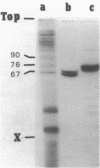
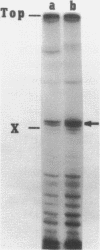
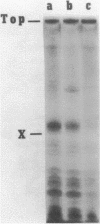
Abstract
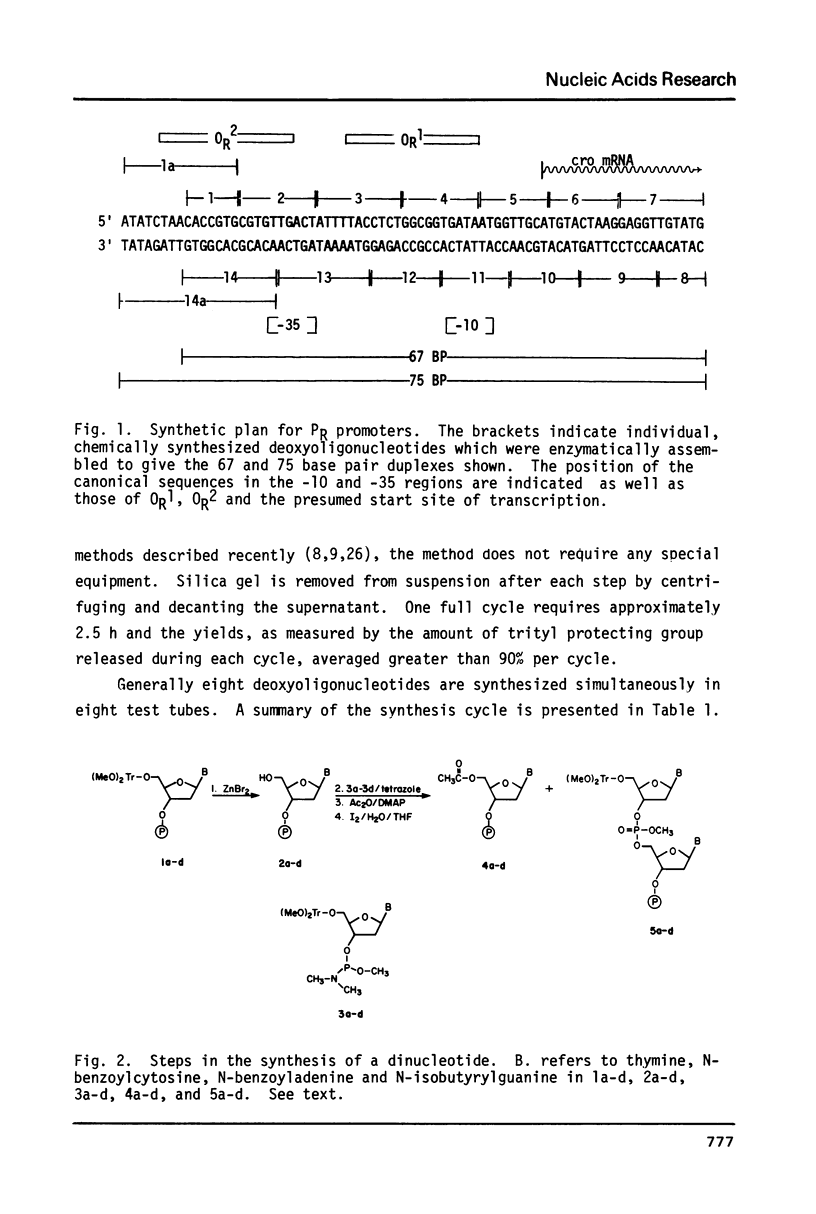
By a combination of chemical and enzymatic methods, a 75 base pair DNA duplex containing the sequence of the lambda PR promoter including the OR1 and OR2 cI repressor binding sites was synthesized. The solid support phosphite triester procedure (Caruthers, M. H. et al., Cold Spring Harbor Symposia on Quantitative Biology XLVII, in press) was used for the synthesis of oligonucleotides comprising the sequence. We report here an adaptation of the method of DNA synthesis in test tubes. Assembly of the oligonucleotides involved the use of T4 polynucleotide kinase and T4 DNA ligase. We show that the synthetic DNA is recognized by RNA polymerase and cI repressor in a manner identical to the same control region contained on a restriction fragment isolated from bacteriophage lambda DNA. Our synthetic approach using chemically synthesized promoter variants is thus suitable for studies probing the function of promoters.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarado-Urbina G., Sathe G. M., Liu W. C., Gillen M. F., Duck P. D., Bender R., Ogilvie K. K. Automated synthesis of gene fragments. Science. 1981 Oct 16;214(4518):270–274. doi: 10.1126/science.6169150. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Blattner F. R., Dahlberg J. E. RNA synthesis startpoints in bacteriophage lambda: are the promoter and operator transcribed? Nat New Biol. 1972 Jun 21;237(77):227–232. doi: 10.1038/newbio237227a0. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Cech C. L., Lichy J., McClure W. R. Characterization of promoter containing DNA fragments based on the abortive initiation reaction of Escherichia coli RNA polymerase. J Biol Chem. 1980 Mar 10;255(5):1763–1766. [PubMed] [Google Scholar]
- Cech C. L., McClure W. R. Characterization of ribonucleic acid polymerase-T7 promoter binary complexes. Biochemistry. 1980 May 27;19(11):2440–2447. doi: 10.1021/bi00552a023. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. J., Nierman W. C., Wiggs J., Neff N. A quantitative assay for bacterial RNA polymerases. J Biol Chem. 1979 Oct 25;254(20):10061–10069. [PubMed] [Google Scholar]
- Chow F., Kempe T., Palm G. Synthesis of oligodeoxyribonucleotides on silica gel support. Nucleic Acids Res. 1981 Jun 25;9(12):2807–2817. doi: 10.1093/nar/9.12.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrynin V. N., Korobko V. G., Severtsova I. V., Bystrov N. S., Chuvpilo S. A., Kolosov M. N. Synthesis of a model promoter for gene expression in Escherichia coli. Nucleic Acids Symp Ser. 1980;(7):365–376. [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. In vitro comparison of initiation properties of bacteriophage lambda wild-type PR and x3 mutant promoters. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6381–6385. doi: 10.1073/pnas.77.11.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Pabo C. O., Sauer R. T. Bacteriophage lambda repressor and cro protein: interactions with operator DNA. Methods Enzymol. 1980;65(1):839–856. doi: 10.1016/s0076-6879(80)65078-2. [DOI] [PubMed] [Google Scholar]
- Kawashima E., Gadek T., Caruthers M. H. Synthesis and biological activity of a lambda pseudo operator. Biochemistry. 1977 Sep 20;16(19):4209–4217. doi: 10.1021/bi00638a013. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McClure W. R., Cech C. L., Johnston D. E. A steady state assay for the RNA polymerase initiation reaction. J Biol Chem. 1978 Dec 25;253(24):8941–8948. [PubMed] [Google Scholar]
- McClure W. R. Rate-limiting steps in RNA chain initiation. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5634–5638. doi: 10.1073/pnas.77.10.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melançon P., Burgess R. R., Record M. T., Jr Nitrocellulose filter binding studies of the interactions of Escherichia coli RNA polymerase holoenzyme with deoxyribonucleic acid restriction fragments: evidence for multiple classes of nonpromoter interactions, some of which display promoter-like properties. Biochemistry. 1982 Aug 31;21(18):4318–4331. doi: 10.1021/bi00261a022. [DOI] [PubMed] [Google Scholar]
- Meyer B. J., Ptashne M. Gene regulation at the right operator (OR) of bacteriophage lambda. III. lambda repressor directly activates gene transcription. J Mol Biol. 1980 May 15;139(2):195–205. doi: 10.1016/0022-2836(80)90304-6. [DOI] [PubMed] [Google Scholar]
- Panet A., van de Sande J. H., Loewen P. C., Khorana H. G., Raae A. J., Lillehaug J. R., Kleppe K. Physical characterization and simultaneous purification of bacteriophage T4 induced polynucleotide kinase, polynucleotide ligase, and deoxyribonucleic acid polymerase. Biochemistry. 1973 Dec 4;12(25):5045–5050. doi: 10.1021/bi00749a003. [DOI] [PubMed] [Google Scholar]
- Ptashne M., Jeffrey A., Johnson A. D., Maurer R., Meyer B. J., Pabo C. O., Roberts T. M., Sauer R. T. How the lambda repressor and cro work. Cell. 1980 Jan;19(1):1–11. doi: 10.1016/0092-8674(80)90383-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U. RNA polymerase unwinds an 11-base pair segment of a phage T7 promoter. Nature. 1979 Jun 14;279(5714):651–652. doi: 10.1038/279651a0. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Stefano J. E., Gralla J. Lac UV5 transcription in vitro. Rate limitation subsequent to formation of an RNA polymerase-DNA complex. Biochemistry. 1979 Mar 20;18(6):1063–1067. doi: 10.1021/bi00573a020. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yansura D. G., Goeddel D. V., Caruthers M. H. Studies on gene control regions. 2. Enzymatic joining of chemically synthesized lactose operator deoxyribonucleic acid segments. Biochemistry. 1977 May 3;16(9):1772–1780. doi: 10.1021/bi00628a002. [DOI] [PubMed] [Google Scholar]