONS AND TOXICOLOGICAL METHODOLOGIES
Xenobiotic particles can be considered in two genres: Air pollution particulate matter (PM) and engineered nanomaterials (ENM). Anthropogenic, or man-made, nanoparticles arise as a by-product of manufacturing or from the breakdown of products produced using ENM. Engineered nanomaterials are purposefully produced at the nanometer scale [78]. Proper dose calculation is essential to all toxicological studies, but is especially critical in particle exposure studies. This section will outline specific characteristics inherent to PM and nanoparticles and considerations that should be weighed (material characterization, route of delivery, dosage and time course) to produce a proper investigation of the microvascular effects of xenobiotic particle exposure.
Particulate Matter
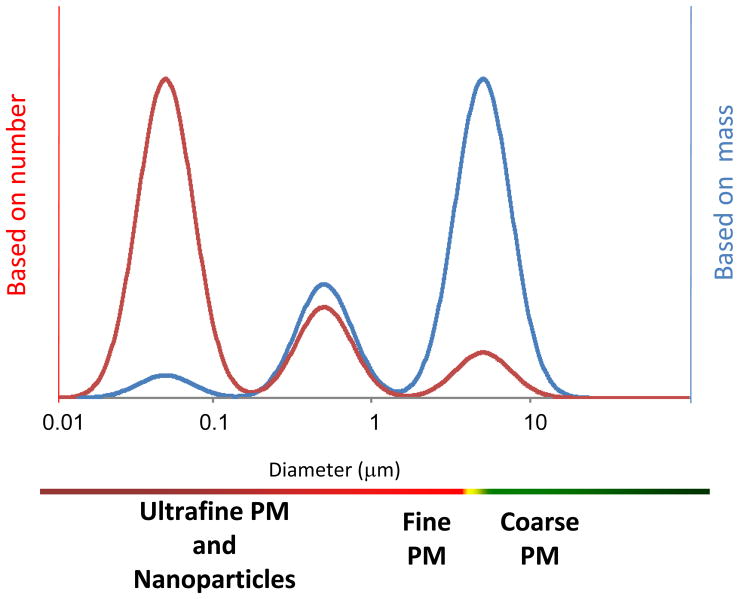
Particulate matter is a mixture of solid particles or liquid droplets suspended in ambient air, resulting in near universal human exposure [35]. Particulate matter is formed through various natural and combustion-related processes such as: volcanic activity, windblown dust, forest fires, and combustion of fossil fuels. Particles are typically described by their size or diameter. Because individual particles of any type tend to agglomerate, the metric used to describe the net aerosol characteristics is extremely important. In Figure 2, aerosol distributions are provided as a function of both particle mass and number. An entire air sample is averaged in order to classify a given net sample of airborne particles. Independent of the source, PM less than 10 μm (PM10) encompasses all respirable particles and is referred to as coarse PM. Particulate matter less than 2.5 μm (PM2.5) encompasses fine PM from which the bulk of cardiovascular health effects emanate. Particles in the ultrafine size range, similar in size to nanoparticles, are <0.1 μm or 100 nm in all dimensions (PM0.1). PM10 will include particles from PM2.5 (fine) and PM0.1 (ultrafine/nanoparticle) size ranges, likewise PM2.5 will include PM0.1, but the net average for any of these definitions does not exceed the indicated size-range. In regard to mass, larger, coarse particles dominant the profile. Whereas, when considering particle number, smaller ultrafine or nanoparticles are the dominant mode. Whether particle mass or number is the appropriate dose metric, is currently a contentious issue that may be dictated by the specific xenobiotic. The reader is encouraged to equally consider exposures in both regards.
Figure 2.
The observed makeup of particle aerosols is a function of the metric of interest. For the mass distribution of particles (blue line) the dominant mode is generally larger than 1 μm, whereas on a number basis (red line) the dominant mode is usually less than 0.1 μm. In between those ranges is a mode where the majority of the surface area occurs, usually around 0.2–0.3 μm. While it is accepted that particles < 1 μm exist (nanoparticles), these particles tend to form aerosol agglomerates, which are represented within this distribution.
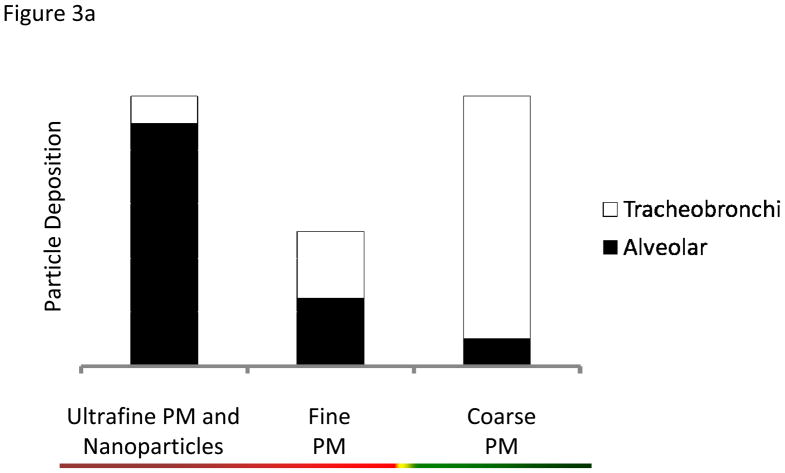
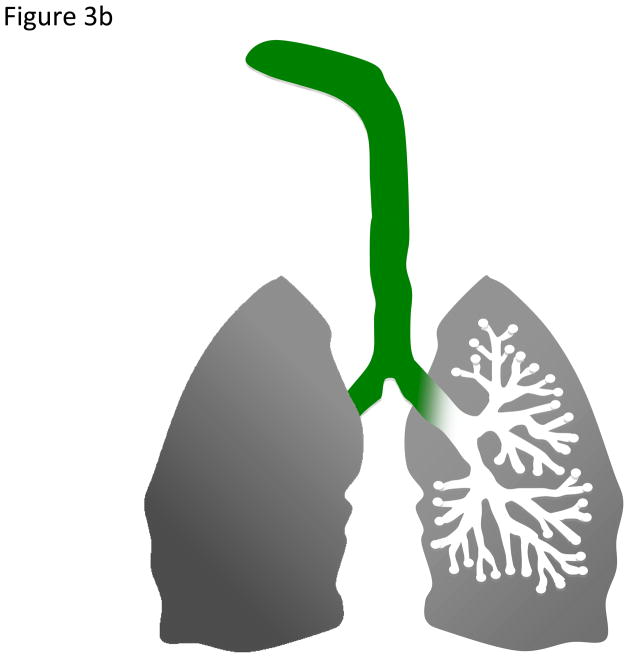
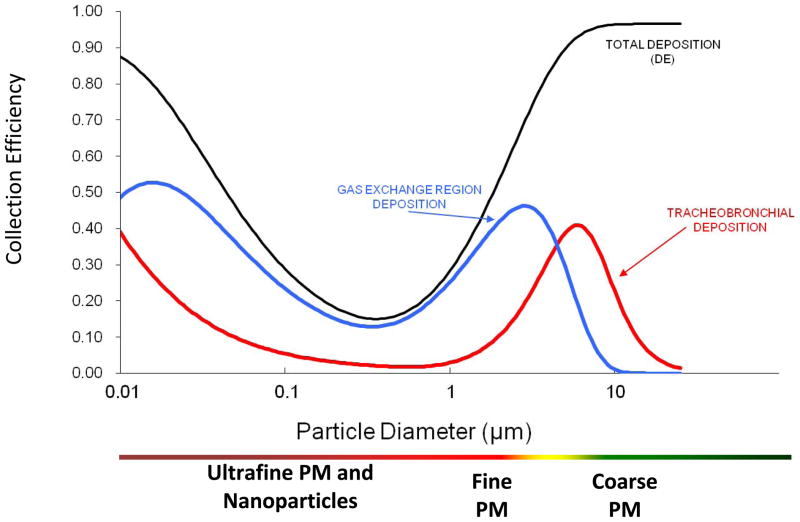
Deposition fractions (df), the proportion of particles deposited in a particular pulmonary region, have been extensively modeled for various aerosol types and reported in the literature for humans [50] and rats [83]. Deposition is greatly affected by particle size (Figure 3A). Particle deposition into the distal airways is greater as particle size decreases, and the converse is true for larger, coarse particles. As a rule of thumb, particles are not distributed equally in all lung regions. For simplicity, representative particle depositions are depicted in Figures 3B and 3C: where coarse particles preferentially deposit in the upper, tracheobronchi regions; while fine and ultrafine/nanoparticles access the entire lung, but deposit preferentially in the alveolar region. For dosing purposes, particle mass deposition can be estimated by the following formula: Particle deposition= df • minute ventilation • Particle concentration • Exposure time. For example, a rat exposed to a particle atmosphere of 6 mg/m3 for 6 hr with a minute ventilation of 200 mL/min and a df of 0.1 will have a total estimated particle deposition of 29 μg of material. The underlying assumption is that as particle diameter decreases, the subsequently increased surface area and particle number enhance the cardiovascular effects stemming from PM exposure. While it is attractive and helpful to dissect out regional deposition (based on particle size and pulmonary anatomy) for illustrative purposes, net lung exposures and depositions must first be calculated as a whole (Figure 4) [135].
Figure 3.
Figure 3a. Particle deposition within the regions of the lung (tracheobronchi and alveolar) with respect to particle size.
Figure 3b. Schematic of course particle (green) preferential deposition within the upper regions of the pulmonary system.
Figure 3c. Schematic of fine and ultrafine/nanoparticle (red) deposition. These smaller particles are found throughout the lung, but preferentially deposit within the alveolar region.
Figure 4.
Deposition and penetration characteristics of inhaled particles. Collection efficiency (y-axis) is the proportion of deposited mass based on particle size (x-axis). Inhaled particles less than ~20 μm in diameter penetrate the airway and deposit in the tracheobronchial (red line) and the gas exchange (i.e. alveolar, blue line) regions of the lungs. Total deposition (black line) is the sum of these regions. Although the mechanisms of particle deposition vary by size, it occurs relative to the particle diameter, with smaller particles penetrating deeper into the airway. Note that the greatest total deposition in either region occurs at the particle size limits (i.e. ultrafine PM and nanoparticles, fine, and course PM).
Nanoparticles
Nanoparticles, are purposefully derived anthropogenic particles <100 nm in one dimension but can be microns in length [7;91]. The study of engineered nanoparticles is termed “nanotoxicology” and is a relatively new sub-specialty of toxicology, proposed in 2004 [33]. Nanoparticles, either as standalone products or as derivatives of ENM, are a disparate group of elements and compounds that range from nano-elements (e.g. silver, gold) to complex forms of carbon (e.g. graphene sheets, buckeyballs, single- and multi-wall carbon nanotubes) to doped core nanoparticles with complex aliphatic side chains (e.g. silicon dioxide doped with polyethylene glycol) [28;47;118].
The uses of nanoparticles are limitless, with many uses in modern society. For example, nanosized titanium dioxide (TiO2), a product that has been on the market for a half century, is used in photocatalysts, antibacterial surface coatings, as well as in cosmetics and sunscreens [1;8;131;138]. A tremendous amount of research effort is currently being directed towards development of novel nanoparticles for medical imaging and drug delivery [8]. Therefore, while it is clear that nanotechnology holds tremendous potential benefit (both realized and unrealized), it also carries great risks as the health effects post-exposure are not fully elucidated.
Very little cardiovascular toxicity data exists for nanomaterials. Our laboratory was the first to demonstrate the effects of particulates and nanoparticle exposure on the systemic microcirculation [70;71;99;100]. However, these remain as the primary investigations into the effects of particle exposure on the microcirculation. Hence, given the lack of data available about the microvascular effects of particulate and nanomaterial exposure, relatively unexplored avenues of microvascular research are available for the pursuit of critically needed data on the health effects of particle exposure.
Material Characterization
Fundamental to the understanding of the health effects of particles is proper characterization of the exposure material. Material characterization is critical to the issues of repeatability and consistency, and is essential to the scientific method. Given the inherent significance in associating exposure with biologic effect, it is strongly encouraged that some form of material characterization be performed. Particle characterization is important to biological toxicity because: (1) not all particle sources are equally toxic, due to the variety in chemical composition [27] (2) particle size greatly affects particle lung deposition (Figure 3) [68] (3) particle surface area and number can alter the toxicity of relatively similar particles (Figure 2) [101], (4) solubility of particles can greatly affect toxicity and systemic exposure [150]. Currently, relaying manufacturer characterization data about nanomaterials is considered a minimum requirement; it is anticipated that this will be insufficient in the near future. Furthermore, material characterization should not be viewed as a necessary evil, but as a means to relate bioactivity with the composition, size, source, and shape of particles, with the ultimate goal of providing critical information to public health and occupational health agencies charged with preventing and mitigating exposure related health effects.
Documenting the particle size within the exposure medium of an experiment (e.g. air, instillate, cell culture medium) should be considered obligatory. Other suggested material characterizations are surface area, charge, dose or dose delivered (mass/m3 or mass/mL; surface-area, #/cm3 or #/mL) per unit time [87], and chemical composition (e.g. metal content, carbon content, sulfate). Many standard reference materials offered by the National Institute of Standards and Technology (NIST) come pre-characterized. However, these materials are expensive and limited in quantity. Alternatively, collaborative efforts with chemistry or environmental toxicology laboratories would eliminate the need for de novo determination of particle composition, characterization, and collection.
Routes of Exposure
Various exposure routes determine the toxicity and amount of material that interacts with cells or tissues. The most obvious routes of exposure are: inhalation/instillation, injection, dermal, and ingestion. Given the importance of the microcirculation and the vigorous development of nanomaterial-based therapies, less obvious exposure routes (e.g. ocular, mucosal, and implanted medical devices) should not be ignored. However, for the sake of clarity, this review will focus on the major routes of particle exposure.
Inhalation/Instillation
Of the exposure routes for which xenobiotic particles can enter the body, pulmonary deposition has been the most extensively investigated. Two experimental methods are generally recognized for the pulmonary deposition of particles: inhalation and instillation.
Inhalation
This method creates an artificial atmosphere/environment through which normal physiology can drive particle deposition. Inhalation exposure requires a considerable amount of trained personnel and specialized equipment. As such, they are costly and labor intensive, but are nevertheless, the “gold standard” for pulmonary exposure studies. The two major exposure systems employed for inhalation studies for rodents are whole body and head/nose-only [152]. Whole-body exposure requires a significant amount of bulk material/particles, and may result in dermal or oral exposure since the animals are unrestrained in the exposure chamber; breathing is “natural” and the technique is efficient for large numbers of animals and longer-term studies [152]. Head/nose-only exposure systems are designed to limit the contact of the animal’s body to the exposure atmosphere via a restraint inserted into a plenum. Though this method more efficiently uses bulk material/particles and prevents exposure via routes other than inhalation restraint may cause stress in the animals [152].
Intratracheal Instillation
Direct pulmonary exposure via instillation is performed by lightly anesthetizing the animal and directly infusing particles suspended in a medium into the lungs via a ball tipped needle. While a number of factors may prohibit inhalation studies, instillation has earned the moniker “poor man’s inhalation”. Moreover, dosing is much simpler, consistent, and often represented as mg/kg in a given volume of vehicle (e.g. 300 μL for rats). The instillation vehicle can vary depending on the particle characteristics. Particle agglomerates are usually dispersed via sonication in a buffer solution and often with protein (e.g. fetal bovine serum) or surfactant [124]. Intratracheal instillation does require some training to avoid accidentally gavaging the animals and is limited as to the number of repeated doses that can be performed by the technician and to the animal [100;152]. Additionally, rats and mice are obligatory nose breathers, which inherently affects particle deposition and distribution in the pulmonary tract. Particle instillation is artificial in that the delivery of particles to the lung by-passes the nose and upper airways; in spite of this, the distribution of particles in the lung by instillation has been shown to be consistent with inhalation as long as the delivered dose is equivalent [49].
Injection
Traditionally, injection of particles has not been a popular theme. Recently, infusion of particles has grown in importance with the development of nanoparticles as drug-delivery vehicles. Therefore, particle intravascular injection has not been investigated thoroughly in the past, it is rapidly becoming prominent. Injection of particles is justified from 2 perspectives: (1) representation of particle translocation after exposure and (2) as a means of clinical drug delivery or imaging for therapeutic and analytic purposes. Other injection types such as intraperitoneal, intramuscular, and subcutaneous are important to consider, although very little data exists for these exposure routes.
Most of the data for particle intravascular effects comes from the particle inhalation field [8]. Unfortunately, these data are only relevant in terms of particle endothelial interactions that result from particle translocation ablumenally to lumenally at a very small fraction of the total inhaled dose. While injection of materials to determine lung involvement following pulmonary particle exposure must be done with the caveat that the intravascular dose will be low compared to physiologically relevant exposures. Furthermore, the time course of particle migration into the systemic circulation is not a highly concentrated bolus introduction of particles but rather slow and concentration dependent.
Nanoparticles are also playing a large role in acting as a vehicle for pharmaceutical transport to the specific site of action via infusion. Experimental nanomaterials in development that have the potential to impact the microcirculation include particles that: are nitric oxide (NO) releasing [18], inhibitory of retinal neovascularization [61], improve drug delivery across the blood brain barrier [80], and enhance tissue angiogenesis [88]. Furthermore, contrast dyes for imaging have been developed with nanomaterials to increase their half-life but may also alter their excretion from the body [17]. While the biological effects of the pharmaceuticals have been scrutinized, the biokinetics, fate, and biological half-life of nanoparticles designed for intravascular use and drug delivery could all play a role in the interactions with blood and microvascular components [68], but remain to be fully defined. Because nanoparticle infusion can modulate known effector molecules and cells of the microcirculation, a better understanding of the direct effects of nanoparticles on the microcirculation is critically needed.
Dermal
Most particle dermal exposures originate from personal care products or from particles designed specifically for transport of compounds into the body through dermal absorption. As such, a considerable amount of research has been directed toward identifying and modeling potential hazards following dermal exposure to particles. Two of the most ubiquitous materials used for dermal application are TiO2 and nanosized zinc oxide (ZnO) [89]. These metal oxides are relatively insoluble and represent absorption through direct penetration into the skin. Penetration of sun-screen formulations of TiO2 and ZnO through the epidermal layers of the skin have been shown to be limited to the stratum cornea [89]. However, hair follicles appear to be the weak point of the skin and may represent an area of enhanced dermal penetration of particles [8;89].
Transdermal delivery of drugs was enhanced in 1990 with the advent of lipid nanoparticles as an alternative to liposomes and other polymer-based carriers [92]. Current technologies under development of transdermal delivery for the delivery of drugs or materials include: microneedles for the delivery of materials [34], nanostructured lipid carriers, and solid lipid nanoparticles [140]. However, while the effects of the drugs the materials are delivering are known, the effects of these new delivery technologies on the skin remain poorly understood. Dermal exposure to particles may affect normal skin microvascular functions such as wound repair and tumor cell metastasis; hence, studies determining the microvascular effects of particle exposure on the skin as well as particle access and route of delivery to the microvasculature are critically needed.
Ingestion
Ingestion represents a direct route of particle exposure through its addition to foodstuffs [134] and a significant secondary route of exposure during the clearance of inhaled particles [67]. As such, exposure to particles via the gut is an ongoing exposure route that has received little attention in the literature. Given the specialized endocytic mechanisms of the intestine, gut exposure to particles may represent a significant point of entry into the systemic circulation via the portal vein. The hepatic microcirculation is an important site of absorption for nutrients and metabolism for subsequent systemic distribution. As such, alterations in hepatic microvascular structure/function by particles may represent a tremendous burden on human health given the importance the liver plays in the maintenance of homeostasis [148].
Toxicological consideration of primary exposures to particles through food stuffs is a relatively new phenomenon [22]. However, much like translocation of particles in the respiratory tract, absorption of particles in the gut is also size dependent with smaller sizes absorbing at a greater rate [55]. Additionally, the uptake of particles appears to be greater in lymphoid tissues than in non-lymphoid tissues [51].
A secondary route of gut exposure to particles is particle clearance following inhalation. Pulmonary exposure to radioactive particles led to a significant burden of particles found in excretia from rats [67]. Similarly, human exposures have also shown a significant burden of particles in the gut following inhalation exposure [107]. These data suggest a significant portion of particles are cleared via the mucociliary escalator and eventually into the gut. However, only a portion of the inhaled particles would be cleared to the gut following inhalation suggesting that the dose of ingested particles would be orders of magnitude greater for particles introduced through the diet. Consistent with pulmonary exposure to particles, translocation of materials from the gut lumen to the portal vein is a slow concentration- and size-dependent movement, which would not represent a bolus infusion into the portal circulation. Therefore, alterations in the hepatic microcirculation following bolus infusion of particle must consider this caveat.
Dose Response and Time Course
Dose response is the establishment of a threshold dose, a maximum effect level, and an effective concentration at 50% (EC50) for a given toxicant in a model system. We have performed this work in this regard to particle-induced attenuation of endothelium-dependent arteriolar dilation, demonstrating a clear dose-response relationship [101]. However, dose-response relationships with other xenobiotics, other microvascular beds, and the mechanisms behind these alterations remain to be fully explored.
Time course is the lag between exposure and the beginning of a measured biologic effect. Epidemiological studies focusing on particles have demonstrated associations between exposure, morbidity, and mortality with a lag of 2 to 48 hours [15;108;109]. Several clinical studies have found strong indicators of cardiovascular alterations (e.g. a preferential increase in diastolic blood pressure) during particle exposure [3] or after a lag of 48 hr [154]. In animals, combustion source particles have been shown to alter pulmonary microvascular permeability during exposure [97], as well as microvascular reactivity in intraseptal coronary arterioles [21;23;24] and mesenteric venules [65] immediately following exposure. Animal exposures to particles have shown a reduction in in vivo vasodilation following active hyperemia [66], a dose-dependent attenuation of endothelium-dependent vasodilation [99–101], as well as cerebral microvessel thrombosis [94] 24 hr post-exposure. Particle exposure has also been shown to attenuate endothelium-dependent dilation in isolated coronary arterioles 24 hr post particle exposure [70;71]. Overall, the effects of particle exposure on the microvasculature have been initially studied during [97], immediately following exposure [21;23;24;65] or 24 hr post-exposure [66;70;71;99–102]. Hence, a critical evaluation of time course of particle-induced microvascular effects remains to be clarified and must be broadened to include all mechanisms of microvascular reactivity and tone.
CURRENT HYPOTHESES
It has been documented that particle exposure has an impact on cardiovascular homeostasis. However, the mechanisms behind these effects are largely unknown. Currently, there are a number of proposed hypotheses on how particle exposure modulates microvascular dysfunction. These hypotheses suggest that after exposure the systemic effects are caused by (1) an innate inflammatory response, (2) alterations in autonomic control, (3) translocation of the particles into the systemic circulation. However, the idea of these mechanisms interacting with one another cannot be ignored. Each of these hypotheses will be discussed in greater detail below.
It should also be noted that attention to experimental methodology should be considered when analyzing the microcirculation. Currently, a popular method used to assess general vascular function is through the monitoring of the changes in force generated by aortic rings in response to various pharmaceutical agents. Ring studies lend themselves well to the particle toxicology field because they are high throughput and do not require extensive surgical preparations or equipment. However, since this method almost exclusively uses conduit arteries, and the need to preconstrict these rings, it does not accurately represent macrovascular or microvascular physiology.
Assessment of vascular function can be more accurately examined with isolated microvessels or intravital microscopy. However, there are inherit limitations and benefits with these methods. In vitro isolated microvessel preparations allows for coincubation of the microvessel with particles and can be a high throughput method (depending on the microvascular bed being investigated and number of isolated microvessel stations); however this method removes the microvessel from the tissue, thereby removing other physiological influences. In the in vivo intravital microscopy preparation the arterioles are examined in an intact tissue bed. This method may be beneficial in determining the mechanisms by which vascular dysfunction is occurring as well as acquiring real-time measurements after particle exposure; nevertheless other physiological influences are present that may or may not be controlled or accounted for. These limitations and benefits need to be considered when determining which procedure or combination of the two should be utilized to evaluate the effects of particle exposure on vascular reactivity.
Inflammatory Response
Microvascular dysfunction may occur through either local inflammation or a systemic cascade following particle exposure. This hypothesis is predicated to particles entering the lungs and activating resident macrophages and epithelial cells; thus increasing local inflammation. This change in the inflammatory state of the lungs can activate or recruit naive circulating polymorphonuclear leukocytes (PMNs) as well as increase the levels of cytokines and chemokines in the blood. These newly activated PMNs and cytokines are able to circulate throughout the body and may trigger the release of additional inflammatory markers capable of increasing leukocyte adherence, and/or reactive stress in the systemic microvascular beds despite no direct interaction with the particles
Both clinical and animal studies have shown that pulmonary particle exposure produces a variety of systemic effects. Clinical studies in children and adults exposed to particles, found increases in circulating pro-inflammatory markers: tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), interleukin-6 (IL-6), endothelin-1 (ET-1), C reactive peptide (CRP); markers of vascular activation and adhesion however are a little more unclear, as there is a marked decrease in vascular adhesion molecule-1, E-, and L- selectin levels, while P-selectin is elevated [20;29;39;77]. Due to the variation within human subjects, some studies that evaluate these inflammation markers found no significant differences after particle exposure. These null results may be due to the health and age of the participants [9]. These vascular adhesion molecules and inflammation markers that are altered clinically after particle exposure can modify endothelial function, thus potentially affecting microvascular reactivity.
Animal studies have also found increases in circulating levels of inflammatory markers (TNF-α, IL-1β, and IL-6), increases in vascular activation markers (E-selectin and leukocyte adherence) and increases in markers indicative of a prothrombogenic environment (platelet activation, fibrin, and von Willebrand Factor (vWF)) [54;96]. These markers have been shown to alter microvascular function in a variety of pathologic states, therefore it is reasonable to speculate that these factors may be altering the microcirculation after particle exposure in a similar manner [26;132].
The possibility of cytokines and chemokines being released as a secondary response to increased lung inflammation has also been shown in cell culture studies. Primary naive cells (cardiac myocytes and endothelial cells) were exposed to monocytes or the cellular media from cultures that had been previously exposed to particles [30;141;142]. The release of inflammatory and vascular activation markers, as well as changes in gene expression were measured in the cells that were not directly exposed to particles [30;141;142]. However, proof of this secondary response is harder to assess in vivo and has not been investigated thoroughly in animals.
Changes in local vascular reactive stresses, which include both oxidative and nitrosative reactive species, have also been evaluated. Significant increases in vascular reactive stresses was shown after various particle exposures [70;71;99;102]. These changes in reactive stress and inflammation may be mediated by changes in monocyte nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase activity, superoxide dismutase, myeloperoxidase, and/or tetrahydrobioprerin [25;56;100]. Further research must clarify the source of systemic reactive stresses and determine if they are primary or secondary to the increases of other cytokines and vascular activation parameters.
One physiological outcome associated with increased local microvascular reactive stress and inflammation is vascular dysfunction. In humans, this vascular dysfunction has been assessed by changes in flow-mediated dilation and reactive hyperemia of the brachial artery and microvasculature; both of which are altered after particle exposure [12;123]. Alterations in vascular reactivity were also evident when patients were infused with endothelial-dependent and -independent agonists after particle exposure [86].
While the clinical standard for non-invasive studies in humans, these techniques are indirect and may not be sensitive enough to measure post particle exposure effects and/or mechanisms. Animal studies provide the opportunity to more directly and extensively test endothelial function and the effects of particle exposure on the microcirculation. Isolated microvessels and intravital microscopy have been used to examine the coronary and spinotrapezius microvascular beds, where a reduction in the ability of arterioles to dilate in response to endothelial agonists and significant increases in local reactive stresses were found [25;71;101]. In the hepatic microcirculation there was an increase in platelet adhesion and fibrinogen, but no changes in pro-inflammatory markers [59;60]. The studies that address microvascular function are limited and need to be broadened and more extensively researched with a wider array of particle exposures, time course, dosages, and microvascular beds (e.g. liver, kidney, lungs, and mesentery).
Autonomic Control
This hypothesis suggests that particle exposure alters autonomic control via sympathetic and parasympathetic innervations in the lungs, heart, vasculature, and other systemic organs [11]. Because these tissues are ripe with a host of receptors, particle exposure may stimulate pulmonary reflex arcs that lead to changes in sympathetic and parasympathetic neural control. Examples of changes in these systems include altered heart rate and blood pressure which are indicative of changes in microvascular control. Therefore, changes in autonomic control can also directly affect the microvascular function of essentially any organ [128]. However, this remains to be definitely shown after particle exposure.
Both clinical and animal studies measure heart rate variability (HRV) through the use of personal electrocardiogram (ECG) monitors or monitoring a subject’s pulse. Changes in the ECG wave readings or pulse changes can be analyzed and used to assess changes in autonomic control. However this measurement is indirect and may lack the specificity and sensitivity needed to determine changes in autonomic control after particle exposure. Additionally, the use of this measurement may be part of the variable results from animal studies and other parameters to assess sympathetic and parasympathetic influence more accurately need to be used.
Clinical studies have shown that after particle exposure there is reduced HRV, an increased heart rate (HR), and/or an increased diastolic blood pressure in healthy young, middle aged, and elderly adults who were exposed to xenobiotic particles [77;79;144]. One study that compared the impact of air pollution in Toronto, Ontario, Canada and Ann Arbor, Michigan on healthy men found very different results between locations [16]. In Toronto, patients had an increased blood pressure, and reduced HRV where as the Ann Arbor patients only had an increased blood pressure [16]. These variations in results highlight potential differences in particle composition and indicate that certain subpopulations maybe more susceptible to autonomic alterations than others. These studies are somewhat limited given that they do not assess the source of these alterations (parasympathetic or sympathetic control).
Several animal studies have been completed to address the source of the alterations in HRV and HR; however, the results are mixed. Studies using rodents attributed the reduced HRV described after particle exposure to changes in sympathetic control [46;110;120;139]. However, these autonomic changes may also be due to alterations in parasympathetic control [120;139]. Studies addressing changes in HR and/or blood pressure (animal and human) are also limited and the alterations in HR were attributed to reduced baroreflex sensitivity [45;73;121]. These data collectively show the need for more experiments with various animal strains and particles to help clarify the control of HRV, HR, and blood pressure differences after exposure.
Currently, research directly examining changes in nerve control after particle exposure in the vasculature are largely absent. A recent study in our laboratory found that after particle exposure an α-adrenergic blockage reduced basal arteriolar tone and upon perivascular nerve stimulation yielded differential results in the presence of adrenergic receptor antagonist, thereby suggesting a change in sympathetic neurotransmitter release [66]. Further research needs to be completed assessing potential changes in nervous control of the vasculature in various microvascular beds, including the lung, mesentery, skeletal muscle, and coronary.
Direct Contact
Direct interaction of the particles with target tissues may also allow particles to exert microvascular effects. This hypothesis assumes that the particles, primarily ultrafine or nanoparticles, translocate into the systemic blood or lymphatic circulation. This exposure may be from dermal exposure through the skin’s surface, inhalation (or instillation experimentally) through the pulmonary epithelium, ingested through the intestinal wall, or simply injected [37;84;85;90;104;147]. In some cases, exposures may impact additional or secondary tissue beds. Particles, cleared from the respiratory tract via the mucosal-cilatory elevator after inhalation or instillation, are then ingested thereby entering and exposing the gastrointestinal system [104]. Moreover, microvascular interaction and subsequent toxicities of particles used as drug delivery carrier molecules or other therapeutic purposes can be considered a serious problem, in addition to incidental exposures. Thus, the role of and impact on the microcirculation within the scope of therapeutic direct tissue-particle interactions or upon secondary impact to unintentional exposures is severely understudied.
Conflicting results emphasize the importance of particle characterization, as crossing through the body’s natural barriers is highly influenced by the size, area, shape, aggregation/agglomeration, solubility, porosity, charge, dosage, time course, and chemical composition of the particle [37;44;105]. Alarmingly, particles have shown the propensity to cross cellular membranes by non-phagocytic mechanisms [86]. Therefore, the same factors influencing toxicity within the tissue of original exposure (lung, skin, GI) and systemic tissues are also associated with a secondary impact (liver, kidney, brain, olfactory bulb, heart, blood, skeleton) [69;103;119;129]. In some cases, secondary impacts may lead to other toxicities and/or inflammatory effects due to increased accumulation. The impact on secondary organs was shown to peak at 7 days post-exposure, with smaller particles (20-nm v 80-nm particles) showing a greater propensity for translocation [69;129]. The overall accumulation of particles can lead to a systemic pro-thrombotic, pro-inflammatory, pro-oxidant environment, capable of many acute and long-term detrimental vascular effects [56;60;151]. As these particles have been readily found in secondary target tissues and within the vascular wall [19;119], the role of the vasculature to aid in the translocation as a transport mechanism and the impact of direct particle interaction with the microcirculation are areas that require further exploration.
While notable, the redistribution of the particles within the systemic circulation is only remarkable if the particle has the propensity to become, or is biologically active, or overwhelms the clearance organ (e.g. liver or kidney) by composition or accumulation [150]. The particles may be composed of or coated with highly reactive surface metals, which may act to catalyze a redox or Fenton reaction, thereby generating high levels of reactive oxygen species (ROS) within a cell or tissue [10]. The concentrations of these highly reactive surface metals can significantly increase above physiologically relevant concentrations, as local intracellular levels of toxic metals continue to rise due to particle uptake, specifically within macrophage populations [82]; for example, a fivefold greater particle deposition in the liver than the lung 18–24 hours after instillation [104]. These local concentrations can not only lead to cellular production of reactive species (both oxidative and nitrosative), but cellular death [147]. While it is attractive to speculate accumulation leading to a more intense microvascular dysfunction, this has yet to be shown.
Given the direct interaction between the particles and the pulmonary epithelium, traditional models considered almost exclusively inhaled particles, presuming that they would exit the lung. However, it has become increasingly clear that nanoparticles are gaining access to the body via non-pulmonary routes intentionally and unintentionally. Therefore, cell culture co-incubation techniques with non-pulmonary cells, are leading the way to initiate the general acceptance of the therapeutic and biological effects of a given particle [2;64;117]. However, while these co-culture experiments are considered novel with regard to the local and immediate biological effect, they do not provide a glimpse into functional or systemic alterations associated with particle exposures found in in vivo conditions. Additionally, morphological changes associated with primary cell culture of endothelial cells may add confounding variables and a misinterpretation of results.
Combined Interactions
It is imperative that the above hypotheses are also considered to work in combination. This combination can be in series for example, direct particle interaction leads to inflammation within the lung, which in turn spills over into the vasculature or in parallel, as direct particle interactions acting along with autonomic alterations. This is most likely to be the biological pathway responsible for alterations to the cardiovascular homeostasis in diverse populations described after exposure and understanding these complex interactions can aid in future prevention and treatment strategies. These diverse populations may give way to the development of interaction based on individual systemic alterations including but not limited to diagnosed comorbidities, age, and sex. This combination may come in multiple forms: (1) that of a time-course of change, where initially an autonomic irregularity leads to altered mechanisms of cardiovascular regulation during the early stage post-exposure and subsequent inflammatory or oxidative mechanisms perpetuate said dysfunction during late stages post-exposure or (2) where direct interactions lead to the development of reactive oxygen species initiating a delayed inflammatory response [10]. While it seems reasonable to hypothesize that oxidative or nitrosative stress (reactive oxygen or nitrogen products) may be the common link between the above hypotheses leading to systemic microvascular dysfunction, it remains speculative at this point and unproven at the level of the microcirculation.
Oxidative stress has been evaluated mechanistically from both ends of the experimental spectrum with respect to particle exposures. The mechanism often associated with in vivo vascular dysfunction and the interruption of microvascular signaling pathways has been shown as an increase in local reactive species [102]. Monocyte recruitment and ensuing release of radicals has also been evaluated as a mechanism of early atherosclerosis due to endothelial dysfunction after nano- and fine particle exposure within a cell culture model, suggesting that the direct interaction hypothesis can give rise to inflammatory and/or reactive stress cascades, activating nearby endothelial cells [155]. Lastly, the oxidative stress may be an effect of direct exposure to the particle or metallic composition as they translocate into the cell, initiating direct contact with mitochondria, leading to greater cellular ROS production [147]. Despite this evidence, it remains to be shown definitively, that any one hypothesized mechanism initiates a second at the microvascular level.
FUTURE DIRECTIONS
Pathology
Studies within the field of epidemiology have shown a link between particle exposures and subsequent elevated rates of cardiovascular mortality and morbidity. The mechanisms behind this connection are not fully elucidated at the tissue of primary exposure (i.e. pulmonary circulation), tissues of secondary impact based on accumulation (i.e. liver or kidney), or due to direct or indirect systemic responses [15;95]. There are primary diseases that stem from particle exposures (i.e. asthma, cancers, fibrosis, chronic obstructive pulmonary disease, etc) currently being investigated; however, targeted microvascular explorations into these post-exposure conditions are also warranted [40;106;146]. However, because of the potential for particle exposure to exacerbate compromised vascular function, this section will focus on interactions between particle exposure and pre-existing conditions.
Individuals with an underlying medical conditions are most susceptible to the effects of particle exposures. These conditions include, but are not limited to, pulmonary disease (e.g. COPD, asthma, emphysema), cancers, diabetes (type 1 and type 2), hypertension, metabolic syndrome, obesity, and cardiovascular disease, may be exacerbated by pulmonary particle exposures [37;41;136]. The pulmonary system is clearly impacted after inhalation or instillation exposures. Many groups are exploring these exposures, which may culminate in a hypersensitivity or acute pulmonary inflammation leading to long-term fibrotic consequences in some cases [81;106].
With respect to the cardiovascular system, shortly after exposure, healthy adults have shown alterations in reactivity of conduit arteries and increases in heart rate during non-strenuous activities [12;13]. During exercise, particle exposures have lead to a greater lung deposition, reduced muscle microvascular blood flow (due to impaired reactive hyperemia) and a reduced exercise work capacity [41;122;130]. Many groups have shown or hypothesized that individuals with preexisting cardiovascular dysfunction may be at greater risk, as vascular reactivity is already compromised; therefore, particle exposure may intensify their symptoms. These findings are consistent with epidemiological studies of cardiovascular episodes reported after high particle exposures [12;41;113;143]. These exposures may acutely lead to extrapulmonary effects of endothelial dysfunction increasing inflammation, plasma viscosity, platelet activity, autonomic activity, and ROS to which existing compensatory mechanisms become overridden [36;38;137;153]. In cell culture, particles have been shown to increase inflammation, decrease NO bioavailability, cause injury, and inhibit growth in endothelial cell lines [153;155]. Additionally, long-term particle exposures representative of an occupational or domestic environment, may also act to aggravate pre-existing atherosclerosis through cytotoxic interactions with oxidized low-density lipoproteins and chronic induction of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) [14;98].
The challenge inherent to any field in the initial stages of development is to study and characterize the microvascular outcomes in the absence of confounding variables, otherwise it is impossible to distinguish the distinct effect and consequence of the initial insult. While these findings are critical to the current understanding of xenobiotic particles at this point, nearly every pathological state includes microvascular involvement. Therefore, it is critical to identify the outcomes associated with particle exposures.
Developmental State (Young/Juvenile/Old)
Age and growth are complex factors involved in microvascular health. Influences include arteriolar tone [75], mechanistic mediators [58;126], myogenic responsiveness [127], hemodynamics [112], and vascular density [4], factors which may also be influenced by particle interactions. How developmental status may affect particle toxicity and clearance from the primary and secondary exposure routes has yet to be determined.
Epidemiological evidence supports the very young and old being at greatest risk to a variety of adverse effects, including those from particles; as cellular and biochemical compensatory mechanisms associated with anti-inflammatory and anti-oxidant handling are altered with aging. While these age-related changes can lead to decline in endothelium-dependent responses and inflated inflammatory effects in older animals, young animals with naive immune systems have not yet been evaluated [37;116]. At this time, we speculate, in the young where vascular reactivity is not as robust as those in mature population, particle exposures may have an augmented effect because compensatory mechanisms are not fully developed. [75;126].
Sex/Hormonal Status
Sex and hormonal status has been shown to affect the microcirculation clinically in cases of arterial compliance [43], intermittent claudication [42], systemic and coronary arteriolar reactivity [5;57], and blood flow [72]. Therefore, the sex and/or hormonal status of the subject exposed to particles may also play a role in the intensity of the vascular effect. It has been shown that females exhibit many mechanistic differences within the endothelial regulation of the cardiovascular system, which are generally associated with age and hormonal status compared with their male counterparts [53]. Particle exposures may exacerbate these differences, as female rats have exhibited a two-fold greater particle accumulation within the kidneys than male counterparts [62;63]. However, after particle inhalation, male rats display a sex-specific endothelial dysfunction at six-months of age, suggesting hormonal protective mechanism within the matched female cohort [116]. As sex differences pertaining to hormonal status play an active role in microvascular function, particle exposure may aggravate these differences with respect to regulation and females may show an even greater protective effect after particle exposures.
The majority of the mechanistic studies performed up to this point focus on representative young, healthy, sexually mature, male rats to determine the methods of vascular disruption associated with particle exposures. The addition of a variety of disease comorbidities, hormonal fluctuations, and/or aging as additional dependent parameters to more accurately describe the vascular pathologies associated with exposure to a variety of particles is within its infancy.
SUMMARY AND CONCLUSION
The definitions, hypotheses and concepts discussed herein represent the most important and current concepts in the field of xenobiotic particle toxicology. We have strived to present them from the cardiovascular perspective, and wherever possible, from the microvascular perspective. As is the case with any subspecialty, exceptions and caveats exist that are outside the scope of this discussion. It is our greater hope that the reader will identify such exceptions or caveats in regard to their specific microvascular research interests, and pursue them. In this regard, we extend our assistance to all interested investigators.
Cardiovascular effects (but not microvascular effects) of human particle exposures have been well-documented for some time. Despite tremendous efforts to increase the quality of ambient as well as indoor air, particle exposures will persist. This may be the result of a variety of reasons ranging from the simple fact that population density continues to increase in some proportion with global energy demands; to the continual introduction or breakdown of new or existing products and protectants (i.e. technology, surfaces, paints, surfactants, insulation, flame retardants, etc.) in our workplaces and homes. Moreover, these statements pertain only to pulmonary exposures, yet we have made a clear case herein that not all particle exposures are pulmonary in origin, or completely contained in the lung. Regardless, an obvious and present threat to human health is developing that has the potential to transcend geographic, environmental, occupational, and domestic as well as socioeconomic boundaries. Whatever form or path this threat takes, we feel it is safe to speculate that the microcirculation will play a critical role, or be a major endpoint.
It is also important to indicate that at this time, the collective threat that particle exposures impose on human health are but a fraction of those generated by heart disease or cancer. With that said, it may be germane to briefly highlight the history of similar research. While research associated with smoking and tobacco use is under the same xenobiotic umbrella, it is an entirely different line of investigation. The series of events associated with tobacco policy are strikingly similar to the pattern that particle research is currently displaying. Obvious health effects associated were identified in 1950 [74]. The mechanisms of these exposure-dependent health effects were elucidated in the following decades, yet the incidence of use increased [145]. While multiple reasons exist for this latter effect, in regard to public impact and policy, the known morbidity and mortality rates were simply not high enough to change the momentum of popular opinion [111]. Furthermore, the gross economic burden of tobacco related morbidities would not become obvious until the 1980’s [145]; and it would not be until the 1990’s that strong public policy started to become commonplace in progressive regions to decrease the impact on human health [111]. In all, it required greater than half a century for research to begin to impact public health policy.
While convenient to compare the two, particle research differs vastly from tobacco research in that the health effects, and epidemiological outcomes of particle exposure are already largely known, and repeatable. The general public opinion in regard to particle exposure is in favor of cleaner air, and safer personal and occupational environments. Research over the past two decades has and continues to assist policy decisions in countries throughout the world. Currently and unfortunately, the weakest link in particle research is definitive mechanisms, and these mechanisms are initially observed in basic research. We have only recently documented some of the mechanisms through which particle exposures induce systemic microvascular effects. However, our studies to date have at best scratched the surface of a very deep and broad field. First, consider that fossil fuels from different geographical or refining sources produce differential emissions. Second, not all emissions are regulated or even considered equally. Third, ENMs are being produced faster than their toxicity can be assessed in even a single vascular bed, let alone a tissue or whole organism. Fourth (but not finally), the environment we live in; either from the global or domestic perspective, changes constantly, and it is only reasonable to anticipate that it will continue to do so. Each of these require a pointed investigation to merely identify a mechanism(s), and only then is it prudent to consider a discovered mechanism in terms of development, sex, co-morbidities or any other possible factor that may interact with the microvascular effects of xenobiotic particle exposures. Therefore, any forward thinking process that considers the impact of particle exposure on future human health, should include a microvascular component. Given analogous history, it is hoped that future mortality rates and fiscal projections are not the rate limiting steps necessary to bring about effective policy changes that will prevent, diminish or treat the health effects of particle exposures. But rather, definitive microvascular studies will characterize the mechanisms though which xenobiotic particles induce biologic effect and thereby, serve as a contributing force that unifies basic, translational, and clinical science in effort to drive greater health effects policies.
Peer review has rightfully requested a “smoking gun” that links pulmonary particle exposures with systemic effects. This has proven to be a warranted, but daunting challenge. However, the scope of future investigations in our opinion has recently broadened with the explosion of ENMs. At a minimum, the production of ENMs and therefore, complex exposures to them has mandated that non-pulmonary routes be considered as important, if not more so in toxicologic assessments. Therefore, traditional dogma that the lung must be part of the physiologic axis in response to particles is no longer present. In essence, this opens virtually any possibility for microvascular scientists of all backgrounds to explore how xenobiotics influence their models.
In conclusion, the microvascular scientist is uniquely poised to examine the biologic changes, differences, and outcomes associated with acute and chronic xenobiotic particle exposures, reduce their intensities, and develop post-exposure treatments in a naive and/or pathological environment. The initial cardiovascular observations/associations have been made. In a period when extramural funding is perhaps at it’s most difficult; substantial Federal and private funding is available in this field. Moreover, there is no better time to initiate investigations than when the number and scope of scientific unknowns are the greatest. Please consider this review an official “call to arms” for microvascular scientists to the field of xenobiotic particle exposures.
Acknowledgments
The authors would like to thank Nathan Minarchick and Carol Grimes for their assistance in the development of the figures, Drs. Lori Kang and Heather O’Leary for their critical analysis of this review, and Dr. Robert Mercer for graciously allowing us to use his image of the MWCNT-epithelial cell interaction shown in Figure 1.
FUNDING
National Institutes of Health, National Institute for Environmental Health Sciences grants: R01-ES015022 (TRN), and RC1-ES018274 (TRN). National Science Foundation (Cooperative Agreement 1003907 to VCM).
References
- 1.Aitken RJ, Chaudhry MQ, Boxall AB, Hull M. Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med (Lond) 2006;56:300–306. doi: 10.1093/occmed/kql051. [DOI] [PubMed] [Google Scholar]
- 2.Apopa PL, Qian Y, Shao R, et al. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part Fibre Toxicol. 2009;6:1. doi: 10.1186/1743-8977-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoli CR, Wellenius GA, Diaz EA, et al. Mechanisms of inhaled fine particulate air pollution-induced arterial blood pressure changes. Environ Health Perspect. 2009;117:361–366. doi: 10.1289/ehp.11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behnke BJ, Prisby RD, Lesniewski LA, Donato AJ, Olin HM, Delp MD. Influence of ageing and physical activity on vascular morphology in rat skeletal muscle. J Physiol. 2006;575:617–626. doi: 10.1113/jphysiol.2006.108431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belfort MA, Saade GR, Snabes M, et al. Hormonal status affects the reactivity of the cerebral vasculature. Am J Obstet Gynecol. 1995;172:1273–1278. doi: 10.1016/0002-9378(95)91492-7. [DOI] [PubMed] [Google Scholar]
- 6.Bell ML, Davis DL. Reassessment of the lethal London fog of 1952: novel indicators of acute and chronic consequences of acute exposure to air pollution. Environ Health Perspect. 2001;109 (Suppl 3):389–394. doi: 10.1289/ehp.01109s3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borm PJ, Muller-Schulte D. Nanoparticles in drug delivery and environmental exposure: same size, same risks? Nanomedicine (Lond) 2006;1:235–249. doi: 10.2217/17435889.1.2.235. [DOI] [PubMed] [Google Scholar]
- 8.Borm PJ, Robbins D, Haubold S, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3:11. doi: 10.1186/1743-8977-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brauner EV, Moller P, Barregard L, et al. Exposure to ambient concentrations of particulate air pollution does not influence vascular function or inflammatory pathways in young healthy individuals. Part Fibre Toxicol. 2008;5:13. doi: 10.1186/1743-8977-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brook RD. You are what you breathe: evidence linking air pollution and blood pressure. Curr Hypertens Rep. 2005;7:427–434. doi: 10.1007/s11906-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 11.Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond) 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- 12.Brook RD, Bard RL, Burnett RT, et al. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. 2011;68:224–230. doi: 10.1136/oem.2009.053991. [DOI] [PubMed] [Google Scholar]
- 13.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105:1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 14.Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep. 2010;12:291–300. doi: 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- 15.Brook RD, Rajagopalan S, Pope CA, III, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 16.Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bui T, Stevenson J, Hoekman J, Zhang S, Maravilla K, Ho RJ. Novel Gd nanoparticles enhance vascular contrast for high-resolution magnetic resonance imaging. PLoS One. 2010:5. doi: 10.1371/journal.pone.0013082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrales P, Han G, Nacharaju P, Friedman AJ, Friedman JM. Reversal of hemoglobin-induced vasoconstriction with sustained release of nitric oxide. Am J Physiol Heart Circ Physiol. 2011;300:H49–H56. doi: 10.1152/ajpheart.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calderon-Garciduenas L, Mora-Tiscareno A, Fordham LA, et al. Canines as sentinel species for assessing chronic exposures to air pollutants: part 1. Respiratory pathology. Toxicol Sci. 2001;61:342–355. doi: 10.1093/toxsci/61.2.342. [DOI] [PubMed] [Google Scholar]
- 20.Calderon-Garciduenas L, Villarreal-Calderon R, Valencia-Salazar G, et al. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal Toxicol. 2008;20:499–506. doi: 10.1080/08958370701864797. [DOI] [PubMed] [Google Scholar]
- 21.Campen MJ, Babu NS, Helms GA, et al. Nonparticulate components of diesel exhaust promote constriction in coronary arteries from ApoE−/− mice. Toxicol Sci. 2005;88:95–102. doi: 10.1093/toxsci/kfi283. [DOI] [PubMed] [Google Scholar]
- 22.Card JW, Jonaitis TS, Tafazoli S, Magnuson BA. An appraisal of the published literature on the safety and toxicity of food-related nanomaterials. Crit Rev Toxicol. 2011;41:22–49. doi: 10.3109/10408444.2010.524636. [DOI] [PubMed] [Google Scholar]
- 23.Cherng TW, Campen MJ, Knuckles TL, Gonzalez BL, Kanagy NL. Impairment of coronary endothelial cell ET(B) receptor function after short-term inhalation exposure to whole diesel emissions. Am J Physiol Regul Integr Comp Physiol. 2009;297:R640–R647. doi: 10.1152/ajpregu.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cherng TW, Jackson-Weaver O, Paffett M, walker B, Campen MJ, Kanagy NL. Mechanisms of diesel-induced endothelial NOS dysfunction in coronary arterioles. Environ Health Persp. 2011;119:98–103. doi: 10.1289/ehp.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherng TW, Paffett ML, Jackson-Weaver O, Campen MJ, Walker BR, Kanagy NL. Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ Health Perspect. 2011;119:98–103. doi: 10.1289/ehp.1002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper D, Stokes KY, Tailor A, Granger DN. Oxidative stress promotes blood cell-endothelial cell interactions in the microcirculation. Cardiovasc Toxicol. 2002;2:165–180. doi: 10.1007/s12012-002-0002-7. [DOI] [PubMed] [Google Scholar]
- 27.Costa DL, Dreher KL. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environ Health Perspect. 1997;105 (Suppl 5):1053–1060. doi: 10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deepak V, Umamaheshwaran PS, Guhan K, et al. Synthesis of gold and silver nanoparticles using purified URAK. Colloids Surf B Biointerfaces. 2011;86:353–358. doi: 10.1016/j.colsurfb.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Delfino RJ, Staimer N, Tjoa T, et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect. 2008;116:898–906. doi: 10.1289/ehp.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.den Hartigh LJ, Lame MW, Ham W, Kleeman MJ, Tablin F, Wilson DW. Endotoxin and polycyclic aromatic hydrocarbons in ambient fine particulate matter from Fresno, California initiate human monocyte inflammatory responses mediated by reactive oxygen species. Toxicol In Vitro. 2010;24:1993–2002. doi: 10.1016/j.tiv.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 31.Dockery DW. Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ Health Perspect. 2001;109 (Suppl 4):483–486. doi: 10.1289/ehp.01109s4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dockery DW, Pope CA, III, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 33.Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJ. Nanotoxicology. Occup Environ Med. 2004;61:727–728. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doraiswamy A, Ovsianikov A, Gittard SD, et al. Fabrication of microneedles using two photon polymerization for transdermal delivery of nanomaterials. J Nanosci Nanotechnol. 2010;10:6305–6312. doi: 10.1166/jnn.2010.2636. [DOI] [PubMed] [Google Scholar]
- 35.Dreher KL. Particulate matter physicochemistry and toxicology: In search of causality - A critical perspective. Inhalation Toxicology. 2000;12(suppl 3):45–57. doi: 10.1080/08958378.2000.11463230. Ref Type: Generic. [DOI] [PubMed] [Google Scholar]
- 36.Elder A, Gelein R, Silva V, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ Health Perspect. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elder AC, Gelein R, Azadniv M, Frampton M, Finkelstein J, Oberdorster G. Systemic effects of inhaled ultrafine particles in two compromised, aged rat strains. Inhal Toxicol. 2004;16:461–471. doi: 10.1080/08958370490439669. [DOI] [PubMed] [Google Scholar]
- 38.Evans SA, Al-Mosawi A, Adams RA, Berube KA. Inflammation, edema, and peripheral blood changes in lung-compromised rats after instillation with combustion-derived and manufactured nanoparticles. Exp Lung Res. 2006;32:363–378. doi: 10.1080/01902140600959671. [DOI] [PubMed] [Google Scholar]
- 39.Fang SC, Cavallari JM, Eisen EA, Chen JC, Mittleman MA, Christiani DC. Vascular function, inflammation, and variations in cardiac autonomic responses to particulate matter among welders. Am J Epidemiol. 2009;169:848–856. doi: 10.1093/aje/kwn405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fireman E. Induced sputum and occupational diseases other than asthma. Curr Opin Allergy Clin Immunol. 2009;9:93–96. doi: 10.1097/aci.0b013e32832921e0. [DOI] [PubMed] [Google Scholar]
- 41.Frampton MW. Does inhalation of ultrafine particles cause pulmonary vascular effects in humans? Inhal. Toxicol. 2007;19 (Suppl 1):75–79. doi: 10.1080/08958370701495071. [DOI] [PubMed] [Google Scholar]
- 42.Gardner AW, Montgomery PS, Blevins SM, Parker DE. Gender and ethnic differences in arterial compliance in patients with intermittent claudication. J Vasc Surg. 2010;51:610–615. doi: 10.1016/j.jvs.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardner AW, Montgomery PS, Blevins SM, Parker DE. Gender and ethnic differences in arterial compliance in patients with intermittent claudication. J Vasc Surg. 2010;51:610–615. doi: 10.1016/j.jvs.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geiser M, Rothen-Rutishauser B, Kapp N, et al. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113:1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, Gonzalez-Flecha B. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol Sci. 2008;102:328–336. doi: 10.1093/toxsci/kfn005. [DOI] [PubMed] [Google Scholar]
- 46.Harder V, Gilmour P, Lentner B, et al. Cardiovascular responses in unrestrained WKY rats to inhaled ultrafine carbon particles. Inhal Toxicol. 2005;17:29–42. doi: 10.1080/08958370590885681. [DOI] [PubMed] [Google Scholar]
- 47.He X, Nie H, Wang K, Tan W, Wu X, Zhang P. In vivo study of biodistribution and urinary excretion of surface-modified silica nanoparticles. Anal Chem. 2008;80:9597–9603. doi: 10.1021/ac801882g. [DOI] [PubMed] [Google Scholar]
- 48.Helfand WH, Lazarus J, Theerman P. Donora, Pennsylvania: an environmental disaster of the 20th century. Am J Public Health. 2001;91:553. doi: 10.2105/ajph.91.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henderson RF, Driscoll KE, Harkema JR, et al. A comparison of the inflammatory response of the lung to inhaled versus instilled particles in F344 rats. Fundam Appl Toxicol. 1995;24:183–197. doi: 10.1006/faat.1995.1022. [DOI] [PubMed] [Google Scholar]
- 50.Heyder J, Gebhart J, Rudolf G, schiller CF, Stahlhofen W. Deposition of particles in the human respiratory tract in the size range 0.005 – 15 μm. Journal of Aerosol Science. 1986;17:811–825. [Google Scholar]
- 51.Hillery AM, Jani PU, Florence AT. Comparative, quantitative study of lymphoid and non-lymphoid uptake of 60 nm polystyrene particles. J Drug Target. 1994;2:151–156. doi: 10.3109/10611869409015904. [DOI] [PubMed] [Google Scholar]
- 52.Hood E. NIEHS strategic plan: new frontiers in environmental science and human health. Environ Health Perspect. 2006;114:A280–A283. [PMC free article] [PubMed] [Google Scholar]
- 53.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation. 2004;11:9–38. doi: 10.1080/10739680490266162. [DOI] [PubMed] [Google Scholar]
- 54.Inoue K, Takano H, Sakurai M, et al. Pulmonary exposure to diesel exhaust particles enhances coagulatory disturbance with endothelial damage and systemic inflammation related to lung inflammation. Exp Biol Med (Maywood) 2006;231:1626–1632. doi: 10.1177/153537020623101007. [DOI] [PubMed] [Google Scholar]
- 55.Jani P, Halbert GW, Langridge J, Florence AT. Nanoparticle uptake by the rat gastrointestinal mucosa: quantitation and particle size dependency. J Pharm Pharmacol. 1990;42:821–826. doi: 10.1111/j.2042-7158.1990.tb07033.x. [DOI] [PubMed] [Google Scholar]
- 56.Kampfrath T, Maiseyeu A, Ying Z, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108:716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang LS, Chen B, Reyes RA, et al. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol. 2011;300:H2105–H2115. doi: 10.1152/ajpheart.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang LS, Chen B, Reyes RA, et al. Aging and estrogen alter endothelial reactivity to reactive oxygen species in coronary arterioles. Am J Physiol Heart Circ Physiol. 2011;300:H2105–H2115. doi: 10.1152/ajpheart.00349.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khandoga A, Stampfl A, Takenaka S, et al. Ultrafine particles exert prothrombotic but not inflammatory effects on the hepatic microcirculation in healthy mice in vivo. Circulation. 2004;109:1320–1325. doi: 10.1161/01.CIR.0000118524.62298.E8. [DOI] [PubMed] [Google Scholar]
- 60.Khandoga A, Stoeger T, Khandoga AG, et al. Platelet adhesion and fibrinogen deposition in murine microvessels upon inhalation of nanosized carbon particles. J Thromb Haemost. 2010;8:1632–1640. doi: 10.1111/j.1538-7836.2010.03904.x. [DOI] [PubMed] [Google Scholar]
- 61.Kim JH, Kim MH, Jo DH, Yu YS, Lee TG, Kim JH. The inhibition of retinal neovascularization by gold nanoparticles via suppression of VEGFR-2 activation. Biomaterials. 2011;32:1865–1871. doi: 10.1016/j.biomaterials.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Kim WY, Kim J, Park JD, Ryu HY, Yu IJ. Histological study of gender differences in accumulation of silver nanoparticles in kidneys of Fischer 344 rats. J Toxicol Environ Health A. 2009;72:1279–1284. doi: 10.1080/15287390903212287. [DOI] [PubMed] [Google Scholar]
- 63.Kim YS, Kim JS, Cho HS, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- 64.Kirkpatrick CJ, Fuchs S, Unger RE. Co-culture systems for vascularization -Learning from nature. Adv Drug Deliv Rev. 2011;63:291–299. doi: 10.1016/j.addr.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 65.Knuckles TL, Lund AK, Lucas SN, Campen MJ. Diesel exhaust exposure enhances venoconstriction via uncoupling of eNOS. Toxicol Appl Pharmacol. 2008;230:346–351. doi: 10.1016/j.taap.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 66.Knuckles TL, Yi J, Frazer DG, et al. Nanoparticle Inhalation alters Systemic Arteriolar Vasoreactivity through Sympathetic and Cyclooxygenase-Mediated Pathways. Nanotoxicology. 2011 doi: 10.3109/17435390.2011.606926. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kreyling WG, Semmler M, Erbe F, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 68.Kreyling WG, Semmler-Behnke M, Moller W. Ultrafine particle-lung interactions: does size matter? J Aerosol Med. 2006;19:74–83. doi: 10.1089/jam.2006.19.74. [DOI] [PubMed] [Google Scholar]
- 69.Kreyling WG, Semmler-Behnke M, Seitz J, et al. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal Toxicol. 2009;21 (Suppl 1):55–60. doi: 10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]
- 70.LeBlanc AJ, Cumpston JL, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs endothelium-dependent vasodilation in subepicardial arterioles. J Toxicol Environ Health A. 2009;72:1576–1584. doi: 10.1080/15287390903232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.LeBlanc AJ, Moseley AM, Chen BT, Frazer D, Castranova V, Nurkiewicz TR. Nanoparticle inhalation impairs coronary microvascular reactivity via a local reactive oxygen species-dependent mechanism. Cardiovasc Toxicol. 2010;10:27–36. doi: 10.1007/s12012-009-9060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.LeBlanc AJ, Reyes R, Kang LS, et al. Estrogen replacement restores flow-induced vasodilation in coronary arterioles of aged and ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1713–R1723. doi: 10.1152/ajpregu.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Legramante JM, Valentini F, Magrini A, et al. Cardiac autonomic regulation after lung exposure to carbon nanotubes. Hum Exp Toxicol. 2009;28:369–375. doi: 10.1177/0960327109105150. [DOI] [PubMed] [Google Scholar]
- 74.Levin ML, Goldstein H, Gerhardt PR. Cancer and tobacco smoking; a preliminary report. J Am Med Assoc. 1950;143:336–338. doi: 10.1001/jama.1950.02910390008002. [DOI] [PubMed] [Google Scholar]
- 75.Linderman JR, Boegehold MA. Modulation of arteriolar sympathetic constriction by local nitric oxide: onset during rapid juvenile growth. Microvasc Res. 1998;56:192–202. doi: 10.1006/mvre.1998.2096. [DOI] [PubMed] [Google Scholar]
- 76.Lippmann M, Frampton M, Schwartz J, et al. The U.S. Environmental Protection Agency Particulate Matter Health Effects Research Centers Program: a midcourse report of status, progress, and plans. Environ Health Perspect. 2003;111:1074–1092. doi: 10.1289/ehp.5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu L, Ruddy T, Dalipaj M, et al. Effects of indoor, outdoor, and personal exposure to particulate air pollution on cardiovascular physiology and systemic mediators in seniors. J Occup Environ Med. 2009;51:1088–1098. doi: 10.1097/JOM.0b013e3181b35144. [DOI] [PubMed] [Google Scholar]
- 78.Lövestam G, Rauscher H, Roebben G, Klüttgen BS, Gibson N, Putaud JP, Stamm H. Considerations on a Definition of Nanomaterial for Regulatory Purposes. 2010. Ref Type: Report. [Google Scholar]
- 79.Magari SR, Hauser R, Schwartz J, Williams PL, Smith TJ, Christiani DC. Association of heart rate variability with occupational and environmental exposure to particulate air pollution. Circulation. 2001;104:986–991. doi: 10.1161/hc3401.095038. [DOI] [PubMed] [Google Scholar]
- 80.Mahajan SD, Roy I, Xu G, et al. Enhancing the delivery of anti retroviral drug “Saquinavir” across the blood brain barrier using nanoparticles. Curr HIV Res. 2010;8:396–404. doi: 10.2174/157016210791330356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maier KL, Alessandrini F, Beck-Speier I, et al. Health effects of ambient particulate matter--biological mechanisms and inflammatory responses to in vitro and in vivo particle exposures. Inhal Toxicol. 2008;20:319–337. doi: 10.1080/08958370701866313. [DOI] [PubMed] [Google Scholar]
- 82.Maynard AD, Warheit DB, Philbert MA. The new toxicology of sophisticated materials: nanotoxicology and beyond. Toxicol Sci. 2011;120 (Suppl 1):S109–S129. doi: 10.1093/toxsci/kfq372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menache MG, Raabe OG, Miller FJ. An empirical dosimetry model of aerodynamic particle deposition in the rat respiratory tract. Inhal Toxicol. 1995;8:539–578. [Google Scholar]
- 84.Mercer RR, Hubbs AF, Scabilloni JF, et al. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part Fibre Toxicol. 2011;8:21. doi: 10.1186/1743-8977-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mercer RR, Hubbs AF, Scabilloni JF, et al. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 2010;7:28. doi: 10.1186/1743-8977-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mills NL, Tornqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 87.MINChar Initiative. Recommended Minimum Physical and Chemical Parameters for Characterizing Nanomaterials on Toxicology Studies. Internet. 2008 6-16-2011. [Google Scholar]
- 88.Mondalek FG, Ashley RA, Roth CC, et al. Enhanced angiogenesis of modified porcine small intestinal submucosa with hyaluronic acid-poly(lactide-co-glycolide) nanoparticles: from fabrication to preclinical validation. J Biomed Mater Res A. 2010;94:712–719. doi: 10.1002/jbm.a.32748. [DOI] [PubMed] [Google Scholar]
- 89.Monteiro-Riviere NA, Wiench K, Landsiedel R, Schulte S, Inman AO, Riviere JE. Safety Evaluation of Sunscreen Formulations Containing Titanium Dioxide and Zinc Oxide Nanoparticles in UVB Sunburned Skin: An In Vitro and In Vivo Study. Toxicol Sci. 2011 doi: 10.1093/toxsci/kfr148. [DOI] [PubMed] [Google Scholar]
- 90.Mortensen LJ, Oberdorster G, Pentland AP, Delouise LA. In vivo skin penetration of quantum dot nanoparticles in the murine model: the effect of UVR. Nano Lett. 2008;8:2779–2787. doi: 10.1021/nl801323y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Muhlfeld C, Gehr P, Rothen-Rutishauser B. Translocation and cellular entering mechanisms of nanoparticles in the respiratory tract. Swiss Med Wkly. 2008;138:387–391. doi: 10.4414/smw.2008.12153. [DOI] [PubMed] [Google Scholar]
- 92.Muller RH, Shegokar R, Keck CM. 20 Years of Lipid Nanoparticles (SLN and NLC): Present State of Development and Industrial Applications. Curr Drug Discov Technol. 2011 doi: 10.2174/157016311796799062. [DOI] [PubMed] [Google Scholar]
- 93.Nemery B, Hoet PH, Nemmar A. The Meuse Valley fog of 1930: an air pollution disaster. Lancet. 2001;357:704–708. doi: 10.1016/S0140-6736(00)04135-0. [DOI] [PubMed] [Google Scholar]
- 94.Nemmar A, Al-Salam S, Dhanasekaran S, Sudhadevi M, Ali BH. Pulmonary exposure to diesel exhaust particles promotes cerebral microvessel thrombosis: protective effect of a cysteine prodrug l-2-oxothiazolidine-4-carboxylic acid. Toxicology. 2009;263:84–92. doi: 10.1016/j.tox.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 95.Nemmar A, Hoet PH, Nemery B. Translocation of ultrafine particles. Environ Health Perspect. 2006;114:A211–A212. doi: 10.1289/ehp.114-a211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nemmar A, Hoylaerts MF, Hoet PH, Vermylen J, Nemery B. Size effect of intratracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicol Appl Pharmacol. 2003;186:38–45. doi: 10.1016/s0041-008x(02)00024-8. [DOI] [PubMed] [Google Scholar]
- 97.Nieman GF, Clark WR, Jr, Goyette D, Hart AK, Bredenberg CE. Wood smoke inhalation increases pulmonary microvascular permeability. Surgery. 1989;105:481–487. [PubMed] [Google Scholar]
- 98.Niwa Y, Iwai N. Nanomaterials induce oxidized low-density lipoprotein cellular uptake in macrophages and platelet aggregation. Circ J. 2007;71:437–444. doi: 10.1253/circj.71.437. [DOI] [PubMed] [Google Scholar]
- 99.Nurkiewicz TR, Porter DW, Barger M, Castranova V, Boegehold MA. Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ Health Perspect. 2004;112:1299–1306. doi: 10.1289/ehp.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nurkiewicz TR, Porter DW, Barger M, et al. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect. 2006;114:412–419. doi: 10.1289/ehp.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nurkiewicz TR, Porter DW, Hubbs AF, et al. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol. 2008;5:1. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nurkiewicz TR, Porter DW, Hubbs AF, et al. Pulmonary nanoparticle exposure disrupts systemic microvascular nitric oxide signaling. Toxicol Sci. 2009;110:191–203. doi: 10.1093/toxsci/kfp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oberdorster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 104.Oberdorster G, Sharp Z, Atudorei V, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 105.Oberdorster G, Utell MJ. Ultrafine particles in the urban air: to the respiratory tract--and beyond? Environ. Health Perspect. 2002;110:A440–A441. doi: 10.1289/ehp.110-1240959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pacurari M, Castranova V, Vallyathan V. Single- and multi-wall carbon nanotubes versus asbestos: are the carbon nanotubes a new health risk to humans? J Toxicol Environ Health A. 2010;73:378–395. doi: 10.1080/15287390903486527. [DOI] [PubMed] [Google Scholar]
- 107.Pery AR, Brochot C, Hoet PH, Nemmar A, Bois FY. Development of a physiologically based kinetic model for 99m-technetium-labelled carbon nanoparticles inhaled by humans. Inhal Toxicol. 2009;21:1099–1107. doi: 10.3109/08958370902748542. [DOI] [PubMed] [Google Scholar]
- 108.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 109.Peters A, Liu E, Verrier RL, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11:11–17. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Pham H, Bonham AC, Pinkerton KE, Chen CY. Central neuroplasticity and decreased heart rate variability after particulate matter exposure in mice. Environ Health Perspect. 2009;117:1448–1453. doi: 10.1289/ehp.0900674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pierce JP, Messer K, White MM, Cowling DW, Thomas DP. Prevalence of heavy smoking in California and the United States, 1965–2007. JAMA. 2011;305:1106–1112. doi: 10.1001/jama.2011.334. [DOI] [PubMed] [Google Scholar]
- 112.Poole D, Behnke B, Musch T. Capillary hemodynamics and oxygen pressures in the aging microcirculation. Microcirculation. 2006;13:289–299. doi: 10.1080/10739680600618793. [DOI] [PubMed] [Google Scholar]
- 113.Pope CA., III Epidemiology of fine particulate air pollution and human health: biologic mechanisms and who’s at risk? Environ. Health Perspect. 2000;108 (Suppl 4):713–723. doi: 10.1289/ehp.108-1637679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pope CA, III, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pope CA, III, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 116.Prisby RD, Muller-Delp J, Delp MD, Nurkiewicz TR. Age, gender, and hormonal status modulate the vascular toxicity of the diesel exhaust extract phenanthraquinone. J.Toxicol. Environ Health A. 2008;71:464–470. doi: 10.1080/15287390701839349. [DOI] [PubMed] [Google Scholar]
- 117.Qu S, Liberda EN, Qu Q, Chen LC. In vitro assessment of the inflammatory response of respiratory endothelial cells exposed to particulate matter. J Toxicol Environ Health A. 2010;73:1113–1121. doi: 10.1080/15287394.2010.484335. [DOI] [PubMed] [Google Scholar]
- 118.Rao CN, Govindaraj A. Carbon nanotubes from organometallic precursors. Acc Chem Res. 2002;35:998–1007. doi: 10.1021/ar0101584. [DOI] [PubMed] [Google Scholar]
- 119.Reddy AR, Krishna DR, Reddy YN, Himabindu V. Translocation and extra pulmonary toxicities of multi wall carbon nanotubes in rats. Toxicol Mech Methods. 2010;20:267–272. doi: 10.3109/15376516.2010.484077. [DOI] [PubMed] [Google Scholar]
- 120.Rhoden CR, Wellenius GA, Ghelfi E, Lawrence J, Gonzalez-Flecha B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochim Biophys Acta. 2005;1725:305–313. doi: 10.1016/j.bbagen.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 121.Routledge HC, Manney S, Harrison RM, Ayres JG, Townend JN. Effect of inhaled sulphur dioxide and carbon particles on heart rate variability and markers of inflammation and coagulation in human subjects. Heart. 2006;92:220–227. doi: 10.1136/hrt.2004.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rundell KW, Caviston R. Ultrafine and fine particulate matter inhalation decreases exercise performance in healthy subjects. J Strength Cond Res. 2008;22:2–5. doi: 10.1519/JSC.0b013e31815ef98b. [DOI] [PubMed] [Google Scholar]
- 123.Rundell KW, Hoffman JR, Caviston R, Bulbulian R, Hollenbach AM. Inhalation of ultrafine and fine particulate matter disrupts systemic vascular function. Inhal Toxicol. 2007;19:133–140. doi: 10.1080/08958370601051727. [DOI] [PubMed] [Google Scholar]
- 124.Sager TM, Kommineni C, Castranova V. Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Part Fibre Toxicol. 2008;5:17. doi: 10.1186/1743-8977-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 126.Samora JB, Frisbee JC, Boegehold MA. Hydrogen peroxide emerges as a regulator of tone in skeletal muscle arterioles during juvenile growth. Microcirculation. 2008;15:151–161. doi: 10.1080/10739680701508497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Samora JB, Frisbee JC, Boegehold MA. Increased myogenic responsiveness of skeletal muscle arterioles with juvenile growth. Am J Physiol Heart Circ Physiol. 2008;294:H2344–H2351. doi: 10.1152/ajpheart.00053.2008. [DOI] [PubMed] [Google Scholar]
- 128.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 129.Semmler M, Seitz J, Erbe F, et al. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal Toxicol. 2004;16:453–459. doi: 10.1080/08958370490439650. [DOI] [PubMed] [Google Scholar]
- 130.Shah AP, Pietropaoli AP, Frasier LM, et al. Effect of inhaled carbon ultrafine particles on reactive hyperemia in healthy human subjects. Environ Health Perspect. 2008;116:375–380. doi: 10.1289/ehp.10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shieh KJ, Li M, Lee YH, Sheu SD, Liu YT, Wang YC. Antibacterial performance of photocatalyst thin film fabricated by defection effect in visible light. Nanomedicine. 2006;2:121–126. doi: 10.1016/j.nano.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Singer G, Granger DN. Inflammatory responses underlying the microvascular dysfunction associated with obesity and insulin resistance. Microcirculation. 2007;14:375–387. doi: 10.1080/10739680701283158. [DOI] [PubMed] [Google Scholar]
- 133.Smith RA. The beginning of a chemical climatology. London: Longmans, Green, and co; 1872. Air and rain. Ref Type: Generic. [Google Scholar]
- 134.Srinivas PR, Philbert M, Vu TQ, et al. Nanotechnology research: applications in nutritional sciences. J Nutr. 2010;140:119–124. doi: 10.3945/jn.109.115048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stahlhofen W, Rudolf G, James A. Intercomparison of experimental regional aerosol deposition data. J Aerosol Med. 1989;2:285–308. [Google Scholar]
- 136.Stewart JC, Chalupa DC, Devlin RB, et al. Vascular effects of ultrafine particles in persons with type 2 diabetes. Environ Health Perspect. 2010;118:1692–1698. doi: 10.1289/ehp.1002237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stone V, Johnston H, Clift MJ. Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions. IEEE Trans Nanobioscience. 2007;6:331–340. doi: 10.1109/tnb.2007.909005. [DOI] [PubMed] [Google Scholar]
- 138.Sun D, Meng TT, Loong TH, Hwa TJ. Removal of natural organic matter from water using a nano-structured photocatalyst coupled with filtration membrane. Water Sci Technol. 2004;49:103–110. [PubMed] [Google Scholar]
- 139.Tankersley CG, Bierman A, Rabold R. Variation in heart rate regulation and the effects of particle exposure in inbred mice. Inhal Toxicol. 2007;19:621–629. doi: 10.1080/08958370701353049. [DOI] [PubMed] [Google Scholar]
- 140.Tiwari R, Pathak K. Nanostructured lipid carrier versus solid lipid nanoparticles of simvastatin: Comparative analysis of characteristics, pharmacokinetics and tissue uptake. Int J Pharm. 2011 doi: 10.1016/j.ijpharm.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 141.Totlandsdal AI, Refsnes M, Skomedal T, Osnes JB, Schwarze PE, Lag M. Particle-induced cytokine responses in cardiac cell cultures--the effect of particles versus soluble mediators released by particle-exposed lung cells. Toxicol Sci. 2008;106:233–241. doi: 10.1093/toxsci/kfn162. [DOI] [PubMed] [Google Scholar]
- 142.Totlandsdal AI, Skomedal T, Lag M, Osnes JB, Refsnes M. Pro-inflammatory potential of ultrafine particles in mono- and co-cultures of primary cardiac cells. Toxicology. 2008;247:23–32. doi: 10.1016/j.tox.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 143.Ulrich MM, Alink GM, Kumarathasan P, Vincent R, Boere AJ, Cassee FR. Health effects and time course of particulate matter on the cardiopulmonary system in rats with lung inflammation. J Toxicol Environ Health A. 2002;65:1571–1595. doi: 10.1080/00984100290071676. [DOI] [PubMed] [Google Scholar]
- 144.Urch B, Silverman F, Corey P, et al. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.US Dept of Health and Human Services. A Report of the Surgeon General. 89–8411. Center for Chrominc Disease Prevention and Health Promotion. Office of Smoking and Health; 1989. Reducing the Health Consequences of Smoking: 25 Years of Progress; pp. 1–713. [Google Scholar]
- 146.van Eeden SF, Sin DD. Chronic obstructive pulmonary disease: a chronic systemic inflammatory disease. Respiration. 2008;75:224–238. doi: 10.1159/000111820. [DOI] [PubMed] [Google Scholar]
- 147.Vanwinkle BA, de Mesy Bentley KL, Malecki JM, et al. Nanoparticle (NP) uptake by type I alveolar epithelial cells and their oxidant stress response. Nanotoxicology. 2009;3:307–318. doi: 10.1080/17435390903121949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339. doi: 10.1152/physrev.00027.2008. [DOI] [PubMed] [Google Scholar]
- 149.Wallace L, Ott W. Personal exposure to ultrafine particles. J Expo Sci Environ Epidemiol. 2011;21:20–30. doi: 10.1038/jes.2009.59. [DOI] [PubMed] [Google Scholar]
- 150.Wallenborn JG, McGee JK, Schladweiler MC, Ledbetter AD, Kodavanti UP. Systemic translocation of particulate matter-associated metals following a single intratracheal instillation in rats. Toxicol Sci. 2007;98:231–239. doi: 10.1093/toxsci/kfm088. [DOI] [PubMed] [Google Scholar]
- 151.Wingard CJ, Walters DM, Cathey BL, et al. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology. 2010 doi: 10.3109/17435390.2010.530004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wong BA. Inhalation exposure systems: design, methods and operation. Toxicol Pathol. 2007;35:3–14. doi: 10.1080/01926230601060017. [DOI] [PubMed] [Google Scholar]
- 153.Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ J. 2006;70:129–140. doi: 10.1253/circj.70.129. [DOI] [PubMed] [Google Scholar]
- 154.Zanobetti A, Canner MJ, Stone PH, et al. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. doi: 10.1161/01.CIR.0000143831.33243.D8. [DOI] [PubMed] [Google Scholar]
- 155.Zhu MT, Wang B, Wang Y, et al. Endothelial dysfunction and inflammation induced by iron oxide nanoparticle exposure: Risk factors for early atherosclerosis. Toxicol Lett. 2011;203:162–171. doi: 10.1016/j.toxlet.2011.03.021. [DOI] [PubMed] [Google Scholar]