Abstract
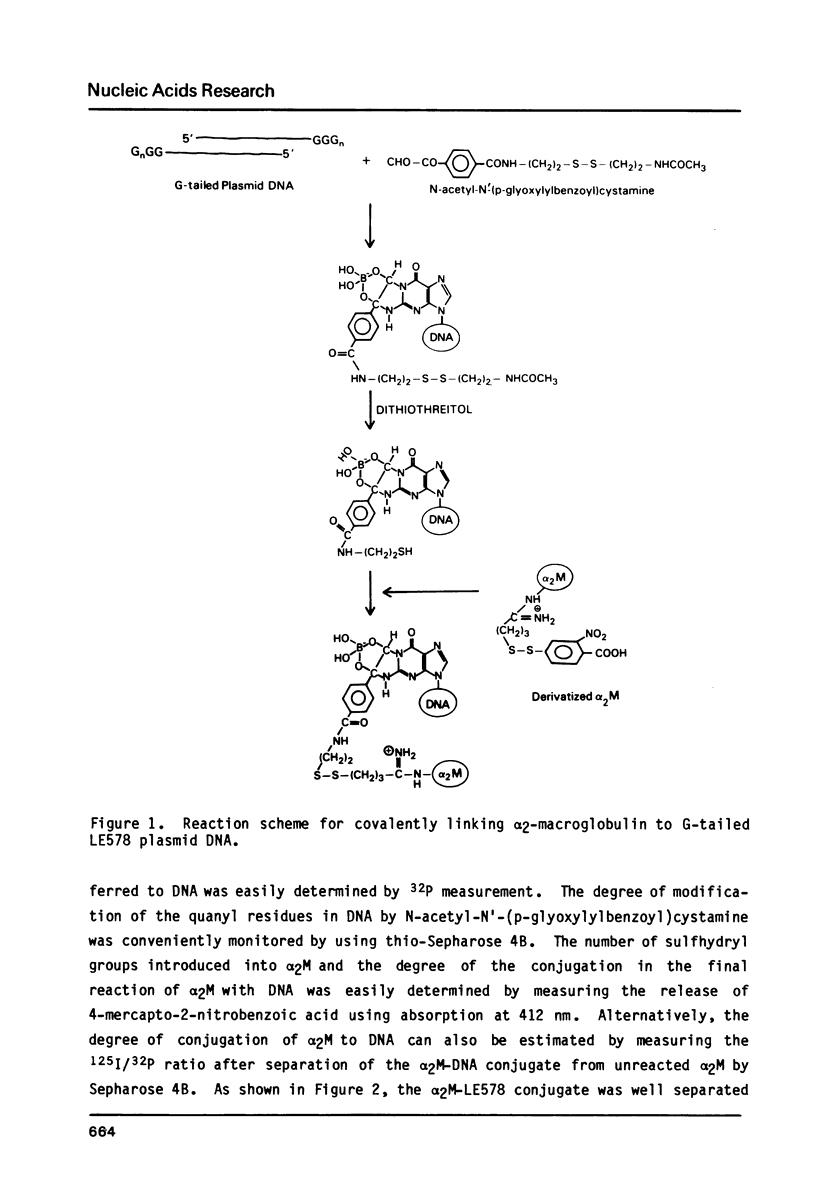
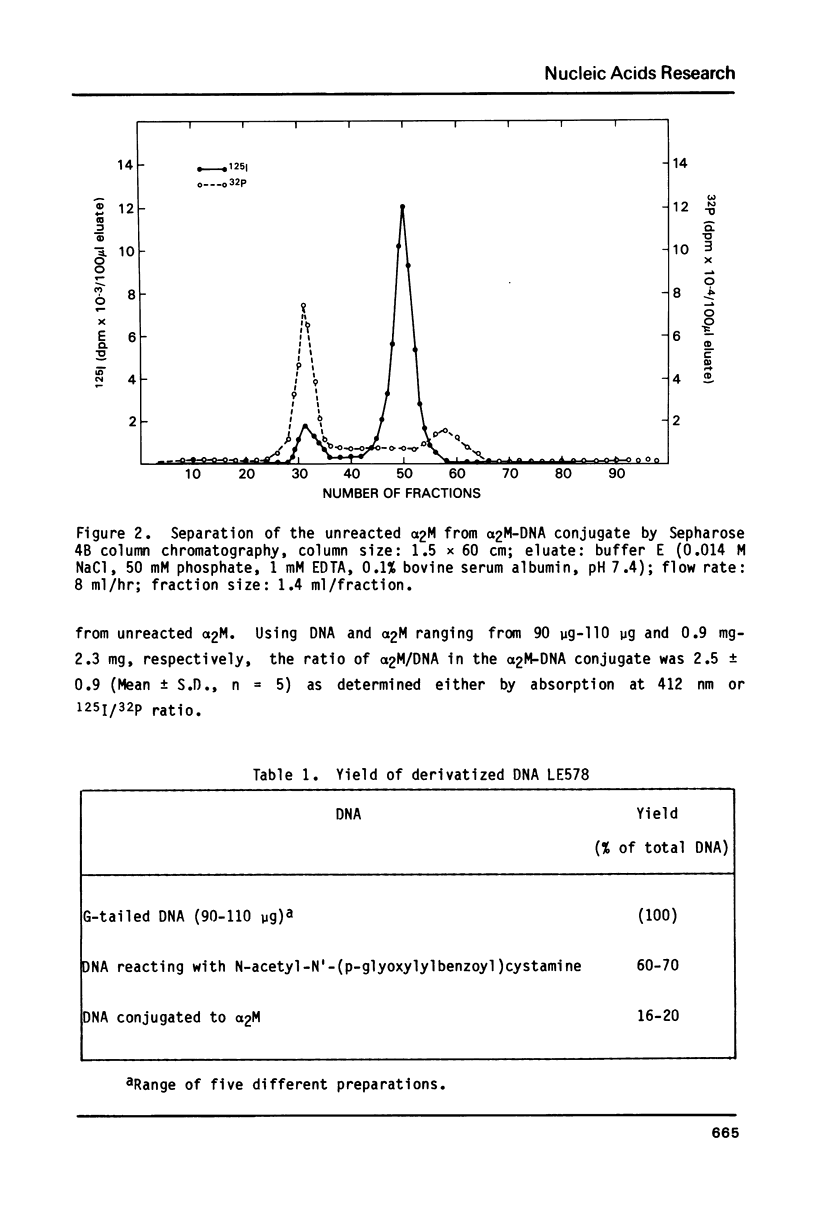
We describe a simple, general method to link proteins covalently to DNA. The method uses two reagents, N-acetyl-N'-(p-glyoxylylbenzoyl)cystamine and 2-iminothiolane. The former reacts specifically with nonpaired quanine residues and upon reduction generates a free sulfhydryl group. The latter reacts with a protein to provide another sulfhydryl group which is subsequently conjugated to DNA by an intermolecular disulfide interchange reaction. Using this method alpha 2-macroglobulin was conjugated to plasmid DNA encoding the Herpes simplex virus-1 thymidine kinase gene or a DNA fragment containing the E. coli chloramphenicol acetyltransferase gene. Up to 20% of the total DNA was conjugated to alpha 2-macroglobulin and the alpha 2-macroglobulin-DNA conjugate had a protein/DNA molar ratio of approximately two. The whole reaction takes place under very mild conditions in aqueous solution. The structure of DNA appears not to be significantly affected by the chemical modification. This method may prove useful in ligand directed gene transfer studies.
Full text
PDF








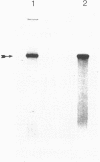
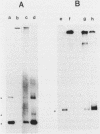
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bäumert H. G., Sköld S. E., Kurland C. G. RNA-protein neighbourhoods of the ribosome obtained by crosslinking. Eur J Biochem. 1978 Sep 1;89(2):353–359. doi: 10.1111/j.1432-1033.1978.tb12536.x. [DOI] [PubMed] [Google Scholar]
- Chapman N. M., Noller H. F. Protection of specific sites in 16 S RNA from chemical modification by association of 30 S and 50 S ribosomes. J Mol Biol. 1977 Jan 5;109(1):131–149. doi: 10.1016/s0022-2836(77)80049-1. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Nicolas J. C., Willingham M. C., Pastan I. Internalization of alpha 2 macroglobulin in receptosomes. Studies with monovalent electron microscopic markers. Exp Cell Res. 1981 Apr;132(2):488–493. doi: 10.1016/0014-4827(81)90127-0. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Vande Woude G. F., Wagner M., Smiley J. R., Summers W. C. Construction and characterization of a recombinant plasmid encoding the gene for the thymidine kinase of Herpes simplex type 1 virus. Gene. 1979 Nov;7(3-4):335–342. doi: 10.1016/0378-1119(79)90052-0. [DOI] [PubMed] [Google Scholar]
- Expert-Bezançon A., Hayes D. Synthesis and properties of N-acetyl-N'-(p-glyoxylylbenzoyl)cystamine, a new reagent for RNA-RNA and RNA-protein cross-linking. Eur J Biochem. 1980 Jan;103(2):365–375. doi: 10.1111/j.1432-1033.1980.tb04323.x. [DOI] [PubMed] [Google Scholar]
- Fiser I., Margaritella P., Kuechler E. Photoaffinity reaction between polyuridylic acid and protein S1 on the Escherichia coli ribosome. FEBS Lett. 1975 Apr 1;52(2):281–283. doi: 10.1016/0014-5793(75)80825-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan J. J., Noller H. F. Altered topography of 16S RNA in the inactive form of Escherichia coli 30S ribosomal subunits. Biochemistry. 1978 Feb 21;17(4):587–593. doi: 10.1021/bi00597a005. [DOI] [PubMed] [Google Scholar]
- Millon R., Olomucki M., Le Gall J. Y., Golinska B., Ebel J. P., Ehresmann B. Synthesis of a new reagent, ethyl 4-azidobenzoylaminoacetimidate, and its use for RNA-protein cross-linking within Escherichia coli ribosomal 30-S subunits. Eur J Biochem. 1980 Sep;110(2):485–492. doi: 10.1111/j.1432-1033.1980.tb04890.x. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Saksela O., Vaheri A. Synthesis and secretion of alpha-2-macroglobulin by cultured adherent lung cells. Comparison with cell strains derived from other tissues. J Clin Invest. 1977 Nov;60(5):1036–1045. doi: 10.1172/JCI108854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Chaires J. B. Functional modification of 16S ribosomal RNA by kethoxal. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3115–3118. doi: 10.1073/pnas.69.11.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Herr W. Letters to the editor: Accessibility of 5 S RNA in 50 S ribosomal subunits. J Mol Biol. 1974 Nov 25;90(1):181–184. doi: 10.1016/0022-2836(74)90266-6. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Topography of 16S RNA in 30S ribosomal subunits. Nucleotide sequences and location of sites of reaction with kethoxal. Biochemistry. 1974 Nov 5;13(23):4694–4703. doi: 10.1021/bi00720a003. [DOI] [PubMed] [Google Scholar]
- Oste C., Brimacombe R. The use of sym-triazine trichloride in RNA-protein cross-linking studies with Escherichia coli ribosomal subunits. Mol Gen Genet. 1979 Jan 5;168(1):81–86. doi: 10.1007/BF00267936. [DOI] [PubMed] [Google Scholar]
- Rinke J., Meinke M., Brimacombe R., Fink G., Rommel W., Fasold H. The use of azidoarylimidoesters in RNA-protein cross-linking studies with Escherichia coli ribosomes. J Mol Biol. 1980 Mar 5;137(3):301–304. doi: 10.1016/0022-2836(80)90318-6. [DOI] [PubMed] [Google Scholar]
- Rinke J., Yuki A., Brimacombe R. Studies on the environment of protein S7 within the 30-S subunit Escherichia coli ribosomes. Eur J Biochem. 1976 Apr 15;64(1):77–89. doi: 10.1111/j.1432-1033.1976.tb10276.x. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Apr;3(4):863–877. doi: 10.1093/nar/3.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAEHELIN M. Inactivation of virus nucleic acid with glyoxal derivatives. Biochim Biophys Acta. 1959 Feb;31(2):448–454. doi: 10.1016/0006-3002(59)90019-8. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Hachmann J. The reaction of guanine derivatives with 1,2-dicarbonyl compounds. Biochemistry. 1966 Sep;5(9):2799–2807. doi: 10.1021/bi00873a004. [DOI] [PubMed] [Google Scholar]
- Sperling J., Sperling R. Photochemical cross-linking of histones to DNA nucleosomes. Nucleic Acids Res. 1978 Aug;5(8):2755–2773. doi: 10.1093/nar/5.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickerhauser M., Hao Y. L. Large scale preparation of macroglobulins. Vox Sang. 1972 Jul-Aug;23(1):119–125. [PubMed] [Google Scholar]
- Willingham M. C., Maxfield F. R., Pastan I. H. alpha 2 Macroglobulin binding to the plasma membrane of cultured fibroblasts. Diffuse binding followed by clustering in coated regions. J Cell Biol. 1979 Sep;82(3):614–625. doi: 10.1083/jcb.82.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]